Abstract
The Ca2+-activated monovalent cation selective transient receptor potential melastatin 4 (TRPM4) channel has been recently identified in detrusor smooth muscle (DSM) of the urinary bladder. Two recent publications by our research group provide evidence in support of the novel hypothesis that TRPM4 channels enhance DSM excitability and contractility. This is a critical question as prior studies have primarily targeted hyperpolarizing currents facilitated by K+ channels, but the depolarizing component in DSM cells is not well understood. For the first time, we utilized the selective TRPM4 channel inhibitor, 9-phenanthrol, to investigate TRPM4 channel functional effects in DSM at both cellular and tissue levels in rodents. Our new data presented here showed that in rat DSM cells, 9-phenanthrol attenuates spontaneous inward currents in the presence of the muscarinic receptor agonist, carbachol, thus reducing DSM cell excitability. In support of our original hypothesis, we found that TRPM4 channel mRNA levels are much higher in DSM vs. vascular smooth muscle and that inhibition of TRPM4 channels can potentially attenuate DSM excitability. Thus, we postulate the novel concept that selective pharmacological inhibition of TRPM4 channels can limit both excitability and contractility of DSM.
Keywords: TRPM4 channel, detrusor smooth muscle, 9-phenanthrol, urinary bladder
Introduction
Contraction and relaxation of detrusor smooth muscle (DSM), which makes up the wall of the urinary bladder, facilitates the storage and voiding of urine. Multiple ion channels that are expressed in DSM control the excitability and contractility of this tissue. However, the mechanisms by which ion channels regulate DSM function are yet to be completely elucidated. This lack of knowledge hinders the efforts aimed at identifying suitable ion channel targets and channel modulators for urinary bladder disorders. Recently, members of the transient receptor potential (TRP) superfamily of ion channels have been implicated in normal and pathologic bladder function.1-3 Mammalian genomes encode 27 human and 28 rodent TRP channel members, respectively, subdivided into 6 subfamilies based on their sequence homology (TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML).4,5 TRP channels respond to physical and chemical stimuli such as temperature, pH, osmolality, pressure, stretch, light, alkaloids, as well as intracellular stimuli including Ca2+; and constitute a fundamental way by which cells perceive and respond to changes in the extracellular environment.5 Until recently, several TRP members have been identified in the bladder urothelium and nerves, but not yet in DSM.1,3,6 One such member is the transient receptor potential melastatin 4 (TRPM4) channel.7,8 TRPM4 channel physiological significance in urothelium remains unknown,6 and its functional role in DSM was recently described by our group when we reported the expression and function of TRPM4 channels in rat and guinea pig DSM.9,10 The function of TRPM4 channels has been studied in non-DSM myocytes and the channel has been identified as an important mediator of smooth muscle cell excitability and contractility.11-16 Novel data included in this addendum indicate that expression of TRPM4 channels is greater in DSM compared with cerebral arterial myocytes, suggesting that the channel may have greater impact in bladder function.
TRPM4, and the related TRPM5 channel, display atypical biophysical properties. TRPM4 is a Ca2+-activated cation channel highly selective for monovalent cations with the rank order of Na+~K+ > Cs+ > Li+, but impermeable to anions and divalent cations such as Ca2+.16 TRPM4 channels exhibit Ca2+ dependency, have single channel conductance ~25 pS, and are voltage-dependent.17-20 Activation of TRPM4 channels is thought to induce cell depolarization via the net entry of Na+ into the cell which in turn activates the L-type voltage-dependent Ca2+ channels (VDCC) favoring Ca2+ entry, thus modulating Ca2+ signaling13,21 and eventually DSM contractility. Collectively, these properties and fundamental roles make TRPM4 channels potentially useful pharmacological targets to control DSM function. However, previous investigations of TRPM4 channel function have been hampered by the lack of selective pharmacological modulators. Recently, a novel selective TRPM4 channel inhibitor, 9-phenanthrol, has been described.15,22,23 Thus, 9-phenanthrol is an important pharmacological tool that can be used to investigate the functional role of TRPM4 channels in DSM excitation-contraction coupling. In this addendum, we provide further evidence for a physiological role of TRPM4 channels in rat DSM using its modulator 9-phenanthrol.
