Hemoglobins modulate embryogenesis by regulating programmed cell death.
Abstract
Programmed cell death (PCD) in multicellular organisms is a vital process in growth, development, and stress responses that contributes to the formation of tissues and organs. Although numerous studies have defined the molecular participants in apoptotic and PCD cascades, successful identification of early master regulators that target specific cells to live or die is limited. Using Zea mays somatic embryogenesis as a model system, we report that the expressions of two plant hemoglobin (Hb) genes (ZmHb1 and ZmHb2) regulate the cell survival/death decision that influences somatic embryogenesis through their cell-specific localization patterns. Suppression of either of the two ZmHbs is sufficient to induce PCD through a pathway initiated by elevated NO and Zn2+ levels and mediated by production of reactive oxygen species. The effect of the death program on the fate of the developing embryos is dependent on the localization patterns of the two ZmHbs. During somatic embryogenesis, ZmHb2 transcripts are restricted to a few cells anchoring the embryos to the subtending embryogenic tissue, whereas ZmHb1 transcripts extend to several embryonic domains. Suppression of ZmHb2 induces PCD in the anchoring cells, allowing the embryos to develop further, whereas suppression of ZmHb1 results in massive PCD, leading to abortion. We conclude that regulation of the expression of these ZmHbs has the capability to determine the developmental fate of the embryogenic tissue during somatic embryogenesis through their effect on PCD. This unique regulation might have implications for development and differentiation in other species.
Selective elimination of overproduced, superfluous, or damaged cells by programmed cell death (PCD) is a common feature of cellular homeostasis. In conjunction with cell division and differentiation, spatial and temporal execution of the death program helps develop the organism’s shape and sculpts its final form. Necrosis, autophagy, and apoptosis are the recognized forms of PCD in vertebrates (for review, see Portt et al., 2011), whereas necrosis and autophagy are the identifiable forms of PCD in plants (for review, see van Doorn et al., 2011). Common features of PCD are loss of osmoregulation in the cell, activation of caspases, nuclear and DNA fragmentation, generation of lysosomal enzymes, and mitochondrial swelling with the release of cytochrome C (for review, see van Doorn et al., 2011). Autophagy differs between vertebrates and plants in that autophagosomes are produced in the former, whereas lytic vacuoles are a common feature in plant cells undergoing PCD. Variations and flexibility in the execution of these events and presence of cross talk among the different death pathways (Whelan et al., 2010) attest to the complexity of the death program. This complexity is further aggravated by the ambiguous involvement of several signal molecules, including Zn2+, in the cell death/survival decision. The participation of Zn2+ in cell death is dichotomous because proapoptotic and antiapoptotic effects have been shown in different systems. Although Zn2+ deficiencies are associated with apoptosis in some systems, including human Burkitt lymphoma B cells (Truong-Tran et al., 2000), elevated Zn2+ levels induce caspase activity in others (Schrantz et al., 2001; Helmersson et al., 2008).
As with vertebrates, plant PCD is an integral component of growth and development, which shapes organs through selective and controlled elimination of cells. Execution of PCD has been reported during different phases of the plant life cycle. It establishes polarity in immature embryos, it eliminates suspensor cells in mature embryos, it contributes to the formation of vascular tissue throughout development, and it is involved in senescence-related processes (Reape et al., 2008). Execution of the death pathway is also a key component of abiotic (aerenchyma formation during hypoxia; Evans, 2003) and incompatible pathogen infection (Pennell and Lamb, 1997). Although a great deal is now known about the molecular and biochemical events regulating the death pathways in vertebrates and plants, information on the mechanisms by which specific cells are targeted to either live or die is scarce.
Hemoglobins (Hbs) are ubiquitous heme-containing proteins found in all nucleated organisms (for review, see Weber and Vinogradov, 2001). Depending on organisms and tissues, they may act as oxygen carriers or stores or bind other small ligands, such as CO2, CO, or NO. Plant Hbs have been cataloged into symbiotic (leghemoglobins) and nonsymbiotic. Leghemoglobins are generally restricted to root nodules of nitrogen-fixing plants, whereas nonsymbiotic Hbs are found in shoots and roots of both monocots and dicots (Hill, 2012). From a phylogenic perspective, Hbs are categorized into three classes (classes 1–3; Hunt et al., 2001). Classes 1 and 2 Hbs share a three-on-three α-helical loop structure surrounding the heme moiety, whereas class 3 Hbs possess a two-on-two α-helical structure, similar to truncated bacterial globins. NO scavenging is one of the major functions of plant Hbs, and evidence of their action in this fashion has been shown in plant development and in response to both biotic and abiotic stress (for review, see Hill, 2012).
Although expressed during embryo development (Smagghe et al., 2007), the majority of information related to the function of plant Hbs has been obtained during postembryonic growth. Embedded in the maternal tissue and difficult to dissect, plant embryos are not readily amenable to physiological and molecular studies. An alternative model to in vivo embryogenesis is somatic embryogenesis, the production of embryos from somatic cells. Other than elucidating key molecular mechanisms governing embryogenesis (Thorpe and Stasolla, 2001), this system has been used to show the requirement of PCD for proper embryo development. In spruce (Picea abies), the activation of PCD is an obligatory step for the formation of somatic embryos, and experimental suppression of the death program compromises embryogenesis (for review, see Bozhkov et al., 2005).
Using maize (Zea mays) somatic embryogenesis as a model system, the relationship between the expression of two maize Hbs (ZmHb1 and ZmHb2), NO, and PCD has been examined during the formation of somatic embryos. The two ZmHbs are shown to regulate the death program through similar mechanisms that interfere with the cascade of events mediated by NO and Zn2+, the mitogen-activated protein kinase (MAPK) cascade, and accumulation of reactive oxygen species (ROS) that lead to PCD. This regulation, which influences the embryogenic pathway in a fashion consistent with the localization patterns of ZmHb1 and ZmHb2, identifies Hbs as unique key regulators of the survival/death decision.
RESULTS
Suppression of ZmHbs Affects Somatic Embryogenesis
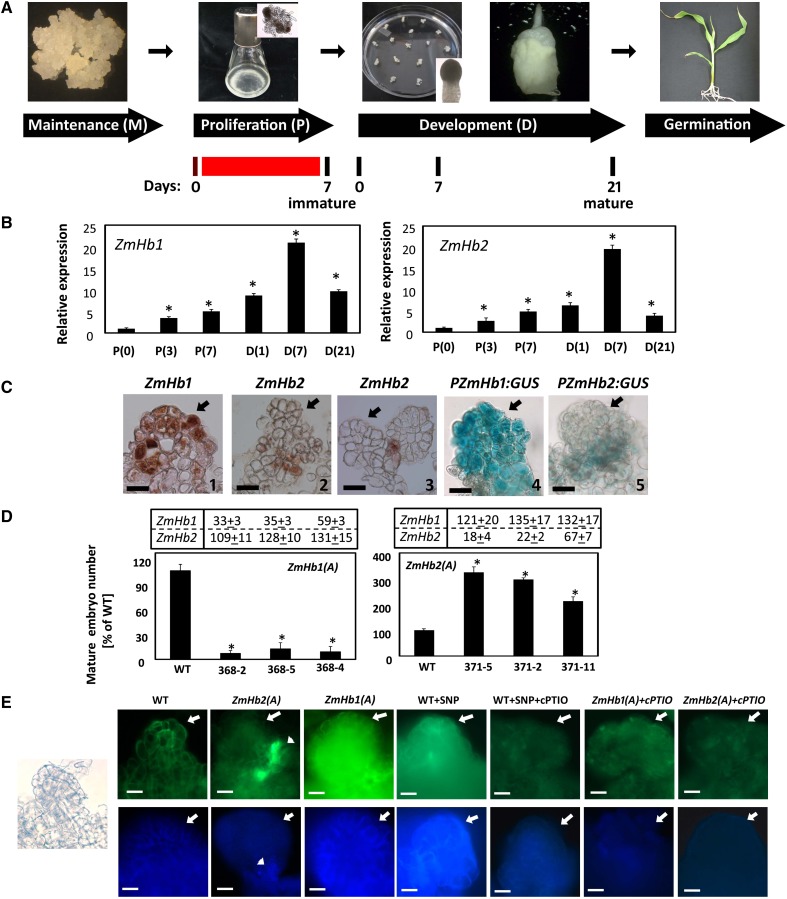
In maize, production of in vitro embryos through somatic embryogenesis is achieved through three distinct culture steps (Garrocho-Villegas et al., 2012; Fig. 1A). Embryogenic tissue grown on solid maintenance medium was transferred in a liquid auxin-containing proliferation (P) medium of similar composition. Cells in the P medium proliferated rapidly, and immature somatic embryos attached to the subtending embryogenic tissue became visible. After 7 d, the tissue was transferred onto a solid auxin-free development (D) medium, which allowed the immature embryos to grow into fully functional mature embryos able to germinate and regenerate viable plants. Pharmacological treatments were applied during 7 d in P medium (Fig. 1A). For the purpose of this study, immature embryos were harvested from P medium at d 7, whereas mature embryos were collected from the D medium at d 21 (Fig. 1A).
Figure 1.
Hb regulation of maize somatic embryogenesis. A, Schematic representation of the maize somatic embryogenic system comprising maintenance (M), P, D, and germination (G) phases. Representative stages of embryo development and days in culture are also depicted. The red line indicates the timing of pharmacological treatments, which were all applied between d 0 and 7 in P medium. B, Expression level by quantitative RT-PCR of ZmHb1 and ZmHb2 during the P and D phases of embryogenesis. Values are means ± ses of three independent biological replicates and normalized to the value of P(0) set at 1. Numbers in parentheses represent days in the respective phase. *, Statistically significant difference (P ≤ 0.05) from P(0). C, Localization of ZmHb1 (1 and 4) and ZmHb2 (2, 3, and 5) in immature embryos (d 7, P medium) using RNA in situ hybridization (1–3) and PZmHb1:GUS and PZmHb2:GUS reporter lines (4 and 5). Arrows indicate the apical domain of the developing embryos. Bars = 20 μm. D, Percentage of mature (d 21, D medium) somatic embryos obtained from antisense lines suppressing ZmHb1 [ZmHb1(A)] or ZmHb2 [ZmHb2(A)]. Values (wild type set at 100%) are means ± ses of four independent biological replicates. *, Statistically significant difference (P ≤ 0.05) from wild type. Numbers in charts indicate the expression levels of ZmHb1 or ZmHb2 in the transgenic lines compared with the wild-type value set at 100%. E, Localization of NO (top) and Zn2+ (bottom) in immature embryos (d 7, P medium). Arrows indicate the apical domain of the embryos, whereas arrowheads indicate the preferential accumulation of NO and Zn2+ in a few cells located at the base of the embryos down-regulating ZmHb2. Left, The micrograph shows the representative development of embryos used for the localization studies. Endogenous NO level was modulated by applications of SNP and/or cPTIO. WT, Wild type. Bars = 20 μm.
