Abstract
Sensory perception modulates aging and physiology across taxa. We found that perception of female sexual pheromones through a specific gustatory receptor expressed in a subset of foreleg neurons in male fruit flies, Drosophila melanogaster, rapidly and reversibly decreases fat stores, reduces resistance to starvation, and limits lifespan together with neurons that express the reward-mediating neuropeptide F. High-throughput RNA-seq experiments revealed a set of molecular processes that were impacted by the activity of the longevity circuit, thereby identifying new candidate cell non-autonomous aging mechanisms. Mating reversed the effects of pheromone perception, suggesting a model where lifespan is modulated through integration of sensory and reward circuits and where healthy aging may be compromised when the expectations defined by sensory perception are discordant with ensuing experience.
Sensory perception can modulate aging and physiology in multiple species (1–6). In Drosophila, exposure to food-based odorants partially reverses the anti-aging effect of dietary restriction, whereas broad reduction in olfactory function promotes longevity and alters fat metabolism (2, 4). Even the well-known relation between body temperature and lifespan may have a sensory component (7, 8).
To identify sensory cues and neuronal circuitry that underlie the effects of sensory perception on aging, we focused on the perception of potential mates. Social interactions are prevalent throughout nature, and the influence of social context on health and longevity is well-known in several species, including humans (9). Such influences include behavioral interactions with mates and broader physiological “costs of reproduction,” which often form the basis for evolutionary models of aging (10, 11).
In Drosophila, the presence of potential mates is perceived largely through non-volatile cuticular hydrocarbons, which are produced by cells called oenocytes and are secreted to the cuticular surface where they function as pheromones (12, 13). To test whether differential pheromone exposure influenced lifespan or physiology, we housed “experimental” flies of the same genotype with “donor” animals of the same sex that either expressed normal pheromone profiles or were genetically engineered to express pheromone profiles characteristic of the opposite sex (Fig. 1A). Donor males with feminized pheromone profiles were generated by targeting expression of the sex determination gene, tra, to the oenocytes (via OK72-GAL4 or Prom-E800-Gal4 (14); Fig. S1), whereas masculinization of female flies was accomplished by expressing tra-RNAi in a similar way (15). This design allowed manipulation of the experimental animals’ perceived sexual environment without introducing complications associated with mating itself.
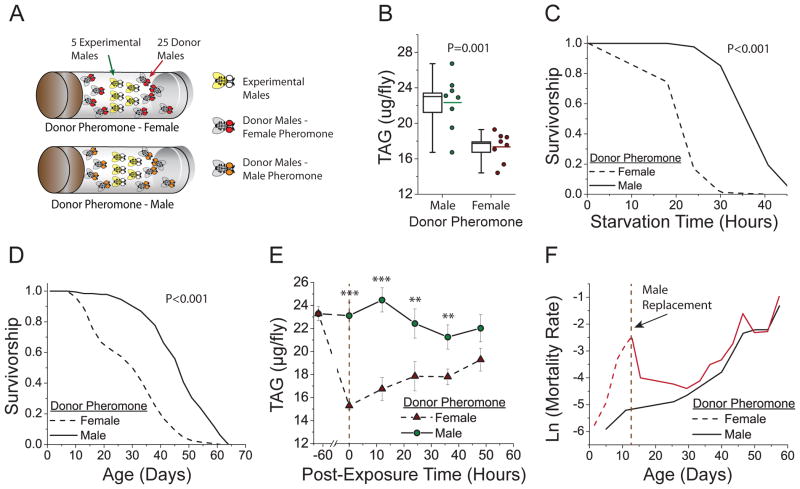
Fig. 1. Exposure to sex-specific pheromones significantly affects physiology, stress resistance, and lifespan in Drosophila.
