Abstract
Here we describe novel forms of structural integration between endo- and episymbiotic microbes and an unusual new species of snail from hydrothermal vents in the Indian Ocean. The snail houses a dense population of γ-proteobacteria within the cells of its greatly enlarged esophageal gland. This tissue setting differs from that of all other vent mollusks, which harbor sulfur-oxidizing endosymbionts in their gills. The significantly reduced digestive tract, the isotopic signatures of the snail tissues, and the presence of internal bacteria suggest a dependence on chemoautotrophy for nutrition. Most notably, this snail is unique in having a dense coat of mineralized scales covering the sides of its foot, a feature seen in no other living metazoan. The scales are coated with iron sulfides (pyrite and greigite) and heavily colonized by ɛ- and δ-proteobacteria, likely participating in mineralization of the sclerites. This novel metazoan-microbial collaboration illustrates the great potential of organismal adaptation in chemically and physically challenging deep-sea environments.
Novel symbiotic relationships have contributed greatly to the nutritional, physiological, behavioral, and anatomical diversification of multicellular organisms (11, 23). The discovery of deep-sea hydrothermal vent ecosystems in 1977 exposed many new animals that derive their nutrition from chemoautotrophic microbes housed in a variety of tissues and structures. For example, adult vestimentiferan tubeworms (family Siboglinidae) lack a digestive tract and derive their nutrition solely from endosymbiotic sulfur-oxidizing (thiotrophic) bacteria housed in a novel organ called the trophosome (9, 20). In contrast, hydrothermal vent mollusks, including clams (family Vesicomyidae), mussels (family Mytilidae), and some snails (family Provannidae), house endosymbionts in hypertrophied gills (3, 5, 39). Likewise, external thiotrophic epibionts are found on vent invertebrates such as the swarming vent shrimp Rimicaris exoculata and the polychete Alvinella pompejana, housed on modified setae of an expanded branchial chamber (38) and on expansions of the epidermis (16, 17), respectively. The surprisingly intimate structural, biochemical, and even molecular integrations between symbiotic partners demonstrate the breadth of organismal diversity that can emerge from the unification of two or more distinct organisms. Remarkably, however, these tightly integrated nutritional symbioses are young on the evolutionary timescale, since many of the invertebrate participants diversified during the late Mesozoic or Cenozoic epoch (27, 43).
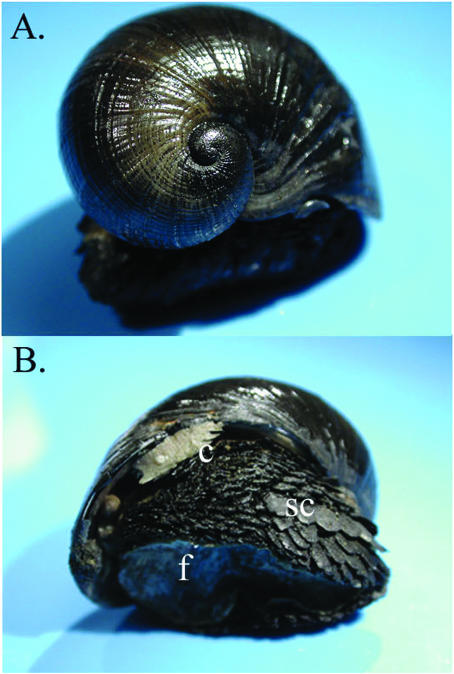
Endo- and episymbioses between bacteria and invertebrates play a critical role in sustaining the high biomass observed at vent fields in all ocean basins. Recently explored Indian Ocean vent sites are no exception, with high abundances of bresiliid shrimp, provannid snails, and bathymodiolin mussels, all shown to have either endo- or epibionts (14, 35, 39, 44). Until recently, gastropod mollusks were known to primarily dominate western Pacific vent sites. Hydrothermal vents on the Central Indian Ridge, however, support diverse and abundant large gastropods, including Phymorhynchus spp., Alvinoconcha sp. nov., and a new genus of snail with a very unusual morphology (Fig. 1) (44). This relatively large snail (∼5-cm shell diameter), tentatively named the scaly snail for its unique pedal morphology, was recently discovered at the base of black smoker chimneys with other gastropods, presumably in a zone of mixing between warm sulfide-rich vent effluents and cold (2°C) oxygen-rich sea water (43, 44, 46). The scaly snail has recently been described as a member of the Neomphalina (family Peltospiridae; genus and species novum), a gastropod order found only at hydrothermal vents (46).
FIG. 1.
Scaly snail anatomy. (A) Whole animal, showing the shell and foot. (B) Whole animal, showing anatomical features, including the underside of the foot (f), metal-rich (pyrite and greigite) scales covering the foot (sc), and the cephalic region (c). Note that the black shell is not pyritized but made of aragonite (46).
The scaly snail is morphologically distinct from known snails in that it lacks a typical operculum but has instead a broad foot covered by hundreds of scale-shaped sclerites. The scales are composed primarily of iron sulfides, both pyrite (FeS2) and greigite (Fe3S4), and exhibit ferrimagnetism (46). In addition, various anatomical characteristics of the scaly snail suggested the potential for an invertebrate-bacterial symbiosis. The scaly snail possessed a robust bipectinate gill, a reduced digestive system (<10% of the volume of typical deposit-feeding gastropods), and a prominent circulatory system, similar to that of provannid gastropods, which are known to host chemoautotrophic symbionts (46). The snail also possessed a weak radula, and iron sulfides were noted as the only gut contents (46). These morphological characteristics are similar to those observed in many invertebrate-bacterial symbioses, but symbionts were not observed in the gills of this animal as they are in all other molluscan hosts of chemoautotrophic bacteria (39, 47).
