Abstract
Apoptosis is the most widely recognized form of physiological programmed cell death. During the past three decades, various nonapoptotic forms of cell death have gained increasing attention, largely because of their potential importance in pathological processes, toxicology, and cancer therapy. A recent addition to the panoply of cell death phenotypes is methuosis. The neologism is derived from the Greek methuo (to drink to intoxication) because the hallmark of this form of cell death is displacement of the cytoplasm by large fluid-filled vacuoles derived from macropinosomes. The demise of the cell resembles many forms of necrosis, insofar as there is a loss of metabolic capacity and plasma membrane integrity, without the cell shrinkage and nuclear fragmentation associated with apoptosis. Methuosis was initially defined in glioblastoma cells after ectopic expression of activated Ras, but recent reports have described small molecules that can induce the features of methuosis in a broad spectrum of cancer cells, including those that are resistant to conventional apoptosis-inducing drugs. This review summarizes the available information about the distinguishing morphological characteristics and underlying mechanisms of methuosis. We compare and contrast methuosis with other cytopathological conditions in which accumulation of clear cytoplasmic vacuoles is a prominent feature. Finally, we highlight key questions that need to be answered to determine whether methuosis truly represents a unique form of regulated cell death.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Commencing with the first descriptions of programmed cell death approximately 50 years ago, intensive research efforts have focused on classifying cell death pathways and defining their underlying mechanisms.1 The most widely recognized form of programmed cell death is apoptosis. Signature morphological features associated with apoptotic cell death include plasma membrane blebbing, shrinkage of the cytoplasm, nuclear chromatin condensation, and eventual fragmentation of the cell to form apoptotic bodies.2,3 Apoptotic signaling pathways converge on the mitochondria, where changes in membrane permeability lead to release of molecules that activate a cascade of cysteine-dependent aspartate-directed proteases (caspases).4 For this reason, apoptosis is often considered to be synonymous with caspase-dependent cell death. However, it is now clear that other proteins released by damaged mitochondria (eg, apoptosis-inducing factor, endonuclease G) can also contribute to apoptosis.5,6
Many forms of nonapoptotic (caspase-independent) cell death have been described.7 The distinguishing characteristics are often morphological, but in some instances, there has been enough progress to permit the use of biochemical and functional criteria to define distinct cell death subroutines. On the basis of such considerations, the Nomenclature Committee on Cell Death has recognized nine regulated cell death modes in addition to intrinsic or extrinsic apoptosis.7 Some of these forms of cell death, such as anoikis, are triggered by unique signals (detachment of adherent cells from the extracellular matrix) but share essentially the same execution pathways as apoptosis.8 Others, such as netosis (granulocytes)9 and cornification (keratinocytes),10 occur only in specific cell types. However, several nonapoptotic cell death subroutines appear to have much broader pathophysiological relevance.
One of the most widely studied alternatives to apoptosis is autophagic cell death, known for many years as type 2 cell death (versus type 1 for apoptosis).11,12 In autophagic cell death, autophagosomes and autophagolysosomes accumulate in the cytoplasmic compartment of the affected cells. Unabated autophagy (self-eating) of proteins and organelles is believed to precipitate the eventual loss of cell viability. Controversy stems from the fact that autophagy is also recognized as a mechanism to promote cell survival in the face of nutrient deprivation, genomic damage, or oxidative stress.13,14 Therefore, in some reported cases of autophagic cell death, the accumulation of autophagosomes may represent an adaptive response to stress, rather than a direct cause of cell death.7
Other forms of cell death that occur in a broad spectrum of cell types appear to fall within the general category of programmed or regulated necrosis.15 Examples include necroptosis16 and parthanatos.17 In contrast to the long held view of necrosis as a passive or accidental cytolytic process provoked by cell injury or metabolic catastrophe,18 these forms of cell death are initiated through defined signaling pathways. For instance, necroptosis is triggered by activation of receptor interacting protein (RIP)1 and/or RIP3 kinases, and can be blocked by a specific inhibitor, necrostatin.19 Parthanatos is induced by activation of poly (ADP-ribose) polymerase (PARP)-1,20 and thus can be ameliorated with the use of PARP inhibitors.
The previously mentioned cell death subroutines have been established as having pathophysiological relevance. That is, they occur in vivo and play roles in either normal developmental tissue remodeling or the responses of cells and tissues to one or more disease processes. Excluded from the list of cell death mechanisms recognized in the last review by the Nomenclature Committee on Cell Death are several distinctive cell death phenotypes that have thus far been confirmed mainly in cells that have been manipulated genetically or pharmacologically in vitro, or in tissues that have been exposed to ischemic injury, cytotoxic compounds, or pathogens. Among these, paraptosis,21,22 oncosis,23 and methuosis (the subject of this review) are characterized by swelling or vacuolization of specific organelles, necrosis-like membrane disruption, and little or no responsiveness to caspase inhibitors. Although the physiological importance of these forms of cell death remains uncertain, they are of significant interest in the fields of toxicology and cancer therapy. Indeed, in a recent review of potential anti-cancer agents that promote nonapoptotic cell death, multiple compounds were featured as inducers of paraptosis, oncosis, or methuosis.24
Methuosis is one of the most recent additions to the list of nonapoptotic cell death phenotypes. The name, which is derived from the Greek methuo (to drink to intoxication), was selected because the most prominent attribute in cells undergoing this form of death is the accumulation of large fluid-filled cytoplasmic vacuoles that originate from macropinosomes. In the present review, we begin by recapping the supporting evidence for classification of methuosis as a distinctive cell death phenotype. We then attempt to place the recent work on methuosis in the context of the extensive literature describing vacuolization of cellular endosomal or lysosomal compartments in response to a variety of toxins and drugs. Finally, we summarize the current knowledge about the underlying mechanisms of methuosis and provide a perspective on the key questions that remain to be addressed.
Cytoplasmic Vacuolization and Cell Death Induced by Activated Ras
Our investigations leading to the identification of methuosis as a novel cell death phenotype were undertaken after a report from Chi et al,25 in which ectopic expression of an activated form of the H-Ras oncoprotein (G12 →V) was shown to induce massive cytoplasmic vacuolization and caspase-independent cell death in cultured glioblastoma (GBM) and gastric carcinoma cells. This form of cell death was initially designated as type 2 (autophagic degeneration). However, as we began to investigate this phenomenon, we noted that the morphological characteristics of the vacuoles, induced by overexpression of Ras, were inconsistent with the morphological characteristics of autophagosomes or autophagolysosomes.26 Specifically, the vacuoles were phase and electron lucent, and were bound by a single membrane, rather than the typical double membrane of autophagosomes (Figure 1, A and C). Analysis of the localization and expression of the autophagosome marker, LC3II, by immunofluorescence microscopy and Western blot analysis revealed that autophagy was, in fact, elevated in GBM cells expressing Ras(G12V), but the vesicles labeled with LC3II were smaller and spatially separate from the much larger-phase lucent vacuoles. Because suppression of the autophagy regulatory protein Beclin-1 had no detectable effect on vacuolization or survival of GBM cells expressing Ras(G12V), we concluded that elevated autophagy was a compensatory stress response rather than a cell death mechanism in this situation.
Figure 1.
