Abstract
Aim: To investigate the association between high myopia (HM) and single nucleotide polymorphisms (SNPs) in the myocilin (MYOC), hepatocyte growth factor (HGF), hepatocyte growth factor receptor (MET), and aggrecan (ACAN) genes in a Han Chinese population. Methods: Sixteen SNPs were genotyped by the SNaPshot method in a subject group composed of 1052 HM patients and 1070 controls. Statistical analysis was performed to determine the association between the SNPs and the susceptibility of HM. Results: Two SNPs (rs3784757 and rs1516794) in ACAN were significantly associated with HM (p=0.0334 and 0.0236, odds ratio [OR]=0.83 and 0.79, respectively). The risk haplotype CA and the protective haplotype TT, generated by rs3784757 and rs1516794, showed significant association with HM (p=0.0327 and 0.0304, OR=1.21 and 0.80, respectively). Two SNPs (rs38857 and rs10215153) in MET and one SNP (rs3784757) in ACAN showed significant association with HM (p=0.0064, 0.0113, and 0.0373; OR=4.14, 5.74 and 0.52; respectively) in the recessive model. None of the other SNPs showed significant association with HM. Conclusions: Our results suggested that genetic variants in ACAN and MET are associated with HM. Functional roles of ACAN and MET in the development of HM need to be further investigated.
Introduction
High myopia (≤−6.0 diopters) is a disorder that can cause poor eyesight by refractive error, bringing about a loss of visual acuity due to severe complications of glaucoma, retinal or choroid detachment (Celorio and Pruett, 1991), and macular degeneration (Burton, 1989). It has the highest prevalence in East Asian populations. The prevalence of high myopia (HM) in a rural Chinese adult population and Singapore adult Chinese population was 1.8% and 9.1% (Wong et al., 2000; Liang et al., 2009). However, the treatments for HM are limited and the pathogenesis of HM remains unclear.
It is believed that myopia is a complex disease caused by the interaction of multiple genetic and environmental factors. However, HM is highly heritable, where genetic predisposition seems to be a dominant factor (Dirani et al., 2006). Identification of genes responsible for myopia, especially HM, is very important but very difficult, although several loci for HM have been mapped (Yang et al., 2009; Ma et al., 2010; Shi et al., 2011a, 2011b; Wojciechowski, 2011). However, no convincing causal genes have yet been identified at these loci. Recently, the candidate gene association studies of HM or refractive error have led to the identification of several susceptible genes including the myocilin (MYOC) gene, the hepatocyte growth factor (HGF) gene, the hepatocyte growth factor receptor (MET) gene, and the aggrecan (ACAN) gene (Han et al., 2006; Tang et al., 2007; Vatavuk et al., 2009; Yanovitch et al., 2009; Yip et al., 2011). To further investigate whether these genes are associated with HM in Han Chinese, 16 single nucleotide polymorphisms (SNPs), which were previously reported to be associated with myopia or HM of the MYOC, HGF, MET, and ACAN genes, have been chosen to be tested in this study in a Han Chinese population composed of 1052 HM cases and 1070 normal controls.
Materials and Methods
Ethics statement
All procedures used in this study conformed to the tenets of the Declaration of Helsinki. The Institutional Review Board and the Ethics Committee of the Affiliated Hospital of Qingdao University School of Medicine of China and Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital approved the protocols used. Informed consent was obtained from all participants.
Subjects
All of the participants in this study were drawn from the Chinese Han population. All individuals (HM patients and normal controls) were recruited at the clinic and ward of the ophthalmic department, the Affiliated Hospital of Qingdao University School of Medicine of China. All subjects underwent standard ophthalmologic examinations, including uncorrected visual acuity testing, best-corrected visual acuity testing, dilated pupillary indirect ophthalmoscopic examination of the fundus, slit-lamp biomicroscopic examination of the anterior chamber, intraocular pressure examination by Goldman applanation tonometer, keratometry by keratometer, optometry, and measurement of axial length by ultrasound (CINE SCAN, French). The diagnosis for HM in this study required the spherical equivalent to be less than or equal to −6.0 diopter sphere (DS) in at least one eye and the axial length of the eye globe to be longer than or equal to 26.0 mm. Individuals were excluded from the study if they had undergone ocular procedures that might alter refraction, or if they had other symptoms besides HM (e.g., besides eye problems, individuals with Stickler syndrome suffer from distinctive facial abnormalities, hearing loss, and joint phenotypes). For the controls, the criteria were a spherical equivalent from −1.0 to +1.0 DS and no evidence of disease in either eye. All of the controls were unrelated individuals to the HM cases and to themselves. A total of 1052 unrelated patients with HM and 1070 normal controls were analyzed in this study (Table 1).
