Abstract
Children with specific reading impairment may have subtle deficits in speech perception related to difficulties in phonological processing. The aim of this study was to examine brain oscillatory activity related to phonological processing in the context of auditory sentence comprehension using magnetoencephalography (MEG) to better understand these deficits. Good and poor readers, 16-18 years of age, were tested on speech perception of sentence-terminal incongruent words that were phonologically manipulated to be similar or dissimilar to corresponding congruent target words. Functional coupling between regions was measured using phase-locking values (PLV). Gamma band (30-45 Hz) PLV between auditory cortex and superior temporal sulcus in the right hemisphere was differentially modulated in the two groups by the degree of phonological contrast between the congruent and incongruent target words in the latency range associated with semantic processing. Specifically, the PLV was larger in the phonologically similar than in the phonologically dissimilar condition in the good readers. This pattern was reversed in the poor readers, whose lower PLV in the phonologically similar condition may be indicative of the group's impaired phonological coding abilities, and consequent vulnerability under perceptually demanding conditions. Overall, the results support the role of gamma oscillations in spoken language processing.
Keywords: speech perception, phonological processing, sentence context, phase-locking value, gamma band oscillations, magnetoencephalography
Introduction
Children with specific reading disability are characterized by difficulties in learning to read despite normal intellectual ability and adequate education. Over the years, deficits in phonological processing have been the single most consistent finding in both children and adults with reading disability [1]. The problem is believed to be caused by a weakness in speech perception rooted in poorly encoded phonological representations, lacking in phonetic detail [2]. In spite of a phonological deficit, the reading-disabled child appears to develop adequate reading comprehension skills [3]. Research suggests that perhaps poor readers rely on contextual clues to facilitate word recognition and reading comprehension [4,5].
Event-related potential and magnetic field studies have also revealed differences between normal and impaired readers on phonological tasks [6,7]. In an auditory comprehension study using magnetoencephalography (MEG), we found significant differences between 7-12-year-old normal and disabled readers’ brain responses to phonologically-manipulated sentence terminal words [8]. The MEG differences were evident in the time range associated with the phonological mismatch (i.e., 200-300 ms), with activation patterns suggestive of impaired word recognition related to the poor readers’ deficit in phonological coding. However, no differences between the groups were found in the time range of the N400 response associated with semantic integration of the incongruous sentence-terminal words into the preceding context. Insofar as poor readers’ phonological deficits tend to persist in later years, it raises questions about neural activation patterns related to auditory language processing in older adolescent disabled readers, in light of the reliance on contextual cues. The present study aims to extend the N400 sentence anomaly paradigm incorporating phonological foils to older children with reading impairment. Additionally, we use time-frequency analysis of brain oscillatory activity, which has been increasingly implicated in linguistic processing [9].
Synchrony in neural oscillatory activity has been suggested to be a general mechanism for integrating information and forming functional networks [10]. Auditory comprehension, involving the integration of sensory, cognitive, and linguistic processes to arrive at the meaning of a word or sentence, lends itself well to study by analyses of oscillatory activity in the brain. Several studies, indeed, have indicated that gamma band oscillations may play an important role in spoken language comprehension [11-13].
In the present study, we used MEG to investigate neural oscillatory activity associated with auditory word processing in sentence context in reading impaired adolescents. We examined phase coupling [14] between cortical activation time-courses of temporal-lobe regions under phonologically demanding conditions in an auditory semantic congruency judgment task. To the extent that children with specific reading disability develop adequate reading comprehension skills, presumably through compensatory mechanisms to overcome their phonological processing problems, we predict that gamma band oscillations will reveal characteristic differences between adolescent good and poor readers in the functional organization of brain activity related to language processing.
Methods
Subjects
Two groups of children, 10 good readers and 10 poor readers, were selected on the basis of their performance on the Word Attack and/or Word Identification subtests of the Woodcock Reading Mastery Tests-Revised. Specifically, poor readers scored below the 25th percentile on Word Attack, a measure of their weakness in nonword decoding (a core characteristic of reading disability) or both subtests, whereas good readers scored above the 39th percentile on both subtests. Thus, the two groups were clearly separable and non-overlapping in their reading ability, with poor readers notably weaker in phonological decoding. Additionally, the children in the poor reader group had also been identified by the school system as reading below grade level. All the participants were right-handed, monolingual English speakers with no neurological or psychological histories, and were screened for attention and hearing problems. The groups were matched on age (good readers 17.2 ± 1.29 years [mean ± s.d.], poor readers 17.2 ± 1.24) and performed within the normal range on verbal and nonverbal IQ (85-125, Wechsler Abbreviated Scale of Intelligence) with no significant difference between the groups (p > 0.05) except on reading (good readers 116 ± 8.2, poor readers 86 ± 6.4; p < 0.05). Written informed consent was obtained from all participants and the protocol was approved by the Human Subjects Committee at Massachusetts General Hospital.
