Abstract
To date, only a single Friend virus (FV) peptide recognized by CD4+ T cells in FV-infected mice of the resistant H-2b haplotype has been described. To more thoroughly examine the repertoire of CD4+ T cell responses in H-2b mice infected with this retrovirus, 18mer peptides spanning the FV gag, pol, and env coding regions with 11mer overlaps were synthesized. The peptides were then used to stimulate whole splenocytes and purified CD4+ T cells from FV-infected mice in an IFN ELISPOT assay. Nine new CD4+ T cell epitopes were identified, 3 encoded by gag, 1 by pol, and 5 by env. The high resistance of H-2b mice could be related to this very broad CD4+ T cell response against multiple peptides during FV infection.
Background
Friend virus (FV) is naturally occurring mouse retrovirus named after Charlotte Friend, who discovered the virus in 1957 (Friend, 1957). Studies with FV have been instrumental in discovering many retroviral resistance genes that function through both immunological and non-immunological mechanisms (Chesebro et al., 1990; Hasenkrug and Chesebro, 1997; Hasenkrug and Dittmer, 2007; Kabat, 1989; Persons et al., 1999; Santiago et al., 2008) (Miyazawa et al., 2008). The major histocompatibility complex (H-2 in the mouse) controls many of the adaptive immune responses necessary to resolve acute FV infections, and mice carrying an H-2b haplotype display the strongest and fastest immune responses described (Chesebro et al., 1990; Hasenkrug and Chesebro, 1997; Miyazawa et al., 2008). It is known that CD4+ T cells play a critical role in both natural and induced immunological resistance to FV infection (Hasenkrug et al., 1998; Hasenkrug and Dittmer, 2000; Miyazawa and Fujisawa, 1997; Miyazawa et al., 1992; Nair et al., 2011; Young et al., 2012) (Nair et al., 2010) (Hasenkrug, 1999; Pike et al., 2009) but surprisingly little is know about the viral epitopes recognized by these cells. A total of only three CD4+ T cell epitopes from FV have been described (Iwashiro et al., 1993; Shimizu et al., 1994; Sugahara et al., 2004) and only one for the resistant H-2b haplotype (Iwashiro et al., 1993; Shimizu et al., 1994). In this study we analyze a large repertoire of peptides spanning the coding regions of FV gag, pol, and env and provide evidence for nine new CD4+ T cell epitopes recognized in the context of the resistant H-2b haplotype.
Results and Discussion
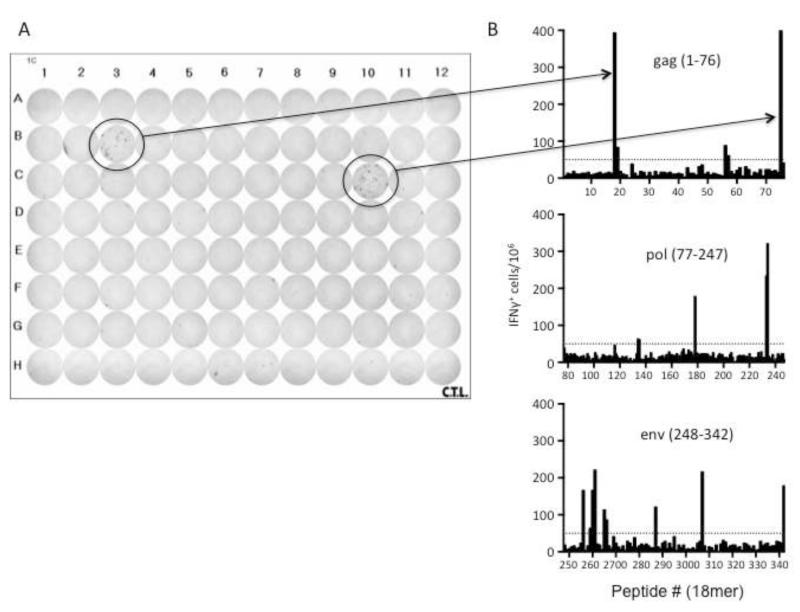
To identify MHC class II-restricted epitopes during FV infection we synthesized three hundred and forty two 18mer peptides with 11mer overlaps beginning at the Met start site of gag and covering the coding regions through env. These peptides were then used to re-stimulate splenocytes harvested from two separate FV-infected mice at 12 days post-infection. A representative plate with two positive gag peptides (#18, #75) is shown in Figure 1A along with bar graphs showing initial results from all of the peptides tested (Figure 1B). All peptides were then tested again in a second experiment with two individual mice. Peptides were considered positive if they elicited greater than twice the number of spots in the negative control wells, elicited a minimum of 50 spots/1 × 106 cells and produced these responses in at least half the infected mice and none of the naïve mice tested in two separate experiments (Dow et al., 2008). None of the positive peptides gave more than 10 spots per 106 cells when reacted with cells from naive mice (data not shown). Positive peptides were then tested in a third experiment with splenocytes from two additional FV-infected mice and the compiled results from all six mice from the three experiments described are presented in Table 1. The number of responding mice out of the six tested as well as the mean number of spots per 106 cells from all six mice is shown for each of the positive peptides.