Here we document the presence of both endo- and episymbionts associated with the scaly snail. These symbioses represent novel forms of structural integration between microbes and this new species of gastropod (Fig. 1). In this study, we show that the scaly snail harbors a single microbial species that appears to provide most of the snail's nutrition within an enlarged esophageal gland. We also show that this snail possesses a diverse array of epibiotic microbes that may be involved in coating the pedal sclerites with iron sulfide minerals.
MATERIALS AND METHODS
Specimens.
In March to May of 2001, the R.V. Knorr, with the R.O.V. Jason, explored the intersection of the southeast, southwest, and central Indian Ridges at the Rodriguez Triple Junction in the Indian Ocean (25°19.23′S, 70°02.42′E) (18, 44). Specimens were collected at depths of 2,415 to 2,460 m and either frozen at −80°C and transported back to the laboratory on dry ice or preserved directly in 10% buffered formalin.
DNA analyses.
The DNEasy kit (QIAGEN, Valencia, Calif.) was used to extract total DNA from frozen tissue (including shell, scales, esophageal gland, and foot tissues from the scaly snail and foot tissue from various additional snail species where noted) following the manufacturer's protocol. A ∼1,500-bp fragment of the 16S rDNA gene was amplified with Taq polymerase (Promega, Madison, Wis.), the Eubacteria-specific primers 27F and 1492R, and the following PCR conditions: initial denaturation at 94°C for 3 min, 25 cycles of 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 7 min. PCR products were either cloned or sequenced directly with an ABI 3100 capillary sequencer and version 3.0 Big Dye according to the manufacturer's recommended protocols (Perkin Elmer/Applied Biosystems, Foster City, Calif.). Bacterial clone libraries were constructed by Topo TA cloning (Invitrogen) according to the manufacturer's instructions. Additional sequence data were obtained from the GenBank database, and all sequences were compiled and aligned with the ARB Fast Aligner (40). Phylogenetic analyses, including parsimony and neighbor-joining methods, were performed with PAUP*4.0b10 (41).
Amplified 16S ribosomal DNA (rDNA) fragments were digested with RsaI and HaeIII according to the manufacturer's recommendations (New England Biolabs, Beverly, Mass.). Restriction fragments were separated on 2% agarose gels and visualized with a Kodak Digital Science imaging system (Kodak, New Haven, Conn.).
Electron microscopy.
Samples for scanning electron microscopy were transferred from 10% buffered formalin to 99.5% ethanol and then air-dried or critical-point dried and examined with a Hitachi S-4300 field emission instrument. Samples for transmission electron microscopy were either rinsed in 0.1 M cacodylate-buffered 2% gluteraldehyde or 0.1 M phosphate buffer with 0.25 M sucrose and postfixed in a 1% osmium tetroxide solution, dehydrated in a graded acetone series, and stained with 2% uranyl acetate. Subsamples of the esophageal gland were infiltrated with Embed 812 epoxy embedding medium, cut into 0.1-μm sections, and stained with lead citrate. Sections were viewed with a Zeiss 109 electron microscope.
Isotopes.
Stable isotope analyses were made by R. Petty, Analytical Laboratory, Marine Science Institute, University of California, Santa Barbara. Carbon stable isotope values are reported in δ notation relative to the Pee Dee Belemnite standard: δ13C = [(13Csample/12Csample)/(13Cstandard/12Cstandard) − 1] × 103. Nitrogen isotopes are reported in δ notation relative to the air standard: δ15N = [(15Nsample/14Nsample)/(15Nstandard/14Nstandard) − 1] × 103.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences acquired during this study are AY310506, AY32787 to AY327886, AY355294 to AY355305, and AY531557 to AY531606.
RESULTS
Anatomy and microscopy.
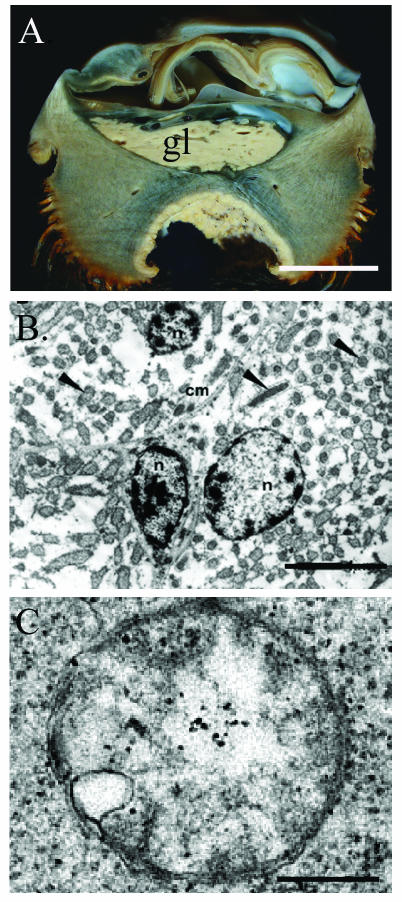
Microscopic examination of scaly snail tissues did not reveal bacterial cells in the gills as they occur in other molluscan hosts of chemoautotrophic bacteria. Transmission electron and epifluorescence microscopy of the enlarged esophageal gland (Fig. 2A), however, revealed what appeared to be bacteriocytes (∼20 to 50 μm in maximum dimensions), densely packed with pleomorphic bacterium-like cells (0.4 to 1.0 μm diameter) within secondary vacuoles (Fig. 2B and C).
FIG. 2.
Esophageal gland of the scaly snail. (A) Cross section through the entire snail, showing the large esophageal gland (gl). Bar, 0.5 cm. (B) Transmission electron micrograph of bacterial cells within the esophageal gland of the scaly snail. The symbionts (arrows) appear to occur within bacteriocytes. cm, cell membrane; n, nucleus. Bar, 2 μm. (C) Single bacterial cell. Bar, 0.5 μm.