Table 1.
Characteristics of High Myopia Cases and Controls in the Study
| Refractive errors (diopter) | Axial length (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Total number | Male (%) | Female (%) | Average age (years)a | Age range (years) | OD | OS | OD | OS |
| Cases | 1052 | 598 (56.8) | 467 (43.2) | 47.65±16.48 | 11–78 | −9.14±2.77 | −9.45±2.93 | 27.82±1.71 | 28.03±1.94 |
| Controls | 1070 | 587 (54.9) | 602 (45.1) | 61.12±12.03 | 40–75 | −0.45±0.41 | −0.48±0.39 | 23.02±0.53 | 23.11±0.47 |
The values are given in±standard deviation.
OD, right eye; OS, left eye.
SNP selection and genotyping
We selected 16 SNPs that were previously reported to be associated with myopia or HM at the loci of the MYOC, HGF, MET, and ACAN genes (Han et al., 2006; Tang et al., 2007; Vatavuk et al., 2009; Yanovitch et al., 2009; Yip et al., 2011). Venous blood from each subject was drawn and collected in an EDTA tube. Genomic DNA was extracted from the blood by serial phenol/chloroform extraction and ethanol precipitation. SNP genotyping was performed with the dye terminator-based SNaPshot method (Applied Biosystems, Foster City, CA). SNP analysis was performed on the ABI 3130 Genetic Analyzer (Applied Biosystems). The SNP reported in this article has a genotyping success rate of 97% accuracy as judged by random re-genotyping of 5% of the samples in the cohort.
Haplotype analysis
The linkage disequilibrium (LD) block structure was examined using the program Haploview (Vision 4.2). The D′ values and r-values for all pairs of SNPs were calculated, and the haplotype blocks were estimated using the program Haploview (Vision 4.2). The haplotype frequencies between cases and controls were compared using χ2 analysis.
Statistical analysis
The Hardy–Weinberg equilibrium (HWE) for the SNPs was calculated by the χ2 test. All analyses were adjusted for age and sex on total cohorts. Allele and genotype frequencies between cases and controls were compared with the χ2 analysis. Statistical significance was defined as p<0.05. All statistical analyses were performed using the software SPSS version 10.0. Correction for multiple testing was analyzed by the Bonfferoni method. Genetic power was calculated by using the software “Power and Sample Size Program” (PS version 3.0.43) (Dupont and Plummer, 1998).
Results
SNP analysis
The 16 selected SNPs were successfully genotyped and were within HWE in both case and control groups (p>0.001, Table 2). The SNP frequencies of the controls in this study were similar to those of Han Chinese Beijing available in HapMap database, which implied reliable genotyping data in the study.
Table 2.