Stimuli and task
The participants were presented 400 spoken sentences, each made up of a sentence stem of 5-10 words and a sentence-terminal critical word. In the incongruent sentences, the critical words of the congruent sentences (e.g., “The boy rolled the ball”) were altered to be either phonologically similar (e.g., ball-doll) or phonologically dissimilar (e.g., ball-hall) to the critical target words. The first phoneme differed by one phonetic feature (voicing or place of articulation) for the phonologically similar and by two or more of the features (voicing, place, or manner of articulation) for the phonologically dissimilar condition. The sentences were piloted with ten adults using a 5-point scale to ensure that there was no difference in semantic plausibility between the sentences in the two incongruent conditions. For more details about the stimuli, see ref. [8].
The participants had to indicate whether a sentence they heard made sense or not by pressing one of two buttons on seeing a question mark, which appeared at the end of each sentence. The subjects were instructed to look at a fixation cross during the presentation of the sentences. The sentences were presented in eight runs of 50 sentences each, with 2-minute breaks between the runs.
MEG data acquisition and source modeling
MEG data were collected with a 306-channel MEG system (VectorView, Elekta-Neuromag). The data were filtered from 0.03 to 200 Hz, sampled at 601 Hz, and segmented into epochs from 100 ms before to 800 ms after the onset of the critical words. Trials containing blinks or large eye movements in the electro-oculogram were rejected from further analysis.
Neural sources of the MEG signals for each trial were estimated using the minimum-norm estimate (MNE) [15] as implemented in the MNE software package (http://www.nmr.mgh.harvard.edu/martinos/userInfo/data/sofMNE.php). The locations of the neural sources were constrained to the cortical surface, which was constructed from high-resolution structural T1-weighted MRIs acquired with a 3 T Siemens scanner (TR = 2,530 ms, TE = 3.25 ms, flip angle = 7°, voxel size = 1.3 × 1.0 × 1.3 mm3) using the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu). Dipole sources were assumed to be on ~3500 cortical vertices in each hemisphere. For the MNE inverse operator, loose orientation constraint and depth weighting were used.
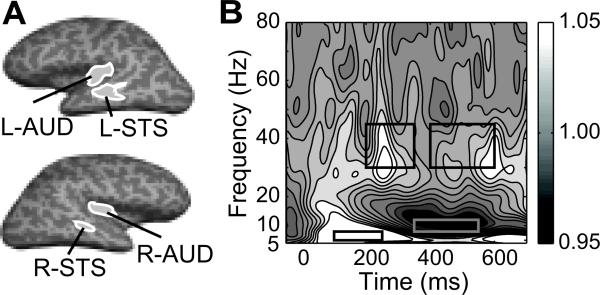
Cortical regions of interest (ROIs) were identified for the time-frequency analysis on the basis of an omnibus source activation map that was computed by averaging noise-normalized MNE solutions across all subjects and conditions in the time window of 200-500 ms [7]. Specifically, four functional ROIs, two in the left and two in the right hemisphere, were drawn around observed activation peaks in the omnibus map. Because the anatomical loci of the peak activations fell within the primary auditory cortex (transverse temporal sulcus) and superior temporal sulcus regions of the sulcal- and gyral-based standard FreeSurfer atlas, these ROIs were labeled as L-AUD, L-STS, R-AUD and R-STS (Fig. 1A). For each trial, the ROI time course was calculated by averaging the MNE solutions for all the dipole elements located within the ROI. The ROIs correspond to areas that are well known to play a role in speech perception [16].
Figure 1. Spatial regions and time-frequency windows of interest.
A. Spatial Regions of Interest (ROIs) in one subject, shown on the inflated representation of the cerebral cortex. The four ROIs, determined from an omnibus function map, were labeled as L-AUD, R-AUD, L-STS, and R-STS. Anatomically, the locations of the ROIs fell in the primary auditory corte and superior temporal sulcus in each hemisphere. B. Time-frequency power spectrum obtained by averaging all trials across all conditions and ROIs in all subjects. The black rectangles indicate the time-frequency windows selected for the phase synchrony analysis.
