Abstract
Reactive oxygen species (ROS) are generated as by-products of normal cellular metabolic activities. Superoxide dismutase, glutathione peroxidase, and catalase are the enzymes involved in protecting cells from the damaging effects of ROS. ROS are produced in response to ultraviolet radiation, cigarette smoking, alcohol, nonsteroidal anti-inflammatory drugs, ischemia-reperfusion injury, chronic infections, and inflammatory disorders. Disruption of normal cellular homeostasis by redox signaling may result in cardiovascular, neurodegenerative diseases and cancer. ROS are produced within the gastrointestinal (GI) tract, but their roles in pathophysiology and disease pathogenesis have not been well studied. Despite the protective barrier provided by the mucosa, ingested materials and microbial pathogens can induce oxidative injury and GI inflammatory responses involving the epithelium and immune/inflammatory cells. The pathogenesis of various GI diseases including peptic ulcers, gastrointestinal cancers, and inflammatory bowel disease is in part due to oxidative stress. Unraveling the signaling events initiated at the cellular level by oxidative free radicals as well as the physiological responses to such stress is important to better understand disease pathogenesis and to develop new therapies to manage a variety of conditions for which current therapies are not always sufficient.
I. INTRODUCTION
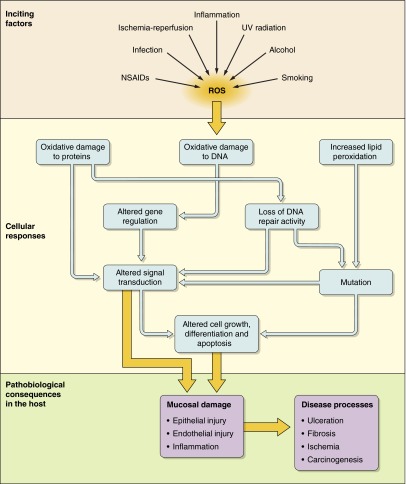
Reactive oxygen species (ROS), also referred to as reactive oxygen intermediates (ROI), are byproducts of normal cellular metabolism. Low and moderate amounts of ROS have beneficial effects on several physiological processes including killing of invading pathogens, wound healing, and tissue repair processes. As discussed in section IV, ROS act as essential signaling molecules. Cancer treatment by chemotherapeutic agents and radiotherapies depend largely on ROS generation to destroy malignant cells by inducing apoptosis. However, disproportionate generation of ROS poses a serious problem to bodily homeostasis and causes oxidative tissue damage. While natural antioxidant pathways can limit the adverse effects of ROS, their levels can be stimulated by many oxidative stressors and maintained such that they contribute to tissue damage. ROS are produced in response to ultraviolet (UV) radiation, cigarette smoking, alcohol consumption, ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs), and many other exogenous agents. Infections, ischemia-reperfusion (I/R) injury, and various inflammatory processes also result in elevated levels of ROS. Disruption of normal cellular homeostasis by redox signaling contributes to disease in virtually every organ including the development of cancer (Figure 1).
FIGURE 1.
Schematic diagram showing the induction of oxidative stress and its pathophysiological effects. Oxidative stress damages internal organs by causing mucosal injury.
The gastrointestinal (GI) tract is a key source of ROS. Despite the protective barrier provided by the epithelial layer, ingested materials and pathogens can cause inflammation by activating the epithelium, polymorphonuclear neutrophils (PMNs), and macrophages to produce inflammatory cytokines and other mediators that contribute further to oxidative stress. Various GI pathological conditions including gastroduodenal ulcers, GI malignancies, and inflammatory bowel disease (IBD) arise in part from oxidative stress. Understanding the signaling events initiated by free radicals as well as the physiological response to such processes is key to furthering our understanding of ROS-mediated GI diseases with the potential to develop novel therapeutic interventions.
II. REACTIVE SPECIES AND THEIR FORMATION
A. ROS and Reactive Nitrogen Species
Molecular oxygen (O2) is not only essential for the survival of aerobic organisms, its reduction to H2O via mitochondrial respiration complexes provides ATP, but paradoxically contributes to cell death (164). Partially reduced O2, collectively named ROS, are highly reactive and continuously produced as by-products of cellular respiration. ROS are also generated during enzymatic reactions. ROS include radical compounds such as superoxide (O2·−), hydroxyl radicals (HO·), lipid hydroperoxides, and reactive nonradical compounds including singlet oxygen (1O2), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), chloramines (RNHCl), and ozone (O3) (22). These oxygen-centered small molecules containing unpaired valence-shell electrons are unstable and highly reactive with proteins, lipids, carbohydrates, and nucleic acids inside the cells. These interactions can irreversibly inactivate target molecules. The redox state of major cellular antioxidants such as glutathione and thioredoxin are affected by the level of intracellular ROS accumulation. Alterations of the balance between ROS production and the capacity to rapidly detoxify reactive intermediates lead to oxidative stress.
Reactive radical compounds such as nitric oxide (·NO), nitrogen dioxide (·NO2), and nonradical compounds, e.g., peroxynitrite (ONOO−) and dinitrogen trioxide (N2O3), are collectively called reactive nitrogen species (RNS). These free radicals are unstable because of the presence of unpaired electrons in their outer electron orbit. RNS is often linked to ROS, e.g., in the formation of peroxynitrite causing nitrosative stress. Oxidative and nitrosative stress have been etiologically implicated in a wide variety of disease processes and states: aging, I/R injury, hypertension, atherosclerosis, diabetic neuropathies, renal diseases, neurological diseases including Alzheimer's disease and other forms of dementia, as well as cancers (20, 44, 92, 93, 110, 227). Oxidative stress also contributes to various GI diseases including gastroduodenal ulcers (226), inflammatory bowel disease (105, 223), and GI malignancies such as gastric (146) and colorectal cancer (130).
B. Mechanisms of ROS Generation
1. Endogenous sources
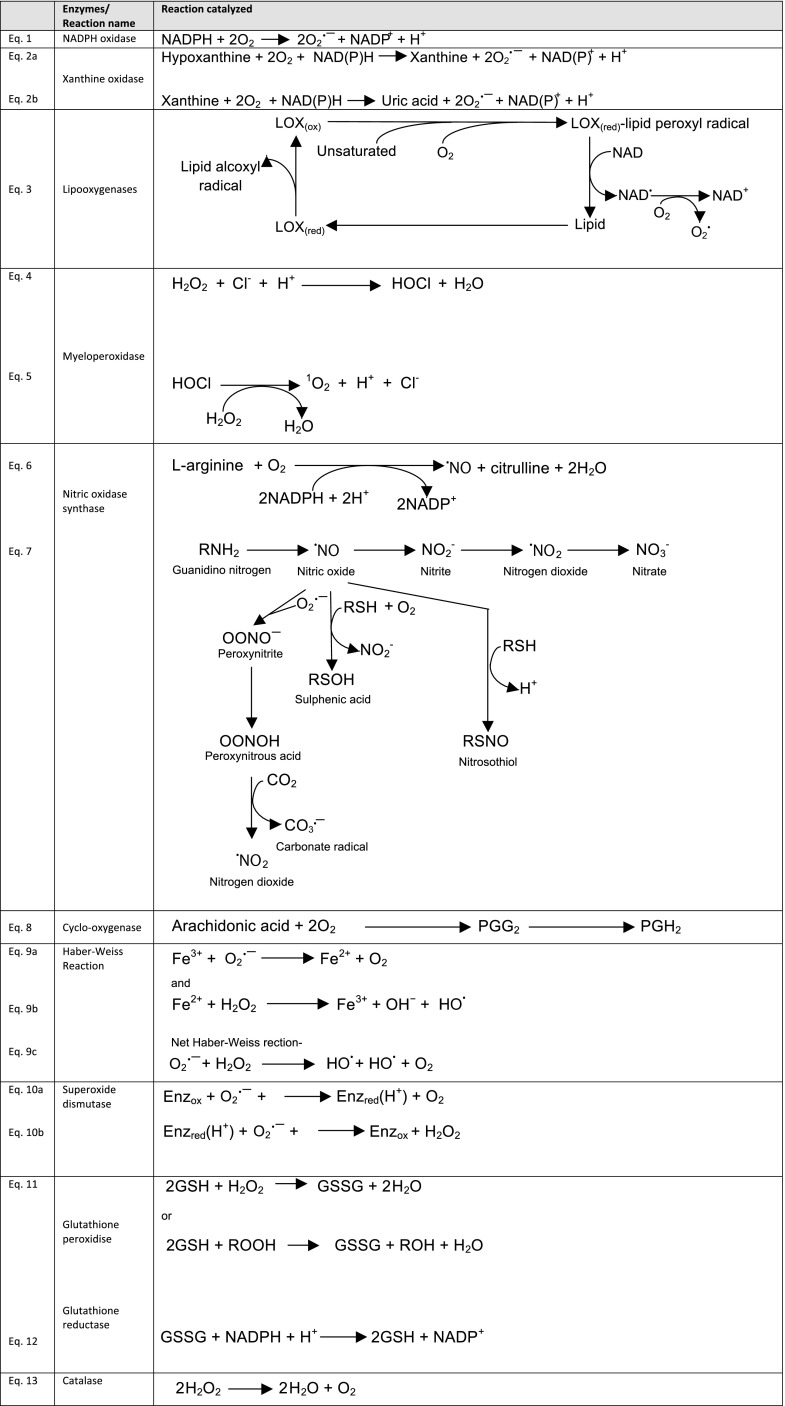
Intracellular compartments including mitochondria, the endoplasmic reticulum, peroxisomes, nuclei, the cytosol, plasma membranes, and even extracellular spaces are capable of ROS generation (13, 238). The mitochondrial electron transport chain is the major site of ROS production in most mammalian cells (237). Enzymes that catalyze ROS-generating chemical reactions are peroxidases, NADPH oxidase, NADPH oxidase isoforms (NOX), xanthine oxidase (XO), lipoxygenases (LOXs), glucose oxidase, myeloperoxidase (MPO), nitric oxide synthase, and cyclooxygenases (COXs) (164, 276).
a) mitochondrial respiratory chain. O2·− is the most crucial ROS as it can give rise to several other forms of reactive oxygen intermediates. The inner mitochondrial membrane (IM) contains a series of enzyme complexes referred to as the mitochondrial respiratory chain (MRC). These include complexes I-IV (NADH-ubiquinone oxidoreductase, succinate dehydrogenase, ubiquinol-cytochrome c oxidoreductase, and cytochrome c oxidase) along with coenzyme Q (CoQ) and a peripheral protein on the outer surface of the inner mitochondrial membrane, cytochrome c, which constitute the MRC. Electron leakage from MRC complexes I and III results in reduction of molecular oxygen, thus forming O2·− (157). Cytochrome c oxidase (complex IV) is the last enzyme component of the MRC which reduces O2 to two molecules of H2O via a four-electron reduction (59). Complex IV is not considered to be a biologically relevant source of ROS (17). Rather, studies indicate that cytochrome c may act as a mitochondrial antioxidant, oxidizing O2·− to O2 (268). At high cellular O2 concentration, cytochrome c oxidase is in an oxidized state and consumes ·NO. However, at low oxygen concentration, ·NO is not used by cytochrome c oxidase, leading to ·NO accumulation in the cell (280).
b) respiratory burst and nadph oxidase. Respiratory burst is the process by which phagocytic cells consume large amounts of oxygen during phagocytosis, mainly via activation of NADPH oxidase and release O2·− into the extracellular space or phagosomes. NADPH oxidase is a multicomponent enzyme present in the plasma membrane and phagosomes of phagocytes such as monocytes, macrophages, neutrophils, and eosinophils (Figure 2, Eq. 1) (12). Phagocytic NADPH oxidase consists of six subunits: membrane-attached gp91PHOX and p22PHOX (PHOX = phagocytic oxidase), cytosolic p67PHOX, p47PHOX and p40PHOX, and Rho GTPases, Rac1 or 2 (258). Activation of NADPH oxidase is caused by relocation of the cytosolic components to the cell membrane. The complex is normally latent in phagocytes but is activated and assembled in the membrane before respiratory burst. p47PHOX, p67PHOX, and either Rac1 or Rac2 can activate the membrane-bound, catalytic core of NADPH oxidase, flavocytochrome b (a heterodimer of gp91PHOX and p22PHOX) (140). p40PHOX also regulates NADPH oxidase activity (66).
FIGURE 2.
Major endogenous oxidative enzymatic reactions.
Six homologs of NADPH oxidase, namely, NOX1, NOX3–5, and DUOX1 and 2 (22, 101) have been identified with diverse intracellular localization. Phagocytic NADPH oxidase (NOX2/gp91phox) and its homologs are collectively called the NOX family of NADPH oxidases. NOX1 and DUOX2 have important roles in GI pathology, especially in Helicobacter pylori-induced gastric inflammation, IBD, and tumor development.
c) xanthine oxidase. Xanthine oxidase (XO), found on the outer surface of the plasma membrane and also in the cytoplasm, is mainly expressed in the liver and small intestinal mucosa within the GI tract (298). It catalyzes oxidation of hypoxanthine (HX) to xanthine and then, to uric acid during purine catabolism (Figure 2, Eqs. 2a and 2b) (114). XDH can be converted to XO by utilizing NAD+. O2·− is generated during oxidation of hypoxanthine to xanthine as well as xanthine to uric acid. Both of these reactions are catalyzed by XO. O2·− is not a highly reactive free radical due to its short half-life and is eventually reduced to H2O2. The charged moiety makes it impermeable to lipid membranes which keeps it restricted to its site of origin.
During ischemia, the production of xanthine and XO is greatly enhanced along with the loss of antioxidant enzymes. O2 is an electron acceptor and cofactor for XO, thus generating O2·− and H2O2. The intestinal mucosa has a tremendous capacity to oxidize hypoxanthine by XO (98). Therefore, it is not unexpected that I/R in the gut produces O2·− and H2O2, the major ROS contributing to GI injury (256).
d) lipooxygenases. Lipooxygenases (LOX) are nonheme iron enzymes catalyzing dioxygenation of polyenoic fatty acids yielding hydroperoxyl derivatives including hydroperoxyeicosatetraenoic acids (HPETEs) (255). Corresponding hydroxyl derivatives hydroxyeicosatetraenoic acid (HETE), leukotrienes (LT), and lipoxins are produced from HPETEs upon reduction (262). ROS can be generated by oxidation of arachidonic acid (AA) by LOX (87, 208) (Figure 2, Eq. 3).
AA is the substrate for LOX in animals while linoleic or linolenic acids serve as substrates in plants (259). Five LOX enzymes have been identified in humans that catalyze four different reactions producing fatty acid hydroperoxides (36) and are named based on position of oxygenated residues in arachidonic acid (277). 5-LOX produce proinflammatory leukotrienes (98, 255) in human monocytes and macrophages (323). The fact that LOX contribute to atherosclerosis (271) illustrates the potential importance of these reactions. Their location is cytosolic in neutrophils and nuclear in macrophages in the resting state, but neutrophilic LOXs also move to the nucleus upon stimulation (322). 12/15-LOX are also expressed in macrophages and are involved in atherosclerosis (162, 332). LTs and HETE can directly activate NADPH oxidase leading to ROS production by translocating the p47PHOX subunit to the plasma membrane (264).
Eicosanoids are produced in various cells of the GI tract including leukocytes, epithelial cells, and other mucosal cells (309). 15-LOX-1/-2 is downregulated in human colorectal tumors (118), and administration of 15-LOX-1 has shown anticarcinogenic effects (28). H. pylori induce 5-LOX-derived LT production in human gastric epithelial cells (GEC) contributing to the neutrophil infiltration characteristic of the inflammation associated with infection (104). 5-LOX-derived LTs contribute in H. pylori-mediated gastric carcinogenesis.
e) myeloperoxidase. Myeloperoxidase (MPO) is a heme-enzyme localized in lysosomes of neutrophils, macrophages, and monocytes. This enzyme chlorinates H2O2 to highly reactive HOCl (Figure 2, Eq. 4). It also catalyzes oxidation of thiocyanate (SCN−) to generate another ROS, hypothiocyanite (OSCN−) via a similar reaction (326). MPO normally exists in the ferric (Fe III) form, although it undergoes different stages of activation depending on the ligands, O2·− or H2O2 (235). Lactoperoxidase present in the airway and digestive tract epithelia is also capable of generating OSCN− (88). HOCl reacts with H2O2 to generate singlet oxygen (1O2) and chloride ion (Cl−) (Figure 2, Eq. 5). 1O2 is not a free radical, but has properties similar to ROS due to its electronic structure.