(A) The protocol used to expose male flies to masculine or feminine pheromones. Five experimental males were housed with either 25 control (donor pheromone = male) or feminized (donor pheromone = female) males. (B) Male flies exposed to male donor pheromone exhibit higher TAG amounts than flies exposed to female donor pheromone (N = 40 for each treatment). Box plots represent the medians and standard error of the mean (box boundaries) for each experimental group. Lines adjacent to the box represent mean values. P-value is by t-test. (C) Male experimental flies exposed to male donor pheromones exhibit greater starvation resistance relative to genetically identical males exposed to female donor pheromones (N=88 and 82, respectively; P-value is by log-rank test). (D) Male experimental flies exposed to male donor pheromones exhibit a longer lifespan relative to flies that are exposed to female donor pheromones (N=184 and 195, respectively; P-value is by log-rank test). (E) TAG amounts are progressively restored after removal (at T=0) of female donor pheromones (N = 50 for each time point). *** = p ≤ 0.001, ** = p ≤ 0.01, no stars= p ≥ 0.05 by t-test. (F) Differences in age-specific mortality caused by pheromone exposure were reversed within days after feminized males were replaced with control males.
In Drosophila, sensory manipulations can affect lifespan, fat storage (as determined by baseline measures of triacylglyceride—TAG), and certain aspects of stress resistance (2, 4). We found that flies exposed to pheromones of the opposite sex showed differences in these phenotypes. Experimental male flies exposed to male donor pheromone had higher amounts of TAG, were substantially more resistant to starvation, and exhibited a significantly longer lifespan than genetically identical male siblings exposed to female donor pheromone (Fig. 1B-D). Females exhibited similar phenotypes in response to male donor pheromone, but the magnitude of the effects was smaller (Fig. S2). Subsequent experiments were therefore focused on males.
The characteristics of pheromone exposure were indicative of a mechanism involving sensory perception. Effects were similar in several genetic backgrounds, including a strain recently collected in the wild (Fig. S3), and were largely unaffected by cohort composition (Fig. S4). Pheromone-induced phenotypes were detected after as little as two days exposure to donor animals (Fig. 1B,C), persisted with longer manipulations (Fig. 1D), and were progressively reversed when female donor pheromone was removed (Figs. 1E, 1F, and S5). Pheromone effects appeared not to be mediated by aberrant or aggressive interactions with donor flies because we did not observe significant differences in such behaviors and because continuous, vigorous agitation of the vials throughout the exposure period, which effectively disrupted observed behaviors, had no effect on the impact of donor pheromone (Fig. S6). Furthermore, exposure of experimental males to the purified female pheromone 7-11-heptacosadiene (7-11 HD) produced physiological changes in the absence of donor animals (mean survival time during starvation of 51.1 ± 1.7hr and 45.4 ± 1.2hr for control and 7,11-HD exposure, respectively, P=0.007, log-rank test).
