Abstract
Abnormal oligomerization and aggregation of α-synuclein (α-syn/WT-syn) has been shown to be a precipitating factor in the pathophysiology of Parkinson's disease (PD). Earlier observations on the induced-alternative splicing of α-syn by Parkinsonism mimetics as well as identification of region specific abnormalities in the transcript levels of 112-synclein (112-syn) in diseased subjects underscores the role of 112-syn in the pathophysiology of PD. In the present study, we sought to identify the aggregation potential of 112-syn in the presence or absence of WT-syn to predict its plausible role in protein aggregation events. Results demonstrate that unlike WT-syn, lack of 28 aa in the C-terminus results in the loss of chaperone-like activity with a concomitant gain in vulnerability to heat-induced aggregation and time-dependent fibrillation. The effects were dose and time-dependent and a significant aggregation of 112-syn was evident at as low as 45°C following 10 min of incubation. The heat-induced aggregates were found to be ill-defined structures and weakly positive towards Thioflavin-T (ThT) staining as compared to clearly distinguishable ThT positive extended fibrils resulting upon 24 h of incubation at 37°C. Further, the chaperone-like activity of WT-syn significantly attenuated heat-induced aggregation of 112-syn in a dose and time-dependent manner. On contrary, WT-syn synergistically enhanced fibrillation of 112-syn. Overall, the present findings highlight a plausible cross-talk between isoforms of α-syn and the relative abundance of these isoforms may dictate the nature and fate of protein aggregates.
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder associated with the loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars compacta [1], [2]. α-Synuclein (α-syn), a pre-synaptic protein is known to play a crucial role in the pathophysiology of PD [3]–[6]. Also, α-syn was found to be the major constituent of Lewy body (LB) aggregates, the intracellular proteinaceous inclusions in PD which are considered as pathological hallmarks [7]. The gene structure of α-syn reveals the presence of 6 exons and so far at least three alternatively spliced transcripts were identified [8]–[10]. α-Syn is a 140-amino acid residue protein consisting of three domains: the N-terminal domain (amino acids 1–60) with five amphipathic helices interacting with lipid membranes, the central hydrophobic NAC domain (amino acids 61–95) and a highly charged acidic C-terminal domain (amino acids 96–140) conferring the negative charge to the protein [11]–[14]. Recently, it was shown that a decrease in the C-terminal charge favors the formation of fibrillar species shifting the equilibrium [15]. In vitro, α-syn aggregates and forms fibrils with similar morphology and properties to that of the amyloid fibrils extracted from Lewy bodies [16], [17]. The initial observation that A30P α-syn may have a tendency to accumulate as oligomers instead of mature fibrils led to the suggestion that α-syn may have a similar toxic mechanism [18], [19]. The small oligomers of α-syn are found to be more toxic when compared to the mature fibrils [20]. It has been suggested that the long range interactions between the NAC and C-terminal domain inhibit the formation of mature fibrils maintaining the stable natively unfolded structure of α-syn [21], [22]. Deletion of the C-terminus has been proposed to enhance the aggregation of α-syn and the C-terminal truncated forms were found to be more prone for fibrillation than the full length protein [23]–[26]. It is now well recognized that the abnormal oligomerization and aggregation of α-syn play a pivotal role in the Lewy body formation and the pathogenesis of Lewy body diseases [27].
Recent discoveries highlight that alternative splicing of α-syn may also play a crucial role in the mechanisms mediating PD [28]–[31]. So far, deletion of exon 3 [126-Syn] or exon 5 [112-Syn] or both exon 3 and 5 [98-Syn] have been identified [32], [33]. The aggregation propensities of these isoforms have been proposed based on the net charge of the protein as compared to wild-type synuclein (WT-syn) [34]. Our earlier study identified an induced-alternative splicing of α-syn by various Parkinsonian mimetics resulting in the generation of 112-syn (112-synuclein) and overexpression of 112-syn was found to be deleterious to dopaminergic cells [35]. Further, region specific transcript abnormalities were also noticed in patients as well as transgenic mouse models of α-synucleinopathies [36], [37]. Hence, in the present study we sought to understand the biological consequences arising out of the increased generation of 112-syn and its functional implications in the presence or absence of WT-syn to understand whether the relative abundance of α-syn isoforms play a crucial role in the events leading to protein aggregation.
Materials and Methods
Materials
Isopropyl-β-D-thiogalactopyranoside (IPTG), Glutathione-Sepharose 4B, Thrombin from bovine plasma, Aldolase (ALD) from rabbit muscle, ThioflavinT (ThT) and all other chemicals were purchased from Sigma, St. Louis, MO, USA. Restriction enzymes were obtained from Fermentas Inc, Fisher Scientific, PA, USA. All other reagents were of analytical grade.