MPO activity is increased in H. pylori-infected subjects (257) and plays a role in the development of H. pylori-induced atrophic gastritis, a potential precursor of gastric cancer (250, 338). Increased MPO activity is also found in inflamed mucosa in ulcerative colitis, and this may contribute to the progression to malignancy associated with this disease (293).
f) nitric oxide synthase. Nitric oxide synthase (NOS) is a heme-containing monooxygenase that generates NO. Three different isozymes of NOS have been identified (274), constitutively expressed neuronal NOS (nNOS or NOS I) as well as endothelial NOS (eNOS or NOS III), and endotoxin or cytotoxin-inducible NOS (iNOS or NOS II) (224). All types of NOS catalyze the oxidation of l-arginine to an intermediate, N-hydroxy-l-arginine, followed by generation of l-citrulline and ·NO (Figure 2, Eq. 6) (37). At low l-arginine concentrations, l-arginine-uncoupled NOS can react with O2·− to generate H2O2.
NO· is a weak oxidant, but when it combines with O2·− to generate OONO−, it becomes a potent ROS (155). ·NO and OONO− generate very stable nitrite (NO2−) and nitrate (NO3−) ions which accumulate in cells, leading to the formation of highly reactive intermediates, such as ·NO2, N2O3, or ·NO (Figure 2, Eq. 7). These intermediates cause nitration and nitrosation of important biological macromolecules such as DNA, RNA, proteins, and lipids, thereby disrupting their function. 8-Nitroguanine, a nitration product of DNA and RNA, is a potent mutagen and pro-oxidant formed within cells (141). Nitrated lipids are capable of eliciting varied physiological responses and can also produce diffusible NO.
NOS are expressed in the GI tract. NO is involved in GI mucosal defense as well as injury. NO maintains normal functions of the GI mucosa and has a cytoprotective role. It maintains GI mucosal integrity by regulating gastric mucosal blood flow, epithelial secretion, and barrier function (16). However, NO can have deleterious effects, and increased iNOS expression is found in chronic ulcerative colitis and peptic ulcer patients (239). As such, RNS generated by iNOS have immense effects on the normal gut as well as pathophysiological conditions of the GI tract.
g) cyclooxygenase. Cyclooxygenase (COX) is a bifunctional enzyme (having both COX and peroxidase activities) that releases arachidonic acid (AA) from membrane phospholipids and catalyzes conversion of AA to prostanoids (Figure 2, Eq. 8). COX has two isoforms: COX-1 and COX-2. A splice variant of COX-1, COX-3 (also called COX-1b or COX-1 variant), has been reported. Initially it was thought to have no physiological role in humans, but recent reports indicate that this enzyme possibly has cytoprotective functions and is induced in human colon cancer cells (205) and gastric cancer cells during high osmotic stress (172). COX adds two O2 molecules to AA by its bioxygenase activity to generate an unstable cyclic hydroperoxide, PGG2. Next, it reduces PGG2 by its peroxidase activity to an endoperoxide, PGH2(266). PGH2 is converted to biologically active and stable prostanoids such as PGE2, prostacyclins, and thromboxane A2 by various synthases. The peroxidase activity of COX generates NAD− and NADP− radicals. These radicals can eventually generate O2·− (163).
COX-1 and COX-2 are expressed in normal human gastric mucosa with increased levels at the edge of ulcers (133). H. pylori can upregulate both COX-1 and COX-2. COX-2 has been associated with precancerous changes in the GI mucosa including Barrett's esophagus, H. pylori-induced gastritis, as well as inflamed colonic mucosa (154) and is implicated in the development of cancers associated with these diseases (207). COX-1 has constitutive expression while COX-2 is upregulated by inflammation and tumorigenesis (179). Accordingly, selective COX-2 inhibitors (coxibs) have been developed as anti-inflammatory and antitumor drugs.
h) transition metals. Transition metal ions such as iron (Fe2+) and copper (Cu) carry out the Fenton reaction that generates HO· and OH· from H2O2 while being oxidized to Fe3+ and Cu2+, respectively. These are reduced back by a reducing agent (Figure 2, Eq. 9b). The net Haber-Weiss reaction is shown in Figure 2 (Figure 2, Eq. 9c). The generation of HO· through this pathway accelerates lipid peroxidation (40). Oxidation of certain biological molecules during exercise generates superoxide anion radicals, and this is mediated by trace amounts of transition metals (63). For example, ferrous ion (Fe2+) can lose its electron to oxygen to produce O2·− and Fe3+ (97). Molecules that undergo such autooxidation are hemoglobin, myoglobin, catecholamines, reduced cytochrome c, and thiols.
2. Exogenous or environmental sources
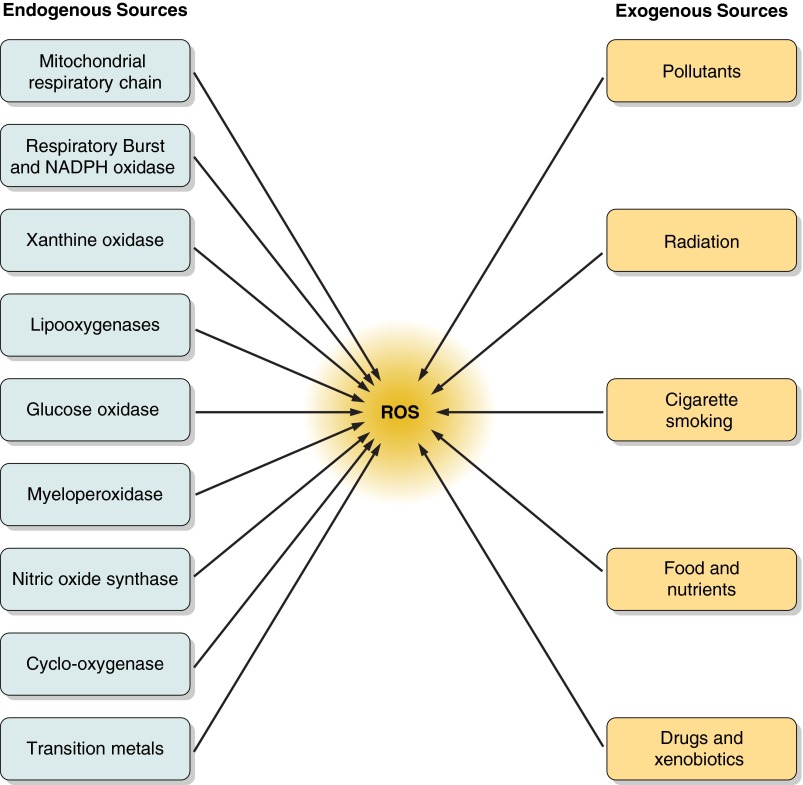
There are multiple external triggers that induce oxidative stress that have direct or indirect effects on responses in the GI tract. Air pollutants, tobacco smoke, ionizing and nonionizing radiations, foods and drugs, as well as xenobiotics can all contribute to oxidative stress. Chemical agents like quinones (33); heavy metals such as lead, arsenic, mercury, chromium, and cadmium; organic solvents; and pesticides are common exogenous sources of ROS (331). Various exogenous and endogenous sources of ROS are included in Figure 3, but this section focuses on those with the most relevance to the GI tract.
FIGURE 3.
Endogenous and exogenous factors leading to reactive oxygen species (ROS) generation. Mitochondrial respiratory chain and various intracellular enzymes are the main generators of endogenous ROS. Environmental pollution, radiation, cigarette smoking, certain foods, and drugs are the major exogenous sources of ROS.
a) radiation and chemotherapy. Ionizing radiation, such as x-rays, neutrons, as well as α, β, and γ rays, can all cause oxidative stress. α Particles have weak penetrative power, but the rest are very penetrating through the human body. Ionizing radiation can produce HO· by radiolysis of water or ROS via secondary reactions (249). High levels of ionizing radiation cause injury to the cerebrovascular, GI, and hematopoietic systems. In the prodromal phase following damage to all of these tissues, GI symptoms appear including anorexia, nausea, vomiting, and diarrhea. When mice receive doses of 6–30 gray (Gy), GI injury syndromes appear, caused in part by p53-mediated death of GI epithelial cells (149). Radiation-induced cell death can be mitigated or even prevented in mice with the antioxidant N-acetyl-cysteine (NAC) (136) which establishes ROS as critical factors in development of radiation-induced GI syndromes.
Cancer chemotherapy is often accompanied by toxic side effects, and ROS generation by chemotherapeutic agents is the primary event leading to induced toxicity. This is evident by increased lipid peroxidation, and reduced antioxidant and tissue GSH levels during chemotherapy. Agents that produce high levels of ROS include anthracyclines (doxorubicin, daunorubicin), alkylating agents, platinum coordination complexes (e.g., cisplatin), epipodophyllotoxins (e.g., etoposide and teniposide), and the camptothecins (60). Methotrexate (MTX) is a widely used chemotherapeutic agent that causes gastrointestinal toxicity leading to diarrhea, nausea, and decreased nutrient absorption. The XO system is involved in MTX-mediated ROS production in an animal model (60).
Both radiation and chemotherapy induce systemic oxidative stress and reduce levels of vitamin E and beta-carotene in patients (57). Antioxidant vitamins have been used to treat these complications (192). Topical application of vitamin E enhances the rate of healing at sites of ulceration. Oral beta-carotene supplementation during the course of radiation and chemotherapy helps in the treatment of oral mucositis (192). Thus understanding the role of ROS in response to these antioxidant vitamins has helped in planning strategies to deal with some ROS-mediated tissue damages.
b) cigarette smoke. Cigarette smoke is another significant generator of ROS (109) and has been shown to modulate GI disease. It is comprised of more than 7,000 chemical compounds and oxidative agents, and tobacco smoke contains 1014-1016 free radicals per puff (329). The active chemicals include aldehydes, quinones, benzo(a)pyrene, epoxides, and peroxides (55). Cigarette smoke has a gas phase which contains ·NO, peroxyl radicals, and carbon-centred radicals as well as a tar phase containing relatively stable polycyclic aromatic hydrocarbons and nitrosamines (319). In the presence of iron, tar semiquinone can generate hydroxyl radicals (HO·) and hydrogen peroxide (H2O2).
Tobacco use is associated with various GI diseases including peptic ulcers, Crohn's disease (296), gastroesophageal reflux disease (GERD) (272), Barrett's esophagus (62), as well as carcinoma in the esophagus, gastric cardia (302), and distal intestine (4). Interestingly, tobacco smoking has a protective effect in ulcerative colitis, which highlights the pathogenic differences between Crohn's disease and ulcerative colitis and reflects the complex mixture of compounds found in tobacco smoke (113, 169). Understanding the mechanisms underlying this difference may provide valuable information for developing new treatments for these two major forms of IBD.
c) foods and alcohol. Ingested food can generate O2·− and H2O2 in the GI tract (67). Humans ingest macronutrients (carbohydrates, proteins, and fats), micronutrients (minerals and vitamins), food preservatives, as well as microorganisms. Dietary iron and also copper generate ROS by the Fenton reaction. Increased intake of Fe2+ generates ROS and RNS, lipid peroxidation, and oxidative stress, and its accumulation in tissues increases the risk of cancer and inflammation (96). Trans fatty acids in processed foods also generate ROS (334). This may in part be attributable to the presence of acrylamide, which can be found in snack foods, breakfast cereals, and crackers. Acrylamide is absorbed mainly via ingestion and reacts with hemoglobin (26). Chronic acrylamide exposure gives rise to oxidative stress in humans by the increased production of ROS.
Lipids from vegetable and animal origin, when heated in microwave ovens, generate free radicals. In addition, foods from plants containing phenols supply oxidants to the body (3) while ethanol at high concentrations can directly damage the mucosal layer of the GI tract. Alcoholic liver disease (324) and alcoholic pancreatitis (217) occur in part due to ROS generated from ethanol. Furthermore, cancers of the oropharynx, larynx, esophagus, and liver are also associated with increased alcohol intake (225). Although these associations may be explained in part by the disruption of the intestinal barrier function due to alcohol-induced NO synthesis (279) and increased production of NF-κB and tumor necrosis factor (TNF)-α (225), further studies are required to better understand the mechanisms of alcohol-induced GI injury.
d) drugs and xenobiotics. Many drugs and xenobiotics contribute to the formation of free radicals in the body. Anticancer drugs such as anthracyclines and analogs, mitoxantrone and other quinones, actinomycin D, enediynes such as bleomycin, chartreusins, elasmin A and related compounds can cause oxidative stress (76). The resultant oxidative stress facilitates their ability to kill tumor cells. Glucocorticoid therapy can lead to O2·− production (131), but its effects on inducing apoptosis in certain leukocyte populations confer a net decrease on oxidative stress. Volatile anesthetics may generate free radicals and change antioxidant levels in patients undergoing surgery (292). However, how these agents impact luminal GI pathophysiology is not well known.
Aspirin and antipyretic, analgesic NSAIDs such as ibuprofen and naproxen, also generate ROS. NSAIDs actions include nonselective inhibition of COX, thereby blocking formation of PGE2 (241). Two main target organs of adverse reactions associated with NSAIDs are the GI tract and the renal system. NSAID-induced gastric injuries, including ulceration (320), occur in part due to induced aggregation of neutrophils in the gastric vascular endothelium (307). This can lead to ROS production and mucosal injury associated with NSAID treatment in rats (294). Acidic NSAID molecules irritate the gastric mucosa directly, but reduction of prostaglandin synthesis in rat gastric mucosa is more significant because this increases gastric acid secretion, and reduces bicarbonate secretion and mucosal blood flow, thereby increasing the risk of gastric ulceration and damage to the small intestine (315). A PGE1 derivative, misoprostol, and agents that inhibit gastric acid secretion such as proton pump inhibitors and histamine receptor blockers are used for the treatment and prevention of NSAID-mediated gastroduodenal injury (229). It is believed that by removing bacterial populations of the stomach and the small intestine, NSAID-induced mucosal damage can be reduced (183). As mitochondrial oxidative phosphorylation mediates NSAID-induced mucosal injury of both of these organs, agents that prevent uncoupling of oxidative phosphorylation may be useful in treating NSAID-mediated GI injuries.
III. ANTIOXIDANT DEFENSE SYSTEMS
Oxidation reactions are crucial for aerobic life, but uncontrolled ROS generation is damaging. Although free radicals are continuously generated, the body is equipped to defend against the harmful effects of ROS with the help of antioxidants, collectively called the antioxidant defense system which comprises both enzymatic and nonenzymatic mechanisms. Antioxidants remove free radicals from the system and inhibit oxidation by being oxidized themselves. Dietary intake is another very important source of antioxidants and points to the potential effects of malnutrition or malabsorption of nutrients on the regulation of these mediators.
A. Endogenous Enzymatic Antioxidants
The major enzymatic antioxidants are superoxide dismutases, glutathione peroxidase, glutathione-reductase, catalase, and superoxide reductases. Superoxide reductase is an oxidoreductase present only in the anaerobic and facultative microorganisms (234). SOD and catalase provide major antioxidant defenses against ROS.
1. Superoxide dismutases
Superoxide dismutases (SOD) are metal ion cofactor-requiring enzymes that catalyze dismutation of O2·− into O2 and H2O2 (Figure 2, Eqs. 10a and 10b). Three isoforms of SOD exist in humans (204): cytosolic copper and zinc-containing enzyme (Cu-Zn-SOD), manganese-requiring mitochondrial enzyme (Mn-SOD), and an extracellular Cu-Zn containing SOD (EC-SOD). Iron-containing SOD (Fe-SOD) is present in bacteria and plants but not in vertebrates and yeast, while nickel-containing SOD (Ni-SOD) is present only in prokaryotes (297). Mn-SOD is essential for survival as Mn-SOD null mice die soon after birth (188).
O2·− formed in the mitochondria is dismuted to H2O2 by Cu-Zn-SOD present in the mitochondrial intermembranous space and Mn-SOD present in the mitochondrial matrix (213). GPX present in the mitochondrial matrix can scavenge H2O2. Uncharged H2O2 crosses the mitochondrial membranes and in the cytosol can be scavenged by either cytosolic Cu-Zn-SOD or catalase (236). Gastrointestinal mucosal injury can be prevented by SOD in the gastrointestinal mucosa (150, 152). Intestinal tissues from IBD patients have increased levels of all three SOD isoforms, particularly in the epithelium (161).