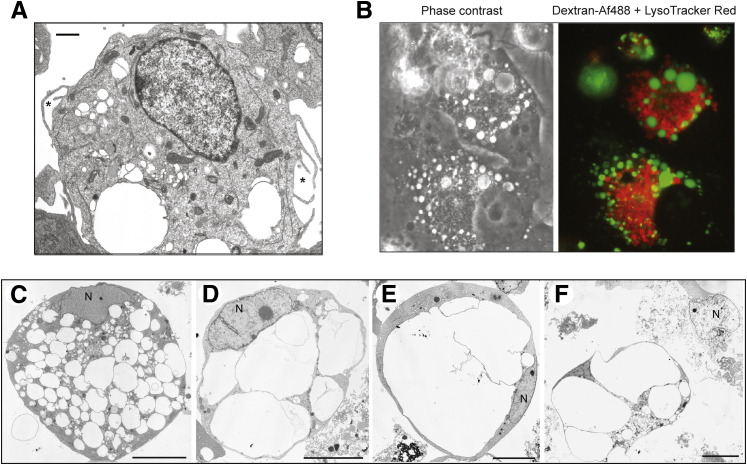
Examples of U251 human GBM cells undergoing methuosis triggered by ectopic expression of activated Ras. Conditional expression of H-Ras(G12V) was induced by addition of doxycycline to a stable cell line (U251-C18). A: The electron micrograph shows the initial stage of methuosis, where large electron-lucent cytoplasmic vacuoles bound by a single membrane can be seen forming from lamellipodial extensions of the plasma membrane (asterisks). B: The macropinosome-derived vacuoles remain separate from lysosomes. Lysosomes were prelabeled for 3 hours with LysoTracker Red. Macropinosome-derived vacuoles were then labeled with dextran–Alexa Fluor 488 (green). The cells were examined 4 hours after addition of the labeled dextran. C–F: Electron micrographs show detached U251-C18 cells in various stages of degeneration between 4 and 6 days after induction of H-Ras(G12V) expression. Vacuoles accumulate and coalesce until they fill almost the entire cytoplasmic space. F: The nuclei (N) generally remain intact, with little or no chromatin condensation, even in cells that have undergone lysis. Images are reprinted with permission from Overmeyer et al.26 Scale bars: 1 μm (A); 10 μm (F).
To identify the origin of the Ras-induced vacuoles, we performed electron microscopy and fluorescence microscopy with fluid-phase tracers and markers for endoplasmic reticulum (ER), mitochondria, endosomes, and lysosomes.26 These studies demonstrated that the Ras-induced vacuoles did not emanate from swollen ER membranes or mitochondria, as is typically the case in paraptosis or oncosis. Instead, we found that the vacuoles were derived from macropinosomes. Macropinocytosis is a clathrin-independent endocytic process by which mammalian cells internalize extracellular fluid, nutrients, and proteins in vesicles (macropinosomes) generated from protrusions of the plasma membrane termed lamellipodia or ruffles.27 After acquiring phosphatidylinositol-3-phosphate and the small GTPase, Rab5, macropinosomes enter the endocytic pathway, where they either recycle back to the cell surface or mature to acquire characteristics of late endosomes [eg, lysosome associated membrane protein (LAMP)1 and Rab7] and ultimately fuse with lysosomes.28,29 In GBM cells overexpressing Ras(G12V), macropinocytosis is stimulated, and large vacuoles can be seen forming from lamellipodial projections (Figure 1A). However, the macropinocytotic process is dysfunctional,26 because the nascent macropinosomes, labeled with fluorescent dextran, do not recycle or fuse with lysosomes (Figure 1B). Instead, they coalesce to form progressively larger vacuoles. The latter acquire both LAMP1 and Rab7, but are not sufficiently acidic to sequester lysosomotropic dyes, such as LysoTracker or acridine orange.26 As vacuoles of increasing size fill the cytoplasmic space, the cells detach from the substratum and lose membrane integrity in a manner reminiscent of necrosis (Figure 1, C–F). However, no protection is afforded by necrostatin, distinguishing this form of cell death from necroptosis. In conjunction with the loss of cell viability, some features common to the execution program of apoptosis can be detected. These include activation of caspase-3 and cleavage of caspase substrates, PARP, and lamin A/C.26 Nevertheless, loss of cell viability is not prevented by the broad-spectrum caspase inhibitor, z-VAD-fmk, and the typical morphological features of apoptosis (cell shrinkage, chromatin condensation, and plasma membrane blebbing) are generally absent (Figure 1, C–F). Given the poor fit of this phenotype with previously described forms of nonapoptotic cell death, and its peculiar association with extreme vacuolization of macropinosome-derived endosomal compartments, we believed that the assignment of a unique descriptive name to the process (ie, methuosis) was warranted.26
Although initial efforts to define the mechanism of methuosis focused on stable U251 GBM cells with H-Ras(G12V) under the control of an inducible promoter, vacuolization and cell death can be triggered by ectopic expression of Ras(G12V) in a variety of other cancer cell lines, including eight genotypically distinct human GBM cell lines,26 U2OS osteosarcoma cells,30 and MKN-1 and TMK-1 gastric carcinoma cells.25 Interestingly, there is some evidence that the potential for cellular vacuolization and disintegration in response to constitutively activated Ras may extend to lower eukaryotic organisms. For example, Fortwendel et al31 recently noted that replacement of wild-type rasA with a dominant-active rasA allele in the filamentous fungus, Aspergillus fumigatus, caused excessive vacuolar expansion reminiscent of methuosis, with swelling and spontaneous lysis of hyphal compartments. Even so, this cellular response is at odds with the large body of evidence indicating that activated forms of Ras are oncogenic. Indeed, not all cell types respond to ectopic expression of activated Ras by undergoing extreme vacuolization.25,26,32 As discussed in the next section, we are beginning to understand some of the factors that determine whether constitutive activation of Ras will exert typical oncogenic activity or anomalous cytotoxicity under particular circumstances.
Mechanism of Ras-Induced Methuosis
Ras GTPases (H-, K-, and N-Ras) have long been known to function as molecular switches in signaling pathways critical for cell growth and survival. The concept that activating mutations in Ras are important in tumorigenesis is firmly established.33,34 Paradoxically, there is some evidence that activation of endogenous Ras pathways can promote cell death instead of cell survival under some circumstances.32,35 In addition to inducing methuosis, overexpression of activated forms of Ras can trigger apoptosis36,37 and autophagy.38 The balance between the prosurvival and prodeath functions of Ras depends on factors such as the relative abundance and subcellular localization of functionally distinct Ras isoforms, the constellation of upstream receptors and nucleotide exchange factors impinging on Ras in a given cell type, and context-specific variations in the status of downstream Ras effector pathways. The general relationship between Ras signaling and apoptosis has been reviewed elsewhere.32,35 Herein, we will focus on what is known about the connection between activated Ras and the vacuolar cytopathological characteristics observed in GBM cells.
Our early studies established that induction of vacuolization in GBM cells by ectopic expression of activated forms of H-Ras or K-Ras4B does not depend on the best known Ras effector pathways: the Raf→mitogen-activated protein kinase/extracellular signal–regulated kinase (ERK)→ERK cascade, the phosphatidylinositol 3-kinase→Akt pathway, or the RalGDS→RalA/RalB pathway.39 In seeking other Ras-dependent mechanisms that could specifically affect vesicular trafficking, we were cognizant of reports indicating the following: i) Ras activation can stimulate macropinocytosis,40,41 ii) a related GTPase, Rac1, serves as a positive mediator of actin assembly required for macropinocytosis,42 and iii) Ras can stimulate guanine-nucleotide exchange factors that promote activation of Rac1.43 Combining these observations, we hypothesized that sustained high-level expression of Ras(G12V) and consequent chronic stimulation of Rac1 might contribute to the extreme vacuolization associated with methuosis. The studies of Bhanot et al30 support this model. They show that when the threshold of ectopic Ras(G12V) expression exceeds the level required for maximal activation of the canonical growth-stimulatory ERK and phosphatidylinositol 3-kinase pathways, the amount of endogenous Rac1 in the active GTP-bound state increases and vacuolization occurs. Most important, the expression of constitutively active Rac1(G12V) mimics the effects of H-Ras(G12V) in GBM cells, and induction of methuosis by H-Ras(G12V) can be blocked by EHT1864, a selective inhibitor of Rac1.
