Abstract
Here, we report an interaction between blood and redox nanoparticles, prepared by self-assembly of amphiphilic block copolymers possessing 2,2,6,6-tetramethylpiperidine-N-oxyls as a side chain of hydrophobic segment. When 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl was added to rat whole blood, its electron spin resonance signal disappeared rapidly. In contrast, the signal from redox nanoparticles remained for a long period of time, indicating that nitroxide radicals were protected in the blood by their compartmentalization in the core of nanoparticle. Although most 2,2,6,6-tetramethylpiperidine-N-oxyls were located in the nanoparticle core, reactive oxygen species-scavenging activity was found outside of blood cells. For example, redox nanoparticles suppressed superoxide anion-induced hemolysis effectively, while 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl did not. It was revealed that redox nanoparticles were not internalized into the healthy blood cells, which was in sharp contrast to 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl. Due to its internalization into healthy platelets, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl induced mitochondrial dysfunction, while redox nanoparticles did not. Redox nanoparticles suppressed platelet adhesion and extended blood coagulation time, in contrast to 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl. These results indicate that redox nanoparticles scavenge reactive oxygen species outside of cells, but do not interfere with normal redox reactions inside of the cell. Based on these results, we determine that an anti-oxidative strategy based on nanotechnology is a rational and safe therapeutic approach.
Keywords: reactive oxygen species, nitroxide radicals, blood activation, redox nanoparticle, mitochondrial dysfunction
Introduction
Oxidative damage within living organisms is governed by the balance between the production of reactive oxygen species (ROS) and endogenous antioxidant-protective systems including vitamin C, vitamin E, glutathione, and antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, etc. Under normal physiological conditions, excessively generated ROS are eliminated by these antioxidant-protective systems. However, under conditions of oxidative stress, the production of ROS increases remarkably and endogenous antioxidants fail to scavenge all of ROS completely, resulting in severe injuries such as atherosclerosis,(1) diabetes,(2) renal failure,(3) Alzheimer’s disease,(4) and myocardial infarction.(5) One of the most plausible ways to prevent these oxidative stress injuries is the administration of an exogenous ROS scavenger. Currently, many ROS scavenging compounds have been reported. Most of these compounds are synthetic and/or natural low-molecular-weight (LMW) compounds. However, LMW ROS scavengers are known to result in various adverse effects within the body, which is hypothesized to be due to non-specific diffusion of LMW drugs throughout the entire body. In particular, LMW drugs are easily internalized into cells, which may result in cellular dysfunction. For example, it has been reported that 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL), which is one of the strongest LMW ROS scavengers, suppresses ROS-induced damage in vitro. However, it is known to induce strong adverse effects such as mitochondrial dysfunction and apoptosis due in part to the excessive uptake of LMW-TEMPOL in mitochondria, which can result in various non-essential redox reactions.(6,7) It has also been reported that when TEMPO derivatives are administered to animals, dramatic reduction in blood pressure can result.(8) As a result, the therapeutic effects of LMW ROS scavengers remain limited. In order to improve therapeutics against oxidative stress injuries, while suppressing their adverse effects, novel strategies must be developed.
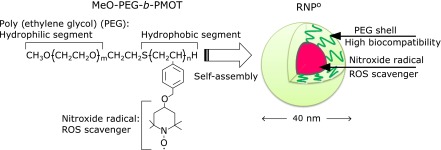
Recently, we have initiated the design of new redox-polymer therapeutics, in which nitroxide radicals were installed covalently to a polymer backbone as a side chain. The polymer which we have synthesized was an amphiphilic methoxy-poly(ethylene glycol)-b-poly[4-(2,2,6,6-tetramethylpiperidine-N-oxyl)oxymethylstyrene] (MeO-PEG-b-PMOT). The core-shell type polymeric micelles [Redox nanoparticles (RNPO)], which are prepared by self-assembling of MeO-PEG-b-PMOT in aqueous media, are several tens nanometers in diameter (Scheme 1). The nitroxide radicals, installed as a side chain to the hydrophobic segment in the block copolymer, were confined within the core of the nanoparticle. As a result, our therapeutic strategy using RNPs has thus far demonstrated remarkable protective and therapeutic effects on renal and cerebral ischemia-reperfusion injuries,(9,10) cerebral hemorrhage,(11) and ulcerative colitis(12) compared to LMW-TEMPOL. In addition, RNPs suppress the toxicity and adverse effects of LMW nitroxide radicals such as anti-hypertensive effects.(10,13) It has confirmed that RNPO have a blood half-life of approximately 18 h after its intravenous administration, which is 4320-fold longer than that of LMW-TEMPOL.(10) In general, it is well known that oxidative stress in blood can result in physiological abnormalities such as the activation of platelets, hemolysis, and blood coagulation. However, the behavior of RNPs or LMW-TEMPOL in blood has not been fully examined. To suppress oxidative stress in blood, a thorough investigation of the influences of RNPs and LMW-TEMPOL in blood is essential as well as blood circulation. In this study, we investigated the behavior of RNPO and LMW-TEMPOL in the blood by measurements of hemolysis, cellular uptake, mitochondrial activity, platelet adhesion, and blood coagulation.
Experimental Section
Synthesis of MeO-PEG-b-PMOT
MeO-PEG-b-PMOT block copolymer was synthesized as described previously.(10) Briefly, methoxy-poly(ethylene glycol)-b-poly(chloromethylstyrene) (MeO-PEG-b-PCMS) was synthesized by performing the radical telomerization of chloromethylstyrene with methoxy-poly(ethylene glycol)-sulphanyl (Mn = 5,000; NOF corporation, Tokyo, Japan) as a telogen. The polymer backbone of MeO-PEG-b-PCMS consisted of PEG for the hydrophilic segment and 14 repeating units of PCMS for the hydrophobic segment, as determined by using 1H NMR data. To obtain MeO-PEG-b-PMOT, chloromethyl groups on the PCMS segment of the block copolymer were converted to nitroxide radicals via Williamson ether synthesis of benzyl chloride in the MeO-PEG-b-PCMS block copolymer with the alkoxide of TEMPOL. After purification of the obtained MeO-PEG-b-PMOT, the substitution ratio of the modified TEMPO moieties per repeating unit of PCMS was 89.5%, as determined by electron spin resonance (ESR) with a standard curve generated from LMW-TEMPOL (MW of MeO-PEG-b-PMOT = 8,800) (the size exclusion chromatography and 1H NMR data of PEG-b-PMOT are described in supporting information as Supplemental Fig. 1*).