Figure 1. IFNγ ELISPOT assay.
A) A representative image of an IFN ELISPOT plate highlighting two positive wells (#18, 75) and corresponding bars in B) depicting the mean number of IFN + cells per 106 cells responding to individual 18mer peptides of the FV gag, pol and env proteins. Each bar represents the mean of two mice from one of three experiments. The dashed line denotes the cutoff of 50 IFN + cells per 106 cells.
Table 1.
Elispot results for positive peptides.
|
|
Whole Splenocy tes |
CD4+ T cells |
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|
| Peptide # (18mer) |
Coding Gene |
18mer Peptide Sequence |
number of positive/t otal |
mean spots/1 06 cells |
number of positive/t otal |
mean spots/1 06 cells |
Predicted Core Peptidea |
IED B scor e |
# of mice reacti ve with core |
|
18 19 |
gag |
PPLSTPPQSSLYPALTS
P |
6/6 |
364 |
2/2 |
429 |
QSSLYPALTSPLN TK |
3.94 |
0/4 |
| gag |
QSSLYPALTSPLNTK PRP |
1/6 |
78 |
1/2 |
128 |
||||
|
56 57 |
gag |
DPGQETNVAMSFIW QSAP |
4/6 |
111 |
2/2 |
195 |
AMSFIWQSAPDI GRK |
7.81 |
1/4 |
| gag |
VAMSFIWQSAPDIG RKLE |
1/6 |
55 |
1/2 |
74 |
||||
|
75
|
gag |
KKPRGPRGPRPQASL LTL |
4/6 |
538 |
2/2 |
107 |
KKPRGPRGPRPQ ASLd |
4.23 |
not done |
|
233/234
|
pol |
QALYLVQHEVWRPL AAAY |
6/6 |
198 |
2/2 |
248 |
LVQHEVWRPLAA AY |
8.25 |
3/4 |
| pol |
HEVWRPLAAAYQE QLDRP |
6/6 |
228 |
2/2 |
233 |
||||
|
256
|
env |
ETVWAISGNHPLWT WWPD |
5/6 |
180 |
2/2 |
269 |
ETVWAISGNHPL WTW |
6.16 |
4/4 |
|
259/260/
261 |
env |
DLCMLALSGPPHWG LEYR |
3/6 |
97 |
1/2 |
223 |
GLEYRAPYSSPPG PP |
0.55 |
2/4 |
| env |
SGPPHWGLEYRAPY SSPP |
3/6 |
105 |
2/2 |
109 |
||||
| env |
LEYRAPYSSPPGPPC CSG |
4/6 |
187 |
2/2 |
174 |
||||
| 265 266e |
env |
DCDEPLTSLTPRCNTA WN |
2/6 |
86 |
2/2 |
107 |
LTSLTPRCNTAW NRLe |
24.3 |
1/4 |
| env |
SLTPRCNTAWNRLK LDQV |
3/6 |
89 |
2/2 |
117 |
||||
|
287
b
|
env |
NPVLADQLSFPLPNPL PK |
3/6 |
112 |
2/2 |
107 |
PRVPIGPNPVLAD QL LADQLSFPLPNPL PK |
1.44 1.45 |
0/4 1/4 |
| 307 b | env |
GKGSYYLVAPAGTM WACN |
6/6 |
165 |
2/2 |
176 |
KGSYYLVAPAGT MWA GSYYLVAPAGTM WAC |
1.99 2.28 |
1/4 2/4 |
|
342
|
env |
LVLTQQYHQLKPLEYE PQ |
4/6 |
174 |
2/2 |
253 |
VLTQQYHQLKPL EYE |
16.2 7 |
3/4 |
| IAb 122- 141c |
env |
DEPLTSLTPRCNTAW NRLKL (purified and optimized positive control) |
6/6 |
1118 |
2/2 |
302 |
|
|
|
Bold=New epitopes
Italics=shared sequences in consecutive reactive sequences
Core peptides were predicted using amino acid sequences of peptides and flanking regions of those peptides that induced IFNy responses in at least one mouse. The core peptide was predicted by the best IAb binder peptide, by IEDB score, as described in the methods.