Although the activation of Rac1 is consistent with the increase in macropinocytotic activity observed in cells expressing Ras(G12V), it does not explain the defect in macropinosome recycling. To address this issue, we considered the impact of chronic Rac1 activation on another GTPase, Arf6, which functions in the recycling pathway for non–clathrin-coated endosomes.44,45 We found that as the relative amount of active Rac1 increases, there is a corresponding decline in the amount of active Arf6.30 This may explain the defect in recycling of newly internalized macropinosomes. The effect of Rac1 on Arf6 appears to be mediated through a stimulatory interaction between Rac1 and GIT-1, a GTPase-activating protein that facilitates hydrolysis of GTP on Arf6.30 Taken together, these findings suggest that unrestricted activation of Rac1 by Ras(G12V) plays a dual role, stimulating the formation of incoming macropinosomes while simultaneously impeding macropinosome recycling by decreasing the pool of active Arf6. The resulting accumulation of macropinosomes may promote abnormal vesicle fusion. In the absence of effective lysosomal clearance, vacuoles eventually fill the cytoplasm and compromise cell viability. Little is known about the molecular basis for the inability of the macropinosome-derived vacuoles to merge with lysosomes or the specific steps that may link cell vacuolization to loss of membrane integrity. As discussed in the next section, the recent development of small molecules that can induce methuosis much more rapidly than manipulation of Ras expression should provide a more tractable experimental model in which to address such questions.
Methuosis Induced by Indole-Based Chalcones
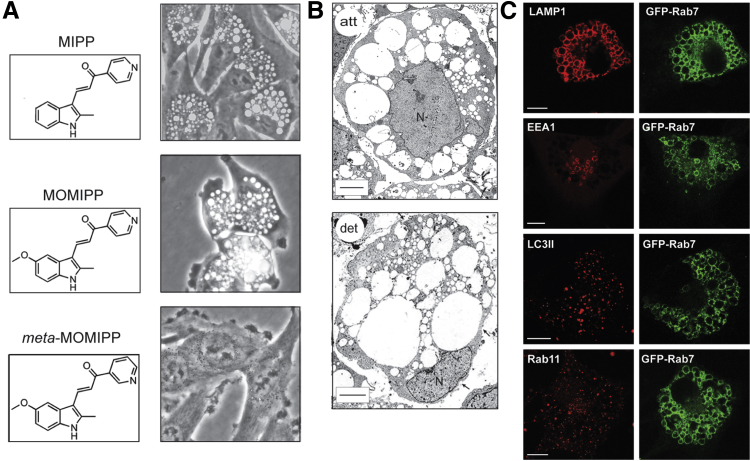
Methuosis caused by overexpression of Ras(G12V) proceeds asynchronously over a prolonged period (4 to 8 days) in cell culture. This makes it difficult to dissect the specific relationship between vacuolization and cell death. Furthermore, we were particularly interested in exploring the possibility that stimulation of this nonconventional cell death pathway might be used as a strategy to kill cancer cells that are resistant to apoptosis. To facilitate such studies, we began to seek small molecules that could rapidly provoke a cellular phenotype closely resembling Ras-induced methuosis. In 2011, we described a synthetic indole-based chalcone 3-(2-methyl-1H indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one (MIPP), which caused a striking accumulation of phase-lucent cytoplasmic vacuoles when applied to U251 GBM cells and a broad spectrum of other cell lines at low micromolar concentrations46 (Figure 2A). We subsequently developed a more potent analog with a 5-methoxy group added to the indole ring (MOMIPP)47 (Figure 2A). These compounds induced vacuolization within 4 hours, and the effect was reversible if the compounds were removed within this time frame. However, if cells were exposed to MIPP or MOMIPP for 48 hours, they detached from the substratum and underwent a necrosis-like membrane disruption. By electron microscopy, the morphological characteristics of the dying cells were similar to the morphological characteristics we had previously observed in cells undergoing Ras-induced methuosis (Figure 2B). Loss of viability was confirmed by a decline in metabolic activity (MTT) and colony formation. A nonapoptotic mode of death was supported by the fact that, although the plasma membrane was disrupted, the nuclear membrane stayed intact and chromatin remained diffuse (Figure 2B). Although there was some evidence of caspase activation (PARP cleavage), the caspase inhibitor, zVAD-fmk, did not prevent loss of viability.46 A final indication that cell death occurs through a novel mechanism came from studies with U251 and MCF7 cell lines selected for their ability to survive in the presence of high levels of the DNA-damaging drugs, temozolomide or doxorubicin, respectively. In these resistant cell lines, MIPP and MOMIPP induced vacuolization and cell death at concentrations comparable to those that were effective in the parental U251 and MCF7 cell lines.46,47
Figure 2.
Examples of U251 GBM cells undergoing methuosis in response to treatment with novel indole-based chalcones. A: The images show representative cells at 24 hours after addition of 10 μmol/L MIPP or MOMIPP. Slight modification of MOMIPP by altering the pyridinyl nitrogen configuration abolishes the formation of vacuoles. B: Electron micrographs show the extreme accumulation of electron-lucent vacuoles in attached (att) and detached (det) cells exposed to MIPP for 48 hours. The detached cells are mostly nonviable (Trypan blue staining), and typically have significant gaps in the plasma membrane (arrows). Nevertheless, the nuclei (N) remain intact, without the characteristic features of apoptosis. C: Vacuoles induced by MIPP in U251 cells expressing GFP-Rab7 are decorated with this late endosomal marker. The same vacuoles contain LAMP1, but not the autophagosome marker, LC3II, or the recycling endosome marker, Rab11. A small subpopulation of the vacuoles contains the early endosome marker, EEA1, possibly representing a transition state between nascent macropinosomes and mature late endosome-like vacuoles. Images in A are reprinted with permission from Overmeyer et al46 and Trabbic et al50 (copyright 2014 American Chemical Society). Images in B and C are reprinted with permission from Overmeyer et al.46 Scale bars: 10 μm (B and C).
The origin of the vacuoles induced by the indole-based chalcones is consistent with methuosis. Time-lapse microscopy performed between 13 and 80 minutes after addition of MIPP revealed waves of phase-lucent macropinosomes entering the cells from regions of active membrane ruffling. The nascent macropinosomes rapidly coalesced to form larger vacuoles that failed to dissipate by recycling or fusing with lysosomes.46 The origination of the vacuoles from endocytic vesicles was confirmed by their incorporation of extracellular Lucifer yellow. Moreover, treatment of cells with filipin (a cholesterol binding agent) blocked vacuole formation, consistent with the essential role of cholesterol-rich membrane microdomains in clathrin-independent endocytic processes.48,49
Mechanism of Methuosis Induced by MIPP and MOMIPP
Studies with a synthetic library of MIPP and MOMIPP analogs revealed exquisite structural specificity in the ability of these molecules to induce vacuolization and cell death. For example, simply shifting the nitrogen in the pyridinyl moiety from the para to meta configuration eliminated vacuolization, growth inhibition, and cytotoxicity47,50 (Figure 2A). This suggests that the methuosis phenotype induced by these molecules is probably due to their interaction with one or more specific protein targets, rather than non-specific electrophilic protein modification. The identities of the specific targets remain to be established, but clues from recent studies suggest that they could include regulatory or structural components of the early and/or late endocytic pathways. Similar to the vacuoles induced by Ras, the MIPP-induced vacuoles acquire late endosomal markers, Rab7 and LAMP1 (Figure 2C), but remain separate from ER, mitochondria, and lysosomes.46 Moreover, once established, the vacuoles are distinct from early endosomes (EEA1), recycling endosomes (Rab11), and autophagosomes (LC3II) (Figure 2C). Despite these striking parallels, the effects of MIPP differ from those induced by Ras in some aspects. First, MIPP-induced cellular vacuolization evolves over a matter of hours rather than days. Second, in the MIPP-treated cells, the initial burst of macropinocytotic activity ceases after a few hours as larger vacuoles accumulate. This could signify that perturbations within the endocytic pathway ultimately disrupt not only the recycling and lysosome fusion steps, but also the machinery for generating new macropinosomes. Finally, the activation states of Rac1 and Arf6 do not change during the initial period of MIPP-induced vacuolization.46 This implies that MIPP and related compounds probably target components of the endocytic apparatus downstream from the steps regulated by Rac1 and Arf6.