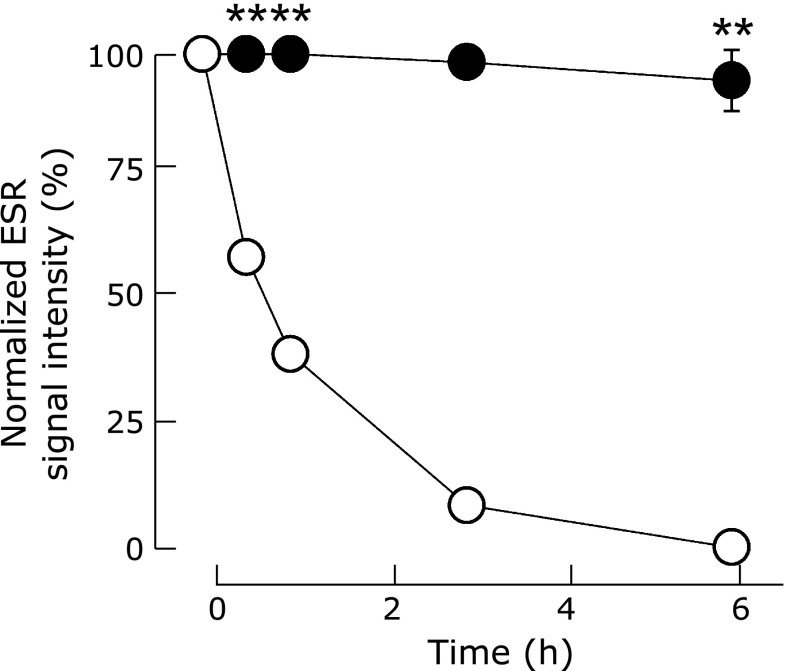
Fig. 1.
Time-course of ESR signal intensities of RNPO (closed circle) and LMW-TEMPOL (open circle) in rat whole blood after RNPO or LMW-TEMPOL was added to rat whole blood (The graphs represent means ± SE for four independent experiments. **p<0.01, Student’s t test).
Preparation of RNPO
RNPO were prepared from MeO-PEG-b-PMOT by using a dialysis method, as described previously.(10) Briefly, 90 mg of MeO-PEG-b-PMOT was dissolved in 6 ml of N,N-dimethylformamide, and the polymer solution was transferred into a membrane tube (molecular-weight cutoff size: 3,500; Spectra/Pro; Spectrum, Houston, TX) and dialyzed for 24 h against 2 l of water, which was changed after 2, 5, 8, and 20 h. Dynamic light scattering (DLS) measurements were carried out to determine the diameter of the obtained RNPO following dialysis (the DLS data are described in supporting information as Supplemental Fig. 2*).
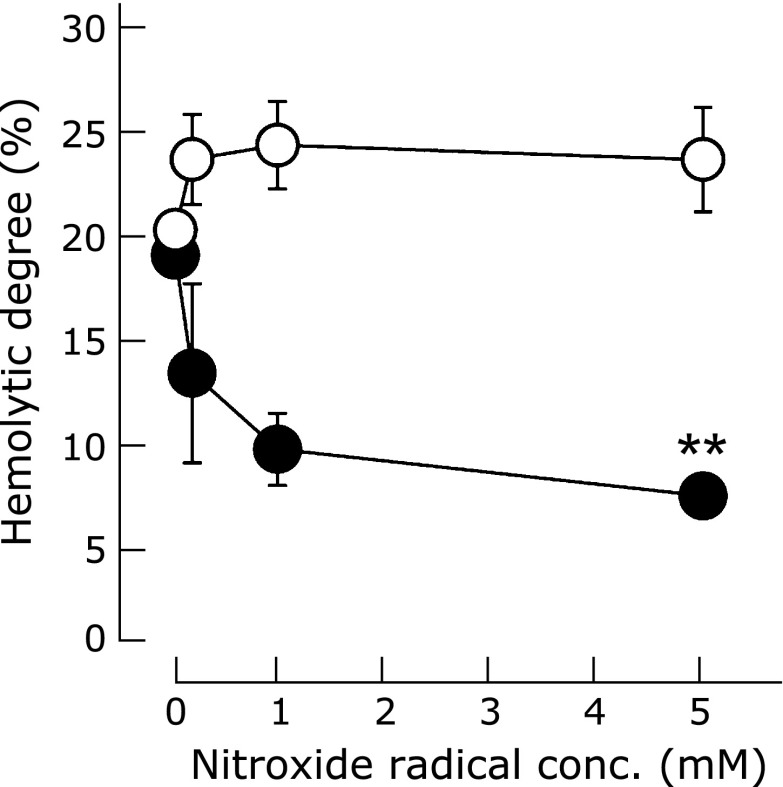
Fig. 2.
The suppressive effect of RNPO (closed circle) and LMW-TEMPOL (open circle) on superoxide anion-induced hemolysis (The graphs represent means ± SE for five independent experiments. **p<0.01, Student’s t test).
Animals
All experiments were performed according to the Guide for the Care and Use of Laboratory Animals at the University of Tsukuba. Male Sprague–Dawley rats (body weight, 150–250 g; age, 5–6 weeks old; Charles River, Yokohama, Japan) were used for all experiments, except for those involving blood coagulation times, which are described in detail in Experimental Section “Measurement of blood coagulation time”, and maintained in the experimental animal facilities at the University of Tsukuba. Rats were anesthetized initially with pentobarbital sodium (20–30 mg/kg) (Kyoritsu Seiyaku Corporation, Tokyo, Japan).
Blood sampling and isolation of red blood cells and platelets from rat whole blood
Collection of rat whole blood.
Blood (9 ml) was collected by performing cardiopuncture from outside the body by using syringes with heparin (Mochida Pharmaceutical, Inc., Tokyo, Japan) (50 IU/ml, 1 ml) except for the measurement of blood coagulation time (the experimental method of blood coagulation time are described in Experimental Section “Measurement of blood coagulation time”), transferred to a siliconized tube, and kept on ice until use, as described previously.(14) The final concentration of heparin was 5 IU/ml.