Association Between High Myopia and 16 Single Nucleotide Polymorphisms of the MYOC, HGF, MET, and ACAN Genes in the Han Chinese Population
| Gene | SNP | Chr | BP | Minor allele | Number (case/control) | MAF (case/control) | p_HWE (case/control) | p_allelica | Odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| MYOC | rs235858 | 1 | 169863125 | C | 1052/1070 | 0.494/0.503 | 0.322/0.087 | 0.5588 | 0.96 (0.86, 1.09) |
| rs2421853 | 1 | 169866576 | A | 1046/1064 | 0.210/0.194 | 0.843/0.033 | 0.1887 | 1.11 (0.95, 1.29) | |
| rs7545646 | 1 | 169873362 | C | 1019/1039 | 0.012/0.013 | 0.704/0.671 | 0.7242 | 0.91 (0.52, 1.57) | |
| rs16864720 | 1 | 169878890 | A | 1048/1064 | 0.012/0.013 | 0.684/0.675 | 0.9341 | 0.98 (0.57, 1.68) | |
| rs7523603 | 1 | 169880993 | C | 1046/1062 | 0.259/0.278 | 0.878/0.363 | 0.1601 | 0.91 (0.79, 1.04) | |
| rs235920 | 1 | 169888598 | C | 1050/1064 | 0.436/0.428 | 0.403/0.001 | 0.6173 | 1.03 (0.91, 1.17) | |
| rs2075537 | 1 | 169890456 | T | 1045/1068 | 0.437/0.459 | 0.266/0.694 | 0.1603 | 0.92 (0.81, 1.04) | |
| HGF | rs2286194 | 7 | 81193385 | A | 1050/1064 | 0.184/0.193 | 0.006/0.490 | 0.4859 | 0.95 (0.81, 1.10) |
| rs3735520 | 7 | 81238875 | T | 1052/1066 | 0.438/0.443 | 0.103/0.709 | 0.7648 | 0.98 (0.87, 1.11) | |
| MET | rs9641562 | 7 | 116110027 | C | 1050/1068 | 0.090/0.104 | 0.004/0.034 | 0.1128 | 085 (0.69, 1.04) |
| rs38857 | 7 | 116152649 | T | 1052/1066 | 0.110/0.096 | 0.295/0.041 | 0.1305 | 1.17 (0.96, 1.42) | |
| rs10215153 | 7 | 116186367 | A | 1052/1070 | 0.105/0.091 | 0.842/0.012 | 0.1147 | 1.18 (0.96, 1.44) | |
| rs2073560 | 7 | 116210397 | A | 1049/1068 | 0.288/0.304 | 0.790/0.434 | 0.2703 | 0.93 (0.81, 1.06) | |
| rs1621 | 7 | 116224842 | G | 1050/1068 | 0.132/0.122 | 0.707/0.579 | 0.3197 | 1.10 (0.91, 1.31) | |
| ACAN | rs3784757 | 15 | 87204408 | T | 1052/1068 | 0.131/0.154 | 0.401/0.390 | 0.0334 | 0.83 (0.70, 0.99) |
| rs1516794 | 15 | 87205907 | T | 1052/1068 | 0.084/0.104 | 0.584/0.418 | 0.0236 | 0.79 (0.64, 0.97) |
p-values were adjusted for age and sex.
BP, base position; Chr, chrosome; MAF, frequency of minor allele; p_HWE, the p_value of Hardy–Weinberg equilibrium; CI, confidence interval; SNP, single nucleotide polymorphism.
We found that rs3784757 and rs1516794 in the ACAN locus were significantly associated with HM in this study (allelic p=0.0334 and 0.0236, respectively; odds ratio [OR] [95% confidence interval (CI)]=0.83 [0.70, 0.99] and 0.79 [0.64, 0.97], respectively; Table 2). None of the other 14 SNPs showed significant difference between the case and control groups (allelic p>0.05, Table 2). We also tested the association between these SNPs and HM by using dominant and recessive models. We found that two SNPs (rs38857 and rs10215153) in the MET gene and one SNP (rs3784757) in the ACAN gene were significantly associated with HM of recessive model (p=0.0064, 0.0113, and 0.0373, respectively; OR=4.14 [95% CI: 1.38, 12.43], 5.74 [95% CI: 1.27, 25.95], and 0.52 [95% CI: 0.28, 0.97], respectively; Table 3), while the other SNPs showed no association between the cases and controls in both dominant and recessive models (p>0.05, Table 3).
Table 3.
Genotype Analysis of rs38857, rs10215153, and rs3784757 in High Myopia Cases and Controls
| Gene | SNP | Chr | BP | Genotype | Case n (%) | Control n (%) | p | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| MET | rs38857 | 7 | 116152649 | TT | 16 (1.5) | 4 (0.4) | 0.0064 | 4.14 (1.38, 12.43) |
| CT | 199 (18.9) | 196 (18.4) | ||||||
| CC | 837 (79.6) | 866 (81.2) | ||||||
| MET | rs10215153 | 7 | 116186367 | AA | 11 (1.0) | 2 (0.2) | 0.0113 | 5.74 (1.27, 25.95) |
| AG | 199 (18.9) | 190 (17.8) | ||||||
| GG | 842 (80.0) | 878 (82.1) | ||||||
| ACAN | rs3784757 | 15 | 87204408 | TT | 15 (1.4) | 29 (2.7) | 0.0373 | 0.52 (0.28, 0.97) |
| CT | 246 (23.4) | 271 (25.4) | ||||||
| CC | 791 (75.2) | 768 (71.9) |
p, the p-value of recessive model; OR, the odds ratio of homozygote.