Reduced SOD activity in the gut causes gastric ulcer, and increased SOD activity has been associated with ulcer healing in patients (200). These responses illustrate both the detrimental effects of ROS on tissue damage and the importance of antioxidant activity in promoting health. Gastric adenocarcinoma and squamous cell esophageal carcinoma tissues exhibit increased expression of Mn-SOD relative to the normal mucosa (134). Colorectal cancer is also associated with enhanced Mn-SOD expression. In contrast, Cu-Zn-SOD is slightly lower in cancer tissues than in normal tissues. Whether these changes are pathogenic or they simply reflect altered homeostasis has yet not been established.
2. Glutathione peroxidase
Glutathione peroxidase (GPX) converts glutathione (GSH), a tripeptide consisting of glutamate, cysteine, and glycine, into oxidized glutathione (also called glutathione disulfide, GSSG) and, during this process, reduces H2O2 to H2O and lipid hydroperoxides (ROOH) to corresponding stable alcohols (Figure 2, Eq. 11). The GPX reaction is coupled to glutathione reductase (GSSG-R), which maintains reduced glutathione (GSH) levels (Figure 2, Eq. 12) (34). Neurons are most vulnerable to free radical damage as they have very low levels of GSH. GPX serves an important role in protecting cells from the harmful effects of peroxide decomposition.
Isozymes of GPX are found in the cytoplasmic, mitochondrial, and extracellular compartments (288). Humans have eight isotypes of GPX, most of which contain selenocysteine residues at their active site (74). GPX1 is ubiquitous, but GPX2 has epithelium-specific expression. GPX2 (originally named GPX-GI) was discovered in the gastrointestinal tract (52) which protects the gut against the absorption of dietary hydroperoxides (318). GPX2 expression is detected in various parts of the GI tract and is induced in gastric cancer cells (153). GPX2 provides a first line of defense against ROS derived from inflammation associated with both pathogenic and nonpathogenic commensal bacteria in the gut (54). GPX1 and GPX2 double-knockout mice suffer from IBD-like symptoms as a result of induced oxidative stress and inflammatory responses (90). Understanding the mechanisms by which GPXs cause IBD and developing GPX-mimetics for future therapeutic approaches could enhance the management of human IBD.
3. Catalase
Catalase dismutates H2O2 to H2O and O2 (Figure 2, Eq. 13) and is found mainly in peroxisomes (261). Catalases are heme enzymes, but a manganese catalase is found in prokaryotes (333). In humans, catalase is found largely in liver, kidney, and erythrocytes, although all organs express this enzyme. Catalase-expressing pathogens such as Campylobacter jejuni (10), H. pylori (198), Helicobacter hepaticus (95), and enterobacteriaceae family bacteria (281) including Escherichia coli, Shigella, and Salmonella synthesize catalase to deactivate H2O2 to evade host response and survive within the host. Less catalase activity is noted in colorectal cancer (47), gastric adenocarcinoma, and H. pylori-infected stomach (196). Crohn's disease patients show permanent suppression of catalase activity in their mononuclear cells (126). Genetically modified Lactobacilli capable of producing catalase have been shown to reduce tumor in colon (75) and colitis in mice (166). Scientists even claim that catalase function is not to detoxify H2O2, but to protect cells from apoptosis (203). Further support for this view comes from a study involving IBD patients (156). However, dissecting out the antiapoptotic and antioxidant roles of catalase in various GI diseases could help in developing more effective treatment strategies for inflammatory GI diseases.
4. Glutathione reductase
Glutathione reductase (GR or GSR) reduces oxidized glutathione disulfide (GSSG) to GSH (Figure 2, Eq. 12). GR is ubiquitously expressed except for Drosophila, Trypanosomes, and gram-negative bacteria (143). This homodimeric enzyme is a flavoprotein disulfide oxidoreductase. Each subunit contains four domains: FAD-binding and NADPH-binding domains, a central domain, and an interface domain. The active site is formed by dimerized interface domains, and only the dimer has catalytic activity (19). GR protects red blood cells, hemoglobin, and cell membranes from oxidative stress by generating GSH (48). Riboflavin deficiency leads to reduced GR activity (100). Increased level of GSH is often associated with drug resistance of various cancers including colon cancer (25, 246). Clinical trials of GR inhibitors and a better understanding of the GST detoxification pathway will further help in developing chemotherapeutic regimens to treat colon cancer.
5. Heme oxygenase
Heme oxygenase (HO) catalyzes degradation of heme and generates CO, biliverdin, and iron (282). Two distinct HO isoforms, HO-1 and HO-2, have been reported (253). HO-2 is constitutively expressed, and HO-1 is inducible. There is a low expression of HO-1 at baseline in nearly all cells, but it is strongly induced by its substrate heme, heat shock, UV radiation, I/R injury, lipopolysaccharide (LPS), cytokines, and oxidative stress (312). Although HO-1 does not have a direct antioxidant enzymatic function, HO-1 and its product CO are believed to have indirect cytoprotective responses against oxidative stress (214, 304). HO-1 overexpression leads to resistance of hyperoxia-induced lung cell death, protein oxidation, and lipid peroxidation injury (228), whereas CO prevents oxidant-induced lung injury (215). HO-1 also has cytoprotective function in GI tumor cells. In an experimental colitis model, HO-1 was significantly upregulated in inflamed colon (310) as was also found in patients with IBD (222). Nrf2-deficient mice, which lack transcriptional regulation of Nrf2 on HO-1 gene, are more susceptible to dextran sodium sulfate, a chemical inducer of colitis, when compared with wild-type mice (147). HO-1 is crucial in modulating cell cycle, apoptosis, as well as oxidative stress in colon cancer cells (210). However, studies to understand HO-1's potential in treating free radical-induced GI diseases are still in their infancy.
B. Endogenous Nonenzymatic Antioxidants
1. Glutathione
Glutathione is found in all eukaryotic cells and is one of the key non-enzyme antioxidants in the body. It is generally present in its reduced form, GSH. This is ubiquitously expressed, and together with three enzymes, glutathione reductase GPX, and glutathione S-transferases (GST) (187), form the glutathione system. In the gut mucosa, the GSH system serves as an antioxidative barrier. High intake of fruits and vegetables stimulate GSH-dependent enzymes (120) which may account for at least some of the reported antioxidative benefit of these food groups.
GSH concentrations are much higher in the glandular gastric tissue, perhaps conferring some additional protection from the effects of gastric acid. While H. pylori infection-induced inflammation causes damage that in part is attributable to the production of ROS, this infection overwhelms the ability of mucosal cells and local glutathione to entirely prevent ROS-mediated damage. Therapeutic regulation of glutathione availability prevents the damage caused by H. pylori infection (184), which illustrates the impact of altering the relative balance of pro- and antioxidants in disease. A very high correlation exists between high GST expression in the GI tract and tumor occurrence. Two isoenzymes of GPX, GPX-1 and GPX-2 or GPX-GI, catalyze reduction of hydroperoxides in the intestinal epithelia. GPX−/− mice are susceptible to infection-induced inflammation and cancer (53). In humans, low GST activity is associated with high tumor incidence, and vice versa. Glutathione/GST causes neoplastic changes in H. pylori-infected gastric mucosa (301). GST activity is reduced in colon cancer. Again, dietary intake of fruits and vegetables reduces the risk of colorectal cancer (107), which may in part be attributable to their ability to favor an antioxidant environment.
2. Thioredoxin
The thioredoxin system is comprised of thioredoxin (Trx) and thioredoxin reductases (TrxR). Trx is disulfide-containing oxidoreductase that modulates activity of redox-sensitive transcription factors. Trx is present in the cytoplasm, membranes, mitochondria, and the extracellular space (151). Its active site contains a conserved sequence Cys-Gly-Pro-Cys. Oxidized Trx (Trx-S-S) is reduced by a flavoenzyme TrxR and NADPH (122) to its active dithiol form which scavenges ROS and helps maintain proteins in their reduced state (8). Several clinical conditions have been shown to involve Trx (21). Trx shields ocular lens from free radical damage (245) and inhibits reperfusion-induced arrhythmias in a rat cardiac tissue (7), indicating a protective effect during acute ischemic heart disease. TRX-1 shows cytoprotective action in various inflammatory conditions. For example, TRX-1 reduces DNA damage and neutrophil aggregation in the Helicobacter felis-infected stomach, suggesting a protective role in murine gastritis (144).
Thioredoxin binding protein-2 (TBP-2) is a negative regulator of Trx and has multiple regulatory functions in cellular redox regulation, growth, apoptosis, and aging. TBP-2−/− mice die from GI bleeding under fasting conditions, indicating a protective role of TBP-2 in gut pathophysiology (212). Anti-ulcer drugs like geranylgeranylacetone can induce Trx production in rat hepatocytes. This drug also promotes secretion of Trx in rat gastric mucosa, suggesting that it has a protective role in at least experimental gastric ulceration (77). Bile acids upregulate TrxR mRNA expression in GI cancers via induced production of ROS (167).
3. Melatonin
Melatonin is a hormone synthesized from serotonin primarily in the mammalian pineal gland but is also found in the retina, lymphocytes, GI tract, and bone marrow (284). It is ubiquitous and can be found in dietary sources such as oats, yeast, and other plants. It is effective in both aqueous and lipid phases in neutralizing HO· and peroxyl radicals, CO3·−, ·NO2, O2·−, and HOCl (247) and can readily cross the blood-brain barrier. Melatonin as an antioxidant is irreversibly oxidized and cannot be reduced. Thus it is referred to as a suicidal or terminal antioxidant (278). During the oxidative reaction, it is converted to several antioxidant intermediate metabolites, 6-hydroxymelatonin being the primary metabolite found in the nuclei and mitochondria. Mitochondria generate most free radicals generated within cells (112) and are particularly prone to oxidative damage as they lack protective histone proteins and have fewer DNA-repair enzymes. As melatonin can directly cross the mitochondrial membranes, it plays a very significant role in protecting mitochondria from oxidative damage. In this manner it protects vital organs including the liver from alcoholic damage (177). Other antioxidants can be converted to free radicals, but melatonin can never become a free radical as its oxidative role involves donation of two electrons. Melatonin's anti-inflammatory effects in animal studies and limited human studies suggest that supplemental melatonin may have a beneficial effect in colitis (284). Further studies are required to fully evaluate its anti-inflammatory and antioxidant functions.
C. Exogenous Antioxidants
1. Vitamin C
Vitamin C or ascorbic acid is the primary antioxidant in plasma and cells (185). It is synthesized from glucose in the liver of most mammalian species, but not by humans and therefore must be ingested to avoid scurvy, a potentially lethal condition (216). Vitamin C can be obtained from fresh fruits and vegetables. Vitamin C donates electrons to other compounds and prevents their oxidation. The many relevant species reduced by vitamin C include various ROS, RNS, sulfur radicals, O3, nitrosating compounds, and HOCl. Vitamin C reduces heavy metal ions (Fe, Cu) that can generate free radicals via the Fenton reaction, and thus it can have pro-oxidant activity (273) although its main function is as an antioxidant.
2. Vitamin E
Vitamin E (the most biologically active form is α-tocopherol) is an important and abundant antioxidant that protects cell membranes from lipid peroxidation (LPO) (289). α-Tocopherol terminates the activity of LPO by scavenging lipid peroxyl radicals (LOO·) but itself is converted into a reactive radical during this reaction (295). α-Tocopherol can also reduce Fe or Cu, as a pro-oxidant (327). The ability of α-tocopherol to act as a pro- or antioxidant depends on the amount of α-tocopherol available to scavenge ROS (327). However, according to some reports, α-tocopherol has no significant role in antioxidant metabolism (11). In one in vitro model, in the presence of Cu2+, α-tocopherol showed an oxidative DNA-damaging effect (328). Epidemiological studies indicate that food rich in fruits and vegetables lowers cancer rates, but supplementation of exogenous vitamin E and other antioxidants have not been shown to prevent gastrointestinal cancers (32). This underscores the complexity of understanding the beneficial effects of foods beyond their individual antioxidant components.
3. Carotenoids including vitamin A
Vitamin A, which is found in food, is referred to as carotenoids or provitamin A. Yellow and orange fruits as well as green leafy vegetables provide most of the carotenoids in our diet. Alpha- and beta-carotene, lycopene, and cryptoxanthin are the main carotenoids in food as well as in the body (103). Beta-carotene and other carotenoids exhibit antioxidant properties depending on the in vitro experimental system used. Antioxidant properties of biological carotenoids depend on retinol-binding proteins and other endogenous antioxidants in vivo (243). Beta-carotene has been shown to suppress lipid peroxidation in mouse models (132). Antioxidant properties can be reversed to pro-oxidant behavior depending on O2 tension or carotenoid concentration (336).
4. Minerals
Zinc (Zn), copper (Cu), manganese (Mn), iron (Fe), and selenium (Se) are key components of enzymes with antioxidant functions and are designated as antioxidant micronutrients. Zn, Mn, and Cu are cofactors of superoxide dismutase (Cu/Zn-SOD) (115). Fe is a component of catalase. Se is a major antioxidant in the form of selenoproteins that mitigates the cytotoxic effects of ROS. Cereals contain selenomethionine, a naturally occurring amino acid that is the most important nutritional source of Se. When Se-GPX is inhibited under physiological conditions, such as during Se deficiency, it leads to toxicity through increased O2·−, ·NO, and lipid peroxidation (189). Thus again, proper nutrition and absorption of these micronutrients is essential to maintain redox homeostasis.
5. Polyphenols including flavonoids
Plant polyphenols are important antioxidants, and dietary intake of these compounds can be up to 50–800 mg/day (232). Polyphenols comprise flavonoids, phenols, phenolic acids, lignins, and tannins. Flavonoid sources include fruits, vegetables, nuts, red wine, beer, tea, seeds, grains, spices, and medicinal plants. Flavonoids prevent superoxide anion production by inhibiting XO (111). In addition, they inhibit COX, LOX, GST, microsomal monooxygenases, and NADH oxidase (39). Many flavonoids chelate free Fe and Cu that could otherwise increase ROS generation, and also reduce ROS such as O2·−, and HO· (41).
IV. ROS AS SIGNALING MOLECULES AND ROS-MEDIATED REGULATION OF CELLULAR SIGNALING
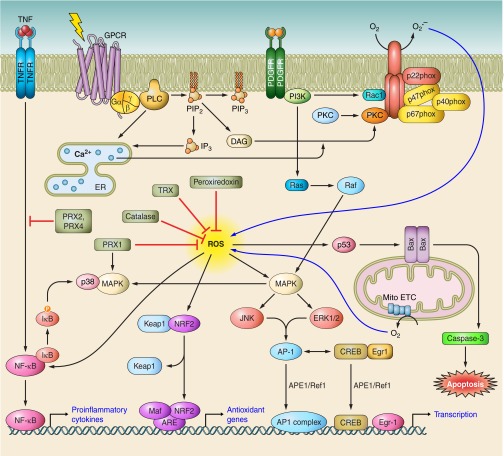
Although often thought of as harmful molecules, ROS act as essential signaling molecules. Despite this function being widely reported, it has remained controversial or perhaps under investigated. Figure 4 illustrates key ROS signaling events within cells. ROS modulate a number of redox-sensitive signaling pathways. Well-characterized targets are the catalytic Cys residues of tyrosine kinases and mitogen-activated protein (MAP) kinase phosphatases (protein tyrosine phosphatases). Oxidation of their Cys residues reversibly abolishes enzymatic activity (287). ROS also cause integration of cellular functions by regulating growth factor-mediated signaling pathways. Specificity of these signaling events is conferred by subcellular compartmentalization of H2O2 (305) and by local modulation of H2O2 concentration by scavengers (321).
FIGURE 4.
Schematic depiction of multiple signaling pathways that generate ROS and the intracellular events activated by ROS accumulation. Upon activation, G protein-coupled receptors (GPCRs) activate phospholipase C (PLC) leading to the activation of protein kinase C (PKC) molecules. Platelet-derived growth factor receptors (PDGFRs) activate phosphoinositide 3-kinase leading to activation of ras-related C3 botulinum toxin substrate 1 (RAC1). Both RAC1 and PKC activate membrane-bound receptors leading to membrane relocation and assembly of various components of phagocytic NADPH oxidases. Mitochondrial electron transport chain (mito ETC) is another robust source of intracellular ROS generation. ROS in turn lead to enhanced production of (APE1/Ref1) and activation of several signaling events including p53-mediated apoptotic events, mitogen-activated protein kinase (MAPK) pathways, NF-E2-related factor (NRF2)-mediated activation of genes containing antioxidant response element (ARE), and nuclear factor-κB (NF-κB). Transcription factors including AP1, NF-κB, cAMP response element-binding (CREB), and early growth response (EGR) protein, induced by these signaling events are kept in the active and reduced form by APE1/Ref1. Thus ROS signaling events play a central role in regulation of proinflammatory events, cell cycle, proliferation, and cell death. Antioxidant defense enzymes such as catalase, thioredoxins (TRX), peroxidases, and peroxiredoxins (PRX) contribute to preventing excessive levels of ROS from accumulating at the cellular and tissue level.