Isolation of red blood cells from rat whole blood.
Isolation of red blood cells (RBCs) from rat whole blood was carried out, according to the method previously reported.(15) Briefly, rat whole blood was centrifuged at 450 × g for 10 min at 5°C. The plasma and buffy coat layers, including platelets and white blood cells, were removed, and the RBCs were washed twice with saline (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) by centrifugation at 450 × g for 3 min at 5°C, followed by resuspension in fresh saline. The final RBCs concentration in saline was approximately 1 × 108 cells/ml.
Isolation of platelets from rat whole blood.
Isolation of platelets from rat whole blood was carried out, according to the method previously reported.(16) Platelet-rich plasma (PRP) was obtained by centrifugation rat whole blood at 200 × g for 10 min at 4°C. The obtained PRP sample was centrifuged at 1,000 × g for 8 min at 4°C and the supernatant was removed. The resulting pellet was washed twice with saline and resuspended in saline to obtain a platelet suspension containing 6.5 × 105 cells/µl.
Time profile of ESR signal intensity of nitroxide radicals of RNPO and LMW-TEMPOL in blood
A total of 240 µl of rat whole blood was added to 60 µl of RNPO or LMW-TEMPOL solution in saline. Following a predetermined incubation time, the mixture was transferred to a capillary tube, followed by measurement of the ESR signal at predetermined time points. The final nitroxide radical concentration of the RNPO was 1 mM. A LMW-TEMPOL solution with the same nitroxide radical concentration in saline was used as a control.
ESR measurement
The ESR signals were recorded at room temperature by using a Bruker EMX-T ESR spectrometer operating at 9.8 GHz with a 100 kHz magnetic field modulation. Signals were collected with the following parameters: sweep width, 200 G; microwave power, 2.0 mW; receiver gain, 1,000; time constant, 40.96 ms; and conversion time, 80 ms.
Hemolysis assay
To hemolyze RBCs by oxidative stress, a hypoxanthine (HX)-xanthine oxidase (XO) system was employed according to a previous report.(17,18) The isolated RBCs (6.4 × 106 cells/µl) were washed three times with ten-fold volumes of suspension buffer (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 5 mM sodium phosphate buffer, pH 7.0). A 1% suspension of washed RBCs was prepared in the suspension buffer. A total volume of 47 ml of the RBCs suspension was placed in a 50 ml centrifuge tube, and 150 µl of 2 units/ml XO (Sigma-Aldrich Japan, Tokyo, Japan) and 2.75 ml of 5 mM HX (Sigma-Aldrich Japan) were added to the suspension. Final concentrations of XO and HX were 6.0 × 10−3 unit/ml and 0.275 mM, respectively. After the suspension was incubated at 37°C in a humidified 5% CO2 incubator for 1 h, 450 µl of the hemolyzed RBCs suspension was placed into a 2 ml tube, and 50 µl of RNPO or LMW-TEMPOL solution in saline was added to the hemolyzed RBCs suspension. After incubation for 2 h, the suspension was centrifuged at 2,000 × g for 10 min at 4°C and the optical density (OD) at 524 nm of the supernatant was measured by using a UV–vis spectrophotometer (Varioskan Flash; Thermo Fisher Scientific Inc., Tokyo, Japan).
Hemolytic degree (HD) of each sample was determined as follow:
HD (%) = [OD – ODsample]/[ODpositive control] × 100,
where ODpositive control was defined as the OD of hemolyzed RBCs supernatant with the addition of distilled water instead of RNPO or LMW-TEMPOL solution in saline at 524 nm, whereas the ODsample was defined as the OD of RNPO and LMW-TEMPOL solution in saline at 524 nm, respectively.
In vitro cellular uptake studies
Rat whole blood (250 µl) was incubated at 37°C with 250 µl of either RNPO or LMW-TEMPOL solution in saline. After a predetermined incubation time, blood cells were separated by centrifugation at 4,500 rpm for 3 min at 4°C. Supernatant (200 µl) was collected from each sample, and its ESR signal was measured following re-oxidation by adding 100 µl of 200 mM K3[Fe(CN)6] (Kanto Chemical, Tokyo, Japan). The uptake into blood cells was expressed relative to ESR signal intensity of RNPO or LMW-TEMPOL without blood cells.
Platelet mitochondrial activity
Platelet mitochondrial activity was determined by the analysis of mitochondrial membrane potential (MMP), which was measured by staining platelets with the lipophilic cationic dye, 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (Biotium, Inc., Tokyo, Japan). A prepared platelet suspension (2 × 106 cells) was incubated with 50 µl of RNPO, LMW-TEMPOL solution in saline, which is an equivalent nitroxide radical concentration, or saline as a control in vial at 37°C for 6 h. After incubation, the supernatant was removed by centrifugation at 4,000 × g for 5 min at room temperature, and the platelets were resuspended in 500 µl of JC-1 reagent solution. Platelets were incubated at 37°C in a humidified 5% CO2 incubator for 15 min. After incubation, the supernatant was removed by centrifugation at 4,000 × g for 5 min at room temperature, and the platelet pellet was washed twice with saline. Platelets were then resuspended in 500 µl of saline, followed by measurement of fluorescence by using a fluorescent spectrophotometer.(19) Both red fluorescence (Ex/Em, 550/600 nm) and green fluorescence (Ex/Em, 485/535 nm) were measured.
Adhesion of platelets on glass beads surfaces
Glass beads (70 mg, 105–125 µm diameter; As one, Tokyo, Japan), which were washed with methanol by using a Soxhlet apparatus for 12 h, followed by vacuum drying overnight, were added to a siliconized tube (Sarstedt, Inc., Tokyo, Japan). RNPO, LMW-TEMPOL solution in saline or saline as a control (30 µl) and 300 µl of heparinized rat whole blood were added to each tube, followed by incubation at room temperature for 30 min with gentle rotation (1 rpm). After incubation, 100 µl of each blood sample was transferred to an EDTA-coated tube and then placed on ice, followed by a measurement of the number of platelets by using a fully automatic hematology analyzer (Celltac α, MEC-6318; Nihon Kohden Co., Tokyo, Japan). Relative platelet count (%) is expressed as the value relative to that without beads under the same experimental procedures.
