Abstract
Objective:
To evaluate the impact of nocturia on the therapeutic response of chronic insomnia to behavioral treatment in older adults.
Methods:
Secondary analysis of a randomized clinical trial designed to assess the efficacy of brief behavioral treatment of insomnia (BBTI) vs. an information-only control (IC) in 79 community-dwelling older adults with chronic insomnia. For the current analysis, participants were stratified into 2 groups: those with self-reported nocturia at baseline i.e., ≥ 1 void/night (N = 30; 16 IC, 14 BBTI) and those without nocturia (N = 49; 24 IC, 25 BBTI). We then determined the impact of BBTI on sleep, sleep quality, and nocturia as assessed by self-report, actigraphy, and polysomnography
Results:
Individuals without baseline nocturia responded well to BBTI with significant decrease in sleep latency, wake after sleep onset, and total sleep time assessed by sleep diary and actigraphy; these changes were significantly greater than those in the IC group. In comparison, changes in the same sleep parameters among participants with nocturia were not significantly different from the IC control. Although BBTI showed significant improvement in sleep quality in groups with and without nocturia (as assessed by PSQI and sleep diary), the effect size of these improvements was larger in those without nocturia than in those with nocturia (PSQI d = 0.82 vs. 0.53, diary sleep quality d = 0.83 vs. 0.51).
Conclusions:
These secondary analyses suggest that brief behavioral treatment of insomnia may be more efficacious in improving insomnia in participants without nocturia. Addressing nocturia may improve the efficacy of behavioral insomnia treatment.
Citation:
Tyagi S; Resnick NM; Perera S; Monk TH; Hall MH; Buysse DJ. Behavioral treatment of chronic insomnia in older adults: does nocturia matter? SLEEP 2014;37(4):681-687.
Keywords: Chronic insomnia, nocturia, elderly
INTRODUCTION
Insomnia is common. It is characterized by persistent difficulty initiating or maintaining sleep or by non-restorative sleep sufficient to cause daytime distress and impaired social or occupational functioning.1 Difficulty maintaining sleep is a particularly common feature among older adults, with a prevalence of 40% to 70%.2,3 There are many reasons for geriatric insomnia, including other sleep disorders (e.g., sleep apnea), spending too much time in bed, and an age-related decrease in sleep continuity and depth, as well as changes in circadian rhythms.4,5 Exogenous factors, such as medications, and excessive light or noise can contribute, as can endogenous factors such as pain, anxiety and stress.6 Reduced arousal threshold also contributes to insomnia in the elderly.7,8
One of the most common causes of insomnia in older adults is nocturia. Nocturia is defined by the International Continence Society (ICS) as waking at least once nightly to void, with each void preceded and followed by sleep.9 Three-quarters of participants in a survey of US residents 18 years or older cited the need to void as the most frequent reason for nocturnal awakenings.10 The proportion of participants reporting nocturia as the primary reason for nighttime awakening increased with age: 39.9% in those aged 18-44 years, 57.6% in those aged 45-64 years, and 77.1% in those aged 65 or above.10 Nocturia is an independent predictor of both insomnia (75% increased risk) and reduced sleep quality (71% increased risk).11 Patients bothered by nocturia also report difficulty returning to sleep after an awakening. In addition, among nocturia patients, wakefulness after sleep onset is increased, subsequent awakenings tend to last longer, and subjective restedness after sleep is significantly decreased.11–14
Despite this growing evidence, the role of nocturia in the etiology and treatment of geriatric insomnia has been largely neglected. In fact, early epidemiologic studies examining sleep complaints in older adults sometimes excluded participants who reported waking to void.2
Although insomnia and nocturia are intricately linked, to our knowledge no study has explored the impact of nocturia on treatment of insomnia. Therefore, we conducted a secondary analysis of a randomized controlled trial of behavioral treatment of chronic insomnia in older adults. We postulated that therapeutic response to behavioral treatment of chronic insomnia would be reduced among individuals with concurrent nocturia.
METHODS
Study Design
The primary study was a randomized controlled trial designed to test the short-term efficacy of brief behavioral treatment of insomnia (BBTI) as compared with an information-only control condition (IC) among older individuals with chronic insomnia.15 After baseline clinical evaluation, participants were randomly assigned to BBTI or IC. Primary outcomes were assessed at the end of a 4-week intervention.