We performed a power analysis to rule out false negatives. We have calculated the genetic power of all the tested SNPs by using the software “Power and Sample Size Program” (PS version 3.0.43) and the power values ranged from 0.951 to 0.986.
Haplotype analysis
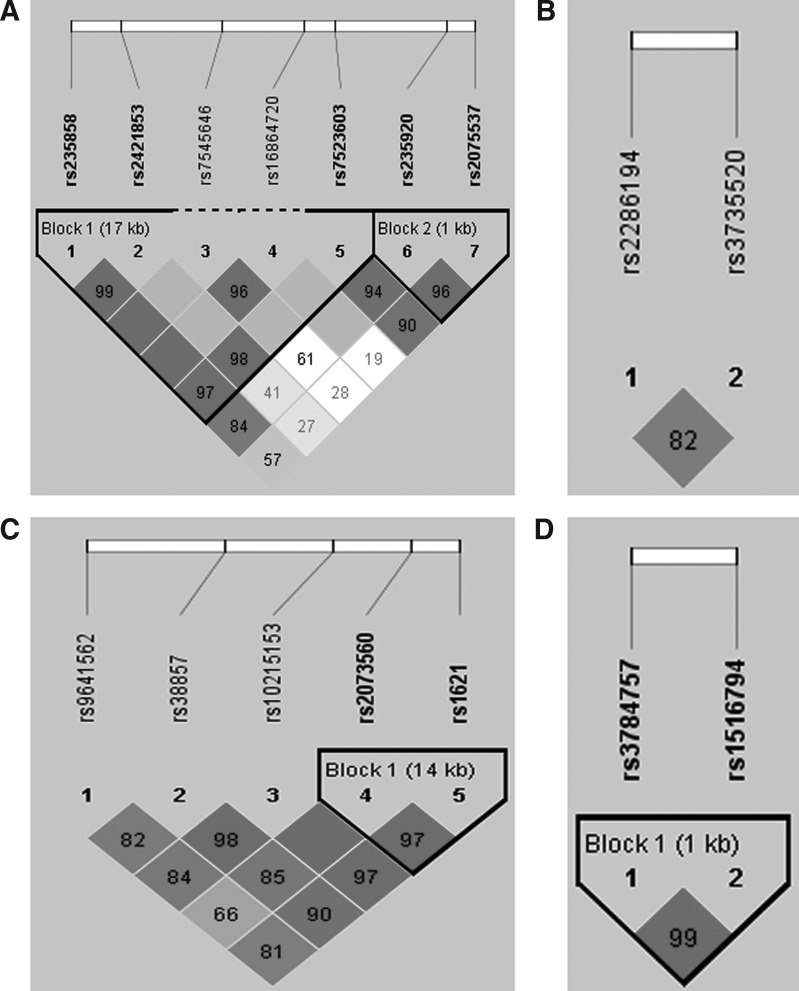
We then performed haplotype analysis by using Haploview 4.2 software to examine the LD block structure of the 16 SNPs in the MYOC, HGF, MET, and ACAN genes. As shown in Figure 1A, two LD blocks were located in the MYOC gene including three and two SNPs, respectively. rs2285194 and rs3735520 of the HGF gene were not in the same LD block with the D′ of 0.82 (Fig. 1B). rs2073560 and rs1621 were in the same LD block of the MET gene with the D′ of 0.97 (Fig. 1C). rs3784757 and rs1516794 in the ACAN gene were in the same LD block with the D′ of 0.99 (Fig. 1D).
FIG. 1.
The linkage disequilibrium (LD) pattern of the 16 SNPs in MYOC, HGF, MET, and ACAN gene (A–D, respectively). The LD block structure was examined using the program Haploview (Vision 4.2) based on the genotype data. The D′ values and r2 values for all pairs of SNPs were calculated, and the haplotype blocks were generated by the program Haploview.