To observe the surfaces of glass beads following incubation with blood, scanning electron microscopy (SEM) (JSM-5610, JEOL, Tokyo, Japan) was employed. The glass beads were incubated with heparinized rat whole blood for 30 min and rinsed with phosphate-buffered-saline (PBS) three times to remove weakly adherent blood cells, and subsequently the adherent blood cells were fixed with a glutaraldehyde solution (Kanto Chemical) (1.25 vol%) in PBS at room temperature for 30 min. The beads were then dehydrated by treating with a graduated ethanol/distilled water series from 50% to 100% ethanol in steps of 10% for 30 min, respectively. Following dehydration, the specimens were rinsed in a mixture (50%/50%) of ethanol/2-methyl-2-propanol (Wako Inc., Tokyo, Japan) for 15 min, followed by incubation with 2-methyl-2-propanol twice for 15 min. The resulting specimens were freeze-dried overnight and then gold-coated by using an ion sputter coater, followed by measurement by SEM under the conditions of an accelerating voltage of 5 kV and magnifications of ×230.
Measurement of blood coagulation time
A blood coagulation test was performed by using male ICR mice (body weight, 32–35 g; age, 6 weeks old; Charles River). Time of blood coagulation was determined as the time required to coagulate blood in vial. A volume of 100 µl of RNPO, LMW-TEMPOL with an equivalent nitroxide radical concentration in RNPO, or saline as a control was added to a 1 ml disposable syringe (TERUMO, Tokyo, Japan). Using the syringe with sample, 900 µl of whole blood was obtained from the inferior vena cava of mice. This blood was then added to 2 ml tubes and incubated at 37°C. It should be noted that heparin was not used in this experiment. The extent of blood coagulation was observed by tapping the tube at a 90° angle.
Statistical analysis
All values are expressed as mean ± SE. Differences between groups were examined for statistical significance by using Student’s t test and ANOVA with Bonferroni post hoc test (SPSS software; IBM Corp, Armonk, NY). p<0.05 was considered significant for all statistical analyses.
Results and Discussion
Reduction of nitroxide radicals in blood
Nitroxide radicals are chemically synthesized organic compounds possessing an unpaired electron, which results in paramagnetic properties and redox reactions.(20) They are known to have numerous applications in biology such as ROS scavengers,(21) and ESR spectroscopic probes(25,26) that are used as spin label/oxymetry and ESR and magnetic resonance (MR) imaging agents.(22) In particular, LMW-TEMPOL is the most extensively utilized nitroxide radical for versatile applications. LMW-TEMPOL has strong redox reactivity,(23) which results in ROS scavenging including superoxide anions, hydroxyl radicals, alkylperoxyl radicals, and so on.
Since most areas of the body are under the reducing conditions,(24,25) TEMPO derivatives are known to be easily reduced to hydroxylamine forms, which has no ESR signal, by endogenous reducing agents such as ascorbic acid and glutathione in vivo.(26) Importantly, ROS scavenging activity of hydroxylamine form is reduced to one-fifth that of a nitroxide radical form.(26) We have previously confirmed that the nitroxide radicals in RNPs show a reduction resistance even in the presence of 3.5 mM ascorbic acid, due to the confinement of nitroxide radicals in the solid core of RNPs, while LMW-TEMPOL is reduced rapidly.(24)
To investigate the profiles of nitroxide radicals in the hydrophobic core of RNPO in blood, ESR signals of RNPO in rat whole blood was measured. As shown in Fig. 1, 60% of the ESR signal of LMW-TEMPOL rapidly decreased within 1 h, and completely disappeared within 6 h in rat whole blood. In contrast, the ESR signal of RNPO did not decrease significantly by 6 h. It should be noted that the broad ESR signal of RNPO was still observed by 6 h, indicating confinement of nitroxide radicals in the hydrophobic core of RNPO in blood (Supplemental Fig. 3* in Supporting Information).
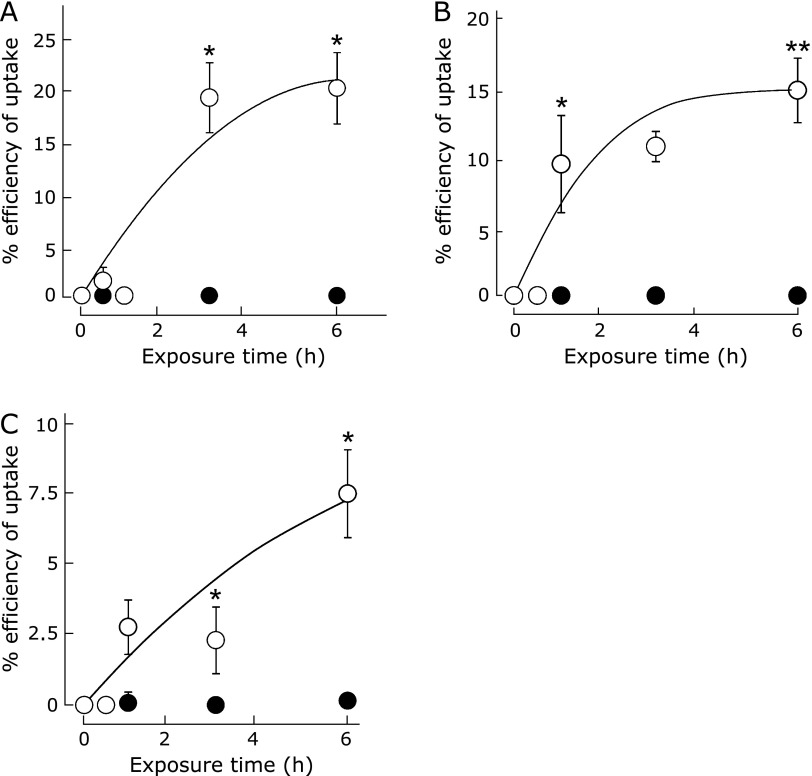
Fig. 3.
The cellular uptake of RNPO (closed circle) and LMW-TEMPOL (open circle) in (A) rat whole blood cells, (B) isolated RBCs and (C) isolated platelets (The graphs represent means ± SE for four independent experiments. *p<0.05 and **p<0.01, Student’s t test).