Expression and localization of two maize Hb genes, ZmHb1 (AF236080) and ZmHb2 (DQ171946; Smagghe et al., 2009; Supplemental Fig. S1), were examined during different phases of the embryogenic process. The expression of both genes gradually increased in the P medium, with a sharp increase occurring between d 1 and 7 in the D medium (Fig. 1B). To verify whether this increase was because of the removal of auxin in the D medium or other differences in chemical composition between the P and D media, the expression of the ZmHb genes was also measured in a D medium devoid of (−) or enriched with (+) auxin. The transcript levels of both ZmHb1 and ZmHb2 increased in the D medium devoid of auxin (Supplemental Fig. S2).
In immature embryos (d 7 in P medium; Fig. 1A), a different localization pattern was observed for the two ZmHbs (Fig. 1C). Unlike ZmHb2, with expression that was restricted to a cluster of basal cells anchoring the embryos to the subtending embryogenic tissue (Fig. 1C, 2 and 5) or other immature embryos (Fig. 1C, 3), ZmHb1 transcripts were detected in extended domains of the embryos (Fig. 1C, 1 and 4).
Figure 2.
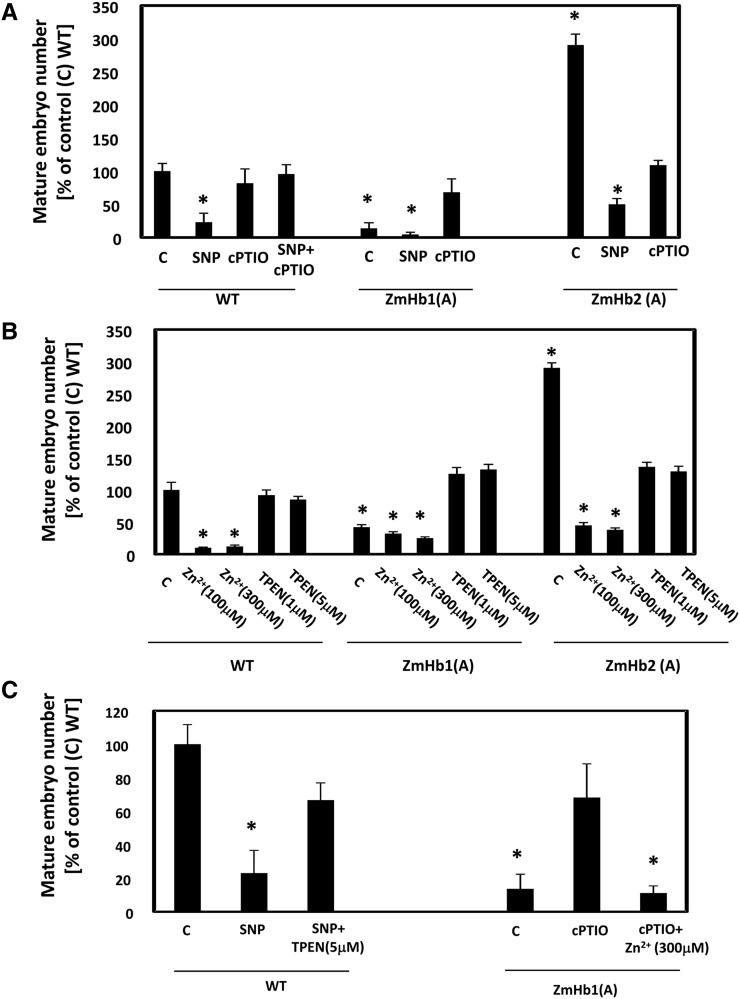
Effects of NO and Zn2+ on the production of mature somatic embryos (d 21, D medium). A, Effects of the NO donor SNP and NO scavenger cPTIO on the number of somatic embryos. B, Effects of the Zn2+ chelator TPEN and Zn2+ supplementations (using ZnSO4) on the number of somatic embryos. C, Effects of combined manipulations of NO and Zn2+ on the number of somatic embryos. Values are means ± ses of at least four biological replicates. *, Statistically significant differences (P ≤ 0.05) from control (C); wild type (WT) set at 100%.
Figure 5.
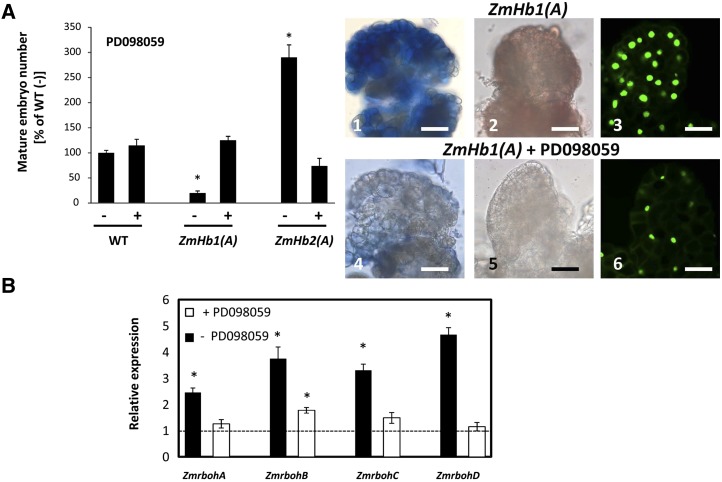
An active MAPKK cascade is required in the ZmHb response. A, Effects of applications of the MAPKK inhibitor PD098059 on mature somatic embryo (d 21, D medium) number, accumulation of superoxide (1 and 4) and hydrogen peroxide (2 and 5), and distribution of TUNEL-positive cells (3 and 6) in immature embryos (d 7, P medium). Values of embryo numbers are means ± ses of at least four biological replicates. *, Statistically significant differences (P ≤ 0.05) from control wild type (−) set at 100%. Bars = 20 μm. B, Expression level of the four NADPH respiratory burst oxidase homologs (ZmrbohA-D) in the ZmHb1(A) tissue (d 7, P medium) cultured in the presence (+) or absence (−) of PD098059. Values are means ± ses of three independent biological replicates and normalized to the expression level of each gene in the wild-type line (set at 1). WT, Wild type. *, Statistically significant difference (P ≤ 0.05) from wild type.
Figure 3.
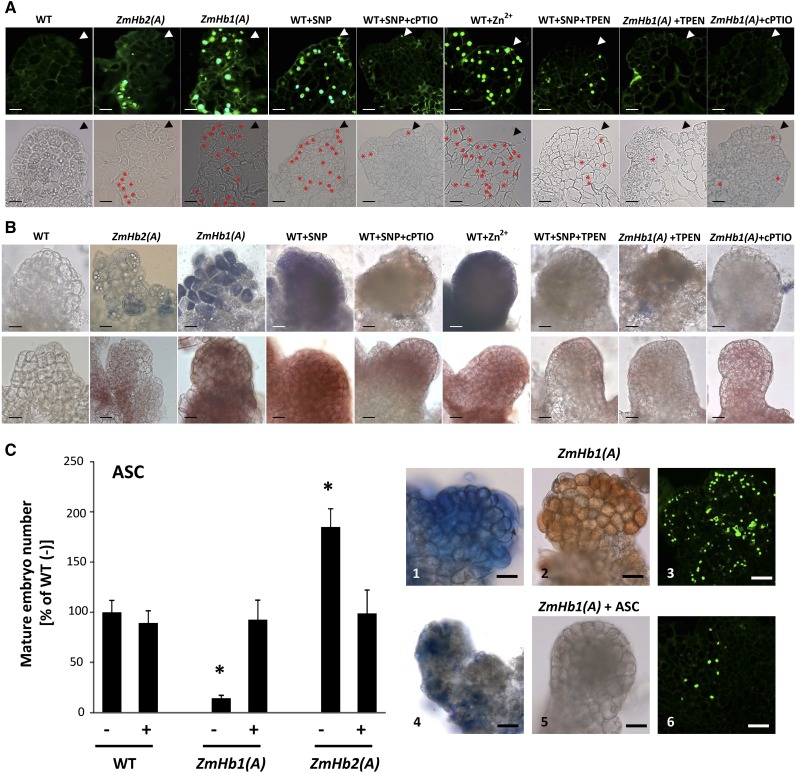
PCD during maize somatic embryogenesis. A, Identification of TUNEL-positive cells in immature embryos (d 7, P medium) of the ZmHb1(A) and ZmHb2(A) lines as well as embryos subjected to alterations in NO and Zn2+ levels using compounds from Figure 2. *, Enhanced visualization of TUNEL-positive cells in the same embryos (bottom). Arrowheads mark the apical domains of the embryos. Bars = 20 μm. B, Localization of superoxide (top) and hydrogen peroxide (bottom) in immature embryos (d 7, P medium) subjected to alterations in NO and/or Zn2+ level. Bars = 20 μm. C, Effects of applications of ASC on mature somatic embryo number (d 21, D medium), accumulation of superoxide (1 and 4) and hydrogen peroxide (2 and 5), and distribution of TUNEL-positive cells (3 and 6) in immature embryos (d 7, P medium). Values of embryo numbers are means ± ses of at least four biological replicates. *, Statistically significant differences (P ≤ 0.05) from control; wild type (WT) set at 100%. Bars = 20 μm.
Figure 4.
