Abstract
Purpose
To evaluate the potential utility of collagen-based corneal implants with anti–Herpes Simplex Virus (HSV)-1 activity achieved through sustained release of LL-37, from incorporated nanoparticles, as compared with cell-based delivery from model human corneal epithelial cells (HCECs) transfected to produce endogenous LL-37.
Methods
We tested the ability of collagen-phosphorylcholine implants to tolerate the adverse microenvironment of herpetic murine corneas. Then, we investigated the efficacy of LL-37 peptides delivered through nanoparticles incorporated within the corneal implants to block HSV-1 viral activity. In addition, LL-37 complementary DNA (cDNA) was transferred into HCECs to confer viral resistance, and their response to HSV-1 infection was examined.
Results
Our implants remained in herpetic murine corneas 7 days longer than allografts. LL-37 released from the implants blocked HSV-1 infection of HCECs by interfering with viral binding. However, in pre-infected HCECs, LL-37 delayed but could not prevent viral spreading nor clear viruses from the infected cells. HCECs transfected with the LL-37 expressed and secreted the peptide. Secreted LL-37 inhibited viral binding in vitro but was insufficient to protect cells completely from HSV-1 infection. Nevertheless, secreted LL-37 reduced both the incidence of plaque formation and plaque size.
Conclusion
LL-37 released from composite nanoparticle-hydrogel corneal implants and HCEC-produced peptide, both showed anti–HSV-1 activity by blocking binding. However, while both slowed down virus spread, neither was able on its own to completely inhibit the viruses.
Translational Relevance
LL-37 releasing hydrogels may have potential utility as corneal substitutes for grafting in HSV-1 infected corneas, possibly in combination with LL-37 producing therapeutic cells.
Keywords: cornea, HSV-1, antiviral peptides; nanoparticles, gene transfer
Introduction
The cathelicidins are innate host defense peptides. In humans, there is only one cathelicidin, the18-kDa human cationic antimicrobial protein (hCAP18), of which LL-37 is a 37 amino acid C-terminal peptide domain with active antimicrobial and antiviral activity.1 In the eye, LL-37 is expressed by the cornea epithelium and has been reported to have potent antiviral activity against herpes simplex virus (HSV)-1,2 the main infectious cause of vision loss and blindness worldwide.3
The global incidence of ocular infection by HSV-1 is roughly 1.5 million per year, including 40,000 new cases of severe monocular visual impairment or blindness each year.4 Once infected with HSV-1, the corneal nerves become sites of latency for the viruses and there is evidence for corneal latency as well.5 Recurrent disease that can lead to uncontrolled stromal inflammation, scarring, and hence, corneal opacity and vision loss, is referred to as herpes simplex keratitis (HSK).3 The only widespread treatment available for a scarred cornea is transplantation. However, this is a high-risk procedure with a low success rate of retaining a clear corneal graft, as the surgery itself has been reported to cause viral reactivation and an inflammation cascade that will affect the new graft.3
Our objective is to develop composite corneal implants as potential alternatives to donor corneas for restoring vision to HSK patients. We have previously shown that corneal implants comprising cross-linked collagen designed to mimic the extracellular matrix of the human cornea is able to promote regeneration of corneal cells and nerves in in a Phase 1 clinical study of 10 patients.6 However, for implantation into corneas with severe immunopathologies such as alkali burns and HSK, we reinforced the implant with a second network of poly(ethylene glycol) diacrylate (PEGDA)-phosphorylcholine. To minimize the effects of reactivated HSV-1 viruses, the implants were loaded with nanoparticles that released LL-37.
Although the antiviral drug acyclovir (ACV) and its prodrugs have been used to treat HSK, penetration through the corneal epithelium is inefficient. Hence, high doses are given systemically to treat HSK. Prophylactic use of ACV reduces disease recurrence in the cornea although not efficiently (e.g., 23.1% of patients on a 12-month treatment regime of 800 mg ACV or 500 mg of its prodrug, valacyclovir, daily still had recurrent disease).7 Therefore, even at high doses, the drugs are not highly effective and side effects of long-term use include nausea, kidney impairment, and headache,7 and even ACV-resistance in patients on chronic antiviral prophylaxis.8 ACV-resistant HSV mutant strains have now been isolated from both immunocompromised and immunocompetent patients.9,10 As a naturally occurring cationic peptide and part of the innate immune system, LL-37 has been proposed as an alternative to ACV for prophylactic use, particularly since it is produced by corneal epithelial cells.
In this study, we first determined the ability of our reinforced corneal implant, comprising a network of collagen fibrils supplemented with a network of 2-methacryloyloxyethyl phosphorylcholine (MPC)-PEGDA to withstand the adverse microenvironment of the HSK cornea, to confirm its suitability for use as graft, and hence, its appropriateness for further development into a potential therapeutic vehicle. We then examined the feasibility for sustained LL-37 release from nanoparticles within our collagen-MPC implants to suppress virus activity. As HSK often damages and depletes the healthy stem cell pool, stem cell replacement therapy will likely be required. Therapeutic replacement cells, however, will require “enhancement” via gene transfer to be virus resistant. As corneal epithelial stem cell transplantation is now a routine procedure,11,12 we also examined the feasibility of LL-37 peptide production by therapeutic cells as a means for conferring resistance to the virus. We used a line of immortalized human corneal epithelial cells (HCECs) with properties similar to normal corneal epithelial cells,13 but with the added advantage of homogeneity for comparative assessments as a model cells.