Analyses of peptides 287 and 307 identified two peptides each, with very low IEDB scores. Both peptides were synthesized and tested.
The IAb 122-141 peptide has been described previously and was included as a postive control. Unlike the other peptides, which are crude preparations, IAb 122-141 has been optimized for reactivity and was HPLC-purified.
The core peptide identified for peptide 75 was not made as the core sequence lacks only the three final amino acids.
The core peptide for peptides 265 and 266 match that of the I-Ab 122-141 positive control peptide
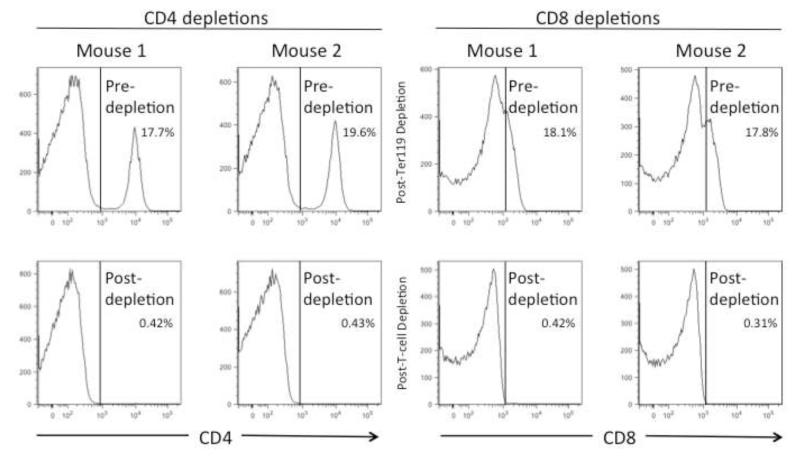
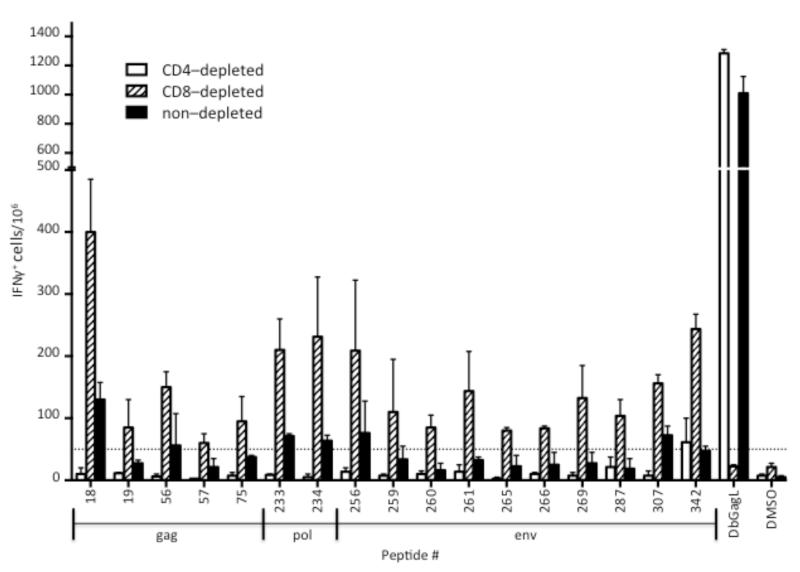
To determine whether the IFN-producing cells were CD4+ or CD8+ T cells, spleen cells were depleted of one subset or the other using microbead columns to produce “untouched” or negatively-selected cells. The pre-depleted cells and the magnetically depleted fractions were analyzed by flow cytometry and the results are shown in Figure 2. The CD8 staining for analysis was not very bright but nonetheless it was obvious that the magentic bead columns had depleted the appropriate subsets from the splenocytes. Furthermore, the efficacy and specificity of the CD8+ T cell depletion is evident in the loss of reactivity to the immunodominant CD8+ T cell epitope, DbGagL (Chen et al., 1996), in the CD8-depleted fraction but not the CD4-depleted fraction (Fig. 3). Interestingly, when the spleen cells were depleted of CD4+ T cells, reactivity to all but one test peptide was lost (Figure 3, open bars). In contrast, splenocytes depleted of CD8+ T cells and enriched for CD4+ T cells showed increased reactivity against the positive peptides relative to the non-depleted fractions (Fig. 3, striped bars). Peptides 265 and 266 contain most or all of the core sequence from the positive control IAb 122-141 (Table 1) and so serve as positive controls for comparison. The IAb 122-141 control peptide elicits very strong responses (Fig. 4 and Table 1) but it has been sequence optimized (Shimizu et al., 1994) and was HPLC-purified. Thus, 265 and 266 are probably better controls for comparison. These results indicated that all of the peptides reactive with the non-depleted spleen cells appeared to be CD4+ T cell epitopes and no new CD8+ T cell epitopes were found. This