Coupling between ROIs: Phase-Locking Value
The time courses corresponding to individual ROIs were transformed to the time-frequency domain using Morlet wavelets, with the width parameter varying linearly from 3 to 10 cycles for frequencies between 3 and 100 Hz. We used epochs from 1100 ms before to 1400 ms after the onset of the critical words for the time-frequency analysis.
The synchrony between the oscillatory signals in two ROIs, X and Y, was quantified for each subject and condition using the phase-locking value (PLV) defined as [14]
where φxk is the phase of the wavelet-transformed complex-value data at time t and frequency f; K is the number of trials. The PLV was normalized using the pre-stimulus period from -500 ms to -100 ms for each subject:
whereμC, μPS are the mean PLV values of the baseline for the congruent, phonologically similar, and phonologically dissimilar conditions, and σc,σPS, and σPD are the corresponding standard deviations.
For the PLV analysis, four time-frequency windows of interest were selected based on an omnibus power spectrum averaged across conditions and ROIs in all subjects: early theta (100-250 ms, 4-8 Hz), early gamma (200-350 ms, 30-45 Hz), late alpha (350-550 ms, 8-12 Hz), and late gamma (400-600 ms, 30-45 Hz) (Fig. 1B). Within each time-frequency window, the PLVn values were averaged separately for each condition and ROI. The mean PLVn values for the congruent condition were subtracted from those for the phonologically similar and phonologically dissimilar conditions. A mixed-model two-way (group × condition) analysis of variance (ANOVA) was conducted, with condition (phonologically similar - congruent, phonologically dissimilar - congruent) as the repeated within-subject factor and group (good readers, poor readers) as the between-subject factor. The p-values were Bonferroni corrected for multiple comparisons with a factor of 24 (6 pairs of ROIs, 4 time-frequency windows).
Results
Behavioral data
Group (good readers, poor readers) × condition (congruent, phonologically similar, phonologically dissimilar) mixed-model two-way ANOVA yielded a main effect of condition for both accuracy (F(2,36) = 61.2, p < 10-11) [percent correct – good readers: congr. 97.6 ± 2.0 (mean ± s.d.), phon. sim. 89.8 ± 4.5, phon. dissim. 97.5 ± 3.0; poor readers: congr. 96.8 ± 1.5, phon. sim. 89.4 ± 3.0, phon. dissim. 97.7 ± 0.9] and reaction time (F(2,36) = 3.611, p = 0.037) [in milliseconds – good readers: congr. 422 ± 150, phon. sim. 454 ± 136, phon. dissim. 440 ± 141; poor readers: congr. 409 ± 102, phon. sim. 449 ± 123, phon. dissim. 441 ± 133]. Post hoc tests (two-tailed paired t-tests using the Bonferroni procedure with a correction factor of 3) indicated that, regardless of group, the subjects were less accurate on the phonologically similar condition than on the phonologically dissimilar (t(19) = 10.9, p < 10-8) or the congruent (t(19) = 7.89, p < 10-6) conditions, consistent with the phonologically similar condition being the most challenging one, as expected. For the reaction times, no pair of conditions was significantly different (p > 0.1). No main effects of group or group × condition interactions were found in the behavioral data.
Coupling between ROIs: PLV
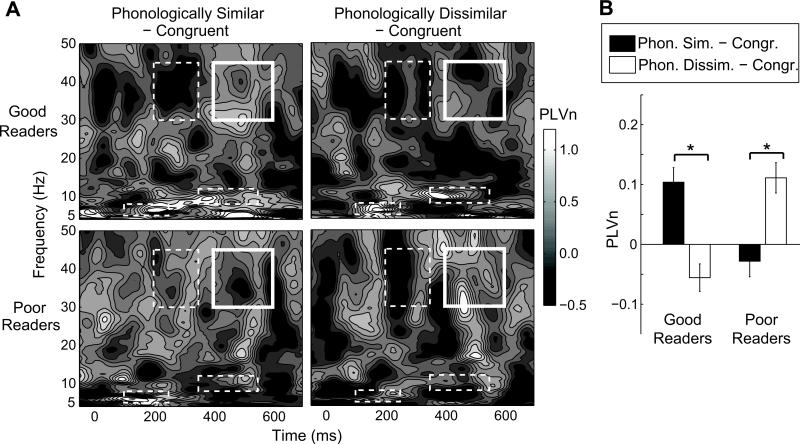
The phase-locking measure PLVn between the two right-hemisphere ROIs is shown in Fig. 2A. Repeated-measures ANOVAs, conducted separately for the six pairs of ROIs and four time-frequency windows, revealed no main effects of group or condition. In the late gamma window (400-600 ms, 30-45 Hz), a significant group × condition interaction was found for the coupling between the right auditory cortex and right superior temporal sulcus (F(1, 18) = 14.1, p = 0.029, corrected). Post hoc within-group two-tailed paired t-tests revealed that for good readers the PLVn was larger in the phonologically similar than in the phonologically dissimilar condition (t(9) = 3.24, p = 0.010) and for poor readers the PLVn was larger in the phonologically dissimilar than in the phonologically similar condition (t(9) = 2.30, p = 0.047) (Fig. 2B). No significant group × condition interactions were found in the remaining time-frequency windows (the early theta, early gamma, and late alpha windows).