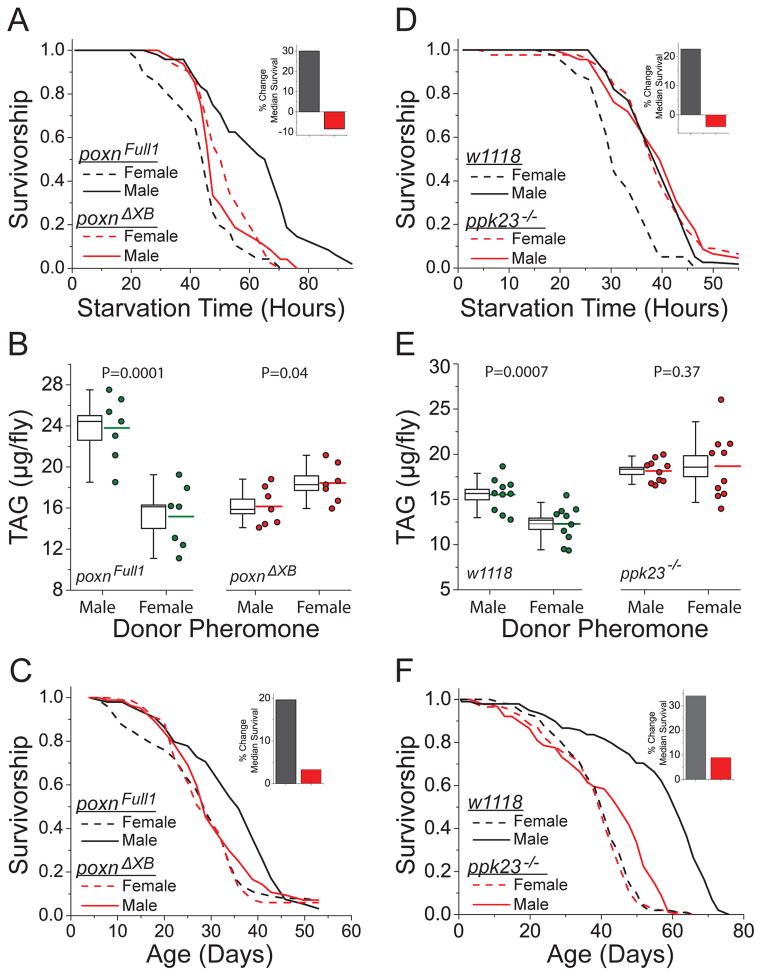
To explore the sensory modality through which donor pheromone exerts its effects, we tested whether a broadly-expressed olfactory co-receptor, Or83b, whose loss of function renders flies largely unable to smell (16), was required for pheromone effects. Or83b mutant flies exhibited similar changes in starvation resistance (Fig. S7) in response to donor pheromone as did control animals, indicating that olfaction was not required. To test whether taste perception was involved, we used flies mutant for the gene Pox neuro (Poxn), a null mutation that putatively transforms all chemosensory neurons into mechanosensory neurons. Drosophila taste neurons are present in the mouthparts and distributed on different body parts including the wings, legs, and genitals, which allow sensation by contact. When the Poxn null mutation is coupled with a partially rescuing transgene, Poxn ΔM22-B5-ΔXB, flies are generally healthy, but gustatory perception is eliminated in the labellum, the legs, and the wing margins (17). Poxn ΔM22-B5-ΔXB flies showed no pheromone-induced changes in starvation resistance, TAG amounts, or lifespan (Fig. 2A–C). However, Poxn mutant flies that carried a transgene that restores taste function to the legs and wing margins (but not labellum; PoxnΔM22-B5-Full1 (17)) responses were similar to those of control flies (Fig. 2A–C). Thus, the effects of pheromone exposure appear to be mediated by taste perception through gustatory neurons outside of the mouthparts.
Fig. 2. The effects of pheromone exposure are mediated by taste perception involving gustatory receptor ppk23.
(A–C) The PoxnΔXB strain lacks taste neurons in the labellum, the legs, and the wing margins, while the PoxnFull1 strain lacks taste neurons in the labellum only. (A) Starvation resistance. N = 46–48 experimental flies for all treatments. Significance values are as follows: P ≤ 0.0001 for PoxnFull1 (exposed to control vs. feminized donor flies); P = 0.56 for PoxnΔXB. (B) TAG amounts. Box plots are presented as in Fig 1. (C) Lifespan. N=100–103 experimental flies for all treatments. Significance values are P = 0.001 for PoxnFull1 exposed to control vs. feminized donor flies and P = 0.30 for PoxnΔXB exposed to control vs. feminized donor flies. (D–F) The gustatory receptor ppk23 is required for pheromone effects. (D) Starvation resistance. N = 40 and 39 for control flies exposed to feminized or control donor males, respectively (P ≤ 0.0001). N = 43 and 46 for ppk23 mutant flies exposed to female or male donor pheromones, respectively (P=0.92). (E) TAG amounts. Box plots are presented as in Fig. 1. (F) Lifespan. N = 98 and 99 for control flies exposed to male or female donor pheromones, respectively. N = 89 and 86 for ppk23 mutant flies exposed to male or female donor pheromones, respectively. P-values were obtained for lifespan and starvation resistance by log-rank test and for TAG amounts by t-test.