Participants
Study procedures have been described previously.15 Briefly, 82 older adults who met the criteria for primary insomnia in the Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR])16 were recruited from the community via advertisement (n = 61) and from primary care practices (n = 21). The diagnosis of primary insomnia was based on response to questionnaires and structured clinician interviews. The criteria for insomnia included difficulty initiating or maintaining sleep for at least one month despite adequate opportunity and circumstances for sleep, as well as associated daytime symptoms causing impairment in social, occupational, or other important areas of functioning. To enhance generalizability, the primary study did not apply quantitative self-report or objective sleep criteria for sleep latency, wakefulness, or total sleep time, or the DSM-IV-TR exclusion criteria for medical or psychiatric disorders. Therefore, the participants would be considered to have “comorbid insomnia.” But those with untreated psychiatric or substance abuse disorder, recent hospitalization (within past 2 weeks), ongoing chemotherapy or other cancer treatment, and life expectancy < 6 months were excluded. Subjects with significant cognitive impairment (Mini-Mental State Examination [MMSE] score ≤ 24) were also excluded because such impairment can limit an individual's ability to comply with behavioral sleep interventions, and some of the interventions (e.g., restricting time in bed) could potentially worsen this underlying condition. People with untreated obstructive sleep apnea syndrome (defined by apneahypopnea index > 15 during full polysomnographic monitoring), narcolepsy, or restless legs syndrome were also excluded. Individuals who were treated for obstructive sleep apnea syndrome, narcolepsy, or restless legs syndrome were not excluded, but the final sample did not include any such participants.
For the current analysis, we stratified all participants by the presence or absence of nocturia (per ICS definition), which was assessed by self-report at the time of recruitment (i.e., reported waking up ≥ 14 times on the 14-day sleep diary completed at the time of inclusion). The numbers of participants analyzed in each subgroup are presented in Figure 1.
Figure 1.
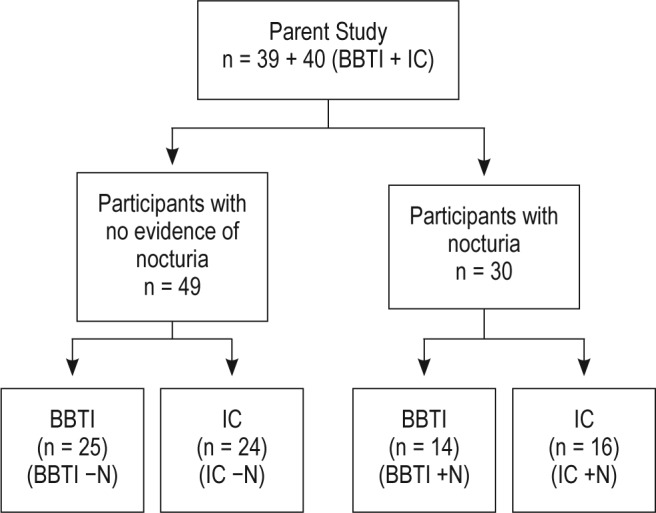
Analytic design. BBTI, brief behavioral treatment of insomnia; IC, information-only control; −N, without nocturia; +N, with nocturia.
Interventions
Participants were randomly assigned to BBTI or IC by permuted block design. BBTI included 2 individual sessions in which a therapist met with the participant; treatment elements were presented in the first session, which lasted 45-60 min, and in a 30-min reinforcement session 2 weeks later. Participants received 20-min telephone calls after 1 and 3 weeks to review sleep-wake times and adherence and to troubleshoot any difficulties.
BBTI constitutes the core of efficacious multimodal behavioral treatments for insomnia. It focuses on sleep education, healthy sleep practices, and behavioral interventions to improve insomnia. The 4 main behavioral components of BBTI are: (1) mildly restricting time in bed (self-reported sleep time plus 30 min, with a minimum of 6 h); (2) establishing a regular wake-up time every day of the week, regardless of sleep duration; (3) going to bed only when sleepy; and (4) getting out of bed if not asleep.
The information control (IC) group received 3 informational brochures developed and published by the American Academy of Sleep Medicine: Insomnia, Sleep as We Grow Older, and Sleep Hygiene. The articles address sleep issues in older adults and discuss behavioral interventions similar to BBTI. This group did not receive any individualized instructions, but did receive 10-min follow-up telephone calls to encourage continued participation. IC participants' queries were not specifically addressed as they were in the BBTI group; rather, they were referred back to the printed material. At the conclusion of the 4-week intervention period, the IC group was offered BBTI. The primary study was approved by institutional review board, and all participants provided written informed consent.