Hemolysis assay
As stated above, we have confirmed that the ESR signal of RNPO was observed for long period of time even in rat whole blood. The next question is how RNPO works under conditions of oxidative stress in blood. Under oxidative stress conditions, damage to RBCs causes hemolysis,(27,28) which releases hemoglobin from the RBCs due to instability of the cell membrane.(27) Here, the effects of RNPO and LMW-TEMPOL on ROS-induced hemolysis were investigated. The HX/XO system, which generates superoxide anions, was employed to induce hemolysis by oxidative stress. As shown in Fig. 2, the extent of hemolysis caused by the HX/XO system was not changed when LMW-TEMPOL was added to hemolyzed RBCs. Though small amount of ROS scavenging activity was remained under the present conditions, it might not affect the hemolysis in this study. In contrast, RNPO dramatically prevented hemolysis in a dose-dependent manner. Taken together with the above section, it was confirmed that nitroxide radicals, which exist in the hydrophobic core of the RNPs, are not easily reduced by reducing agents such as ascorbic acid, but rather strongly scavenge HX/XO-induced superoxide anions. As a result of this character, it was found that RNPO prevented ROS-induced hemolysis effectively. Therefore, these results indicate that the gaseous superoxide anion diffuses into the hydrophobic core of RNPO and reacts with nitroxide radicals, while hydrophilic ascorbic acid might not penetrate into the hydrophobic core of RNPO.
Cellular uptake of RNPO
Here, the cellular uptakes of RNPO and LMW-TEMPOL into blood cells were carried out. LMW-TEMPOL is a cell membrane-permeable compound(29); this might be attributed to an inessential redox reaction of LMW-TEMPOL in mitochondria because LMW-TEMPOL induces inhibition of the activity of complex I, and it contributes to ROS formation in the mitochondria.(6) In fact, it has been reported previously that LMW-TEMPOL reduces the activity of the electron transfer chain in mitochondria and induces apoptosis.(6) Since nitroxide radicals in RNPO are covalently conjugated to polymers with about 9 kDa, it is hard to internalize cell interior across cell membranes. It should be noted that there is no risk of leakage of LMW nitroxide radicals in vivo. Moreover, self-assembled nanoparticles are anticipated to avoid internalization in mitochondria, through the cell membrane, due to their size. If RNPO avoids uptake into blood cells, it may not cause mitochondrial dysfunction.
As shown in Fig. 3A, the cellular uptake of LMW-TEMPOL increased in a time-dependent manner, and it was confirmed that a 20% uptake of LMW-TEMPOL was attained by 6 h. On the contrary, no uptake of RNPO into blood cells was observed under the same conditions. This tendency was further confirmed by similar experiments with isolated RBCs and platelets viz.; internalization of RNPO into these healthy blood cells was not observed as shown in Fig. 3B and C, while an uptake of 15% and 7.5% LMW-TEMPOL was attained by 6 h into RBCs and platelets, respectively. Several tens nanometer size and PEG shell layer might prevent internalization in healthy blood cells. PEG, which is the hydrophilic segment of PEG-b-PMOT, is one of the most widely used polymers as remarkable biocompatible materials,(30) which possesses the ability to repel biomolecules, including cell surfaces, due to its strong hydration ability,(31) conformational flexibility,(32) and repulsive force of the PEG tethered chain.(33) In addition, size of several nanometers reduces the permeability of RNPO, which is in sharp contrast to the LMW compound. Such characters might prevent internalization to blood cells. Taken together with the results of the suppression of superoxide anion-induced hemolysis, these findings suggest that RNPO must scavenge ROS outside of the blood cells.
Platelet mitochondrial activity
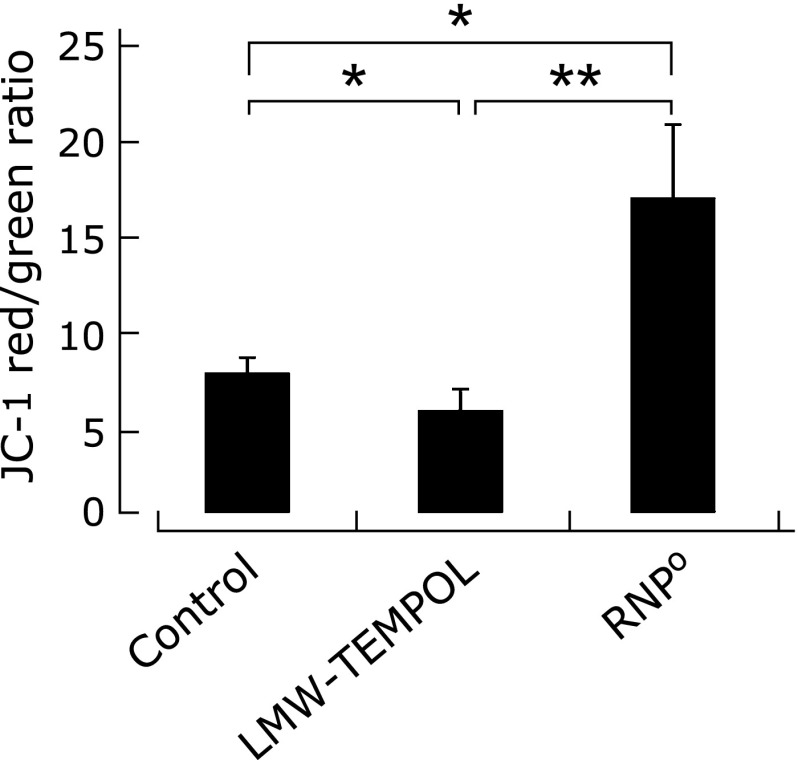
It has been reported that LMW-TEMPOL reduces mitochondrial activity due to internalization into healthy cells. To examine whether RNPO suppresses reduction in mitochondrial activity, mitochondrial activity of platelets was measured compared to LMW-TEMPOL, by using JC-1 dye, which has been used as an indicator of MMP in a variety of cell types. JC-1 shows intense red fluorescence in healthy cells with high MMP, while it shows green fluorescence in apoptotic or unhealthy cells with low MMP.(34) As shown in Fig. 4, it was confirmed that, when platelets were exposed to LMW-TEMPOL, its red/green ratio of fluorescent intensity of JC-1 was lower than that of control; this means that LMW-TEMPOL induces mitochondrial dysfunction. In contrast, in the case of RNPO, its red/green ratio of fluorescent intensity of JC-1 was higher than that of LMW-TEMPOL, indicating that RNPO does not significantly cause mitochondrial dysfunction, unlike LMW-TEMPOL. Note that the red/green ratio of JC-1 into RNPO treated platelet is significantly greater than that of control. We emphasize that the isolation of platelets was carried out according to the previously reported method.(35–37) The possibility exists that under these incubation conditions, platelet mitochondrial activity without any treatments might gradually decrease due to oxidative stress and effective ROS scavenging activity of RNPO suppresses this oxidative stress under incubation.