Results
Expression levels of TRPM4 channel mRNA are greater in DSM compared with cerebral artery smooth muscle
Quantitative RT-PCR experiments showed that TRPM4 channel mRNA expression level is greater in rat DSM compared with cerebral artery smooth muscle (2.6 ± 0.7 fold greater; n = 3, N = 3; p < 0.05, Fig. 1), suggesting that TRPM4 channels may have a more important functional impact in the bladder as compared with vasculature.
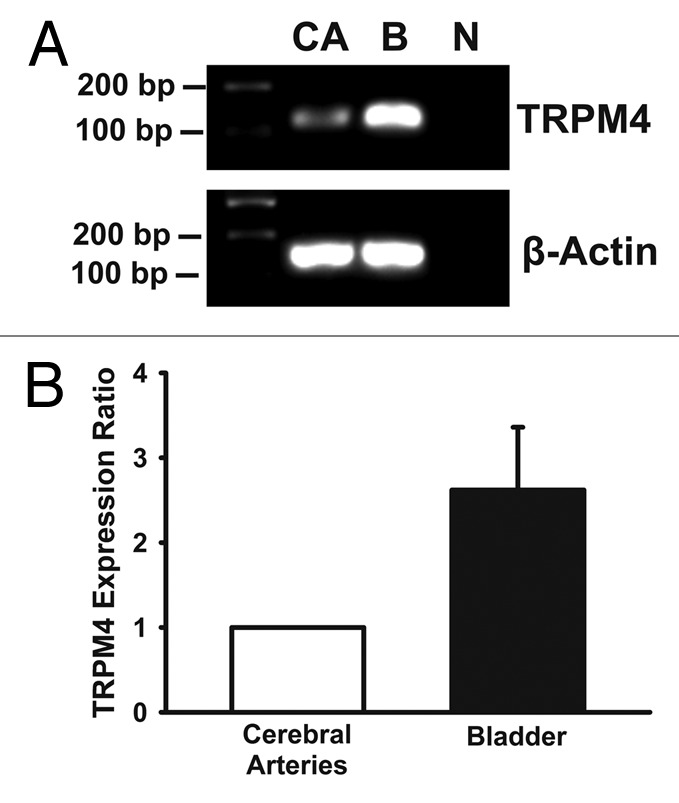
Figure 1. TRPM4 channel mRNA is expressed at higher levels in DSM compared with cerebral artery smooth muscle in rats. (A) Agarose gel of PCR products from either bladder (B) or cerebral artery (CA) cDNA for TRPM4 (117 bp) or β-actin (145 bp); no products were detected when cDNA template was absent (N). (B) Summary data showing that TRPM4 channel mRNA expression is 2.6 ± 0.7 fold higher in DSM relative to cerebral arteries as determined by quantitative RT-PCR in Sprague Dawley rats (n = 3, N = 3; p < 0.05).
TRPM4 channel blocker 9-phenanthrol reduces spontaneous inward current in freshly-isolated rat DSM cells
We used the amphotericin-B perforated whole cell patch-clamp technique and the selective inhibitor of TRPM4 channels, 9-phenanthrol, to evaluate the functional role of TRPM4 channels in the regulation of DSM cell excitability. As shown in Figure 2, freshly-isolated DSM cells at a holding potential of –60 mV exhibited spontaneous basic activity along with numerous inward currents as originally reported in rat DSM cells at -70 mV.10 9-phenanthrol (30 µM) significantly attenuated the spontaneous inward current from NPo of 4.0 ± 0.6 under control conditions to 2.2 ± 0.4 in the presence of 30 µM 9-phenanthrol (n = 13, N = 6; p < 0.05, Fig. 2). The inhibitory effect of 9-phenanthrol on spontaneous inward currents completely recovered to the control level upon the washout of 9-phenanthrol from the bath solution (NPo = 4.0 ± 1.0; n = 8, N = 5; p > 0.05 washout vs. control; Fig. 2). Our experimental data confirms the concept that TRPM4 channels are tonically active under physiological conditions and are key regulators of DSM cell excitability.
