Abstract
Study Objectives:
The effect of common sedatives on upper airway physiology and breathing during sleep in obstructive sleep apnea (OSA) has been minimally studied. Conceptually, certain sedatives may worsen OSA in some patients. However, sleep and breathing could improve with certain sedatives in patients with OSA with a low respiratory arousal threshold. This study aimed to test the hypothesis that trazodone increases the respiratory arousal threshold in patients with OSA and a low arousal threshold. Secondary aims were to examine the effects of trazodone on upper airway dilator muscle activity, upper airway collapsibility, and breathing during sleep.
Design:
Patients were studied on 4 separate nights according to a within-subjects cross-over design.
Setting:
Sleep physiology laboratory.
Patients:
Seven patients with OSA and a low respiratory arousal threshold.
Interventions:
In-laboratory polysomnograms were obtained at baseline and after 100 mg of trazodone was administered, followed by detailed overnight physiology experiments under the same conditions. During physiology studies, continuous positive airway pressure was transiently lowered to measure arousal threshold (negative epiglottic pressure prior to arousal), dilator muscle activity (genioglossus and tensor palatini), and upper airway collapsibility (Pcrit).
Measurements and Results:
Trazodone increased the respiratory arousal threshold by 32 ± 6% (-11.5 ± 1.4 versus -15.3 ± 2.2 cmH2O, P < 0.01) but did not alter the apnea-hypopnea index (39 ± 12 versus 39 ± 11 events/h sleep, P = 0.94). Dilator muscle activity and Pcrit also did not systematically change with trazodone.
Conclusions:
Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold without major impairment in dilator muscle activity or upper airway collapsibility. However, the magnitude of change in arousal threshold was insufficient to overcome the compromised upper airway anatomy in these patients.
Citation:
Eckert DJ; Malhotra A; Wellman A; White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. SLEEP 2014;37(4):811-819.
Keywords: Arousal, lung, muscles, respiratory physiology, sedative, sleep-disordered breathing, upper airway
INTRODUCTION
Obstructive sleep apnea (OSA) is an increasingly common sleep related breathing disorder.1 OSA is characterized by the occurrence of repetitive respiratory events, each lasting > 10 sec, in which the upper airway narrows or closes restricting airflow during sleep. Termination of respiratory events is typically associated with a brief awakening from sleep (cortical arousal). In other instances, sufficient recruitment of pharyngeal dilator muscles occurs to restore airflow in the absence of cortical arousal.2
In addition to some degree of upper airway compromise, other nonanatomical traits importantly contribute to OSA pathogenesis in most patients with OSA.2–7 Under some circumstances, cortical arousal likely serves as a last line of defense to assist in rapidly terminating, severe respiratory events.2,8 However, awakening too easily to airway narrowing, i.e., having a low respiratory arousal threshold, may perpetuate breathing instability and subsequent respiratory events. Indeed, a low arousal threshold is likely to be an important nonanatomical contributor to the pathogenesis of OSA in as many as one third of all patients with OSA.2,3,5,9
A low respiratory arousal threshold could contribute to OSA via several mechanisms. First, repetitive cortical arousals disrupt sleep continuity and prevent deeper stages of sleep that are often associated with stable breathing.10 Second, a brisk ventilatory response can occur with arousal, which could perpetuate fluctuations in CO2 and lead to respiratory control instability.5,6,11,12 Lastly, periods of breathing stability during sleep, mediated by an increase in the arousal threshold and increased pharyngeal dilator muscle activation,13,14 occur intermittently in most patients with OSA.4 Thus, waking up prematurely to a relatively modest level of airway narrowing can limit the ability to build up sufficient respiratory stimuli to recruit the pharyngeal dilator muscles to open the upper airway, thereby achieving breathing stability.2,11 Importantly, if sleep could be maintained without arousal, relatively small increases in respiratory stimuli are predicted to stabilize breathing in many patients with OSA.11,15
Accordingly, pharmacologically increasing the threshold for cortical arousal to respiratory stimuli may facilitate breathing stability during sleep in some patients with OSA. Recent proof-of-concept clinical data provide support for this concept.9 In considering this approach, it is important to optimize the balance between retaining the beneficial components of arousal for potential times of need (e.g., during marked hypoxemia) versus minimizing the undesirable effects of repetitive arousals to relatively mild episodes of airway narrowing. In addition, sedative-induced decrements in pharyngeal dilator muscle activity also could worsen apnea. Given the high rates of sedative use in the community, particularly in the obese16, it is important to determine the effects of sedatives on upper airway physiology and breathing during sleep.
Trazodone, a serotonin antagonist and reuptake inhibitor, is the most commonly used sedative in the United States. Trazodone has been shown to reduce breathing disturbances without impairing upper airway muscle activity in an English bulldog model of OSA.17 However, the effects of trazodone on upper airway muscle activity, airway collapsibility, and breathing during sleep in humans has not been studied. A standard dose of trazodone (100 mg) increases the arousal threshold to chemical (hypercapnia) but not mechanical (transient continuous positive airway pressure [CPAP] reductions) stimuli in unselected patients with OSA (with a wide range of arousal thresholds).18 In the current detailed physiology study, we targeted the clinically relevant group of patients with OSA with low arousal thresholds, and hypothesized that trazodone would increase the arousal threshold to transient CPAP reductions. Secondary aims were to examine the effects of trazodone on pharyngeal dilator muscle activity and responsiveness, upper airway collapsibility, and breathing during sleep on an individual patient basis to provide mechanistic insight.