In mammals, thiol-based peroxidases, peroxiredoxins (PRXs, a family of peroxidases), and GPX play important roles in ROS signaling. PRXs modulate H2O2 signaling downstream of growth factor tyrosine kinases and cytokine receptors (142). PRX1 is induced in macrophages by oxidized low-density lipoprotein and functions not only as an antioxidant and a reducer of ROS, but activates p38 MAPK, and enhances cell survival (61). PRX2 blocks H2O2 and TNF-α-induced upregulation of NFκB (61). Overexpression of PRX4 also inhibits the above-mentioned event (138). Control of inflammatory responses by ROS is of course very relevant to GI diseases and is discussed in more detail in section V.
Another level of regulation of mammalian ROS is achieved by modulating mammalian antioxidant systems in a process involving several factors. p53 can be activated by NO, which decreases H2O2 accumulation by upregulating GPX1 (254). S-nitrosylation of the p53 inactivator Hdm2, a ubiquitin ligase (260), inhibits its interaction with p53, thereby blocking p53 ubiquitination and proteolysis. ·NO downregulates Mdm2 (mouse equivalent of Hdm2), similarly decreasing p53 ubiquitination (311). c-Myc can induce GSH formation, which potentiates its oncogenic functions (24). The class O forkhead box (FOXO) family of transcripton factors is activated by H2O2 and imparts tolerance to oxidative stress by enhancing SOD1 expression (43). NRF2 transcription factor modulates the expression of defensive genes coding detoxifying enzymes and antioxidant proteins. KEAP1, the inhibitor of NRF2, helps in its retention in the cytosol. The NRF2-KEAP1 pathway is committed to xenobiotic and oxidant elimination. In response to attack by electrophiles or ROS, NRF2 is switched on and off via distinct mechanisms. Oxidative modification of KEAP1 at Cys residues and NRF2 phosphorylation results in release of NRF2 from KEAP1 (335). Stabilized NRF2 translocates to the nucleus, interacts with various proteins, and binds with antioxidant response elements involved in activation of gene expression, thereby protecting cells from free radical damage.
Eukaryotic ROS sensing transcription factors, AP-1, and NF-κB act as potent redox sensors due to the presence of single Cys in their DNA-binding domains (1). Oxidation of these Cys residues blocks their binding to the respective consensus DNA sequences. Apurinic/apyrimidinic (AP) endonuclease 1 (APE1), also known as Redox effector factor 1 (Ref1), functions as a reducing agent for various transcription factors (91). This ubiquitous multifunctional protein is induced by ROS (242) and is involved in base excision repair (78). The DNA-binding activity of transcription factors are restored by Trx (180). Although reducing condition is favorable for DNA binding, both AP-1 and NF-κB can be activated by oxidative stress via induction of APE1/Ref1. A Zn-finger DNA-binding protein, early growth response gene-1 (Egr-1), is activated by ROS, and a positive feedback loop between APE1/Ref1 and Egr-1 regulates their early transcriptional activation after oxidative stress (233). Egr-1 also induces SOD1 and thus reduces free radical-induced damage (194). The functions of these signaling molecules will be discussed further in the next section.
V. PATHOPHYSIOLOGY OF OXIDATIVE STRESS IN THE GI MUCOSAL DISEASES
The GI tract is prone to ROS attack as it is accessed by the outside environment with resident immune cells and intestinal flora as well as dietary factors, all potential sources of ROS. Two main enzymatic reactions generate ROS in the GI tract-the HX/XO system and the NADPH oxidase system. In fact, the GI tract has the highest concentration of XO in the body, which along with numerous phagocytic cells (and a large number of catalase-negative bacteria in the colon), combine to generate large amounts of O2·− (181). ROS have been linked with various inflammatory GI disorders such as gastroduodenal inflammation, ulceration, and gastric cancer (230). Excessive levels of ROS damage cellular proteins (244) including cytoskeletal proteins (15) and, ultimately, disrupt GI tract barrier to increase gut permeability which contributes to inflammation in a variety of GI diseases. Furthermore, excess ROS induce inflammation by stimulating PMNs, thereby causing further damage to the tissue. As many GI diseases are initiated and promoted by oxidative stress, the mechanisms underlying the development of these pathophysiological conditions need to be elaborated.
A. Esophageal Diseases
1. Reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma
GERD occurs due to prolonged contact of the esophageal mucosa with refluxed acidic gastric contents and is a very common disorder especially in developed nations. Acid, bile salts, and esophagitis (inflammation of the esophagus) that are associated with GERD lead to increased ROS, reduce the amount of antioxidants (for example, GSH and vitamin C), and increase expression of ROS-inducible genes. Reflux esophagitis can lead to erosions or ulceration of the esophagus and also Barrett's esophagus (BE). In BE, specialized intestinal-type columnar epithelium replaces the normal squamous epithelial lining of the esophagus possibly due to the chronic exposure to gastroesophageal refluxate (316).
Bile acids, one of the major constituents of duodenogastrooesophageal refluxate, are also believed to promote BE and esophageal adenocarcinoma (EA). Injection of SOD or buthionine sulfoximine reduces esophagitis in rat duodenogastroesophageal reflux model, suggesting that EA development is mediated by inflammation and oxidative stress (314). EA starts with metaplasia, followed by dysplasia, which ultimately develop into carcinoma (5). Individuals with BE are prone to develop EA, although more recent studies show a lower association between the two (121, 125). Prolonged contact with acid and bile of the gastroesophageal refluxate damages esophageal epithelium and induces inflammation. The amount of bile salts and acid in the esophageal refluxate is proportional to the degree of esophageal mucosal injury (202). Unconjugated bile acids are robust COX-2 inducers and activate PI3K/AKT and ERK1/2 pathways in BE and EA cells through ROS induction (270).
Comparison of mucosal biopsies from erosive gastritis and BE revealed that O2·− is the main oxidant responsible for reflux esophagitis (137). O2·−, along with H2O2, HO·, ONOO− produced by mitochondrial complexes, NADPH oxidase and NOS in inflammatory cells and the epithelium are linked with reflux esophagitis, BE, and EA (128). ROS activate varied signal transduction pathways such as AP1, NF-κB, insulin receptor kinase, MAPKs, and Src kinases which regulate proliferation, differentiation, and apoptosis of epithelial cells (46, 102).
Various processes of reflux-induced free radical generation in the esophageal epithelium contribute towards BE pathogenesis. For example, lipid peroxidation is enhanced in BE (313), and along with reactive lipid-derivatives, NO and HOCl are also enhanced in BE and EA (317). Other changes include an increase in iNOS that is associated with inflammation and cell proliferation in BE and EA (56). COX-2 is overexpressed in human EA and is induced in biopsies of acid or bile acid-treated columnar-lined esophagus (252).
An inverse correlation exists between antioxidant supplementation and EA development (168). ROS scavengers can reduce esophageal mucosal damage. For example, SOD prevents the development to BE and EA in rats (231). Supplementation of α-tocopherol decreases EA progression in rats (50). Aspirin and NSAIDs reduce the risk of BE and EA, and the protective effect of NSAIDs targets an early stage of the metaplasia-dysplasia-carcinoma sequence (5). Aspirin may provide protection against BE and EA by inhibiting COX-2, while NSAIDs might serve as chemopreventive agents by reducing neoplastic progression in BE (299), but further studies are needed to prove the purported benefits.
2. Esophageal squamous cell cancer
A second major type of esophageal cancer is squamous cell carcinoma (ESCC). ESCC is associated with a poor prognosis due to its typically late stage at the time of diagnosis and propensity for metastasis. It is a relatively aggressive form of squamous cell cancer compared with other tissues such as skin, head/neck, lung, and urogenital tract. Oxidative stress markers such as 8-hydroxydeoxyguanosine (8-OhdG) and thiobarbituric acid-reactive substances are elevated in tissues from ESCC (79). Alcoholism, cigarette smoking, as well as mineral and antioxidant vitamin deficiencies seem to promote ESCC (121), while consumption of antioxidants like vitamin C, β-carotene, and α-tocopherol are associated with a reduced risk of ESCC (283). Thus, by association, oxidative stress appears to be a major player in development of ESCC. Diakowska et al. (80) found that 8-OhdG level is high in advanced ESCC patients and total antioxidant levels are decreased (80). Thus the authors proposed that estimation of serum 8-OhdG and total antioxidant status can be used as diagnostic markers of ESCC progression. In vitro experiments showed that oxidative stress and radiation cause nuclear accumulation of FOXO3a, and its downregulation reduced the radiosensitivity of esophageal cancers (49). The same study also reported that patient survival proportionately increases with nuclear FOXO3a accumulation and thus FOXO3a can act as a therapeutic marker for ESCC. Further studies in animals and other models are required to identify ROS-induced ESCC-associated proteins that could be successfully used as diagnostic and therapeutic markers of ESCC.
B. Gastroduodenal Diseases
1. Peptic ulcer disease and gastritis
Gastritis is defined as inflammation of the stomach mucosal lining and occurs in several conditions including H. pylori infection, NSAID use, alcohol consumption, and stress. Peptic ulcer disease (PUD) occurs in the proximal GI tract and is often associated with chronic gastritis. Gastric and duodenal ulcers represent the most common and chronic PUDs. On the basis of pathophysiology, PUD can be broadly classified into the following etiologic groups: 1) excessive acid secretion type (e.g., Zollinger-Ellison syndrome), 2) associated with infections, and 3) NSAID induced (174, 275). Gastritis and peptic ulcer are caused by multiple factors, both endogenous and exogenous, and free radicals are closely linked to both conditions.
There are several factors contributing to the accumulation of ROS in the stomach. Reduced antioxidant enzyme SOD levels (200) and antioxidant vitamin intake (206) contribute to the accumulation of ROS associated with gastroduodenal inflammatory diseases. Ethanol-induced gastric inflammation is associated with increased O2·− generation (117). Phagocytic leukocytes are the main source of ROS in chronic inflammation such as one observes in H. pylori-induced gastritis and IBD. Significant numbers of neutrophils and/or macrophages infiltrate the gastric mucosa during inflammation, generating large amounts of ROS.
Another cause of gastritis is ischemic injury, which is known to involve free radicals such as O2·−, H2O2, and HO· (191). Exogenous factors are also important. Smoking increases MPO activity in neutrophils (38) and in the extracellular spaces, thus enhancing gastric damage. XO activity is also higher in cigarette smoke-exposed rats (51). All these lead to neutrophil aggregation and vascular damage. Apoptosis, oxidative damage by ROS, and reduction of angiogenesis in the gastric mucosa lead to arrest of cell proliferation, and these events in turn induce ulceration (173). ROS-mediated increased lipid peroxidation, lowered GSH level, and antioxidant systems are involved in the pathogenesis of almost all forms of gastric ulcer (71, 108).
The role of H. pylori in development of gastritis and PUD deserves special mention as it is a major contributor to peptic ulcer and gastritis. When first determined to be a cause of peptic ulcer, this organism was found in ∼95% of cases of duodenal ulcers and up to 70% of cases of gastric ulcers (94). Rates of infection in both types of ulcers in developed countries have decreased but still remain high in many developing nations. H. pylori strains isolated from duodenal ulcer patients induce higher neutrophil activity relative to gastritis strains (70). In the acute stage of H. pylori infection, neutrophil infiltration is observed, but unlike other bacterial infections, active inflammation persists throughout this lifelong infection that characterizes the majority of infected subjects worldwide. Chronic inflammatory cells including macrophages/monocytes, lymphocytes, and plasma cells are also present in the gastric mucosa of chronically infected humans, and thus these stromal cells contribute significantly to the development of gastritis and PUD. Gastric epithelial pit cells also produce ROS by activating nonphagocytic NADPH oxidase in response to H. pylori (286). Davies et al. (72) reported that mucosal samples from patients with duodenal ulcer and severe duodenitis generated significantly higher ROS levels than those from control subjects. This pathogen also enhances gastric antral ROS production which is correlated with bacterial load (73). The fact that infected individuals have significant reduction in vitamin E and C levels likely also contribute in the oxidative stress during infection (83), and low antioxidant levels are also linked to gastric ulcer disease (206). Therefore, the balance of factors that enhance or attenuate the local concentration of oxidants regulates disease progression.
Host and environmental factors such as genetics, diet, stress, tobacco, and levels of hygiene contribute significantly to the accumulation of ROS and the pathogenesis of H. pylori infection (173, 211). H. pylori possibly reduces GEC's ability to protect from ROS-mediated damage; however, the mechanisms that account for this are still elusive (269). High levels of lipid peroxidation and decreased mucosal GSH levels are noted in patients with H. pylori infection or uninfected peptic ulcer and gastritis (139). A chemotactic peptide from H. pylori, N-formyl-methionyl-leucyl-phenylalanine (fMLP) contributes to neutrophil accumulation and activation (197). The severity of H. pylori-induced GI diseases is often associated with the cag (cytotoxin associated gene) pathogenicity island (PAI), a 40-kb stretch of DNA encoding several components of a type IV secretion system (263). The cag(+) strains exhibit enhanced oxidative burst in PMNs (337). Intracellular GSH levels are lowered by H. pylori infection (23) and impairs GSH metabolism in the gastric epithelium (148). Kimura et al. reported that H. pylori vacuolating cytotoxin A (VacA) decreases GSH efflux and suppresses intracellular GSH turnover rate (148). Catalase and SOD released by H. pylori are insufficient to remove excess extracellular ROS but play important roles in the elimination of ROS generated by bacteria (198). NH3 derived from H. pylori reacts with HOCl to generate monochloramine (NH2Cl). NH2Cl can penetrate cell membranes and damages intracellular components (106). Lipid peroxidation is increased in H. pylori-infected patients and is significantly lessened in the mucosa of patients after successful eradication of infection. (84).
H. pylori and ROS collaborate to activate the transcription factors NF-κB and AP-1 in the gastric epithelium which upregulate the expression of chemokines including CXCL8 (IL-8) (69, 145). IL-8 also enhances neutrophil migration into the gastric epithelial layer and their subsequent activation (65, 145), thereby further contributing to inflammation. We have reported that the expression of APE1/Ref1, which reductively activates transcription factors, is induced in H. pylori-infected human gastric epithelia (82). We have also shown that APE1/Ref1 expressed in gastric epithelial cells enhances transcriptional activity of AP1 and NF-κB and induces H. pylori-mediated IL-8 expression (209). As discussed, APE1/Ref1 reductively activates transcription factors to enhance their ability to bind to DNA. Thus these observations point to one potential mechanism whereby the oxidative stress signals are transduced to regulate the inflammatory response to H. pylori.
H. pylori colonization of the gastric pits is a major risk factor that predicts the severity of pathogenesis. Host cell death and survival depends on ROS produced in the infected stomach. H. pylori activate the intrinsic pathway of apoptosis (45). Induced expression of pro-apoptotic factors Bax and Bid and reduced expression of the anti-apoptotic factor bcl-2 have been reported in H. pylori infection (9). Suppression of pit cell death by H. pylori is also reported and is believed to favor persistent bacterial colonization in the stomach as the rapid self-renewal of progenitor cells and apoptosis of gastric pit cells limit bacterial colonization. Infection with cagA(+) H. pylori but not with a mutant increases the survival factors phospho-ERK and antiapoptotic protein Mcl1 expression in the gastric pits (193). As APE1/Ref1 activates the tumor suppressor apoptotic protein p53, but forced overexpression of APE1/Ref1 prevents apoptosis (6), we examined this “paradoxical role” of APE1/Ref1 in H. pylori-mediated GEC apoptosis. We observed that acetylation of APE1/Ref1 as a result of H. pylori infection suppresses Bax expression, thereby preventing p53-mediated apoptosis in infected gastric epithelium (29). ROS are known to activate and stabilize hypoxia-inducible factor 1α (HIF1α) (248). Gastric epithelial ROS enhance normoxic stabilization of HIF1α (218). We showed that APE1/Ref1 increases HIF1α level in H. pylori-infected gastric mucosa, and in conjunction with transcriptional co-activator p300 induces transcriptional activity of HIF1α (30). Further studies are needed to determine the role of HIF1α in regulating oxidative stress in mucosal inflammation due to H. pylori infection and other factors.
