Abstract
Whey protein intake is associated with the modulation of energy metabolism and altered body composition both in human subjects and in animals, but the underlying mechanisms are not yet elucidated. We fed obesity-prone C57BL/6J mice high-fat diets with either casein (HF casein) or whey (HF whey) for 6 weeks. At equal energy intake and apparent fat and nitrogen digestibility, mice fed HF whey stored less energy as lipids, evident both as lower white adipose tissue mass and as reduced liver lipids, compared with HF-casein-fed mice. Explorative analyses of 48 h urine, both by 1H NMR and LC–MS metabolomic platforms, demonstrated higher urinary excretion of tricarboxylic acid (TCA) cycle intermediates citric acid and succinic acid (identified by both platforms), and cis-aconitic acid and isocitric acid (identified by LC–MS platform) in the HF whey, relative to in the HF-casein-fed mice. Targeted LC–MS analyses revealed higher citric acid and cis-aconitic acid concentrations in fed state plasma, but not in liver of HF-whey-fed mice. We propose that enhanced urinary loss of TCA cycle metabolites drain available substrates for anabolic processes, such as lipogenesis, thereby leading to reduced lipid accretion in HF-whey-fed compared to HF-casein-fed mice.
Keywords: obesity, adipose tissue, metabolomics, whey, casein, tricarboxylic acid cycle, citric acid cycle, Krebs cycle, lipogenesis, glutaminolysis
Introduction
Obesity has reached pandemic proportions, and between 1980 and 2008 the mean body mass index (BMI) worldwide was estimated to have increased by 0.4 and 0.5 kg/m2 per decade for men and women, respectively.1 Understanding how environmental factors, such as diet, affect the development of obesity is important. From cross-sectional studies, an inverse relationship between dairy consumption and BMI has been suggested.2,3 More specifically, inclusion of dairy products during energy-restricted weight loss resulted in higher fat mass reduction while maintaining lean body mass.4−6 In further support of a fat-reducing effect of milk-proteins, a randomized controlled study with overweight subjects showed that intake of milk protein for 20 weeks reduced both visceral and subcutaneous fat more than intake of soy protein.7
Milk protein consists of about 80% casein and 20% whey, but casein and whey proteins are likely to affect metabolism differently. A randomized, double-blinded intervention study with crossover design demonstrated that after ingestion of a liquid test meal consisting of 50% protein, 40% carbohydrate, and 10% fat, whey protein induced a higher postprandial thermic effect than both casein and soy protein.8 In another intervention study with free-living overweight and obese subjects, intake of whey protein but not soy protein (both ∼56g protein/day for 23 weeks) resulted in a significant reduction in body mass, fat mass, and waist circumference, relative to the carbohydrate (maltodextrin) control treatment.9 Thus, data from human studies indicate that whey protein induces different effects on energy metabolism than do both casein and soy protein.
This notion is further supported by studies using the obesity prone C57BL/6J mice fed high-fat diets with either whey or casein as protein sources. In a study including a period of 50 days with energy restriction before 50 days with free access to feed (weight regain period), whey protein dose-dependently prevented weight regain and protected against fatty liver development relative to casein during the weight regain period.10 Importantly, neither energy intake nor apparent fat digestibility was reduced by whey protein feeding.10 Two other studies have confirmed the ability of whey protein, relative to casein, to reduce adiposity in high-fat-fed C57BL/6J mice at equal energy intake.11,12
On the basis of existing data from human and mice studies, dietary whey protein has been shown to elicit a weight-reducing effect, with specific reduction in fat mass. The underlying mechanisms governing reduced adiposity by whey ingestion are yet to be established. Therefore, the aim of the present study was to further elaborate our understanding of the antiobesity effects of whey protein intake. For this purpose, we fed obesity-prone C57BL/6J mice high-fat diets (20 energy % protein, 60 energy % fat) with either casein or whey protein as the sole protein source. Samples from the mice were analyzed by biochemical and molecular methods, explorative NMR-based and LC–MS-based metabolomics, as well as targeted LC–MS metabolomics. In whey-fed mice, our results identify a potential mechanism by loss of energy through enhanced urinary excretion of tricarboxylic acid (TCA) intermediates. Our metabolomic analyses provided novel knowledge on how different protein sources may affect energy metabolism, indicating that at equal energy intake and with equal fat and nitrogen digestibility, HF whey feeding prevented body fat gain by increasing urinary excretion of TCA intermediates, thereby reducing the availability of these for anabolic processes in the body as compared with HF casein feeding.