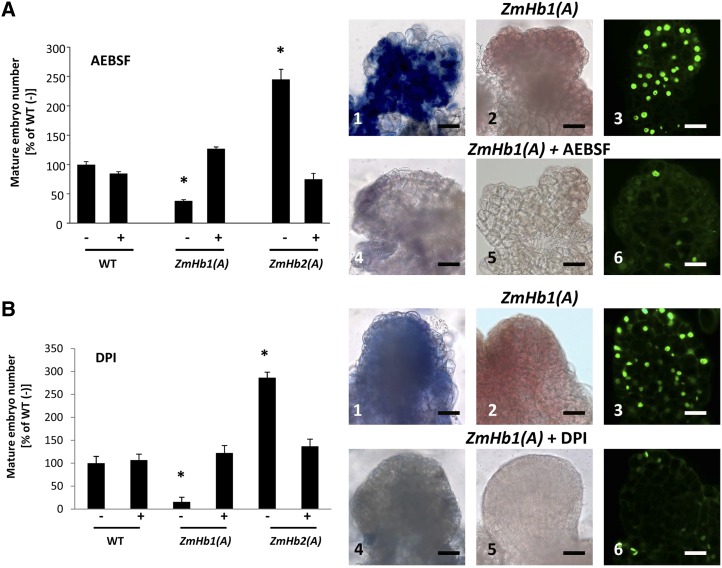
Requirement of NADPH oxidase in the ZmHb response. Effects of applications of the NADPH oxidase inhibitors AEBSF (A) and DPI (B) on mature (d 21, D medium) somatic embryo number, accumulation of superoxide (1 and 4) and hydrogen peroxide (2 and 5), and distribution of TUNEL-positive cells (3 and 6) in immature embryos (d 7, P medium). Values of embryo numbers are means ± ses of at least four biological replicates. *, Statistically significant differences (P ≤ 0.05) from wild type (WT; −) set at 100%. Bars = 20 μm.
The functional role of ZmHb1 and ZmHb2 in the embryogenic process was further analyzed through antisense-mediated suppression studies. Competence to produce mature somatic embryos (d 21 on D medium; Fig. 1A) was highly repressed in lines down-regulating ZmHb1 [ZmHb1(A) lines] and induced in lines down-regulating ZmHb2 [ZmHb2(A) lines; Fig. 1D]. These embryogenic differences were solely ascribed to a repression of the respective ZmHb gene, because the antisense suppression of one ZmHb gene did not significantly alter the expression of the other (charts in Fig. 1D). Additional studies were performed using the 368-2 ZmHb1(A) line and the 371-5 ZmHb2(A) line, because they showed the greatest differences in embryo number and the most pronounced repression of the respective ZmHb.
ZmHbs Regulate Intracellular Zn2+ through the NO-Dependent Metal Release from Metallothioneins
The documented ability of Hbs to scavenge NO (Weber and Vinogradov, 2001) was retained by ZmHb1 and ZmHb2 as revealed by the fluorescent signal of 4,5-diaminofluorescein diacetate (DAF-2DA), which is routinely used to detect cellular NO (Hebelstrup and Jensen, 2008; Elhiti et al., 2013). In the antisense lines, NO accumulation was elevated in areas where the specific ZmHb was detected in wild-type tissue (i.e. small clusters of basal cells in the ZmHb2(A) line; arrowhead in Fig. 1E, top) and a larger group of embryonic cells in the ZmHb1(A) line (compare Fig. 1, C with E). Specificity of the signal was verified in the wild-type line with the commonly used NO donor, sodium nitroprusside (SNP), and/or an NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; Hebelstrup and Jensen, 2008; Elhiti et al., 2013). Applications of cPTIO reduced NO and Zn2+ signals in the transformed lines.
In vertebrates, Zn2+ homeostasis regulates several cell responses, some of which are modulated by NO (Lee and Koh, 2010). Intracellular Zn2+ was detected using the membrane-permeable Zinquin-ethyl-ester (Zalewski et al., 1993), which is highly sensitive to variations in Zn2+ in maize embryos (Supplemental Fig. S3). Zinquin is often used to image free Zn2+ for its strong specificity because it does not react with other divalent cations (Helmersson et al., 2008). An increased intracellular Zn2+ pool was observed in cells accumulating NO, whereas cells with reduced NO content had less intense Zn2+ signal (Fig. 1E, bottom). The relationship between Zn2+ levels and NO presence was substantiated by pharmacological manipulations of NO content using SNP or cPTIO. Free Zn2+ level increased after applications of the NO donor SNP, whereas it decreased in cells treated with the NO scavenger cPTIO (Fig. 1E).
Despite the lack of information relative to the regulation of intracellular Zn2+ pool by NO in plants, the role of metallothioneins (MTs) in the storage and homeostasis of Zn2+ and other heavy metal is becoming more evident. Based on the arrangements of Cys residues, four types of plant MTs (MT1–MT4) have been identified (Cobbett and Goldsbrough, 2002). The expression and localization of MTs vary between tissues and organs. In both Arabidopsis (Arabidopsis thaliana) and barley (Hordeum vulgare), MT4 isoforms contribute to the storage and release of Zn2+ during embryogenesis (Hegelund et al., 2012; Ren et al., 2012). To investigate whether the maize ZmMT4 is capable of storing Zn2+ and releasing it in a high-NO environment, a recombinant maltose binding protein (MBP) fusion with ZmMT4 was constructed and expressed in Escherichia coli. In vivo E. coli feeding with ZnSO4 yielded a recombinant ZmMT4 protein loaded with Zn2+ (Table I), which was assessed by acid release and dithizone (DTZ) measurement. Treatments with the NO donor diethylamine NONOate (DEA/NO) released a substantial percentage of the Zn2+ contained in the recombinant ZmMT4 during the 45-min incubation time (Table I). No Zn2+ was detected on DEA/NO treatment of MBP alone. The amount of Zn2+ released was dependent on the amount of NO provided (Supplemental Fig. S4). This unloading of Zn2+ clearly shows that NO can regulate intracellular Zn2+ level through its release from MTs, a process that has not been described in plants.
Table I. Effects of the NO donor DEA/NO on the release of Zn2+ from the recombinant MBP5 and the MBP5-ZmMT4 fusion protein.
Medium was supplemented with 0.4 mm ZnSO4. Values are means ± ses of three biological replicates. Zn2+ measurements were performed by the dithizone method (Paradkar and Williams, 1994).
To further investigate the requirement of NO in modulating intracellular Zn2+ during the ZmHb response, we examined embryo production in the transformed lines cultured in a medium enriched with or depleted of NO and/or Zn2+ (Fig. 2). In the wild-type line, increased NO levels by SNP repressed the production of mature somatic embryos, whereas removal of NO by cPTIO had no effects (Fig. 2A). The possibility that SNP had toxic effects on embryogenesis was excluded by the combined applications of SNP and cPTIO. A similar embryogenic inhibition by SNP was also observed in the ZmHb1(A) and ZmHb2(A) lines, whereas cPTIO had opposite effects, promoting the production of mature somatic embryos in the former and repressing it in the latter (Fig. 2A).
Alterations in Zn2+ using a pharmacological procedure described by Helmersson et al. (2008) also affected embryogenesis. Supplementation of Zn2+ was detrimental for the production of mature somatic embryos in all lines (Fig. 2B). Sequestration of Zn2+ by the Zn2+ chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) promoted embryogenesis in the ZmHb1(A) line and repressed embryo yield in the ZmHb2(A) line (Fig. 2B).
To assess the effects of combined alterations of NO and Zn2+ levels on embryogenesis, the cell lines were subjected to different treatments. The wild-type line, characterized by the lowest accumulation of NO (Fig. 1E), was used to test the effects of an experimental increase of NO in a low Zn2+ environment, whereas the NO-accumulating ZmHb1(A) line (Fig. 1E) was used to investigate the effects of a depletion of NO levels in a Zn2+-enriched environment. The repressive effect of NO (SNP treatment) on wild-type embryo number was reversed by decreasing Zn2+ levels (SNP and TPEN treatment; Fig. 2C). Consistent with this observation, the increased production of mature embryos in the ZmHb1(A) line with low NO levels (cPTIO treatment) was abolished by elevating intracellular Zn2+ levels (cPTIO and Zn2+ treatment; Fig. 2C). These observations place Zn2+ as a downstream component of NO in the ZmHb response.
ZmHb Modulation of Embryogenesis Involves PCD and Production of ROS
In vertebrates, apoptosis and autophagy are influenced by essential trace elements. The role of Zn2+ homeostasis in both processes is ambiguous. Although Zn2+ deficiencies are associated with apoptosis in some systems (Truong-Tran et al., 2000; Helmersson et al., 2008), elevated Zn2+ levels induce caspase activity in others (Schrantz et al., 2001). Controlled cell death is essential in both vertebrates and plants because it shapes the body of the organism by eliminating specific cells and tissues. To verify whether the ZmHb regulation of somatic embryogenesis is executed through PCD, we assayed nuclear DNA fragmentation using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL; Fig. 3A). Although no or little PCD was observed in immature wild-type embryos, the distribution of the TUNEL signals in the ZmHb2(A) line was restricted to small clusters of basal cells, which was in contrast to the ZmHb1(A) line, where PCD targeted a large number of embryonic cells (Fig. 3A). Cells undergoing PCD are most likely those suppressing ZmHbs and with elevated levels of NO and Zn2+, which are shown by the similar distribution patterns of the TUNEL-positive nuclei, ZmHb expression, and NO and Zn2+ signals (compare Fig. 1, C and E with Fig. 3A). The requirement of NO and Zn2+ for the ZmHb regulation of PCD was confirmed pharmacologically in the wild-type line. Although increasing levels of either NO- (by SNP) or Zn2+-induced PCD, depletion of NO (by cPTIO) or Zn2+ (by TPEN) reduced the frequency of TUNEL-positive cells (Fig. 3A). Using the ZmHb1(A) line characterized by the highest levels of NO and Zn2+ (Fig. 1E), it is shown that a reduction of NO (by cPTIO) or Zn2+ (by TPEN) was sufficient to reduce PCD (Fig. 3A). These results were also verified by calculating the percentage of TUNEL-positive cells (Supplemental Fig. S5).
Manifestation of PCD in plant cells is characterized by biochemical and molecular hallmarks, such as fragmentation of nuclei, increased expression of metacaspases, and caspase-like activities (Reape et al., 2008). Both antisense lines showed signs of fragmentations in the nuclei undergoing PCD and increasing caspase 3-like activity (Supplemental Fig. S6). The expression of the metacaspase ZmMCII-3 increased significantly in the ZmHb1(A) line but only slightly in the ZmHb2(A) line. In the transgenic lines, the ZmMCII-3 transcripts localized in similar areas, where the respective ZmHb was detected in wild-type tissue (compare Fig. 1C with Supplemental Fig. S6). These results suggest that expression of ZmMCII-3 and caspase 3-like activity might be involved in the ZmHb mediation of PCD.