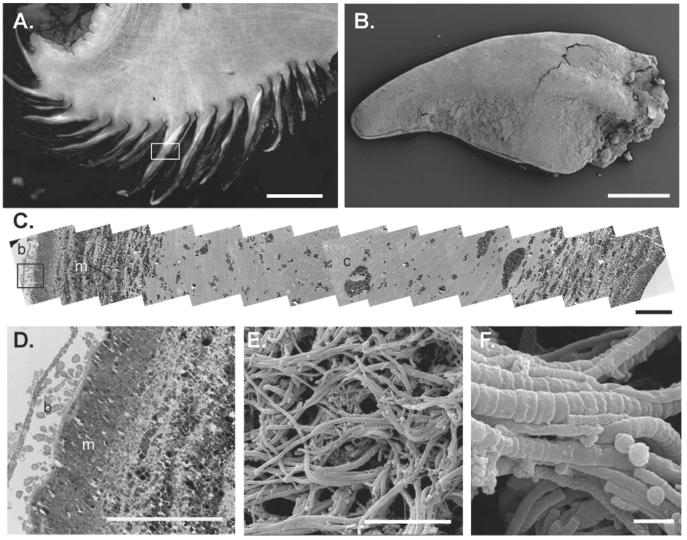
Transmission electron microscopy of the iron sulfide-mineralized sclerites (Fig. 3A and B) revealed heavy mineralization along the outside of the scales, with some diffuse mineralized particles within the snail tissue itself (Fig. 3C). Similarly, scanning electron microscopy revealed colonization of the outer surface of the scales by a diverse microflora, dominated by filamentous forms of bacteria but with some cocci and rods (Fig. 3D to F). Additionally, scanning electron microscopy observations suggested that the abundance of microflora observed on the shell of the scaly snail was lower than that on the sclerites.
FIG. 3.
Sclerites of the scaly snail. (A) Cross section through the foot and scales, showing snail tissue (white and brown layers) and iron sulfide layers (black). Bar, 4 mm. The boxed area is enlarged in panel C. (B) Scanning electron micrograph showing an individual sclerite. Bar, 1 mm. (C) Mosaic of 15 transmission electron micrographs through a scale in cross section (the boxed area is enlarged in panel D). Bar, 10 μm. (D) Transmission electron micrograph through a scale in cross section (corresponds to far-left box in panel C). Bar, 10 μm. (E) Scanning electron micrograph showing filamentous and coccoid bacteria on the scale surface. Bar, 10 μm. (F) Scanning electron micrograph showing filamentous and coccoid bacteria on the scale surface. Bar, 1 μm.
Phylogenetic analysis of the esophageal gland microbes.
To assess whether the bacterium-like cells found in the esophageal gland were phylogenetically related to known endosymbionts, we examined 16S ribosomal DNA sequences amplified from the esophageal gland (25). Examination of restriction fragment length polymorphism patterns for 75 clones generated from a 16S rDNA library from the esophageal gland of a single scaly snail with the restriction enzyme RsaI revealed a dominance of one phylotype. Multiple clones (9 and 16) from two snails and four direct PCR products from the esophageal glands of four additional snails were sequenced. Only one ribotype was recovered among the 29 sequences from the six snails (GenBank accession no. AY310506).
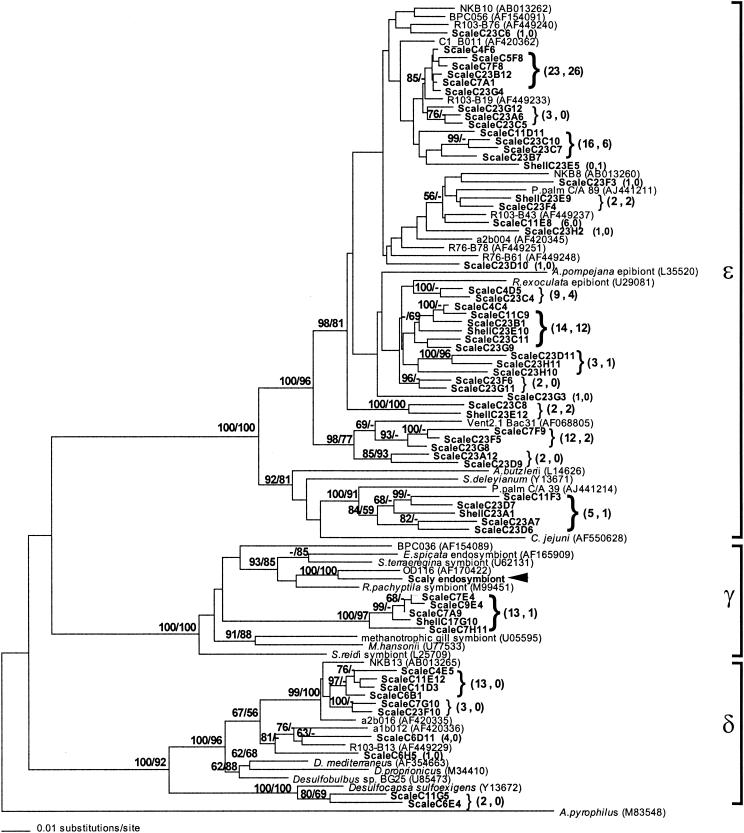
Phylogenetic analysis (distance and parsimony methods) (Fig. 4) placed this microbial ribotype within a well-supported clade of the γ-Proteobacteria subdivision (bootstrap value, >95) that contains thiotrophic endosymbionts from a number of invertebrates. Within this clade, however, the ribotype from the esophageal gland was most closely related (96% sequence similarity) to a free-living thiotrophic hydrothermal vent bacterium (Milos strain OD1116) (24). The scaly snail microbe was also similar in 16S rDNA sequence to the symbionts of the clam Solemya reidi (92%) and the vestimentiferan Riftia pachyptila (93%) (Fig. 4). In contrast, the closest Indian Ocean microbial relative of the scaly snail symbiont, the thiotrophic endosymbiont found in the mussel Bathymodiolus aff. brevior (49), showed only 86% similarity in 16S rDNA sequence.
