Abstract
The interactive effects of HIV-1 infection and methamphetamine (METH) abuse in producing cognitive dysfunction represent a serious medical problem; however, the neural mechanisms underlying this interactive neurotoxicity remain elusive. In this study, we report that a combination of low, sub-toxic doses of METH + HIV-1 Tat 1–86 B, but not METH + HIV-1 gp120, directly induces death of rodent midbrain neurons in vitro. The effects of D1- and NMDA-receptor specific antagonists (SCH23390 and MK-801, respectively) on the neurotoxicity of different doses of METH or HIV-1 Tat alone and on the METH + HIV-1Tat interaction in midbrain neuronal cultures suggest that the induction of the cell death cascade by METH and Tat requires both dopaminergic (D1) and N-methyl D-aspartate (NMDA) receptor-mediated signaling. This interactive METH+Tat neurotoxicity does not occur in cultures of hippocampal neurons, which are predominately glutamatergic, express very low levels of dopamine receptors, and have no functional dopamine transporter (DAT). Thus, the presence of a subpopulation of neurons capable of dopamine release/uptake is essential for METH+Tat induction of the cell death cascade. Overall, our results support the hypothesis that METH and HIV-1 Tat disrupt the normal conjunction of signaling between D1 and NMDA receptors, resulting in neural dysfunction and death.
Keywords: Cell culture, HIV-1 Tat, HIV-1 gp120, SCH23390, MK-801
Introduction
Consequences of methamphetamine (METH) use and HIV-1 infection are major public health problems in the world today (Reiner et al. 2009; Bao et al. 2010; Marshall and Werb 2010). Clinical studies indicate that HIV-1 infection (Grant 2008) and METH dependence (Gouzoulis-Mayfrank and Daumann 2009; Scott et al. 2007) are associated with neuropsychological deficits, suggesting that these factors in combination may produce additive or even synergistic adverse effects on neural function (Langford et al. 2003). While potential mechanisms of adverse independent effects of METH abuse or HIV-1 infection on neuronal functioning have been the subject of many experimental reports (Cadet and Krasnova 2007; Cadet and Krasnova 2009), fewer studies have been reported on the combined effects of METH and HIV-1 in the brain (Ferris et al. 2008; Rippeth et al. 2004). This cooperation between HIV-1 and METH may reflect common pathways to neural injury involving both cytotoxic and apoptotic mechanisms (Purohit et al. 2011).
Indeed, the attempt to integrate recent findings on the individual effects of METH or HIV to gain insight into potential converging pathways of their co-morbid effects on the brain has produced a fairly complex picture of METH+HIV-1 cooperation (Reiner et al. 2009). The major emphasis related to the co-morbid effect of METH and HIV-1 in the central nervous system (CNS) has been on the concurrent toxicity of METH and HIV-1 viral proteins (Cadet and Krasnova 2007). In particular, recent studies have focused on interactions between METH and either the HIV-1Tat protein, the key transactivating regulatory protein (Langford et al. 2004) or on HIV-1 gp120, a viral envelope glycoprotein (Banerjee et al. 2010; Bennett et al. 1995; Roberts et al. 2010).
In the brain, a primary action of METH is to elevate the levels of extracellular dopamine (DA) by redistribution of DA from synaptic vesicles (via vesicular monoamine transporter, VMAT2) to the neuronal cytoplasm and the reverse transport through the plasma membrane (via the membrane dopamine transporter, DAT) into the extracellular space (Raiteri et al. 1979; Riddle et al. 2006). Interestingly, our laboratory has found that HIV-1 Tat protein also influences DAT controlled uptake/release of dopamine, thereby producing alterations in functioning of the dopaminergic transmission system (Wallace et al. 2006; Ferris et al. 2009a; Zhu et al. 2009; Zhu et al. 2011) and synergistic Tat+cocaine interactions (Aksenov et al. 2006; Ferris et al. 2009b; Ferris et al. 2010). Thus, interactions between METH and Tat may also involve dopaminergic alterations (Ferris et al. 2008).
Although the understanding of these mechanisms remains incomplete, the involvement of aberrant glutamate transmission, mitochondrial disruption and the subsequent increased production of free radical species is evident (Riddle et al. 2006) in studies of METH neurotoxicity and in combined Tat+METH toxicity (Flora et al. 2003). Glutamate signaling may be an important component of METH-induced DAergic deficits. High doses of METH were shown to acutely activate the nigrostriatal pathway to increase glutamate release and mediate long-term DA toxicity in the striatum (Mark et al. 2004). Antagonists of NMDA receptors, such as MK-801 (dizocilpine) were reported to block METH neurotoxicity in mice by mechanisms independent from thermoregulation (Bowyer et al. 2001). The HIV-1 viral proteins Tat and gp120 also interact with the glutamate system, producing excitotoxic neural effects (Shin et al. 2011; Xu et al. 2011; Li et al. 2008). In sum, interactions between METH and Tat have also been found to involve glutamate signaling in producing neurotoxicity, in addition to dopaminergic interactions.
We hypothesized that the adverse effects of METH and HIV-1 proteins on dopaminergic and glutamatergic neurotransmission may converge/interact via a common substrate, leading to facilitated neurodegeneration. In the present study, two assessments were made: 1) the potential for low, individually non-cytotoxic, doses of METH, HIV-1 Tat or gp120 to produce cell death in combination, and 2) the involvement of D1 and NMDA receptors in combined METH + HIV-1 protein neurotoxicity. Our results suggest that interactions between dopamine and glutamate systems play a key role in the neurotoxicity associated with METH use in HIV-1 positive populations.