METHODS
Patients
Seven patients with OSA (1 female) who took part in a larger study investigating the multifactorial causes of OSA3 participated in the current subprotocol. Patients who were estimated to have a low respiratory arousal threshold (≥ 15 cmH2O) following preliminary visual inspection of the epiglottic pressure swings prior to arousal during a baseline physiology night were invited to participate in the current protocol. All patients had no history of allergy or an adverse reaction to trazodone or any other sedative, had been treated with CPAP for ≥ 3 mo, were otherwise healthy, and were not taking any medications known to affect sleep or the other variables measured in the study. There were no other specific inclusion/exclusion criteria. OSA was defined as an apnea-hypopnea index (AHI) ≥ 10 events/h sleep. Each participant provided informed written consent to participate in the protocol, which was approved by the Partners HealthCare Institutional Review Board.
Measurements and Equipment
Polysomnography
Electroencephalograms, electrooculograms, and surface electromyograms (EMG) were applied to score arousals, leg movements, and stage sleep.19,20 Abdominal and chest bands, a pulse oximeter, a position sensor, and airflow monitoring devices (nasal pressure plus thermistor) were applied to detect respiratory events according to standard criteria.21
Physiological Measurements
The nostrils were decongested (0.05% oxymetazoline HCl) and the clearer nostril was anesthetized (4% lidocaine HCl). An epiglottic pressure transducer (model MCP-500, Millar, Houston, TX) was advanced 1 to 2 cm below the base of the tongue. The transducer was taped to the nostril and passed through a port in a nasal CPAP mask (Gel Mask, Philips Respironics, Murrysville, PA). A pneumotachograph (model 3700A, Hans Rudolf Inc, Kansas City, MO) with a differential pressure transducer (Validyne Corporation, Northbridge, CA) in series was attached to the mask for accurate quantification of airflow. Bipolar EMG recordings of genioglossus and tensor palatini were obtained via two stainless steel fine-wire intramuscular electrodes (for each muscle) coated with Teflon (Cooner Wire Company, Chatsworth, CA). Two mm of Teflon was removed from the tip. Electrodes were inserted into the genioglossus muscle via a 25-gauge needle and into the tensor palatini at a 45° angle along the lateral surface of the medial pterygoid plate as described previously.22 Signals were acquired on a 1401-plus interface and Spike 2 software (Cambridge Electronic Design Ltd., Cambridge, UK).
Protocol
Each patient was studied overnight on four separate occasions at least 1 w apart. The five conditions were: (1) a standard in-laboratory overnight polysomnogram off CPAP to quantify the apnea-hypopnea index (AHI) at baseline; (2) a repeat polysomnogram off CPAP following 100 mg of trazodone immediately prior to sleep; (3) a detailed baseline physiology night to quantify the respiratory arousal threshold, upper airway dilator muscle activity and responsiveness, respiratory parameters (minute ventilation, upper airway resistance, and peak flow), and the critical closing pressure of the upper airway (Pcrit); and (4) a repeat detailed physiology night after receiving 100 mg of trazodone immediately prior to sleep.
Following instrumentation during the detailed physiology studies, maneuvers including swallows and tongue protrusions were performed to determine maximal genioglossus and tensor palatini EMG.23,24 Wakefulness upper airway muscle activity and respiratory parameters were acquired during quiet breathing on and off therapeutic CPAP prior to lights out at approximately 10:30. If required, the CPAP level was increased throughout the night to eliminate any sign of inspiratory flow limitation (according to the epiglottic pressure-flow relationship), yielding the holding pressure.
The respiratory arousal threshold, upper airway muscle responsiveness during sleep, and Pcrit were measured as described previously.3 Briefly, during stable, supine, non-rapid eye movement (NREM) sleep, the CPAP level was transiently reduced for up to 3 min to induce varying degrees of upper airway collapse using a modified CPAP device capable of delivering ± 20 cmH2O (Philips Respironics). Upper airway muscle activity and respiratory parameters at the CPAP holding pressure were acquired for 1 min prior to each transient CPAP reduction. The methods used to quantify these metrics are outlined below.
Data Analysis
CPAP usage was quantified objectively via a built-in compliance meter. Arousal, sleep scoring, and respiratory event detection were performed blinded to the study intervention. Raw genioglossus and tensor palatini EMG were rectified, moving-time averaged (100 ms), and expressed as a percentage of maximum activity.23,24 Peak (maximum during inspiration) and tonic EMG (nadir during expiration), and respiratory parameters were quantified on a breath-by-breath basis using custom-designed semi-automated software as described previously.25 Upper airway resistance (RUA) was quantified as the difference in mask versus epiglottic pressure during inspiration at a flow rate of 200 mL/s.25 Artifact-free respiratory and upper airway EMG variables were averaged for data collection periods of at least 5 min during quiet wakefulness with and without therapeutic CPAP and for the 60 sec prior to each CPAP drop while on therapeutic CPAP during NREM sleep.
Physiology parameters derived from transient reductions in CPAP were quantified as described previously.3 Briefly, the respiratory arousal threshold was quantified as the average nadir epiglottic pressure immediately prior to cortical arousal (> 3 sec of high-frequency activity on the EEG) for CPAP drops ≥ 10 sec combined with a ≥ 2 cmH2O decrement in epiglottic pressure preceding arousal. Genioglossus and tensor palatini muscle responsiveness during sleep was defined as the average slope of the relationship between peak EMG and nadir epiglottic pressure derived from all artifact-free breaths during CPAP drops. To quantify Pcrit, linear regression was performed between peak inspiratory flow and mask pressure for breaths three to five after each CPAP drop in cases where the breaths were flow limited.
Statistical Procedures
Statistical comparisons between baseline and trazodone nights for polysomnography and key physiological variables were performed using Student paired t-tests. Analysis of variance (ANOVA) for repeated measures was used to examine trazodone, condition (wakefulness no CPAP, wakefulness on CPAP, and NREM on CPAP) and trazodone × condition interaction effects on respiratory and upper airway EMG muscle activity (SPSS version 21, SPSS Inc., Chicago, IL). Where significant ANOVA effects were observed, post hoc comparisons were performed using Student paired t-tests. Statistical significance was inferred when P < 0.05. All data are reported as mean ± standard error of the mean.