Additionally, cells programmed to die accumulate ROS (Van Breusegem and Dat, 2006). To examine whether maize cells targeted by PCD accumulated ROS, we stained with nitroblue tetrazolium and 3,3′-diaminobenzidine, which detect superoxide and hydrogen peroxide, respectively (Thordal-Christensen et al., 1997; Lin et al., 2009b). Although the specificity of each individual dye should be interpreted with due caution, the use of both ensures an accurate localization of ROS in plant cells (Lin et al., 2009b). The staining signal of superoxide and hydrogen peroxide coincided with the distribution pattern of TUNEL-positive nuclei (compare Fig. 3A with Fig. 3B), thus suggesting that ROS accumulate in cells targeted to die by the suppression of ZmHbs. As observed for PCD, accumulation of ROS required NO and Zn2+, whereas a chelation of Zn2+ by TPEN decreased ROS levels in an environment enriched with NO (by SNP). These results suggest that Zn2+ is a necessary downstream component in the NO production of ROS (Fig. 3B).
ROS Accumulation in Maize Cells Is Required for the Execution of PCD
In plant cells, ROS homeostasis is regulated by antioxidant molecules, including reduced ascorbic acid (ASC). Through the ascorbate-glutathione redox system, ROS are scavenged in a series of sequential reactions converting ASC to dehydroascorbate (Potters et al., 2002). Depletion of cellular ASC occurs in cells overproducing ROS (Potters et al., 2002), a characteristic also observed in our system (Supplemental Fig. S7). The antioxidant properties of ASC were used to further investigate the requirement of ROS for the activation of the death program (Fig. 3C). Using the ZmHb1(A) line, which accumulated higher levels of NO and ROS and displayed extensive PCD (Figs. 1E and 3, A and B), it is shown that ASC reduced both NO and ROS signals as well as decreased the number of TUNEL-positive nuclei (Fig. 3C). Furthermore, exogenous ASC applications modulated embryogenesis by increasing the production of mature somatic embryos in the ZmHb1(A) line and decreasing it in the ZmHb2(A) line to wild-type values (Fig. 3C). This finding was consistent with the opposite effects of the ZmHb-induced PCD on embryogenesis.
Collectively, these data suggest that suppression of the two ZmHbs triggers identical molecular responses, but their different localization pattern results in opposite embryogenic behavior. Repression of ZmHb2, which is normally expressed in the basal cells anchoring the immature embryos to the embryogenic mass (Fig. 1C), elevates NO levels, which, in turn, increase free intracellular Zn2+ (Fig. 1E). A rise in the Zn2+ pool triggers ROS-mediated PCD (Fig. 3), eliminating these anchor cells and allowing the embryos to develop further at a higher frequency (Fig. 1D). Induction of PCD through similar events also occurs in ZmHb1 down-regulating cells. However, the enlarged localization pattern of ZmHb1, encompassing most of the embryo proper (Fig. 1C), results in the elimination of numerous cells (Fig. 3A), leading to embryo lethality and suppression of embryogenesis (Fig. 1D).
The Oxidative Burst Contributes to ROS Production
In plants, the ROS-generating oxidative burst is mainly controlled by NADPH oxidases, a family of membrane-bound enzymes activated in a variety of stress and developmental responses (Sagi and Fluhr, 2006). In mammalian systems, NADPH oxidase is a large protein complex comprising cytosolic regulatory proteins, which include the p47phox and a membrane-bound NADPH-binding cytochrome consisting of the glycosylated transmembrane protein gp91phox and the nonglycosylated p22phox (Torres and Dangl, 2005). Investigations on the function of NADPH oxidases have been limited by the reduced specificity of pharmacological inhibitors, such as 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF; Bayraktutan, 2005) and diphenylene iodonium (DPI; Bindschedler et al., 2006). Although effective in preventing the activation of the ROS-producing NADPH oxidase in cell-free systems and intact stimulated macrophages by interfering with the translocation of p47phox to the plasma membrane (Diatchuk et al., 1997), AEBSF is a Ser protease inhibitor (Wind et al., 2010). Undesirable side effects have also been reported for DPI, which other than inhibiting NADPH oxidase in many systems (Bindschedler et al., 2006; Davies et al., 2006), also inhibits other flavoenzymes (Wind et al., 2010). In light of these side effects, although the efficacy of each individual inhibitor must be interpreted with due caution, the use of both should provide a solid indication on the participation of NADPH oxidase in the production of ROS.
Applications of both AEBSF and DPI reduced the intensity of superoxide and hydrogen peroxide signal as well as the number of TUNEL-positive nuclei in the ZmHb1(A) line (Fig. 4) used for its elevated levels of ROS and extensive PCD (Fig. 3, A and B). Furthermore, both inhibitors modulated embryogenesis in a pattern mimicking that resulting from depletion of ROS (compare Fig. 3C with Fig. 4) and consistent with the regulation of PCD by the two ZmHbs (compare Fig. 3A with Fig. 4). The identical responses obtained with both inhibitors suggest that NADPH oxidase is involved in the accumulation of ROS during maize somatic embryogenesis.
To further validate this notion, the expression pattern of four maize respiratory burst oxidase (ZmrbohA-D) homologs to gp91phox, a component of the mammalian NADPH oxidase complex, was measured in immature embryos. All genes, with expression patterns that correlate to the activation of NADPH oxidase (Lin et al., 2009a), were induced in lines suppressing ZmHb1(A) and ZmHb2(A) in a fashion consistent with a regulation mediated by NO and Zn2+ (Supplemental Fig. S8).
Collectively, these data confirm the requirement of the oxidative burst in the ZmHb regulation of PCD and strongly suggest the participation of NADPH oxidases in the accumulation of ROS.
The Production of ROS Necessitates an Active MAPK Cascade
The MAPK cascade transduces many stimuli, some of which are mediated by NO (Keshet and Seger, 2010), into cellular responses. This cascade, triggered by the sequential activation of the mitogen-activated protein kinase kinase (MAPKK) and the MAPKK kinase by MAPK, culminates in downstream events, including protein phosphorylation and gene expression (Keshet and Seger, 2010). The participation of the MAPK cascade in the production of ROS was investigated pharmacologically using 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD098059), an effective MAPK cascade inhibitor that prevents the phosphorylation of MAPKK in animals and plants (Favata et al., 1998; Samuel and Ellis, 2002; Wang et al., 2010). Inactivation of the MAPK cascade by PD098059 abolished the accumulation of ROS and reduced PCD in the ZmHb1(A) line as well as modulated embryogenesis by increasing mature embryo yield in the ZmHb1(A) line and decreasing it in the ZmHb2(A) line (Fig. 5A). These results, which phenocopy closely those obtained by depleting ROS with ASC or inhibiting NADPH oxidase with AEBSF or DPI (compare Fig. 3C with Fig. 4), identify the MAPK cascade as a requirement for the production of ROS and activation of PCD.
Suppression of the MAPK cascade by PD098059 also repressed the expression of the four maize respiratory burst oxidase homologs (ZmrbohA-D; Fig. 5B), a result consistent with the requirement of an active MAPK cascade for the function of NADPH oxidase (Lin et al., 2009a).
Targeted PCD in ZmHb-Expressing Cells Phenocopies Suppression of Hbs
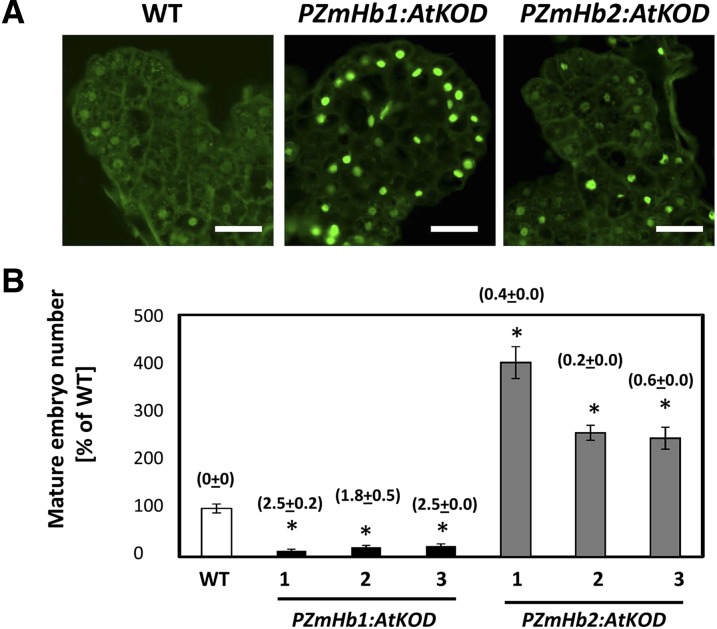
Evidence presented so far suggests that the opposite embryogenic response of the ZmHb1(A) and ZmHb2(A) lines is caused by the execution of PCD in different domains encompassing a large number of cells in the ZmHb1(A) embryos and a few cells anchoring the embryos to the embryogenic tissue in the ZmHb2(A) line. To show more precisely that cell death in these domains is a sufficient factor responsible for the observed embryogenic behavior, we induced death in ZmHb-expressing cells by driving the Arabidopsis gene KISS OF DEATH (KOD), a known executor of PCD (Blanvillain et al., 2011), under the control of ZmHb1 or ZmHb2 promoters. Expression of AtKOD was sufficient to induce death in ZmHb-expressing domains (compare Fig. 1C with Fig. 6A). Furthermore, the embryogenic response in the PZmHb1:AtKOD and PZmHb2:AtKOD lines mimicked that of the ZmHb1(A) and ZmHb2(A) lines, respectively (compare Fig. 1D with Fig. 6B), thereby showing the sufficient role of PCD in the ZmHb regulation of embryogenesis.
Figure 6.
Induced cell death in ZmHb-expressing domains regulates embryogenesis. A, Identification of TUNEL-positive cells in immature embryos (d 7, P medium) obtained from the PZmHb2:AtKOD lines, the PZmHb1:AtKOD lines, and the wild-type line. Bars = 20 μm. B, Number of mature embryos (d 21, D medium) produced by three PZmHb1:AtKOD lines and three PZmHb2:AtKOD lines. Numbers in parentheses indicate the KOD-actin ratio in the respective lines. Values are means ± ses of four biological replicates and expressed as a percentage of wild type (set at 100%). WT, Wild type. *, Statistically significant difference (P ≤ 0.05) from wild type.