Clinical Assessment
Detailed demographic information and participant history were obtained by self-report questionnaires and clinician-administered interviews. All subjects completed 14-day sleep diaries and all underwent actigraphy and in-home polysomnography (PSG) prior to treatment and 4 weeks after the start of intervention. BBTI responders were contacted 6 months later for follow-up in the primary study, but these data were not used for our secondary analysis.
Measures
The measures obtained in the primary study have been described previously in detail.15 Briefly, demographic information included age, sex, and medical status which was evaluated based on response to a comorbidity questionnaire adapted from the Charlson Comorbidity Index,17,18 with an expanded range of health conditions including hypertension, hyperlipidemia, cardiovascular disease, diabetes, and genitourinary conditions. Medication use was collected via clinician interview and medications were classified into 15 categories: we examined those relevant to nocturia, including cardiac drugs, anti-hyper-tensives, and diuretics. Neuropsychiatric status was characterized using the 17-item Hamilton Rating Scale for Depression (HRSD).19 For our analysis we excluded the sleep questions of HRSD to avoid redundancy with sleep outcomes. Subjective sleep quality was characterized with the Pittsburgh Sleep Quality Index (PSQI).20
Each subject completed the Pittsburgh sleep diary at inclusion and during weeks 3 and 4 following the start of the intervention.21 The Pittsburgh sleep diary is a self-report measure of bed time, rise time, sleep onset latency (SOL), wakefulness after sleep onset (WASO), and sleep quality (visual analogue scale). Reported sleep parameters were used to calculate total sleep time (TST) and sleep efficiency (SE calculated as 100 × TST/time in bed). Diaries were used to stratify subjects with and without nocturia. Every morning over the 14-day period, the participants documented in the diary how many of their awakenings were to void. The sleep diary did not differentiate between a convenience void and an urge resulting in an awakening; however, the diary did specifically ask the reason for each awakening, options being: to use the bathroom, noises, discomfort or physical complaint, another reason, or no special reason.21
The primary outcomes of the original study15 were 4 categorical outcomes assessed using PSQI scores and sleep diary SE. These outcomes were: remission (response criterion plus final PSQI score < 5 and sleep diary SE > 85%); response (decrease in PSQI score > 3 points or increase in sleep diary SE of > 10%); partial response (improvement in PSQI or SE but worsening in other measures); and nonresponse (decrease in PSQI < 3 points and increase in sleep diary SE < 10%).
As an objective measure of sleep-wake patterns, wrist actigraphy data (Actiwatch-64; Minimitter, Bend, Oregon) were collected concurrently with the sleep diaries for 14 days. One-min epochs were analyzed with Actiware software, version 5.04 using sleep diary data to identify bed time and wake time. Actigraphy-assessed variables analyzed in the present report include SOL, WASO, TST, and SE.
Polysomnography was conducted in participants' homes at their usual sleep times using Compumedics Siesta (Compu-medics Limited, Abbotsford, Victoria, Australia) monitors. One screening PSG was obtained to quantify apnea and periodic limb movements, and 2 additional consecutive nights were collected at both pretreatment baseline and after treatment. Details of PSG recording and scoring have been described previously.15 The polysomnography outcome measures included SOL, WASO, TST, and SE averaged over 2 nights.
Statistical Analysis
We used independent sample t-tests and χ2 tests, respectively, to compare continuous and categorical participant characteristics and baseline measures between the 2 intervention arms. We used paired sample t-tests to measure statistical significance of pre- to post-intervention change in each of the 2 arms and independent sample t-tests to make unadjusted comparisons between intervention arms of pre- to post-intervention change. To make adjusted comparisons, we fitted a series of analysis of covariance models with pre- to post-intervention change in each outcome as the dependent variable; intervention arm (BBTI/ IC), baseline presence of nocturia (yes/no) and their interaction as independent factors of interest; and the pre-intervention value of the outcome as a covariate. Appropriately constructed means contrasts were used to obtain adjusted between-intervention differences separately for those with and without nocturia at baseline. Effect sizes were computed as standardized means differences (Cohen's d) and interpreted as 0.2-0.3 = small, 0.5-0.6 = moderate and 0.8-0.9 = large using Cohen's (1977) criteria. Four categorical outcomes, which were the primary outcomes in the original study,15 were assessed across groups using χ2 tests. SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses.