Materials and methods
Primary midbrain and hippocampal cell cultures were prepared from 18-day-old Sprague–Dawley rat fetuses as previously described (Aksenov et al. 2006; 2008; Aksenova et al. 2006; 2009). In brief, both the midbrain and hippocampal regions were rapidly microdissected and incubated for 15 min in a solution of 2 mg/ml trypsin in Ca2+- and Mg2+- free Hanks’ balanced salt solution (HBSS) buffered with 10 mM HEPES (Invitrogen, Carlsbad, CA). Cells were dissociated by trituration and distributed to poly-L-lysine coated culture plates wells containing DMEM/F12 medium (Invitrogen) supplemented with 100 mL/L fetal bovine serum (Sigma Chemicals, St. Louis, MO). After a 24 h period, the DMEM/F12 medium was replaced with 2 %v/v B-27 Neurobasal medium supplemented with 2 mM GlutaMAX and 0.5 % w/v D-(+) glucose (Invitrogen), which was continued throughout the experiment. All experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina.
Cultures were used for experiments at 14–21 days in vitro (DIV) and were >90 % neuronal as determined by anti-MAP-2/anti-GFAP/Hoechst fluorescent staining. Cell populations in primary rat fetal brain cultures were further characterized with standard immunocytochemical techniques (Aksenova et al. 2009) using the antibodies as detailed in Table 1 for the current study. For all immunocytochemistry experiments, cultures were fixed in 4 % paraformaldehyde for 10 min and washed with D-PBS 3 × 5 min. Following fixation, the cultures were treated with 10 % normal horse serum and processed with the appropriate primary antibodies (Table 1). Primary antibodies were incubated overnight at 4 °C. Cells were incubated with secondary antibodies for 1 h at room temperature and then washed 3 × PBS. The presence of microglia in the cultures was assessed using DyLight 594 labeled Tomato lectin (DL-1177, Vector Laboratories, Burlingame, CA). Hoechst was used as a general counterstain to identify cell nuclei in culture.
Table 1.
Information on primary antibodies
| Antibody | Host | Antibody type |
Source | Dilution | Study |
|---|---|---|---|---|---|
| Anti-MAP 2 | rabbit | polyclonal | Santa Cruz, CA | 1: 500 | Bertrand et al. 2010; Current study |
| Anti-GFAP | chicken | polyclonal | AbCam, MA | 1:5000 | Bertrand et al. 2010; Current study |
| Anti-TH | chicken | polyclonal | Aves, OR | 1:200 | Current study |
| Anti-DAT | rabbit | polyclonal | Chemicon, CA | 1:500 | Aksenov et al. 2008; Current study |
| Anti-NR1 | rabbit | monoclonal | Chemicon, CA | 1:500 | Aksenova et al. 2009; Current study |
| Anti-D1AR | mouse | monoclonal | Chemicon, CA | 1:200 | Current study |
Treatments of primary rat fetal neuronal cell cultures with METH, Tat, and gp120 was carried out by addition of freshly-prepared stock solutions (separately or in combination) or vehicle (control) into the cell culture growth medium. Recombinant Tat 1–86 B (LAI/Bru strain of HIV-1 clade B, GenBank accession no. K02013) was purchased from Diatheva (Italy). Recombinant full length gp120 LAV (T-tropic) was purchased from Protein Sciences (Meriden, CT). Methamphetamine was obtained from Sigma Chemicals (St. Louis, MO). A range of METH concentrations from 1 µM to 5 mM were used to determine the threshold for drug cytotoxicity in primary cultures of rat fetal neurons. Non-cytotoxic doses of Tat (10 nM) and gp120 (30 pM) were selected based on numerous previous studies using primary CNS cultures (Turchan et al. 2001; Aksenov et al. 2006, 2009; Aksenova et al. 2006, 2009).
Neuronal cell viability was assessed using a microplate reader formatted (Aksenov et al. 2006, 2008, 2009; Aksenova et al. 2006, 2009; Adams et al. 2010) variant of the Live/Dead assay (Molecular Probes, Inc., Eugene, OR). In brief, the cleavage product of calcein AM produces a green fluorescence (F530 nm) when exposed to 494-nm light and is used to identify live cells. Bound ethidium homodimer-1 produces a red fluorescence (F645 nm) when exposed to 528-nm light, allowing the identification of dead cells. Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). For each individual cell culture (well), ratios between corrected green and red fluorescence (F530 nm/F645 nm, Live/Dead ratios) were calculated. All individual relative numbers of live and dead cells were expressed in terms of percentages of average maximum Live/Dead ratio determined for the set of non-treated control cell cultures (8–16 wells) from the same plate: [F530 nm/F645 nm]well n/[F530 nm/F645 nm]average max × 100 %. All Live/Dead assays were conducted 72 h after initiating the cell culture treatments.