Host defense peptides like LL-37 have been shown to inhibit viral infection by blocking either viral entry into host cells or replication by suppressing viral gene expression.14 Other natural and synthetic cationic peptides including cell penetrating peptides have also been found to inhibit viruses. Although their exact antiviral mechanism is poorly understood, it is believed that viral envelope disruption and viral entry blocking are involved.2,15 One such cationic peptide with documented anti-HSV activity is the entry blocker (EB) peptide. It comprises the fibroblast growth factor 4 (FGF4) signal sequence and an additional N-terminal RRKK sequence. Like LL-37, EB has been reported to have antiviral activity against several viruses including HSV-1 viruses.16–18 Here we compare the efficacy of LL-37, whose mechanism of antiviral activity is still unclear, with EB, which has been reported to directly interact with HSV-1 virions and inactivate them irreverrsibly.17
Materials and Methods
Collagen-MPC Hydrogels
Collagen-MPC hydrogels were prepared as we previously described.19 Briefly, 500 mg of 14% (wt/wt) porcine type I acidic atelocollagen solution (Nippon Meat Packers, Inc., Tokyo, Japan) was buffered with 150 μL of 0.625 M morpholinoethanesulfonic acid (MES; EMD Chemicals, Gibbstown, NJ) buffer in a syringe mixing system. Fifteen microliters of 2 M NaOH was added to adjust pH to 5.0. Then, N-hydroxyl-succinimide (NHS), MPC (Paramount Fine Chemicals Co. Ltd, Dalian, China), PEGDA (Sigma-Aldrich Sweden AB, Stockholm, Sweden), ammonium persulphate (APS; Sigma-Aldrich), N,N,N′,N′-tetramethyl ethylene diamine (TEMED; Sigma-Aldrich), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC; Sigma-Aldrich) were sequentially added into the syringe mixing system and mixed with the collagen solution at 0°C. The NHS:EDC:collagen ratio was 0.35:0.7:1 while the MPC:collagen ratio (wt/wt) was 1:2. PEGDA:MPC ratio (wt/wt) equaled 1:3, APS:MPC ratio was 0.03:1, APS:TEMED equaled 1:0.77. The final mixture was immediately dispensed into glass-plate molds. The hydrogels were cured at 100 % humidity under nitrogen at room temperature for 16 hours, and then demolded in phosphate-buffered saline (PBS) solution.
For in vivo experiments we substituted recombinant human collagen type III (RHC; FibroGen Inc., San Francisco, CA) for the porcine collagen. An 18% RHCIII starting solution was used for final preparation of hydrogels comprising RHCIII-MPC that were 150- to 200-μm thick. We have previous shown that the commercially available RHCIII is functionally equivalent to recombinant human collagen type I (discontinued by manufacturer) as implants in pigs over a 12-month observation period.20
Implantation of Collagen-MPC Hydrogels Into Herpetic Murine Corneas
The ability of collagen-MPC hydrogels to survive in the herpetic cornea was assessed by comparing the rate of rejection with that of mismatched allograft donor corneas in a murine model of graft rejection.
All in vivo studies were performed according to the guidelines described in the ARVO Statement for the Use of Animals in Vision and Ophthalmic Research and Animal License Act (United Kingdom). To determine the ability of RHC-MPC hydrogels to withstand the HSK cornea microenvironment compared with mismatched allograft corneas, hydrogels were grafted into the right cornea of 12-week-old BALB/c mice (obtained from the Medical Research Facility, University of Aberdeen, Scotland) that were previously infected with HSV-1 strain RE 6 weeks before grafting procedure (n = 13)21 and had gone on to develop extensive corneal scarring and neovascularization indicative of HSK. Controls (n = 6) were animals that were grafted with donor corneas that differed across major and minor MHC antigens. Prior to grafting in HSK recipients, the RHC-MPC hydrogel sheets were washed in sterile PBS and gentamicin overnight and 1.5-mm diameter implants were trephined out for full thickness grafting procedure. Each implant was secured by one 11-0 continuous suture (11-0 Ethilon, Ethicon, UK), as we previously described.22 Mice with postoperative bleeding and severe inflammation were excluded from the experiment. The implants were examined under the operating microscope on alternate days and graded according the clinical signs of increased opacity as described previously or extrusion/melt of the implant was clinically obvious.23 The time to rejection of implants (corneal opacity score) compared with allograft corneas was recorded.
EB and LL-37 Peptide Synthesis
LL-37 (CSGLLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2) and EB (RRKKAAVALLPAVLLALLAP-NH2) peptides were synthesized on a Symphony automated peptide synthesizer (Protein Technologies Inc., Tucson, AZ) using standard fluorenylmethoxycarbonyl (Fmoc) chemistry with HCTU (2-(6-Chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate) (ChemPep Inc., Wellington, FL) as the activating reagent. The synthesis was performed on a 0.1-mmol scale with Fmoc-PAL-PS resin (Applied Biosystems, Life Technologies Europe BV, Stockholm, Sweden) using 4-fold excess of amino acid in each coupling. The peptides were cleaved from the resin by treatment with a mixture of trifluoroacetic acid (TFA), water and tri-isopropylsilane (TIS) (95:2.5:2.5 vol/vol; 10 mL/g of polymer) for 2 hours at room temperature (RT). After filtration, TFA was evaporated and the peptides were precipitated by the addition of cold diethyl ether, centrifuged, and lyophilized. The crude products were purified by reversed-phase HPLC on a semi-preparative C-18 column (Grace Vydac, Helsingborg, Sweden), and identified from their MALDI-TOF spectra. Scrambled LL-37 (sc-LL-37) peptide (RP20332; GenScript USA Inc., Piscataway, NJ) was used as a control.
Effect of EB and LL-37 on Corneal Epithelial Cells
Immortalized HCEC13 were cultured in 96-well tissue culture plates supplemented with Keratinocyte Serum Free Medium containing L-glutamine, human epidermal growth factor (EGF) and bovine pituitary extract (BPE; Life Technologies Europe BV). As the corneal epithelium is continuously renewed, we examined the effects of increasing concentrations of EB and LL-37 peptides on HCEC proliferation. A colorimetric WST-1–based cell proliferation assay (Roche Applied Science, Mannheim, Germany) was performed according to the manufacturer's instructions. The cells were incubated with the WST-1 reagent at 37°C for about 30 minutes and then read using an ELISA reader at 450 nm (N = 3/group; Molecular Devices, Sunnyvale, CA).
