Abstract
Bacteremia caused by methicillin-resistant Staphylococcus aureus (MRSA) USA600 has been associated with increased patient mortality. We found that USA600 MRSA exhibited significantly increased resistance to human cathelicidin LL-37 killing and daptomycin MIC creep compared to non-USA600 MRSA. Virulent health care-associated MRSA strains may coevolve innate host defense peptide and antibiotic resistances.
TEXT
Methicillin-resistant Staphylococcus aureus (MRSA) is responsible for 10,000 to 20,000 annual deaths in the United States and is no longer confined to the health care setting (1). While USA100 is the most common health care-associated MRSA genotype and USA300 predominates in community-onset infections, considerable overlap is emerging (2). In addition, significant differences in local epidemiology complicate the attempt to generalize recommendations for therapy. For example, a gradual increase in the central tendency of vancomycin MICs (MIC creep) is recognized in some centers as a predictor of vancomycin treatment failure, but it is not seen in other hospitals. Such local differences are often lost in large susceptibility analyses (3). Furthermore, some hospitals report specific clones that are associated with worse patient outcomes.
To initiate a study of the fundamental biological differences of problematic MRSA clones at specific hospitals, we focused on USA600 MRSA at Henry Ford Hospital, a tertiary 800-bed hospital in Detroit, MI. In a prior study, the USA600 MRSA lineage was a significant independent risk factor for vancomycin treatment failure in bacteremia, with a mortality rate of 60%, compared to 20% with USA100 MRSA (4, 5). Interestingly, these strains showed 53% heteroresistance to vancomycin (heteroresistant vancomycin-intermediate S. aureus [hVISA]), a rate much higher than that previously reported for MRSA, yet only 25% of patients had received vancomycin in the previous 90 days (5). This led us to pursue the possibility that resistance to a host immune defense factor was increased in USA600 MRSA compared to that of non-USA600 MRSA bloodstream isolates from the same hospital.
Sixty-eight MRSA clinical bloodstream isolates (45 USA600 and 23 non-USA600 isolates consisting of the USA100 and USA300 strains) were subjected in duplicate to 90-min in vitro killing assays (6) with 16 μM human cathelicidin LL-37, a critical antimicrobial host defense peptide produced by phagocytic and epithelial cells (7, 8). The assays were performed by K. Guram, who was blinded to all genotypic, susceptibility, and clinical data surrounding the isolates that were previously determined by the Detroit investigators (4, 5).
Figure 1A displays bacterial survival stratified by USA600 versus other MRSA (consisting of the USA100 and USA300 lineages), noting a highly significant increased resistance to LL-37 killing of the USA600 strain (P < 0.001). Stratification by individual USA types showed significant increased resistance to killing of USA600 by LL-37 compared individually to the USA100 (P = 0.021) and USA300 (P = 0.017) strains (see Fig. S1A in the supplemental material).
FIG 1.
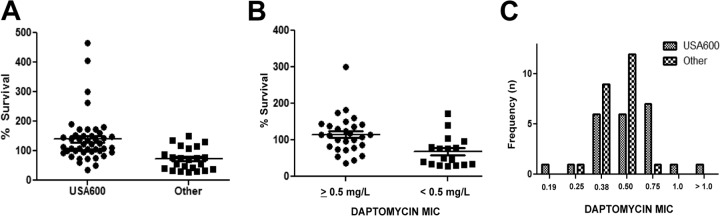
LL-37 (16 μM) killing of MRSA bloodstream isolates at 90 min, stratified by USA600 versus other MRSA isolates (A) or daptomycin MIC (B). USA600 was more resistant to killing (P < 0.0001, Mann-Whitney U test), and MRSA isolates with a daptomycin MIC of ≥0.5 were significantly more resistant to killing (P < 0.001, Mann-Whitney U test). (C) Daptomycin MIC distribution of MRSA bloodstream isolates demonstrating an increased MIC distribution among USA600 versus other MRSA strains (P < 0.01, Mann-Whitney U test). Horizontal lines denote the mean value for the population.
We previously reported cross-susceptibility between daptomycin and thrombin-induced platelet microbicidal proteins (tPMP), another class of cationic defense peptides (9, 10). A similar relationship was noted with the current MRSA collection and LL-37. Figure 1B shows the percent survival in LL-37 assays of 46 MRSA isolates with daptomycin MIC data (Etest) from the prior study stratified by daptomycin MIC (<0.5 versus ≥0.5 mg/liter), as determined by Etest (4, 5). MRSA isolates with a daptomycin MIC of <0.5 mg/liter were significantly more susceptible to killing by LL-37 than were those with MICs of ≥0.5 mg/liter (P < 0.001).