result likely reflects technical aspects of the experimental design more than the physiological situation in vivo. The structure of the MHC class II peptide-binding groove is relatively open on the ends and able to accommodate and bind to long peptides such as the 18mers synthesized for this study (Castellino et al., 1997). In contrast, MHC class I molecules such as H-2Kb and H-2Db are more closed on the ends and generally require much shorter peptides in the range of 8 or 9mers (Bjorkman et al., 1987; Rotzschke et al., 1990; Theodossis et al., 2010). With regard to MHC class I molecules, the synthesis of all possible 8 and 9mers would be thousands of peptides, which is not currently feasible. Furthermore, previous attempts to predict MHC class I epitopes in FV using algorithms have not been successful (data not shown). That said, the identification of nine new class II-restricted peptides provides an important tool set for future studies of CD4+ T cell responses, including the creation of new tetramers.
Figure 2. CD4 and CD8 cell depletions.
Spleen cells from FV-infected mice at 12 dpi were depleted of Ter119+ erythroblasts and either CD4+ cells (left) or CD8+ cells (right). The pre- and post-depletion fractions were then stained with CD4 and CD8 fluorescent antibodies and analyzed by flow cytometry using a live lymphocyte gate as determined by forward and side scatter. Fractions of cells prior to depletions are shown on the top and fractions after depletion are shown on the bottom. Percentages of CD4+ and CD8+ cells are given.
Figure 3. Reactivity of CD4- and CD8-depleted splenocytes against positive peptides.
Splenocytes from FV-infected mice at 12 dpi were depleted of either CD4+ cells (open bars), CD8+ cells (striped bars), or neither cell type (solid bars) as described in the methods. All samples were depleted of Ter119+ erythroid cells. The bar graph depicts the mean number (± s.d.) of IFN-producing cells per 106 spleen cells responding to the indicated 18mer peptide from two FV infected mice. The dashed line represents the threshold of 50 spots per 106 spleen cells. DbGagL is the positive control CD8+ T cell epitope. DMSO is the diluent control with no peptide.
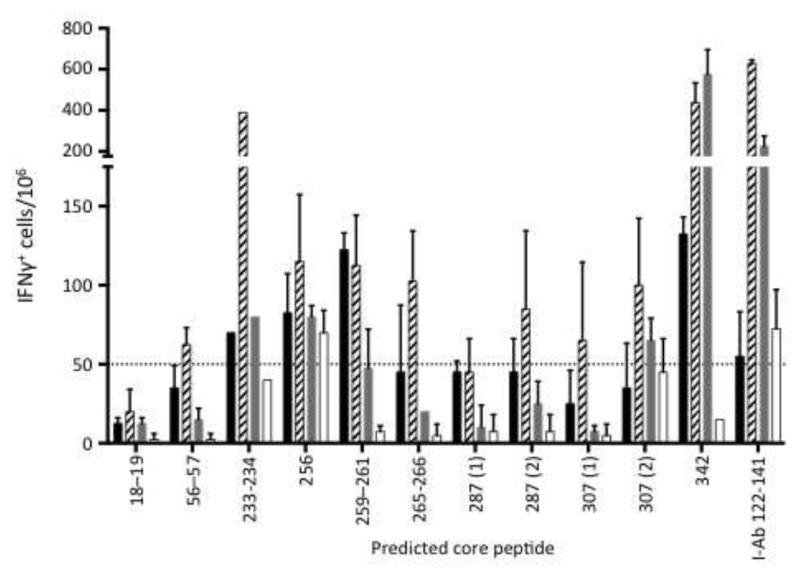
Figure 4. Reactivity against predicted core peptides.