RNS generation, in contrast, has been found to be helpful for maintaining gastric mucosal health. ·NO is a free radical that may inhibit pathogenic mechanisms of gastric ulcer by slowing disease progression (171). ·NO stimulates mucus secretion by gastric mucosal cells (170) and inhibits expression of adhesion molecule in the epithelium such as P-selectin, thereby reducing the ability of neutrophil to adhere in the gastric mucosa (306) and preventing neutrophil-released ROS-mediated ulceration. ·NO inhibits mast cell degranulation, thus providing another level of gastroprotection (306). Thus ROS or RNS species are important to identify as different entities can either enhance or attenuate gastric tissue damage.
2. Gastric adenocarcinoma and gastric cancer
Currently, gastric cancer is the fourth most common cancer in the world and second most common cause of mortality from cancer. Gastric cancer involves a number of genetic alterations of tumor-regulatory genes as well as epigenetic factors. H. pylori infection is the major cause of gastric cancer and a useful biomarker for this disease. So far, H. pylori gastritis is the only universal precursor condition for the diffuse form of gastric cancer. Correa (64) postulated that hyperproliferation found in H. pylori gastritis initiates a sequence of events leading to gastric cancer. This hyperproliferation possibly favors mutations which transform normal gastric mucosa to gastric carcinoma. Histopathologically, the sequence starts with superficial gastritis followed by multifocal atrophy, intestinal metaplasia and, lastly, dysplasia or cancer (64). H. pylori colonize the stomachs of ∼50% of humans, and those who are infected have at least a twofold increased risk of gastric cancer relative to the uninfected population (123). The cag+ H. pylori strains are more highly associated with gastric carcinogenesis than strains that do not have cag (221). Significantly high ROS or RNS production occur in H. pylori-infected gastric mucosa, vascular endothelium, as well as in neutrophils accumulated in the inflamed mucosa (199). Phagocytes accumulated in gastric mucosa after H. pylori infection produce O2·−, HO·, and HOCl. O2·− is not a very reactive molecule. The exact role of HO· is still not well understood, but OCl− produced by phagocytic neutrophils reacts with NH3 generated by the urease activity of H. pylori in the stomach lining and generates highly reactive molecule NH2Cl. NH2Cl has been reported to induce apoptosis in rat gastric mucosal cells (201). Epstein-Barr virus (EBV) is one of the major etiological agents of gastric cancer and represents 7% of gastric cancer cases (129). NH2Cl derived from infiltrating neutrophils in H. pylori-infected stomach is able to convert latent EBV into lytic EBV which can further contribute in gastric carcinogenesis (195). Although the role of ROS produced in infected GECs is not clearly understood, those are believed to initiate signaling events in GECs which determine the course of H. pylori pathogenesis. In addition to stimulating host responses that contribute to ROS, H. pylori infection induces oxidative stress in GECs directly through the generation of ROS and regulates proinflammatory cytokine production, inflammation, and cell death (29, 81, 209). Persistent ROS causes proto-oncogene activation, oncogene/tumor suppressor gene mutations, and chromosomal aberrations, as a result of oxidative genome damage including oxidation of guanine to generate 8-OhdG and 8-oxo,7,8-dihydroguanosine (8-OHG) in DNA and RNA, respectively (68, 85).
Gastric adenoma and gastric cancer tissues (H. pylori-infected or uninfected) have increased mucosal expression of ROS and APE1/Ref1 compared with normal mucosa (99). Infection with H. pylori is associated with reduced amount of ascorbic acid in the gastric lumen and lowers its amount in the gastric juice. This antioxidant impairs effects of carcinogens, as it can reduce mutagenic agents such as nitrosamines and ROS. As the conventional therapeutic approach to kill cancer cells is via generation of ROS, depletion of cellular antioxidants increases the efficiency of ROS in killing cancer cells. Studies to inhibit various antioxidant mechanisms during neoadjuvant therapies will help us in controlling the disease.
C. Intestinal Diseases
1. IBDs (ulcerative colitis, Crohn's disease)
IBDs, both Crohn's disease and ulcerative colitis, involve chronic inflammation of the GI tract. In ulcerative colitis, only the colon is affected, whereas Crohn's disease may occur anywhere in the GI tract. Ulcerative colitis generally begins in the rectum, advancing proximally as the disease progresses with continuous inflammation that affects only the mucosal layer of the gut wall. In contrast, Crohn's disease inflammation may occur in a segmental fashion and is transmural, involving all layers of the GI tract wall. The exact causes of IBD are not completely understood but are believed to result from inappropriate inflammatory response to commensal gut microbiota, which may be genetically regulated. Altered mesenteric circulation, intestinal microcirculation, and intestinal ischemia are potential etiologic factors in IBD, although their involvement could be secondary (127). The association of ROS with IBD is evident from the observation that increased ROS and decreased antioxidant levels contribute toward major pathogenic mechanisms in IBD (58, 126). ROS also potentiate immune reactions in IBD by inflammatory leukocytes, mainly PMNs, further augmenting tissue damage. O2·−, H2O2, and HO· secreted by phagocytes accumulate at the site of inflammation resulting in lipid peroxidation. No wonder that McCord described the GI tract as a “free radical time bomb” (186) and “in IBD, the fuse appears to be lit” (42).
Defects in mucosal antioxidant defenses are a contributing factor in ulcerative colitis as the redox status of mucosal glutathione is associated with inflammation and disease progression. The severity of ulcerative colitis in mice is related to SOD (159). Dextran sodium sulfate (DSS) is used to induce inflammatory responses in mouse models of ulcerative colitis. This results from activation of IκBα and NF-κB pathways via ROS generation. DSS increases sulfate load of cells which induces ROS, leading to activation of inflammatory response. Likewise, diets rich in sulfur in human ulcerative colitis induce ROS-mediated inflammation (31). Iron, which induces ROS production via the Fenton reaction, contributes to induction of colorectal tumor in a murine ulcerative colitis model (265). The antioxidant resveratrol significantly reduces inflammatory responses of ulcerative colitis in mice (330), further establishing the importance of ROS in ulcerative colitis.
Understanding the role of ·NO in intestinal inflammation is less straightforward. While some reports suggest ·NO's role in inflammation, other studies indicate that it has a protective role in the intestine (308). ·NO reacts with O2·−, produced by activated neutrophils, to form another potent oxidant, peroxynitrite (ONOO−). ONOO− administration to the colon results in tissue injury (240). Thus iNOS might contribute in tissue injury via generation of ONOO−, and iNOS inhibitors have been shown to reduce colonic damage and inflammation (190). Oral administration of pre- and probiotics in DSS-induced acute murine colitis decreases NO levels in peritoneal macrophages and thus is reported to reduce colonic lesion (2). Further experiments to identify anti-inflammatory properties of probiotics that have been used to treat patients with IBD could determine underlying mechanisms of these potentially beneficial agents.
While the two forms of IBD share similar pathophysiology, HO· and O2·− are found to be responsible for Crohn's disease, while H2O2 and HOCl are more associated with ulcerative colitis (160). Increased XO and Mn-SOD activity are reported in inflamed mucosa of Crohn's disease patients (160). Crohn's disease patients exhibit elevated TNF-α in the colonic mucosa, and inflamed mucosa shows induction of iNOS, similar to ulcerative colitis (267). Enhanced oxidative stress and inflammation in conjunction with decreased antioxidant levels have been reported in patients with active Crohn's disease. However, with improvement of the patient's condition, these parameters of oxidative stress go back to normal levels (178). As NF-κB is a major inducer of inflammatory cytokines, the effect of a potent NF-κB inhibitor, vanillin, has been studied in mouse model of colitis (325). Vanillin was shown to suppress the production of Th1 cytokines as well as scavenges 1O2, underscoring its potential as a future treatment for IBD.
Lower plasma levels of vitamins A and E as well as beta-carotene and decreased antioxidant enzymes in the intestinal mucosa of IBD patients may determine the severity of IBD (116). Further studies are required to establish whether these vitamins affect the course of the disease. Antioxidants, to some extent, are effective in treating experimental colitis. SOD has had some success when used in the treatment of murine ulcerative colitis (89) and lecithinized SOD (PC-SOD), which overcomes the clinical limitations of SOD, treats ulcerative colitis more effectively. Further evidence that ROS contribute to the pathogenesis of IBD comes from the discovery that sulfasalazine, a drug with antioxidant properties, has some beneficial effects in the treatment of IBD. Despite these advancements, none could be claimed as a cure of IBD even with combinations of such agents. Disruption of intestinal homeostasis is now believed to be the major event in the development of IBD (176). Specifically, the role of gut microbiota in this process is developing as our knowledge in this field advances. Treating UC patients with fecal bacteriotherapy (infusion of fecal microbiota from healthy donors) is an emerging field of research and has been found to improve UC (35). Substantial research in this field is required to understand how fecal transplantation benefits patients with IBD and potentially other gastroenteric diseases.
2. Enteric infections
Intestinal epithelia produce ROS in response to microbial signals. The human large intestine contains ∼1014 prokaryotes from over 500 species (86). Epithelia contacted by enteric commensal bacteria rapidly generate ROS (165). Commensal bacteria-induced ROS generated in the intestinal epithelium modulate the protein degradation machinery of various signaling molecules and thus regulate diverse physiological events within the host cells (165). The early response to S. enterica serovar Typhimurium infection is ROS generation by NADPH oxidase with potent bactericidal effects (300). During the later stages of Salmonella infection, RNS are also generated (182). The antioxidant N-acetylcysteine (NAC) and NADPH oxidase inhibitor diphenyliodonium (DPI) have been reported to attenuate disease, implicating ROS generation as an important host response to gastrointestinal infection (165). A key issue to ascertain is whether therapeutic inhibition of ROS will protect the host from enteric infection-induced tissue damage or if any benefit would be offset by the impairment of antimicrobial host responses.
3. Ischemic intestinal injury
Intestinal I/R occurs in surgical and trauma patients and arises when blood flow to the intestine is interrupted due to various circumstances (291). I/R activates Toll-like receptors (TLRs) leading to acute intestinal and lung injury and inflammation observed during gut trauma (303). The tissue damage due to reperfusion is primarily caused by reentry of oxygen, rather than by ischemia itself. Ischemia followed by reperfusion is more damaging than ischemia without reperfusion (220). One explanation for this damage is that ATP becomes metabolized to HX during ischemia, while during reperfusion, oxygen reacts with HX to form xanthine and O2·−. Reperfusion enhances the damaging effects of ischemic injury due to accumulation of activated neutrophils and generation of ROS (175). These events are collectively called reperfusion injury. I/R-mediated GI injury is significantly reversed by both SOD and allopurinol. Observations that SOD prevents I/R-induced GI injury and oxidized glutathione is found in I/R-exposed GI mucosa establish the crucial role of ROS in the pathogenesis of I/R-mediated GI injury.
Blockage of blood supply to the colon leads to ischemic colitis. Ischemic colitis is of two types: occlusive and nonocclusive. Occlusive ischemic colitis occurs when a blood clot diminishes blood flow to the colon; nonocclusive ischemia develops because of narrowing of blood vessels or low systemic blood pressure (119). In its milder form, ischemic colitis leads to mild necrosis or ulceration, whereas severe ischemic colitis is characterized by sepsis, ulceration, and in some cases gangrene. Ischemic colitis has some pathological features in common with IBD. Enhanced lipid peroxidation is evident in ischemic colitis (135). Treatment of feline experimental I/R GI injury with antioxidants like SOD and drugs that attenuate the effect of inflammatory mediators have shown promising effects (219). While the morbidity and mortality of intestinal ischemia is best mitigated by preventing the ischemic insult, once injury occurs better strategies to reduce anoxia and oxidative stress are helpful, and further research in this field is needed.
4. Colorectal cancer
The development of colorectal cancer (CRC) is dependent on several mechanisms. Genes that affect the control of cell growth are frequently mutated in colon cancer. In CRC, free radicals produced by the colonic bacteria Enterococcus faecalis may directly cause mutations in colonic DNA resulting in colon cancer (14). Free radicals convert dietary procarcinogens to carcinogens that may contribute to CRC (124). Lipid peroxidation of polyunsaturated fatty acids results in production of reactive metabolites that have also been implicated in the pathogenesis of CRC (18).
Chronic inflammation in IBD results in persistent oxidative stress and inflammation that contribute to dysplasia (251). A population-based study and one meta-analysis have shown that Crohn's disease and ulcerative colitis patients have an increased risk of developing small bowel carcinoma in Crohn's disease and colon cancer in both forms of IBD, compared with a non-IBD population (27). The risk of CRC increases with the duration of clinical disease, the severity of the inflammatory response, and the extent of IBD (290). CRC in IBD patients generally begins with low-grade dysplasia, progressing to indefinite dysplasia, then to high-grade dysplasia and eventually, invasive adenocarcinoma. DNA damage caused by ROS is a major contributor to CRC development in ulcerative colitis patients. Thus oxidative stress-induced cellular damage may provide a mechanistic basis for colon cancer by causing genetic instability, specific gene alterations, and aberrant methylation. Inflammation-induced ROS interact with cancer-regulating genes, transcription factors, as well as DNA mismatch repair genes (158). Cytokines such as IL-6, IL-8, TGF-β1, and mediators such as COX-2 are pivotal factors in the development of CRC (158). Excess iron intake may also lead to CRC by inducing ROS and inflammation-mediated epithelial changes (265a). There is emerging information that chronic inflammation and host microbes may play key roles in not only IBD-related carcinogenesis but also in sporadic and other forms of CRC (285).
VI. CONCLUSIONS
Many cell types within the mucosa of the GI tract produce ROS as part of normal physiology, yet the gut mucosa also is a target of various oxidants that can lead to pathological conditions. Oxidative stress makes a substantial contribution to the pathogenesis of many GI mucosal diseases, and despite recent progress, mechanisms of ROS-mediated GI diseases are not well established. Understanding the cellular and molecular mechanisms including altered signaling caused by ROS are critical to developing future therapies for GI diseases mediated by oxidative stress. As discussed, major GI diseases in which ROS play a major role (e.g., gastritis, gastric cancer, IBD, colonic inflammation, and cancer) are substantially linked to the microbiota as well as disruption of the antioxidant balance in the mucosa. Future treatment strategies for these important mucosal GI diseases lie in a better understanding of free radical biology of the GI mucosa.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 DK61769 (to S. E. Crowe), American Gastroenterological Society Funderburg Research Scholar Award (to S. E. Crowe), NIH Grant R01 ES08457 (to S. Mitra), and Department of Biotechnology (DBT) India RGYI grant (to A. Bhattacharyya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful for assistance from Dr. Arup Sarkar in figure preparation.
Address for reprint requests and other correspondence: S. E. Crowe, Dept. of Medicine, Univ. of California, San Diego, San Diego, CA 92093-0063 (e-mail: secrowe@ucsd.edu).