Viral Propagation and Titration
The HSV-1, strain F,24 (a gift from Earl Brown, University of Ottawa, Ontario, Canada) was used for the majority of this study. Virus propagation was carried out in Vero cells (ATCC, CCL-81) maintained in culture flasks supplemented with Dulbecco's modified Eagle's medium (DMEM; Life Technologies) containing 10% fetal bovine serum (FBS). To harvest virus, the flasks were frozen and thawed, and the culture supernatants were clarified by centrifugation at 400× g for 5 minutes at 4°C. The virus was then split into small aliquots and stored at −80°C until used.
To determine virus titers, culture media was collected for standard plaque forming assays. A range of virus dilutions were added onto 80% confluent Vero cells and incubated at 37°C for 1 hour. After adsorption, the cells were overlain with DMEM containing 5% FBS and 1% agarose and incubated for 3 days, after which they were fixed with 10% formaldehyde and stained with 0.5% crystal violet. The number of plaques formed were counted.
Effect of Free EB and LL-37 on HSV-1 Activity
To determine the efficacy of free EB, LL-37, or sc-LL-37 in blocking HSV-1 activity, HCECs were infected with viruses at a multiplicity of infection (MOI) of 1 for 1 hour in the presence of varying concentrations of peptide from 1 to 20 μg/ml. After 1 hour of adsorption, the inoculated virus was washed away and the cells were cultured in fresh medium containing EB, LL-37, or sc–LL-37 peptides. At 24 and 48 hours postinfection (hpi), the culture medium were collected for plaque-forming assays. HSV-1 infected cells were visualized using an immunofluorescence assay, as described below. A MOI of 1 (1 virus per 1 cell) was used to produce an infection that will be sustained over the 48 hours, by infecting over 63% of the HCECs initially and allow for the infection to progress.
Immunofluorescence Assay (IFA)
Cells were fixed with 4% paraformaldehyde for 30 minutes at 4°C, permeabilized with PBS containing 0.5% Triton X-100 for 5 minutes at RT, and stained with primary antibodies at RT for 1 hour. The antibodies used in this assay were anti–LL-37 (sc-166,770; Santa Cruz Biotechnology Inc., Dallas, TX), anti–HSV-1 Infected Cell Protein 4 (ICP4; sc-69,809; Santa Cruz Biotechnology Inc., Dallas, TX), and biotinylated anti–HSV-1/2 antibodies. After a PBS/Tween wash, the cells were reacted with Cy3-conjugated goat anti-mouse secondary antibody and Alexa Fluor 594-conjugated or Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody, respectively. Cells were incubated with 4′,6-diamidino-2-phenylindole (DAPI) to stain for nuclei and examined with a Zeiss fluorescent microscope (Zeiss Axio Observer Z1, Carl Zeiss AB, Stockholm, Sweden).
Composite Collagen-MPC Hydrogels Containing SiNP Encapsulated LL-37
The preparation of SiO2 nanoparticles (SiNPs) has been previously described.25 Briefly, 4 mL cyclohexane (Acros Organics, Morris Plains, NJ) was combined with 1 mL Triton (Sigma-Aldrich), followed by the addition of a 1 mL LL-37 solution (14 mg/mL). Under magnetic stirring, 0.75 mL tetraethyl orthosilicate (TEOS; Sigma-Aldrich) was added dropwise, followed by slow neutralization of the mixture using concentrated ammonia hydroxide (target pH was 4.0–6.0). The mixture was stirred for 2 days at 50°C. The resulting nanoparticles (NPs) were washed twice with 50% ethanol, and then vacuum dried. Particle size and morphology were checked on a JEOL1230 transmission electron microscope (TEM; JEOL USA, Inc., Peabody, MA). Encapsulation efficacy of LL-37 was calculated at 54.4%. The release kinetics were followed over 2 weeks.
To incorporate free LL-37 into collagen-MPC hydrogels, 300 μL of LL-37 solution (500 μg/mL) was added to the hydrogel solution prior to addition of APS to cure. To incorporate LL-37 SiNPs into collagen-MPC, 300 μL of LL-37 SiNPs solution, (7 mg/mL) was added to the mixture prior to APS curing.
Effects of Prophylactic Release of LL-37 on HSV-1 Activity
To simulate the prophylactic use of LL-37 in suppressing HSV-1 activity, HCECs were cultured in 24-well plates until 90% confluence. Collagen-MPC hydrogels incorporating LL-37 SiNPs, free LL-37, or hydrogels only were placed on top of the cells for 24 to 72 hours, during which LL-37 would be released. The cells were then exposed to HSV-1 viruses for 1 hour by introduction into the culture medium. A MOI of 0.1 was used to produce an infection that will progress over a further 48 hours. The presence of viral protein in the cells was detected by immunofluorescence as described above. Culture supernatant containing shed viruses were tittered by a plaque formation assay.
Effects of LL-37 Release on HSV-1 Spreading
To determine the efficacy of the released LL-37 to prevent viral spread, HCECs cultured in 24-well plates were exposed viruses for 1 hour at a MOI of 0.05. This MOI selected to infect a small proportion of HCECs to allow for observations of cell-to-cell virus spread. After virus removal and a medium change, hydrogels with and without LL-37 (free and SiNP encapsulated) were introduced to the infected cells. As previously described, presence of HSV-1 protein in the cells was detected by immunofluorescence, while a plaque formation assay was used to measure virus titer.