RESULTS
Anthropometric and Polysomnographic Characteristics
The mean age and body mass index of the participants was 45 ± 3 y (range, 31-56) and 33 ± 2 kg/m2 (range, 25-39), respectively. Objective CPAP compliance during the 3 mo prior to the study was high at 5.9 ± 0.4 h (range, 5-8.2) per night. On average, OSA was severe (Table 1), although there was a wide range in severity between participants from 10 to 101 events per hour of sleep.
Table 1.
Polysomnography parameters during baseline and trazodone off continuous positive airway pressure
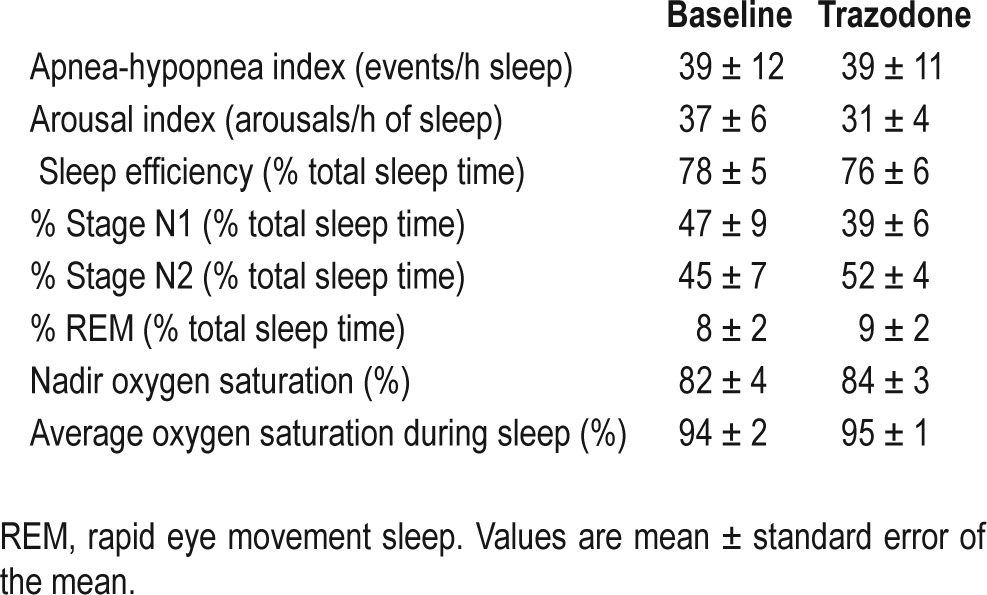
As a group, polysomnographic parameters were similar at baseline and following 100 mg of trazodone prior to sleep (Table 1). On average, the arousal index decreased following trazodone compared to baseline, but this difference was not statistically significant (P = 0.09). Average values for percent time spent in N1 sleep decreased, whereas percent N2 sleep increased (Table 1). However, these changes also were not statistically significant (P = 0.19 and P = 0.12, respectively).
Physiological Variables
Respiratory variables (minute ventilation, peak flow, and upper airway resistance) and upper airway dilator muscle activity during wakefulness (on and off CPAP) and during stable NREM sleep on therapeutic CPAP during baseline and trazodone nights are displayed in Table 2. There was no signifi-cant trazodone or trazodone by condition (wakefulness no CPAP, wakefulness on CPAP, and NREM on CPAP) interaction effects for respiratory or upper airway EMG activity.
Table 2.
Respiratory and upper airway muscle activity during wakefulness and sleep during baseline and trazodone
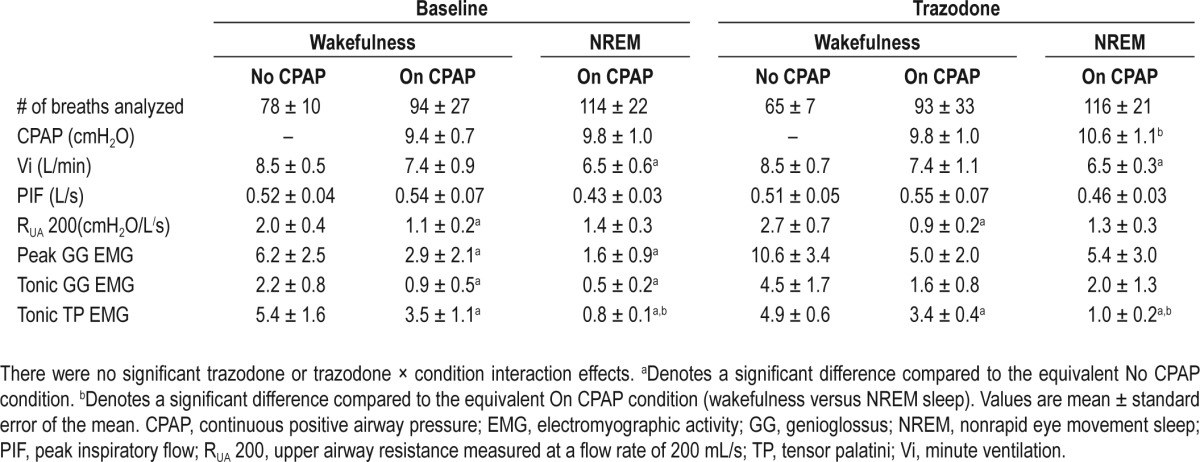
An additional 0.7 ± 0.2 cmH2O of CPAP was applied during NREM sleep compared to the wakefulness CPAP condition during the trazodone night. Minute ventilation decreased from wakefulness off CPAP to NREM sleep on CPAP during baseline and trazodone nights. Upper airway resistance decreased with CPAP compared to without CPAP during wakefulness on both study nights. Compared to the no-CPAP wakefulness condition, peak and tonic genioglossus EMG decreased with CPAP application during wakefulness and NREM sleep during the baseline night. Similar non-significant reductions in peak (P = 0.1 and P = 0.1, respectively) and tonic (P = 0.08 and P = 0.07, respectively) genioglossus EMG were observed during the trazodone night. Tensor palatini EMG decreased on CPAP compared to off CPAP during wakefulness, with further reductions in NREM sleep during both the baseline and trazo-done nights (Table 2).