In accord with the foregoing idea, we noted that bafilomycin A1 (Baf-A), an inhibitor of the vacuolar H+-ATPase,51 blocked the induction of vacuolization by MIPP.46 This is similar to what happens in cells exposed to Helicobacter pylori vacuolating toxin A (VacA), where Baf-A prevents vacuolization of late endosomes.52 The mechanistic basis for the effect of Baf-A on MIPP-induced vacuolization remains to be clarified. In addition to its ability to increase the pH of lysosomes53 and interfere with fusion between autophagosomes and lysosomes,54 Baf-A can disrupt trafficking between early and late endosomes.55,56 Recent studies suggest that the latter could be mediated through perturbation of the regulatory cycle of the Rab5 GTPase.57 Rab5 influences vesicle fusion, maturation, and recycling in both the clathrin-dependent and the clathrin-independent endocytic pathways.57,58 Macropinosomes typically recruit Rab5 soon after they are formed, in concert with changes in their membrane phosphoinositide composition.41 Activation of Rab5 transiently stabilizes macropinosomes, whereas dissociation of Rab5 after GTP hydrolysis allows recruitment of Rab7 and subsequent fusion with lysosomes.57 When we quantified the active GTP-bound forms of Rab5 and Rab7 in cells treated with MIPP, we detected a significant decline in Rab5-GTP and an increase in Rab7-GTP.46 Consistent with these observations, the membranes of MIPP-induced vacuoles were decorated with Rab7, but not Rab5.46 This could indicate that MIPP directly or indirectly affects components of the Rab5 complex,58,59 potentially destabilizing nascent macropinosomes and allowing premature recruitment of Rab7. The increase in Rab7-GTP might reflect an impairment of Rab7 interaction with its downstream GTPase-activating protein or effectors, perhaps related to the inability of the vacuoles to fuse with lysosomes. The possibility that MIPP exerts pleiotrophic effects at various points in the endocytic pathway is reinforced by the finding that neither constitutively active Rab5 nor dominant-negative Rab7 was able to prevent vacuolization when expressed in MIPP-treated cells.46
Relationship of Methuosis to Other Cellular Phenotypes Involving Endosomal Vacuolization
In 1946, Trowell60 described the formation of large watery vacuoles in the cell cytoplasm in liver, after a period of anoxia. Since then, numerous reports have documented the accumulation of clear vacuoles in tissues or cultured cells in response to a variety of environmental changes or toxic compounds. In an excellent review, Henics and Wheatley61 traced the historical progression of research on cellular vacuolization from the early view that this was an artifact of microscopy or “an adaptive physiological response”pp485 to the more current concept that “the demise which occurs via the vacuolation route may, in fact, be a distinct form of cell death which is difficult to fit into the conventional lytic and apoptotic modes.”pp485 In his often-cited review of developmental cell death, Clarke11 emphasized distinct cell morphological characteristics that did not fit with the descriptions of apoptosis (type 1) and autophagic (type 2) cell death. Collectively, these were termed nonlysosomal vesiculate degradation (type 3 cell death), and they typically involved swelling of organelles and vacuolization of the cytoplasm, without the nuclear changes associated with apoptosis. It now seems likely that some of these early descriptions of type 3 cell death may correspond to instances of oncosis, paraptosis, or necroptosis, where, as noted earlier, cytoplasmic vacuoles originate from swollen ER or mitochondria. Nevertheless, as discussed later, the literature is replete with reports of cellular degeneration, where large vacuoles originate from macropinosomes, endosomes, or lysosomes. This raises a question as to whether the cell death phenotype we have dubbed methuosis might be applicable to a range of cytopathological features in which extreme vacuolization of endolysosomal compartments is associated with cell death. In the following sections, we discuss several examples and comment on potential morphological and mechanistic similarities to methuosis induced by Ras or the indolyl chalcones.
Ectopic Expression of TrkA
Li et al62 described a novel caspase-independent form of cell death in medulloblastoma cells, in which ectopically expressed TrkA receptor tyrosine kinase was stimulated by nerve growth factor. Cell death involved hyperstimulation of macropinocytosis and accumulation of large phase-lucent vacuoles that could be labeled with fluid-phase tracers and antibodies against LAMP1. As in our studies, cell death was not prevented by suppression of autophagy. There are some differences between cells overexpressing TrkA and cells undergoing Ras- or MIPP-induced cell death. In the latter case, the vacuoles exhibit late endosome characteristics but fail to fuse with lysosomes, whereas the vacuoles induced via stimulation of TrkA retain some ability to merge with lysosomes. Nevertheless, the overall resemblance of this cell death phenotype to methuosis is striking.
Meth-Induced Neurotoxicity
In addition to altering the integrity of dopaminergic nerve terminals and the release of neurotransmitters, methamphetamine (Meth) can cause lysosomal alkalinization and vacuolization of endosomal and lysosomal compartments.63 In recent studies with cultured neuroblastoma cells, Nara et al64 demonstrated that Meth can induce macropinocytosis and promote accumulation of large LAMP1-positive cytoplasmic vacuoles, culminating in cell death without caspase activation. As in our studies of methuosis, the Meth-induced vacuoles incorporated fluid-phase tracers but did not display markers for autophagosomes or ER. The resemblance to Ras- and MIPP-induced methuosis was further underlined by the apparent disruption of endolysosomal trafficking in Meth-treated cells, as evidenced by impaired processing of the lysosomal protease, cathepsin-L. Finally, as in Ras-induced methuosis, formation of the vacuoles was shown to be dependent on the activation of Ras and Rac1.65 Despite these conspicuous similarities, the Meth-induced cell death phenotype is not identical to the Ras- and MIPP-induced phenotypes. For example, in the former, there is no evidence of caspase activation, whereas in the latter, caspase activation occurred but was not essential for cell death. Nevertheless, Meth-induced cell death could be a form of methuosis.
Organic Amines
A wide variety of tertiary amines (eg, procaine, procainamide, disobutamide, and metoclopramide) and other weakly basic drugs (eg, ephedrine, atropine, and pilocarpine) can induce prominent cytoplasmic vacuolization in cultured cells and tissues, generally when applied in the millimolar range.66,67 Vacuolization induced by these compounds is not uniformly linked to cell death. In some cases, cell proliferation or migration is affected,68,69 whereas in other cases, prolonged exposure at high concentrations is associated with modest cytotoxicity.70 It is generally thought that these compounds penetrate the cell membrane as nonionized weak bases, and then become trapped inside acidic organelles when they are protonated. The resulting high intravesicular drug concentration causes an influx of water to maintain osmotic balance, leading to the expansion of clear vacuoles derived from lysosomes, late endosomes, or trans-Golgi vesicles.69–71 Given the key roles of low intravesicular pH and osmotic swelling in this type of vacuolization, it is not surprising that proton pump inhibitors (eg, Baf-A) and extracellular dehydrating agents (eg, mannitol) can mitigate the morphological effects of the basic compounds.72 In contrast to the organic amines, the indolyl and pyridinyl nitrogens in MIPP and MOMIPP are not predicted to behave as weak bases at physiological pH. Moreover, vacuolization induced by these compounds is not inhibited by 200 mmol/L mannitol (J. Overmeyer, unpublished data). Unlike vacuolization induced by organic amines, which entails vesicle swelling without coalescence, methuosis begins with abnormal fusions of peripheral macropinosomes and clathrin-independent endosomes. On the basis of these notable differences, we conclude that methuosis induced by the indolyl chalcones is mechanistically distinct from the vacuolization of acidic organelles induced by millimolar concentrations of organic amines.