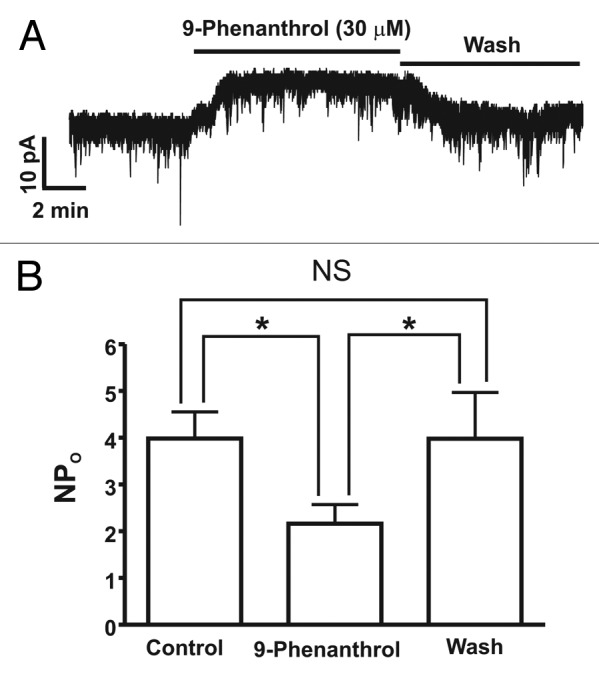
Figure 2. Reduction of spontaneous inward current by the TRPM4 channel inhibitor 9-phenanthrol in freshly-isolated rat DSM cells. (A) An original recording illustrating the inhibitory effect of 9-phenanthrol (30 μM) on spontaneous inward current recorded at −60 mV in a single DSM cell using the perforated whole cell patch-clamp technique. (B) Summary data for the inhibitory effect of 9-phenanthrol on TRPM4 spontaneous inward current, presented as NPo before (control) and after its application at 30 μM (n = 13, N = 6; *p < 0.05), and following the washout of 9-phenanthrol (n = 8, N = 5; p > 0.05 washout vs control); NS = non-significant for the comparison shown.
TRPM4 channel blocker 9-phenanthrol inhibits spontaneous inward current in freshly-isolated rat DSM cells in the presence of muscarinic receptor agonist carbachol
In the next experimental series, we sought to evaluate whether pharmacological inhibition of TRPM4 channels with 9-phenanthrol affects the spontaneous inward current in freshly-isolated DSM cells in the presence of 1 µM carbachol. As illustrated in Figure 3, in the presence of 1 µM carbachol, spontaneous basic current activity along with numerous inward currents at a holding potential of -70 mV were measured. Treatment of DSM cells with 30 µM 9-phenanthrol significantly suppressed the spontaneous inward currents from NPo of 4.5 ± 0.7 in the presence of 1 µM carbachol alone to 2.5 ± 0.7 in the presence of both 1 µM carbachol and 30 µM 9-phenanthrol (n = 11, N = 6; p < 0.05, Fig. 3). Taken together, our results show that the TRPM4 channel inhibitor 9-phenanthrol attenuates the inward currents in the presence of the muscarinic receptor agonist carbachol, thus regulating DSM cell excitability.
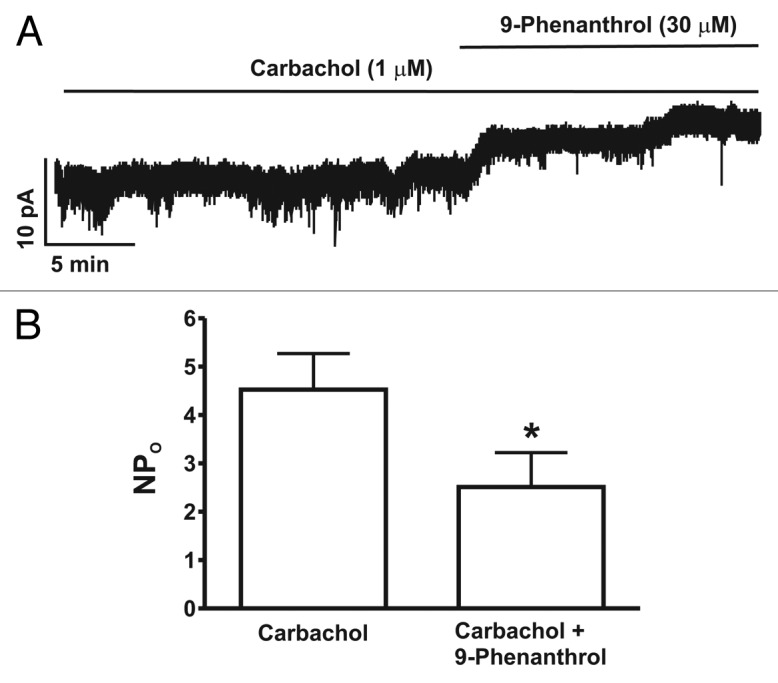
Figure 3. Reduction of spontaneous inward current by the TRPM4 channel inhibitor 9-phenanthrol in the presence of the muscarinic receptor agonist carbachol in freshly-isolated rat DSM cells. (A) A representative recording illustrating the inhibitory effect of 9-phenanthrol (30 μM) on spontaneous inward current recorded in the presence of carbachol (1 µM) at −70 mV in a single DSM cell using the perforated whole cell patch-clamp technique. (B) Summary data for the inhibitory effect of 9-phenanthrol on TRPM4 spontaneous inward current in the presence of carbachol (1 µM), presented as NPo before (control) and after application of 30 μM 9-phenanthrol (n = 11, N = 6; *p < 0.05).