RESULTS
Of the 79 participants enrolled in the primary study, 30 (38 %) met the ICS definition of nocturia at baseline (16 in IC group and 14 in BBTI group). Table 1 displays their baseline characteristics, pertinent medical conditions, and medication use patterns. At baseline, subjects in BBTI and IC groups were comparable in age, BMI, medical conditions, and medication use. Among those with nocturia, there were significantly more males in the BBTI group (43% vs. 19%); the BBTI group also had a higher prevalence of self-reported genitourinary disorders at baseline (86% vs. 31%), including benign prostatic hypertrophy, prostatectomy, poor stream, urinary retention, frequency, urgency, urinary incontinence, erectile dysfunction, and nephrolithiasis. Among those with nocturia, 43% had only nocturia without any self-reported lower urinary tract symptoms. Among those without nocturia, the gender distribution and prevalence of genitourinary disorders between BBTI and IC groups were comparable.
Table 1.
Baseline demographics and clinical characteristics of participants with and without nocturia
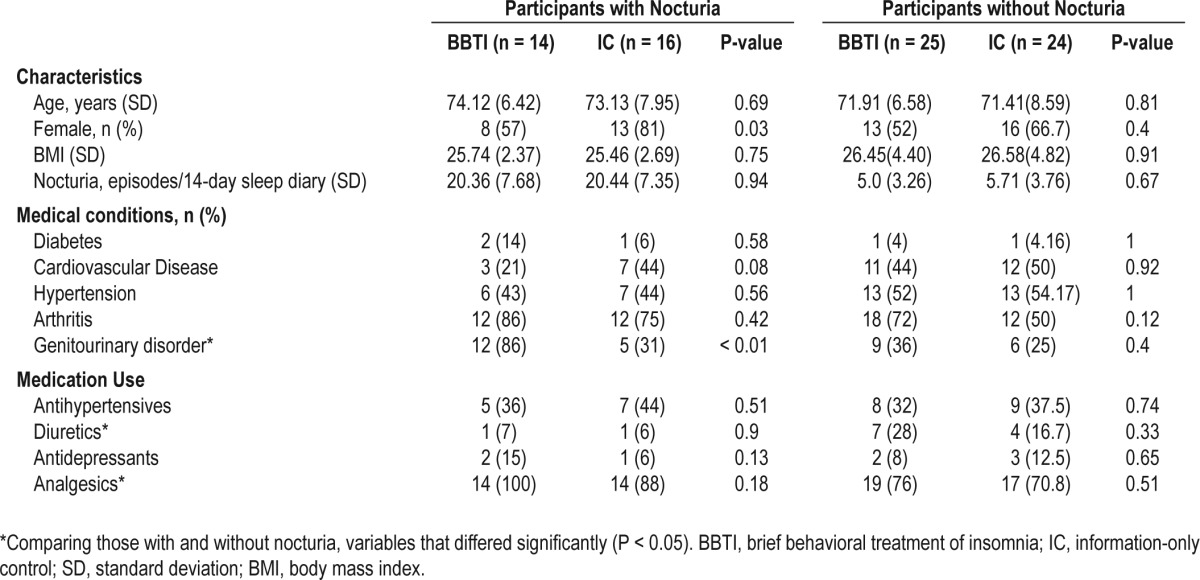
At baseline, nocturia status was comparable between the 2 intervention groups. Among those with nocturia, the average number of self-reported nighttime awakenings to void (summed over the 14 nights) among the IC group was 20.44 (SD 7.35), compared to 20.36 (SD 7.68) in the BBTI group (P = 0.94). Among participants without nocturia, the average number of self-reported nighttime awakenings to void over 14 days among the IC group was 5.71 (SD 3.76), compared to 5.0 (SD 3.26) in the BBTI group (P = 0.67).