A similar quantity of CPAP drops was delivered during the baseline versus the trazodone physiology night (17 ± 3 versus 20 ± 2, P = 0.18). The number of artifact-free CPAP drops and the change from the holding level during CPAP drops are displayed in Table 3. The respiratory arousal threshold increased by 3.8 ± 1 cmH2O during NREM sleep following trazodone. This equates to a 32 ± 6% increase from baseline (Figure 1). For CPAP drops that triggered an arousal, the average time to arousal from stimulus onset was not different following trazodone compared to baseline (52 ± 11 versus 35 ± 5 sec, P = 0.23). Similarly, the nadir oxygen saturation associated with CPAP drops that triggered arousal was not different following trazodone versus baseline (88 ± 1 versus 89 ± 1%, P = 0.27). Minute ventilation and peak inspiratory flow tended to be lower on the breath immediately prior to arousal during the trazodone night compared to baseline (Table 3). However, these differences were not statistically significant (P = 0.08 and P = 0.10, respectively). Upper airway EMG activity on the breath prior to arousal was not different during trazodone compared to the baseline night (all P > 0.5) (Table 3).
Table 3.
CPAP drop stimulus characteristics, respiratory, and upper airway muscle activity during the breath immediately prior to arousal
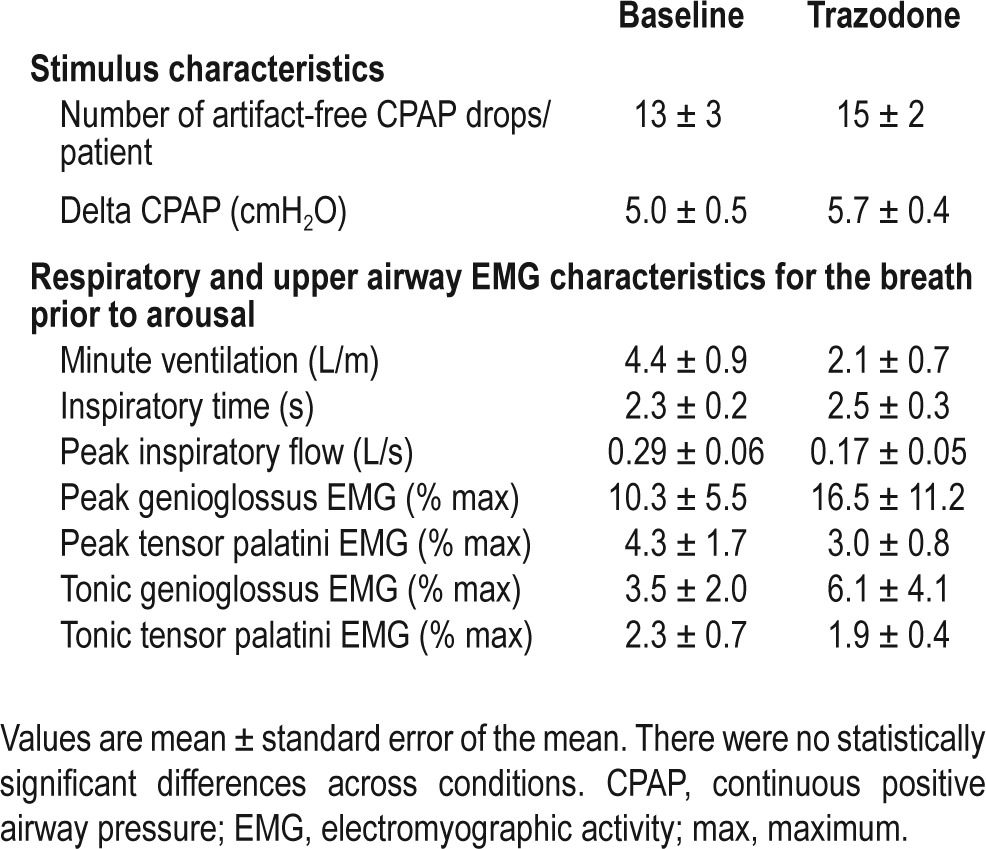
Figure 1.
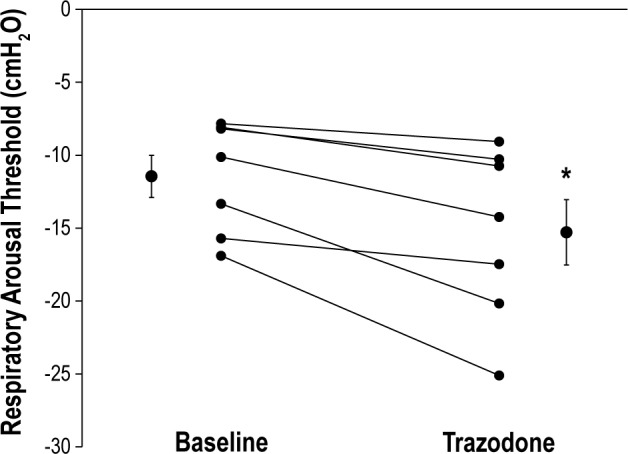
Respiratory arousal threshold values during baseline and following 100 mg of trazodone prior to sleep. Individual and mean ± standard error of the mean data are presented during both conditions. The asterisk denotes a significant difference between baseline and trazodone study nights.
Genioglossus and tensor palatini muscle responsiveness (%max EMG/-cmH20) during CPAP drops were not systematically different between baseline and trazodone conditions (Figure 2A and 2B, respectively). There were insufficient flow-limited breaths for the three to five breaths post-CPAP reduction to estimate Pcrit in one patient. Of the remaining six-paired comparisons, airway collapsibility was not different during the trazodone night compared to baseline (0.2 ± 0.8 versus -0.1 ± 1.5 cmH2O, P = 0.84).