The bar graph depicts the number of IFN +-spot forming cells per 106 spleen cells responding to the indicated core peptide from four individual mice infected with FV at 12 dpi (core sequences are shown in Table 1). The different shaded bars represent individual mice. Values represent the mean (± s.d.) of duplicate samples. The core peptides were designed using IEDB scores for predictions of binding to IAb for amino acid sequences surrounding the peptides identified in Table 1. Predictions for two of the sequences, 287 and 307, both resulted in two core peptides with very low IEDB scores so both were synthesized and tested. The I-Ab 122-141 peptide is the previously identified positive control peptide. The dashed line denotes the cutoff of 50 IFN + cells per 106 cells.
All of the positive peptides were analyzed for predicted binding to the H-2 IAb molecule, which is the only MHC class II molecule expressed in mice with the H-2b haplotype (Mathis et al., 1983; Ozato et al., 1980). The MHCII binding predictions were made using the IEDB analysis resource consensus tool (Wang et al., 2008; Wang et al., 2010), and the best binding peptides from each 18mer are shown in Table 1 along with the binding scores. In several cases two or three consecutive peptides were positive suggesting that the shared sequence in the overlap contained the reactive peptide. In each of those cases the lowest scored peptide (best H-2IAb binder) was in a shared sequence. The predicted core sequences were then synthesized and tested for their stimulatory activity in an IFN ELISPOT assay. Four mice were tested and demonstrated considerable variation in their reactivity to the predicted core peptides (Figure 4). Mouse number 4 (Figure 4, open bars) only reacted to the 256 core peptide and the positive control, whereas mouse number two (striped bars) reacted to nine of the core peptides. Only one of the core peptides, #256, elicited responses in all four of the mice tested. The 342 core was very strong in three of the four mice but the 18-19, 56-57, 287, and 307 (1) cores were poorly reactive. The reactivity of the core peptides did not correlate with their IEDB scores. In fact, the control peptide, to which all four mice reacted, had one of the lowest predicted binding to IAb (Table 1). All four of these mice reacted strongly to the purified MHC class I Db GagL peptide eliciting 988 – 2,572 IFN + cells per 106 cells (data not shown).
Conclusions
This study identified nine new CD4+ T cell epitopes encoded by FV and restricted by the H-2b haplotype, most likely by IAb molecules. These included three peptides encoded by gag (18, 56, 75), one by pol (233/234), and five by env (256, 259-261, 287,307, 342). All of these peptides elicited ELISPOT responses as strong or stronger than peptides 265 and 266, which contain most or all of the core of the previously described IAb 122-141 peptide (Shimizu et al., 1994). This unexpectedly broad response could be one reason why mice carrying an H-2b haplotype are so resistant to FV-induced erythroleukemia. The core sequences that gave strong reactivity may be used to create new MHC class II tetramers for studying CD4+ T cell responses to FV infection and immunization.
Methods
Mice
(A.BY × B10)F1 female mice, 3-5 months of age, were used for all experiments. All mice were treated in accordance with the regulations and guidelines of the Animal Care and Use Committee of RML, NIAID, NIH.
Virus
Mice were inoculated with a Friend virus swarm comprised of replication competent, B-tropic Friend murine leukemia virus (F-MuLV) and a polycythemia-inducing, spleen focus-forming virus. Infections were by intravenous inoculation with 6,000 spleen focus-forming units (sffu) of FV in 0.5mL of a phosphate-buffered balance salt solution. Mice were euthanized and spleens harvested at 12 days post-infection.
Peptides
Peptides were synthesized using Sigma-Aldrich PEPscreen® based on the sequence of the FB29 strain of FV (Perryman et al., 1991) NCBI GenBank Accession # Z11128. Peptides were 18 amino acids in length with an eleven amino acid overlap, resulting in 76 gag, 170 pol and 95 env peptides. Peptide stocks were made in DMSO at 20mg/mL. As positive controls, the previously identified H-2 IAb FV env 122-141 peptide DEPLTSLTPRCNTAWNRLKL and the H-2 Db restricted peptide CCLCLTVFL, substituting aminobutyric acid for the three cysteine residues as previously described (Schepers et al., 2002) were used. The positive control peptides were HPLC-purified large preps while the test peptides were small, unpurified preps.