Methods
Construction of WT and 112-syn expression plasmids
Human SH-SY5Y dopaminergic cells were treated with 2 mM MPP+ for 24 h, for the induction of 112-syn as described previously [35]. Following the termination of the experiment, the medium was aspirated and 1 ml of TRIzol reagent (Invitrogen Inc, USA) was added to the cells in 6-well plates and total RNA was extracted using the manufacturer's protocol. 5 µg of RNA was used for the first strand cDNA synthesis using a first strand cDNA synthesis kit (Thermo Scientific, USA) according to the manufacturer's protocol. Full-length WT-syn and 112-syn mRNA were amplified using high-fidelity PCR supermix (Hi FI PCR supermix, Invitrogen Inc, USA) using the forward (5′-AACCCGGGCATGGATGTATTCATGAA AGGACTTTCA-3′) and reverse (5′-AACTCGAGAGATATTTCTTAGGCTTCAGGTT CTAGT-3′) primers containing SmaI and Xho I restriction sites (shown in bold). Following PCR, the amplified product was digested with SmaI and Xho I and ligated into pGEX-4T-1 plasmid (GE Healthcare Life Sciences, USA) that was predigested with the same restriction enzymes in order to obtain the plasmids expressing the GST (Glutathione-S-transferase) fusion constructs of WT-syn and 112-syn respectively.
Expression and purification of α-syn isoforms
The GST-synuclein fusion constructs were transformed into BL21 (DE3) pLysS strain of Escherichia coli and protein expression was induced with 1 mM IPTG. The recombinant GST-synuclein fusion proteins of WT and 112-syn were purified by affinity chromatography using glutathione sepharose-4B beads as described previously [38]. The fusion proteins were subjected to thrombin cleavage and subsequently mixed with glutathione sepharose-4B beads and p-aminobenzamidine agarose to trap any cleaved-off GST and thrombin. The proteins were concentrated using Centricon concentrators (Millipore, USA) of molecular mass cut-off 10 kDa and further purified using Superdex75 (GE Healthcare) gel-filtration column equilibrated with phosphate-buffered saline, pH 7.4. The protein concentrations were determined by bicinchoninic acid (BCA) protein assay kit [Thermo Fisher Scientific, IL, USA] using bovine serum albumin as a standard and the purity of the proteins was verified by SDS-PAGE. Protein samples were centrifuged at 12,000×g, for 30 min at 4°C prior to the experiments to remove any possible particulates.
Measurement of chaperone like activity of α-syn
The ability of WT-syn and 112-syn in suppressing the heat-induced aggregation of substrate proteins like aldolase was monitored as described previously with slight modifications [39], [40]. Briefly, individual protein solutions of aldolase (0.1 mg/mL), WT and 112-syn, or mixtures of both WT and 112-syn with aldolase at varying concentrations were prepared in phosphate buffered saline (PBS) pH 7.4 and heated at 65°C for a period of 10 min in a quartz cuvette. The light scattering of the solution was monitored at 360 nm as a function of time using a thermostatted spectrophotometer (Jasco).
Turbidometric analysis of 112-syn
Turbidity measurement as an assay for protein aggregation was performed according to the established protocols [40]. Briefly, 112-syn at varying concentrations (0.2, 0.4, 0.6 mg/mL in a final volume of 100 µl) in phosphate buffered saline (PBS) pH 7.4 was incubated individually at three different temperatures of 45°C, 55°C, and 65°C for a period of 10 min in a quartz cuvette. The light scattering of the solution was monitored at 360 nm as a function of time using a spectrophotometer equipped with thermostatted cuvette holder (Jasco). In experiments where interdependency of isoforms was analyzed, 112-syn (0.4 mg/mL) was incubated with WT-syn at 1∶0.5, 1∶1 and 1∶2 ratios and kinetics of aggregation was analyzed by turbidometry.
Fibril formation
Solutions of both WT and 112-syn (0.5 mg/mL) in 20 mM Tris buffer, pH 7.5 were incubated under shaking conditions (1000 rpm) at 37°C in a thermo mixer (Eppendorf) as described previously [41]. Aliquots from the incubation mixtures were withdrawn at different time intervals for fluorescence measurements and transmission electron microscopy (TEM) analysis. In experiments where interdependency of isoforms was analyzed, WT-syn (0.25, 0.5 and 1.0 mg/mL) and 112-syn (0.5 mg/mL) were incubated at different ratios and kinetics of fibrillation was analyzed by Thioflavin (ThT) staining.
Analysis of fibril formation by ThT binding assay
Fibril formation was monitored with ThT fluorescence as described previously with slight modifications [42]. Briefly, aliquots of 5 µl from the incubation mixtures were withdrawn at various time points, diluted to 100 µl with 25 µM ThT in 50 mM Tris buffer (pH 8.0). The fluorescence emission spectrum (470–600 nm) of the samples was recorded following the excitation of samples at 450 nm in an Enspire 800 model Perkin-Elmer multimode reader. The blank measurement recorded prior to the addition of proteins was subtracted from the signal obtained from each sample.