Figure 2.
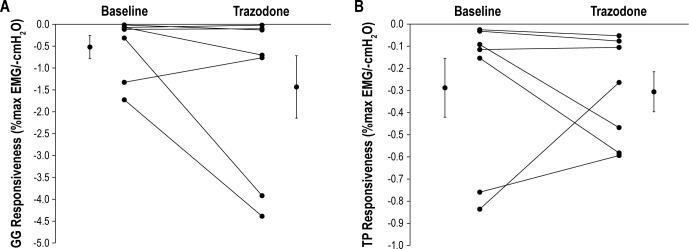
Slope of the relationship between (A) peak genioglossus (GG) and (B) peak tensor palatini (TP) muscle responsiveness (as a % of maximal activation) versus negative epiglottic pressure during continuous positive airway pressure reductions at baseline and following 100 mg of trazodone prior to sleep. Individual and mean ± standard error of the mean data are presented during both study nights. Note: no change in muscle responsiveness between conditions. Refer to text for further details.
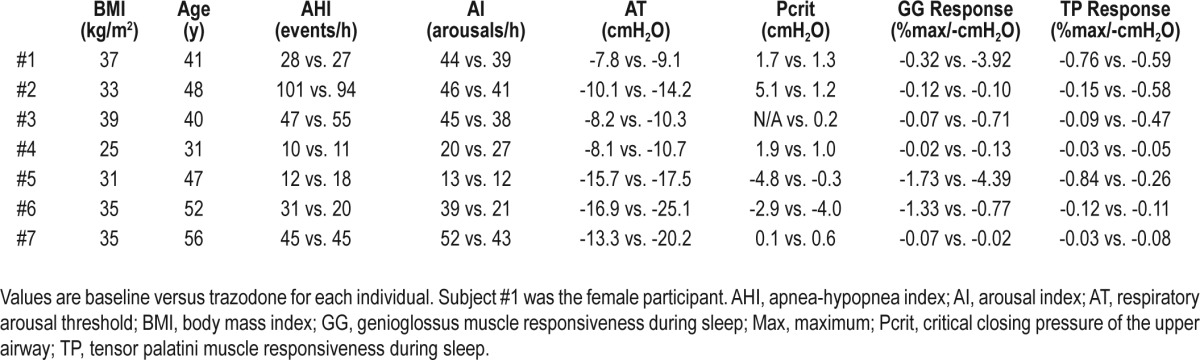
Individual anthropometric, AHI, arousal index, arousal threshold, Pcrit, and genioglossus and tensor palatini muscle responsiveness data at baseline and following trazodone are displayed in Table 4. All participants were estimated to have a low respiratory arousal threshold (≥ 15 cmH2O) after their baseline study. However, detailed post hoc analyses revealed that one participant had a slightly higher baseline value (-16.9 cmH2O, Table 4). Three of the participants had poor genioglossus muscle responsiveness at baseline according to previously defined criteria (< 0.1% of maximum EMG increase per negative cmH2O of epiglottic pressure).3 Of the six participants in whom baseline Pcrit data were available, four had positive values (Table 4).
Table 4.
Individual patient anthropometric, key polysomnographic, and physiology parameters during baseline versus trazodone
DISCUSSION
Approximately one third of patients with OSA have a low arousal threshold.2,3,5,9 The main finding of this detailed physiology study is that, on average, 100 mg of trazodone increases the respiratory arousal threshold by more than 30% compared to baseline in patients with OSA and a low arousal threshold. Conversely, 100 mg of trazodone does not cause major reductions in upper airway dilator muscle activity/ responsiveness during sleep, upper airway collapsibility (Pcrit), or breathing during sleep in patients with OSA and a low arousal threshold.
Effect of Trazodone on the Respiratory Arousal Threshold
An elevated respiratory arousal threshold with trazodone is consistent with the findings of an earlier study showing that 100 mg of trazodone increases the respiratory arousal threshold by almost 50% to elevated CO2 in nine unselected patients with OSA.18 However, in that same study, a nonsignificant 9% increase in the arousal threshold to CPAP drops was noted during the trazodone night. Several factors may account for the apparent disparity between the current findings and those of the previous study, including patient selection and study design. First, in the prior study, on average, OSA was more severe (mean AHI 52 events/h) and arousal thresholds were also higher, varying between ∼ -10 to -30 cmH2O compared to between ∼ -8 to -17 cmH2O in the current study. Second, in the current study we focused on CPAP drops (combined mechanoreceptor and chemical stimuli). The previous study used both CPAP drops and elevated CO2 stimuli during the same night. This combined approach resulted in approximately 50% fewer CPAP drops being delivered compared to the current study. The reduced number of replicate trials may have made it more difficult to observe a change in arousal threshold with trazodone in the prior study. Nonetheless, together these findings indicate that 100 mg of trazodone is capable of increasing the arousal threshold to respiratory stimuli in obstructive sleep apnea by 30-50%.
An increase in the arousal threshold with trazodone of this magnitude is consistent with changes in the arousal threshold observed with standard doses of other common sedatives. For example, 0.25 mg of triazolam increases the respiratory arousal threshold to airway occlusion by ∼ 33% in healthy individuals26 and by ∼ 24% in patients with severe OSA.27 Similarly, 3 mg of eszopiclone increases the arousal threshold during naturally occurring respiratory events by ∼ 29% in patients with OSA who have predominantly low to moderate arousal thresholds (mean ∼ -17 cmH2O).9
Effects of Trazodone on Upper Airway Muscle Activity and Airway Collapsibility
Upper airway muscle activity decreases during the transition from wakefulness to sleep.28–30 Similarly, with increasing concentrations of propofol anesthesia, a point is reached after which there is a sudden reduction in genioglossus muscle activity and airway collapsibility increases.31 However, when light sedation using midazolam is used to induce a sleeplike state, upper airway collapsibility is similar to natural sleep.32 This finding is consistent with the current study in which upper airway collapsibility and sleep parameters were similar before versus after trazodone.