The co-localization of D1R and NMDAR in cultured rat fetal midbrain neurons was analyzed in midbrain cultures prepared in 24-well plates. Cultured neurons were fixed in 4 % paraformaldehyde, and analyzed using immunofluorescent double-labeling with anti-D1R and anti-NR1 antibodies. A mouse monoclonal antibody generated against the D1A dopamine receptor (MAB5290, Chemicon, Temecula, CA; 1:200) was used to immunolabel D1R. NMDAR immunolabeling was performed as previously described (Aksenova et al. 2009), using a rabbit monoclonal primary antibody specific for the NR1 subunit (AB1516, Chemicon, Temecula, CA; 1:500). Secondary antibodies were AlexaFluor Dye-conjugated IgGs (goat anti-rabbit AlexaFluor 488 (green) or anti-mouse Alexa Fluor 594 (red) Molecular Probes, Inc; Eugene OR) against the particular host animal (rabbit or mouse; 1:500). To assess the specificity of fluorescent signals in double-labeling experiments, one or both of the specific primary antibodies were excluded from the labeling procedure. Images were acquired via a CCD digital camera connected to the Nikon Eclipse 2000 inverted fluorescent microscope under 20X objective magnification and processed with NIS Elements (Nikon) software package. Assessment of cell populations (percentage of single vs. double labeling) was conducted using 3 captured images/well. The specificity of the anti-D1R and anti-NR1 primary antibodies were confirmed (using Western blots of both adult rat brain tissue extracts and cell culture extracts) prior to experimentation.
The involvement of D1 and/or NMDA receptors in METH or METH/HIV-1 protein neurotoxicity was tested using varying concentrations of D1R-specific (SCH23390) and NMDAR-specific (MK-801) antagonists. SCH 23390 and MK 801 were obtained from Sigma Chemicals (St. Louis, MO).
Statistical comparisons were made using ANOVA and planned comparisons were used to determine specific treatment effects. Significant differences were set at P<0.05. The time course of Tat and Tat + METH cell viability curves were best fit with four-parameter sigmoid equations (R2>0.99) using SigmaPlot 8.0 software package (Systat Software, Inc., San Jose, CA).
Results
METH cytotoxicity and the effect of concurrent exposure of primary rat fetal midbrain cell cultures to individually non-cytotoxic doses of METH and HIV-1 proteins (Tat or gp120) Midbrain primary cell cultures (14–21 DIV) were composed of 90–95 % neurons, 5 %–10 % astrocytes, (determined by anti-MAP2/anti-GFAP immunolabeling) and no microglia (as determined by DyLight 594 labeled tomato lectin). The midbrain neuronal population included tyrosine hydroxylase (TH)-positive (≈52 %), DAT-positive (22–25 %), and D1R-positive (≈84 %) cells.
The Live/Dead cell viability assay was carried out in midbrain cell cultures subjected to varying doses of METH (Fig.1). No statistically significant change in the viability of rat fetal midbrain neurons was detected when METH concentrations in the growth medium were less than 1 mM. However, the maximum METH dose (5 mM) resulted in a pronounced (≈50 %) decrease in neuronal viability 72 h after treatment.
Fig. 1.
Dose–response for METH toxicity in midbrain cell cultures. Cell viability measurements after 72-h treatment of midbrain cell cultures with varying concentrations (1 µM – 5 mM) of METH. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. *- indicates the significant (P<0.05) differences in the Live/Dead ratios (compared to control cultures)