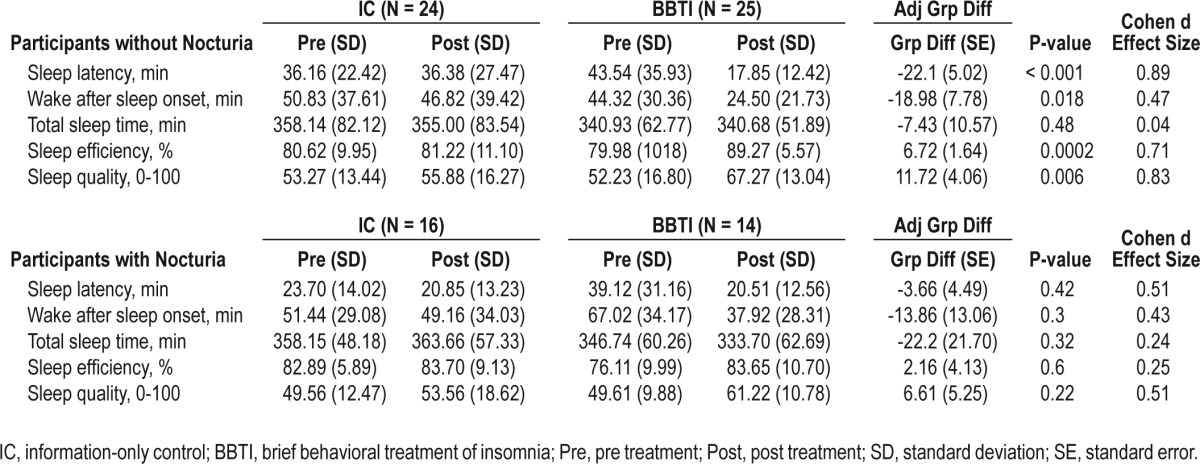
There was a significant nocturia × intervention interaction for sleep latency as assessed by sleep diary (P = 0.02). Statistical significance of the interaction term for other outcome measures ranged from P = 0.10-0.65. Among those without nocturia, BBTI participants reported a significantly greater improvement than IC participants in sleep diary measures of sleep latency, wake after sleep onset, and sleep quality (all P < 0.05), but not in total sleep time (P = 0.48). Improvement in sleep efficiency was most notable with a large effect size (P < 0.01, d = 0.71). Among those with nocturia, improvement in the BBTI group was attenuated with a smaller effect size for every parameter, and the differences between the BBTI and IC groups were not statistically significant (Table 2).
Table 2.
Sleep diary measures
Similar results emerged from analyses using actigraphy (Table 3). Polysomnographically assessed sleep outcomes showed contrary results. Participants without nocturia experienced an increased WASO in the BBTI group compared to IC, though the change did not reach statistical significance. On the contrary, despite increase in sleep latency, WASO was significantly improved in the BBTI group with nocturia compared to IC (Table 4). Given the discrepant results between diary and PSG measures, we investigated whether PSG procedures themselves may have affected sleep behavior and thereby nocturia. We compared diary reports of time in bed on nights with and without PSG and found that time in bed was less on PSG nights than on non-PSG nights in both the BBTI and IC groups at the post-treatment assessment, but these differences did not reach statistical significance in either group.
Table 3.
Actigraphy measures
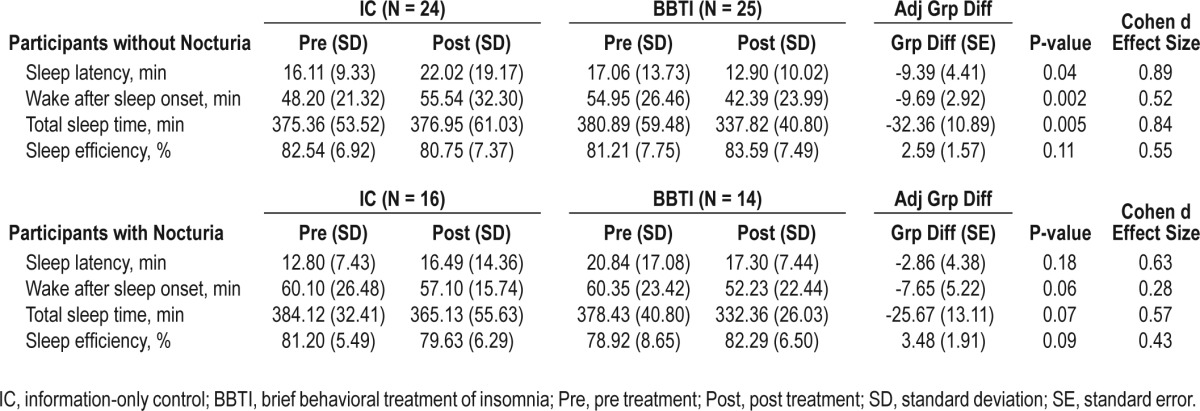
Table 4.