REFERENCES
- 1.Abate C, Patel L, Rauscher IIIFJ, Curran T. Redox regulation of Fos and Jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Abdelouhab K, Rafa H, Toumi R, Bouaziz S, Medjeber O, Touil-Boukoffa C. Mucosal intestinal alteration in experimental colitis correlates with nitric oxide production by peritoneal macrophages: effect of probiotics and prebiotics. Immunopharmacol Immunotoxicol 34: 590–597, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JC, Latreille M, Messina C, Alpern Z, Grimson R, Martin C, Hubbard P, Shaw RD. Smokers as a high-risk group: data from a screening population. J Clin Gastroenterol 43: 747–752, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Anderson LA, Johnston BT, Watson RG, Murphy SJ, Ferguson HR, Comber H, McGuigan J, Reynolds JV, Murray LJ. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res 66: 4975–4982, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ 9: 717–725, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Aota M, Matsuda K, Isowa N, Wada H, Yodoi J, Ban T. Protection against reperfusion-induced arrhythmias by human thioredoxin. J Cardiovasc Pharmacol 27: 727–732, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ashktorab H, Frank S, Khaled AR, Durum SK, Kifle B, Smoot DT. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut 53: 805–813, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atack JM, Kelly DJ. Oxidative stress in Campylobacter jejuni: responses, resistance and regulation. Future Microbiol 4: 677–690, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radic Biol Med 43: 16–21, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52: 741–744, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 160: 2253–2257, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther 291: 1075–1085, 1999 [PubMed] [Google Scholar]
- 16.Barrachina MD, Panes J, Esplugues JV. Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: perspective for drugs development. Curr Pharm Des 7: 31–48, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Barros MH, Netto LE, Kowaltowski AJ. H2O2 generation in Saccharomyces cerevisiae respiratory pet mutants: effect of cytochrome c. Free Radic Biol Med 35: 179–188, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis 20: 2209–2218, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Bashir A, Perham RN, Scrutton NS, Berry A. Altering kinetic mechanism and enzyme stability by mutagenesis of the dimer interface of glutathione reductase. Biochem J 312: 527–533, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baud L, Ardaillou R. Involvement of reactive oxygen species in kidney damage. Br Med Bull 49: 621–629, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Becker K, Gromer S, Schirmer RH, Muller S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem 267: 6118–6125, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Beil W, Obst B, Sewing KF, Wagner S. Helicobacter pylori reduces intracellular glutathione in gastric epithelial cells. Dig Dis Sci 45: 1769–1773, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M, Zupi G, Biroccio A. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell 21: 509–519, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Berger SJ, Gosky D, Zborowska E, Willson JK, Berger NA. Sensitive enzymatic cycling assay for glutathione: measurements of glutathione content and its modulation by buthionine sulfoximine in vivo and in vitro in human colon cancer. Cancer Res 54: 4077–4083, 1994 [PubMed] [Google Scholar]
- 26.Bergmark E, Calleman CJ, He F, Costa LG. Determination of hemoglobin adducts in humans occupationally exposed to acrylamide. Toxicol Appl Pharmacol 120: 45–54, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 91: 854–862, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharya S, Mathew G, Jayne DG, Pelengaris S, Khan M. 15-Lipoxygenase-1 in colorectal cancer: a review. Tumour Biol 30: 185–199, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya A, Chattopadhyay R, Cross JV, Ernst PB, Mitra S, Bhakat KK, Crowe SE. Acetylation of apurinic/apyrimidinic endonuclease-1 regulates Helicobacter pylori-mediated gastric epithelial cell apoptosis. Gastroenterology 136: 2258–2269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharyya A, Chattopadhyay R, Hall EH, Mebrahtu ST, Ernst PB, Crowe SE. Mechanism of hypoxia-inducible factor 1 alpha-mediated Mcl1 regulation in Helicobacter pylori-infected human gastric epithelium. Am J Physiol Gastrointest Liver Physiol 299: G1177–G1186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharyya S, Dudeja PK, Tobacman JK. ROS, Hsp27, and IKKbeta mediate dextran sodium sulfate (DSS) activation of IkappaBa, NFkappaB, and IL-8. Inflamm Bowel Dis 15: 673–683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364: 1219–1228, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol 13: 135–160, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Bompart GJ, Prevot DS, Bascands JL. Rapid automated analysis of glutathione reductase, peroxidase, and S-transferase activity: application to cisplatin-induced toxicity. Clin Biochem 23: 501–504, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 37: 42–47, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 274: 23679–23682, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682–685, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridges RB, Fu MC, Rehm SR. Increased neutrophil myeloperoxidase activity associated with cigarette smoking. Eur J Respir Dis 67: 84–93, 1985 [PubMed] [Google Scholar]
- 39.Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J 330: 1173–1178, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucher JR, Tien M, Aust SD. The requirement for ferric in the initiation of lipid peroxidation by chelated ferrous iron. Biochem Biophys Res Commun 111: 777–784, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300: 535–543, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Bulger EM, Helton WS. Nutrient antioxidants in gastrointestinal diseases. Gastroenterol Clin North Am 27: 403–419, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci 27: 352–360, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA, Reed TT, Perluigi M, De MC, Coccia R, Keller JN, Markesbery WR, Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer's disease. Brain Res 1148: 243–248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvino-Fernandez M, ito-Martinez S, Parra-Cid T. Oxidative stress by Helicobacter pylori causes apoptosis through mitochondrial pathway in gastric epithelial cells. Apoptosis 13: 1267–1280, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Cerutti PA. Prooxidant states and tumor promotion. Science 227: 375–381, 1985 [DOI] [PubMed] [Google Scholar]
- 47.Chang D, Hu ZL, Zhang L, Zhao YS, Meng QH, Guan QB, Zhou J, Pan HZ. Association of catalase genotype with oxidative stress in the predication of colorectal cancer: modification by epidemiological factors. Biomed Environ Sci 25: 156–162, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Chang JC, van der Hoeven LH, Haddox CH. Glutathione reductase in the red blood cells. Ann Clin Lab Sci 8: 23–29, 1978 [PubMed] [Google Scholar]
- 49.Chen MF, Fang FM, Lu CH, Lu MS, Chen WC, Lee KD, Lin PY. Significance of nuclear accumulation of Foxo3a in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 71: 1220–1229, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Mikhail SS, Ding YW, Yang G, Bondoc F, Yang CS. Effects of vitamin E and selenium supplementation on esophageal adenocarcinogenesis in a surgical model with rats. Carcinogenesis 21: 1531–1536, 2000 [PubMed] [Google Scholar]
- 51.Chow JY, Ma L, Cho CH. Involvement of free radicals and histamine in the potentiating action of cigarette smoke exposure on ethanol-induced gastric mucosal damage in rats. Free Radic Biol Med 24: 1285–1293, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem 268: 2571–2576, 1993 [PubMed] [Google Scholar]
- 53.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res 64: 962–968, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Chu FF, Esworthy RS, Doroshow JH. Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic Biol Med 36: 1481–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark GW, Smyrk TC, Mirvish SS, Anselmino M, Yamashita Y, Hinder RA, DeMeester TR, Birt DF. Effect of gastroduodenal juice and dietary fat on the development of Barrett's esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol 1: 252–261, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Clemens MR, Ladner C, Ehninger G, Einsele H, Renn W, Buhler E, Waller HD, Gey KF. Plasma vitamin E and beta-carotene concentrations during radiochemotherapy preceding bone marrow transplantation. Am J Clin Nutr 51: 216–219, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collman JP, Devaraj NK, Decreau RA, Yang Y, Yan YL, Ebina W, Eberspacher TA, Chidsey CE. A cytochrome c oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux. Science 315: 1565–1568, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther 3: 294–300, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Conway JP, Kinter M. Dual role of peroxiredoxin I in macrophage-derived foam cells. J Biol Chem 281: 27991–28001, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Cook MB, Shaheen NJ, Anderson LA, Giffen C, Chow WH, Vaughan TL, Whiteman DC, Corley DA. Cigarette smoking increases risk of Barrett's esophagus: an analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology 142: 744–753, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper CE, Vollaard NB, Choueiri T, Wilson MT. Exercise, free radicals and oxidative stress. Biochem Soc Trans 30: 280–285, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Correa P. A human model of gastric carcinogenesis. Cancer Res 48: 3554–3560, 1988 [PubMed] [Google Scholar]
- 65.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJD. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastoduodenal mucosa. J Clin Pathol 47: 61–66, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cross AR. p40(phox) participates in the activation of NADPH oxidase by increasing the affinity of p47(phox) for flavocytochrome b(558). Biochem J 349: 113–117, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cross CE, Halliwell B, Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet 1: 1328–1330, 1984 [DOI] [PubMed] [Google Scholar]
- 68.Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol 21: 32–38, 2005 [PubMed] [Google Scholar]
- 69.Crowe SE, Alvarez L, Sherman PM, Jin Y, Dytoc M, Hunt RH, Patel J, Muller MJ, Ernst PB. Expression of interleukin-8 and CD54 by human gastric epithelium after H. pylori infection in vitro. Gastroenterology 108: 65–74, 1995 [DOI] [PubMed] [Google Scholar]
- 70.Danese S, Cremonini F, Armuzzi A, Candelli M, Papa A, Ojetti V, Pastorelli A, Di Caro S, Zannoni G, De Sole P, Gasbarrini G, Gasbarrini A. Helicobacter pylori CagA-positive strains affect oxygen free radicals generation by gastric mucosa. Scand J Gastroenterol 36: 247–250, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem 125: 115–125, 1993 [DOI] [PubMed] [Google Scholar]
- 72.Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut 33: 1467–1472, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies GR, Simmonds NJ, Stevens TRJ, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut 35: 179–185, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dayer R, Fischer BB, Eggen RI, Lemaire SD. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 179: 41–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Moreno de LA, LeBlanc JG, Perdigon G, Miyoshi A, Langella P, Azevedo V, Sesma F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J Med Microbiol 57: 100–105, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol 2012: 645460, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dekigai H, Nakamura H, Bai J, Tanito M, Masutani H, Hirota K, Matsui H, Murakami M, Yodoi J. Geranylgeranylacetone promotes induction and secretion of thioredoxin in gastric mucosal cells and peripheral blood lymphocytes. Free Radic Res 35: 23–30, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair 4: 1442–1449, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Diakowska D, Krzystek-Korpacka M, Lewandowski A, Grabowski K, Diakowski W. Evaluation of 8-hydroxydeoxyguanosine, thiobarbituric acid-reactive substances and total antioxidant status as possible disease markers in oesophageal malignancies. Clin Biochem 41: 796–803, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Diakowska D, Lewandowski A, Kopec W, Diakowski W, Chrzanowska T. Oxidative DNA damage and total antioxidant status in serum of patients with esophageal squamous cell carcinoma. Hepatogastroenterology 54: 1701–1704, 2007 [PubMed] [Google Scholar]
- 81.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, rden-Kramer B, Boldogh I, Ernst PB, Crowe SE. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun 75: 4030–4039, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding SZ, O'Hara AM, Denning TL, Dirden-Kramer B, Mifflin RC, Reyes VE, Ryan KA, Elliott SN, Izumi T, Boldogh I, Mitra S, Ernst PB, Crowe SE. Helicobacter pylori and H2O2 increases AP endonuclease-1/redox factor-1 expression in human gastric epithelial cells. Gastroenterology 127: 845–858, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KLM, Chalmers DM, Axon ATR. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 17: 559–562, 1996 [DOI] [PubMed] [Google Scholar]
- 84.Drake IM, Mapstone NP, Schorah CJ, White KLM, Chalmers DM, Dixon MF, Axon ATR. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H. pylori eradication. Gut 42: 768–771, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du MQ, Carmichael PL, Phillips DH. Induction of activating mutations in the human c-Ha-ras-1 proto-oncogene by oxygen free radicals. Mol Carcinog 11: 170–175, 1994 [DOI] [PubMed] [Google Scholar]
- 86.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, Buchler MW, Lerch MM, Pandol SJ, Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol 289: G1137–G1147, 2005 [DOI] [PubMed] [Google Scholar]
- 88.El-Chemaly S, Salathe M, Baier S, Conner GE, Forteza R. Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med 167: 425–430, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Emerit J, Loeper J, Chomette G. Superoxide dismutase in the treatment of post-radiotherapeutic necrosis and of Crohn's disease. Bull Eur Physiopathol Respir 17 Suppl: 287, 1981 [PubMed] [Google Scholar]
- 90.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 281: G848–G855, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res 461: 83–108, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Faraci FM. Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke 36: 186–188, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev CD003840, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr, Gorelick PL, Ward JM. Helicobacter hepaticus sp nov, a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol 32: 1238–1245, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology 180: 23–32, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23: 239–257, 1983 [DOI] [PubMed] [Google Scholar]
- 98.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875, 2001 [DOI] [PubMed] [Google Scholar]
- 99.Futagami S, Hiratsuka T, Shindo T, Horie A, Hamamoto T, Suzuki K, Kusunoki M, Miyake K, Gudis K, Crowe SE, Tsukui T, Sakamoto C. Expression of apurinic/apyrimidinic endonuclease-1 (APE-1) in H. pylori-associated gastritis, gastric adenoma, and gastric cancer. Helicobacter 13: 209–218, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Garcia SC, Moragon AC, Lopez-Fernandez ME. Frequency of glutathione reductase, pyruvate kinase and glucose-6-phosphate dehydrogenase deficiency in a Spanish population. Hum Hered 29: 310–313, 1979 [DOI] [PubMed] [Google Scholar]
- 101.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 279: 51715–51718, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal 19: 1807–1819, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Gerster H. The potential role of lycopene for human health. J Am Coll Nutr 16: 109–126, 1997 [DOI] [PubMed] [Google Scholar]
- 104.Go MF, Crowe SE. Virulence and pathogenicity of Helicobacter pylori. Gastroenterol Clin North Am 29: 649–670, 2000 [DOI] [PubMed] [Google Scholar]
- 105.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet 344: 859–861, 1994 [DOI] [PubMed] [Google Scholar]
- 106.Grisham MB, Jefferson MM, Thomas EL. Role of monochloramine in the oxidation of erythrocyte hemoglobin by stimulated neutrophils. J Biol Chem 259: 6757–6765, 1984 [PubMed] [Google Scholar]
- 107.Grubben MJ, Nagengast FM, Katan MB, Peters WH. The glutathione biotransformation system and colorectal cancer risk in humans. Scand J Gastroenterol Suppl 68–76, 2001 [DOI] [PubMed] [Google Scholar]
- 108.Guslandi M. A radical view of Helicobacter pylori. Am J Gastroenterol 94: 2797–2798, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102 Suppl 10: 5–12, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer's disease. Exp Physiol 93: 116–120, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med 16: 845–850, 1994 [DOI] [PubMed] [Google Scholar]
- 112.Harman D. The aging process. Proc Natl Acad Sci USA 78: 7124–7128, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harries AD, Baird A, Rhodes J. Non-smoking: a feature of ulcerative colitis. Br Med J 284: 706, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harris CM, Sanders SA, Massey V. Role of the flavin midpoint potential and NAD binding in determining NAD versus oxygen reactivity of xanthine oxidoreductase. J Biol Chem 274: 4561–4569, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Harris ED. Regulation of antioxidant enzymes. FASEB J 6: 2675–2683, 1992 [DOI] [PubMed] [Google Scholar]