The combination of 20 µM METH+10 nM Tat produced a statistically significant (P<0.05) decrease (≈20 %) in cell viability after 72 h of the exposure (Fig. 2a). In contrast, the treatment of midbrain cultures for 72 h with the combination of 20 µM METH+30 pM gp120 did not affect cell viability (Fig. 2b).
Fig. 2.
Combined sub-lethal doses of Tat + METH (a) or gp120+ METH (b) and cell viability. a Combined toxicity of 10 nM Tat+ 20 µM METH in midbrain cell cultures. Live/Dead ratios were measured after 72 h of treatment. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. *- indicates the significant (P<0.05) differences in the Live/Dead ratios (compared to control cultures). b The effect of 30 pM gp120+20 µM METH after 72 h of treatment. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. No significant changes in the Live/Dead ratios were observed
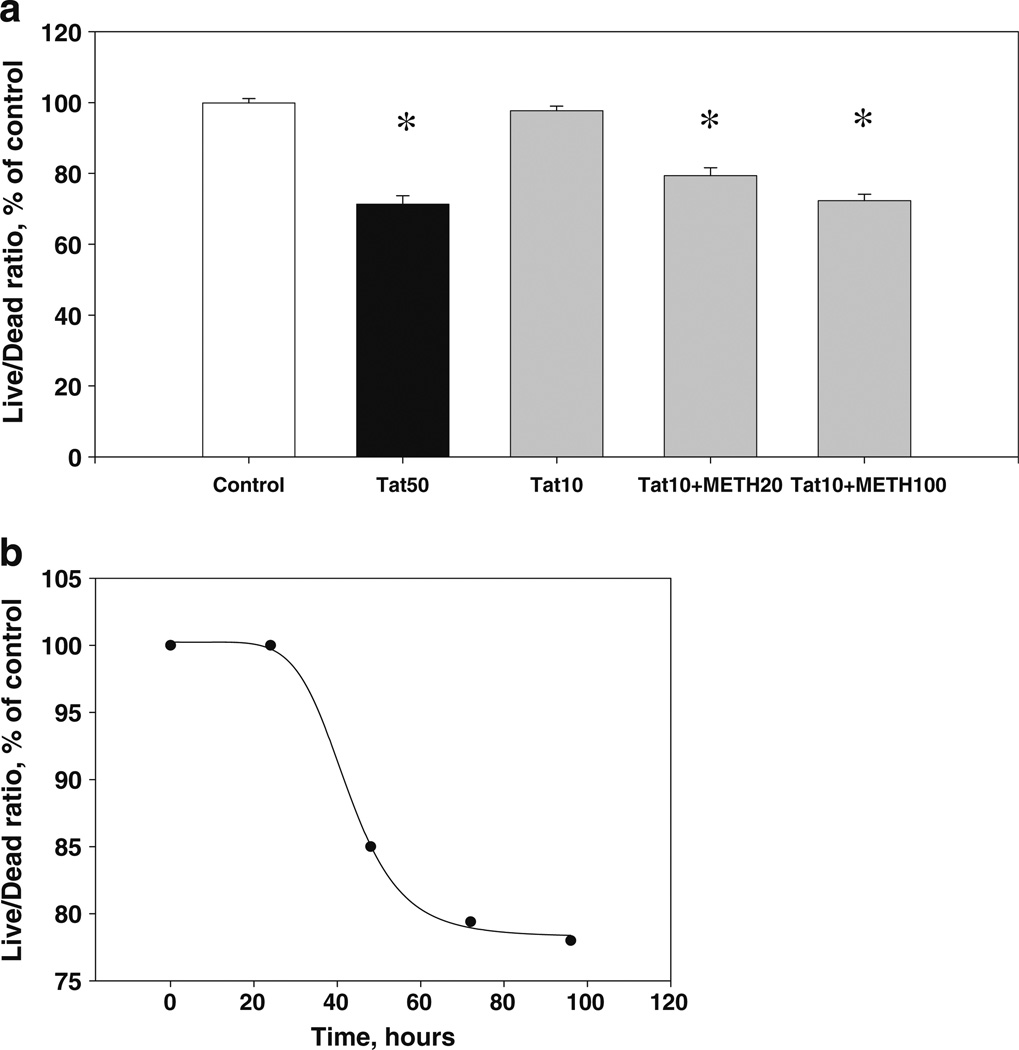
The time course of combined Tat + METH cytotoxicity in midbrain cell cultures Consistent with our previous results (Aksenov et al. 2009; Aksenova et al. 2009), the Live/Dead ratio in midbrain cultures treated for 72 h with 50 nM Tat 1–86 B was 71.3±2.4 % of control (P<0.05), which approximates the cell viability decrease produced by combination of individually non-cytotoxic doses of Tat and METH. The Live/Dead ratios measured after 72-h treatment of midbrain cells with the combination of 10 nM Tat +20 µM METH and 10 nM Tat +100 µM METH were 79.4±2.2 % and 72.3±1.8 % of control, respectively (Fig. 3a). The difference between the decrease in Live/Dead ratios induced by 72 h-long exposure of midbrain cells to either 10 nM Tat in combination with two different individually non-toxic doses of METH or by 50 nM Tat alone was not statistically significant (P>0.05). The time course for cell viability declines in midbrain cell cultures treated by either the combination of individually non-toxic doses of Tat and METH (10 nM Tat +20 µM METH) (Fig. 3b) was similar to that previously reported for 50 nM Tat alone (Aksenova et al. 2009).
Fig. 3.
Cell viability decrements resulting from a toxic dose of Tat and a combination of non-toxic doses of Tat + METH. a The maximal cytotoxic effect (after 72 h of treatment) of 50 nM Tat and combinations of 10 nM dose of Tat with sub-lethal (20 and 100 µM) doses of METH in midbrain cell cultures. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. *- indicates the significant (P<0.05) differences in the Live/Dead ratios (compared to control cultures). b The time course of Live/Dead ratio decline produced by 10 nM Tat+20 µM METH in midbrain cell cultures. Plot was best fit with a four-parameter sigmoid equation (R2>0.99)
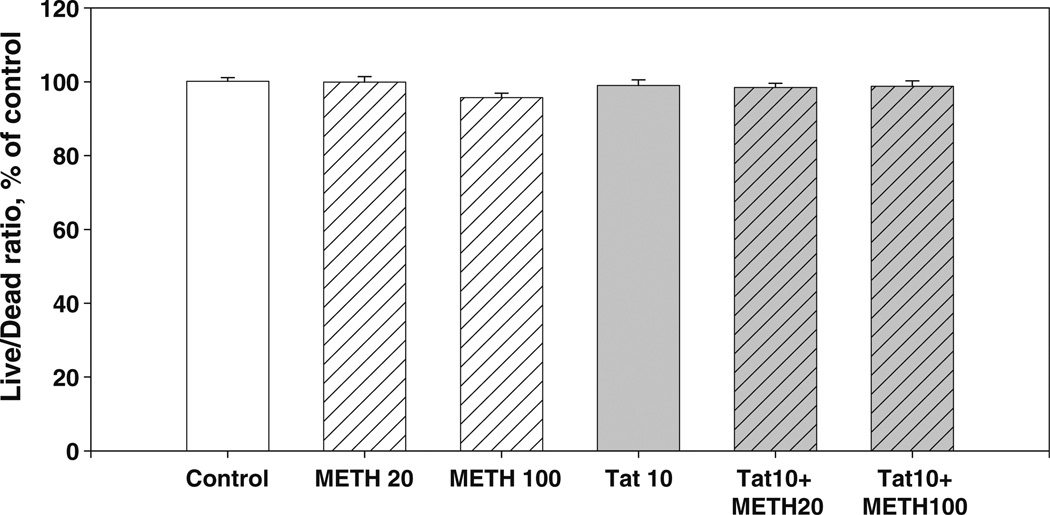
The effect of METH and Tat + METH combinations on the cell viability in hippocampal cell cultures To determine whether METH could potentiate Tat neurotoxicity in a hippocampal primary cell culture predominantly composed of glutamatergic neurons (Mattson 1988), we exposed 14–21 DIV-old hippocampal cell culture to METH (20 and 100 µM). The 10 nM Tat 1–86 B, 20 or 100 µM METH alone were not cytotoxic in this cell culture model. As shown in Fig. 6, neither the 20 µM METH+10 nM Tat, nor the 100 µM METH+10 nM Tat produced any significant alterations in cell viability in hippocampal cell cultures (Fig. 4).