EB and LL-37 Vector Construction
The pSin backbone plasmid was a gift from Jeffrey Dilworth (Ottawa Hospital Research Institute, Ontario, Canada). Briefly, the vectors used were constructed using the elongation factor 1-alpha (EF1-α) promoter to drive EB (5′-CGAAGGAAGAAGGCCGCGGTAGCGCTGCTCCCGGCGGTCCTGCTGGCCTTGCTGGCGCCCTAG-3′), or LL-37 (5′-CTGCTGGGTGATTTCTTCCGGAAATCTAAAGAGAAGATTGGCAAAGAGTTTAAAAGAATTGTCCAGAGAATCAAGGATTTTTTGCGGAATCTTGTACCCAGGACAGAGTCCTAG-3′) expression. The immunoglobulin kappa chain (Igκ) leader sequence (5′-ATGGAGACAGACACACTCCTGCTATGGGTACTGCTGCTCTGGGTTCCAGGTTCCACTGGTGAC-3′) and a strong artificial signal peptide (HMM-38) (5′-ATGTGGTGGCGCCTGTGGTGGCTGCTGCTGCTGCTGCTGCTGCTGTGGCCCATGGTGTGGGCC-3′) were used to direct resulting peptides into the cellular secretory pathway.26,27
EB and LL-37 Expressing Cell Lines
To establish stable lines that expressed EB or LL-37, HCECs were transfected with vectors containing a puromycin-resistant gene together with EB or LL-37 (Lipofectamine 2000 Transfection Reagent; Life Technologies). Stable, puromycin-resistant transfectants were selected (2 μg/mL of puromycin; Life Technologies).
Microtiter plate–based ELISA was used for the detection of secreted LL-37. Briefly, the culture supernatants from HCECs expressing LL-37, Igκ-LL-37, HMM-LL-37, or mock transfected cells were coated onto Immulon 2 HB 96-Well Microtiter EIA Plate (ImmunoChemistry Technologies, Bloomington, MN). The nonspecific antibody-binding sites were blocked with PBS containing 5% bovine serum albumin and 0.1% Tween-20. Anti–LL-37 antibody (sc-166,770; Santa Cruz Biotechnology Inc., Dallas, TX) was added to the plate and then reacted with horseradish peroxidase-conjugated anti-mouse IgG antibody (Biorad, Hercules, CA). Finally, 3,3′,5,5′-tetramethylbenzidine liquid substrate (Sigma-Aldrich) was used to develop the plates.
Effects of Endogenous LL-37 Expression on HSV-1 Binding and Infectivity
The antiviral effects of endogenous EB and LL-37 expression in HCECs was tested by exposing the cells to HSV-1 at an MOI equaling 2 (where about 86% of cells are infected and the infection can be sustained for 24 hours). After 24 hours, cells were stained for expressed HSV viral protein ICP4 using IFA as described above.
The ability of LL-37 producing HCECs to block HSV-1 uptake, and hence, be resistant to HSV-1 infection was examined by quantifying the binding of viruses to the surface of HCECs expressing LL-37, Igκ–LL-37, HMM–LL-37, or non–LL-37 expressing HCECs. Green fluorescent protein (GFP)-labelled HSV-1 (K26-GFP), which has fluorescent GFP fused to the capsid, but otherwise behaves like wild-type virus,28 was used to quantify virus binding onto the cell surface and uptake into HCECs lines expressing LL-37. Briefly, 105 PFU of HSV-1–GFP (K26-GFP) (∼MOI = 10) was added into the well for 1 hour on ice. A high MOI was used to enable detection of the GFP signal from the viruses.29 The cells were then washed with ice-cold PBS three times. Green fluorescent protein fluorescence was detected using a POLARstar Omega plate reader (N = 4/group; BMG Labtech, Fisher Scientific, Nepean, Canada).
To characterize the extent to which LL-37 secreting cells were resistant to HSV-1, HCECs expressing LL-37 or unmodified controls in 6-well plates were incubated with 100∼200 PFU of HSV-1 at 37°C for 1 hour. Cells were washed three times with PBS, overlaid with DMEM medium containing 5% FBS and 1% agarose, and incubated at 37°C for 5 days for plaque formation. The percentage of plaque formation was calculated as 100 X (the plaque numbers of LL-37 groups divided by the plaque numbers of HCEC controls; N = 7 per group). The diameters of the plaques were measured using an Axio Vert. A1 microscope and Zen lite software (Carl Zeiss AB, Stockholm, Sweden).
Data and Statistical Analysis
All data are presented as mean ± SD. Statistical analysis (t-test) was performed using Microsoft Excel software. Graft or hydrogel survival was analyzed by nonparametric logrank test (Mantel-Cox test; GraphPrizm). Statistical significance was set at P less than 0.05.
Results
Performance of Collagen-MPC Hydrogels Compared With Corneal Allografts
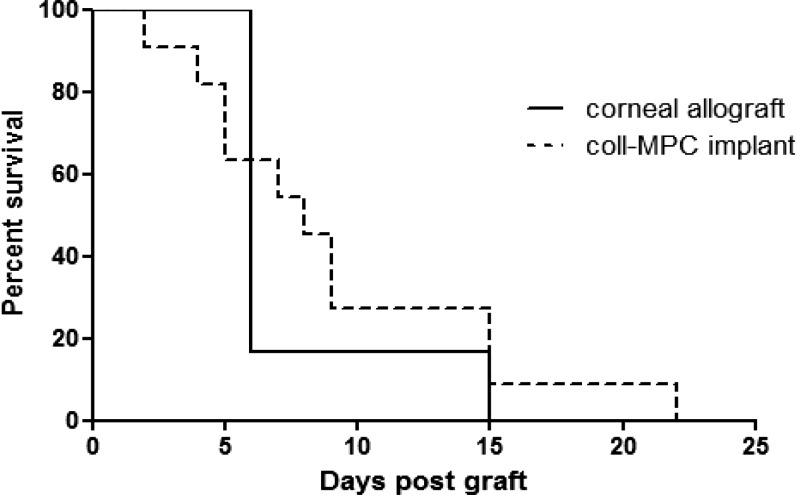
In HSK cornea transplantation, the rate of graft rejection is high despite the use of topical or systemic immunosuppression and anti-inflammatory medication.30 While the mouse grafting model is a rejection model that does not provide direct efficacy information, the results nevertheless show that RHC-MPC grafts were able to persist until 22 days post operation in HSK corneas, In contrast, all donor cornea allografts were rejected by day 15 post operation (Fig. 1). The differences in survival rate between the two groups, however, was not significantly different (P > 0.05).