The risk haplotype CA and the protection haplotype TT generated by rs3784757 and rs1516794 were significantly associated with HM (p=0.0327 and 0.0324, respectively; Table 4) with OR of 1.21 (95% CI: 1.02, 1.43) and 0.80 (95% CI: 0.65, 0.98), respectively. However, the other haplotypes showed no significant association between the case and control groups (p>0.05, Table 5 and 6).
Table 4.
ACAN Haplotype Association with High Myopia in the Han Chinese Population
| Haplotype | Frequency | |||||
|---|---|---|---|---|---|---|
| ACAN | rs3784757 | rs1516794 | Case | Control | p | OR (95% CI) |
| 1 | C | A | 0.869 | 0.846 | 0.0327 | 1.21 (1.02, 1.43) |
| 2 | T | T | 0.084 | 0.103 | 0.0304 | 0.80 (0.65, 0.98) |
| 3 | T | A | 0.048 | 0.051 | 0.5881 | 0.93 (0.70, 1.22) |
Table 5.
MYOC Haplotype Association with High Myopia in the Han Chinese Population
| Haplotype | Frequency | ||||||
|---|---|---|---|---|---|---|---|
| Block 1 | rs235858 | rs2421853 | rs7523603 | Case | Control | p | OR (95% CI) |
| 1 | T | G | T | 0.293 | 0.303 | 0.475 | 0.95 (0.84, 1.09) |
| 2 | C | G | C | 0.254 | 0.276 | 0.1055 | 0.89 (0.78, 1.02) |
| 3 | C | G | T | 0.24 | 0.226 | 0.2848 | 1.08 (0.94, 1.25) |
| 4 | T | A | T | 0.207 | 0.192 | 0.2292 | 1.10 (0.94, 1.28) |
| Block 2 | rs235920 | rs2075537 | Case | Control | p | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| 1 | T | T | 0.433 | 0.452 | 0.2064 | 0.93 (0.82, 1.04) | |
| 2 | C | G | 0.432 | 0.421 | 0.4758 | 1.05 (0.93, 1.18) | |
| 3 | T | G | 0.131 | 0.120 | 0.2958 | 1.10 (0.92, 1.32) | |
Table 6.
MET Haplotype Association with High Myopia in the Han Chinese Population
| Haplotype | Frequency | |||||
|---|---|---|---|---|---|---|
| MET | rs2073560 | rs1621 | Case | Control | P | OR (95% CI) |
| 1 | G | A | 0.581 | 0.574 | 0.6833 | 1.03 (0.91, 1.16) |
| 2 | A | A | 0.287 | 0.303 | 0.2427 | 0.92 (0.81, 1.05) |
| 3 | G | G | 0.131 | 0.122 | 0.3668 | 1.09 (0.91, 1.30) |
Discussion
Recently, MYOC, HGF, MET, and ACAN SNPs were reported to lead susceptibility for HM (Han et al., 2006; Tang et al., 2007; Vatavuk et al., 2009; Yanovitch et al., 2009; Yip et al., 2011). However, controversy exists in the previous studies regarding the association between them and HM (Khor et al., 2009; Schache et al., 2009; Zayats et al., 2009). In this study, we found that SNPs in the MET and ACAN genes but not in the MYOC and HGF genes were associated with HM in a Han Chinese population.
The myocilin gene (MYOC; OMIM 601652), located on chromosome 1q24-q25, is expressed in many human eye tissues, including the trabecular meshwork cells (TMC), sclera, ciliary body, iris, choroids, and retina (Adam et al., 1997), and the secreted protein is present in the aqueous humor (Tamm, 2002). MYOC is well known for its role in glaucoma (Polansky, 2003; Gong et al., 2004). From clinical statistics, myopia patients are susceptible to open-angle glaucoma (Perkins and Phelps, 1982; Mastropasqua et al., 1992; Grødum et al., 2001), and there is evidence of higher intraocular pressure in myopic eyes compared with emmetropic eyes (Wu et al., 2000). Additionally, some factors, such as bFGF, that stimulate myocilin expression in TMC have also been implicated in the regulation of postnatal eye growth and myopia (Rohrer et al., 1997; Lin et al., 2006; Andrew et al., 2008). Association between MYOC and HM was first reported in a population-based case–control study of Chinese ethnicity in Singapore (Wu et al., 1999). However, the results of the following studies between MYOC and HM were inconsistent and were even conflicting in the same ethical origin populations (Leung et al., 2000; Zayats et al., 2009). In the present study, we examined the association between seven SNPs in the MYOC gene and their haplotypes and HM in a Han Chinese population. We found no evidence to support a significant association between MYOC polymorphisms and HM (Table 2). More work should be carried out to verify the influence of MYOC on HM susceptibility in Han Chinese.