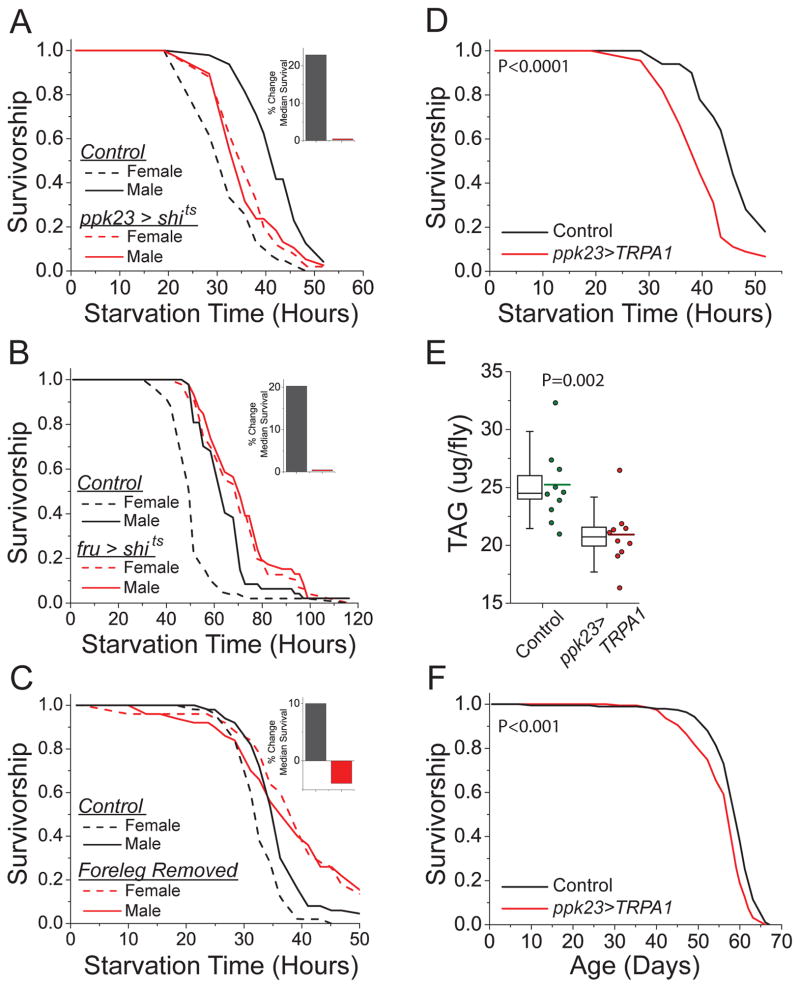
To identify specific gustatory receptors and neurons that might mediate the pheromone effects, we tested candidate pheromone receptors. Of the mutants that we examined, only flies that carried a loss of function mutation in the gene pickpocket 23 (ppk23) were resistant to the effects of pheromone exposure (Fig. S8). Further analysis verified that ppk23 was required for the effects of pheromone exposure on starvation resistance, TAG amounts, and lifespan (Fig. 2D–F). Silencing ppk23-expressing neurons only during exposure to donor males by expressing a temperature-sensitive dominant negative allele of the dynamin gene shibire (via ppk23-GAL4; UAS-shits) also eliminated the differential response to pheromones (Fig 3A). In male Drosophila, the transcription factor fruitless (fru) is expressed with ppk23 in pheromone-sensing neurons located in the animals’ forelegs (18), and silencing fru-expressing neurons during exposure (via fru-GAL4;UAS-shits) abrogated pheromone effects (Fig. 3B). Consistent with a requirement for these neurons, we found that surgical amputation of the forelegs, but not injury alone, was sufficient to reproducibly eliminate the effects of pheromone exposure (Figs. 3C and S9). Moreover, acute, targeted activation of ppk23-expressing neurons using a temperature-sensitive TRPA1 channel (ppk23-GAL4;UAS-TRPA1) was sufficient to mimic the effects of female pheromone without exposure (Fig. 3D–F). Together, these data indicate that pheromone-sensing neurons in the foreleg of the male fly that express the gustatory receptor, ppk23, and the transcription factor, fruitless, influence stress resistance, physiology, and lifespan in response to perception of female pheromones.