Transmission Electron Microscopy
An aliquot of 5 µl was withdrawn from the incubation mixtures at different time intervals and adsorbed onto a glow-discharged carbon-coated copper Formvar EM grid. After 1–2 min of adsorption, the grids were rinsed for 1 min with a few drops of 1% uranyl acetate, blotted with filter paper and air dried as described earlier [42]. The images were recorded with a TECNAI FE12 TEM (Eindhoven, The Netherlands) instrument operating at 120 kV. The photomicrographs were analyzed with SIS imaging software.
Results
Increased propensity of 112-syn to heat-induced aggregation is associated with the lack of its chaperone activity
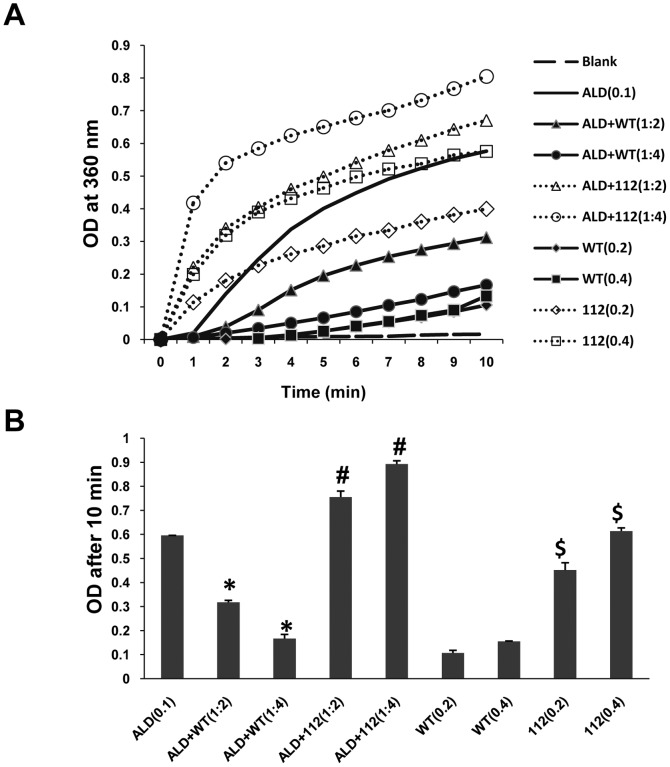
The ability of α-syn to suppress the aggregation of non-native conformations of substrate proteins like aldolase has been widely employed as a measure of its chaperone like activity in vitro. Previously, it was shown that chaperone-like activity of α-syn was lost due to the lack of its acidic C-terminus [40]. Concomitant with the earlier findings, our initial studies aimed at understanding the functional consequence of deleted exon-5 in 112-syn also noticed that unlike WT-syn, 112-syn did not demonstrate any inhibitory effect on the heat-induced aggregation of aldolase [Fig. 1 A and B]. Results indicate that incubation of aldolase alone (0.1 mg/mL) at 65°C induced its aggregation and the absorbance values reached from 0 to ∼0.6 during the incubation period of 10 min [Fig. 1A and B]. Moreover, WT-syn suppressed the aggregation of aldolase in a dose dependent manner [Fig. 1A and B]; which corroborates with the earlier observations [39]. However, the absorbance of incubation mixtures, wherein, a standard amount of aldolase (0.1 mg/mL) when incubated with increasing concentrations of 112-syn demonstrated a dose dependent increase in the absorbance at 360 nm which is significantly more than values exhibited by aldolase alone [Fig. 1A and B]. Under similar conditions, incubation mixture consisting of 112-syn alone exhibited a dose dependent increase in the absorbance at 360 nm and this phenomena was not observed with WT-syn [Fig. 1A and B]. The obtained results prompted us to study the heat-induced aggregation propensity of 112-syn.
Figure 1. Effect of WT and 112-syn on heat induced aggregation of aldolase (ALD).
(A) In vitro protein aggregation assay employing aldolase (0.1 mg/mL in PBS) was performed at 65°C in the absence or presence of WT and 112-syn at different ratios (1∶2 and 1∶4) and the light scattering was monitored at 360 nm for a period of 10 min. (B) The net change in the absorbance of reaction mixtures of (A) before and after 10 min of incubation at 65°C. Data are the mean ± SD of three separate experiments. * indicates p<0.01 as compared to aldolase (decrease); #p<0.01 as compared to aldolase (increase) and $ p<0.01 as compared to the respective controls (before heating).