Polysomnography measures
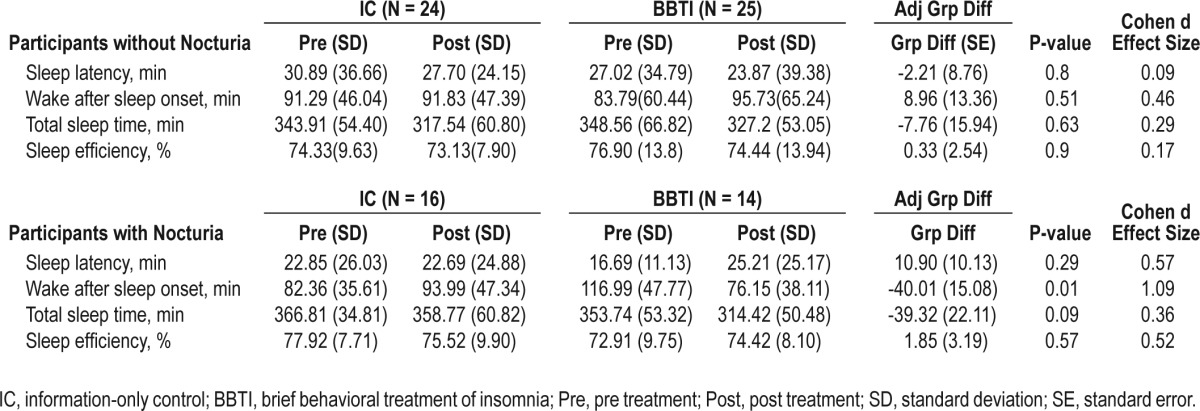
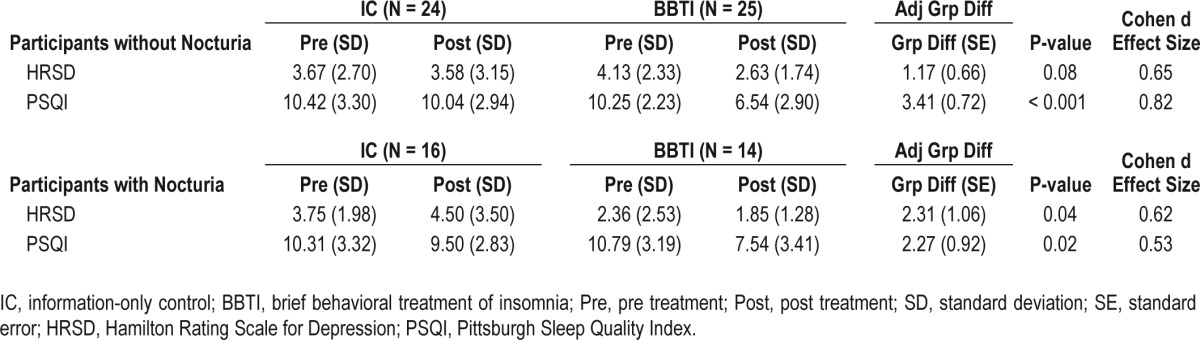
Although BBTI improved sleep quality assessed by PSQI in both those with and without nocturia, the improvement was larger in those without nocturia (Table 5). Participants in both the nocturic and non-nocturic groups reported significantly greater improvement in their scores on the Hamilton Rating Scale for Depression (excluding sleep questions).
Table 5.
General clinical and sleep measures
Using the 4 categorical outcomes, we found that outcomes for BBTI were significantly better than those for the IC group, both in those with and without nocturia. BBTI participants also had significantly better outcomes as evaluated by 2 categories of remission or response vs. partial response or nonresponse among both groups (without nocturia χ2 = 9.05, P = 0.006; with nocturia χ2 = 4.99, P = 0.04; Figure 2).
Figure 2.
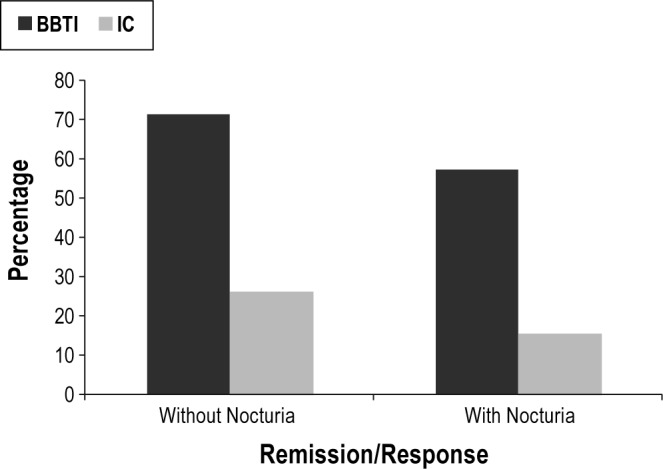
Categorical outcomes. Outcomes for participants assigned to the brief behavioral treatment for insomnia (BBTI) and information-only control (IC) among those with and without nocturia groups. Shown in the graph are the percentages of remitters/responders (without nocturia χ2 = 9.05, P = 0.006; with nocturia χ2 = 4.99, P = 0.04).