- 116.Hengstermann S, Valentini L, Schaper L, Buning C, Koernicke T, Maritschnegg M, Buhner S, Tillinger W, Regano N, Guglielmi F, Winklhofer-Roob BM, Lochs H. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin Nutr 27: 571–578, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Hernandez-Munoz R, Montiel-Ruiz C, Vazquez-Martinez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest 80: 1161–1169, 2000 [DOI] [PubMed] [Google Scholar]
- 118.Heslin MJ, Hawkins A, Boedefeld W, Arnoletti JP, Frolov A, Soong R, Urist MM, Bland KI. Tumor-associated down-regulation of 15-lipoxygenase-1 is reversed by celecoxib in colorectal cancer. Ann Surg 241: 941–946, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 19: 729–738, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Hoensch H, Morgenstern I, Petereit G, Siepmann M, Peters WH, Roelofs HM, Kirch W. Influence of clinical factors, diet, and drugs on the human upper gastrointestinal glutathione system. Gut 50: 235–240, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol 17: 2–9, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Holmgren A. Thioredoxin. Annu Rev Biochem 54: 237–271, 1985 [DOI] [PubMed] [Google Scholar]
- 123.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 125: 1636–1644, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 23: 529–536, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 365: 1375–1383, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Iborra M, Moret I, Rausell F, Bastida G, Aguas M, Cerrillo E, Nos P, Beltran B. Role of oxidative stress and antioxidant enzymes in Crohn's disease. Biochem Soc Trans 39: 1102–1106, 2011 [DOI] [PubMed] [Google Scholar]
- 127.Ibrahim CB, Aroniadis OC, Brandt LJ. On the role of ischemia in the pathogenesis of IBD: a review. Inflamm Bowel Dis 16: 696–702, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Iijima K, Henry E, Moriya A, Wirz A, Kelman AW, McColl KE. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology 122: 1248–1257, 2002 [DOI] [PubMed] [Google Scholar]
- 129.Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA 91: 9131–9135, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Inokuma T, Haraguchi M, Fujita F, Tajima Y, Kanematsu T. Oxidative stress and tumor progression in colorectal cancer. Hepatogastroenterology 56: 343–347, 2009 [PubMed] [Google Scholar]
- 131.Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res 92: 81–87, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Iyama T, Takasuga A, Azuma M. β-Carotene accumulation in mouse tissues and a protective role against lipid peroxidation. Int J Vitam Nutr Res 66: 301–305, 1996 [PubMed] [Google Scholar]
- 133.Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut 47: 762–770, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Janssen AM, Bosman CB, van DW, Oostendorp-van de Ruit MM, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van d V, Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res 6: 3183–3192, 2000 [PubMed] [Google Scholar]
- 135.Jarry A, Bach-Ngohou K, Masson D, Dejoie T, Lehur PA, Mosnier JF, Denis MG, Laboisse CL. Human colonic myocytes are involved in postischemic inflammation through ADAM17-dependent TNFalpha production. Br J Pharmacol 147: 64–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC). Radiat Res 173: 579–589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jimenez P, Piazuelo E, Sanchez MT, Ortego J, Soteras F, Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett's esophagus. World J Gastroenterol 11: 2697–2703, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem 272: 30952–30961, 1997 [DOI] [PubMed] [Google Scholar]
- 139.Jung HK, Lee KE, Chu SH, Yi SY. Reactive oxygen species activity, mucosal lipoperoxidation and glutathione in Helicobacter pylori-infected gastric mucosa. J Gastroenterol Hepatol 16: 1336–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Kakinuma K, Kaneda M, Chiba T, Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem 261: 9426–9432, 1986 [PubMed] [Google Scholar]
- 141.Kaneko K, Akuta T, Sawa T, Kim HW, Fujii S, Okamoto T, Nakayama H, Ohigashi H, Murakami A, Akaike T. Mutagenicity of 8-nitroguanosine, a product of nitrative nucleoside modification by reactive nitrogen oxides, in mammalian cells. Cancer Lett 262: 239–247, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem 273: 6297–6302, 1998 [DOI] [PubMed] [Google Scholar]
- 143.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Muller HM, Botella-Munoz J, Schneuwly S, Schirmer R, Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 291: 643–646, 2001 [DOI] [PubMed] [Google Scholar]
- 144.Kawasaki K, Nishio A, Nakamura H, Uchida K, Fukui T, Ohana M, Yoshizawa H, Ohashi S, Tamaki H, Matsuura M, Asada M, Nishi T, Nakase H, Toyokuni S, Liu W, Yodoi J, Okazaki K, Chiba T. Helicobacter felis-induced gastritis was suppressed in mice overexpressing thioredoxin-1. Lab Invest 85: 1104–1117, 2005 [DOI] [PubMed] [Google Scholar]
- 145.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kB in gastric epithelial cells. Gastroenterology 113: 1099–1109, 1997 [DOI] [PubMed] [Google Scholar]
- 146.Kekec Y, Paydas S, Tuli A, Zorludemir S, Sakman G, Seydaoglu G. Antioxidant enzyme levels in cases with gastrointesinal cancer. Eur J Intern Med 20: 403–406, 2009 [DOI] [PubMed] [Google Scholar]
- 147.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66: 11580–11584, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Kimura M, Goto S, Ihara Y, Wada A, Yahiro K, Niidome T, Aoyagi H, Hirayama T, Kondo T. Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb Pathog 31: 29–36, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Kirsch DG, Santiago PM, di TE, Sullivan JM, Hou WS, Dayton T, Jeffords LB, Sodha P, Mercer KL, Cohen R, Takeuchi O, Korsmeyer SJ, Bronson RT, Kim CF, Haigis KM, Jain RK, Jacks T. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327: 593–596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Klinowski E, Broide E, Varsano R, Eshchar J, Scapa E. Superoxide dismutase activity in duodenal ulcer patients. Eur J Gastroenterol Hepatol 8: 1151–1155, 1996 [DOI] [PubMed] [Google Scholar]
- 151.Koharyova M, Kolarova M. Oxidative stress and thioredoxin system. Gen Physiol Biophys 27: 71–84, 2008 [PubMed] [Google Scholar]
- 152.Kohut A, Mojzis J. Effect of allopurinol and superoxide dismutase on indomethacin-induced gastric lesions in the rat. Physiol Res 42: 273–276, 1993 [PubMed] [Google Scholar]
- 153.Komatsu H, Okayasu I, Mitomi H, Imai H, Nakagawa Y, Obata F. Immunohistochemical detection of human gastrointestinal glutathione peroxidase in normal tissues and cultured cells with novel mouse monoclonal antibodies. J Histochem Cytochem 49: 759–766, 2001 [DOI] [PubMed] [Google Scholar]
- 154.Konturek PC, Kania J, Burnat G, Hahn EG, Konturek SJ. Prostaglandins as mediators of COX-2 derived carcinogenesis in gastrointestinal tract. J Physiol Pharmacol 56 Suppl 5: 57–73, 2005 [PubMed] [Google Scholar]
- 155.Koppenol WH. The basic chemistry of nitrogen monoxide and peroxynitrite. Free Radic Biol Med 25: 385–391, 1998 [DOI] [PubMed] [Google Scholar]
- 156.Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci 49: 1433–1437, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol 9: 405–410, 2009 [DOI] [PubMed] [Google Scholar]
- 159.Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med 194: 1207–1218, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol 201: 28–36, 2003 [DOI] [PubMed] [Google Scholar]
- 161.Kruidenier L, Kuiper I, van DW, Marklund SL, van Hogezand RA, Lamers CB, Verspaget HW. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J Pathol 201: 7–16, 2003 [DOI] [PubMed] [Google Scholar]
- 162.Kuhn H, Heydeck D, Hugou I, Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J Clin Invest 99: 888–893, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59: 612–619, 1986 [DOI] [PubMed] [Google Scholar]
- 164.Kulkarni AC, Kuppusamy P, Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal 9: 1717–1730, 2007 [DOI] [PubMed] [Google Scholar]
- 165.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J 26: 4457–4466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.LeBlanc JG, del CS, Miyoshi A, Azevedo V, Sesma F, Langella P, Bermudez-Humaran LG, Watterlot L, Perdigon G, de Moreno LA. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn's disease in mice. J Biotechnol 151: 287–293, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Lechner S, Muller-Ladner U, Schlottmann K, Jung B, McClelland M, Ruschoff J, Welsh J, Scholmerich J, Kullmann F. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis 23: 1281–1288, 2002 [DOI] [PubMed] [Google Scholar]
- 168.Li SD, Mobarhan S. Association between body mass index and adenocarcinoma of the esophagus and gastric cardia. Nutr Rev 58: 54–56, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126: 1504–1517, 2004 [DOI] [PubMed] [Google Scholar]
- 170.Ma JJ, Hou DQ, Zhang QB, Korsten MA. Reversal of the gastric effects of nicotine by nitric oxide donor treatment. Digestion 63: 102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Ma L, Chow JY, Cho CH. Cigarette smoking delays ulcer healing: role of constitutive nitric oxide synthase in rat stomach. Am J Physiol Gastrointest Liver Physiol 276: G238–G248, 1999 [DOI] [PubMed] [Google Scholar]
- 172.Mahkonen A, Putaala H, Mustonen H, Rautonen N, Puolakkainen P. Lactobacillus acidophilus 74–2 and butyrate induce cyclooxygenase (COX)-1 expression in gastric cancer cells. Immunopharmacol Immunotoxicol 30: 503–518, 2008 [DOI] [PubMed] [Google Scholar]
- 173.Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer–recent mechanistic update. Mol Cell Biochem 253: 329–338, 2003 [DOI] [PubMed] [Google Scholar]
- 174.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 374: 1449–1461, 2009 [DOI] [PubMed] [Google Scholar]
- 175.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 49: 1359–1377, 2004 [DOI] [PubMed] [Google Scholar]
- 176.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306, 2011 [DOI] [PubMed] [Google Scholar]
- 177.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther 298: 737–743, 2001 [PubMed] [Google Scholar]
- 178.Maor I, Rainis T, Lanir A, Lavy A. Oxidative stress, inflammation and neutrophil superoxide release in patients with Crohn's disease: distinction between active and non-active disease. Dig Dis Sci 53: 2208–2214, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol 49: 265–290, 2009 [DOI] [PubMed] [Google Scholar]
- 180.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J 14: 1889–1900, 2000 [DOI] [PubMed] [Google Scholar]
- 181.Martin HM, Hancock JT, Salisbury V, Harrison R. Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun 72: 4933–4939, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med 2: 393–406, 2002 [DOI] [PubMed] [Google Scholar]
- 183.Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 48: 107–111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matthews GM, Butler RN. Cellular mucosal defense during Helicobacter pylori infection: a review of the role of glutathione and the oxidative pentose pathway. Helicobacter 10: 298–306, 2005 [DOI] [PubMed] [Google Scholar]
- 185.May JM. Is ascorbic acid an antioxidant for the plasma membrane? FASEB J 13: 995–1006, 1999 [DOI] [PubMed] [Google Scholar]
- 186.McCord JM. Radical explanations for old observations. Gastroenterology 92: 2026–2028, 1987 [DOI] [PubMed] [Google Scholar]
- 187.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 52: 711–760, 1983 [DOI] [PubMed] [Google Scholar]
- 188.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet 18: 159–163, 1998 [DOI] [PubMed] [Google Scholar]
- 189.Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17: 235–248, 1994 [DOI] [PubMed] [Google Scholar]
- 190.Miller MJ, Thompson JH, Zhang XJ, Sadowska-Krowicka H, Kakkis JL, Munshi UK, Sandoval M, Rossi JL, Eloby-Childress S, Beckman JS. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology 109: 1475–1483, 1995 [DOI] [PubMed] [Google Scholar]
- 191.Miller TA. Mechanisms of stress-related mucosal damage. Am J Med 83: 8–14, 1987 [DOI] [PubMed] [Google Scholar]
- 192.Mills EE. The modifying effect of beta-carotene on radiation and chemotherapy induced oral mucositis. Br J Cancer 57: 416–417, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mimuro H, Suzuki T, Nagai S, Rieder G, Suzuki M, Nagai T, Fujita Y, Nagamatsu K, Ishijima N, Koyasu S, Haas R, Sasakawa C. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2: 250–263, 2007 [DOI] [PubMed] [Google Scholar]
- 194.Minc E, de CP, Masson P, Thiery L, Dutertre S, mor-Gueret M, Jaulin C. The human copper-zinc superoxide dismutase gene (SOD1) proximal promoter is regulated by Sp1, Egr-1, and WT1 via non-canonical binding sites. J Biol Chem 274: 503–509, 1999 [DOI] [PubMed] [Google Scholar]
- 195.Minoura-Etoh J, Gotoh K, Sato R, Ogata M, Kaku N, Fujioka T, Nishizono A. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol 55: 905–911, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Monari M, Foschi J, Calabrese C, Liguori G, Di FG, Rizzello F, Gionchetti P, Trinchero A, Serrazanetti GP. Implications of antioxidant enzymes in human gastric neoplasms. Int J Mol Med 24: 693–700, 2009 [DOI] [PubMed] [Google Scholar]
- 197.Mooney C, Keenan J, Munster D, Wilson I, Allardyce R, Bagshaw P, Chapman B, Chadwick V. Neutrophil activation by Helicobacter pylori. Gut 32: 853–857, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mori M, Suzuki H, Suzuki M, Kai A, Miura S, Ishii H. Catalase and superoxide dismutase secreted from Helicobacter pylori. Helicobacter 2: 100–105, 1997 [DOI] [PubMed] [Google Scholar]
- 199.Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med 33: 323–336, 2002 [DOI] [PubMed] [Google Scholar]
- 200.Naito Y, Yoshikawa T, Ando T, Kishi A, Ueda S, Oyamada H, Kondo M. Changes in superoxide dismutase activity in the gastric mucosa of peptic ulcer patients. J Clin Gastroenterol 14 Suppl 1: S131–S134, 1992 [DOI] [PubMed] [Google Scholar]
- 201.Naito Y, Yoshikawa T, Fujii T, Boku Y, Yagi N, Dao S, Yoshida N, Kondo M, Matsui H, Ohtani-Fujita N, Sakai T. Monochloramine-induced cell growth inhibition and apoptosis in a rat gastric mucosal cell line. J Clin Gastroenterol 25: S179–S185, 1997 [DOI] [PubMed] [Google Scholar]
- 202.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44: 598–602, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nenoi M, Ichimura S, Mita K, Yukawa O, Cartwright IL. Regulation of the catalase gene promoter by Sp1, CCAAT-recognizing factors, and a WT1/Egr-related factor in hydrogen peroxide-resistant HP100 cells. Cancer Res 61: 5885–5894, 2001 [PubMed] [Google Scholar]
- 204.Nozik-Grayck E, Suliman HB, Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol 37: 2466–2471, 2005 [DOI] [PubMed] [Google Scholar]
- 205.Nurmi JT, Puolakkainen PA, Rautonen NE. Intron 1 retaining cyclooxygenase 1 splice variant is induced by osmotic stress in human intestinal epithelial cells. Prostaglandins Leukot Essent Fatty Acids 73: 343–350, 2005 [DOI] [PubMed] [Google Scholar]
- 206.O'Connor HJ, Schorah CJ, Habibzedah N, Axon AT, Cockel R. Vitamin C in the human stomach: relation to gastric pH, gastroduodenal disease, and possible sources. Gut 30: 436–442, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.O'Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis 16: 1411–1420, 2010 [DOI] [PubMed] [Google Scholar]
- 208.O'Donnell VB, Azzi A. High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid-metabolizing enzyme. Biochem J 318: 805–812, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.O'Hara AM, Bhattacharya A, Bai J, Mifflin RC, Smith MF, Jr, Ryan KA, Scott KG-E, Naganuma M, Casola A, Izumi T, Mitra S, Ernst PB, Crowe SE. Interleukin-8 induction by Helicobacter pylori in human gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J Immunol 177: 7990–7999, 2006 [DOI] [PubMed] [Google Scholar]
- 210.Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol 12: 4281–4295, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Oh TY, Yeo M, Han SU, Cho YK, Kim YB, Chung MH, Kim YS, Cho SW, Hahm KB. Synergism of Helicobacter pylori infection and stress on the augmentation of gastric mucosal damage and its prevention with alpha-tocopherol. Free Radic Biol Med 38: 1447–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 212.Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, Yoshida T, Nakamura H, Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J 20: 121–123, 2006 [DOI] [PubMed] [Google Scholar]
- 213.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001 [DOI] [PubMed] [Google Scholar]
- 214.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279: L1029–L1037, 2000 [DOI] [PubMed] [Google Scholar]
- 215.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol 163: 2555–2563, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22: 18–35, 2003 [DOI] [PubMed] [Google Scholar]
- 217.Palmieri VO, Grattagliano I, Palasciano G. Ethanol induces secretion of oxidized proteins by pancreatic acinar cells. Cell Biol Toxicol 23: 459–464, 2007 [DOI] [PubMed] [Google Scholar]
- 218.Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res 9: 433–440, 2003 [PubMed] [Google Scholar]
- 219.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 82: 9–15, 1982 [PubMed] [Google Scholar]
- 220.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol Gastrointest Liver Physiol 250: G749–G753, 1986 [DOI] [PubMed] [Google Scholar]
- 221.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40: 297–301, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rummele P, Farkas S, Scholmerich J, Fleck M, Rogler G, Herfarth H. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol 140: 547–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 33: 311–322, 2002 [DOI] [PubMed] [Google Scholar]
- 224.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev 90: 831–858, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pelucchi C, Gallus S, Garavello W, Bosetti C, La VC. Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev 17: 340–344, 2008 [DOI] [PubMed] [Google Scholar]