Figure 2. Normalized phase-locking values (PLVn) between the two right-hemisphere regions of interest (right auditory cortex and right superior temporal sulcus).
A. Time-frequency maps of PLVn for phonologically similar (left) and phonologically dissimilar (right) incongruent conditions, from which the values for congruent condition were subtracted. Upper row: good readers; lower row: poor readers.
B. Mean PLVn values for the late gamma band time-frequency window (400-600 ms, 30-45 Hz; solid white rectangles in A), where significant (p < 0.05, Bonferroni corrected) group × condition interaction was observed; ‘*’ indicates p < 0.05 on post hoc paired t-tests.
Discussion
We investigated the role of brain oscillations in spoken language processing in adolescent children with and without reading impairment using an N400 sentence anomaly paradigm incorporating phonological foils. The PLV analysis of MEG data revealed that the degree of phonological contrast of the sentence-terminal critical words differentially modulated neural synchrony between right-hemisphere auditory and language-homologous areas in the gamma band about 400-600 ms post critical word onset in good vs. poor readers. The results support a role for gamma-band activity in spoken language comprehension. Furthermore, the difference between the two groups’ oscillatory response to the semantic violations as a function of the degree of phonological manipulations may point to poor readers’ subtle deficits in speech perception rooted in weakly coded phonological representations. The brain activation data suggest that, despite a lack of behavioral performance differences, the groups were not performing the task identically.
The PLV effect was found in the N400 latency range associated with semantic processing. The PLV was higher in the phonologically-demanding phonologically similar incongruent condition, compared with the phonologically dissimilar incongruent condition, in the good readers. This may reflect the good readers’ superior coding of, and hence, sensitivity to subtle phonological differences between stimuli, as well as their consequent effort to resolve between perceptually confusable terminal words in a semantically-conflicting context. In contrast, for poor readers, who were selected based on impaired decoding abilities, the PLV was lower in the phonologically similar than in the phonologically dissimilar condition. This may relate to the group's weak speech perception abilities in detecting subtle phonological differences from the expected critical word, and reliance on sentence context under confusing auditory word recognition conditions. The present findings are in keeping with a modulation of gamma-band activity in the semantic time range that has been found in several studies using both word-level [12,13,17] as well as sentence-level [11,18] language comprehension tasks.
Listeners often use contextual clues to disambiguate speech sounds under perceptually challenging conditions. The current study was designed to take advantage of this phenomenon in order to examine differences in the phonological processing abilities of normal versus impaired readers. That this type of top-down influence on phonemic processing is mediated by gamma band activity [17] may be taken as evidence of the involvement of the gamma frequency band in integration of multiple processes during auditory comprehension in the present study. Modulation of gamma oscillations has also been observed to be related to cognitive processes other than language, including verbal memory and attention [19]. These functions may have been recruited by our task. As such, the observed effects of gamma synchronization in the present study may reflect the harnessing of a number of cognitive processes in the service of comprehension under phonologically-demanding speech perception conditions.
The observed group-by-condition interaction in the gamma-band PLV was in the right hemisphere. While the left-hemisphere dominance in language processing is widely accepted, involvement of the right hemisphere has also been reported [20]. As in the present study, the patterns of activation depend on task demands and the groups under study. Activation of the right hemisphere for language tasks has been seen in young children as well as dyslexic readers in a number of studies [21,22]. Additionally, poor readers tend to show atypical cortical activity and/or reduced activation in the left hemisphere on a variety of event-related potential and magnetic field studies of language processing [7,8,23]. Brain oscillation studies of children with reading impairment, though limited in number, too, have shown atypical activation compared with normal controls [24,25]. Taken together, the findings in these studies suggest that individuals with reading disability are impaired in the use of their left hemisphere and present with right hemisphere differences during phonological processing.