DISCUSSION
Nocturia is a frequently overlooked cause of insomnia in the elderly.12 The effect of concurrent nocturia on chronic insomnia treatment has never been evaluated. To address the gap we conducted a secondary, subgroup analysis of a randomized controlled trial of behavioral treatment for chronic insomnia in older adults. In the present analysis we found evidence of reduced treatment effect among participants with concurrent nocturia as compared to those without nocturia. Among participants without nocturia at baseline, the BBTI group reported a significant decrease in wake after sleep onset and sleep latency measured by sleep diary and actigraphy. Changes in sleep latency and wake after sleep onset differed significantly by treatment group. Sleep outcome measures assessed by polysomnography did not show a similar pattern of findings, however. Differential improvements between with nocturia and without nocturia groups were also seen in sleep quality as measured by sleep diary and PSQI score. In contrast to these differences assessed by continuous univariate outcomes, categorical outcomes showed that BBTI was associated with better outcomes than IC in both groups (with and without nocturia).
To our knowledge, our study is the first to explore the impact of concurrent nocturia on the response of insomnia to treatment. The results of this secondary analysis of a randomized controlled trial suggest that the presence of concurrent nocturia attenuates the benefit of BBTI on chronic insomnia in older adults. BBTI focuses on sleep consolidation, but if arousal is precipitated by perception of an urge to void, it may negatively affect the insomnia treatment. Therefore in patients with chronic insomnia, addressing comorbid nocturia may improve the efficacy of behavioral treatment of insomnia. Moreover, behavioral interventions focused on restricting fluid intake, moderate daily exercise, keeping warm in bed, pelvic floor exercises, urge suppression techniques, delayed voiding, and increased support and education from nurse practitioners have shown reduction in nocturia.22–25 This raises the possibility that multicomponent behavioral interventions targeting both nocturia and insomnia may act synergistically to improve both conditions.
We noted a discrepancy between some outcome measures depending on the modality of sleep assessment (diary, actigraphy, polysomnography), but each method has its own advantages and disadvantages. Sleep diaries provide important patient-reported outcomes. Individuals with insomnia may overestimate sleep latency and wake after sleep onset, and underestimate total sleep time relative to objective methods. By contrast, by tracking sleep over two weeks, sleep diaries are more likely to capture the night-to-night variability.21,26,27 Actigraphy is “objective” and measures sleep in the home environment, but it may overestimate sleep when a person is lying quietly awake.26,28 In our analyses, diary- and actigraphy-measured sleep outcomes were congruent. PSG is the “gold standard” for some things (i.e., diagnosing apnea, measuring particular sleep stages), but the instrumentation itself and the small number of recording nights (2 nights in the primary study) may provide skewed assessments of “usual” sleep.29 PSG is, thus, not routinely recommended for clinical assessment of insomnia, but it is often included among insomnia trial outcomes.26,29 We previously reported no change in PSG outcomes in response to BBTI over a four-week treatment period. Therefore, the absence of confirmatory PSG findings in the present secondary analysis is not surprising.15
We recognize the limitations of subgroup analyses. It could be argued that formal assessment of a nocturia by intervention group interaction would be a more powerful test of differential treatment effects.30 Nonetheless, our results, especially combined with the presence of a significant interaction between nocturia and BBTI with respect to sleep latency and a similar trend for other interactions despite the low statistical power, suggest that the adverse impact of nocturia on therapeutic response may be real and warrants further investigation.
Our study has some limitations. First, there is the potential that unmeasured confounders could have affected the association detected between the intervention and our outcome. Second, the primary outcome of the parent study focused on insomnia, and thus the study was neither designed nor powered to detect differential improvements in the sleep measures between interventions in those with and without nocturia. Moreover, the participants with nocturia at baseline had an average of 1.46 episodes of nocturia per night which is not as severe as seen in an average geriatric clinic practice.31 Yet our hypothesis was generated prior to data analysis and despite these limitations, large effect sizes were observed in this initial investigation. Prospective studies will be needed to confirm our observations and evaluate the effect of concurrent nocturia on the efficacy of behavioral treatment of insomnia, which will help elucidate the intricate relationship of insomnia and nocturia and guide the management of these coexisting conditions in older adults. Our findings should be viewed as the necessary first step in justifying such studies.