Whether changes in upper airway dilator muscle activity with sedatives arise due to changes in sedation depth or via a direct inhibitory effect on upper airway muscle activity is uncertain. In vagotomized, decerebrate cats the benzodiazepine diazepam preferentially reduces hypoglossal nerve activity without changing phrenic nerve activity.33 Similar observations have been reported in lambs.34 Conversely, in rats, other sedatives (including pentobarbital, ketamine, lorazepam, and zolpidem) may actually increase genioglossus muscle activity under certain conditions.35–37 Phasic activity of the sternohyoid muscle during the transition from wakefulness to sleep was not impaired compared to placebo across three doses of trazodone in five English bulldogs.17
Few studies have examined the effects of common sedatives on upper airway muscle activity in humans. Ten mg of diazepam has been reported to reduce genioglossus muscle activity during CO2 rebreathing in otherwise healthy older males.38 However, this study was conducted during wakefulness and it is not clear if sleep intrusion confounded this observation. Compared with placebo, 100 mg of pentobarbital does not impair the genioglossus negative pressure reflex during wakefulness confirmed by EEG and is associated with increased phasic activity during sleep albeit with increased upper airway resistance and decreased airflow in healthy individuals.39 Similarly, in the current study, after trazodone administration, participants tended to tolerate a greater reduction in CPAP and an approximately 50% greater reduction in ventilation and peak airflow immediately prior to arousal. These findings presumably were mediated by the increase in arousal threshold although changes were not sufficient to worsen accompanying hypoxemia.
Minute ventilation, upper airway resistance, and muscle activity on therapeutic CPAP also were not different after trazo-done compared to baseline, nor was the responsiveness of the genioglossus and tensor palatini to combined mechanical and chemical stimuli over time during use of CPAP drops in the current study. These findings are consistent with data obtained in nine patients with OSA in whom minute ventilation and genioglossus muscle activity measured on therapeutic CPAP (and in response to chemical stimuli and a brief reduction in CPAP) were not different in the first half of the night compared to the second half of the night after receiving 10 mg of zopiclone.40 Interestingly, similar to observations in the English bulldog,17 genioglossus activity during NREM sleep remained similar to wakefulness levels on CPAP in the current study on the trazo-done night. Together, these findings suggest that standard doses of zopiclone and trazodone increase the respiratory arousal threshold but do not impair upper airway muscle activity/responsiveness, airway collapsibility, or basal breathing. However, the potential for higher doses and certain classes of sedatives (e.g., benzodiazepines) to reduce upper airway muscle activity preferentially and to increase airway collapsibility remains.
Mechanistic Insight Into the Effects of Trazodone on Sleep and Breathing: Potential Interindividual Differences
The lack of a systematic change in the AHI with a standard dose of a sedative is consistent with previous studies in unselected patients.41–47 In preselecting patients with OSA with low respiratory arousal thresholds, we anticipated that trazodone would reduce apnea severity similar to recent findings with eszopiclone whereby eight patients with OSA and a low arousal threshold had invariable reductions in their AHI.9 There are several possibilities that may account for the apparent disparity between studies, outlined in the next paragraphs.
As stated, there are a number of possible mechanisms by which a low respiratory arousal threshold may contribute to OSA pathogenesis, including: (1) sleep fragmentation preventing deeper, more stable sleep; (2) repetitive arousals leading to respiratory control instability; and (3) inadequate respiratory stimuli to recruit pharyngeal dilator muscles. Although both sedatives increased the respiratory arousal threshold by ∼ 30%, eszopiclone (a nonbenzodiazepine gamma-aminobutyric acid receptor agonist) and trazodone (a serotonin antagonist and reuptake inhibitor) are pharmacologically distinct. Accordingly, standard doses of these two agents may affect the aforementioned mechanisms and other key causes of OSA differently as will be discussed in the next paragraphs. Physiological differences between the patient groups studied also may be important.
First, although neither drug led to an increase in the proportion of N3 sleep, eszopiclone significantly decreased the proportion of lighter N1 sleep, improved sleep efficiency, and reduced the arousal index. Although similar nonsignificant reductions in N1 sleep and the arousal index were observed for trazodone in the current study, sleep efficiency did not change. Thus, trazo-done may be less effective than eszopiclone in stabilizing sleep in patients with OSA who have a low arousal threshold.
Second, the effects of eszopiclone on upper airway muscle activity, airway collapsibility, and respiratory control during sleep remain unknown. However, as mentioned, similar to the current findings with trazodone, zopiclone (which is pharmacologically and functionally similar to eszopiclone) recently has been shown not to reduce genioglossus muscle activity on CPAP during sleep in OSA.40 This observation suggests that a drug-specific effect of eszopiclone increasing genioglossus activity during sleep appears unlikely, although this possibility requires further investigation. Although trazodone did not cause major reductions in the neural output to the upper airway muscles as measured by EMG, it remains possible that, similar to recent observations with propofol anaesthesia,48 muscle effectiveness may have been reduced, mitigating any beneficial effects of an increase in the arousal threshold on breathing stability.