Experimental Procedures
Ethical Statement
The animal experiments were approved by the National Animal Health Authorities. Care and handling were in accordance with local institutional recommendations and rules. Adverse events were not observed.
Animal Studies and Diets
Male C57BL/6JBomTac aged 8 to 9 weeks were purchased from Taconic, Denmark. After 5 days of acclimatization, the mice were assigned to one of three dietary treatments: (I) HF casein (n = 14), a casein-based very high-fat diet (60en% fat and 20en% protein, D12492, Research Diets, USA), (II) HF whey (n = 14), a whey-protein-based very high-fat diet (60 en% fat and 20 en% protein), or (III) LF reference (n = 12), a casein-based low-fat diet, (D12450B, Research Diets, USA) (Table S1 Supporting Information). The mice were housed in individual cages and had free access to food and water. The mice were maintained on a 12:12 h light-dark cycle at thermoneutrality, 28 ± 1 °C. The mice were fed Mondays, Wednesdays, and Fridays, and body mass was recorded once a week. Leftover feed was quantitatively collected from the cages every other week. During the fourth week of the feeding period, a subpopulation of the mice (LF reference, n = 6; HF casein and HF whey, n = 8/treatment) spent 48 h in wire-bottomed cages for urine collection for metabolomic studies. Urine was collected in 0.5 mL of 1 mol/L HCl and frozen after 24 and 48 h. After feeding for 42–46 days in the nonfasted state (free access to food until termination), the mice were anesthetized with isoflurane (Isoba vet, Schering-Plough, Denmark) and euthanized by cardiac puncture. Blood was collected, and heparin plasma was prepared and frozen at −80 °C. Tissues were quickly dissected out and weighted. From a subpopulation of mice (LF, n = 6; HF casein and HF whey, n = 8), a part of the liver was homogenized for ex vivo hepatic fatty acid oxidation capacity measurements. Samples were taken from the epididymal and inguinal white adipose tissues (eWAT and iWAT, respectively) and fixed for histology. The rest of the tissues were quickly snap-frozen in liquid nitrogen and stored at −80 °C. From all mice (n = 12 or 14/group), liver, skeletal muscle tibialis anterior, eWAT, iWAT, and interscapular brown adipose tissue (iBAT) were dissected out. Additionally, from a subpopulation of the mice (n = 6–8/group), kidneys, heart, and skeletal muscle soleus were dissected out. An overview of the study design is presented in Figure S1 (Supporting Information). A separate set of mice (n = 10/diet) was fed the HF casein or the HF whey diet to determine apparent fat and nitrogen digestibility. Food intake and feces excretion was quantified during the last 5 days.
Gross Energy in the Experimental Diets
The gross energy contents were determined in a bomb calorimeter following the manufacturer’s instruction (Parr Instruments, Moline, IL).
Amino Acid Composition in the HF Diets
Amino acids in the diets were analyzed by standard methodology, as described in detail in the Supporting Information.
Total Fat in Diets and Feces
Total fat content was determined gravimetrically after extracting samples with organic solutions before and after acidic hydrolysis of the samples, as described in detail elsewhere.13
Nitrogen in Diets and Feces
The nitrogen (N) content was determined by the Dumas method in a Leco FP-528 nitrogen analyzer (LECO Corp., St. Joseph, MI). The crude protein content in the diets was calculated as N × 6.25.
Apparent Fat and Nitrogen Digestibility
Feces were weighted and analyzed for nitrogen and total fat content. Fat and nitrogen apparent digestibility were calculated as 100((eaten (mg) – fecal output (mg))/(eaten (mg)).
Histology
Sections of adipose tissue were fixed in 4% formaldehyde in 0.1 mol/L phosphate buffer overnight at 4 °C, washed in 0.1 mol/L PB, dehydrated in ethanol, and embedded in paraffin after clearing with xylene. Then, 5 μm thick sections of the embedded tissue were stained with eosin and hematoxylin.
Quantitative Reverse-Transcriptase PCR
From a subpopulation of the mice (n = 6–8/diet), RNA was purified, cDNA was synthesized, and quantitative reverse-transcriptase PCR (qRT-PCR) was run as previously described in detail.14 GeNorm was used to determine the most suitable normalization gene for each tissue; therefore, Tbp (TATA box binding protein) was used for normalization of gene expression in iWAT and iBAT, Actb (β-actin) was used for normalization in soleus skeletal muscle, and Canx (calnexin) was used in liver. Primers for qRT-PCR were designed using Primer Express 2.0 (Applied Biosystems, USA) and are presented in Table S2 in the Supporting Information.