Given the small number of subjects, the results of the present study should be viewed with some caution, and would be important to replicate with a larger sample. However, our findings do support a role for gamma band oscillations in auditory sentence comprehension. The gamma-band PLV revealed differences in the patterns of neural activity between good and poor readers related to spoken language processing under phonologically challenging conditions.
Acknowledgements
Supported by NIH grants NS057500, HD046171, and The National Center for Research Resources (P41RR14075). The authors thank Surina Basho and Shira Schwartz for various aspects of data collection and analysis.
Footnotes
Conflicts of Interest and Source of Funding: none declared
References
- 1.Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Curr Opin Neurobiol. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 2.Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: auditory processing or phonological coding? J Exp Child Psychol. 1997;64:199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- 3.Shaywitz SE, Fletcher JM, Holahan JM, Shneider AE, Marchione KE, Stuebing KK, et al. Persistence of dyslexia: the Connecticut Longitudinal Study at adolescence. Pediatrics. 1999;104:1351–1359. doi: 10.1542/peds.104.6.1351. [DOI] [PubMed] [Google Scholar]
- 4.Chiappe P, Chiappe DL, Gottardo A. Vocabulary, context, and speech perception among good and poor readers. Educational Psychology. 2004;24:825–843. [Google Scholar]
- 5.Perfetti CA, Goldman SR, Hogaboam TW. Reading skill and the identification of words in discourse context. Mem Cognit. 1979;7:273–282. doi: 10.3758/bf03197600. [DOI] [PubMed] [Google Scholar]
- 6.Bonte ML, Blomert L. Developmental dyslexia: ERP correlates of anomalous phonological processing during spoken word recognition. Brain Res Cogn Brain Res. 2004;21:360–376. doi: 10.1016/j.cogbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Wehner DT, Ahlfors SP, Mody M. Effects of phonological contrast on auditory word discrimination in children with and without reading disability: a magnetoencephalography (MEG) study. Neuropsychologia. 2007;45:3251–3262. doi: 10.1016/j.neuropsychologia.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mody M, Wehner DT, Ahlfors SP. Auditory word perception in sentence context in reading-disabled children. Neuroreport. 2008;19:1567–1571. doi: 10.1097/WNR.0b013e328311ca04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 11.Obleser J, Kotz SA. Multiple brain signatures of integration in the comprehension of degraded speech. Neuroimage. 2011;55:713–723. doi: 10.1016/j.neuroimage.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Shahin AJ, Picton TW, Miller LM. Brain oscillations during semantic evaluation of speech. Brain Cogn. 2009;70:259–266. doi: 10.1016/j.bandc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavabi K, Embick D, Roberts TP. Spectral-temporal analysis of cortical oscillations during lexical processing. Neuroreport. 2011;22:474–478. doi: 10.1097/WNR.0b013e3283476b84. [DOI] [PubMed] [Google Scholar]
- 14.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- 16.Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 17.Hannemann R, Obleser J, Eulitz C. Top-down knowledge supports the retrieval of lexical information from degraded speech. Brain Res. 2007;1153:134–143. doi: 10.1016/j.brainres.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 18.Hald LA, Bastiaansen MC, Hagoort P. EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang. 2006;96:90–105. doi: 10.1016/j.bandl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. Neuroreport. 2005;16:207–211. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- 20.Federmeier KD, Wlotko EW, Meyer AM. What's “right” in language comprehension: ERPs reveal right hemisphere language capabilities. Lang Linguist Compass. 2008;2:1–17. doi: 10.1111/j.1749-818X.2007.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, et al. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- 22.Simos PG, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, et al. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci Lett. 2000;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- 23.Heim S, Eulitz C, Elbert T. Altered hemispheric asymmetry of auditory P100m in dyslexia. Eur J Neurosci. 2003;17:1715–1722. doi: 10.1046/j.1460-9568.2003.02596.x. [DOI] [PubMed] [Google Scholar]
- 24.Klimesch W, Doppelmayr M, Wimmer H, Gruber W, Rohm D, Schwaiger J, et al. Alpha and beta band power changes in normal and dyslexic children. Clin Neurophysiol. 2001;112:1186–1195. doi: 10.1016/s1388-2457(01)00543-0. [DOI] [PubMed] [Google Scholar]
- 25.Spironelli C, Penolazzi B, Vio C, Angrilli A. Inverted EEG theta lateralization in dyslexic children during phonological processing. Neuropsychologia. 2006;44:2814–2821. doi: 10.1016/j.neuropsychologia.2006.06.009. [DOI] [PubMed] [Google Scholar]