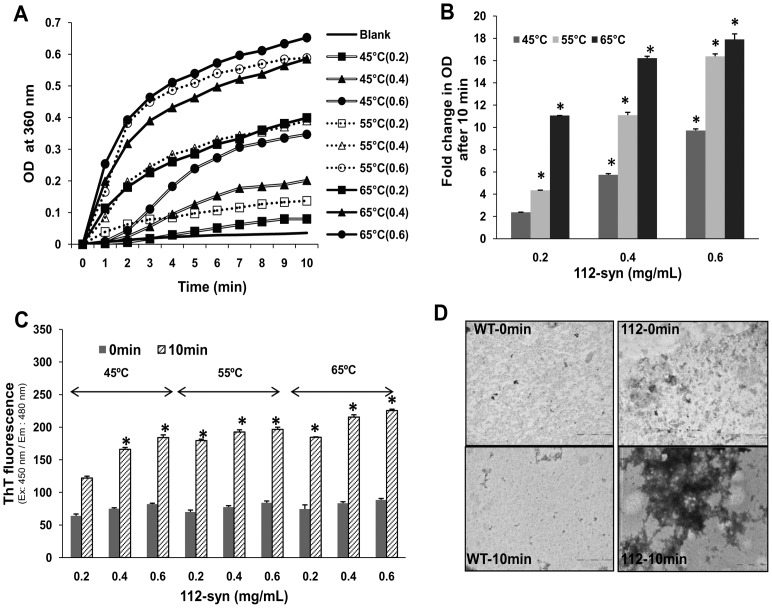
Next, we analyzed the kinetics of apparent light scattering of 112-syn (0.2, 0.4, 0.6 mg/mL) against heat-induced aggregation at three different temperatures (45, 55 and 65°C) over a period of 10 min. Results indicate a dose-dependent increase in the turbidity of 112-syn at all the temperatures examined [Fig. 2A and B]. While, 0.2 mg/mL of 112-syn displayed nearly 2-fold increase in the absorbance following 10 min of incubation at 45°C, the corresponding values for 0.4 and 0.6 mg/mL of protein were found to be 5 and 9 fold respectively. Similarly at 55°C, the three different concentrations of 112-syn (0.2, 0.4, 0.6 mg/mL) exhibited 4, 11 and 16 fold increase in the absorbance and at 65°C the fold increase in absorbance was found to be 11, 16 and 18 fold respectively [Fig. 2B]. The obtained data clearly demonstrate the enhanced vulnerability of 112-syn to variations in temperature and a significant increase in turbidity was evident at as low as 45°C. The binding of ThT to the crossed β-sheet structures (characteristic feature of amyloid fibrils of proteins) enhances its fluorescence and is being widely used to study protein fibrillation [43]. ThT staining of 112-syn before and after exposure to different temperatures though found significantly increased [Fig. 2C], but, the fold increase was not proportional to that of the observed turbidity under same experimental conditions (Fig. 2A and B). TEM images of 112-syn exposed to heat (65°C for 10 min) revealed the presence of ill-defined aggregates, however, under similar conditions no aggregates were found with WT-syn [Fig. 2D].
Figure 2. Temperature dependent aggregation of 112-syn.
(A) 112-syn at different concentrations (0.2, 0.4, 0.6 mg/mL in PBS) was heated at 45°C, 55°C, and 65°C and the light scattering was monitored at 360 nm for a period of 10 min as described under “Methods” section. (B) Fold change in the net absorbance values from (A) at 360 nm before and after heating the reaction mixtures. (C) ThT fluorescence of reaction mixtures as indicated in (A) before (0 min) and after exposing the samples at indicated temperatures for 10 min.*p<0.01 as compared to 0 min readings for both (B) and (C). (D) Negatively stained TEM images of WT and 112-syn before and after exposure to 65°C for 10 min.
WT-syn attenuates heat-induced aggregation of 112-syn
Based on the above observations on distinctly different property of 112-syn as compared to WT-syn, we surmised whether the chaperone-like activity of WT-syn confers any protection towards the heat-induced aggregation of 112-syn. To examine this, we have exposed 112-syn to 65°C for 10 min in the presence or absence of WT-syn (in the ratios of 1∶0.5, 1∶1 and1∶2). Results demonstrate that WT-syn dose-dependently inhibited the heat-induced aggregation of 112-syn. At 1∶0.5, 1∶1 and 1∶2 ratios of 112-syn: WT-syn, the percent inhibition was found to be nearly 46, 62 and 72% respectively [Fig. 3A and B]. Under similar experimental conditions no gross changes in the absorbance were noticed in incubation mixtures consisting of WT-syn alone [Fig. 3B].
Figure 3. Effect of WT-syn on heat induced aggregation of 112-syn.
(A) The chaperone-like activity of WT-syn was monitored by incubating 112-syn (0.4 mg/mL) in the presence or absence of different ratios of WT-syn as described in the “Methods” section. The time dependent increase in absorbance at 360 nm was recorded over a period of 10 min. (B) Fold change in the net absorbance values from (A) before and after 10 min of incubation at 65°C recorded at 360 nm.*p<0.01 as compared to 112-syn before heating.