Nonetheless, in the absence of marked changes in basal tone of the upper airway muscles, passive collapsibility of the upper airway also would not be expected to increase with a sedative. Indeed, this assertion is consistent with the trazodone findings in the current study. Thus, differences in induced changes in airway collapsibility between the two agents also would appear unlikely. This is important because Pcrit is an essential determinant of the importance of nonanatomical contributors to OSA.3 Altering one non-anatomical trait such as the arousal threshold in isolation is not predicted to yield a major change in breathing stability unless Pcrit is below -2 cmH2O.3 In support of this concept, six of the seven participants had Pcrit values near or above zero following trazodone, with minimal change in their AHI following trazodone. Conversely, the patient (subject #6, Table 4) who had a post-trazodone Pcrit less than -2 cmH2O, had an approximately 35% reduction in his AHI with trazo-done. Ultimately, simple, noninvasive tools to identify patients with a low arousal threshold and the other key anatomical and non-anatomical contributors to OSA will be required to translate these concepts to clinical practice.7,49
Another important consideration is the extent to which a sedative increases the respiratory arousal threshold beyond what is considered to be low. On average, although there were between-patient differences, arousal thresholds post-trazodone remained low (∼ -15 cmH2O). Conversely, average arousal thresholds increased to the moderate range in the prior eszopi-clone study (∼ -20 cmH2O). Combining two classes of sedatives that act on different components of the arousal system in patients with OSA and a low arousal threshold may yield more pronounced changes in the respiratory arousal threshold and accompanying breathing stability, although this possibility remains untested. Interestingly, subject #6 in the current study had the biggest increase in arousal threshold with trazo-done to ∼ -25 cmH2O and the largest reduction in the arousal index (∼ 45%, Table 4). Had trazodone consistently led to a greater absolute shift in arousal threshold, this change may have promoted increased breathing stability, although the balance between preventing unnecessary repetitive arousals and retaining their protective role during times of need (e.g., severe hypoxemia) may begin to be comprised. Differences in plasma concentration levels of the drug also are likely to be important in determining their effects on sleep and breathing. For example, plasma concentrations following a standard dose of morphine can vary twentyfold between individuals, and this variable predicts morphine-related changes in key polysomno-graphic variables during sleep in OSA.50
Finally, the effect of trazodone and other sedatives on respiratory control, including hypercapnic and hypoxic ventila-tory responses during sleep, remains untested and may differ between agents. However, basal breathing during sleep on CPAP appears to be minimally affected by zopiclone40 or trazo-done in patients with OSA. Other sedatives, including benzodiazepines, trazodone, and zolpidem, have been shown to reduce the severity of certain forms of central sleep apnea that may be due, at least in part, to a reduction in respiratory control instability (loop gain), although the precise underlying mechanisms remain uncertain.51–55 Thus, the effects of sedatives on respiratory control are worthy of future investigation.
Methodological Considerations
Given the complexity of the measurements performed, a relatively small number of participants were tested. Thus, the sample size for the current study is insufficient to permit generalization of the findings to all patients with OSA who have a low arousal threshold. Nonetheless, the study was appropriately powered (> 80%) to detect a 3.5- cmH2O difference with trazodone in our primary outcome measure of arousal threshold based on a sample size of seven with a between-condition standard deviation of 2.7 cmH2O. In addition, although AHI has previously been shown to decrease in eight patients with OSA who had a low arousal threshold following administration of eszopiclone,9 the primary goal of collecting polysomnographic data in the current protocol was to provide insight into potential changes in sleep and breathing with trazodone by linking such changes with detailed physiological parameters on an individual patient basis. Thus, determining the effects of trazodone on OSA severity per se may well require larger, more appropriately designed studies. Nonetheless, these data provide important mechanistic insight into the ability of a standard dose of trazodone to increase the respiratory arousal threshold and the physiological determinants that are likely to be important in mediating its effects on sleep and breathing in OSA.
Data were also acquired during NREM sleep and predominantly in men with a wide range in body weight/body mass indices. Recent studies have shown that a standard dose of zolpidem may be longer lasting and more potent in women than in men.56 Accordingly, the effects of REM sleep, body weight/ body mass index, and potential sex differences in the effects of common sedatives on sleep and breathing in OSA will be important to investigate in future studies. Finally, this was a nonrandomized physiological study and participants were not blinded to the study intervention. Thus, order and subjective influences could have biased the findings. However, study conditions were standardized between visits and sleep efficiency was similar, suggesting a lack of an order effect. Polysomnographic records also were scored blinded to the study condition and the key physiological variables were derived during sleep. Thus, subjective influences would appear highly unlikely under these conditions.
SUMMARY
The findings of this study provide novel mechanistic insight into the effects of a common sleeping agent on the propensity for respiratory arousal and its physiological effects on the upper airway and sleep disordered breathing for which data are scarce. Specifically, the findings show that 100 mg of trazodone increases the respiratory arousal threshold in patients with OSA who have a low respiratory arousal threshold without major impairment in upper airway dilator muscle activity/responsiveness, airway collapsibility, and breathing during sleep. Given the high rates of sedative use in the community, particularly in the obese, these findings are important.
DISCLOSURE STATEMENT
This study was funded by NIH 5R01HL048531. The authors have also received funding support from AHA (10SDG3510018), NHMRC of Australia (510392, 1049814), and NIH (RO1 102231, R01 HL090897, NIH K24 HL 093218, NIH P01 HL 095491, NIH R01HL110350, and NIH R01 HL085188). The Harvard Catalyst is funded by UL1 RR 025758-01. A modified continuous positive airway pressure machine was provided by Philips Respironics and was used to obtain the physiological measurements performed in this study. Dr. Eckert has consulted for Apnex. Dr. Malhotra has consulted for and/or received research support from Philips Respironics, Pfizer, SHC, SGS, Apnex, Apnicure, but has relinquished all personal outside income since May 2012. Dr. Wellman has consulted for Apnex, Apnicure, Philips Respironics, Galleon, and Sova. His interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr. White was Chief Medical Officer of Philips Respironics until December 2012 and is now Chief Scientific Officer of Apnicure. Work for this study was performed at Brigham and Women's Hospital, Division of Sleep Medicine, Sleep Disorders Program and Harvard Medical School, Boston, MA.