H. pylori VacA Toxin
The accumulation of large acidic vacuoles derived from late endosomes was one of the first cytopathological effects documented for the VacA toxin.52 VacA enters endothelial cells via a Rac1-dependent, clathrin-independent, endocytic pathway.73,74 In the late endosomes, VacA oligomers form anion-selective channels that promote influx of Cl−, thereby stimulating the activity of the H+-ATPase and decreasing the intraluminal pH.75,76 With the subsequent entry, protonation, and trapping of NH3 and other basic molecules, osmotic swelling occurs, resulting in the formation of large-phase lucent vacuoles. In this respect, the swelling of endosomes induced by VacA is similar to what occurs with organic amines. Because VacA has pleiotrophic effects separate from endosomal vacuolization,75,76 including direct interactions with mitochondria,77,78 the specific steps that lead to cell death remain unclear. However, on the basis of the available information, it seems likely that VacA-induced cytotoxicity is mechanistically distinct from methuosis.
Therapeutic mAbs
Monoclonal antibodies (mAbs) used for treatment of lymphoid malignancies, such as the type II anti-CD20 mAb, tositumomab, have been reported to induce nonapoptotic, nonautophagic cell death in human leukemia and lymphoma cells. This form of cell death was characterized by actin rearrangement and “gross vacuolization of cytoplasm with relatively intact nuclei.”79,pp2153 Although the origin of the vacuoles was not studied in detail, they were presumed to arise from swelling of lysosomes. This was based on the finding that cell death was associated with release of cathepsin B from acidic vacuoles and could be averted by addition of a cathepsin inhibitor. It remains to be determined if methuosis involves lysosomal membrane permeabilization, but the characteristic phase-lucent vacuoles in methuosis do not stain with lysosomotropic agents or substrates for cathepsin B.46 Thus, the cell death phenotype induced by therapeutic mAbs appears to be distinct from methuosis.
Vacuolins
Cerny et al80 described a series of triazine-based compounds termed vacuolins that induced extensive cytoplasmic vacuolization in HeLa cells and other cell lines. The most potent of these, vacuolin-1, caused the formation of large vacuoles with characteristics of early endosomes (EEA1 positive) and smaller vacuoles with features of late endosomes or lysosomes (LAMP1 positive). Receptor-mediated endocytic trafficking and sorting were not affected, but vacuolin-1 markedly inhibited Ca+-dependent exocytosis of lysosomes. The authors reported that extensive exposure to vacuolin-1 (7 to 21 days) did not affect cell viability. Therefore, despite the notable accumulation of phase-lucent endosomal vacuoles, the cellular phenotype induced by vacuolin-1 does not qualify as methuosis.
Topoisomerase II Inhibitor
F14512 is a polyamine-modified topoisomerase II inhibitor, which is selectively targeted to cancer cells via the polyamine transport system.81 It is more potent and tumor selective than the parent compound, etoposide. Brel et al82 reported that cytotoxicity of F14512 is not due to apoptosis or autophagy, but rather the accumulation of numerous multilamellar vesicular bodies and large, electron-lucent (methuosis-like) vacuoles. Despite the provocative morphological resemblance to methuosis, the entry of F14512-treated cancer cells into a senescent state, detected by β-galactosidase staining, does not fit the methuosis profile. Moreover, because the origin of the vacuoles from macropinosomes or endosomes was not confirmed, it remains possible that the F14512-induced structures could arise from swelling of ER or mitochondria, possibly signifying paraptosis or oncosis. Further studies will be required to classify this form of cell death.
Anticancer Oligonucleotide Aptamer
AS1411 is a guanine-rich oligodeoxynucleotide that selectively inhibits proliferation and induces death in many types of cancer cells.83,84 The anticancer activity of AS1411 is related, at least in part, to its ability to bind to nucleolin and alter its subcellular localization.85 Most recently, Reyes-Reyes et al86 reported that AS1411causes hyperstimulation of macropinocytosis in cancer cells. This is accompanied by cell swelling and cytoplasmic vacuolization (P. Bates, personal communication). Further definition of the origin of the vacuoles and their connection to loss of viability in cells treated with AS1411 will be required to determine whether this may represent a form of methuosis.
Summary
Table 1 summarizes the features associated with the cell death phenotype we have termed methuosis, compared with other recognized forms of nonapoptotic cell death. We propose that the following criteria can be used to broadly define the methuosis phenotype: i) Loss of cell viability is preceded by extreme cytoplasmic vacuolization, caused by dysfunctional trafficking of macropinosomes and/or non–clathrin-coated endosomes. Although this may include an initial acceleration of macropinosome formation, the vacuolar cytopathology is primarily related to abnormal coalescence of nascent macropinosomes that lack the capacity to recycle and/or merge with lysosomes. ii) The vacuoles formed in the early stages of methuosis are decorated with late endosomal markers (eg, LAMP1 and Rab7), but they are insensitive to dehydrating agents and do not sequester acidotropic agents, such as acridine orange or LysoTracker. These features can help to distinguish methuosis from endosomal or lysosomal swelling induced by weak bases or bacterial toxins. iii) Loss of cell viability occurs without the classic morphological features of apoptosis. For example, cells swell rather than shrink, plasma membrane blebbing is absent, and chromatin condensation and nuclear fragmentation do not occur before cell lysis. iv) Protection from death is not afforded by caspase inhibitors, necrostatin, or suppression of autophagy genes.
Table 1.
Features of Methuosis Compared with Other Forms of Nonapoptotic Cell Death
| Features | Methuosis26,46 | Oncosis23,87 | Paraptosis21 | Necroptosis16,19 | Ferroptosis88 | Pyroptosis89 | Parthanatos17 | Autophagic death7,12,14 |
|---|---|---|---|---|---|---|---|---|
| Visible vacuoles (origin) | Yes (macropinosomes and endosomes) | Yes (ER and mitochondria) | Yes (ER and mitochondria) | Yes (ER and mitochondria) | No | No | No | Yes (autophagosomes) |
| Rupture of plasma membrane | Yes | Yes | Yes | Yes | No | Yes | No | Varies |
| Cell swelling | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Membrane blebbing | No | Yes | No | No | No | No | No | No |
| Chromatin condensation | No | Yes | No | Yes | Yes | Yes | Yes | Yes (late) |
| DNA fragmentation | Varies | No | No | No | No | Yes | Yes | Yes (late) |
| Activation of executioner caspases (occurs but not required) | Yes | No | No | No | No | No | Yes | Varies |
| Accumulation of autophagosomes | Yes | Yes | Yes | No | No | Yes | ||
| Mitochondrial membrane permeability | Yes | Yes | Yes | No | No | Yes | Varies | |
| Lysosomal membrane permeability | Yes | No | Yes | No | No | Varies | ||
| ATP depletion | Yes | Yes | Yes | Yes | Yes | No | Yes | Varies |
| Inhibitors | Glycine PARP inhibitors | Necrostatin | Ferrostatin iron chelators | Caspase-1 inhibitors | PARP inhibitors | 3-Methyl-adenine siRNA vs atg-5, beclin-1 |
Perspective
Our current working model to describe the underlying cellular perturbations contributing to methuosis is presented in Figure 3. A limitation of the model is that it is based primarily on our own studies with GBM cells expressing activated Ras or treated with indole-based chalcones. However, at least some of the mechanistic features may also apply to previously mentioned cytopathological conditions characterized by dysfunctional macropinocytosis, as described by other laboratories.62–64,86 Because the characterization of methuosis as a novel cell death phenotype is a relatively recent development, a potential point of controversy centers on whether it truly represents a unique form of regulated cell death or just a subtype of necrosis or oncosis. Ultimately, the resolution of this conundrum will require answers to the following key questions.