Time-dependent fibrillation of 112-syn
Earlier reports demonstrated that α-syn aggregates in a time dependent manner forming large network of fibrils and a significant increase in ThT staining was reported following prolonged incubation (three days) at room temperature [42]. In order to understand the observed differences in ThT reactivity, we have incubated individually WT and 112-syn (0.5 mg/mL) under shaking conditions for 24 h at 37°C and subjected to ThT staining. The fluorescence emission spectra of ThT at different time points (0, 12, 18 & 24 h) for WT-syn and 112-syn are shown in Figure 4A and B. Results indicate that incubation mixtures containing WT-syn did not show any gross changes in the ThT emission spectrum during the initial 24 h time period [Fig. 4A]. Nevertheless, a significant increase in the aggregation of WT-syn was evident following prolonged incubation as reported in earlier studies (data not shown) [42]. Under similar incubation conditions, 112-syn exhibited a time dependent increase in the fibrillation based on the ThT emission spectrum [Fig. 4B]. Nearly, three, five and ten-fold-increase in ThT fluorescence of 112-syn was observed at 12, 18 and 24 h respectively, whereas, ThT fluorescence of WT-syn remained the same during the initial 24 h incubation [Fig. 4C]. TEM images of 112-syn under the above incubation conditions showed the presence of long extended network of fibrils and this effect was not observed with WT-syn [Fig. 4D], which is in coherence with the obtained ThT signal [Fig. 4A and B]. The data demonstrates that 112-syn possess enhanced vulnerability towards fibrillation as compared to WT-syn.
Figure 4. Time dependent fibrillation of WT and 112-syn.
Time dependent fibrillation of WT-syn (A) and 112-syn (B) was monitored by the enhancement of ThT fluorescence. WT and 112-syn (0.5 mg/mL) at 37°C were incubated in 20 mM Tris buffer, pH 7.5 for indicated time intervals as described in the “Methods” section and the ThT fluorescence emission spectrum was recorded. (C) Fold change in the ThT fluorescence of WT and 112-syn incubated for different time intervals. Data presented are the mean ± SD of three separate experiments. (D) Aliquots from reaction mixtures incubated at 37°C for 24 h were adsorbed onto carbon coated grids and then stained with 1% uranyl acetate for 1–2 min. Negatively stained TEM images of WT and 112-syn are shown. Scale bars, 500 nm and data is a representative of three different fields of view. *p<0.01 as compared to 0 h readings for 112-syn.
WT-syn exacerbates fibrillation of 112-syn
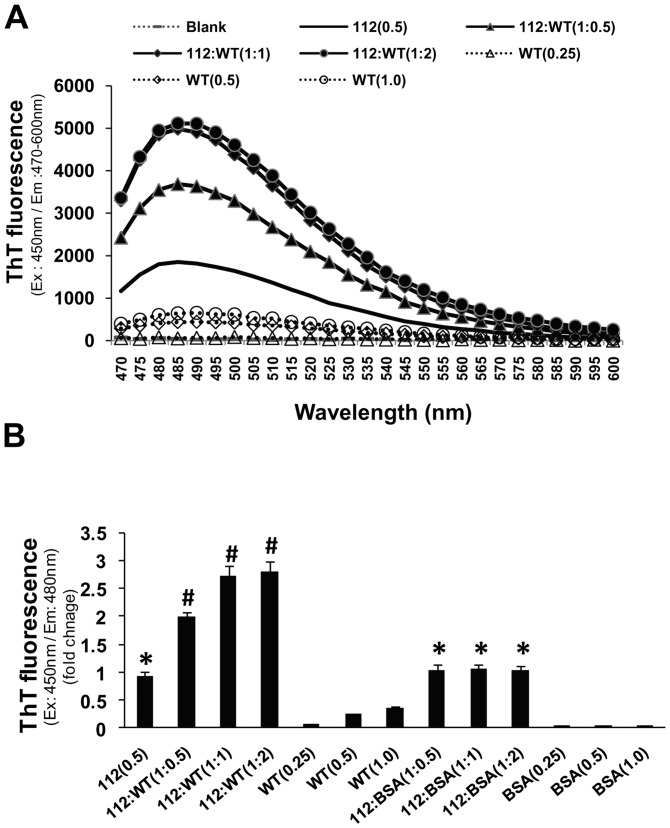
Since, WT-syn was found to inhibit heat-induced aggregation of 112-syn, we next analyzed whether similar effects would also be evident on the fibrillation of 112-syn. To examine this, fibrillation of 112-syn was monitored by ThT staining following 12 h incubation at 37°C in reaction mixture containing 112-syn in the presence or absence of WT-syn at different ratios. The obtained data indicates that WT-syn dose-dependently increased the fibrillation of 112-syn as measured by ThT staining [Fig. 5A]. 112-syn when incubated with WT-syn at 1∶0.5 and 1∶1 ratio resulted in the enhancement of ThT fluorescence by nearly 1.9 and 2.7 fold respectively and the values remained nearly the same even at 1∶2 ratio [Fig. 5A and B]. A similar enhancement in ThT staining was not observed when 112-syn was incubated with different concentrations of bovine serum albumin, which rules out the possibility of differences in protein concentration between samples for the observed effects.