Figure 1. .
The survival curves of mismatched corneal allografts (n = 6), and collagen-MPC hydrogels (n = 13) in HSK recipients.
Effects of EB and LL-37 Peptides on HSV-1 and Human Corneal Epithelial Cells
The WST-1 results showed that exposure of HCECs to 0, 1, 5, 10, 15, 20 μg/mL of EB, LL-37, or sc-LL-37 for 24 hours and 48 hours had no significant effect (P > 0.05) on cell proliferation (Fig. 2A) and no cytotoxic effects were observed. The cells had a normal morphology with clear nuclei. At a higher dose of 50 μg/ml; however, both EB and LL-37 inhibited cell proliferation.
Figure 2. .
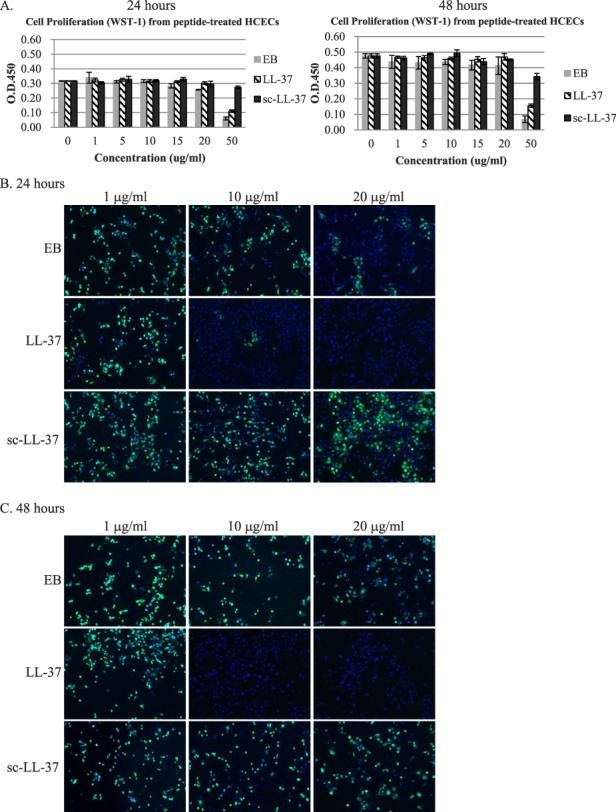
(A) Effect of increasing concentrations of EB, LL-37, or sc-LL-37 peptide on cell proliferation at 24 and 48 hours, as determined by WST-1. (B) Concentration-dependent effect of EB, LL-37, or sc–LL-37 peptides in blocking HSV-1 infection (MOI = 1) in HCECs. The presence of viral protein is indicated by green immunofluorescence, while cell nuclei are stained with DAPI (blue).
When HCECs were exposed to HSV-1 (MOI = 1) in the presence of EB and LL-37 peptide, at 24 and 48 hpi, viral protein expression was reduced by the presence of peptides, particularly LL-37 (Fig. 2B, 2C). The presence of sc–LL-37 peptide at all three tested doses did not produce noticeable changes in viral protein expression. In addition, LL-37 peptide treatment resulted in a marked decrease in virus titer, compared with the more marginal effects of EB. However, it was noted that the reduction in infectivity was more dramatic at 24 than 48 hpi (Table 1). LL-37 was therefore selected for release studies from nanocomposite corneal implants.
Table 1. .
Fold Decrease in Viral Titer in HCECS at 24 and 48 Hours After Treatment With 10 and 20 μg/mL of Either LL-37 or EB Peptide, Compared With Untreated Controls

Effects of Prophylactic Release of LL-37 on HSV-1 Infection
LL-37 release from SiNP within collagen-MPC gels was sustained over 14 days (data not shown). The cumulative, protective effect of LL-37 release was observed in cultures of HCECs infected with HSV-1 (MOI = 0.1). LL-37 released over 24 hours substantially reduced HSV-1 infectivity but the reduction was more dramatic after 72 hours of cumulative drug release. In contrast, hydrogels that contained free LL-37 had very little protective effect against HSV-1 (Figs. 3A, 3C).
Figure 3. .

(A) The prophylactic effects of collagen-MPC hydrogels that incorporated LL-37 SiNPs, free LL-37, or nothing in HSV-1 infection (MOI = 0.1). Red immunofluorescence indicated localization of HSV-1 infected cells; cell nuclei are stained with DAPI (blue). (B) Effects of LL-37 release on virus spreading, after inoculation with HSV-1 (MOI = 0.05). (C) Quantification of HSV-1 titers from (A). (D) Quantification of HSV-1 titers corresponding (B).
Effect of LL-37 Release on HSV-1 Spreading
When HCECs were already infected with HSV-1 viruses (MOI = 0.05), the released LL-37 was able to block HSV-1 activity up to 24 hours of culture (Fig. 3B). By 72 hours, however, the viral titer had increased. Nevertheless, infected HCECs exposed to released LL-37 from the SiNP-hydrogel still showed distinctly less HSV-1 spreading when compared with nontreated HCECs and to the cells treated with hydrogels containing free LL-37 or hydrogels alone (Fig. 3D).
Expression of Endogenous LL-37 in Transfected Cells
The efficacy of two different secretion signals (Igκ and HMM-38)26,27 used to drive EB or LL-37 expression were compared in stable HCECs transfectants. As there are no antibodies against EB, we only evaluated the expression of LL-37 in HCECs. All cells stably transfected with LL-37, Igκ-LL-37, or HMM-LL-37 expressed the peptide, as detected by an anti–LL-37 antibody (Fig. 4A). However, quantitation of peptide expression showed that HCEC transfected with HMM–LL-37 (HCEC/HMM–LL-37) had the highest level of secreted LL-37 (Fig. 4B). These data indicated that HCECs stably transfected with antiviral peptide genes such as LL-37 were able to express and secrete antiviral peptides. However, the amount of secreted LL-37 was very low (Fig. 4B)
Figure 4. .