Discussion
Aside from our just published reports,9,10 the functional role of the TRPM4 channel in DSM cell excitability has not been studied. Recently, a novel selective TRPM4 channel inhibitor, 9-phenanthrol, has been described.9,10,15,22,23 Thus, 9-phenanthrol is an important pharmacological tool that could be used to investigate the TRPM4 channel physiological role in DSM excitability.9,10 Use of this novel selective pharmacological inhibitor has allowed us to show that TRPM4 channels contribute to the electrical activity of freshly-isolated rat and guinea pig DSM cells.9,10 These studies provide novel mechanistic insights and better our understanding of the functional roles of TRPM4 channels in urinary bladder. Our recent papers9,10 provide strong, initial evidence for a key role of the TRPM4 channels in regulating DSM excitability and contractility. Major strengths of our studies include the use of only native, freshly-isolated (not cultured) DSM cells, and a combination of experimental approaches encompassing TRPM4 mRNA and protein detection, patch-clamp electrophysiology, and tissue contractility.
New data presented here provide a significant rationale for further study of the role of TRPM4 channels in bladder function. This question is critical as prior studies have primarily targeted hyperpolarizing currents, such as K+ currents,24 but the depolarizing component in DSM cells is not completely understood. In support of our original hypothesis, we found that inhibition of TRPM4 channels with 9-phenanthrol diminished DSM excitability in the presence or absence of the muscarinic receptor agonist, carbachol (Figs. 2–3), complementing our prior experimental evidence.9,10 Most importantly, the new quantitative RT-PCR data showed much higher TRPM4 channel mRNA expression levels in DSM compared with vascular smooth muscle in rat (Fig. 1). This key finding suggests TRPM4 channels might have a more prominent physiological role in DSM compared with the vasculature, and therefore, could be novel pharmacological targets for bladder dysfunction with minimal vascular effects.
Bladder dysfunction, such as overactive bladder (OAB), is a significant medical problem that affects ~17% of the Western population.25-27 The current pharmacological treatment for OAB is based primarily on antimuscarinics.25,28 The clinical use of these drugs is associated with dose-related side effects including dry mouth, dry eyes, constipation, and tachycardia.25,28 Thus, there is a significant need to identify novel therapeutic treatments for OAB, which directly target DSM while minimizing unwanted side effects. A critical step in the development of more effective therapies for OAB involves a better understanding of the basic mechanisms that control DSM excitability and contractility under normal and OAB conditions. We hypothesize that TRPM4 channels are major determinants of DSM excitability and contractility, and therefore, TRPM4 channels represent novel therapeutic targets for OAB. However, there are substantial gaps in our knowledge regarding the functional roles of TRPM4 channels in DSM regulation, especially in humans. Future investigations in this area may lead to the development of selective pharmacological therapies for OAB.24 Targeting TRPM4 channels with novel highly selective inhibitors has the potential to decrease DSM contractility, and thus may have clinical application for the treatment of OAB, which drives the significance of future studies in this area.