FIG. 4.
Phylogenetic relationships, based on partial 16S rDNA gene sequences (∼958 bp), of the scaly snail endosymbiont (bold with arrow) and epibionts (bold) within the three major proteobacterial subdivisions (101 taxa included). Numbers in parenthesess following boldfaced taxa indicate the number of clones represented by each particular ribotype on the scales (first number) and the shell (second number). A neighbor-joining tree with Kimura two-parameter distances is shown. The numbers at the nodes represent bootstrap values from both neighbor-joining (first value) and parsimony (second value) methods obtained from 1,000 and 100 replicate samplings, respectively. In addition to microbes associated with the scaly snail, sequences were obtained from GenBank as noted.
Phylogenetic analysis of microbes on the mineralized scales.
The 16S ribosomal DNA sequences were also examined to determine the identity of microbes associated with the mineralized scales (25). Multiple clones (n = 155) recovered from the scales of two individual snails were sequenced. The majority of sclerite-associated 16S rDNA sequence types clustered mainly within three bacterial subdivisions (based on both distance and parsimony methods) (Fig. 4): the ɛ-proteobacteria (comprising 67% of the 155 total recovered environmental sequences), the sulfate-reducing lineages of the δ-proteobacteria (15%), and the γ-proteobacteria (8%, distinct from the esophageal gland bacteria). The Cytophaga-Flavobacterium-Bacteroides group represented 8% of the recovered environmental sequences (Fig. 5). Within the ɛ-Proteobacteria subdivision, nine ribotypes (6%) were closely related to epibionts on the shrimp Rimicaris exoculata and 60 ribotypes (39%) were similar to microbes observed on the tubes and mucus secretions of Riftia pachyptila and Paralvinella sp. polychetes (1, 29, 33).
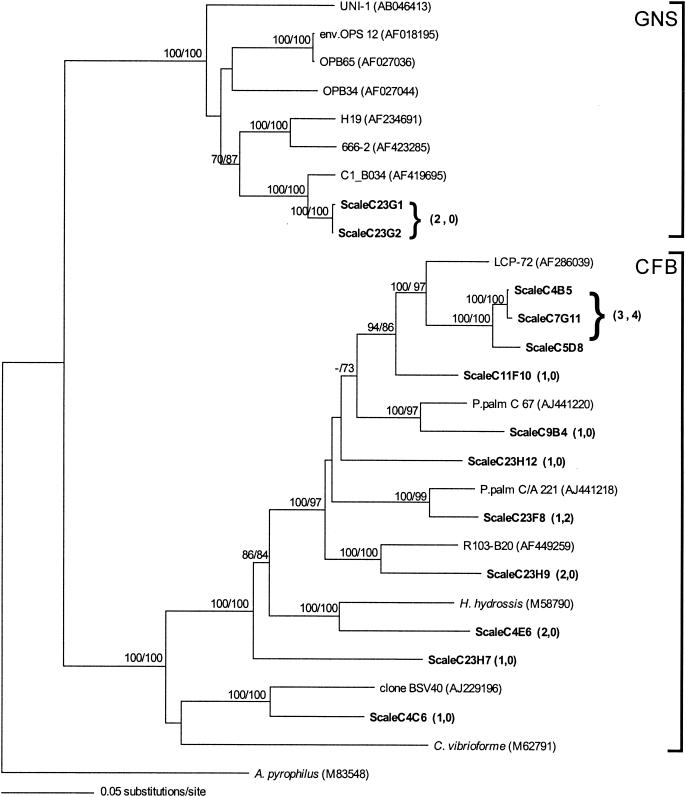
FIG. 5.
Phylogenetic relationships, based on partial 16S rDNA gene sequences (1,236 bp), of the scaly snail epibionts (in bold) within the Cytophaga-Flavobacterium-Bacteroides (CFB) and green nonsulfur (GNS) microbial groups (28 taxa included). Numbers in parentheses following boldfaced taxa indicate the number of clones represented by each particular ribotype on the scales (first number) and the shell (second number). A neighbor-joining tree with Kimura two-parameter distances is shown. The numbers at the nodes represent bootstrap values from both neighbor-joining (first value) and parsimony (second value) methods obtained from 1,000 replicate samplings. The GenBank accession numbers for sequences acquired during this study are listed in the text. In addition to microbes associated with the scaly snail, sequences were obtained from GenBank as noted.
Within the δ-Proteobacteria subdivision, almost all representatives (22 ribotypes, 14% of total recovered sequences) clustered with members of the family Desulfobulbaceae, related to Desulfobulbus and Desulfocapsa spp., commonly found in marine sediments and microbial mats (7, 31, 37). Additionally, the green nonsulfur bacteria and spirochetes were each represented by two clones (∼1%) (Fig. 5). Although the percentage of each sequence type is not indicative of its abundance in the environment, rarefaction curves with sequence ribotypes (155 total sequences) and restriction fragment length polymorphism patterns (89 clones cut with RsaI or HaeIII) suggested adequate sampling of environmental microbial variability.
Phylogenetic analysis indicated that the microflora found on the snail's shell, primarily ɛ-proteobacteria (89% of 63 clones recovered and sequenced), Cytophaga-Flavobacterium-Bacteroides group organisms (∼10%), and γ-proteobacteria (∼2%), comprised only a subset of ribotypes associated with the sclerites. Attempts to amplify bacteria from the foot tissue of co-occurring hydrothermal vent gastropod species, including Alvinoconcha sp. nov., Phymorhynchus sp. nov., and two species of Desbruyeresia from the Indian Ocean, were negative.
Isotopes.