CD4, CD8, and Ter119 depletions
Spleen cells were depleted of Ter119+ erythroid cells and either CD4+ cells, or CD8+ cells using MACS Microbeads (Miltenyi Biotec Inc.) following the manufacturers instructions. Briefly, splenocytes were reacted with anti-Ter119 microbeads, passed over LD columns and the negative fractions were split into three parts. The first part received no further treatment, the second part was reacted with anti-CD4 microbeads, and the third part reacted with anti-CD8 microbeads. Parts two and three were passed over LD columns and the negative fractions saved. Aliquots of the splenocytes were collected prior to and after depletion to examine the quality of the depletions. Cells were stained with anti-CD4 FITC (BD Biosciences), and anti-CD8 Alexa Fluor 700 and anti-Ter119 PE-Cy7 (eBiosciences).
Interferon-gamma ELISPOT Assays
Splenocytes from whole spleens or fractionated spleens of mice at 12 days post-infection with FV were assayed for IFN producing cells using the mouse IFN gamma ELISPOT Ready-SET-Go!® kit from eBioscience following the manufacturers instructions. Briefly, 0.45μm Immobilon-P MultiScreen filter plates (Millipore) were coated with anti-IFN overnight at 4°C. The following day, after blocking with complete RPMI (RPMI supplemented with 10% fetal bovine serum and pen/strep), 2 × 105 cells per well were added to each well in complete RPMI. FV peptides were added to each well at 4μg/mL and the plates were incubated at 37°C, 5% CO2. After two days, the plates were stained with biotinylated anti-mouse IFN followed by avidin-horseradish peroxidase, and developed with 3-amino-9-ethyl carbazole substrate. Spots were read on a Cellular Technologies Ltd. plate reader (Shaker Heights, OH) using ImmunoSpot 5.0 Professional DC Software. Peptides were considered positive if they: 1. elicited greater than twice the number of spots in the negative control wells, 2. elicited a minimum of 50 spots/1 × 106 cells, 3. produced these responses in at least half the infected mice tested in two separate experiments and none of the naïve mice and 4. elicited responses equivalent or greater than peptides 265 and 266, which contain most or all of the core sequence of the previously reported IAb 122-141 positive control.
MHC-II binding predictions
The MHCII binding predictions were made on 9/5/2013 using the IEDB analysis resource Consensus tool (Wang et al., 2008; Wang et al., 2010). http://tools.immuneepitope.org/mhcii/
Highlights.
-
-
Nine new CD4+ T cell epitopes from Friend virus were identified
-
-
CD4+ T cell epitopes include 3 encoded by gag, 1 by pol, and 5 by env
-
-
The high resistance of H-2b mice could be related to a very broad CD4+ T cell response
Acknowledgements
This work was funded by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: All authors (RJM, KJL and KJH) helped design the study. RJM performed the experiments. RJM and KJH analyzed and interpreted the data and wrote the paper.
References
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol. 1997;54:159–169. doi: 10.1016/s0198-8859(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Chen W, Qin H, Chesebro B, Cheever MA. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 1996;70:7773–7782. doi: 10.1128/jvi.70.11.7773-7782.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Miyazawa M, Britt WJ. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Dow C, Oseroff C, Peters B, Nance-Sotelo C, Sidney J, Buchmeier M, Sette A, Mothe BR. Lymphocytic choriomeningitis virus infection yields overlapping CD4+ and CD8+ T-cell responses. Journal of Virology. 2008;82:11734–11741. doi: 10.1128/JVI.00435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp. Med. 1957;105:307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ. Lymphocyte deficiencies increase susceptibility to friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J Virol. 1999;73:6468–6473. doi: 10.1128/jvi.73.8.6468-6473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Brooks DM, Dittmer U. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. Journal of Virology. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci U S A. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug KJ, Dittmer U. The Role of CD4 and CD8 T Cells in Recovery and Protection from Retroviral Infection: Lessons from the Friend Virus Model. Virology. 2000;272:244–249. doi: 10.1006/viro.2000.0387. [DOI] [PubMed] [Google Scholar]