Plasma Measurements
Plasma measurements were made on a subpopulation of the mice (n = 6–8/diet). Leptin and insulin concentrations were analyzed by ELISA kits (Leptin: BioVendor, Czech Republic; Insulin: DRG Diagnostics, Germany). Glucagon and adiponectin we measured by radioimmunoassay kits (Glucagon: Millipore, USA; Adiponectin: LINCO Research, USA). Plasma β-hydroxybutyrate (OH-butyrate), triacylglycerol (TAG), free fatty acids (FFA), glucose, and lactate concentrations were analyzed by conventional enzymatic kits (β-hydroxybutyrate: Randox, United Kingdom; TG, FFA, and glucose: DIALAB, Austria; lactate: Sentinel Diagnostics, Italy) using a MaxMat PL II (MAXMAT S.A., Montpellier, France).
Liver Lipid Analysis
From a subpopulation of the mice (n = 6/diet), total lipids were extracted from liver samples with chloroform/methanol 2:1 (v:v). The lipid classes were analyzed on an automated Camaq HPTLC system and separated on HPTLC silica gel 60 F plates, as previously described.15
Liver β-Oxidation Capacity Measurement
From a subpopulation of the mice (LF n = 6; HF casein and HF whey, n = 8), one part of liver tissue was immediately after sampling homogenized in nine parts (w/v) ice-cold homogenization buffer (200 mM mannitol, 50 mM sucrose, 10 mM HEPES-KOH, 1 mM EDTA, 0.1% fatty acid free BSA, pH 7.4).16 Fatty acid oxidation capacity was measured in the whole homogenate by the acid-soluble product method using radiolabeled palmitate as the substrate, as previously described in detail17 and in the Supporting Information.
Metabolomic Analyses by 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
From a subpopulation of mice (LF ref, n = 6; HF diets, n = 8/diet), urine samples were analyzed by NMR spectroscopy. The NMR measurements were performed using a Bruker Avance 600 NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany), operating at a 1H frequency of 600.13 MHz and equipped with a 5 mm TXI inverse probe. Urine samples were prepared by mixing 200 μL of urine with 400 μL of D2O containing 0.05% sodium trimethylsilyl-[2,2,3,3-2H4]-1-propionate (TSP). Prior to NMR measurements, the urine samples were adjusted to pH 5.5, as this value was close to the actual pH of the urine. Standard 1D 1H NMR spectra were obtained using single 90° degree pulse experiment with water suppression achieved by irradiating the water peak during the relaxation delay. The spectra were acquired by 64 scans, 32k data points, a spectral width of 17.34 ppm, and a temperature of 310 K. An exponential line-broadening of 0.3 Hz was applied prior to the Fourier transformation. Each spectrum was manually phased, baseline-corrected, and referenced to TSP. Two-dimensional (2D) NMR experiments were performed on selected samples to assist tentative assignment by online databases such as the Human Metabolome Database (HMDB).18 The 2D-NMR experiments confirmed that the difference in pH between urine samples in the present study (5.5) and the pH of the compounds in the HMDB set to 7.0 did not influence on compound assignments. Prior to the multivariate data analysis, spectra were aligned using the icoshift procedure19 in MATLAB (version R2009b, The Mathworks, Natick, MA). The water signal was excluded by removing the region from 4.7 to 4.9 ppm. Subsequently, the spectra were subdivided into 0.007 ppm spectral regions and integrated. The data sets were normalized to total area to remove any overall effects of differential dilution of the samples. Supervised orthogonal partial least-squares discriminant analysis (O-PLS-DA) was performed using the SIMCA-P+ software version 13.0 (Umetrics AB, Umeå, Sweden) on the mean-centered and Pareto-scaled data and with full cross-validation (leave-one-out). The quality of the models was evaluated by R2X, describing how much of the variation that is explained by the model (goodness of fit), and Q2, representing the predictive ability of the model (goodness of prediction) and the cross-validation ANOVA feature in SIMCA-P+.