Given the above findings, we hypothesized that a trend toward a higher daptomycin MIC may be present in USA600 MRSA than in non-USA600 MRSA. Figure 1C demonstrates a notable upward shift in the daptomycin MIC in USA600 MRSA, as the daptomycin MIC90 of non-USA600 MRSA was 0.5 mg/liter, whereas the modal daptomycin MIC for USA600 MRSA was 0.75 mg/liter.
Our finding that USA600 MRSA was more resistant to killing by LL-37 than USA300 and USA100 MRSA prompted us to examine whether the LL-37 resistance phenotype might translate directly to important metrics of clinical outcome: patient mortality and bacteremia duration. We stratified the bacteremia durations of <4 days versus ≥4 days based on our prior data showing this to be a critical marker for patient outcome (11). Focusing on the USA600 cohort in order to eliminate clonal type as a variable, we found no statistically significant relationship between patient mortality or bacteremia duration and resistance to killing by LL-37 (see Fig. S1B and C, respectively, in the supplemental material).
Recent data have demonstrated that antibiotic resistance in S. aureus is intimately intertwined with resistance to endogenous cationic host defense peptides produced by the innate immune system in these ways: (i) vancomycin selection pressure on MRSA in vitro and in vivo results in resistance to tPMP killing (12), (ii) the loss of daptomycin susceptibility in MRSA is accompanied by an increased cationic host defense peptide resistance phenotype (10), (iii) reduced tPMP killing is observed among MRSA isolates from daptomycin-naive patients with a daptomycin MIC of 1 mg/liter versus those with an MIC of <0.5 mg/liter (9), and (iv) daptomycin-nonsusceptible S. aureus emerges in vivo in the absence of any administered antibiotics, presumably under selection pressure by innate host defense peptides (13).
While several studies have examined the impact of vancomycin MICs on clinical outcome in S. aureus bacteremia (14, 15), data are limited on examinations of the impact of resistance to host defense peptides on outcomes. Recent findings that susceptibility to vancomycin has an influence even on the outcomes of methicillin-susceptible S. aureus (MSSA) bacteremia treated with β-lactams strongly suggests that an elevated vancomycin MIC can be a marker of a more broadly encompassing virulence property of these strains (15), such as antimicrobial peptide resistance, and that coresistance to innate host defense peptides may be a direct factor influencing treatment outcome in S. aureus bacteremia. Supporting evidence of this is found in unpublished data from our laboratory of LL-37 killing assays (64 μM) performed against random samples of VISA (n = 5), hVISA (n = 6), and MRSA (n = 6) bloodstream isolates showing significantly increased resistance to LL-37 killing among VISA isolates (G. Sakoulas, unpublished observations).
To determine if poor response to antimicrobial therapy and increased mortality associated with specific clonal lineages can be explained by resistance to killing by host defense peptides, like LL-37, we focused on USA600 MRSA bloodstream isolates and compared their susceptibility to killing by the cationic host defense peptide human cathelicidin, LL-37, to those of USA100 and USA300 MRSA bloodstream isolates from a single hospital, in which USA600 was a strong predictor of mortality. LL-37 was chosen due to increased appreciation of its importance in patient survival from infection among hemodialysis patients, who are particularly vulnerable to invasive S. aureus disease (16). The results observed in this subset of MRSA bloodstream isolates suggest that higher mortality may be related to increased resistance to killing by LL-37. Interestingly, this phenotype also confers an increased trend in daptomycin MIC, suggesting that these high-risk MRSA isolates may have already begun on the path toward resistance to antimicrobial therapy. Our attempts to further link this phenotype directly to patient outcome were unsuccessful, as we found no statistically significant relationship between patient all-cause mortality and bacteremia duration; this suggests that other factors may be more important in driving individual patient outcome in S. aureus bacteremia.
Despite the limitation of being a single-center study, the data from this study suggest that predominant health care-associated clones at different hospitals warrant a careful examination as to how they differ not only with respect to their MICs to vancomycin and daptomycin but also in their innate host defense peptides. We anticipate that reduced susceptibility to host defense peptide killing may be a fitness property common to health care-associated MRSA isolates that, in addition to host comorbidities, contributes to higher mortality of MRSA bacteremia in these settings (17). Emerging evidence exists that reduced susceptibility to host defense peptides may be a feature of other S. aureus clones (e.g., clonal complex 30 [CC30]) associated with adverse outcomes (18–20). What will be more challenging but important to determine is whether bacterial resistance to host defense peptides is being driven by patient exposure to multiple antibiotics, which itself poses an additional risk of adverse consequences to patients (21), or if these clones have intrinsic differences that render them a selective advantage in the antibiotic-selective health care setting.
Supplementary Material
ACKNOWLEDGMENTS
G.S. has received speaking honoraria from Cubist, Forest, and Novartis Pharmaceuticals, consulting fees from Cubist and Forest Pharmaceuticals, and research grant support from Forest Pharmaceuticals. K.R. and M.Z. have received research grant support from Cubist Pharmaceuticals.