Carbon and nitrogen isotope values were depleted for all tissues of the scaly snail (δ13C range = −20.7 to −16.7‰, δ15N range = 3.3 to 3.9‰, n = 4 for each tissue type) compared to those for the gastropods Phymorhynchus spp. nov. (δ13C = −14.5 ± 1.8‰, δ15N = 8.8 ± 0.6‰, n = 3) and Lepetodrilus sp. nov. (δ13C = −14.0 ± 1.0‰, δ15N = 6.8 ± 0.8‰, n = 9) collected from the same habitat (Table 1).
TABLE 1.
Stable isotope compositions of Indian Ocean hydrothermal vent gastropods
| Sample | Tissue | No. of samples tested | Avg ‰ ± SD
|
|
|---|---|---|---|---|
| δ13C | δ15N | |||
| Scaly snaila | Esophageal gland | 4 | −20.7 ± 0.9 | 3.3 ± 1.8 |
| Gill | 4 | −18.3 ± 0.6 | 3.9 ± 0.6 | |
| Foot | 4 | −18.2 ± 0.6 | 3.8 ± 0.5 | |
| Scales | 4 | −16.7 ± 0.6 | 3.8 ± 0.9 | |
| Phymorhynchus spp. nov.b | Whole animal | 3 | −14.5 ± 1.8 | 8.8 ± 0.6 |
| Lepetodrilus sp. nov. | Whole animal | 9 | −14.0 ± 1.0 | 6.8 ± 0.8 |
Comparable whole-animal bulk measurements (δ13C = −17.7±0.4‰, δ15N = 3.1 ± 0.5‰) were made by Van Dover et al. (44).
Identified by A. Waren.
DISCUSSION
Esophageal gland and endosymbionts.
The anatomical characteristics of the scaly snail, including a reduced digestive system, prominent circulatory system, and reduced ability to feed via radular function, suggested the potential for an invertebrate-bacterial symbiosis (46). Likewise, depleted carbon and nitrogen isotope values in the tissues of the scaly snail (Table 1) provided evidence for a unique carbon and nitrogen source compared with that used by predatory (Phymorhynchus spp. nov.) or grazing (Lepetodrilus sp. nov.) gastropods found in the same habitat.
Microscopic examination of the scaly snail did not reveal bacterial cells in the gill tissues as they occur in other molluscan hosts of chemoautotrophic bacteria, such as Alvinoconcha and Ifremeria spp. (39, 47). The esophageal gland, which is typically used for digestion in other gastropods, was highly vascularized and disproportionately large in the scaly snail (by approximately two orders of magnitude over the dimensions in other snails) (46). A unique network of blood vessels between the snail's foot and the esophageal gland provides a potential path for gas and metabolite exchange with the environment. These are characteristics of a tissue that may have evolved to house symbionts. Microscopy of the esophageal gland revealed bacteriocytes densely packed with bacterium-like cells within secondary vacuoles. Although this arrangement differs from that of other molluscan hosts, it is reminiscent of vestimentiferan worms, including Riftia pachyptila, which house symbionts in a vascularized and centralized tissue (9, 21).
Phylogenetic analysis of the bacterium-like cells found within the esophageal gland placed the single recovered microbial ribotype within a well-supported clade of the γ-proteobacteria that contains thiotrophic endosymbionts of mollusks and tubeworms. The closest relative of the scaly snail endosymbiont was a free-living thiotrophic bacterium (Milos strain OD1116) isolated from a shallow-water hydrothermal vent in the Aegean Sea (24). The OD1116 isolate might, in fact, be the first free-living representative to fall within a lineage of known chemoautotrophic sulfur-utilizing endosymbionts. This may be important when considering the mode of symbiont acquisition, either environmental or maternal inheritance, by invertebrate hosts.
Epibionts and mineralization of the scales.
The scaly snail is unique among gastropods because of its coat of mineralized sclerites. The scales, which are assumed to be homologous with the gastropod operculum, consisted of typical organic molluscan conchiolin tissue, such as the periostracum covering the interior of most mollusk shells (46). The exterior region was covered by layers of pyrite (FeS2) and greigite (Fe3S4) (46). Scanning electron microscopy revealed colonization of the outer surface of the scales by a morphologically diverse microflora that is visually similar to the epigrowth observed on rimicarid shrimp, alvinellid polychetes, ciliates, and nematodes from vents and other reducing environments (8, 12, 16, 17, 33, 34).
The majority of 16S rDNA sequence types recovered from members of this diverse microflora suggest a dominance of bacteria involved in sulfur cycling (Fig. 4). The sclerite-associated microflora clustered mainly within ɛ-proteobacteria found in hydrothermal vents, sulfate-reducing lineages of the δ-proteobacteria, and γ-proteobacteria (distinct from the esophageal gland bacteria). Evidence suggests that the episymbiotic bacteria of the scaly snail were not common on available surfaces or among gastropods within the same habitat. The microfloral composition of the scaly snail sclerites was different from the microflora found on hydrothermal vent chimney sulfides (from the scaly snail habitat), which consisted mostly of ɛ-proteobacteria and Aquificales spp. (19). Similarly, scanning electron microscopy observations suggested that the abundance of microflora observed on the shell of the scaly snail was lower than that on the sclerites and comprised only a subset of ribotypes of ɛ- and δ-proteobacteria. Additionally, PCR amplifications of bacterial sequences from the foot tissue of co-occurring gastropod species were negative.
ɛ-Proteobacteria are common within hydrothermal vent environments and can represent up to 40 to 98% of all phylotypes in environmental clone libraries, particularly in microbial mats, on sulfide edifices, and as epibionts on animals (28, 32, 35). Many cultured ɛ-proteobacteria are known to be involved in sulfur cycling (6, 48), although this metabolic pathway remains unconfirmed for a majority of the lineage. Filamentous forms of ɛ-proteobacteria in particular are common epibionts. For example, 65 to 96% of the microbes present on the vent polychete Alvinella pompejana and the shrimp Rimicaris exoculata were filamentous ɛ-proteobacteria (17, 35).