ACKNOWLEDGMENTS
The authors are grateful for contributions and valuable technical support on this project provided by Pamela DeYoung, Louise Dover, Lauren Hess, Geoffrey Kehlmann, Salonee Parikh, Erik Smales, Scott Smith, Karen Stevenson, and Patricia Kelly of the Brigham and Women's Hospital Investigational Drug Service who prepared the trazodone for this study. Dr Eckert is supported by a R.D. Wright Biomedical Fellowship from the National Health and Medical Research Council of Australia (1049814).
REFRENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Eckert DJ, Younes M. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol. 2014;116:302–13. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 3.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 5.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 6.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 7.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–37. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White DP, Younes M. Obstructive Sleep Apnea. Comprehensive Physiology. 2012;2:2541–94. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 9.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 12.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–9. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 13.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratnavadivel R, Stadler D, Windler S, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010;65:107–12. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 15.Younes M, Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol. 2012;112:249–58. doi: 10.1152/japplphysiol.00312.2011. [DOI] [PubMed] [Google Scholar]
- 16.Vozoris NT, Leung RS. Sedative medication use: prevalence, risk factors, and associations with body mass index using population-level data. Sleep. 2011;34:869–74. doi: 10.5665/SLEEP.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160:1659–67. doi: 10.1164/ajrccm.160.5.9812007. [DOI] [PubMed] [Google Scholar]
- 18.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 20.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 21.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Eckert DJ, Saboisky JP, Jordan AS, White DP, Malhotra A. A secondary reflex suppression phase is present in genioglossus but not tensor palatini in response to negative upper airway pressure. J Appl Physiol. 2010;108:1619–24. doi: 10.1152/japplphysiol.01437.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol. 2011;111:1644–53. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–64. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–60. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 27.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 28.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas CL, Jordan AS, Heckel L, et al. Discharge patterns of human tensor palatini motor units during sleep onset. Sleep. 2012;35:699–707. doi: 10.5665/sleep.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogel RB, Trinder J, White DP, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005;564:549–62. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology. 2009;111:63–71. doi: 10.1097/ALN.0b013e3181a7ec68. [DOI] [PubMed] [Google Scholar]
- 32.Genta PR, Eckert DJ, Gregorio MG, et al. Critical closing pressure during midazolam-induced sleep. J Appl Physiol. 2011;111:1315–22. doi: 10.1152/japplphysiol.00508.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–5. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 34.Wood GA, Harding R. The effects of pentobarbitone, diazepam and alcohol on oral breathing in neonatal and mature sheep. Respir Physiol. 1989;75:89–104. doi: 10.1016/0034-5687(89)90089-3. [DOI] [PubMed] [Google Scholar]
- 35.Park E, Younes M, Liu H, Liu X, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep. 2008;31:355–65. doi: 10.1093/sleep/31.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 37.Eikermann M, Grosse-Sundrup M, Zaremba S, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leiter JC, Knuth SL, Krol RC, Bartlett D., Jr The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–9. doi: 10.1164/arrd.1985.132.2.216. [DOI] [PubMed] [Google Scholar]
- 39.Eikermann M, Eckert DJ, Chamberlin NL, et al. Effects of pentobarbital on upper airway patency during sleep. Eur Respir J. 2010;36:569–76. doi: 10.1183/09031936.00153809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34:1061–73. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–70. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–5. [PubMed] [Google Scholar]
- 43.Wang D, Marshall NS, Duffin J, et al. Phenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulness. J Sleep Res. 2011;20:526–32. doi: 10.1111/j.1365-2869.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 44.Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007;11:159–64. doi: 10.1007/s11325-006-0096-4. [DOI] [PubMed] [Google Scholar]
- 45.Gooneratne NS, Gehrman P, Gurubhagavatula I, Al-Shehabi E, Marie E, Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo-controlled pilot study. J Clin Sleep Med. 2010;6:572–80. [PMC free article] [PubMed] [Google Scholar]
- 46.Frase L, Schupp J, Sorichter S, Randelshofer W, Riemann D, Nissen C. Sodium oxybate-induced central sleep apneas. Sleep Med. 2013;14:922–4. doi: 10.1016/j.sleep.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 47.George CF, Feldman N, Inhaber N, et al. A safety trial of sodium oxybate in patients with obstructive sleep apnea: Acute effects on sleep-disordered breathing. Sleep Med. 2010;11:38–42. doi: 10.1016/j.sleep.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Dotan Y, Pillar G, Tov N, et al. Dissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesia. Eur Respir J. 2013;41:74–84. doi: 10.1183/09031936.00159611. [DOI] [PubMed] [Google Scholar]
- 49.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–22. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Somogyi AA, Yee BJ, et al. The effects of a single mild dose of morphine on chemoreflexes and breathing in obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185:526–32. doi: 10.1016/j.resp.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Grimaldi D, Provini F, Vetrugno R, et al. Idiopathic central sleep apnoea syndrome treated with zolpidem. Neurol Sci. 2008;29:355–7. doi: 10.1007/s10072-008-0995-1. [DOI] [PubMed] [Google Scholar]
- 53.Nickol AH, Leverment J, Richards P, et al. Temazepam at high altitude reduces periodic breathing without impairing next-day performance: a randomized cross-over double-blind study. J Sleep Res. 2006;15:445–54. doi: 10.1111/j.1365-2869.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 54.Carley DW, Trbovic SM, Radulovacki M. Diazepam suppresses sleep apneas in rats. Am J Respir Crit Care Med. 1998;157:917–20. doi: 10.1164/ajrccm.157.3.9710006. [DOI] [PubMed] [Google Scholar]
- 55.Salazar-Grueso EF, Rosenberg RS, Roos RP. Sleep apnea in olivopontocerebellar degeneration: treatment with trazodone. Ann Neurol. 1988;23:399–401. doi: 10.1002/ana.410230417. [DOI] [PubMed] [Google Scholar]
- 56.FDA requires lower dosing of zolpidem. Med Lett Drugs Ther. 2013;55:5. [PubMed] [Google Scholar]