DISCUSSION
ZmHbs Modulate Somatic Embryogenesis through PCD
Somatic embryogenesis, the ability to generate embryos from somatic cells, has been often used as a model system to investigate basic aspects of plant growth and development because the embryogenic pathway can be easily controlled, manipulated, and/or redirected (Thorpe and Stasolla, 2001). In many plant species, including maize, an obligatory event of this process is the differentiation of immature somatic embryos from the surface of the embryogenic tissue consisting of a heterogeneous cell population. This differentiation step is facilitated by removal of plant growth regulators (Fig. 1A), and in spruce, it requires the selective elimination of embryogenic cells through PCD (Filonova et al., 2000). Abrogation of the death program using either a pharmacological approach or PCD-deficient cell lines decreases the number of somatic embryos (Bozhkov et al., 2005). The programmed nature of cell death during spruce embryogenesis was confirmed by the requirement of metacaspases, proteases with similar functions to animal caspases that are components of the late phases of the apoptotic pathway (Bozhkov et al., 2005). However, identification and characterization of molecular components participating in the early phases of the developmental PCD process, not only in embryogenesis but also during advanced stages of animal and plant development, have been elusive (Elmore, 2007).
Here, we show that the spatial regulation of ZmHbs is an early factor of the death program, which influences maize somatic embryogenesis. Suppression of both ZmHb1 and ZmHb2 triggers the death program in specific domains of the embryogenic tissue, resulting in embryogenic outcomes consistent with their respective localization patterns. Although suppression of ZmHb1 triggers death in extended domains of embryogenic tissue, leading to embryo abortion, suppression of ZmHb2 eliminates selected cells (i.e. the anchor cells) connecting the developing embryos to the subtending tissue and increases the formation of mature somatic embryos. Although the effects of the ZmHb2-mediated dismantling of anchor cells on embryo formation are beyond the scope of this work, they might be required for shaping the embryo body and promoting growth, which was documented in animal embryogenesis (Elmore, 2007) or, most likely, reducing embryonic density, an impeding factor during in vitro embryogenesis (Thorpe and Stasolla, 2001), through the physical separation of immature embryos from the embryogenic tissue.
The possibility that factors other than PCD are accountable for the embryogenic behavior of the ZmHbs-suppressing lines was dismissed by triggering death in ZmHb1- and ZmHb2-expressing domains using KOD. Identified as an early inducer of the death pathway in Arabidopsis, where it contributes to the depolarization of the mitochondria membrane in the Bax-mediated death pathway, heterologous expression of KOD is sufficient to activate the suicide mode (Blanvillain et al., 2011). Expression of KOD in ZmHbs domains closely phenocopied the death pattern and the embryogenic response of lines suppressing ZmHbs (compare Figs. 1D, 3A, and 6), a similarity that proves unequivocally the sufficient requirement of the death program for the observed behavior of the transformed lines. In addition to regulating the cell death/survival decision during embryogenesis, as evidenced by our results, plant Hbs might fulfill a similar function during postembryonic development in an analogous fashion to animal neuroglobins. Expressed at high levels in metabolically active cells, neuroglobins protect the brain from Alzheimer’s disease by preventing neuron PCD (Khan et al., 2007; Greenberg et al., 2008) through mechanisms interfering with apoptotic events, such as the release of mitochondrial cytochrome C (Brittain et al., 2010). In plants, Hbs are regulated by many abiotic and biotic stress responses characterized by the manifestation of PCD (Hill, 2012). Independent studies show that overexpression of Hbs reduces aerenchyma formation (Dordas et al., 2003) and represses cell death after pathogen infection (Mur et al., 2012).
ZmHb Regulation of PCD Is Mediated by Cellular NO and Zn2+ and Requires ROS
A key role played by plant Hbs during development and stress responses is to scavenge NO through an NADH-dependent dioxygenase (Igamberdiev et al., 2014). In harmony with previous studies documenting that suppression of AtHbs in Arabidopsis increased cellular NO and applications of NO donors had an antagonistic effect to AtHbs (Hebelstrup and Jensen, 2008), our results showed a rise in NO in cells suppressing ZmHb1 or ZmHb2 (Fig. 1E). Elevated NO levels were apparent in many embryonic cells of the ZmHb1(A) line and a few basal embryonic cells of the ZmHb2(A) line. These NO-enriched domains coincided with the localization patterns of the respective ZmHb in the wild-type line (compare Fig. 1C with Fig. 1E). DAF-2DA fluorescence, as an indicator of NO presence, must be interpreted with caution (Mur et al., 2011). DAF-2DA fluorescence is quenched at basic pH, whereas PCD is associated with acidification of the cytoplasm (Bosch and Franklin-Tong, 2007). Fluorescence quenching caused by cellular basic pHs should not, therefore, be an issue in this circumstance, and in any event, fluorescence quenching should reduce the observed fluorescence, not increase it. Ascorbate and dehydroascorbate can also react with DAF-2DA, resulting in fluorescence similar to that observed with NO. Previous work (Igamberdiev et al., 2006) has shown that expression of Hb increases cell ascorbate and dehydroascorbate. In this work, no fluorescence was observed in cells with suppressed levels of NO. Furthermore, lower levels of reduced ASC were observed in cells of ZmHb1(A) and ZmHb2(A) lines (Supplemental Fig. S7). Increases in ascorbate/dehydroascorbate cell levels resulting in false NO detection are unlikely in this situation.
Many studies suggest that, in both animals and plants, accumulation of NO modulates several developmental and physiological responses, including PCD (Brüne, 2003). The participation of NO in the death program is dichotomous because both antiapoptotic and proapoptotic pathways have been shown to be regulated by NO (Brüne, 2003). Proapoptotic pathways are triggered by NO through a variety of mechanisms, including the inhibition of respiration and reduction in membrane potential, the activation of caspase activity (Brüne, 2003), and the covalent modification of target proteins by nitrosylation (for review, see Igamberdiev et al., 2014). During maize embryogenesis, the increased levels of NO in ZmHb-suppressing cells induce PCD (Fig. 3A), and modulation of NO levels affects embryogenesis in a pattern consistent with the expression of ZmHbs. Specifically, a reduction of NO with cPTIO increases the number of mature somatic embryos in the ZmHb1(A) line, whereas it reduces it in the ZmHb2(A) line (Fig. 2).
Accumulation of NO elevated the levels of cellular Zn2+, an important regulator of cellular integrity and response to endogenous and exogenous stimuli (Roohani et al., 2013). Elevated Zn2+ signals were detected in domains enriched in NO and suppressing ZmHbs (Fig. 1E) as well as in cells treated with the NO donor SNP (Fig. 1E). Furthermore, depletion of NO with cPTIO reduced Zn2+ levels. The requirement of NO for Zn2+ accumulation can be ascribed, at least in part, to the ability of NO to release Zn2+ from MTs (Table I; Supplemental Fig. S4), small heavy-metal binding proteins able to regulate cellular Zn2+ homeostasis (St Croix et al., 2002). The participation of MTs in NO signaling by sequestration or release of Zn2+ is a unique concept in plants, which other than having potential central roles in other NO-mediated responses unrelated to PCD, provide evidence of similar NO-regulatory mechanisms operating across organisms. In both hippocampus and endothelial cells, NO increases the amount of labile Zn2+ (Berendji et al., 1997; Cuajungco and Lees, 1998), and elevated Zn2+ levels can be generated from the destruction of the Zn2+-sulfur clusters of MTs by NO (Aravindakumar et al., 1999). In animal cells, the contribution of the MT-mediated Zn2+ release to the overall cellular Zn2+ pool can be significant and is detectable with the same Zn2+-specific fluorophore Zinquin (St Croix et al., 2002) used in this study.
Unequivocal experimental evidence indicates that elevation of Zn2+ levels in ZmHb-suppressing maize cells mediates the NO activation of PCD. First, chelation of Zn2+ abolishes death by NO, whereas increasing Zn2+ levels induce PCD in environments depleted of NO (Fig. 3). Second, manipulations of Zn2+ in the culture medium override the effects of NO on embryo yield (Fig. 2). The proapoptotic role of Zn2+ reported in our study agrees with work on animal systems (Wätjen et al., 2002; Land and Aizenman, 2005), which show that the death-triggering accumulation of Zn2+ originates solely from MTs (Lee et al., 2003), but not with others documenting PCD in Zn2+-deficient cells (Rogers et al., 1995; Helmersson et al., 2008). This discrepancy is possibly caused by differences in perception of Zn2+ homeostasis operating across organisms. When manifested, the proapoptotic role of Zn2+ in animal cells is often mediated by ROS (Zhang et al., 2007), key regulators of developmental and environmental death programs (Elmore, 2007). This finding is in agreement with our results showing the accumulation of ROS in Zn2+-supplemented cells, their depletion in Zn2+-deficient cells (Fig. 3B), and the abolishment of the death program after scavenging of ROS by ASC in the ZmHb1(A) line characterized by high levels of Zn2+ (Fig. 3C). Furthermore, the observation that Zn2+ manipulations (supplementation or chelation) override the effects of NO on ROS production (Fig. 3B) places ROS downstream of NO and Zn2+ in the Hb response.
Inhibition of the Oxidative Burst and MAPK Cascade Abolishes ROS Formation
Generation of ROS in plants occurs through an oxidative burst triggered by diverse mechanisms, some of which involve NADPH oxidases, membrane-bound enzymes implicated in several responses, including PCD (Sagi and Fluhr, 2006). A reduction in ROS production during maize embryogenesis was achieved pharmacologically using both AEBSF and DPI (Diatchuk et al., 1997; Tiwari et al., 2002). Other than decreasing superoxide and hydrogen peroxide, a result consistent with previous studies (Bindschedler et al., 2006; Davies et al., 2006), these inhibitors reduced PCD in the ZmHb1(A) line characterized by elevated levels of ROS and TUNEL-positive nuclei (Fig. 4). Furthermore, both AEBSF and DPI abolished the effects of ZmHb suppression on embryogenesis, consistent with the reduction of ROS and PCD (compare Fig. 3C with Fig. 4).