The hepatocyte growth factor (HGF) and its receptor (MET), broadly expressed in retina, pigment epithelium, and choroid, play an important role in matrix metalloproteinases and tissue inhibitors of metalloproteinase pathways, and may play an active role in scleral remodeling, axial elongation, and myopia development as well (Hamasuna et al., 1999; Gong et al., 2003; Han et al., 2006). It was initially shown as a candidate for myopia in an analysis of mice eye weight (Zhou and Williams, 1999) and was confirmed to have significant effects on eye size, lens weight, and retinal area. HGF had previously been reported to be associated with HM in a Han Chinese population by family-based analysis (128 nuclear families with 133 severely myopic offspring) (Han et al., 2006). A strong association has been found between the mild to moderate myopia group and the HGF SNP rs3735520 and the HGF haplotypes rs2286194-rs3735520-rs17501108 and rs12536657-rs2286194, and a moderate association of the extreme HM with rs2286194 in a Caucasian family dataset (649 individuals) (Yanovitch et al., 2009). However, no evidence of association between the MET gene and myopia was found in the same study (Yanovitch et al., 2009). Analysis of a Singaporean children cohort (1126 individuals) suggested that carriage of rs2073560 (MET+110703) A allele was associated with increased susceptibility to myopia and was also associated with a faster rate of change in refractive error (Khor et al., 2009). A current study (Schache et al., 2009) found that there is likely no genetic association of the MET gene with myopia, axial length, anterior chamber depth, and corneal curvature in a Caucasian population. In our study, no genetic association between the HGF gene and HM has been found (Table 2). Two SNPs (rs38857 and rs10215153) in the MET gene showed significant association with HM in the recessive model (Table 3). Because of the relatively larger sample used in this study, we suggested it is not HGF but MET that is more likely to be susceptible for HM in Han Chinese. However, given our findings and the previous studies together, it indicated that HGF and MET might play different role in different populations and in different type of myopia. We need to examine the association of HGF and MET between moderate, high, and extreme HM (≤−10.0 diopters) respectively in the future.
Aggrecan (ACAN) is a proteoglycan gene in eye sclera. It was first reported in form-deprivation myopia animal models. In chicks, it accumulated in increased amounts in the presence of increased turnover rate in the cartilaginous layer of the posterior sclera, in parallel with the scleral growth of the myopic eye (Rada et al., 1994; Rada et al., 1998). However, in mammals such as tree shrew and monkey, the changes were opposite (Marzani and Wallman, 1997; McBrien et al., 2000). In the last year, a Hong Kong group reported an evaluation of proteoglycan gene polymorphisms as risk factor in the genetic susceptibility to HM (Yip et al., 2011). They found that rs3784757 and rs1516794 in the ACAN gene showed a weak association in group 1 subjects (300 patients with HM and 300 emmetropic controls) but was not replicated in group 2 subjects (356 HM patients and 354 controls). To our knowledge, the allele frequency of an SNP may be changed in different cohorts, even those from the same ethnic origin; thus, the results of association studies need to be further replicated in different cohorts or increase the sample size. In this study, our results showed borderline association between these two SNPs and HM (Table 2). However, after multiple testing by using the Bonferroni correction, rs3784757 and rs1516794 showed no significant differences between HM and control groups (p=0.534 and 0.378, respectively). Nevertheless, haplotype frequencies of these SNPs in the ACAN gene were statistically associated with HM (Table 4). Therefore, to avoid filtering real myopia genes, the role of ACAN in the pathogenesis of myopia requires more refinement in both animal models and human genetic epidemiologic studies.
Taking our data and previous studies together, the association between genetic variants and the MYOC, ACAN, HGF, and MET genes were inconsistent in different populations. This may be explained by the genetic heterogeneity of HM among different ethnicities. It is also suggested that HM genetics are still not clearly defined and that a multifactorial model as yet to be determined will likely be the etiology.