Fig. 6.
Inhibition of combined Tat + METH toxicity by the D1R-selective antagonist SCH23390. The protective effect of 10 µM SCH23390 against decreased midbrain cell viability after 72-h exposure to 10 nM Tat + 20 µM METH. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. *- indicates the significant (P<0.05) differences in the Live/Dead ratios (compared to control cultures). SCH = SCH23390
Fig. 4.
Combined sub-lethal doses of Tat and METH and hippocampal cell viability. The effect of 10 nM Tat and (20 µM and 100 µM) METH after 72 h of treatment. Results (Live/Dead ratio, % of control) are presented as mean values ± SEM, n of sister cultures analyzed =8 per group. No significant changes in the Live/Dead ratios were observed in hippocampal cultures
Immunolocalization of NMDAR and D1R in midbrain cell cultures NR1 and D1R immunoreactivities overlap in a subset (≈30 %) of primary midbrain neurons (Fig. 5). Co-localization of NMDARs and D1Rs was observed in both the cell body and in the neuronal processes of midbrain cells (14–21 DIV).
Fig. 5.
Immunolocalization of NMDAR and D1R in midbrain neurons. Images of midbrain neurons showing colocalization of NR1 (green) and D1R (red), with Hoechst counterstained nuclei (blue). Midbrain cell cultures showed co-localization of NMDARs and D1Rs in cell bodies (left panels) and neuronal processes (right panels). Boxed selections were digitally magnified - scale bars: upper micrographs, 8 µm; lower micrographs, 20 µm
The effect of D1-selective antagonist, SCH 23390, on METH and Tat-induced cytotoxicity in midbrain cell cultures Exposure of 10 µM of SCH23390 (72 h) to midbrain cells was not toxic (Live/Dead ratio = 100±1.3 % of control). Cell cultures treated with 10 nM Tat + SCH23390 or with 20 µM METH + SCH23390 failed to exhibit any statistically significant changes in the Live/Dead ratios compared to control (99.4±1.2 % or 98.7±1.5 %, P>0.05). The 10 µM dose of SCH23390 inhibited (P<0.05) the decrease of Live/Dead ratios in cultures exposed to 10 nM Tat +20 µM METH (Fig. 6).
The effect of NMDAR-specific blocker, MK 801, on METH and Tat-induced cytotoxicity in midbrain cell cultures The expression of NMDAR subunits (NR1, NR2A, NR2B) was previously determined in midbrain cell culture lysates (Aksenova et al. 2009). Different (0.1, 1 and 10 µM) concentrations of MK801 alone or in combination with 10 nM Tat 1–86 B did not produce changes in midbrain cell viability following 72 h of exposure. Co-treatment of cultures with 0.1 or 1 µM MK 801 and 20 µM METH also did not affect the viability of midbrain neurons. However, the combination of 10 µM MK801 with 20 µM METH produced a statistically significant (P<0.05) decrease in Live/Dead ratios. The 0.1 and 1 µM doses of MK801 ameliorated the combined toxicity of 10 nM Tat + 20 µM METH (Fig. 7).
Fig. 7.
Inhibition of combined Tat + METH toxicity by the NMDAR-specific antagonist MK-801. The protective effect of 0.1 and 1.0 µM MK-801 against decreased midbrain cell viability after 72-h exposure to 10 nM Tat+20 µM METH. Results (Live/Dead ratio, % of control) presented as mean values ± SEM, n of sister cultures analyzed =8 per group. *- indicates the significant (P<0.05) differences in the Live/Dead ratios (compared to control cultures). MK = MK-801
Discussion
The current study found that the neurotoxic effects produced by combined (sub-lethal) concentrations of Tat 1–86+ METH in midbrain neuronal cell cultures are sensitive to changes in D1R- and NMDAR-dependent signaling. Moreover, the sub-lethal concentration (30 pM) of gp120, did not synergize with METH in our predominantly neuronal midbrain cell cultures (containing <10 % astrocytes and few microglia). The inability to observe direct gp120/METH combined cytotoxicity in a relatively pure neuronal rat fetal midbrain cell culture is consistent with the known importance of glial cells in producing gp120-induced neuronal apoptosis (Lipton 1994a, b). The cellular pathways to neuronal degeneration mediated by these two viral proteins have common steps, but are not identical. Gp120 has been shown to act primarily via binding to cytokine receptors, particularly, CXCR4, and its ability to cause neuronal death strongly depends on non-neuronal (astrocytic or microglial) cell responses (Meucci and Miller 1996; Dong and Xiong 2006).