Sample Preparation for Liquid Chromatography–Mass Spectrometry (LC–MS)
From a subpopulation of mice (n = 6–8) LC–MS metabolomic analyses were performed in urine, liver and plasma samples. Urine was diluted 5 times with 0.1% formic acid prior to LC–MS analysis. Heparin plasma samples were mixed with methanol to a final concentration of 80% methanol and precipitation proceeded for 30 min at room temperature. After centrifugation (20 000g, 20 min, 4 °C), the supernatant was taken out and evaporated using a rotary centrifuge at 35 °C. Metabolites were redissolved in HPLC buffer to obtain the original sample concentration. Freeze-dried liver samples (50 mg) were extracted with 1.5 mL of methanol (80%). Samples were sonicated for 30 min at room temperature and centrifuged (20 000g, 20 min, 4 °C), supernatants were evaporated as previously described, and then metabolites were redissolved in 200 μL of HPLC buffer. One μL sample was injected in the entire LC–MS analysis.
LC–MS Operation
The LC–MS analyses were performed with a MicrOTOF-Q mass spectrometer (Bruker Daltonics, Bremen, Germany) coupled to an Agilent 1200 series capillary HPLC (Agilent, Santa Clara, CA), as previously described.20 In brief, separation was performed on a ZORBAX SB C18 column (0.5 × 150 mm, 3.5 μm, Agilent Technologies, Waldbronn, Germany) at 25 °C using a gradient of water and acetonitrile containing 0.1% formic acid. All analyses were carried out in duplicate, and the MS was operated in negative mode. For MS/MS analysis, an ion intensity threshold of 2000 ion counts was set, and the three most intense peaks were fragmented. Peaks that appeared in three consecutive spectra were excluded from fragmentation for 1 min. Fragmentation was obtained with collision energy of 15–50 eV.
LC–MS Data Analysis
The mass spectra were automatically calibrated in Hystar (Bruker Daltonics, Bremen, Germany) and processed using MZmine (version 2.2),21 as previously described.20 For urine mass spectral data, compound features extracted with MZmine were subjected to multivariate data analysis using SIMCA-P+ (Umetrics AB, Umeå, Sweden). Peak areas were normalized to the total peak area and each variable was mean-centered and autoscaled. PC analysis revealed a clear separation between the three groups. Principal component (PC) 1, describing 14.1% of the variation, discriminated between the low-fat and high-fat diets, while the whey and casein diets could be discriminated along PC 3 (9.8% of the variation). To select the metabolites that contributed most significantly to the discrimination between the whey and casein diet, an orthogonal partial least-squares discriminant analysis (OPLS-DA) was performed, and features with variable importance (VIP) significantly higher than 1 were selected (in total 92 features). Tentative identifications were obtained by search against Human Metabolome Database (HMDB) using MZmine. The resulting molecular formulas were further checked by SmartFormula software (Bruker Daltonics) on the basis of high mass accuracy (<2 mDa) and well-matched isotopic pattern (mSigma score <20). Structures were determined using tandem mass spectrometry and comparison with pure standards or data posted on metabolite databases (HMDB, Metlin).22,18 The software Metaboanalyst23 was used for the identification of significant metabolic pathways and reports the pathway significance (p) values calculated by the software. (See the Supporting Information.)
Statistical Analyses
All data represent mean ± SE. All data sets were tested for homogeneity of variances using the Levene’s test. Data with heterogeneous variances were log-transformed before statistical analyses. Growth curves were analyzed using repeated measurement ANOVA using a Fisher LSD test for post hoc comparisons. The rest of the data were analyzed using one-way ANOVA with Fisher LSD as post hoc test or Student’s t test (targeted LC–MS metabolomics in liver and plasma). In the case of heterogeneous variances after log-transformation, metabolomic data were analyzed by Kruskal–Wallis test, as indicated in Table 1. A value of P < 0.05 was considered as statistically significant. Statistics were performed with STATISTICA 9.0 (StatSoft, USA).