Figure 5. Effect of WT-syn on time dependent fibrillation of 112-syn.
(A) 112-syn (0.5 mg/mL) was incubated in the presence or absence of different ratios of WT-syn at 37°C in 20 mM Tris buffer, pH 7.5 for 12 h. The increase in ThT fluorescence emission spectrum (470–600 nm) was recorded following excitation at 450 nm. (B) Fold change in the ThT fluorescence of 112-syn, incubated for 12 h in the presence or absence of different ratios of WT-syn. Data presented are the mean ± SD of three separate experiments. *p<0.01 as compared to 0 h reading for 112-syn; #p<0.01 as compared to112-syn alone.
Discussion
The presynaptic protein, α-syn is being considered as one of the precipitating factor in the pathophysiology of PD and gaining significance for its promising role in dementia with Lewy bodies (DLB) and related synucleinopathies [7], [44]–[45]. Earlier studies have identified the regulation of α-syn at various levels, such as, protein-protein interactions, post translational modifications effects on dopamine transporters, gene dosage or multiplication and alternative splicing [9], [46]. However, the events leading to the oligomerization and aggregation of α-syn were considered to be crucial in understanding the formation of LB aggregates, the hallmark of PD and related synucleinopathies [16], [17]. Amongst the proposed mechanisms, posttranslational modifications were shown to enhance the aggregation propensity of α-syn due to the formation of covalent links and stabilization of α-syn filaments [47]–[49]. Recent reports suggest that alternative splicing of α-syn is yet another regulation by which specific domains of proteins are deleted resulting in the generation of smaller isoforms with altered properties [33]–[35]. Also, PD-associated α-syn gene variants at the 3′ region influencing the expression of 112-syn and identification of region specific transcript abnormalities in related synucleinopathies emphasize the need to understand the role of these smaller isoforms in the pathophysiology of PD and related ailments [23]–[26] [36]–[37] [50].
Though the precise mechanism contributing to the generation of alternatively spliced isoforms of α-syn are not completely understood, our recent study identified the induced-alternative splicing of α-syn by Parkinsonian mimetics resulting in the generation of 112-syn. Also, overexpression of 112-syn was found to be deleterious to dopaminergic cells [35]. Based on these previous observations, the present study was conceived to understand the role of 112-syn by examining the behavior of this protein both in vitro and in situ in order to predict its plausible role in the events leading to protein aggregation/fibrillation.
The protein domain structure of α-syn reveals primarily three main regions, the first half from the N-terminus contains several repeat regions of the consensus sequence KTKEGV and is considered as the membrane interaction domain; the middle region, referred to as a non-amyloid-component region (NAC), is the region prone for aggregation; and the highly acidic C-terminus region maintains the protein in the solubilized form and functions as a chaperone [6], [39]. Consistent with this model, we found that unlike WT-syn, 112-syn loses chaperone-like activity as analyzed by the heat induced aldolase aggregation assay and this finding corroborates with earlier report [Fig. 1A and B][40]. Moreover, 112-syn alone exhibited an enhancement of absorbance in a dose dependent manner, which was further increased in presence of aldolase and this unique observation prompted us to study in detail its aggregation potential [Fig. 2].
The present study employing purified proteins of WT and 112-syn demonstrated that112-syn possess a temperature dependent aggregation propensity and this feature was lacking in WT-syn. In fact, a significant increase in 112-syn aggregation was evident at as low as 45°C within 10 min and the effects were observed to be dose-dependent. To our knowledge, the present study happens to be the first one to identify the temperature-dependent aggregation of 112-syn in a cell free assay system [Fig. 2 A and B].