Sulfate reducers within the δ-proteobacteria are phylogenetically and morphologically diverse, and many are believed to be capable of biologically induced mineralization of iron sulfides (2, 10, 13, 15, 42). Desulfovibrio desulfuricans, closely related to microbial representatives on the scaly snail sclerites, has been shown to produce greigite (2). Similarly, Desulfocapsa sulfoexigens, within the family Desulfobulbaceae, can produce iron sulfides as a consequence of elemental sulfur disproportionation (13, 42). The production of exopolymers by sulfate reducers and the ability to influence the microenvironmental pH can also result in iron sulfide precipitation by members of the δ-proteobacteria (15). Pyritization of the scales, if microbial in nature, would likely result from extracellular production of pyrite, as observed in δ-proteobacteria, rather than intracellular production, as observed in magnetotactic bacteria (2). Microscopic examination (i.e., absence of magnetite chains) and the absence of known magnetotactic bacteria in our existing clone libraries argue against their involvement in mineralization of the scaly snail sclerites.
The relationship between the microflora of the scaly snail sclerites and the mineralized layer of iron sulfide and the greater relative abundance of sulfate-reducing, iron sulfide-producing δ-proteobacteria on the sclerites compared to other surfaces lead us to infer that the fortification of the sclerites is a result of the δ-proteobacterial episymbiotic microflora. The involvement of the snail is also likely, however, based on the specialized internal structure of the scales, the presence of mineralized particles within the snail conchiolin itself, the position of the microbes (i.e., fewer bacteria on the shell and other external tissues of the scaly snail itself), and the lack of microbes on the foot and opercular tissues of gastropods found within the same environment. We hypothesize that the snail facilitates the attachment of bacteria to the sclerites by the secretion of organic compounds (i.e., mucus), and, in turn, the community of bacteria produces the observed veneer of pyrite and greigite.
Unfortunately, iron sulfides produced by microbial sulfate reducers have not been investigated by high-resolution electron microscopy, and thus it is difficult to determine biogenic particles from those produced abiotically (2). Our observations therefore do not preclude abiotic precipitation of iron sulfides. The factors that control the abiotic formation of pyrite (and greigite) and the associated chemical pathways are controversial (4). The formation of pyrite can be facilitated by sulfur intermediates, such as polysulfides or elemental sulfur (4), or by hydrogen sulfide alone (45). Abiotic production of pyrite on the scales, however, is unlikely to occur at the presumed environmental temperatures surrounding the scaly snail (5 to 15°C) (36).
Mineralization of the sclerites by the host snail itself remains a possibility. Substantial iron sulfide particles were noted within the foot tissue of the scaly snail, suggesting possible internal precipitation of the minerals (Fig. 3C) (46). Numerous aquatic invertebrates mineralize metal compounds, such as magnetite, copper chloride, and zinc (26, 30). Controlled biomineralization of iron sulfides, however, has not been demonstrated in metazoans.
Previously described epibiotic associations have not been known to result in the formation of novel mineralized structures. Thus, the purpose of the sclerites remains an important question. Protection for the snail seems the most likely reason for the pyrite-covered pedal sclerites. Pyrite layers adjacent to the tubes of the hydrothermal polychete Paralvinella sulfincola are thought to provide a protective barrier from temperature fluctuations (22). Additionally, a coat of pyrite-covered scales may confer protection from predators, such as the predatory snails of the genus Phymorhynchus.
Conclusion.
The endo- and episymbioses observed in the scaly snail represent novel forms of structural integration between microbes and metazoans. This newly discovered snail demonstrates partnerships between two distinct assemblages of bacteria: a diverse community of epibionts and a single endosymbiont. The snail is morphologically and presumably physiologically different from other gastropod hosts of chemoautotrophic symbionts, and its metal-rich sclerites and enlarged esophageal gland are unique among animals. The symbiotic associations described here appear to be functional and persistent, although the obligate nature of the association, the degree of specificity, and the nutritional integration require further investigation. Many questions remain concerning this unusual animal, including the evolution and function of the pedal scales, the origin and control of mineralization of the scales, and the specific function of the diverse associated microbes (nutrition and/or detoxification). Despite these unanswered questions, close associations between the scaly snail and at least three major groups of bacteria, including γ-, δ-, and ɛ-proteobacteria, appear to be necessary for the success of this newly discovered species of mollusk.
Acknowledgments
We thank the scientific party on Knorr leg KN162-13 and the captains and crew of the R.V. Knorr and the R.O.V. Jason. We also thank Todd Walsh for photographic assistance, J. Salerno and S. Bengtson for original transmission and scanning electron micrographs, R. Lee for isotopic measurements, and C. Cavanaugh, A. L. Reysenbach, and R. Popa for helpful discussions.
This work was supported by the David and Lucile Packard Foundation, the U.S. National Science Foundation (R.C.V. and C.L.V.D.), and the M. Bergvall Foundation (A.W.).
REFERENCES
- 1.Alain, K., M. Olagnon, D. Desbruyeres, A. Page, G. Barbier, S. K. Juniper, J. Querellou, and M. Cambon-Bonavita. 2002. Phylogenetic characterization of the bacterial assemblage associated with mucous secretions of the hydrothermal vent polychaete Paralvinella palmiformis. FEMS Microbiol. Ecol. 1-14. 1440: [DOI] [PubMed]
- 2.Bazylinski, D. A., and B. M. Moskowitz. 1997. Microbial biomineralization of magnetic iron minerals: microbiology, magnetism and environmental significance, p. 181-223. In J. F. Banfield and K. H. Nealson (ed.), Reviews in mineralogy, vol. 35. Geomicrobiology: interactions between microbes and minerals. Mineralogical Society of America, Washington, D.C.