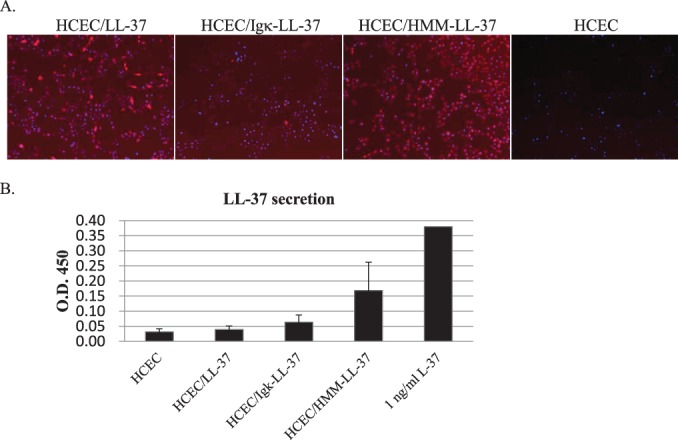
(A) LL-37 expression in stable transfected HCECs. LL-37 peptide within HCECs is fluorescently-labeled red using an anti–LL-37 antibody. The cell nuclei were stained with DAPI (blue). (B) Secreted levels of LL-37 in the culture medium as determined by ELISA.
Effect of Endogenously Expressed Antiviral Peptides on HSV-1
All HCECs expressing EB and LL-37 endogenously, when infected with HSV-1 at a MOI 2 showed the presence of viral ICP4 protein (Fig. 5A). There was no significant reduction (P > 0.05) in infectivity, as determined by plaque formation assays (Fig. 5B).
Figure 5. .
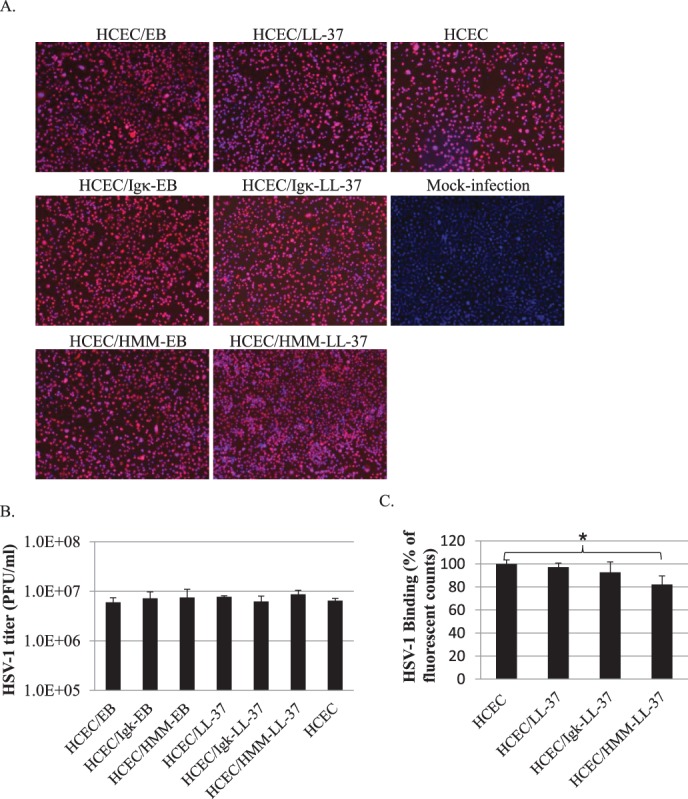
(A) The effect of endogenous expression of EB and LL-37 in HCECs during HSV-1 infection (MOI = 2). At 24 hours after viral exposure, all infected cells expressing viral protein ICP4 were fluorescently-labeled red. (B) Quantification of viral titers (expressed as plaque forming units (PFU/mL) in the presence of cells expressing endogenous EB or LL-37. (C) Relative fluorescent counts of GFP-labelled HSV-1 particles bound to LL-37 producing HCECs. Only HCECs secreting LL-37 (HCEC/HMM–LL-37) showed a 20% decrease in GFP virus fluorescence; *P < 0.05.
However, when challenged with GFP–HSV-1 in a binding assay, we found a significant decrease (P < 0.05) in the binding of HSV-1 to HCECs that were secreting expressing LL-37, compared with non–LL-37 producing or cells with cytosolic LL-37. There was an approximately 20% reduction in overall viral-source GFP fluorescence (Fig. 5C). These results showed that LL-37 expression within HCEC was not able to prevent HSV-1 infection. However, the binding efficiency of HSV-1 to LL-37 secreting cells was dramatically lowered.
With a low MOI, the decrease in binding of HSV-1 to HCEC was more marked. Plaque formation assays showed that HCEC/HMM–LL-37 cells, which secreted the most LL-37 had a 60% reduction in numbers of plaques formed compared with control naïve HCECs (Fig. 6A). In contrast, cells that only had cytosolic LL-37 expression (HCEC/LL-37) had similar numbers of plaques formed as naïve HCECs. Furthermore, sizes of plaques formed in HCEC/HMM–LL-37 but not in other cells were dramatically reduced (from 919 ± 84 μm to 629 ± 102 μm, P < 0.001), indicated that the plaque forming ability of HSV-1 was interfered with the secreted LL-37 (Fig. 6B).
Figure 6. .