CONCLUSION
Our results suggest that BBTI may be more efficacious in improving insomnia in older adults without nocturia. Further controlled trials are warranted to determine whether efficacy of behavioral treatment of chronic insomnia may be further enhanced by addressing concurrent nocturia.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding sources: Pittsburgh Training in Geriatrics and Gerontology. Grant #: T32 AG021885; John A. Hartford Center of Excellence in Geriatric Medicine; AgeWise Program Project Grant P01 AG020677; and University of Pittsburgh Clinical and Translational Science Institute RR 024153. Dr. Perera has received salary support from grants awarded to the University of Pittsburgh by Merck, Eli Lilly, Ortho Biotech, and Teva. Dr. Buysse has served as a paid consultant on scientific advisory boards for Merck, Phillips Respironcs, and Purdue Pharma. He has also spoken at single-sponsored educational meetings for Servier and has spoken for single-sponsored lecture for Astellas. The other authors have indicated no financial conflicts of interest. There is no off label or investigational use.
ACKNOWLEDGMENTS
The authors acknowledge all the participants of AgeWise project, and the research team at Western Psychiatric Institute and Clinic. Reported secondary analysis was performed at the Division of Geriatrics and Gerontology, Department of Medicine, School of Medicine University of Pittsburgh, Pittsburgh, PA. Primary study (AgeWise) was performed at the Western Psychiatric Institute & Clinic, University of Pittsburgh, Pittsburgh, PA.
Footnotes
A commentary on this article appears in this issue on page 631.
REFERENCES
- 1.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Van Someren EJ. Circadian rhythms and sleep in human aging. Chronobiol Int. 2000;17:233–43. doi: 10.1081/cbi-100101046. [DOI] [PubMed] [Google Scholar]
- 4.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep-disordered breathing and nocturia in older adults. J Am Geriatr Soc. 2004;52:957–60. doi: 10.1111/j.1532-5415.2004.52264.x. [DOI] [PubMed] [Google Scholar]
- 7.Schnelle JF, Cruise PA, Alessi CA, Al-Samarrai N, Ouslander JG. Individualizing nighttime incontinence care in nursing home residents. Nurs Res. 1998;47:197–204. doi: 10.1097/00006199-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Zepelin H, McDonald CS, Zammit GK. Effects of age on auditory awakening thresholds. J Gerontol. 1984;39:294–300. doi: 10.1093/geronj/39.3.294. [DOI] [PubMed] [Google Scholar]
- 9.Van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90(Suppl 3):11–5. doi: 10.1046/j.1464-410x.90.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon MM. Nocturnal awakenings and comorbid disorders in the American general population. J Psychiatr Res. 2008;43:48–54. doi: 10.1016/j.jpsychires.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 12.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10:540–8. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Zeitzer JM, Bliwise DL, Hernandez B, Friedman L, Yesavage JA. Nocturia compounds nocturnal wakefulness in older individuals with insomnia. J Clin Sleep Med. 2013;9:259–62. doi: 10.5664/jcsm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th ed. Washington, DC: American Psychiatric Association; 2000. Text Revision ed. [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 22.Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102:62–6. doi: 10.1111/j.1464-410X.2008.07463.x. [DOI] [PubMed] [Google Scholar]
- 23.Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. J Urol. 2010;184:1000–4. doi: 10.1016/j.juro.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Burgio KL, Goode PS, Johnson TM, et al. Behavioral versus drug treatment for overactive bladder in men: the Male Overactive Bladder Treatment in Veterans (MOTIVE) Trial. J Am Geriatr Soc. 2011;59:2209–16. doi: 10.1111/j.1532-5415.2011.03724.x. [DOI] [PubMed] [Google Scholar]
- 25.Cho SY, Lee SL, Kim IS, Koo DH, Kim HJ, Oh SJ. Short-term effects of systematized behavioral modification program for nocturia: a prospective study. Neurourol Urodyn. 2012;31:64–8. doi: 10.1002/nau.21186. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 27.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 28.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 29.Chesson A, Jr., Hartse K, Anderson WM, et al. Practice parameters for the evaluation of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2000;23:237–41. [PubMed] [Google Scholar]
- 30.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 31.Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. 2013;189:S86–92. doi: 10.1016/j.juro.2012.11.033. [DOI] [PubMed] [Google Scholar]