Additional evidence supporting the involvement of NADPH oxidase in the accumulation of ROS during the ZmHbs regulation of embryogenesis is apparent by the behavior of the four ZmrbohA-D genes, homologs of the human neutrophil pathogen-related gp91PHOX (a component of the NADPH oxidase) that are expressed at high levels during active production of ROS by NADPH oxidases (Lin et al., 2009a). Suppression of ZmHb1 or ZmHb2 induces the expression of the four genes through the mediation of NO by Zn2+ (Supplemental Fig. S8).
Activation of the oxidative burst is linked to several transduction mechanisms, including the MAPK cascade (Lin et al., 2009a), which transduces many stimuli, some of which are mediated by NO (Browning et al., 2000). Inhibition of the MAPK cascade by the cell-permeable PD098059, which prevents the phosphorylation of MAPKK (Favata et al., 1998; Samuel and Ellis, 2002; Wang et al., 2010), reduces ROS production, abolishes the death program, and modulates embryogenesis in a pattern consistent with the depletion of ROS (Fig. 5A). The requirement of active MAPK for the accumulation of ROS might be exercised through the transcription of the four ZmrbohA-D (Fig. 5B), the expressions of which have been shown to correlate to the activation of NADPH oxidase (Lin et al., 2009a).
A Unique Model Linking ZmHbs to Cell Death/Survival Decision during in Vitro Embyrogenesis
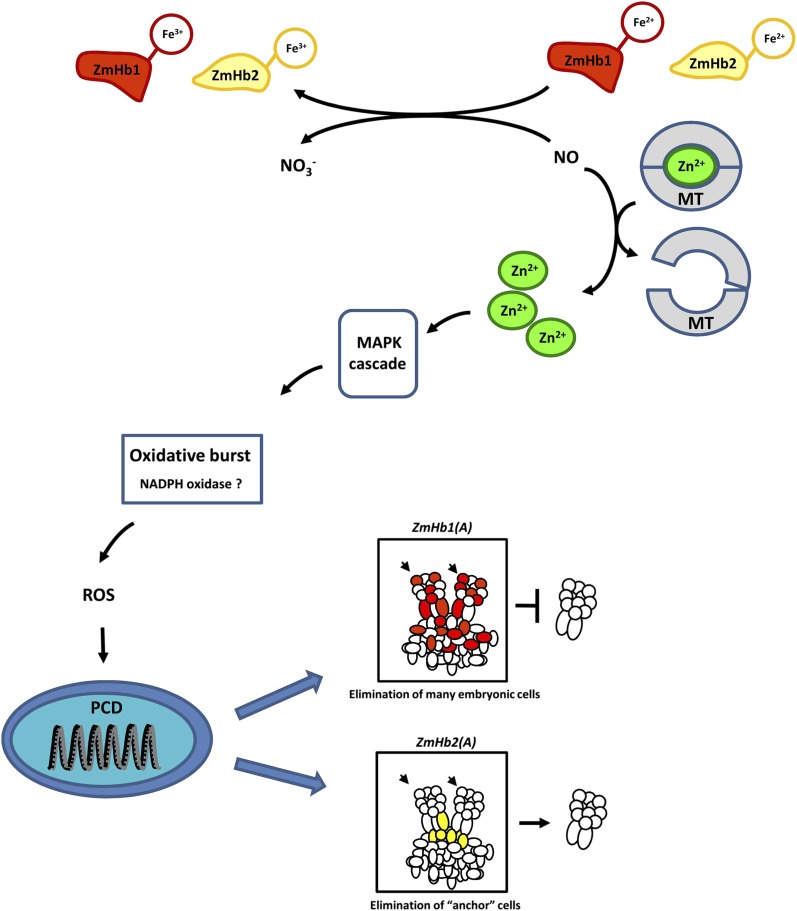
Results presented here fit a model in which ZmHb controls PCD by regulating cellular NO during maize somatic embryogenesis (Fig. 7). NO is produced as an initiator of the cascade of events ending in death of the cell. Most of the PCD pathway has been previously been described, but this work has shown the unique finding that Zn2+, like in vertebrate systems, is a critical component in this cascade and released from MTs. Depending on characteristics within a specific maize embryonic cell, ZmHb1 or ZmHb2 may be expressed to inhibit this process. The oxygenated form of the Hb reacts with cellular NO, producing nitrate and methemoglobin (Hb [Fe3+]). Therefore, the cellular expression of a specific Hb gene has the capability to determine the fate of that cell under conditions where mobile or transportable hormones or effectors, such as auxin, ethylene, or NO, are present in an organ. Expression of ZmHb1 during maize somatic embryogenesis protects a large number of embryonic cells from PCD by scavenging NO; therefore, when this gene’s expression is suppressed [ZmHb1(A)], significant embryo abortion occurs. Expression of ZmHb2 protects a few basal embryonic cells from PCD. Suppression of expression of this gene [ZmHb2(A)] results in death of these anchor cells, increasing the number of mature somatic embryos produced. Programming of PCD in suspensor cells to establish embryo polarity plays a critical role in survival and development during zygotic embryogenesis (Smertenko and Bozhkov, 2014). Our results would suggest that the two maize Hbs may function to both protect the developing maize embryo from PCD (ZmHb1) and orchestrate the timing of suspensor PCD (ZmHb2) during zygotic embryogenesis.
Figure 7.
Proposed model of ZmHb action during somatic embryogenesis. Suppression of ZmHbs releases the inhibitory effects of NO on PCD through a cascade involving intracellular Zn2+, the MAPK cascade, and accumulation of ROS, which are possibly mediated by NADPH oxidase. The distinct expression domains of ZmHb1 and ZmHb2 identify cells targeted by PCD and, ultimately, embryo formation. Cells undergoing PCD in the two lines [red, ZmHb1(A); yellow, ZmHb2(A)] are outlined. Small arrows indicate the apical domain of developing embryos embedded in the embryogenic tissue.
Manifestation of PCD is also important for key events during the in vivo reproductive phase. The dismantling of the suspensor during the middle late phases of embryogenesis, the selective elimination of nucellus and aleurone cells during endosperm development, pollen-stigma interaction in the initial steps of fertilization, male and female organ sculpturing in developing flowers, and parthenogenesis are all examples of events modulated by PCD. Based on the premises of this model, it is possible that Hbs are intimately linked with these events, and their manipulation can be used as a tool to enhance the reproductive outcome.
It cannot be excluded that the regulation of PCD by Hbs can also operate across species. The majority of plants that have had Hbs identified contain at least two Hb genes, with rice (Oryza sativa) having as many as five genes (Vázquez-Limón et al., 2012). Cytoglobin and neuroglobin are two Hbs that potentially could function in a similar fashion in vertebrates (Burmester and Hankeln, 2009). They are up-regulated during hypoxia, capable of scavenging NO, and prevent PCD. We conclude that ZmHbs modulate somatic embryogenesis in maize through PCD and identify Hbs as potential regulators of morphogenesis in other systems.
MATERIALS AND METHODS
Plant Material and Treatments
Maize (Zea mays) Hi II Type II embryogenic tissue was obtained from the Plant Transformation Facility at Iowa State University (http://agron-www.agron.iastate.edu/ptf/). Somatic embryogenesis was induced as reported by Wang and Frame (2009) by transferring embryogenic tissue maintained on a solid 2,4-dichlorophenoxyacetic acid-containing maintenance medium into a liquid P medium of similar composition. After 7 d, the tissue was plated on solid hormone-free D medium to allow embryo maturation, which lasted 21 d. Pharmacological treatments were carried out for 7 d in the P medium. The NO donor SNP was applied at a concentration of 10 µm, and the NO scavenger cPTIO was applied at a concentration of 100 µm (Elhiti et al., 2013). ZnSO4 was used as a Zn2+ source and applied at a concentration of 100 or 300 µm (Helmersson et al., 2008). In the Zn2+ supplementation experiments, control cells were also grown in a medium devoid of ZnSO4. The membrane-permeable Zn2+ chelator TPEN was applied at a concentration of 1 or 5 µm (Helmersson et al., 2008). The specific inhibitor of MAPKK PD098059 was administered at a concentration of 50 µm (Wang et al., 2010). The NADPH oxidase inhibitors AEBSF and DPI were applied at concentrations of 1 mm and 5 μm, respectively (Diatchuk et al., 1997; Drummond et al., 2011; Ryder et al., 2013). Reduced ASC was used at a concentration of 100 µm (Stasolla and Yeung, 1999). Treatments were interrupted at d 7 in the P medium, and the tissue was either cultured on solid D medium to assess embryo yield or collected for gene expression, immunological, and histological analyses. When not specified, ZnSO4 and TPEN were applied at concentrations of 300 and 5 µm, respectively.
Plant Transformation
For construction of antisense maize lines, the two Hbs, ZmHb1 (AF236080) and ZmHb2 (DQ171946), were amplified from complementary DNA (cDNA) prepared from maize embryogenic tissue by reverse transcription (RT)-PCR using gene-specific primers and subcloned into a pGEM-T Easy vector (Promega). Sequences were confirmed by DNA sequencing, and BamHI sites were added to both 5′ and 3′ ends of both genes by PCR. The maize polyubiquitin1 (ubi-1) promoter was isolated from pUbiSXR vector, and KpnI restriction sites were added to the 5′ and 3′ ends by PCR. The nopaline synthase terminator was isolated from pBI121 with SacI restriction sites and added to the 5′ and 3′ ends. The entire cassette was assembled through sequential ligation of the ubi-1 promoter, nos-t, and ZmHb1 or ZmHb2 into pBluescript SK−. Confirmed antisense constructs were cobombarded into immature maize embryos and selected on glufosinate-containing media (Wang and Frame, 2009).
For the promoter constructs, putative PZmHb1 and PZmHb2 were isolated by PCR from maize Hi II Type II callus genomic DNA using specific primers (MaizeGDB, http://www.maizegdb.org/; BDGP, http://www.fruitfly.org/; Zhao et al., 2008). The promoters (898 and 1,251 bp, respectively) were subcloned into pGEM-T Easy vector and confirmed by DNA sequencing. Using Gateway Cloning Technology (Invitrogen), both promoters were assembled into the destination vector pKGWFS7 (Karimi et al., 2002) to generate PZmHb1:GUS and PZmHb2:GUS lines.