Fig. 4.
Measurement of mitochondrial membrane potential of platelet after treatment of RNPO, LMW-TEMPOL solution or saline as a control, as determined by JC-1 staining (The graphs represent means ± SE for five independent experiments. *p<0.05 and **p<0.01, Bonferroni post hoc test).
Suppression of platelet activation
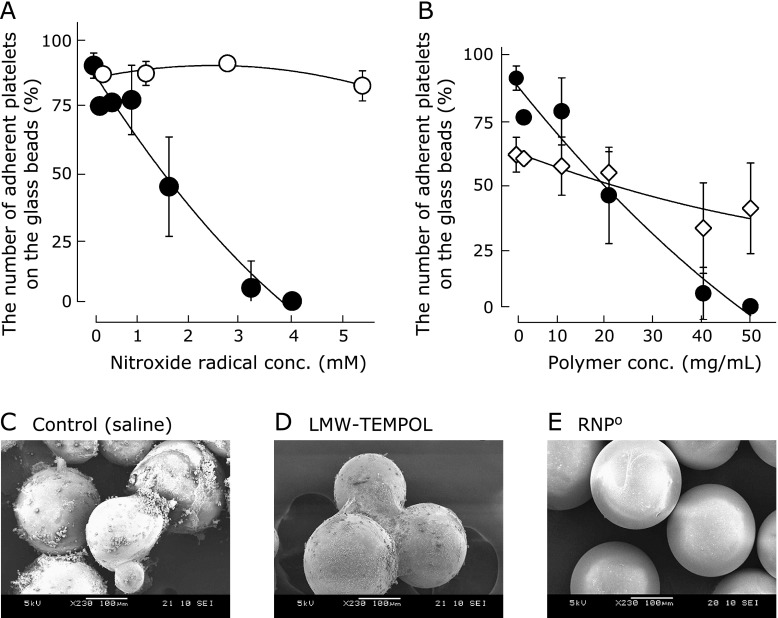
It has been reported that superoxide anions and hydroxyl radicals promote platelet adhesion to thrombin and result in platelet aggregation.(38) In our previous report, it has been proved that a glass bead surface coated with the nitroxide radical-containing polymer results in the activation of blood cells and suppressed blood coagulation due to the scavenging of ROS.(14) Therefore, to investigate the suppressive effect of RNPO on platelet activation, rat whole blood was incubated with glass beads to cause contact activation of blood in a vial. Simultaneously, RNPO or LMW-TEMPOL was added to the vial, followed by a measurement of the change in the number of platelets in the blood using a cytometer. As shown in Fig. 5A, more than 90% of platelets disappeared from blood when the blood was contacted with glass beads, indicating that contact activation of blood to bead surfaces took place. When LMW-TEMPOL was added to blood along with glass beads, the number of adherent platelets did not change, even at high concentrations of the nitroxide radical. In contrast, when RNPO was added to blood along with glass beads, the number of adherent platelets decreased in a dose-dependent manner. Note that the drastic decrease in the number of adherent platelets was not observed when nRNP, which is prepared by self-assembly of MeO-PEG-b-PCMS, thus no nitroxide radicals in the core, was added to blood along with glass beads (Fig. 5B). From these results, it is indicated that the scavenging of extracellular ROS by nitroxide radicals in the core of RNPO is attributed to the suppression of platelet activation.
Fig. 5.
Change in the numbers of platelets in rat whole blood stimulated by placing glass beads as a function of (A) nitroxide radical concentration of RNPO (closed circle) and LMW-TEMPOL (open circle) and (B) the extent of nRNP prepared by PEG-b-PCMS (open squares) and RNPO prepared by PEG-b-PMOT (closed circles). (n = 4) (C–E) SEM images of glass beads after contact of rat whole blood with (C) saline, (D) LMW-TEMPOL and (E) RNPO for 30 min. The concentration of nitroxide radicals was 5 mM.
SEM images of the surfaces of glass beads were recorded to observe the adhesion of blood cells. As shown in Fig. 5C and D, numerous blood cell adhesions were observed on the surfaces of glass beads incubated with rat whole blood in the presence of saline and LMW-TEMPOL, respectively. On these surfaces, platelet filopodia, fibrin, and blood cell coagulation were observed. In contrast, in the case of RNPO, the surfaces were remarkably smooth, and almost no adhesion of blood cells was observed (Fig. 5E). A significant difference was observed between LMW-TEMPOL and RNPO, and it is suggested that in case of LMW-TEMPOL, the combination of the incomplete ROS scavenge outside of cells and unwanted disturbance of the redox reaction inside of cells might cause platelet activation and promote platelet adhesion on the glass beads surfaces as shown in Fig. 3 and 4. From these results, it was revealed that RNPO can prevent platelet activation, resulting in suppression of platelet adhesion to the glass beads surfaces.