Earlier reports demonstrated the tendency of WT-syn to form fibrillar structures at room temperatures following prolonged incubation [42]. However, the present study identified the enhanced fibrillation potential of 112-syn as compared to its wild-type counterpart and the fibrillar structures exhibited enhanced ThT fluorescence unlike the heat-induced aggregates. Though fibrillation of WT-syn was noticed following prolonged incubation corroborating with earlier findings (data not shown) [42], no gross fibrillation was evident during the initial 24 h time period [Fig. 4A]. These findings clearly indicate that 112-syn possesses accelerated potential towards fibrillation in addition to heat-induced aggregation. The inherent chaperone-like activity of WT-syn in preventing the non-native conformations of other proteins has been reported previously [39], [40]. Further, we also noticed that WT-syn dose-dependently inhibits the heat-induced aggregation of 112-syn [Fig. 3A and B]. Recently, it was reported that the C-terminus truncated form of α-syn induced the aggregation of WT-syn [25]. In the present study, we observed that WT-syn exacerbated fibrillation of 112-syn in vitro in a dose dependent manner [Fig 5A and B], however, the implications of the observed findings needs to be examined in vivo. Overall, the present results clearly indicate a possible cross-talk between α-syn isoforms in protein aggregation and provide us with new information on the possible biological consequences especially when the ratios of α-syn isoforms are altered. Moreover, our earlier study identified induced-alternative splicing of α-syn resulting in the generation of 112-syn by various Parkinsonian mimics leading to gross alterations in the ratios of WT-syn: 112-syn in cellular models of PD [35]. In addition, the existence of altered levels of α-syn isoforms in patients suffering from PD, DLB and synucleinopathies have been recently reported [36], [37]. In light of the above findings, the observed aggregation potential of 112-syn in conjunction with its inter-dependence on the relative abundance of WT-syn might bear serious biological consequences and underscores the importance of plausible cross-talk between α-syn isoforms in the pathophysiology of PD or related pathologies. However, further in vivo studies are warranted to pin-point the relative contribution of individual isoforms of α-syn in disease pathology.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
S.V.K. acknowledges funding from the Department of Biotechnology (DBT), India and miND project (BSC-115) from CSIR, India. The senior research fellowship from Council of Scientific and Industrial Research (CSIR), India, is gratefully acknowledged by K.M.M. D.Y. acknowledges financial support from DENOVA project by CSIR, INDIA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beitz JM (2014) Parkinson's disease: a review. Front Biosci (Schol Ed) 1(6): 65–74. [DOI] [PubMed] [Google Scholar]
- 2. Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122(Pt 8): 1437–1448. [DOI] [PubMed] [Google Scholar]
- 3. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645): 839–840. [DOI] [PubMed] [Google Scholar]
- 4. Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson's disease. Science 302(5646): 819–822. [DOI] [PubMed] [Google Scholar]
- 5. Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, et al. (1998) Abnormal accumulation of NACP/alpha-synuclein in neurodegenerative disorders. Am J Pathol 152(2): 367–372. [PMC free article] [PubMed] [Google Scholar]
- 6. Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, et al. (2004) Alpha synuclein and Parkinson's disease. FASEB J 18(6): 617–626. [DOI] [PubMed] [Google Scholar]
- 7. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) Alpha Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95(11): 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia Y, Saitoh T, Ueda K, Tanaka S, Chen X, et al. (2001) Characterization of the human alpha-synuclein gene: Genomic structure, transcription start site, promoter region and polymorphisms. J Alzheimers Dis 3(5): 485–494. [DOI] [PubMed] [Google Scholar]
- 9. Beyer K (2006) Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol 112(3): 237–251. [DOI] [PubMed] [Google Scholar]
- 10. Surguchov A (2013) Synucleins: are they two-edged swords? J Neurosci Res 91(2): 161–166. [DOI] [PubMed] [Google Scholar]
- 11. Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273(16): 9443–9449. [DOI] [PubMed] [Google Scholar]
- 12. Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, et al. (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 90(23): 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han H, Weinreb PH, Lansbury PT Jr (1995) The core Alzheimer's peptide NAC forms amyloid fibrils which seed and are seeded by beta-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem Biol 2(3): 163–169. [DOI] [PubMed] [Google Scholar]
- 14. Uversky VN (2007) Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem 103(1): 17–37. [DOI] [PubMed] [Google Scholar]
- 15. Levitan K, Chereau D, Cohen SI, Knowles TP, Dobson CM, et al. (2011) Conserved C-terminal charge exerts a profound influence on the aggregation rate of α-synuclein. J Mol Biol 411(2): 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA (2000) Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc Natl Acad Sci U S A 97(9): 4897–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conway KA, Harper JD, Lansbury PT Jr (2000) Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry 39(10): 2552–2563. [DOI] [PubMed] [Google Scholar]
- 18. Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, et al. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A 97(2): 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldberg MS, Lansbury PT Jr (2000) Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson's disease? Nat Cell Biol 2(7): E115–E119. [DOI] [PubMed] [Google Scholar]