- Hasenkrug KJ, Dittmer U. Immune control and prevention of chronic Friend retrovirus infection. Front Biosci. 2007;12:1544–1551. doi: 10.2741/2167. [DOI] [PubMed] [Google Scholar]
- Iwashiro M, Kondo T, Shimizu T, Yamagishi H, Takahashi K, Matsubayashi Y, Masuda T, Otaka A, Fujii N, Ishimoto A, et al. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J. Virol. 1993;67:4533–4542. doi: 10.1128/jvi.67.8.4533-4542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Mathis DJ, Benoist C, Williams VE, 2nd, Kanter M, McDevitt HO. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci U S A. 1983;80:273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M, Fujisawa R. Restriction of Friend virus-induced erythroid cell proliferation by CD4+ T-lymphocytes that recognize a single gp70 epitope. Leukemia. 1997;11(Suppl 3):227–229. [PubMed] [Google Scholar]
- Miyazawa M, Nishio J, Chesebro B. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J Virol. 1992;66:4497–4507. doi: 10.1128/jvi.66.7.4497-4507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M, Tsuji-Kawahara S, Kanari Y. Host genetic factors that control immune responses to retrovirus infections. Vaccine. 2008;26:2981–2996. doi: 10.1016/j.vaccine.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Nair S, Bayer W, Ploquin MJ, Kassiotis G, Hasenkrug KJ, Dittmer U. Distinct roles of CD4+ T cell subpopulations in retroviral immunity: lessons from the Friend virus mouse model. Retrovirology. 2011;8:76. doi: 10.1186/1742-4690-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SR, Zelinskyy G, Schimmer S, Gerlach N, Kassiotis G, Dittmer U. Mechanisms of control of acute Friend virus infection by CD4+ T helper cells and their functional impairment by regulatory T cells. J Gen Virol. 2010;91:440–451. doi: 10.1099/vir.0.015834-0. [DOI] [PubMed] [Google Scholar]
- Ozato K, Lunney JK, El-Gamil M, Sachs DH. Evidence for the absence of I-E/C antigen expression on the cell surface in mice of the H-2b or H-2s haplotypes. Journal of immunology. 1980;125:940–945. [PubMed] [Google Scholar]
- Perryman S, Nishio J, Chesebro B. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 1991;19:6950. doi: 10.1093/nar/19.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DA, Paulson RF, Loyd MR, Herley MT, Bodner SM, Bernstein A, Correll PH, Ney PA. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- Pike R, Filby A, Ploquin MJ, Eksmond U, Marques R, Antunes I, Hasenkrug K, Kassiotis G. Race between retroviral spread and CD4+ T-cell response determines the outcome of acute Friend virus infection. Journal of Virology. 2009;83:11211–11222. doi: 10.1128/JVI.01225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee HG. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Toebes M, Sotthewes G, Vyth-Dreese FA, Dellemijn TA, Melief CJ, Ossendorp F, Schumacher TN. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J. Immunol. 2002;169:3191–3199. doi: 10.4049/jimmunol.169.6.3191. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Uenishi H, Teramura Y, Iwashiro M, Kuribayashi K, Tamamura H, Fujii N, Yamagishi H. Fine structure of a virus-encoded helper T-cell epitope expressed on FBL-3 tumor cells. J Virol. 1994;68:7704–7708. doi: 10.1128/jvi.68.12.7704-7708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara D, Tsuji-Kawahara S, Miyazawa M. Identification of a protective CD4+ T-cell epitope in p15gag of Friend murine leukemia virus and role of the MA protein targeting the plasma membrane in immunogenicity. J Virol. 2004;78:6322–6334. doi: 10.1128/JVI.78.12.6322-6334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodossis A, Guillonneau C, Welland A, Ely LK, Clements CS, Williamson NA, Webb AI, Wilce JA, Mulder RJ, Dunstone MA, Doherty PC, McCluskey J, Purcell AW, Turner SJ, Rossjohn J. Constraints within major histocompatibility complex class I restricted peptides: presentation and consequences for T-cell recognition. Proc Natl Acad Sci U S A. 2010;107:5534–5539. doi: 10.1073/pnas.1000032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010;11:568. doi: 10.1186/1471-2105-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GR, Ploquin MJ, Eksmond U, Wadwa M, Stoye JP, Kassiotis G. Negative selection by an endogenous retrovirus promotes a higher-avidity CD4+ T cell response to retroviral infection. PLoS Pathog. 2012;8:e1002709. doi: 10.1371/journal.ppat.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]