Moreover, we found that low doses of METH were unable to enhance the effects of low dose of Tat in hippocampal cell cultures. The neuronal population in primary hippocampal cell cultures is predominantly glutamatergic (Mattson 1988). As such, primary hippocampal neurons exhibit very low levels of D1R- and DAT-specific ligand binding compared to primary midbrain neurons. At 14–21 DIV, 85–90 % of total cell population in rat fetal hippocampal cultures is composed of large pyramidal neurons (Bertrand et al. 2010). Hippocampal cultures are characterized by very low D1R-specific ligand binding (5±1 fmol/mg protein of [3H]SCH23390 (Silvers et al. 2007)). In contrast, the specific binding of [3H] SCH23390 was previously determined to be 82.6± 5 fmol/mg protein in our 14-day old midbrain cell cultures (Silvers et al. 2007). Thus, the current findings in hippocampal cell cultures indicate that the presence of glutamate signaling and a functional DA-dependent signaling system are necessary for the ability of sub-lethal doses of Tat and METH to cooperatively activate the cell death cascade.
The neurotoxic effects resulting from METH-induced changes in the DA uptake/release can be modulated by selective DA receptor ligands (Riddle et al. 2006). We previously reported that in rat fetal midbrain cultures -where the majority of neurons expresses DA receptors and a significant portion of the neuronal population possess fully-functional DAT protein - a 10 µM dose of SCH23390 prevented cell death induced by Tat 1–86 B (Silvers et al. 2007). Although Tat protein is not known to interact directly with D1R, Tat may influence DA receptor activities via direct inhibition of the DAT protein in vitro (Zhu et al. 2009; 2011) and in vivo (Ferris et al. 2009a, b), thereby altering synaptic DA concentrations(Ferris et al. 2010). Studies in primary cultures containing DAergic neurons suggested the existence of neuronal subsets selectively sensitive to neurodegeneration induced by Tat (Aksenova et al. 2009) or METH (Larsen et al. 2002).
Additionally, studies have suggested that METH can influence the functioning of the glutamatergic transmission system (Yamamoto et al. 1999; Mark et al. 2007; Simões et al. 2007; Swant et al. 2010). However, it is unclear exactly how METH alters glutamatergic transmission (Swant et al. 2010). The generally understood mechanism of METH action in the brain is linked to its ability to reverse the carrier-mediated dopamine (DA) uptake system (Raiteri et al. 1979). High doses of the drug can cause the selective damage and death of DA neurons via oxidative stress-mediated apoptosis (Larsen et al. 2002; Riddle et al. 2006). The free radical-mediated demise of DA neurons in vitro, in various CNS cell culture models, is typically observed at millimolar METH concentrations (Choi et al. 2002; Larsen et al. 2002; Jiménez et al. 2004; Kanthasamy et al. 2006; Kanthasamy et al. 2011). Indeed, we also found a significant decrease in cell viability occurred in midbrain neuronal cell cultures exposed to 1 mM or higher concentrations of METH; however, we found interactive toxicity with Tat at much lower METH doses. Direct interactions of the Tat cysteine-rich domain with the NMDAR (Song et al. 2003; Li et al. 2008) results in abnormal activation of the receptor complex and may lead to apoptotic death of Tat-affected neurons (Aksenova et al. 2009). The NR1 subunit of the NMDA receptor complex (which co-localized with D1R in the current study) is thought to play a critical role in D1R interactions with NMDA receptors (Jocoy et al. 2011); D1-NR1 physical interaction occurs via a direct protein-protein interaction mediated by the carboxyl tail of both receptors (Pei et al. 2004).
Most studies that have investigated cooperative cytotoxicity of METH and HIV-1 proteins have been conducted using CNS cell cultures (Langford et al. 2004; Cadet and Krasnova 2007; Cai and Cadet 2008; Nath et al. 2002). In contrast, fewer studies of METH + Tat/gp120 toxicity have been conducted in vivo. In a series of animal studies, stereotaxic microinjections of Tat and gp120 into the rat brain were accompanied by the exposure of animals to METH (Maragos et al. 2002; Theodore et al. 2006; Banerjee et al. 2010), producing neurotoxicity. Using transgenic animal models of HIV-1 protein expression, METH has been demonstrated to have neurotoxic properties and alter behavioral endpoints (Kass et al. 2010; Liu et al. 2009; Silverstein et al. 2011). Recent studies from our lab, suggest that METH can exacerbate behavioral deficits in HIV-1 transgenic animals (Moran et al. 2012). Specifically, we found prominent behavioral and neurochemical changes in the adolescent HIV-1 Tg rat, especially consequent to METH challenge. HIV-1 Tg animals that received METH displayed higher MAO-A protein expression and lower TH protein expression relative to control animals. Thus, expression of HIV-1 proteins in animals had significant effects on behavioral and neurochemical measures reflecting integrity of the DA system, and these alterations were especially evident consequent to in vivo METH challenge. These findings suggest that HIV-1 protein + METH interactions may occur in chronic exposure models. Long-term effects of HIV-1 Tat on D1 and NMDA receptor signaling might be anticipated to have a powerful influence on neuroadaptations and learning (Castner and Williams 2007); however, interactions between the D1R and NMDAR have yet to be examined in METH-treated HIV-1 transgenic animals.