Figure 3.
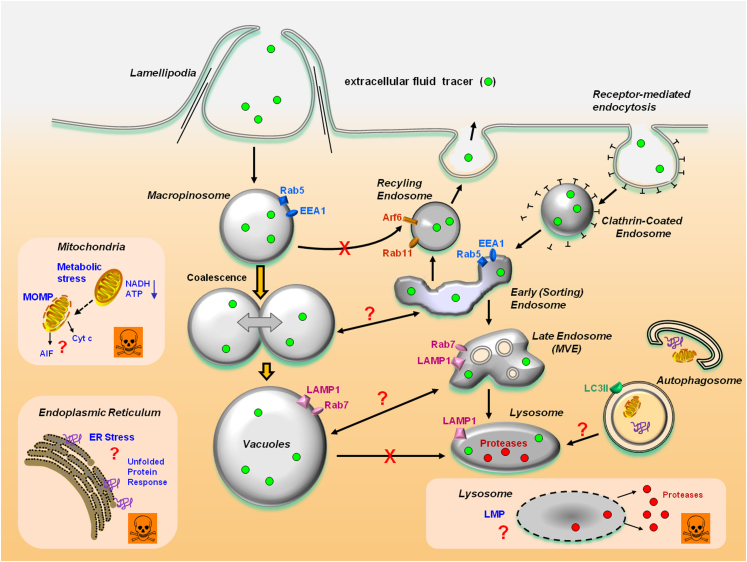
A working model of methuosis: Nascent macropinosomes generated from lamellipodial membrane projections enter the cell and coalesce to form large fluid-filled vacuoles. The latter are unable to recycle like normal macropinosomes, but rapidly mature to form a stable vacuole population with some characteristics of late endosomes (Rab7 and LAMP1). In contrast to functional late endosomes, the vacuoles do not sequester acidotropic markers (LysoTracker and acridine orange) or fuse with pre-existing lysosomes. It remains to be determined if the vacuoles have the capacity to disrupt protein trafficking in the clathrin-dependent pathway or merge with early endosomes or late multivesicular endosomes. Although there is good evidence that the macropinosome-derived vacuoles are distinct from autophagosomes, it will be important to determine whether the accumulation of vacuoles or associated changes in the metabolic state of the cell may have an impact on macroautophagy. A central question for future study is how vacuolization of macropinosomes or late endosomes leads to loss of membrane integrity and necrotic cell death. Contributing factors, which are not mutually exclusive, could include compromised mitochondrial energy metabolism, mitochondrial outer membrane permeability (MOMP), lysosomal membrane permeability (LMP), or ER stress. AIF, apoptosis-inducing factor; Cyt c, cytochrome c.
What Is the Connection between Vacuolization and Cell Death?
At present, we have only a rudimentary understanding of how the vacuolization of macropinosomal and endosomal compartments may be linked to molecular pathways or metabolic events that ultimately lead to the loss of cellular integrity. In particular, it remains to be determined whether there is a direct causal relationship between the accumulation of vacuoles and cell death. This is a challenging problem because compounds that are typically used to block macropinocytosis or endosomal vacuolization (eg, cytochalasins, amiloride, filipin, and Baf-A) are themselves cytotoxic when applied for more than a few hours. At present, the main evidence that vacuolization contributes to cell death comes from correlative studies. For instance, in early studies of Ras-induced methuosis, mutagenesis of the C-terminal farnesylation motif on Ras(G12V) prevented the induction of vacuolization and eliminated the cytotoxic effect.39 In more recent studies with a directed library of MIPP-related compounds, minor structural alterations that abolished vacuolization also eliminated effects on cell proliferation and viability.47 Despite this type of circumstantial evidence, there is reason to suspect that endosomal vacuolization, by itself, may not be sufficient to precipitate nonapoptotic cell death. Compounds such as vacuolin-180 and procaine66 induce prominent endosomal or lysosomal vacuolization but are not immediately cytotoxic. In some normal cell lines (human skin fibroblasts or mammary epithelial cells), MIPP and MOMIPP induce robust vacuolization, but cytotoxicity is substantially reduced in comparison to tumor cells. Recently, we reported that substituting longer hydrocarbons for the 2-methyl on the MOMIPP indolyl moiety markedly attenuated cytotoxicity but did not prevent the induction of vacuolization in GBM cells.50 This suggests that cytotoxicity of these compounds may be related to a unique combination of effects on macropinosome/endosome trafficking and other cellular processes that remain to be defined. Ongoing efforts to identify the specific protein targets of compounds that are active in inducing methuosis should shed light on this important issue.
What Is the Execution Program in Cells Undergoing Methuosis?
As noted earlier, cells undergoing extreme vacuolization in response to activated Ras or treatment with MIPP or MOMIPP eventually detach from the substratum and undergo a necrosis-like disruption of membrane integrity. A decline in ATP is temporally correlated with cell death.46 Although these events are suggestive of metabolic catastrophe, this remains to be proved. Failure of mitochondrial bioenergetic function91 and release of proteases from damaged lysosomes92,93 are hallmarks of necrotic cell death, but so far, we know little about potential changes in mitochondrial or lysosomal membrane permeability during methuosis. There is some evidence that disruption of metabolic activity, perhaps reflecting altered mitochondrial function, may be an early event in GBM cells exposed to MOMIPP. For instance, in an unpublished study, we observed that the capacity of the vacuolated cells to reduce MTT to formazan declines by approximately 50% after only 4 hours, whereas loss of cell viability (Trypan blue uptake) is not detected until 24 to 48 hours. Conversion of MTT to formazan reflects the activity of succinate and NADH-dependent dehydrogenases in mitochondria and other subcellular compartments.94,95 Thus, these preliminary observations raise the possibility that defects in cellular bioenergetics may contribute to the cellular demise associated with methuosis. As suggested in Figure 3, changes in energy status might also lead to cell death via the accumulation of unfolded proteins (ER stress) or alterations in lysosomal membrane permeability. Further examination of the relationship between vacuolization and oxidative metabolism should prove extremely useful for establishing the basis for the necrotic changes that occur during methuosis, as well as the differential sensitivity of normal versus cancer cells.
What Is the Pathophysiological Relevance of Methuosis?
We anticipate that recognition of cell death phenotypes similar or identical to methuosis will increase as more investigators begin to define the origins of cytoplasmic vacuoles frequently observed in cultured cells treated with small molecules and toxins. However, an important unresolved question is whether methuosis can occur in vivo in response to intrinsic signals under normal or pathological conditions. There is no shortage of morphological evidence for the occurrence of clear nonlysosomal vacuoles in necrotic cells during developmental tissue remodeling11,96 and in post-mortem tissues from animals exposed to anoxia,60,97 ischemia,87 or toxic chemicals.98 Although such observations are often attributed to oncosis, in the absence of information about the origins of such vacuoles or data to causally link vacuolization with cell death, it remains possible that some of these observations might represent instances of methuosis. Ultimately, the identification of molecular markers or specific inhibitors of methuosis will be required to determine whether this form of cell death occurs in animal tissues. Regardless of the eventual answer to this question, there is substantial value in continuing to characterize this and other non-physiological modes of cell death because of their relevance in toxicology,99 and because of the real prospect that artificially stimulating such forms of cell death may be therapeutically useful for treating cancers in which mutations in tumor-suppressor genes decrease susceptibility to agents that depend on induction of classic apoptosis.24,100
Note Added in Proof
While this review was in press, Kitambi et al101 published an article describing a small molecule termed Vacquinol-1, which induces nonapoptotic cell death characterized by massive macropinocytosis, catastrophic vacuolization, and membrane rupture in human glioblastoma cells. We believe that this form of cell death meets the general criteria for methuosis summarized herein. In particular, the authors demonstrate that Vacquinol-1 is able to suppress tumor growth and prolong survival in an orthotopic xenograft model of human glioblastoma. This reinforces the concept that small molecules with the capacity to induce methuosis may be of considerable interest as potential therapeutics for cancers that are resistant to apoptosis.