For the KOD constructs, AtKOD was isolated by PCR from genomic DNA of Arabidopsis (Arabidopsis thaliana) ecotype Colombia-0 using gene-specific primers (Blanvillain et al., 2011), subcloned into pGEM-T Easy vector, and confirmed by DNA sequencing. The cloning fragments were amplified by SpeI-AtKOD-F and puc19-M13-R primers and ligated into the SpeI-digested and -dephosphorylated pGEM-T:PZmHb1 and pGEM-T:PZmHb2, respectively. Using Gateway Cloning Technology (Invitrogen), both PZmHb1:AtKOD and PZmHb2:AtKOD were transferred into the destination vector pEarleygate 301 (Earley et al., 2006).
All primers used are listed in Supplemental Table S1.
Isolation of the Maize Embryo-Specific ZmMT4
Total RNA was isolated from wild-type maize tissue using the Dynabeads Kit (Life Technologies). The cDNA of ZmMT4 embryo-specific U10696 (protein accession no. CAA84233; Leszczyszyn et al., 2013) was obtained by RT-PCR. The amplified product was purified and cloned into the pCR4-blunt TOPO vector (Life Technologies) and confirmed by sequencing. An MBP fusion construct was assembled in pMAL-c5X vector (NEB) using an Infusion strategy (Clontech) by linearizing the vector with XmnI and EcoRI followed by amplification with the primers MT4_fw and MT4_rev (Supplemental Table S1).
ZmMT4 Expression and Purification
A single colony of NEB expression Escherichia coli harboring the pMAL-c5X vector alone or the positive vector containing ZmMT4 fusion was used to inoculate an overnight starter culture in 4 mL of Luria-Bertani broth incubated at 37°C with vigorous shaking. Two milliliters of the starter was used to inoculate 200 mL of double-concentrated Luria-Bertani broth grown at 37°C. When the optical density600 reached 4.5, the expression was induced by 0.5 mm isopropyl-β-d-1-thiogalactoside in a medium supplemented with or without 0.4 mm ZnSO4. The cells were grown for an additional 3 h, harvested by centrifugation, and then resuspended in 8 mL of extraction buffer (0.1 m Tris-HCl, pH 8.0, 0.5 m NaCl, 1 mm EDTA, 1 mm AEBSF, 10 µm trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane, 20 µm Bestatin, 0.1 mm Phosphoramidon, 4 mm dithiothreitol, 0.01% [v/v] Triton X-100, and 20% [v/v] glycerol). After sonication, the lysate was clarified by centrifugation and purified on 10 mL of amylose resin according to the manufacturer’s specifications (NEB). Eluted control MBP and MBP-ZmMT4 fusion proteins were dialyzed and concentrated using vivaspin 20 centrifugal devices (GE Healthcare). Approximately 10 mg of recombinant MBP was recovered from 200 mL of culture. Protein quantification was performed with the method by Bradford (1976), whereas purity was confirmed by SDS-PAGE with Coomassie staining.
Release of Zn2+ from the Purified ZmMT4
The NO donor DEA/NO (sodium salt hydrate) was prepared as a 0.5 m stock solution in 10 mm NaOH. To quantify the presence of Zn2+ in the purified MBP or MBP-ZmMT4 proteins, 0.2 to 0.26 mg of each protein, in triplicate, was incubated with 1 n HCl for 45 min at room temperature and centrifuged at 20,000g for 2 min. The release of Zn2+ was quantitatively evaluated by DTZ (diphenylthiocarbazone) by following published protocols (Paradkar and Williams, 1994).
For the Zn2+ release by DEA/NO, 1 concentration of ZmMT4 and MBP (as negative control), in triplicate, was incubated at room temperature for 45 min in 0.05 m Tris-HCl (pH 7.5) with 3 mm DEA/NO or the same amount of water (negative control). The amount of Zn2+ released was directly assayed by the DTZ method described above.
The choice of different NO donors (SNP for embryogenesis and DEA/NO for the MTs experiment) was based on their different characteristics. SNP is the NO donor used most routinely in vivo (Hebelstrup and Jensen 2008; Elhiti et al., 2013). NO release from SNP is not spontaneous but relies on chemical reactions after SNP is absorbed by the cells (Miller and Megson, 2007). Therefore, SNP was used only for the embryogenesis studies but not the MTs experiment. The effectiveness of SNP was always verified by monitoring NO levels. DEA/NO spontaneously dissociates, releasing NO at neutral or slight alkaline pH. The release of NO from DEA/NO is more homogeneous and more suitable for in silico studies because it can be regulated/controlled by adjusting the pH of the buffer used. A pH of 7.5 was used for the MT experiment. Because of its pH-dependent properties, the use of DEA/NO is not recommended in vivo, especially in culture, where changes in pH often occur over time.
In Situ Localization of NO, Zn2+, and PCD
NO was assayed using DAF-2DA (Elhiti et al., 2013). Zinquin-ethyl-ester was used to localize Zn2+ (Helmersson et al., 2008). Nuclear DNA fragmentation was detected with the In Situ Cell Death Detection Kit-Fluorescein (Roche). Tissue was fixed in 4% (w/v) paraformaldehyde, dehydrated in ethanol series, and embedded in wax. Sections (10 µm) were dewaxed in xylene and labeled with the TUNEL kit (Roche) according to the manufacturer’s protocol, with the exclusion of the permeabilization step by proteinase K. Omission of terminal deoxynucleotidyl transferase was used for negative controls.
Localization of Superoxide, Hydrogen Peroxide, and Reduced ASC
Localization of hydrogen peroxide in maize cells was performed as described by Thordal-Christensen et al. (1997). Suspension cells (0.2 mL) were stained with 3,3′-diaminobenzidine (pH 3.8) for 3 h in the dark. Superoxide was visualized by incubating cells in a solution containing 0.05% (w/v) nitroblue tetrazolium, 10 mm sodium azide, and 50 mm phosphate-buffered saline (pH 7.6; Lin et al., 2009b). Reduced ASC was localized following the procedure by Liso et al. (2004).
Expression Studies
TRI Reagent Solution was used to extract RNA following the manufacturer’s instruction (Invitrogen), and the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for cDNA synthesis. Gene expression studies were performed by quantitative RT-PCR. All primers used for gene expression studies are listed in Supplemental Table S1. The relative gene expression level was analyzed with the 2−∆∆CT method (Livak and Schmittgen, 2001) using actin as the reference gene.
Expression of AtKOD was detected by quantitative RT-PCR, Taqman technology using AtKOD-F/R with AtKOD-FAM probe, and Actin1-F/R with Actin1-HEX probe (Supplemental Table S1).
Caspase-Like Activity
Caspase 3-like activity was assayed using the substrate Ac-DEVD-AFC (Coffeen and Wolpert, 2004; Ye et al., 2013). The assay was conducted using a fluorescence spectrophotometer (excitation of 400 nm and emission of 505 nm).
RNA in Situ Hybridization
RNA in situ hybridization studies were conducted as described (Elhiti et al., 2010). The two full-length ZmHb1 and ZmHb2 cDNAs were cloned into a pGEM-T Easy Vector System (Promega). The two cDNAs were subsequently amplified from the vector using T7 and SP6 primers and used for the preparation of digoxigenin-labeled sense and antisense riboprobes following the procedure outlined in the DIG Application Manual (Roche Diagnostics). Slide preparation, hybridization conditions, and color development were performed as documented previously (Elhiti et al., 2010).
Statistical Analysis
Tukey’s post hoc test (Zar, 1999) was used for comparison and analysis of significant differences.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Amino acid sequence alignment of ZmHb1 and ZmHb2 with Clustal W using BLOSUM protein weight matrix (Larkin et al., 2007).
Supplemental Figure S2. Regulation of ZmHb1 and ZmHb2 by auxin.
Supplemental Figure S3. Detection of Zn2+ in maize embryos by Zinquin.
Supplemental Figure S4. Effects of increasing concentrations of the NO donor (DEA/NO) on the release of Zn2+ from a constant concentration of recombinant ZmMT4 protein (Table I).
Supplemental Figure S5. Percentage of TUNEL-positive cells in immature (d 7, P medium) embryos cultured in environments with altered levels of NO and Zn2+.
Supplemental Figure S6. Cytological and molecular hallmarks of PCD in ZmHb-suppressing embryos.
Supplemental Figure S7. Localization of reduced ASC in immature (d 7, P medium) maize embryos produced by the wild-type line and ZmHb1(A) and ZmHb2(A) lines.
Supplemental Figure S8. Expression level of four respiratory burst oxidase homologs (ZmrbohA-D) in immature embryos (d 7, P medium) produced from the wild-type lines and ZmHb1(A) and ZmHb2(A) lines.
Supplemental Table S1. List of primers used in this study.
Supplementary Material
Acknowledgments
We thank Doug Durnin for technical assistance.