Fig. 3. Activation of ppk23-positive pheromone-sensing neurons in the foreleg of male flies is necessary and sufficient for changes in physiology and lifespan.
(A) Targeted inhibition of ppk23-expressing neurons abrogates differences in starvation caused by pheromone exposure. N = 48 and 39 for control (ppk23-GAL4;w1118) flies exposed to male or female donor pheromones, respectively (P ≤ 0.0001). N=38 and 50 for treatment (ppk23-GAL4; UAS-shits) flies exposed to male or female donor pheromones, respectively (P = 0.96). (B) Targeted inhibition of fruitless-expressing neurons abrogates the effect of pheromone exposure on starvation resistance. N = 47 and 49 for control (fru-GAL4 X w1118) flies exposed to male or female donor pheromones, respectively (P ≤ 0.0001). N = 46 and 47 for treatment (fru-GAL4; UAS-shits) flies exposed to male or female donor pheromones, respectively (P=0.65). (C) Surgical removal of the forelegs abrogates the effects of pheromone exposure on starvation resistance. N = 50 for each genotype/treatment. P=0.0008 for unmanipulated and P=0.66 for amputee flies exposed to either male or female donor pheromones. See also Fig. S9. (D–F) Activation of ppk23-expressing neurons via heat-activated TRPA1 phenocopies the effects of pheromone exposure. (D) Starvation resistance. N = 50 for control (ppk23-GAL4;w1118), N=45 for flies with activated neurons (ppk23-GAL4;UAS-TRPA1) (P ≤ 0.0001). (E) TAG amounts. Box plots are presented as in Fig 1. (F) Lifespan. N=193 (ppk23-GAL4;w1118) and N=191 (ppk23-GAL4;UAS-TRPA1). P-values were obtained for lifespan and starvation resistance by log-rank test and for TAG amounts by t-test.
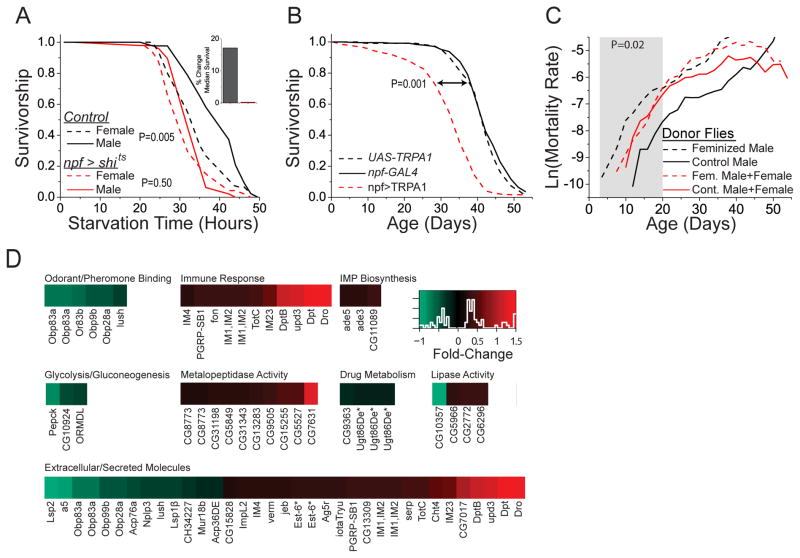
To examine brain circuits that may function in transducing pheromone perception, we selectively expressed UAS-shits to block synaptic transmission in various neuro-anatomical regions with the goal of disrupting the physiological effects of donor pheromone exposure. The effects were abrogated when UAS-shits was driven in neurons characterized by expression of neuropeptide F (NPF, as represented by npf-GAL4) (Fig. S10). Further analysis verified that pheromone-induced changes in starvation resistance and TAG abundance were lost following silencing of npf-expressing neurons (Fig. 4A). Consistent with a possible role in transducing pheromone information, npf expression was significantly increased by 30% in experimental males after exposure to feminized donor males (Fig. S11), and activation of npf-expressing neurons was sufficient to decrease lifespan in the absence of pheromone exposure (Fig. 4B).