Acknowledgments
We thank all the subjects for their participation. This work was supported by the following grants: the Outstanding Young Scientist Research Award Foundation of Shandong Province (BS2010SF009, to X.Y.); the National Basic Research Program of China (973 Program, 2011CB504604 to Z.Y.); the Natural Science Foundation of China (81170882 to Y.S.; 81271047 to X.L.; 81300768 to J.P.); the Department of Science and Technology of Sichuan Province, China (2012JQ0023 to Y.S.).
Declaration of Funding Sources
The funding organization had no role in the design or conduct of this research.
Author Disclosure Statement
All authors have declared that no competing interests exist.
References
- Adam MF, Belmouden A, Binisti P, et al. (1997) Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 6:2091–2097 [DOI] [PubMed] [Google Scholar]
- Andrew T, Maniatis N, Carbonaro F, et al. (2008) Identification and replication of three novel myopia common susceptibility gene loci on chromosome 3q26 using linkage and linkage disequilibrium mapping. PLoS Genet 4:e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton TC. (1989) The influence of refractive error and lattice degeneration on the incidence of RD. Trans Am Ophthalmol Soc 87:143–155 [PMC free article] [PubMed] [Google Scholar]
- Celorio JM, Pruett RC. (1991) Prevalence of lattice degeneration and its relation to axial length in severe myopia. Am J Ophthalmol 111:20–23 [DOI] [PubMed] [Google Scholar]
- Dirani M, Chamberlain M, Shekar SN, et al. (2006) Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci 47:4756–4761 [DOI] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD. (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601 [DOI] [PubMed] [Google Scholar]
- Gong G, Kosoko-Lasaki O, Haynatzki GR, et al. (2004) Genetic dissection of myocilin glaucoma. Hum Mol Genet 13 Spec No 1:R91–R102 [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Tolbert EM, et al. (2003) Hepatocyte growth factor modulates matrix metalloproteinases and plasminogen activator/plasmin proteolytic pathways in progressive renal interstitial fibrosis. J Am Soc Nephrol 14:3047–3060 [DOI] [PubMed] [Google Scholar]
- Grødum K, Heijl A, Bengtsson B. (2001) Refractive error and glaucoma. Acta Ophthalmol Scand 79:560–566 [DOI] [PubMed] [Google Scholar]
- Hamasuna R, Kataoka H, Moriyama T, et al. (1999) Regulation of matrix metalloproteinase-2 (MMP-2) by hepatocyte growth factor/scatter factor (HGF/SF) in human glioma cells: HGF/SF enhances MMP-2 expression and activation accompanying up-regulation of membrane type-1 MMP. Int J Cancer 82:274–281 [DOI] [PubMed] [Google Scholar]
- Han W, Yap MK, Wang J, et al. (2006) Family-based association analysis of hepatocyte growth factor (HGF) gene polymorphisms in high myopia. Invest Ophthalmol Vis Sci 47:2291–2299 [DOI] [PubMed] [Google Scholar]
- Khor CC, Grignani R, Ng DP, et al. (2009) cMET and refractive error progression in children. Ophthalmology 116:1469–1474, 1474.e1. [DOI] [PubMed] [Google Scholar]
- Leung YF, Baum L, Lam DS, et al. (2000) TIGR/MYOC proximal promoter GT-repeat polymorphism is not associated with myopia. Hum Genet 107:404–405 [DOI] [PubMed] [Google Scholar]
- Liang YB, Wong TY, Sun LP, et al. (2009) Refractive errors in a rural Chinese adult population the Handan eye study. Ophthalmology 116:2119–2127 [DOI] [PubMed] [Google Scholar]
- Lin HJ, Wan L, Tsai Y, et al. (2006) The TGFbeta1 gene codon 10 polymorphism contributes to the genetic predisposition to high myopia. Mol Vis 12:698–703 [PubMed] [Google Scholar]
- Ma JH, Shen SH, Zhang GW, et al. (2010) Identification of a locus for autosomal dominant high myopia on chromosome 5p13.3-p15.1 in a Chinese family. Mol Vis 16:2043–2054 [PMC free article] [PubMed] [Google Scholar]
- Marzani D, Wallman J. (1997) Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci 38:1726–1739 [PubMed] [Google Scholar]