Glossary
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- ASC
ascorbic acid
- cDNA
complementary DNA
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3 oxide
- D
development
- DEA/NO
diethylamine NONOate
- DPI
diphenylene iodonium
- DTZ
dithizone
- Hb
hemoglobin
- KOD
KISS OF DEATH
- MAPK
mitogen-activated protein kinase
- MAPKK
mitogen-activated protein kinase kinase
- MBP
maltose binding protein
- MT
metallothionein
- P
proliferation
- PCD
programmed cell death
- PD098059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- ROS
reactive oxygen species
- RT
reverse transcription
- SNP
sodium nitroprusside
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
Footnotes
This work was supported by the National Sciences and Engineering Research Council (to R.D.H. and C.S.) and the University of Manitoba (Graduate Fellowship to S.H.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Aravindakumar CT, Ceulemans J, De Ley M. (1999) Nitric oxide induces Zn2+ release from metallothionein by destroying zinc-sulphur clusters without concomitant formation of S-nitrosothiol. Biochem J 344: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktutan U. (2005) Coronary microvascular endothelial cell growth regulates expression of the gene encoding p22-phox. Free Radic Biol Med 39: 1342–1352 [DOI] [PubMed] [Google Scholar]
- Berendji D, Kolb-Bachofen V, Meyer KL, Grapenthin O, Weber H, Wahn V, Kröncke KD. (1997) Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett 405: 37–41 [DOI] [PubMed] [Google Scholar]
- Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, et al. (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47: 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanvillain R, Young B, Cai YM, Hecht V, Varoquaux F, Delorme V, Lancelin JM, Delseny M, Gallois P. (2011) The Arabidopsis peptide kiss of death is an inducer of programmed cell death. EMBO J 30: 1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Franklin-Tong VE. (2007) Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen. Proc Natl Acad Sci USA 104: 18327–18332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhkov PV, Filonova LH, Suarez MF. (2005) Programmed cell death in plant embryogenesis. Curr Top Dev Biol 67: 135–179 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brittain T, Skommer J, Raychaudhuri S, Birch N. (2010) An antiapoptotic neuroprotective role for neuroglobin. Int J Mol Sci 11: 2306–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning DD, McShane MP, Marty C, Ye RD. (2000) Nitric oxide activation of p38 mitogen-activated protein kinase in 293T fibroblasts requires cGMP-dependent protein kinase. J Biol Chem 275: 2811–2816 [DOI] [PubMed] [Google Scholar]
- Brüne B. (2003) Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ 10: 864–869 [DOI] [PubMed] [Google Scholar]
- Burmester T, Hankeln T. (2009) What is the function of neuroglobin? J Exp Biol 212: 1423–1428 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, Goldsbrough PB. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Coffeen WC, Wolpert TJ. (2004) Purification and characterization of serine proteases that exhibit caspase-like activity and are associated with programmed cell death in Avena sativa. Plant Cell 16: 857–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. (1998) Nitric oxide generators produce accumulation of chelatable zinc in hippocampal neuronal perikarya. Brain Res 799: 118–129 [DOI] [PubMed] [Google Scholar]
- Davies DR, Bindschedler LV, Strickland TS, Bolwell GP. (2006) Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: implications for basal resistance. J Exp Bot 57: 1817–1827 [DOI] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. (1997) Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301 [DOI] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD. (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35: 763–770 [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C. (2013) Function of type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J 74: 946–958 [DOI] [PubMed] [Google Scholar]
- Elhiti M, Tahir M, Gulden RH, Khamiss K, Stasolla C. (2010) Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J Exp Bot 61: 4069–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE. (2003) Aerenchyma formation. New Phytol 161: 35–49 [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632 [DOI] [PubMed] [Google Scholar]
- Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S. (2000) Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci 113: 4399–4411 [DOI] [PubMed] [Google Scholar]
- Garrocho-Villegas V, de Jesús-Olivera MT, Quintanar ES. (2012) Maize somatic embryogenesis: recent features to improve plant regeneration. Methods Mol Biol 877: 173–182 [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K, Khan AA. (2008) Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol 8: 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebelstrup KH, Jensen EO. (2008) Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta 227: 917–927 [DOI] [PubMed] [Google Scholar]
- Hegelund JN, Schiller M, Kichey T, Hansen TH, Pedas P, Husted S, Schjoerring JK. (2012) Barley metallothioneins: MT3 and MT4 are localized in the grain aleurone layer and show differential zinc binding. Plant Physiol 159: 1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmersson A, von Arnold S, Bozhkov PV. (2008) The level of free intracellular zinc mediates programmed cell death/cell survival decisions in plant embryos. Plant Physiol 147: 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RD. (2012) Non-symbiotic haemoglobins: What's happening beyond nitric oxide scavenging? AoB Plants 2012: pls004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, Peacock WJ. (2001) Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol 47: 677–692 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Stasolla C, Hill RD. (2014) Low oxygen stress, nonsymbiotic hemoglobins, NO and programmed cell death. In van Dongen JT, Licausi F, eds, Oxygen Stress in Plants. Springer-Verlag, Berlin, pp 41–58 [Google Scholar]
- Igamberdiev AU, Stoimenova M, Seregélyes C, Hill RD. (2006) Class-1 hemoglobin and antioxidant metabolism in alfalfa roots. Planta 223: 1041–1046 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Keshet Y, Seger R. (2010) The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol 661: 3–38 [DOI] [PubMed] [Google Scholar]
- Khan AA, Mao XO, Banwait S, Jin K, Greenberg DA. (2007) Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci USA 104: 19114–19119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land PW, Aizenman E. (2005) Zinc accumulation after target loss: an early event in retrograde degeneration of thalamic neurons. Eur J Neurosci 21: 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. (2003) Zinc released from metallothionein-III may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol 184: 337–347 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Koh JY. (2010) Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol Brain 3: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczyszyn OI, Imam HT, Blindauer CA. (2013) Diversity and distribution of plant metallothioneins: a review of structure, properties and functions. Metallomics 5: 1146–1169 [DOI] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong W, Jiang M. (2009a) Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J Exp Bot 60: 3221–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Liu N, Lin GZ, Peng CL. (2009b) In situ localisation of superoxide generated in leaves of Alocasia macrorrhiza (L.) Shott under various stresses. J Plant Biol 52: 340–347 [Google Scholar]
- Liso R, De Tullio MC, Ciraci S, Balestrini R, La Rocca N, Bruno L, Chiappetta A, Bitonti MB, Bonfante P, Arrigoni O. (2004) Localization of ascorbic acid, ascorbic acid oxidase, and glutathione in roots of Cucurbita maxima L. J Exp Bot 55: 2589–2597 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Miller MR, Megson IL. (2007) Recent developments in nitric oxide donor drugs. Br J Pharmacol 151: 305–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Mandon J, Cristescu SM, Harren FJ, Prats E. (2011) Methods of nitric oxide detection in plants: a commentary. Plant Sci 181: 509–519 [DOI] [PubMed] [Google Scholar]
- Mur LA, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH. (2012) Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot 63: 4375–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar RP, Williams RR. (1994) Micellar colorimetric determination of dithizone metal chelates. Anal Chem 66: 2752–2756 [Google Scholar]
- Pennell RI, Lamb C. (1997) Programmed cell death in plants. Plant Cell 9: 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. (2011) Anti-apoptosis and cell survival: a review. Biochim Biophys Acta 1813: 238–259 [DOI] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. (2002) Ascorbate and glutahione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40: 537–548 [Google Scholar]
- Reape TJ, Molony EM, McCabe PF. (2008) Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59: 435–444 [DOI] [PubMed] [Google Scholar]
- Ren Y, Liu Y, Chen H, Li G, Zhang X, Zhao J. (2012) Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in Arabidopsis. Plant Cell Environ 35: 770–789 [DOI] [PubMed] [Google Scholar]
- Rogers JM, Taubeneck MW, Daston GP, Sulik KK, Zucker RM, Elstein KH, Jankowski MA, Keen CL. (1995) Zinc deficiency causes apoptosis but not cell cycle alterations in organogenesis-stage rat embryos: effect of varying duration of deficiency. Teratology 52: 149–159 [DOI] [PubMed] [Google Scholar]
- Roohani N, Hurrell R, Kelishadi R, Schulin R. (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci 18: 144–157 [PMC free article] [PubMed] [Google Scholar]
- Ryder LS, Dagdas YF, Mentlak TA, Kershaw MJ, Thornton CR, Schuster M, Chen J, Wang Z, Talbot NJ, Talbot NJ. (2013) NADPH oxidases regulate septin-mediated cytoskeletal remodeling during plant infection by the rice blast fungus. Proc Natl Acad Sci USA 110: 3179–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Ellis BE. (2002) Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14: 2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, Vazquez A. (2001) Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ 8: 152–161 [DOI] [PubMed] [Google Scholar]
- Smagghe BJ, Blervacq AS, Blassiau C, Decottignies JP, Jacquot JP, Hargrove MS, Hilbert JL. (2007) Immunolocalization of non-symbiotic hemoglobins during somatic embryogenesis in chicory. Plant Signal Behav 2: 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagghe BJ, Hoy JA, Percifield R, Kundu S, Hargrove MS, Sarath G, Hilbert JL, Watts RA, Dennis ES, Peacock WJ, et al. (2009) Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 91: 1083–1096 [DOI] [PubMed] [Google Scholar]
- Smertenko A, Bozhkov PV. (2014) Somatic embryogenesis: life and death processes during apical-basal patterning. J Exp Bot 65: 1343–1360 [DOI] [PubMed] [Google Scholar]
- Stasolla C, Yeung EC. (1999) Ascorbic acid improves conversion of white spruce somatic embryos. In Vitro Cell Dev Biol Plant 35: 316–319 [Google Scholar]
- St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. (2002) Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol 282: L185–L192 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Thorpe TA, Stasolla C. (2001) Somatic embryogenesis. In Bhojwani SS, Soh WY, eds, Current Trends in the Embryology of Angiosperms. Kluwer Academic Publisher, Dordrecht, pp 279–336 [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128: 1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Ho LH, Chai F, Zalewski PD. (2000) Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J Nutr (5SSuppl) 130: 1459S–1466S [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. (2006) Reactive oxygen species in plant cell death. Plant Physiol 141: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, et al. (2011) Morphological classification of plant cell deaths. Cell Death Differ 18: 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Limón C, Hoogewijs D, Vinogradov SN, Arredondo-Peter R. (2012) The evolution of land plant hemoglobins. Plant Sci 191-192: 71–81 [DOI] [PubMed] [Google Scholar]
- Wang K, Frame B. (2009) Biolistic gun-mediated maize genetic transformation. Methods Mol Biol 526: 29–45 [DOI] [PubMed] [Google Scholar]
- Wang PC, Du YY, Li Y, Ren DT, Song CP. (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22: 2981–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wätjen W, Cox M, Biagioli M, Beyersmann D. (2002) Cadmium-induced apoptosis in C6 glioma cells: mediation by caspase 9-activation. Biometals 15: 15–25 [DOI] [PubMed] [Google Scholar]
- Weber RE, Vinogradov SN. (2001) Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev 81: 569–628 [DOI] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN. (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44 [DOI] [PubMed] [Google Scholar]
- Wind S, Beuerlein K, Eucker T, Müller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. (2010) Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Li Z, Xing D. (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ 36: 1–15 [DOI] [PubMed] [Google Scholar]
- Zalewski PD, Forbes IJ, Betts WH. (1993) Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem J 296: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. (1999) Biostatistical Analysis, Ed 4 Prentice-Hall, Upper Saddle River NJ, pp 177–230 [Google Scholar]
- Zhang Y, Aizenman E, DeFranco DB, Rosenberg PA. (2007) Intracellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death. Mol Med 13: 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Gu RL, Gao P, Wang GY. (2008) A nonsymbiotic hemoglobin gene from maize, ZmHb, is involved in response to submergence, high-salt and osmotic stresses. Plant Cell Tissue Organ Cult 95: 227–237 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.