- 20. Volles MJ, Lansbury PT Jr (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry 42(26): 7871–7878. [DOI] [PubMed] [Google Scholar]
- 21. Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, et al. (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci U S A 102(5): 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hong DP, Xiong W, Chang JY, Jiang C (2011) The role of the C-terminus of human α-synuclein: intra-disulfide bonds between the C-terminus and other regions stabilize non-fibrillar monomeric isomers. FEBS Lett 585(3): 561–566. [DOI] [PubMed] [Google Scholar]
- 23. Hoyer W, Cherny D, Subramaniam V, Jovin TM (2004) Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro. Biochemistry 43(51): 16233–16242. [DOI] [PubMed] [Google Scholar]
- 24. Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, et al. (2005) A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem 280(24): 22670–22678. [DOI] [PubMed] [Google Scholar]
- 25. Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M (2010) Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur J Neurosci 32(3): 409–422. [DOI] [PubMed] [Google Scholar]
- 26. Murray IV, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, et al. (2003) Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry 42(28): 8530–8540. [DOI] [PubMed] [Google Scholar]
- 27. Shults CW (2006) Lewy bodies. Proc Natl Acad Sci U S A 103(6): 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLean PJ, Hyman BT (2002) An alternatively spliced form of rodent alpha-synuclein forms intracellular inclusions in vitro: role of the carboxy-terminus in α-synuclein aggregation. Neurosci Lett 323(3): 219–223. [DOI] [PubMed] [Google Scholar]
- 29. Beyer K, Domingo-Sàbat M, Humbert J, Carrato C, Ferrer I, et al. (2008) Differential expression of alpha-synuclein, parkin, and synphilin-1 isoforms in Lewy body disease. Neurogenetics 9(3): 163–172. [DOI] [PubMed] [Google Scholar]
- 30. Beyer K, Domingo-Sábat M, Lao JI, Carrato C, Ferrer I, et al. (2008) Identification and characterization of a new alpha-synuclein isoform and its role in Lewy body diseases. Neurogenetics 9(1): 15–23. [DOI] [PubMed] [Google Scholar]
- 31. Beyer K, Humbert J, Ferrer A, Lao JI, Carrato C, et al. (2006) Low alpha-synuclein 126 mRNA levels in dementia with Lewy bodies and Alzheimer disease. Neuroreport 17(12): 1327–1330. [DOI] [PubMed] [Google Scholar]
- 32. Campion D, Martin C, Heilig R, Charbonnier F, Moreau V, et al. (1995) The NACP/synuclein gene: chromosomal assignment and screening for alterations in Alzheimer disease. Genomics 26(2): 254–257. [DOI] [PubMed] [Google Scholar]
- 33. Ueda K, Saitoh T, Mori H (1994) Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid. Biochem Biophys Res Commun. 205(2): 1366–1372. [DOI] [PubMed] [Google Scholar]
- 34. Beyer K, Ariza A (2013) α-Synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol Neurobiol 47(2): 509–524. [DOI] [PubMed] [Google Scholar]
- 35. Kalivendi SV, Yedlapudi D, Hillard CJ, Kalyanaraman B (2010) Oxidants induce alternative splicing of alpha-synuclein: implications for Parkinson's disease. Free Radic Biol Med 48(3): 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mc LeanJR, Hallett PJ, Cooper O, Stanley M, Isacson O (2012) Transcript expression levels of full-length alpha-synuclein and its three alternatively spliced variants in Parkinson's disease brain regions and in a transgenic mouse model of alpha-synuclein overexpression. Mol Cell Neurosci 49(2): 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardo LF, Coto E, de Mena L, Ribacoba R, Mata IF, et al. (2014) Alpha-synuclein transcript isoforms in three different brain regions from Parkinson's disease and healthy subjects in relation to the SNCA rs356165/rs11931074 polymorphisms. Neurosci Lett 562(6): 45–49. [DOI] [PubMed] [Google Scholar]
- 38.Andrekopoulos C, Zhang H, Joseph J, Kalivendi S, Kalyanaraman B (2004) Bicarbonate enhances alpha-synuclein oligomerization and nitration: intermediacy of carbonate radical anion and nitrogen dioxide radical. Biochem J. 378(Pt 2): 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim TD, Paik SR, Yang CH, Kim J (2000) Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci 9(12): 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim TD, Paik SR, Yang CH (2002) Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry 41(46): 13782–1379. [DOI] [PubMed] [Google Scholar]
- 41. Uversky VN, Li J, Fink AL (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276(14): 10737–10744. [DOI] [PubMed] [Google Scholar]
- 42. Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, et al. (2003) Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem 278(5): 3235–3240. [DOI] [PubMed] [Google Scholar]
- 43. LeVine H 3rd (1999) Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309: 274–284. [DOI] [PubMed] [Google Scholar]
- 44. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276(5321): 2045–2047. [DOI] [PubMed] [Google Scholar]
- 45. Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, et al. (2002) Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99(22): 14524–14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee FJ, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15(6): 916–926. [DOI] [PubMed] [Google Scholar]
- 47. Ischiropoulos H (2003) Oxidative modifications of alpha-synuclein. Ann N Y Acad Sci 991: 93–100. [DOI] [PubMed] [Google Scholar]
- 48. Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, et al. (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290(5493): 985–989. [DOI] [PubMed] [Google Scholar]
- 49.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H (2000) Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 275(24): 18344–18349. [DOI] [PubMed] [Google Scholar]
- 50. McCarthy JJ, Linnertz C, Saucier L, Burke JR, Hulette CM, et al. (2011) The effect of SNCA 3′ region on the levels of SNCA-112 splicing variant. Neurogenetics 12(1): 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.