- 3.Belkin, S., D. C. Nelson, and H. W. Jannasch. 1986. Symbiotic assimilation of CO2 in two hydrothermal vent animals, the mussel B. thermophilus and the tube worm R. pachyptila. Biol. Bull. 170:110-121. [Google Scholar]
- 4.Benning, L. G., R. T. Wilkin, and H. L. Barnes. 2000. Reaction pathways in the Fe-S system below 100°C. Chem. Geol. 167:25-51. [Google Scholar]
- 5.Boss, K. J., and R. D. Turner. 1980. The giant white clam from the Galapagos Rift, Calyptogena magnifica species novum. Malacologia 20:161-194. [Google Scholar]
- 6.Campbell, B. J., C. Jeanthon, J. E. Kostka, G. W. Luther III, and S. C. Cary. 2001. Growth and phylogenetic properties of novel bacteria belonging to the epsilon subdivision of the Proteobacteria enriched from Alvinella pompejana and deep-sea hydrothermal vents. Appl. Environ. Microbiol. 67:4566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canfield, D. E., B. Thamdrup, and S. Fleischer. 1998. Isotopic fractionation and sulfur metabolism by pure and enrichment cultures of elemental sulfur-disproportionating bacteria. Limnol. Oceanogr. 43:253-264. [Google Scholar]
- 8.Cary, S. C., M. T. Cottrell, J. T. Stein, F. Camacho, and D. Desbruyères. 1997. Molecular identification and localization of filamentous symbiotic bacteria associated with the hydrothermal vent annelid Alvinella pompejana. Appl. Environ. Microbiol. 63:1124-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh, C. M., S. L. Gardiner, M. L. Jones, H. W. Jannasch, and J. B. Waterbury. 1981. Procaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science 213:340-342. [DOI] [PubMed] [Google Scholar]
- 10.DeLong, E. F., R. B. Frankiel, and D. A. Bazylinski. 1993. Multiple evolutionary origins of magnetotaxis in bacteria. Science 259:803-806. [DOI] [PubMed] [Google Scholar]
- 11.Distel, D. L. 1998. Evolution of chemoautotrophic endosymbioses in bivalves. Bioscience 48:277-286. [Google Scholar]
- 12.Fenchel, T., and B. J. Finlay. 1989. Kentrophoros: a mouthless ciliate with a symbiotic kitchen garden. Ophelia 30:75-93. [Google Scholar]
- 13.Finster, K., W. Liesack, and B. Thamdrup. 1998. Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediments. Appl. Environ. Microbiol. 64:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher, C. R., J. J. Childress, R. S. Oremland, and R. R. Bidigare. 1987. The importance of methane and thiosulphate in the metabolism of the symbionts of two deep-sea mussels. Mar. Biol. 96:59-71. [Google Scholar]
- 15.Fortin, D., F. G. Ferris, and T. J. Beveridge. 1997. Surface-mediated mineral development by bacteria, p. 161-180. In J. F. Banfield and K. H. Nealson (ed.), Reviews in mineralogy, vol. 35. Geomicrobiology: interactions between microbes and minerals. Mineralogical Society of America, Washington, D.C.
- 16.Gaill, F., D. Desbruyères, and L. Laubier. 1988. Relationships between the Pompeii worms and their epibiotic bacteria. Oceanol. Acta 8(Suppl.):147-154. [Google Scholar]
- 17.Haddad, A., F. Camacho, P. Durand, and S. C. Cary. 1995. Phylogenetic characterization of the epibiotic bacteria associated with the hydrothermal vent polychete Alvinella pompejana. Appl. Environ. Microbiol. 61:1679-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto, J., S. Ohta, T. Gamo, H. Chiba, T. Yamaguchi, S. Tsuchida, T. Okudaira, H. Watabe, T. Yamanaka, and M. Kitazawa. 2001. First hydrothermal vent communities from the Indian Ocean discovered. Zool. Sci. 18:717-721. [Google Scholar]
- 19.Hoek, J., A. Banta, F. Hubler, and A.-L. Reysenbach. 2003. Microbial diversity of a sulphide spire located in the Edmond deep-sea hydrothermal vent field on the Central Indian Ridge. Geobiology 1:119-127.
- 20.Jones, M. L. 1981. Riftia pachyptila Jones: observations on the vestimentiferan worm from the Galapagos Rift. Science 213:333-336. [DOI] [PubMed] [Google Scholar]
- 21.Jones, M. L. 1981. Riftia pachyptila, new genus, new species, the vestimentiferan worm from the Galapagos Rift geothermal vents (Pogonophora). Proc. Biol. Soc. Wash. 93:1295-1313. [Google Scholar]
- 22.Juniper, S. K., and P. Martineau. 1995. Alvinellids and sulfides at hydrothermal vents of the eastern Pacific: a review. Am. Zool. 35:174-185. [Google Scholar]
- 23.Khakhina, L. 1992. Evolutionary significance of symbiosis; developments of the symbiogenesis concept. Symbiosis 14:217-228. [Google Scholar]
- 24.Kuever, J., S. M. Sievert, H. Stevens, T. Brinkhoff, and G. Muyzer. 2002. Microorganisms of the oxidative and reductive part of the sulphur cycle at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Cahiers Biol. Mar. 43:413-416. [Google Scholar]
- 25.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, N.Y.