Fig. 4. Aging and physiology are modulated by neural mechanisms of expectation and reward.
(A) Inhibition of npf-expressing neurons abrogates differences in starvation caused by pheromone exposure. N = 43 and 45 for control (npf-GAL4;w1118) flies exposed to male or female donor pheromones, respectively (P = 0.005 by log-rank test). N = 48 and 47 for treatment (npf-GAL4; UAS-shits) flies exposed to male or female donor pheromones, respectively (P = 0.50 by log-rank test). (B) Activation of npf-expressing neurons causes decreased longevity in the absence of pheromone exposure. UAS-dTrpA1/+; npf-GAL4/+ males (N=239) exhibit significantly shorter lifespan compared to UAS-dTrpA1/+ (N=235; p≤0.001 by log-rank test) and npf-GAL4/+ (N=179; p≤0.001 by log-rank test) male transgene controls. (C) Mortality rates are reduced when males exposed to female donor pheromone (dashed black line) are given access to excess females (dashed red line; P=0.02 through 20 days of age by Aalen regression). Cohorts consisted of five experimental males together with either (i) 30 control donor males (solid black), (ii) 30 feminized donor males (dashed black), (iii) 5 feminized donor males + 25 females (dashed red), or (iv) 5 control donor males + 25 females (solid red). 20 replicate cohorts, totaling 100 experimental flies, were measured for each treatment. (D) Significantly enriched Gene Ontology pathways and functions whose genes are differentially regulated following pheromone exposure. A complete list of genes with significant changes in expression is provided in Table S1.
NPF may function as a mediator of sexual reward in Drosophila (19), and its mammalian counterpart, neuropeptide Y (NPY), has been associated with sexual motivation and psychological reward (20, 21). We tested whether the effects of pheromone perception might be rescued by allowing males to successfully mate with females. Neither a small number of conjugal visits with virgin females nor housing with wild-type females in a 1:1 ratio was sufficient to ameliorate the effects of pheromone exposure (Fig. S12). In this context, decreased longevity may be a consequence of pheromone perception and not of mating itself. Male Drosophila are willing and able to copulate up to five times in rapid succession before requiring a refractory period (22). We found that supplementing donor cohorts with an excess of mating females (in a 5:1 ratio) was sufficient to significantly reduce the effects on mortality and TAG caused by female donor pheromone early in life (Figs. 4C and S13). The benefits of mating on age-specific mortality decreased with age, suggesting that aging may reduce mating efficiency or may diminish effective mating reward.
To identify how sexual perception and reward may alter physiological responses in peripheral tissues, we examined changes in gene expression using whole-genome RNA-seq technology. We found 195 genes with significantly different expression (using an experiment-wise error rate of 0.05) in control male flies that were exposed to feminized or control donor males for 48 hours. Nearly all (188/195=96%) of the changes appeared to be due to pheromone perception because they were not observed in identical experiments using ppk23 mutant flies (Table S1). Males exposed to female pheromones decreased transcription of genes encoding odorant-binding proteins and increased transcription of several genes with lipase activity (Fig. 4E). A significant enrichment was observed in secreted molecules, which includes genes encoding proteins mediating immune- and stress-responses. Many of these genes and pathways were highlighted in a recent meta-analysis of gene expression changes in response to stress and aging (23).
Activities of insulin and target of rapamycin (TOR) signaling, which modulate aging across taxa, increase sexual attractiveness in flies (24). Our current demonstration that perception of sexual characteristics is sufficient to modulate lifespan and physiology suggests aging pathways in one individual may modulate health and lifespan in another (Fig. S14). These types of indirect genetic effects have the potential to be influential agents of natural selection (25), suggesting that expectation/reward imbalance may have broad effects on health and physiology in humans and may present a potent evolutionary force in nature.