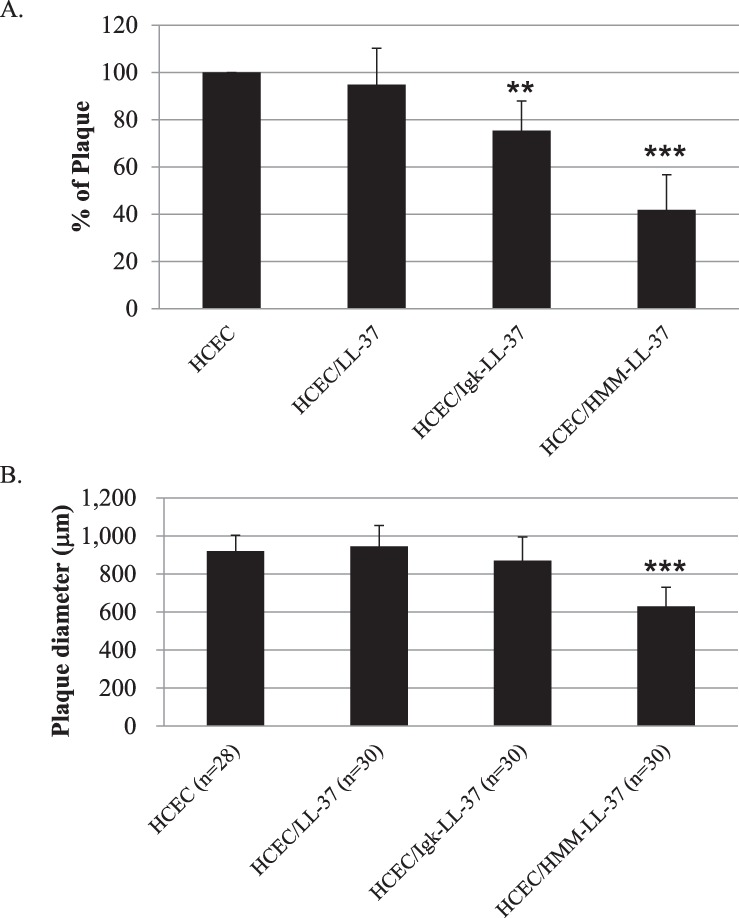
Effects of endogenous LL-37 expression on susceptibility of HCEC to HSV-1 infection, as shown by changes in (A) percentage of plaque formation, and (B) plaque size (calculated from plaque diameters). The data shown here are the average and standard error of three independent experiments. The results of each treatment were compared to their untreated control by Student's t-test, **P < 0.01 and ***P < 0.001.
Discussion
The mouse model of fully mismatched corneal grafting in naïve animals in general has a high rate of rejection with only approximately 20% of acceptance of grafts in long-term.31 The survival of fully mismatched corneal allograft in “high-risk” of rejection graft beds is even worse, the rapid and fulminant rejection occurring in 100% of experimental animals (manuscript in preparation). However, using this model for testing of artificially prepared corneal equivalents (collagen-based hydrogels) as a comparison may indicate the potential for graft retention in comparison to the normal allograft corneal tissue. The collagen-MPC implants survived in HSK recipients for 7 days longer than allografts (Fig. 1), although this difference was not statistically significant. Nevertheless, collagen-MP implants are at least comparable with allograft tissues in their ability to tolerate the adverse environment in HSK corneas, and an appropriate base material to use for delivering therapeutic agents or cells.
While both LL-37 and EB peptides have documented HSV-1 inhibition activity, we found that LL-37 was significantly more potent than EB. Since cationic peptides with antiviral properties have been reported to perturb the viral coat,32 LL-37 most likely worked by disrupting the viral coat, like EB. However, the pleiotropic effects of LL-37 could have also enabled cell survival, contributing to the observed higher survival rate of HCECs in the presence of LL-37 and not EB.
LL-37 could also be effectively encapsulated for sustained release over 20 days. The released peptide retained its activity against HSV-1. However, our results showed that once the cells were infected with virus, even at low MOI (of 0.1) to simulate a mild primary infection, doses of 15 and 20 μg/mL of LL-37 were only able to slow down the virus spreading in a dose-dependent manner. Treatment with LL-37 was not able to clear the virus from already infected cells. This suggests that both exogenously applied free and released LL-37 were only able to affect HSV-1 viruses within the culture medium, but were unable to affect intracellular virus transmission. This is different from the case of HIV, where LL-37 was reported to bind directly to HIV-1 reverse transcriptase to block its activity.33
In the present study, LL-37 peptide was not able to clear HSV-1 from infected cells and hence, would not be effective as a therapeutic agent on its own. However, as LL-37 was able to block viral activity when co-administered, there is potential for use as a prophylactic agent. The results also showed that LL-37 is able to slow down viral spread for up to 72 hours, potentially providing a sufficient margin of time for the infected patient to receive appropriate antiviral drug therapy (e.g., ACV).
We therefore explored the possibility of introducing the LL-37 gene into corneal cells, to allow the cells to produce LL-37 and potentially be sufficiently virus resistant to tolerate recurrent disease. We compared the responses of HCEC produced LL-37 that remained within the cytosol versus cells that secreted LL-37. The use of a strong artificial signal peptide (HMM-38) together with the LL-37 enabled stably transfected corneal epithelial cells to secrete peptide, although the amounts were very low. We should also point out that although LL-37 transfected HCECs had been through a selection process, not all cells would have been transfected equally. Hence, there may be a difference in LL-37 production by different subpopulations of HCECs that could account for the overall low level of LL-37 production reported by the bulk assay used. Nevertheless, the cells that secreted endogenously produced LL-37 were able to reduce viral binding, as visualized by the 20% fluorescence reduction when infected with a high MOI. Infection with a low MOI on the other hand, resulted in a marked decrease in plaque formation by 60% and concomitant reduction in the size of plaques formed. It might be possible that transfer of multiple copies of peptide DNA would result in a stronger block of HSV-1 activity.
As LL-37 is highly cationic and we have shown that high concentrations of peptide were cytotoxic, cells that overproduce LL-37 are not likely to survive. Hence, there is a built-in safeguard against cells whose biosynthetic machinery may get out of control to overproduce LL-37.