- 226.Peng YC, Hsu CL, Tung CF, Chou WK, Huang LR, Hung DZ, Hu WH, Yang DY. Chemiluminescence assay of mucosal reactive oxygen species in gastric cancer, ulcer and antral mucosa. Hepatogastroenterology 55: 770–773, 2008 [PubMed] [Google Scholar]
- 227.Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol 2: 120–123, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol 278: L312–L319, 2000 [DOI] [PubMed] [Google Scholar]
- 229.Peura DA. Prevention of nonsteroidal anti-inflammatory drug-associated gastrointestinal symptoms and ulcer complications. Am J Med 117 Suppl 5A: 63S–71S, 2004 [DOI] [PubMed] [Google Scholar]
- 230.Phull PS, Green CJ, Jacyna MR. A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol 7: 265–274, 1995 [PubMed] [Google Scholar]
- 231.Piazuelo E, Cebrian C, Escartin A, Jimenez P, Soteras F, Ortego J, Lanas A. Superoxide dismutase prevents development of adenocarcinoma in a rat model of Barrett's esophagus. World J Gastroenterol 11: 7436–7443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pietta PG. Flavonoids as antioxidants. J Nat Prod 63: 1035–1042, 2000 [DOI] [PubMed] [Google Scholar]
- 233.Pines A, Bivi N, Romanello M, Damante G, Kelley MR, Adamson ED, D'Andrea P, Quadrifoglio F, Moro L, Tell G. Cross-regulation between Egr-1 and APE/Ref-1 during early response to oxidative stress in the human osteoblastic HOBIT cell line: evidence for an autoregulatory loop. Free Radic Res 39: 269–281, 2005 [DOI] [PubMed] [Google Scholar]
- 234.Pinto AF, Rodrigues JV, Teixeira M. Reductive elimination of superoxide: structure and mechanism of superoxide reductases. Biochim Biophys Acta 1804: 285–297, 2010 [DOI] [PubMed] [Google Scholar]
- 235.Podrez EA, bu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med 28: 1717–1725, 2000 [DOI] [PubMed] [Google Scholar]
- 236.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab 20: 332–340, 2009 [DOI] [PubMed] [Google Scholar]
- 237.Poyton RO, Castello PR, Ball KA, Woo DK, Pan N. Mitochondria and hypoxic signaling: a new view. Ann NY Acad Sci 1177: 48–56, 2009 [DOI] [PubMed] [Google Scholar]
- 238.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001 [DOI] [PubMed] [Google Scholar]
- 239.Rachmilewitz D, Karmeli F, Eliakim R, Stalnikowicz R, Ackerman Z, Amir G, Stamler JS. Enhanced gastric nitric oxide synthase activity in duodenal ulcer patients. Gut 35: 1394–1397, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, Xavier RJ, Podolsky DK. Peroxynitrite-induced rat colitis–a new model of colonic inflammation. Gastroenterology 105: 1681–1688, 1993 [DOI] [PubMed] [Google Scholar]
- 241.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem 42: 3–27, 2007 [DOI] [PubMed] [Google Scholar]
- 242.Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci 95: 5061–5065, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 55: 207–216, 2007 [DOI] [PubMed] [Google Scholar]
- 244.Rao R, Baker RD, Baker SS. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol 57: 685–695, 1999 [DOI] [PubMed] [Google Scholar]
- 245.Reddy PG, Bhuyan DK, Bhuyan KC. Lens-specific regulation of the thioredoxin-1 gene, but not thioredoxin-2, upon in vivo photochemical oxidative stress in the Emory mouse. Biochem Biophys Res Commun 265: 345–349, 1999 [DOI] [PubMed] [Google Scholar]
- 246.Redmond SM, Joncourt F, Buser K, Ziemiecki A, Altermatt HJ, Fey M, Margison G, Cerny T. Assessment of P-glycoprotein, glutathione-based detoxifying enzymes and O6-alkylguanine-DNA alkyltransferase as potential indicators of constitutive drug resistance in human colorectal tumors. Cancer Res 51: 2092–2097, 1991 [PubMed] [Google Scholar]
- 247.Reiter RJ, Tan DX, Manchester LC, El-Sawi MR. Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann NY Acad Sci 959: 238–250, 2002 [DOI] [PubMed] [Google Scholar]
- 248.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 275: 26765–26771, 2000 [DOI] [PubMed] [Google Scholar]
- 249.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65: 27–33, 1994 [DOI] [PubMed] [Google Scholar]
- 250.Roe I, Nam S, Kim J, Shin J, Bang W, Yang M. Association of the myeloperoxidase -463G–>A polymorphism with development of atrophy in Helicobacter pylori-infected gastritis. Am J Gastroenterol 97: 1629–1634, 2002 [DOI] [PubMed] [Google Scholar]
- 251.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract 204: 511–524, 2008 [DOI] [PubMed] [Google Scholar]
- 252.Rustgi AK. Models of esophageal carcinogenesis. Semin Oncol 33: S57–S58, 2006 [DOI] [PubMed] [Google Scholar]
- 253.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 254.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med 11: 1306–1313, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237: 1171–1176, 1987 [DOI] [PubMed] [Google Scholar]
- 256.Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J Clin Biochem Nutr 40: 1–12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sasayama Y, Kawano S, Tsuji S, Fusamoto H, Kamada T, Fukui H, Yoneda S, Okishio T. Relationship between interleukin-8 levels and myeloperoxidase activity in human gastric mucosa. J Gastroenterol Hepatol 12: 104–108, 1997 [DOI] [PubMed] [Google Scholar]
- 258.Schalk I, Zeng K, Wu SK, Stura EA, Matteson J, Huang M, Tandon A, Wilson IA, Balch WE. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature 381: 42–48, 1996 [DOI] [PubMed] [Google Scholar]
- 259.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol 14: 473–488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schonhoff CM, Daou MC, Jones SN, Schiffer CA, Ross AH. Nitric oxide-mediated inhibition of Hdm2-p53 binding. Biochemistry 41: 13570–13574, 2002 [DOI] [PubMed] [Google Scholar]
- 261.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta 1763: 1755–1766, 2006 [DOI] [PubMed] [Google Scholar]
- 262.Schweiger D, Furstenberger G, Krieg P. Inducible expression of 15-lipoxygenase-2 and 8-lipoxygenase inhibits cell growth via common signaling pathways. J Lipid Res 48: 553–564, 2007 [DOI] [PubMed] [Google Scholar]
- 263.Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA 96: 14559–14564, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106: 1067–1075, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci 47: 1266–1278, 2002 [DOI] [PubMed] [Google Scholar]
- 265a.Seril DN, Liao J, West AB, Yang GY. High-iron diet: foe or feat in ulcerative colitis and ulcerative colitis-associated carcinogenesis. J Clin Gastroenterol 40: 391–397, 2006 [DOI] [PubMed] [Google Scholar]
- 266.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387–437, 2004 [DOI] [PubMed] [Google Scholar]
- 267.Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111: 871–885, 1996 [DOI] [PubMed] [Google Scholar]
- 268.Skulachev VP. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett 423: 275–280, 1998 [DOI] [PubMed] [Google Scholar]
- 269.Smoot DT, Elliott TB, Verspaget HW, Jones D, Allen CR, Vernon KG, Bremner T, Kidd LC, Kim KS, Groupman JD, Ashktorab H. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis 21: 2091–2095, 2000 [DOI] [PubMed] [Google Scholar]
- 270.Song S, Guha S, Liu K, Buttar NS, Bresalier RS. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett's oesophagus and oesophageal adenocarcinoma. Gut 56: 1512–1521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci USA 100: 1238–1243, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Stanghellini V. Relationship between upper gastrointestinal symptoms and lifestyle, psychosocial factors and comorbidity in the general population: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol Suppl 231: 29–37, 1999 [PubMed] [Google Scholar]
- 273.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18: 321–336, 1995 [DOI] [PubMed] [Google Scholar]
- 274.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta 1411: 217–230, 1999 [DOI] [PubMed] [Google Scholar]
- 275.Sung JJ. Management of nonsteroidal anti-inflammatory drug-related peptic ulcer bleeding. Am J Med 110: 29S–32S, 2001 [DOI] [PubMed] [Google Scholar]
- 276.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev 217: 186–205, 2007 [DOI] [PubMed] [Google Scholar]
- 277.Takahashi Y, Zhu H, Yoshimoto T. Essential roles of lipoxygenases in LDL oxidation and development of atherosclerosis. Antioxid Redox Signal 7: 425–431, 2005 [DOI] [PubMed] [Google Scholar]
- 278.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept 9: 137–159, 2000 [DOI] [PubMed] [Google Scholar]
- 279.Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res 33: 1220–1230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Taylor CT, Moncada S. Nitric oxide, cytochrome c oxidase, and the cellular response to hypoxia. Arterioscler Thromb Vasc Biol 30: 643–647, 2010 [DOI] [PubMed] [Google Scholar]
- 281.Taylor WI, Achanzar D. Catalase test as an aid to the identification of Enterobacteriaceae. Appl Microbiol 24: 58–61, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Terry P, Lagergren J, Ye W, Nyren O, Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. Int J Cancer 87: 750–754, 2000 [PubMed] [Google Scholar]
- 284.Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis 15: 134–140, 2009 [DOI] [PubMed] [Google Scholar]
- 285.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 138: 2101–2114, 2010 [DOI] [PubMed] [Google Scholar]
- 286.Teshima S, Tsunawaki S, Rokutan K. Helicobacter pylori lipopolysaccharide enhances the expression of NADPH oxidase components in cultured guinea pig gastric mucosal cells. FEBS Lett 452: 243–246, 1999 [DOI] [PubMed] [Google Scholar]
- 287.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121: 667–670, 2005 [DOI] [PubMed] [Google Scholar]
- 288.Toppo S, Vanin S, Bosello V, Tosatto SC. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid Redox Signal 10: 1501–1514, 2008 [DOI] [PubMed] [Google Scholar]
- 289.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43: 4–15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 29: 2727–2737, 2009 [PubMed] [Google Scholar]
- 291.Tunc T, Uysal B, Atabek C, Kesik V, Caliskan B, Oztas E, Ersoz N, Oter S, Guven A. Erdosteine and ebselen as useful agents in intestinal ischemia/reperfusion injury. J Surg Res 155: 210–216, 2009 [DOI] [PubMed] [Google Scholar]
- 292.Turkan H, Aydin A, Sayal A. Effect of volatile anesthetics on oxidative stress due to occupational exposure. World J Surg 29: 540–542, 2005 [DOI] [PubMed] [Google Scholar]
- 293.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 140: 1807–1816, 2011 [DOI] [PubMed] [Google Scholar]
- 294.Vaananen PM, Meddings JB, Wallace JL. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol Gastrointest Liver Physiol 261: G470–G475, 1991 [DOI] [PubMed] [Google Scholar]
- 295.Van Acker SA, Koymans LM, Bast A. Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radic Biol Med 15: 311–328, 1993 [DOI] [PubMed] [Google Scholar]
- 296.Van der HF, Nolte IM, Kleibeuker JH, Wijmenga C, Dijkstra G, Weersma RK. Differences in genetic background between active smokers, passive smokers, and non-smokers with Crohn's disease. Am J Gastroenterol 105: 1165–1172, 2010 [DOI] [PubMed] [Google Scholar]
- 297.Van CW, Inze D, Van MM. The regulation and function of tobacco superoxide dismutases. Free Radic Biol Med 23: 515–520, 1997 [DOI] [PubMed] [Google Scholar]
- 298.Van DV, Tuinstra TJ, Bast A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem Pharmacol 38: 2807–2818, 1989 [DOI] [PubMed] [Google Scholar]
- 299.Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol 6: 945–952, 2005 [DOI] [PubMed] [Google Scholar]
- 300.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192: 227–236, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Verhulst ML, Hopman WP, Peters WH, Jansen JB. Effects of Helicobacter pylori infection on endocrine and exocrine mucosal functions in the upper gastrointestinal tract. Scand J Gastroenterol Suppl 21–31, 2000 [PubMed] [Google Scholar]
- 302.Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res 182: 1–17, 2010 [DOI] [PubMed] [Google Scholar]
- 303.Victoni T, Coelho FR, Soares AL, de FA, Secher T, Guabiraba R, Erard F, de Oliveira-Filho RM, Vargaftig BB, Lauvaux G, Kamal MA, Ryffel B, Moser R, Tavares-de-Lima W. Local and remote tissue injury upon intestinal ischemia and reperfusion depends on the TLR/MyD88 signaling pathway. Med Microbiol Immunol 199: 35–42, 2010 [DOI] [PubMed] [Google Scholar]
- 304.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA 91: 2607–2610, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Vilhardt F, van DB. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J 23: 739–748, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wallace JL, Ma L. Inflammatory mediators in gastrointestinal defense and injury. Exp Biol Med 226: 1003–1015, 2001 [DOI] [PubMed] [Google Scholar]
- 307.Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, Granger DN, Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol Gastrointest Liver Physiol 265: G993–G998, 1993 [DOI] [PubMed] [Google Scholar]
- 308.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119: 512–520, 2000 [DOI] [PubMed] [Google Scholar]
- 309.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology 128: 1445–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 310.Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol 281: G586–G594, 2001 [DOI] [PubMed] [Google Scholar]
- 311.Wang X, Michael D, de MG, Oren M. p53 Activation by nitric oxide involves down-regulation of Mdm2. J Biol Chem 277: 15697–15702, 2002 [DOI] [PubMed] [Google Scholar]
- 312.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets 11: 1551–1570, 2010 [DOI] [PubMed] [Google Scholar]
- 313.Wetscher GJ, Hinder RA, Klingler P, Gadenstatter M, Perdikis G, Hinder PR. Reflux esophagitis in humans is a free radical event. Dis Esophagus 10: 29–32, 1997 [DOI] [PubMed] [Google Scholar]
- 314.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, Adrian TE, Hinder RA. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci 40: 1297–1305, 1995 [DOI] [PubMed] [Google Scholar]
- 315.Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology 80: 94–98, 1981 [PubMed] [Google Scholar]
- 316.Wild CP, Hardie LJ. Reflux, Barrett's oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer 3: 676–684, 2003 [DOI] [PubMed] [Google Scholar]
- 317.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res 58: 2929–2934, 1998 [PubMed] [Google Scholar]
- 318.Wingler K, Muller C, Schmehl K, Florian S, Brigelius-Flohe R. Gastrointestinal glutathione peroxidase prevents transport of lipid hydroperoxides in CaCo-2 cells. Gastroenterology 119: 420–430, 2000 [DOI] [PubMed] [Google Scholar]
- 319.Witschi H. Carcinogenic activity of cigarette smoke gas phase and its modulation by beta-carotene and N-acetylcysteine. Toxicol Sci 84: 81–87, 2005 [DOI] [PubMed] [Google Scholar]
- 320.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340: 1888–1899, 1999 [DOI] [PubMed] [Google Scholar]
- 321.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003 [DOI] [PubMed] [Google Scholar]
- 322.Woods JW, Coffey MJ, Brock TG, Singer II, Peters-Golden M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J Clin Invest 95: 2035–2046, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Woods JW, Evans JF, Ethier D, Scott S, Vickers PJ, Hearn L, Heibein JA, Charleson S, Singer II. 5-Lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med 178: 1935–1946, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis 29: 141–154, 2009 [DOI] [PubMed] [Google Scholar]
- 325.Wu SL, Chen JC, Li CC, Lo HY, Ho TY, Hsiang CY. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J Pharmacol Exp Ther 330: 370–376, 2009 [DOI] [PubMed] [Google Scholar]
- 326.Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA 106: 20515–20519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yamamoto K, Niki E. Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim Biophys Acta 958: 19–23, 1988 [DOI] [PubMed] [Google Scholar]
- 328.Yamashita N, Murata M, Inoue S, Burkitt MJ, Milne L, Kawanishi S. Alpha-tocopherol induces oxidative damage to DNA in the presence of copper(II) ions. Chem Res Toxicol 11: 855–862, 1998 [DOI] [PubMed] [Google Scholar]
- 329.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38: 689–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, Jia CH, Wei XX, Feng JL, Zhao L, Wang LS. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res 41: 288–294, 2010 [DOI] [PubMed] [Google Scholar]
- 331.Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba l.), and black tea (Camellia sinensis) extracts. J Agric Food Chem 48: 5030–5034, 2000 [DOI] [PubMed] [Google Scholar]
- 332.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Glass CK, Sigal E, Witztum JL, Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci USA 87: 6959–6963, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zamocky M, Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog Biophys Mol Biol 72: 19–66, 1999 [DOI] [PubMed] [Google Scholar]
- 334.Zapolska-Downar D, Kosmider A, Naruszewicz M. Trans fatty acids induce apoptosis in human endothelial cells. J Physiol Pharmacol 56: 611–625, 2005 [PubMed] [Google Scholar]
- 335.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23: 8137–8151, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang P, Omaye ST. Beta-carotene and protein oxidation: effects of ascorbic acid and alpha-tocopherol. Toxicology 146: 37–47, 2000 [DOI] [PubMed] [Google Scholar]
- 337.Zhang QB, Nakshabendi IM, Mokhashi MS, Dawodu JB, Gemmell CG, Russell RI. Association of cytotoxin production and neutrophil activation by strains of Helicobacter pylori isolated from patients with peptic ulceration and chronic gastritis. Gut 38: 841–845, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhu H, Yang L, Zhou B, Yu R, Tang N, Wang B. Myeloperoxidase G-463A polymorphism and the risk of gastric cancer: a case-control study. Carcinogenesis 27: 2491–2496, 2006 [DOI] [PubMed] [Google Scholar]