Funding for this research was provided by NICHD Developmental and Translational Pharmacology of Pediatric Antimicrobial Therapy grant U54 HD071600-01 09/26/2011-06/30/2016 (to G.S. and V.N.) and Great Lakes Regional Center for Excellence in Biodefense and Emerging Infectious Disease Research grant AI057153 (to V.N.). The funding bodies had no role in study design, data collection or analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 19 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00189-14.
REFERENCES
- 1.Klevens RM, Morrison MA, Nadie J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core Surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 2.Prosperi M, Veras N, Azarian N, Rathore M, Nolan D, Rand K, Cook RL, Johnson J, Morris JG, Jr, Salemi M. 2013. Molecular epidemiology of community-associated methicillin-resistant Staphylococcus aureus in the genomic era: a cross-sectional study. Scientific Reports. 3:1902–1909. 10.1038/srep01902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal JJ, Rybak MJ, Snydman DR, Steed LL, Waites K, Jones RN. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127–4132. 10.1128/AAC.00616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CL, Lu M, Cheema F, Osaki-Kiyan P, Perri MB, Donabedian S, Haque NZ, Zervos MJ. 2011. Prediction of failure in vancomycin-treated methicillin-resistant Staphylococcus aureus bloodstream infection: a clinically useful risk stratification tool. Antimicrob. Agents Chemother. 55:4581–4588. 10.1128/AAC.00115-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore CL, Osaki-Kiyan P, Perri MB, Donabedian S, Haque NZ, Chen A, Zervos MJ. 2010. USA600 (ST45) methicillin-resistant Staphylococcus aureus bloodstream infections in urban Detroit. J. Clin. Microbiol. 48:2307–2310. 10.1128/JCM.00409-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of daptomycin binding. Clin. Infect. Dis. 53:158–163. 10.1093/cid/cir340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijnik A, Hancock RE. 2009. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 16:41–47. 10.1097/MOH.0b013e32831ac517 [DOI] [PubMed] [Google Scholar]
- 8.Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 280:22–35. 10.1016/j.cellimm.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 9.Mishra NN, Bayer AS, Moise PA, Yeaman MR, Sakoulas G. 2012. Reduced susceptibility to host-defense cationic peptides and daptomycin coemerge in MRSA from daptomycin-naive bacteremic patients. J. Infect. Dis. 206:1160–1167. 10.1093/infdis/jis482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra NN, McKinnell J, Yeaman MR, Rubio A, Nast CC, Chen L, Kreiswirth BN, Bayer AS. 2011. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4012–4018. 10.1128/AAC.00223-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose WE, Eickhoff JC, Shukla SK, Pantrangi M, Rooijakkers S, Cosgrove SE, Nizet V, Sakoulas G. 2012. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J. Infect. Dis. 206:1604–1611. 10.1093/infdis/jis552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakoulas G, Eliopoulos GM, Fowler VG, Jr, Moellering RC, Jr, Novick RP, Lucindo N, Yeaman MR, Bayer AS. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 49:2687–2692. 10.1128/AAC.49.7.2687-2692.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra NN, Yang SJ, Chen L, Muller C, Saleh-Mghir A, Kuhn S, Peschel A, Yeaman MR, Nast CC, Kreiswirth BN, Crémieux AC, Bayer AS. 2013. Emergence of daptomycin resistance in daptomycin-naïve rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS One 8:e71151. 10.1371/journal.pone.0071151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hal SJ, Fowler VG., Jr 2013. Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin. Infect. Dis. 56:1779–1788. 10.1093/cid/cit178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Gao W, Christiansen KJ, Coombs GW, Johnson PD, Howden BP. 2011. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J. Infect. Dis. 204:340–347. 10.1093/infdis/jir270 [DOI] [PubMed] [Google Scholar]
- 16.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R. 2009. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin. Infect. Dis. 48:418–424. 10.1086/596314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubenstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliot TS, Levine DP, Bayer AS, ICE Investigators 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 18.Moise PA, Forrest A, Bayer AS, Xiong YQ, Yeaman MR, Sakoulas G. 2010. Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: role of platelet microbicidal protein killing and agr genotypes. J. Infect. Dis. 201:233–240. 10.1086/649429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler VG, Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J. Infect. Dis. 196:738–747. 10.1086/520088 [DOI] [PubMed] [Google Scholar]
- 20.Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 190:1140–1149. 10.1086/423145 [DOI] [PubMed] [Google Scholar]
- 21.Shorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, Micek ST, Kollef MH. 2013. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin. Infect. Dis. 57:362–367. 10.1093/cid/cit254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