Footnotes
Supported by NIH grant R01 CA115495 (W.A.M.) and the Harold and Helen McMaster Endowment (W.A.M.).
Disclosures: None declared.
References
- 1.Lockshin R.A., Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–550. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- 2.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 4.Tait S.W., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 5.Joza N., Susin S.A., Daugas E., Stanford W.L., Cho S.K., Li C.Y., Sasaki T., Elia A.J., Cheng H.Y., Ravagnan L., Ferri K.F., Zamzami N., Wakeham A., Hakem R., Yoshida H., Kong Y.Y., Mak T.W., Zuniga-Pflucker J.C., Kroemer G., Penninger J.M. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 6.Li L.Y., Luo X., Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., Gottlieb E., Green D.R., Hengartner M.O., Kepp O., Knight R.A., Kumar S., Lipton S.A., Lu X., Madeo F., Malorni W., Mehlen P., Nunez G., Peter M.E., Piacentini M., Rubinsztein D.C., Shi Y., Simon H.-U., Vandenabeele P., White E., Yuan J., Zhivotovsky B., Melino G., Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valentijn A.J., Zouq N., Gilmore A.P. Anoikis. Biochem Soc Trans. 2004;32:421–425. doi: 10.1042/BST0320421. [DOI] [PubMed] [Google Scholar]
- 9.Remijsen Q., Kuijpers T.W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckhart L., Lippens S., Tschachler E., Declercq W. Cell death by cornification. Biochim Biophys Acta. 2013;1833:3471–3480. doi: 10.1016/j.bbamcr.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Clarke P.G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 12.Lockshin R.A., Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–2419. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Karantza-Wadsworth V., Patel S., Kravchuk O., Chen G., Mathew R., Jin S., White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G., Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edinger A.L., Thompson C.B. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Hitomi J., Christofferson D.E., Ng A., Yao J., Degterev A., Xavier R.J., Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrabi S.A., Dawson T.M., Dawson V.L. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker N.I., Harmon B.V., Gobe G.C., Kerr J.F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- 19.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Kim N.S., Haince J.F., Kang H.C., David K.K., Andrabi S.A., Poirier G.G., Dawson V.L., Dawson T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos) Sci Signal. 2011;4:ra20. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperandio S., de Belle I., Bredesen D.E. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasik A.M., Almestrand S., Wang X., Hultenby K., Dackland A.L., Andersson P., Kimby E., Christensson B., Sander B. WIN55,212–2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell Death Dis. 2011;2:e225. doi: 10.1038/cddis.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weerasinghe P., Buja L.M. Oncosis: an important non-apoptotic mode of cell death. Exp Mol Pathol. 2012;93:302–308. doi: 10.1016/j.yexmp.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Kornienko A., Mathieu V., Rastogi S.K., Lefranc F., Kiss R. Therapeutic agents triggering nonapoptotic cancer cell death. J Med Chem. 2013;56:4823–4839. doi: 10.1021/jm400136m. [DOI] [PubMed] [Google Scholar]
- 25.Chi S., Kitanaka C., Noguchi K., Mochizuki T., Nagashima Y., Shirouzu M., Fujita H., Yoshida M., Chen W., Asai A., Himeno M., Yokoyama S., Kuchino Y. Oncogenic Ras triggers cell suicide through the activation of a caspase-independent cell death program in human cancer cells. Oncogene. 1999;18:2281–2290. doi: 10.1038/sj.onc.1202538. [DOI] [PubMed] [Google Scholar]
- 26.Overmeyer J.H., Kaul A., Johnson E.E., Maltese W.A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol Cancer Res. 2008;6:965–977. doi: 10.1158/1541-7786.MCR-07-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson J.A., Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 28.Racoosin E.L., Swanson J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donaldson J.G., Porat-Shliom N., Cohen L.A. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhanot H., Young A.M., Overmeyer J.H., Maltese W.A. Induction of non-apoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol Cancer Res. 2010;8:1358–1374. doi: 10.1158/1541-7786.MCR-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fortwendel J.R., Juvvadi P.R., Rogg L.E., Steinbach W.J. Regulatable Ras activity is critical for proper establishment and maintenance of polarity in Aspergillus fumigatus. Eukaryot Cell. 2011;10:611–615. doi: 10.1128/EC.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overmeyer J.H., Maltese W.A. Death pathways triggered by activated Ras in cancer cells. Front Biosci (Landmark Ed) 2011;16:1693–1713. doi: 10.2741/3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karnoub A.E., Weinberg R.A. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young A., Lyons J., Miller A.L., Phan V.T., Alarcon I.R., McCormick F. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 35.Cox A.D., Der C.J. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- 36.Vos M.D., Ellis C.A., Bell A., Birrer M.J., Clark G.J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J Biol Chem. 2000;275:35669–35672. doi: 10.1074/jbc.C000463200. [DOI] [PubMed] [Google Scholar]
- 37.Khokhlatchev A., Rabizadeh S., Xavier R., Nedwidek M., Chen T., Zhang X.F., Seed B., Avruch J. Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol. 2002;12:253–265. doi: 10.1016/s0960-9822(02)00683-8. [DOI] [PubMed] [Google Scholar]
- 38.Elgendy M., Sheridan C., Brumatti G., Martin S.J. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42:23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Kaul A., Overmeyer J.H., Maltese W.A. Activated Ras induces cytoplasmic vacuolation and non-apoptotic death in glioblastoma cells via novel effector pathways. Cell Signal. 2007;19:1034–1043. doi: 10.1016/j.cellsig.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Sagi D., Feramisco J.R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 41.Porat-Shliom N., Kloog Y., Donaldson J.G. A unique platform for H-Ras signaling involving clathrin-independent endocytosis. Mol Biol Cell. 2008;19:765–775. doi: 10.1091/mbc.E07-08-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 43.Lambert J.M., Lambert Q.T., Reuther G.W., Malliri A., Siderovski D.P., Sondek J., Collard J.G., Der C.J. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 44.Radhakrishna H., Al Awar O., Khachikian Z., Donaldson J.G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 45.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overmeyer J.H., Young A.M., Bhanot H., Maltese W.A. A Chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol Cancer. 2011;10:69. doi: 10.1186/1476-4598-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson M.W., Overmeyer J.H., Young A.M., Erhardt P.W., Maltese W.A. Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J Med Chem. 2012;55:1940–1956. doi: 10.1021/jm201006x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimmer S., van Deurs B., Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–2962. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- 49.Naslavsky N., Weigert R., Donaldson J.G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trabbic C.J., Dietsch H.M., Alexander E.M., Nagy P.I., Robinson M.W., Overmeyer J.H., Maltese W.A., Erhardt P.W. Differential induction of cytoplasmic vacuolization and methuosis by novel 2-indolyl-substituted pyridinylpropenones. ACS Med Chem Lett. 2014;5:73–77. doi: 10.1021/ml4003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowman E.J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papini E., Bugnoli M., de Bernard M., Figura N., Rappuoli R., Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7:323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 54.Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 55.Clague M.J., Urbe S., Aniento F., Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- 56.Bayer N., Schober D., Prchla E., Murphy R.F., Blaas D., Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J Virol. 1998;72:9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feliciano W.D., Yoshida S., Straight S.W., Swanson J.A. Coordination of the Rab5 cycle on macropinosomes. Traffic. 2011;12:1911–1922. doi: 10.1111/j.1600-0854.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galvez T., Gilleron J., Zerial M., O'Sullivan G.A. SnapShot: mammalian Rab proteins in endocytic trafficking. Cell. 2012;151:234. doi: 10.1016/j.cell.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Poteryaev D., Datta S., Ackema K., Zerial M., Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Trowell O.A. The experimental production of watery vacuolation of the liver. J Physiol. 1946;105:268–297. [PMC free article] [PubMed] [Google Scholar]