- Mastropasqua L, Lobefalo L, Mancini A, et al. (1992) Prevalence of myopia in open angle glaucoma. Eur J Ophthalmol 2:33–35 [DOI] [PubMed] [Google Scholar]
- McBrien NA, Lawlor P, Gentle A. (2000) Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci 41:3713–3719 [PubMed] [Google Scholar]
- Perkins ES, Phelps CD. (1982) Open angle glaucoma, ocular hypertension, low-tension glaucoma, and refraction. Arch Ophthalmol 100:1464–1467 [DOI] [PubMed] [Google Scholar]
- Polansky JR. (2003) Current perspectives on the TIGR/MYOC gene (Myocilin) and glaucoma. Ophthalmol Clin North Am 16:515–527 [DOI] [PubMed] [Google Scholar]
- Rada JA, Achen VR, Rada KG. (1998) Proteoglycan turnover in the sclera of normal and experimentally myopic chick eyes. Invest Ophthalmol Vis Sci 39:1990–2002 [PubMed] [Google Scholar]
- Rada JA, Matthews AL, Brenza H. (1994) Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res 59:747–760 [DOI] [PubMed] [Google Scholar]
- Rohrer B, Tao J, Stell WK. (1997) Basic fibroblast growth factor, its high- and low-affinity receptors, and their relationship to form-deprivation myopia in the chick. Neuroscience 79:775–787 [DOI] [PubMed] [Google Scholar]
- Schache M, Chen CY, Dirani M, et al. (2009) The hepatocyte growth factor receptor (MET) gene is not associated with refractive error and ocular biometrics in a Caucasian population. Mol Vis 15:2599–2605 [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Li Y, Zhang D, et al. (2011a) Exome sequencing identifies ZNF644 mutations in high myopia. PLoS Genet 7:e1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Qu J, Zhang D, et al. (2011b) Genetic variants at 13q12.12 are associated with high myopia in the Han Chinese population. Am J Hum Genet 88:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER. (2002) Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res 21:395–428 [DOI] [PubMed] [Google Scholar]
- Tang WC, Yip SP, Lo KK, et al. (2007) Linkage and association of myocilin (MYOC) polymorphisms with high myopia in a Chinese population. Mol Vis 13:534–544 [PMC free article] [PubMed] [Google Scholar]
- Vatavuk Z, Skunca Herman J, Bencić G, et al. (2009) Common variant in myocilin gene is associated with high myopia in isolated population of Korcula Island, Croatia. Croat Med J 50:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski R. (2011) Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet 79:301–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Foster PJ, Hee J, et al. (2000) Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 41:2486–2494 [PubMed] [Google Scholar]
- Wu H, Yu XH, Yap EP. (1999) Allelic association between trabecular meshwork-induced glucocorticoid response (TIGR) gene and severe myopia. Invest Ophthalmol Vis Sci 40:S600 [Google Scholar]
- Wu SY, Nemesure B, Leske MC. (2000) Glaucoma and myopia. Ophthalmology 107:1026–1027 [DOI] [PubMed] [Google Scholar]
- Yang Z, Xiao X, Li S, et al. (2009) Clinical and linkage study on a consanguineous Chinese family with autosomal recessive high myopia. Mol Vis 15:312–318 [PMC free article] [PubMed] [Google Scholar]
- Yanovitch T, Li YJ, Metlapally R, et al. (2009) Hepatocyte growth factor and myopia: Genetic association analyses in a caucasian population. Mol Vis 15:1028–1035 [PMC free article] [PubMed] [Google Scholar]
- Yip SP, Leung KH, Ng PW, et al. (2011) Evaluation of proteoglycan gene polymorphisms as risk factors in the genetic susceptibility to high myopia. Invest Ophthalmol Vis Sci 52:6396–6403 [DOI] [PubMed] [Google Scholar]
- Zayats T, Yanovitch T, Creer RC, et al. (2009) Myocilin polymorphisms and high myopia in subjects of European origin. Mol Vis 15:213–222 [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Williams RW. (1999) Eye1 and Eye2: gene loci that modulate eye size, lens weight, and retinal area in the mouse. Invest Ophthalmol Vis Sci 40:817–825 [PubMed] [Google Scholar]