The 20 µM concentration of METH that was used for the majority of experiments in this study was beneath threshold for inducing death of primary DA neurons (Larsen et al. 2002) and corresponds with the drug concentration limits reported in the study of unsupervised recreational METH users (Melega et al. 2007). Additionally, concentrations of Tat that do not induce cell death are comparable with reported circulating concentrations of these viral proteins in the blood of HIV-infected individuals – soluble Tat levels in HIV-1+ patients CSF and plasma have been reported as up to 40 ng/ml (Westendorp et al.; 1995; Xiao et al. 2000). These low Tat levels may be sufficient to activate NMDARs (Bennett et al. 1995; Self et al. 2009) thereby altering the release/uptake of catecholamines, particularly, DA (Wallace et al. 2006) and interact with METH to produce localized neurotoxicity.
In the present study, we found that approximately 30 % of our cultured midbrain neurons were immunolabeled for both D1 and NR1. D1 and NMDAR are known to co-localize extensively at synaptic, parasynaptic, and nonsynaptic sites in dendritic spines and shafts (Pickel et al. 2006). Direct physical interactions between D1 and NMDA receptors have been reported (Zhang et al. 2009; Nai et al. 2010; Pei et al. 2004). The existence of close interactions between D1R and NMDAR (Missale et al. 2010; Zorumski and Izumi 2012) indicates that alterations in control of DA uptake/release concurrently by METH and Tat may converge with the pathway of HIV protein-mediated NMDAR hyperactivation and, thereby, make D1R/NMDAR-expressing midbrain neurons a highly sensitive target for combined HIV-1 Tat+METH neurotoxicity. Moreover, given that NMDAR activation is key in regulating the number of D1 receptors present in dendritic spines (Scott et al. 2006), and that maintaining synaptic integrity is critical in preventing HIV-1 neurotoxicity (Bellizzi et al. 2006; Gelman et al. 2012; Ellis et al. 2007), the D1/NMDA complex may represent a novel target for HIV-1 neurotherapeutics.
In sum, we suggest that the conjunction of signaling between D1 and NMDA receptors contributes to HIV-1 Tat neurotoxicity in midbrain cell cultures that include significant numbers of D1/NR1-expressing neurons. Further investigation of altered D1R-NMDAR interactions in concurrent METH/HIV-1 neurotoxicity may help to understand the neural basis of cognitive deficits produced by HIV-1 and drugs of abuse such as methamphetamine.
Acknowledgements
This work was supported by NIH grants DA031604, DA011337, DA013137, HD043680.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Michael Y. Aksenov, Email: aksenov@mailbox.sc.edu.
Rosemarie M. Booze, Email: booze@mailbox.sc.edu.
References
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER-β mediates 17β-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse. 2010;64:829–838. doi: 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27(2):217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29(6):971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. Attenuated neurotoxicity of the transactivation-defective HIV-1 Tat protein in hippocampal cell cultures. Exp Neurol. 2009;219(2):586–590. doi: 10.1016/j.expneurol.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395(3):235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Exp Neurol. 2009;215(2):253–263. doi: 10.1016/j.expneurol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med. 2010;48(10):1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YP, Liu ZM, Lu L. Review of HIV and HCV infection among drug users in China. Curr Opin Psychiatry. 2010;23(3):187–194. doi: 10.1097/YCO.0b013e328338658b. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Shao-Ming L, Gelbard HA. Protecting the synapse: Evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmun Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995;705(1–2):168–176. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- Bertrand SJ, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Endogenous amyloidogenesis in long-term rat hippocampal cell cultures. BMC Neurosci. 2010;12:38. doi: 10.1186/1471-2202-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Holson RR, Miller DB, JP O’Callaghan. Phenobarbital and dizocilpine can block methamphetamine-induced neurotoxicity in mice by mechanisms that are independent of thermoregulation. Brain Res. 2001;919:179–183. doi: 10.1016/s0006-8993(01)03051-7. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12(3):181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai NS, Cadet JL. The combination of methamphetamine and of the HIV protein, Tat, induces death of the human neuroblastoma cell line, SH-SY5Y. Synapse. 2008;62(7):551–552. doi: 10.1002/syn.20512. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. Tuning the engine of cognition: A focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cognition. 2007;63:94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Yoo TM, Chung SY, Yang JS, Kim JI, Ha ES, Hwang O. Methamphetamine-induced apoptosis in a CNS-derived catecholaminergic cell line. Mol Cells. 2002;13(2):221–227. [PubMed] [Google Scholar]
- Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in Neuro-AIDS. Neurosci Biobehav Rev. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009a;63(3):181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat1–86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux micro-dialysis study. Neurosci. 2009b;159(4):1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115(4):885–896. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Henning B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neruol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9345-4. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of drugs of abuse-the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines. Dialogues Clin Neurosci. 2009;11(3):305–317. doi: 10.31887/DCNS.2009.11.3/egmayfrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20(1):33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Jordà EG, Verdaguer E, Pubill D, Sureda FX, Canudas AM, Escubedo E, Camarasa J, Camins A, Pallàs M. Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicol Appl Pharmacol. 2004;196(2):223–234. doi: 10.1016/j.taap.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Jocoy EL, Andre VM, Cummings DM, Rao SP, Wu N, Ramsey AJ, Caron MG, Cepeda C, Levine MS. Dissecting the contribution of individual receptor subunits to the enhancement of N-methyl-D-aspartate currents by dopamine D1 receptor activation in striatum. Front Sys Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00028. article 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy A, Anantharam V, Ali SF, Kanthasamy AG. Meth-amphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin-D in methamphetamine-induced apoptotic cell death. Ann N Y Acad Sci. 2006;1074:234–244. doi: 10.1196/annals.1369.022. [DOI] [PubMed] [Google Scholar]