Blood coagulation
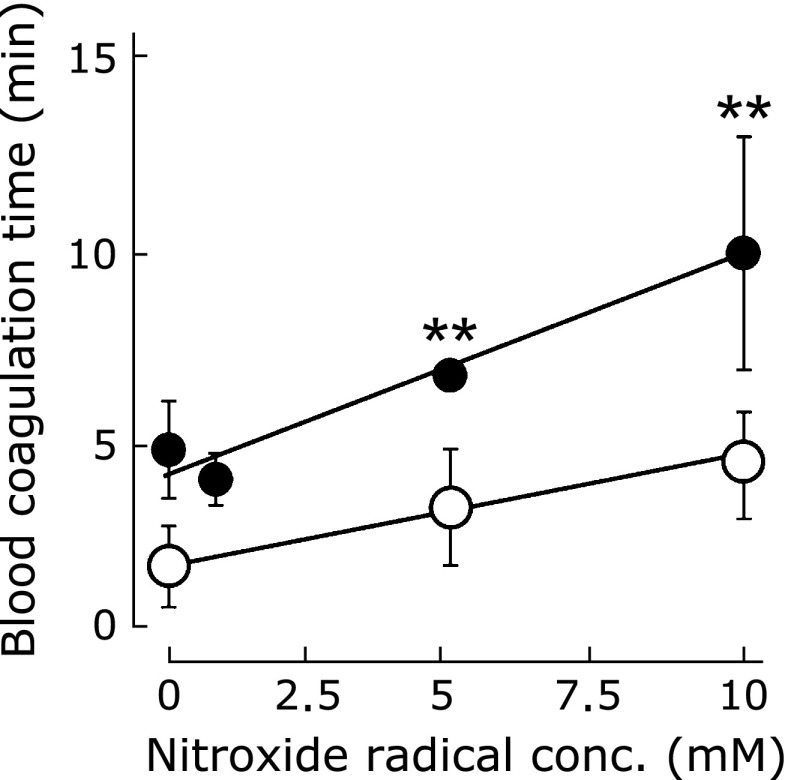
As discussed previously, platelet activation leads to blood coagulation.(39,40) Here, we hypothesized that RNPO suppresses blood coagulation by prevention of activation of blood cells via scavenging of ROS outside the blood cells.
To investigate the suppressive effect of RNPO on blood coagulation, blood coagulation time was measured. Fig. 6 shows blood coagulation time as a function of nitroxide radical concentration in RNPO and LMW-TEMPOL solution in saline. In the case of saline as a control, blood coagulated at about 3.5 min. LMW-TEMPOL slightly prolonged blood coagulation time, resulting in blood coagulation by 4.6 min even at 10 mM. In contrast, RNPO prolonged blood coagulation time in a dose-dependent manner, and suppressed blood coagulation for 10 min at 10 mM (polymer concentration; 30 mg/ml). Blood coagulation time in the presence of nRNP under the same polymer concentration as RNPO (polymer concentration; 30 mg/ml), was about 5.3 min, indicating that nRNP did not work significantly unlike RNPO.
Fig. 6.
The effect of RNPO (closed circle) and LMW-TEMPOL (open circle) on blood coagulation time (The graphs represent means ± SE for five independent experiments. **p<0.01, Student’s t test).
It is known that the blood coagulation mechanism includes intrinsic and extrinsic system. In the case of the intrinsic system, blood coagulation is accelerated by activation of contact factors such as a high molecular weight kininogen-prekallikrein complex when blood is contacted with the foreign materials such as the glass beads. Recently, ROS have been recognized as intracellular messengers. We have hypothesized that ROS are involved in the process of blood coagulation as a key player. Since LMW-ROS scavengers have so far used in the field of biological studies, however, it has been difficult to reveal the relationship between ROS and blood coagulation.
Our designed RNPO avoids cellular uptake and do not cause mitochondrial dysfunction, then RNPO can scavenge extracellular ROS in blood effectively without activation of blood. By its characteristics, we revealed that ROS scavenging in blood leads to the suppression of platelet activation and blood coagulation. Moreover, so far, it has been confirmed that treatment with our designed RNPs is useful in therapy for various oxidative stress injuries including stroke and hemorrhage. Taken together with the results in this study, we assure that suppression of oxidative stress in blood is also attributed to the therapeutic/protective effect of RNPs on oxidative stress injuries, avoiding adverse effects.
Conclusions
We have so far developed nitroxide radical-containing nanoparticles (RNPs) for novel antioxidative nanotherapeutics and confirmed the efficiency for versatile oxidative stress injuries. Most of these investigations were carried out via intravenous administration of RNPs. In this study, we investigated the behavior of RNPO in the blood. One of the principal findings was no internalization of RNPO into healthy blood cells, which is in sharp contrast to LMW nitroxide compounds such as TEMPOL. Because RNPO surrounds the outside of blood cells, no adverse effects inside of cells, such as mitochondrial dysfunction, were observed unlike LMW-TEMPOL. RNPO also prolonged the circulation of nitroxide radicals in the blood stream, which protects damage and activation of blood cells from oxidative stress. On the basis of these results, RNPO suppressed ROS-induced hemolysis, mitochondrial dysfunction and platelet activation. RNPO thus provided novel safety therapeutic system.
Scheme 1.
Acknowledgments
A portion of this work was supported by a Grant-in-Aid for Scientific Research S (No. 25220203) and the World Premier International Research Center Initiative (WPI Initiative) on Materials Nanoarchitronics of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Martinet W, de Meyer GR, Herman AG, Kockx MM. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur J Clin Invest. 2004;34:323–327. doi: 10.1111/j.1365-2362.2004.01343.x. [DOI] [PubMed] [Google Scholar]
- 2.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 3.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 4.Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743:56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monti E, Supino R, Colleoni M, Costa B, Ravizza R, Gariboldi MB. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J Cell Biochem. 2001;82:271–276. doi: 10.1002/jcb.1160. [DOI] [PubMed] [Google Scholar]
- 7.Suy S, Mitchell JB, Samuni A, Mueller S, Kasid U. Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer. 2005;103:1302–1313. doi: 10.1002/cncr.20898. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marushima A, Suzuki K, Nagasaki Y, et al. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia-reperfusion injury. Neurosurgery. 2011;68:1418–1425. doi: 10.1227/NEU.0b013e31820c02d9. [DOI] [PubMed] [Google Scholar]
- 10.Yoshitomi T, Hirayama A, Nagasaki Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials. 2011;32:8021–8028. doi: 10.1016/j.biomaterials.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Chonpathompikunlert P, Fan CH, Ozaki Y, Yoshitomi T, Yeh CK, Nagasaki Y. Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine (Lond) 2012;7:1029–1043. doi: 10.2217/nnm.12.2. [DOI] [PubMed] [Google Scholar]
- 12.Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027–1036.e3. doi: 10.1053/j.gastro.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Yoshitomi T, Nagasaki Y. Nitroxyl radical-containing nanoparticles for novel nanomedicine against oxidative stress injury. Nanomedicine (Lond) 2011;6:509–518. doi: 10.2217/nnm.11.13. [DOI] [PubMed] [Google Scholar]