Supplementary Material
Acknowledgments
We thank the members of the Pletcher laboratory for Drosophila husbandry, N. Linford for comments on the revision, P.J. Lee for figure illustration, and members of the Dierick and Pletcher laboratories for the suggestions on experiments and comments on the manuscript. The research was supported by US National Institutes of Health (R01AG030593, TR01AG043972, and R01AG023166), the Glenn Foundation, the American Federation for Aging Research, the Ellison Medical Foundation (to S.D.P.), a Ruth L. Kirschstein National Research Service Award from the National Institute on Aging (F32AG042253) (to B.Y.C.), a Glenn/AFAR Scholarship for Research in the Biology of Aging (to Z.M.H.), and the Alexander von Humboldt foundation, Singapore National Research Foundation (RF001-363) (to J.Y.Y.). This work utilized the Drosophila Aging Core of the Nathan Shock Center of Excellence in the Biology of Aging funded by the National Institute of Aging (P30-AG-013283). RNA-seq expression data are provided in Table S1 of the Supporting Online Material.
Footnotes
Financial Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
CMG, THK, ZH and SDP conceived and designed the experiments. CMG, THK, ZH, BC, JYY, HAD, and SDP performed the experiments. CMG, THK, ZH, BC, JYY, and SDP analyzed the data. CMG, THK, JYY, HAD, and SDP wrote the paper.
References and Notes
- 1.Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999 Dec 16;402:804. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- 2.Libert S, et al. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007 Feb 23;315:1133. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 3.Linford NJ, Kuo TH, Chan TP, Pletcher SD. Sensory perception and aging in model systems: from the outside in. Annu Rev Cell Dev Biol. 2011 Nov 10;27:759. doi: 10.1146/annurev-cellbio-092910-154240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8:e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith ED, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC developmental biology. 2008;8:49. doi: 10.1186/1471-213X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004 Jan 8;41:45. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009 May 12;19:715. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao R, et al. A Genetic Program Promotes C. elegans Longevity at Cold Temperatures via a Thermosensitive TRP Channel. Cell. 2013;152:1. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005 Apr 29;308:648. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 10.Partridge L, Barton NH. Optimality, mutation and the evolution of ageing. Nature. 1993 Mar 25;362:305. doi: 10.1038/362305a0. [DOI] [PubMed] [Google Scholar]
- 11.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005 Feb 25;120:461. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009 Oct 15;461:987. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 13.Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005 May;35:279. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferveur JF, et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997 Jun 6;276:1555. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez MD, et al. Pheromonal and Behavioral Cues Trigger Male-to-Female Aggression in Drosophila. Plos Biology. 2010 Nov;8 doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004 Sep 2;43:703. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Boll W, Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002 Dec;129:5667. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- 18.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012 May 25;149:1140. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012 Mar 16;335:1351. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalra SP, Clark JT, Sahu A, Dube MG, Kalra PS. Control of feeding and sexual behaviors by neuropeptide Y: physiological implications. Synapse. 1988;2:254. doi: 10.1002/syn.890020313. [DOI] [PubMed] [Google Scholar]
- 21.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004 Aug;38:213. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner M, Golic K, Hawley RS. Drosophila: A laboratory handbook. 2. Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- 23.Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging. 2012 Nov;4:768. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo TH, et al. Insulin signaling mediates sexual attractiveness in Drosophila. PLoS Genet. 2012;8:e1002684. doi: 10.1371/journal.pgen.1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGlothlin JW, Moore AJ, Wolf JB, Brodie ED., 3rd Interacting phenotypes and the evolutionary process. III. Social evolution. Evolution; international journal of organic evolution. 2010 Sep;64:2558. doi: 10.1111/j.1558-5646.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 26.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012 Mar;7:562. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dierick HA. A method for quantifying aggress in male Drosophila melanogaster. Nat Protoc. 2007;2:2712–2718. doi: 10.1038/nprot.2007.404. [DOI] [PubMed] [Google Scholar]
- 28.Aalen OO. A linear regression model for the analysis of life times. Stat Med. 1989;8:907. doi: 10.1002/sim.4780080803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.