- Kanthasamy K, Gordon R, Jin H, Anantharam V, Ali S, Kanthasamy AG, Kanthasamy A. Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Curr Neuropharmacol. 2011;9:49–53. doi: 10.2174/157015911795017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2010;5(4):566–573. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. HIV Neurobehavioral Research Group Patterns of selective neuronal damage in methamphetamine- user AIDS patients. J Acquir Immune Defic Syndr. 2003;34(5):467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol. 2004;10(6):327–337. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22(20):8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28:12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. HIV coat protein induces soluble neurotoxins in culture medium. Neurosci Res Commun. 1994a;15:31–37. [Google Scholar]
- Lipton SA. Ca2+, N -methyl-d-aspartate receptors, and AIDS-related neuronal injury. Int Rev Neurobiol. 1994b;36:1–27. doi: 10.1016/s0074-7742(08)60301-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4(3):309–316. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83(4):955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27(25):6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Werb D. Health outcomes associated with meth-amphetamine use among young people: a systematic review. Addiction. 2010;105(6):991–1002. doi: 10.1111/j.1360-0443.2010.02932.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988;472:179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61(4):216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. Gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16(13):4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Collo G, Spano P. The neurobiology of dopamine receptors: evolution from the dual concept to heterodimer complexes. J Recept Signal Transduct Res. 2010;30(5):347–354. doi: 10.3109/10799893.2010.506192. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012 doi: 10.2174/157016212802138788. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Li S, Wang SH, Liu J, Lee FJ, Frankland PW, Liu F. Uncoupling the D1-N-methyl-D-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biol Psychiatry. 2010;67(3):246–254. doi: 10.1016/j.biopsych.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Pei L, Lee FJS, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24(5):1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala. Neuroscience. 2006;142(3):671–690. doi: 10.1016/j.neuroscience.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine and HIV-associated neurocognitive disorders/HIV-associated dementia. 2011;44:102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Cerrito F, Cervoni AM, Levi G. Dopamine can be released by two mechanisms differentially affected by the dopamine transport inhibitor nomifensine. J Pharmacol Exp Ther. 1979;208(2):195–202. [PubMed] [Google Scholar]
- Reiner BC, Keblesh JP, Xiong H. Methamphetamine abuse, HIV infection, and neurotoxicity. Int J Physiol Pathophysiol Pharmacol. 2009;1:162–179. [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8(2):E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore JD, Gonzalez R, Wolfson IG. The HNRC Group. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Maung R, Sejbuk NE, Ake C, Kaul M. Alteration of methamphetamine-induced stereotypic behaviour in transgenic mice expressing HIV-1 envelope protein gp120. J Neurosci Methods. 2010;186:222–225. doi: 10.1016/j.jneumeth.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Nat Acad Sci. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JC, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Self RL, Smith KJ, Butler TR, Pauly JR, Prendergast MA. Intra-cornu ammonis 1 administration of the human immunodeficiency virus-1 protein trans-activator of transcription exacerbates the ethanol withdrawal syndrome in rodents and activates N-methyl-D-aspartate glutamate receptors to produce persisting spatial learning deficits. Neuroscience. 2009;163(3):868–876. doi: 10.1016/j.neuroscience.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA. Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01805.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: Involvement of D1 dopamine receptor. NeuroToxicology. 2007;28:1184–1190. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, Kumar A. Methamphetamine toxicity and its implications during HIV-1 infection. J Neurovirol. 2011;17(5):401–415. doi: 10.1007/s13365-011-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões PF, Silva AP, Pereira FC, Marques E, Grade S, Milhazes N, Borges F, Ribeiro CF, Macedo TR. Methamphetamine induces alterations on hippocampal NMDA and AMPA receptor subunit levels and impairs spatial working memory. Neuroscience. 2007;150(2):433–441. doi: 10.1016/j.neuroscience.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9(3):399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Swant J, Chirwa S, Stanwood G, Khoshbouei H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS One. 2010;5(6):e11382. doi: 10.1371/journal.pone.0011382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Steiner J, Young K, Maragos WF. Inhibition of tumor necrosis factor-alpha signaling prevents human immunodeficiency virus-1 protein Tat and methamphetamine interaction. Neurobiol Dis. 2006;23(3):663–668. doi: 10.1016/j.nbd.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1–72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59(1):51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Bae M, Tovar-y-Romo LB, Patel N, Bandaru VV, Pomerantz D, Steiner JP, Haughey NJ. The human immunodeficiency virus coat protein sp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci. 2011;31:17074–17090. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kitamura N, Lin XH, Ikeuchi Y, Hashimoto T, Shirakawa O, Maeda K. Differential changes in glutamatergic transmission via N-methyl-D-aspartate receptors in the hippocampus and striatum of rats behaviourally sensitized to methamphetamine. Int J Neuropsychopharmacol. 1999;2(3):155–163. doi: 10.1017/S1461145799001480. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu TX, Hallett PJ, Watanabe M, Grant SG, Isacson O, Yao WD. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J Neurosci. 2009;29(9):2948–2960. doi: 10.1523/JNEUROSCI.4424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3 H]dopamine uptake: dissociation of [3 H]dopamine uptake and [3 H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329(3):1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription (1–86) allosterically modulates dopamine transporter activity. Synapse. 2011;65(11):1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: Mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]