- 26.Lichtenegger, H. C., T. Schoberl, M. H. Bartl, H. Waite, and G. D. Stucky. 2002. High abrasion resistance with sparse mineralization: copper biomineral in worm jaws. Science 298:389-392. [DOI] [PubMed] [Google Scholar]
- 27.Little, C. T. S., and R. C. Vrijenhoek. 2003. Are hydrothermal vent animals living fossils? Trends Ecol. Evol. 582-588. 18:
- 28.Longnecker, K., and A. L. Reysenbach. 2001. Expansion of the geographic distribution of a novel lineage of epsilon-Proteobacteria to a hydrothermal vent site on the Southern East Pacific Rise. FEMS Microbiol. Lett. 35:287-293. [DOI] [PubMed] [Google Scholar]
- 29.López-García, P., F. Gaill, and D. Moreira. 2002. Wide bacterial diversity associated with tubes of the vent worm Riftia pachyptila. Environ. Microbiol. 4:204-215. [DOI] [PubMed] [Google Scholar]
- 30.Lowenstam, H. A. 1981. Minerals formed by organisms. Science 211:1126-1131. [DOI] [PubMed] [Google Scholar]
- 31.Minz, D., J. L. Flax, S. J. Green, G. Muyzer, Y. Cohen, M. Wagner, B. E. Rittmann, and D. A. Stahl. 1999. Diversity of sulfate-reducing bacteria in oxic and anoxic regions of a microbial mat characterized by comparative analysis of dissimilatory sulfate reductase genes. Appl. Environ. Microbiol. 65:4666-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1995. Phylogenetic diversity of the bacterial community from a microbial mat at an active hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 61:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polz, M. F., and C. M. Cavanaugh. 1995. Dominance of one bacterial phylotype at a mid-Atlantic Ridge hydrothermal vent site. Proc. Natl. Acad. Sci. USA 92:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polz, M. F., J. A. Ott, M. Bright, and C. M. Cavanaugh. 2000. When bacteria hitch a ride: associations between sulfur-oxidizing bacteria and eukaryotes represent spectacular adaptations to environmental gradients. ASM News 66:531-539. [Google Scholar]
- 35.Polz, M. F., J. J. Robinson, C. M. Cavanaugh, and C. L. Van Dover. 1998. Trophic ecology of massive shrimp aggregations at a mid-Atlantic Ridge hydrothermal vent site. Limnol. Oceanogr. 43:1631-1638. [Google Scholar]
- 36.Rickard, D. 1997. Kinetics of pyrite formation by the H2S oxidation of iron(II) monosulfide in aqueous solutions between 25 and 125 C: the rate equation. Geochim. Cosmochim. Acta 61:115-134. [Google Scholar]
- 37.Rooney-Varga, J. N., B. R. Genthner, R. Devereux, S. G. Willis, S. D. Friedman, and M. E. Hines. 1998. Phylogenetic and physiological diversity of sulphate-reducing bacteria isolated from a salt marsh sediment. Syst. Appl. Microbiol. 21:557-568. [DOI] [PubMed] [Google Scholar]
- 38.Segonzac, M., M. de Saint Laurent, and B. Casanova. 1993. The enigma of the trophic behaviour of Alvinocaridid shrimps from hydrothermal vent sites on the Mid-Atlantic Ridge. Cahiers Biol. Mar. 34:535-571. [Google Scholar]
- 39.Stein, J. L., S. C. Cary, R. R. Hessler, S. Ohta, R. D. Vetter, J. J. Childress, and H. Felbeck. 1988. Chemoautotrophic symbiosis in a hydrothermal vent gastropod. Biol. Bull. 174:373-378. [Google Scholar]
- 40.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckman, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K.-H. Schleifer, and W. Ludwig. 1999. ARB: a software environment for sequence data. Department of Microbiology, Technische Universitat München, Munich, Germany.
- 41.Swofford, D. L. 1998. PAUP*: phylogenetic analysis using parsimony (*and other methods), 4th ed. Sinauer, Sunderland, Mass.
- 42.Thamdrup, B., K. Finster, J. W. Hansen, and F. Bak. 1993. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl. Environ. Microbiol. 59:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Dover, C. L., C. R. German, K. G. Speer, L. M. Parson, and R. C. Vrijenhoek. 2002. Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295:1253-1257. [DOI] [PubMed] [Google Scholar]
- 44.Van Dover, C. L., S. E. Humphris, D. Fornari, C. M. Cavanaugh, R. Collier, S. K. Goffredi, J. Hashimoto, C. M. Lilley, A. L. Reysenbach, T. M. Shank, K. L. Von Damm, A. Banta, R. M. Gallant, D. Götz, D. Green, J. Hall, T. L. Harmer, L. A. Hurtado, P. Johnson, Z. P. McKiness, C. Meredith, E. Olson, I. L. Pan, M. Turnipseed, Y. Won, C. R. Young III, and R. C. Vrijenhoek. 2001. Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science 294:818-823. [DOI] [PubMed] [Google Scholar]
- 45.Wächtershäuser, G. 1993. The cradle chemistry of life: on the origin of natural products in a pyrite-pulled chemoautotrophic origin of life. Pure Appl. Chem. 65:1343-1348. [Google Scholar]
- 46.Warén, A., S. Bengtson, S. K. Goffredi, and C. L. Van Dover. 2003. A hydrothermal-vent gastropod with iron sulfide biomineralized dermal sclerites. Science 302:1007. [DOI] [PubMed]
- 47.Windoffer, R., and O. Giere. 1997. Symbiosis of the hydrothermal vent gastropod Ifremeria nautilei (Provannidae) with endobacteria—structural analyses and ecological considerations. Biol. Bull. 193:381-392. [DOI] [PubMed] [Google Scholar]
- 48.Wirsen, C. O., S. M. Sievert, C. M. Cavanaugh, S. J. Molyneaux, A. Ahmad, L. T. Taylor, E. F. Delong, and C. D. Taylor. 2002. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl. Environ. Microbiol. 68:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Won, Y.-J. 2003. Molecular systematics, population structure, and gene flow of deep-sea hydrothermal vent mussels (Mytilidae: Bathymodiolus) and associated sulfur-oxidizing endosymbionts. Ph.D. thesis. Rutgers University, New Brunswick, N.J.