Materials and Methods
Patch-clamp electrophysiology was performed as previously described.9,10,29 Quantitative RT-PCR experiments were performed as previously described.29,30 N is the number of animals; n is the number of individual samples/cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the NIH R01 DK084284 and South Carolina Clinical and Translational Research Institute NIH/NCATS UL1TR000062 grants to Petkov GV, and NIH R01 HL091905 and a Monfort Excellence Award from the Monfort Family Foundation to Earley S.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/26289
References
- 1.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int. 2010;106:1114–27. doi: 10.1111/j.1464-410X.2010.09650.x. [DOI] [PubMed] [Google Scholar]
- 2.Birder LA. TRPs in bladder diseases. Biochim Biophys Acta. 2007;1772:879–84. doi: 10.1016/j.bbadis.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skryma R, Prevarskaya N, Gkika D, Shuba Y. From urgency to frequency: facts and controversies of TRPs in the lower urinary tract. Nat Rev Urol. 2011;8:617–30. doi: 10.1038/nrurol.2011.142. [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE, Montell C, Schultz G, Julius D, International Union of Pharmacology International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev. 2003;55:591–6. doi: 10.1124/pr.55.4.6. [DOI] [PubMed] [Google Scholar]
- 5.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol. 2011;300:F49–59. doi: 10.1152/ajprenal.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/S0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 8.Xu XZS, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A. 2001;98:10692–7. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, Malysz J, Petkov GV. Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol. 2013;304:C467–77. doi: 10.1152/ajpcell.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AC, Parajuli SP, Hristov KL, Cheng Q, Soder RP, Afeli SA, Earley S, Xin W, Malysz J, Petkov GV. TRPM4 channel: a new player in urinary bladder smooth muscle function in rats. Am J Physiol Renal Physiol. 2013;304:F918–29. doi: 10.1152/ajprenal.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer L, Rhee PL, Lowe V, Zheng H, Peri L, Ro S, Sanders KM, Koh SD. Basally activated nonselective cation currents regulate the resting membrane potential in human and monkey colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2011;301:G287–96. doi: 10.1152/ajpgi.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–9. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C279–88. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales AL, Earley S. Endogenous cytosolic Ca(2+) buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium. 2012;51:82–93. doi: 10.1016/j.ceca.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol. 2010;299:C1195–202. doi: 10.1152/ajpcell.00269.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinamard R, Demion M, Launay P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 2010;25:155–64. doi: 10.1152/physiol.00004.2010. [DOI] [PubMed] [Google Scholar]
- 17.Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005;280:22899–906. doi: 10.1074/jbc.M501686200. [DOI] [PubMed] [Google Scholar]
- 18.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–33. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–78. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Yoo JC, Yarishkin OV, Hwang EM, Kim E, Kim DG, Park N, Hong SG, Park JY. Cloning and characterization of rat transient receptor potential-melastatin 4 (TRPM4) Biochem Biophys Res Commun. 2010;391:806–11. doi: 10.1016/j.bbrc.2009.11.142. [DOI] [PubMed] [Google Scholar]
- 21.Fliegert R, Glassmeier G, Schmid F, Cornils K, Genisyuerek S, Harneit A, Schwarz JR, Guse AH. Modulation of Ca2+ entry and plasma membrane potential by human TRPM4b. FEBS J. 2007;274:704–13. doi: 10.1111/j.1742-4658.2006.05614.x. [DOI] [PubMed] [Google Scholar]
- 22.Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R. 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol. 2008;153:1697–705. doi: 10.1038/bjp.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simard C, Sallé L, Rouet R, Guinamard R. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol. 2012;165:2354–64. doi: 10.1111/j.1476-5381.2011.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol. 2012;9:30–40. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 26.Ganz ML, Smalarz AM, Krupski TL, Anger JT, Hu JC, Wittrup-Jensen KU, Pashos CL. Economic costs of overactive bladder in the United States. Urology. 2010;75:526–32, e1-18. doi: 10.1016/j.urology.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 27.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 28.Andersson KE. Pharmacotherapy of the overactive bladder. Discov Med. 2009;8:118–24. [PubMed] [Google Scholar]
- 29.Hristov KL, Afeli SAY, Parajuli SP, Cheng Q, Rovner ES, Petkov GV. Neurogenic detrusor overactivity is associated with decreased expression and function of the large conductance voltage- and Ca(2+)-activated K(+) channels. PLoS One. 2013;8:e68052. doi: 10.1371/journal.pone.0068052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afeli SA, Rovner ES, Petkov GV. SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol. 2012;303:F559–68. doi: 10.1152/ajprenal.00615.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