In conclusion, we have shown that antiviral effects of the LL-37 peptide observed is most likely due to the blocking of HSV-1 binding to cells. The peptide was ineffective against the viruses once they have been internalized by the cells. Corneal epithelial cells can be transfected to express antiviral peptides such LL-37. Endogenously produced LL-37, even in low titers, was able to reduce viral binding and consequently plaque formation. Delivery of antiviral peptide in this case, by endogenous production by the target cells, appears to be a viable option for continuous delivery of peptide. With further optimization of the gene transfer and copy number of transferred LL-37 genes into HCECs, and/or combinations of different antiviral gene sequences, more complete viral resistance may be possible in the future.
Acknowledgments
The authors thank Martin Mak for technical assistance.
Supported by an EU ERAnet Nanomedicine project grant “I-CARE” (funded by the Swedish Research Council and Vinnova [MG, BL]), and Vironova AB (JA) ; in vivo work was supported by grants from National Health Science Grampian Endowment grant, Saving Sight in Grampian and the IGEN Centre, Linköping University (LK, VR, JVF); and by a post-doctoral fellowship from the Swedish Institute (OB).
*Oleksiy Buznyk and Lucia Kuffova contributed equally to this work.
Disclosure: C.-J. Lee, None; O. Buznyk, None; L. Kuffova, None; V. Rajendran, None; J.V. Forrester, None; J. Phopase, None; M.M. Islam, None; M. Skog, None; J. Ahlqvist, Employed by Vironova AB; M. Griffith, None
Footnotes
Oleksiy Buznyk and Lucia Kuffova contributed equally to this work.
References
- 1.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary A, Higgins GT, Kaye SB. Herpes Simplex Keratitis and Related Syndromes. In: Reinhard T, Larkin F, editors. Cornea and External Eye Disease. Berlin Heidelberg, Germany: Springer;; 2008. pp. 115–152. In. eds. [Google Scholar]
- 4.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy DP, Clement C, Arceneaux RL, Bhattacharjee PS, Huq TS, Hill JM. Ocular herpes simplex virus type 1: is the cornea a reservoir for viral latency or a fast pit stop? Cornea. 2011;30:251–259. doi: 10.1097/ICO.0b013e3181ef241d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001022. 46ra61. [DOI] [PubMed] [Google Scholar]
- 7.Miserocchi E, Modorati G, Galli L, Rama P. Efficacy of valacyclovir vs acyclovir for the prevention of recurrent herpes simplex virus eye disease: a pilot study. Am J Ophthalmol. 2007;144:547–551. doi: 10.1016/j.ajo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 8.van Velzen M, van de Vijver DA, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 2013;208:1359–1365. doi: 10.1093/infdis/jit350. [DOI] [PubMed] [Google Scholar]
- 9.Nugier F, Colin JN, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 10.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 11.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 12.Tsai RJ-F, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 13.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 14.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow PG, Svoboda P, Mackellar A, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6:e25333. doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann SE, Jones JC, Schultz-Cherry S, Brandt CR. Inhibition of Vaccinia virus entry by a broad spectrum antiviral peptide. Virology. 2009;388:248–259. doi: 10.1016/j.virol.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bultmann H, Busse JS, Brandt CR. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J Virol. 2001;75:2634–2645. doi: 10.1128/JVI.75.6.2634-2645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JC, Turpin EA, Bultmann H, Brandt CR, Schultz-Cherry S. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol. 2006;80:11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Deng C, McLaughlin CR, et al. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551–1559. doi: 10.1016/j.biomaterials.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Merrett K, Fagerholm P, McLaughlin CR, et al. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: performance of type I versus type III collagen. Invest Ophthalmol Vis Sci. 2008;49:3887–3894. doi: 10.1167/iovs.07-1348. [DOI] [PubMed] [Google Scholar]
- 21.Frank GM, Divito SJ, Maker DM, Xu M, Hendricks RL. A novel p40-independent function of IL-12p35 is required for progression and maintenance of herpes stromal keratitis. Invest Ophthalmol Vis Sci. 2010;51:3591–3598. doi: 10.1167/iovs.09-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn JI, Kuffova L, Merrett K, et al. Crosslinked collagen hydrogels as corneal implants: effects of sterically bulky vs. non-bulky carbodiimides as crosslinkers. Acta Biomater. 2013;9:7796–7805. doi: 10.1016/j.actbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Kuffova L, Lumsden L, Vesela V, et al. Kinetics of leukocyte and myeloid cell traffic in the murine corneal allograft response. Transplantation. 2001;72:1292–1298. doi: 10.1097/00007890-200110150-00019. [DOI] [PubMed] [Google Scholar]
- 24.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 25.Bareiss B, Ghorbani M, Li F, et al. Controlled release of acyclovir through bioengineered corneal implants with silica nanoparticle carriers. Open Tissue Eng Regen Med J. 2010;3:10–17. [Google Scholar]
- 26.Coloma MJ, Hastings A, Wims LA, Morrison SL. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 27.Barash S, Wang W, Shi Y. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem Biophys Res Com. 2002;294:835–842. doi: 10.1016/S0006-291X(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 28.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology. 2010;397:389–398. doi: 10.1016/j.virol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertelmann E, Jaroszewski J, Pleyer U. Corneal allograft rejection: current understanding. Ophthalmologica. 2002;216:2–12. doi: 10.1159/000048289. [DOI] [PubMed] [Google Scholar]
- 31.Plskova J, Kuffova L, Holan V, Filipec M, Forrester JV. Evaluation of corneal graft rejection in a mouse model. Br J Ophthalmol. 2002;86:108–113. doi: 10.1136/bjo.86.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van ‘t Hof W, Veerman EC, Helmerhorst EJ, Amerongen AV. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 33.Wong JH, Legowska A, Rolka K, et al. Effects of cathelicidin and its fragments on three key enzymes of HIV-1. Peptides. 2011;32:1117–1122. doi: 10.1016/j.peptides.2011.04.017. [DOI] [PubMed] [Google Scholar]