- 14.Yoshitomi T, Yamaguchi Y, Kikuchi A, Nagasaki Y. Creation of a blood-compatible surface: a novel strategy for suppressing blood activation and coagulation using a nitroxide radical-containing polymer with reactive oxygen species scavenging activity. Acta Biomater. 2012;8:1323–1329. doi: 10.1016/j.actbio.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Klebanoff SJ, Clark RA. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975;45:699–707. [PubMed] [Google Scholar]
- 16.Awabdy D, Bryan-Lluka LJ, Wanstall JC. 5-Hydroxytryptamine and platelets: uptake and aggregation in hypoxic pulmonary hypertensive rats. Eur J Pharmacol. 2003;459:1–7. doi: 10.1016/s0014-2999(02)02734-6. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi M, Aikawa M, Sakagami T. A simple and effective method for hemolysis with a hypoxanthine-xanthine oxidase system and alteration of erythrocyte phospholipid composition during the hemolysis. J Biochem. 1981;89:795–800. doi: 10.1093/oxfordjournals.jbchem.a133261. [DOI] [PubMed] [Google Scholar]
- 18.Tamai H, Miki M, Mino M. Hemolysis and membrane lipid changes induced by xanthine oxidase in vitamin E deficient red cells. J Free Radic Biol Med. 1986;2:49–56. doi: 10.1016/0748-5514(86)90123-6. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Shi C, Li Q, Wu J, Forster EL, Yew DT. Mitochondrial dysfunction in platelets and hippocampi of senescence-accelerated mice. J Bioenerg Biomembr. 2007;39:195–202. doi: 10.1007/s10863-007-9077-y. [DOI] [PubMed] [Google Scholar]
- 20.Nagasaki Y. Nitroxide radicals and nanoparticles: a partnership for nanomedicine radical delivery. Ther Deliv. 2012;3:165–179. doi: 10.4155/tde.11.153. [DOI] [PubMed] [Google Scholar]
- 21.Risso-de Faverney C, Devaux A, Lafaurie M, Girard JP, Bailly B, Rahmani R. Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygene species. Aquat Toxicol. 2001;53:65–76. doi: 10.1016/s0166-445x(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Kazama S, Tanizawa H, et al. ESR imaging on a solid-tumor-bearing mouse using spin-labeled dextran. Biosci Biotechnol Biochem. 2001;65:787–794. doi: 10.1271/bbb.65.787. [DOI] [PubMed] [Google Scholar]
- 23.Kato F, Kikuchi A, Okuyama T, Oyaizu K, Nishide H. Nitroxide radicals as highly reactive redox mediators in dye-sensitized solar cells. Angew Chem Int Ed Engl. 2012;51:10177–10180. doi: 10.1002/anie.201205036. [DOI] [PubMed] [Google Scholar]
- 24.Yoshitomi T, Miyamoto D, Nagasaki Y. Design of core—shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules. 2009;10:596–601. doi: 10.1021/bm801278n. [DOI] [PubMed] [Google Scholar]
- 25.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 26.Samuni A, Goldstein S, Russo A, Mitchell JB, Krishna MC, Neta P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J Am Chem Soc. 2002;124:8719–8724. doi: 10.1021/ja017587h. [DOI] [PubMed] [Google Scholar]
- 27.Jarolim P, Lahav M, Liu SC, Palek J. Effect of hemoglobin oxidation products on the stability of red cell membrane skeletons and the associations of skeletal proteins: correlation with a release of hemin. Blood. 1990;76:2125–2131. [PubMed] [Google Scholar]
- 28.Batko J. The effect of experimental neoplastic disease on malondialdehyde level and glutathione status in erythrocytes of rats. Acta Biochim Pol. 1997;44:767–769. [PubMed] [Google Scholar]
- 29.Di Paola R, Mazzon E, Zito D, et al. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J Clin Periodontol. 2005;32:1062–1068. doi: 10.1111/j.1600-051X.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 30.Amiji M, Park K. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Ed. 1993;4:217–234. doi: 10.1163/156856293x00537. [DOI] [PubMed] [Google Scholar]
- 31.Cao Q, Li S, He C, Li K, Liu F. Extraction and determination of papaverin in pericarpium papaveris using aqueous two-phase system of poly(ethylene glycol)-(NH4)2SO4 coupled with high-performance liquid chromatography. Anal Chim Acta. 2007;590:187–194. doi: 10.1016/j.aca.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 32.Lee BS, Yoon OJ, Cho WK, Lee NE, Yoon KR, Choi IS. Construction of protein-resistant pOEGMA films by helicon plasma-enhanced chemical vapor deposition. J Biomater Sci Polym Ed. 2009;20:1579–1586. doi: 10.1163/092050609X12464345079969. [DOI] [PubMed] [Google Scholar]
- 33.Nagasaki Y. Construction of a densely poly(ethylene glycol)-chain-tethered surface and its performance. Polymer Journal. 2011;43:949–958. [Google Scholar]
- 34.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 35.Gordon JL, Olverman HJ. 5-Hydroxytryptamine and dopamine transport by rat and human blood platelets. Br J Pharmacol. 1978;62:219–226. doi: 10.1111/j.1476-5381.1978.tb08449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakano K, Tarashima M, Tachikawa E, et al. Platelet mitochondrial evaluation during cytochrome c and dichloroacetate treatments of MELAS. Mitochondrion. 2005;5:426–433. doi: 10.1016/j.mito.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Thushara RM, Hemshekhar M, Santhosh MS, et al. Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol Cell Biochem. 2013;373:73–83. doi: 10.1007/s11010-012-1476-7. [DOI] [PubMed] [Google Scholar]
- 38.Salvemini D, de Nucci G, Sneddon JM, Vane JR. Superoxide anions enhance platelet adhesion and aggregation. Br J Pharmacol. 1989;97:1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorlach A. Redox regulation of the coagulation cascade. Antioxid Redox Signal. 2005;7:1398–1404. doi: 10.1089/ars.2005.7.1398. [DOI] [PubMed] [Google Scholar]
- 40.Gregg D, de Carvalho DD, Kovacic H. Integrins and coagulation: a role for ROS/redox signaling? Antioxid Redox Signal. 2004;6:757–764. doi: 10.1089/1523086041361604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.