- 61.Henics T., Wheatley D.N. Cytoplasmic vacuolation, adaptation and cell death: a view on new perspectives and features. Biol Cell. 1999;91:485–498. doi: 10.1016/s0248-4900(00)88205-2. [DOI] [PubMed] [Google Scholar]
- 62.Li C., Macdonald J.I., Hryciw T., Meakin S.O. Nerve growth factor activation of the TrkA receptor induces cell death, by macropinocytosis, in medulloblastoma Daoy cells. J Neurochem. 2010;112:882–899. doi: 10.1111/j.1471-4159.2009.06507.x. [DOI] [PubMed] [Google Scholar]
- 63.Cubells J.F., Rayport S., Rajendran G., Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nara A., Aki T., Funakoshi T., Uemura K. Methamphetamine induces macropinocytosis in differentiated SH-SY5Y human neuroblastoma cells. Brain Res. 2010;1352:1–10. doi: 10.1016/j.brainres.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 65.Nara A., Aki T., Funakoshi T., Unuma K., Uemura K. Hyperstimulation of macropinocytosis leads to lysosomal dysfunction during exposure to methamphetamine in SH-SY5Y cells. Brain Res. 2012;1466:1–14. doi: 10.1016/j.brainres.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Yang W.C.T., Strasser F.F., Pomerat C.M. Mechanism of drug-induced vacuolization in tissue culture. Exp Cell Res. 1965;38:495–506. doi: 10.1016/0014-4827(65)90373-3. [DOI] [PubMed] [Google Scholar]
- 67.Ref Ty Ruben Z., Fuller G.C., Knodle S.G. Disobutamide-induced cytoplasmic vacuoles in cultured dog coronary artery muscle cells. Arch Toxicol. 1984;55:206–212. doi: 10.1007/BF00316131. [DOI] [PubMed] [Google Scholar]
- 68.Henics T., Wheatley D.N. Vacuolar cytoplasmic phase separation in cultured mammalian cells involves the microfilament network and reduces motional properties of intracellular water. Int J Exp Pathol. 1997;78:343–354. doi: 10.1046/j.1365-2613.1997.320367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morissette G., Moreau E., Gaudreault R., Marceau F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther. 2004;310:395–406. doi: 10.1124/jpet.104.066084. [DOI] [PubMed] [Google Scholar]
- 70.Morissette G., Moreau E., Gaudreault R., Marceau F. N-substituted 4-aminobenzamides (procainamide analogs): an assessment of multiple cellular effects concerning ion trapping. Mol Pharmacol. 2005;68:1576–1589. doi: 10.1124/mol.105.016527. [DOI] [PubMed] [Google Scholar]
- 71.Aki T., Nara A., Uemura K. Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biol Toxicol. 2012;28:125–131. doi: 10.1007/s10565-012-9212-3. [DOI] [PubMed] [Google Scholar]
- 72.Morissette G., Lodge R., Marceau F. Intense pseudotransport of a cationic drug mediated by vacuolar ATPase: procainamide-induced autophagic cell vacuolization. Toxicol Appl Pharmacol. 2008;228:364–377. doi: 10.1016/j.taap.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Hotchin N.A., Cover T.L., Akhtar N. Cell vacuolation induced by the VacA cytotoxin of Helicobacter pylori is regulated by the Rac1 GTPase. J Biol Chem. 2000;275:14009–14012. doi: 10.1074/jbc.c000153200. [DOI] [PubMed] [Google Scholar]
- 74.Ricci V., Galmiche A., Doye A., Necchi V., Solcia E., Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol Biol Cell. 2000;11:3897–3909. doi: 10.1091/mbc.11.11.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cover T.L., Blanke S.R. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 76.Boquet P., Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20:165–174. doi: 10.1016/j.tim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Galmiche A., Rassow J., Doye A., Cagnol S., Chambard J.C., Contamin S., de Thillot V., Just I., Ricci V., Solcia E., Van O.E., Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willhite D.C., Cover T.L., Blanke S.R. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- 79.Ivanov A., Beers S.A., Walshe C.A., Honeychurch J., Alduaij W., Cox K.L., Potter K.N., Murray S., Chan C.H., Klymenko T., Erenpreisa J., Glennie M.J., Illidge T.M., Cragg M.S. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerny J., Feng Y., Yu A., Miyake K., Borgonovo B., Klumperman J., Meldolesi J., McNeil P.L., Kirchhausen T. The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2004;5:883–888. doi: 10.1038/sj.embor.7400243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barret J.M., Kruczynski A., Vispe S., Annereau J.P., Brel V., Guminski Y., Delcros J.G., Lansiaux A., Guilbaud N., Imbert T., Bailly C. F14512, a potent antitumor agent targeting topoisomerase II vectored into cancer cells via the polyamine transport system. Cancer Res. 2008;68:9845–9853. doi: 10.1158/0008-5472.CAN-08-2748. [DOI] [PubMed] [Google Scholar]
- 82.Brel V., Annereau J.P., Vispe S., Kruczynski A., Bailly C., Guilbaud N. Cytotoxicity and cell death mechanisms induced by the polyamine-vectorized anti-cancer drug F14512 targeting topoisomerase II. Biochem Pharmacol. 2011;82:1843–1852. doi: 10.1016/j.bcp.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 83.Choi E.W., Nayak L.V., Bates P.J. Cancer-selective antiproliferative activity is a general property of some G-rich oligodeoxynucleotides. Nucleic Acids Res. 2010;38:1623–1635. doi: 10.1093/nar/gkp1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bates P.J., Laber D.A., Miller D.M., Thomas S.D., Trent J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teng Y., Girvan A.C., Casson L.K., Pierce W.M., Jr., Qian M., Thomas S.D., Bates P.J. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 2007;67:10491–10500. doi: 10.1158/0008-5472.CAN-06-4206. [DOI] [PubMed] [Google Scholar]
- 86.Reyes-Reyes E.M., Teng Y., Bates P.J. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70:8617–8629. doi: 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Majno G., Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 88.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., III, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Overholtzer M., Mailleux A.A., Mouneimne G., Normand G., Schnitt S.J., King R.W., Cibas E.S., Brugge J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 91.Kroemer G., Dallaporta B., Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 92.Boya P., Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 93.Kirkegaard T., Jaattela M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Peterson D.A., Kimura H., Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 95.Berridge M.V., Herst P.M., Tan A.S. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 96.Schweichel J.-U., Merker H.J. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7:253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 97.Tapp R.L., Trowell O.A. The experimental production of watery vacuolation in the acinar cells of the submandibular gland. J Physiol. 1967;188:191–205. doi: 10.1113/jphysiol.1967.sp008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walker R.M., McElligott T.F. Furosemide induced hepatotoxicity. J Pathol. 1981;135:301–314. doi: 10.1002/path.1711350407. [DOI] [PubMed] [Google Scholar]
- 99.Orrenius S., Nicotera P., Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- 100.Nelson D.A., White E. Exploiting different ways to die. Genes Dev. 2004;18:1223–1226. doi: 10.1101/gad.1212404. [DOI] [PubMed] [Google Scholar]
- 101.Kitambi S.S., Toledo E.M., Usoskin D., Wee S., Harisankar A., Svensson R., Sigmundsson K., Kalderén C., Niklasson M., Kundu S., Aranda S., Westermark B., Uhrbom L., Andäng M., Damberg P., Nelander S., Arenas E., Artursson P., Walfridsson J., Forsberg Nilsson K., Hammarström L.G., Ernfors P. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell. 2014;157:313–328. doi: 10.1016/j.cell.2014.02.021. [DOI] [PubMed] [Google Scholar]