Table 1. Explorative LC–MS Metabolomic Analysis of 48 h Urine Collected from Mice Fed HF Casein, HF Whey, or LF Reference Dietsa.
| ion count |
|||||||
|---|---|---|---|---|---|---|---|
| metabolite | m/z | RT | HF casein | HF whey | LF reference | change (%)b | pathwayc |
| pyruvic acid | 87.007 | 3.5 | 62 ± 32B | 304 ± 29A | 141 ± 16B | 385 | 1–8, 10, 11, 15–24 |
| citric acid | 191.020 | 3.6 | 9069 ± 3531B | 37231 ± 3936A | 17000 ± 815B | 311 | 1, 2 |
| cis-aconitic acidd | 173.009 | 5.9 | 2077 ± 293B | 7625 ± 1525A | 4893 ± 409A | 267 | 1, 2 |
| isocitric acide | 191.020 | 2.8 | 2480 ± 373B | 4085 ± 364A | 3139 ± 61AB | 65 | 1, 2 |
| succinic acidd | 117.019 | 4.1 | 58 ± 13B | 921 ± 182A | 123 ± 11B | 1488 | 1, 2, 9, 11, 15, 16 |
| citramalic acide | 147.030 | 3.2 | 209 ± 23B | 1323 ± 309A | 339 ± 34B | 533 | |
| N-acetylglutamined | 187.071 | 3.1 | 468 ± 32B | 1052 ± 113A | 967 ± 49A | 124 | 26 |
| N-acetyl-l-glutamic acid | 188.057 | 3.8 | 387 ± 59B | 740 ± 94A | 549 ± 26AB | 91 | 26, 27 |
| hippuric acid | 178.051 | 10.8 | 3806 ± 218B | 2130 ± 128C | 7055 ± 201A | –44 | 10 |
| 4-OH-benzoic acid | 137.025 | 10.4 | 317 ± 20A | 232 ± 12B | 316 ± 8A | –27 | 10 |
| p-OH-phenylacetic acid | 151.039 | 11.9 | 612 ± 35B | 439 ± 28C | 819 ± 27A | –28 | 10, 11 |
| 4-OH-phenyllactic acid | 181.051 | 9.4 | 10001 ± 918A | 5663 ± 947B | 7901 ± 429AB | –43 | 11 |
| kynurenic acid | 188.036 | 11.0 | 900 ± 36C | 1475 ± 77A | 1216 ± 36B | 64 | 13 |
| xanthurenic acid | 204.030 | 10.3 | 6685 ± 434B | 12368 ± 1520A | 6719 ± 212B | 85 | 13 |
| glutaconic acid | 129.018 | 6.4 | 1452 ± 157C | 5101 ± 183A | 2858 ± 378B | 251 | 13, 25 |
| orotic acidd | 155.008 | 3.5 | 163 ± 14C | 522 ± 68A | 293 ± 18B | 220 | 12 |
| ureidopropionic acid | 131.045 | 3.0 | 176 ± 23B | 359 ± 61A | 311 ± 22AB | 103 | 5, 12, 13 |
| 3-furoic acid | 111.009 | 6.7 | 296 ± 46B | 1053 ± 157A | 689 ± 48A | 256 | |
| 4-sulfobenzyl alcohol | 187.007 | 20.6 | 94596 ± 7417A | 50780 ± 7844B | 76237 ± 1683A | –46 | |
| p-cresol glucuronide | 283.083 | 12.2 | 14458 ± 1154A | 8382 ± 1686B | 15082 ± 491A | –42 | |
| trans-aconitic acid | 173.009 | 5.1 | 212 ± 44B | 504 ± 50A | 479 ± 26A | 137 | |
LC–MS data were obtained in negative mode, and mass to charge ratio (m/z) and retention times (RT) for the given metabolites are given. Ion counts are reported as mean ± SEM (n = 6–8, except for glutaconic acid, n = 3–7). Numbers with different capital letters across columns are significantly different, p < 0.05.
The change represents percent change in the HF Whey group relative to the HF casein group, with negative values for decrease and positive values for increase.
Numbers refer to metabolic pathways that the metabolites participate in 1, TCA cycle; 2, glyoxylate and dicarboxylate; 3, pyruvate; 4, glycolysis and gluconeogenesis; 5, pantothenate and CoA biosynthesis; 6, Val, Leu, Ile; 7, Cys, Met; 8, Gly, Ser, Thr; 9, propanoate; 10, Phe; 11, Tyr; 12, pyrimidines; 13, Trp; 14, purines; 15, butanoate; 16, Ala, Asp, and Glu; 17, taurine and hypotaurine; 18, vitamin B6; 19, ascorbate and aldarate; 20, pentose phosphate pathway; 21, pentose and glucuronate interconversions; 22, nicotinate and nicotinamide; 23, terpenoid backbone biosynthesis; 24, β-Ala; 25, Lys, OH-Lys; 26, Gln and Glu; 27, urea cycle; 28, Acyl-CoA and Gly. (−) Metabolites without known pathway annotation.
Fisher LSD test performed on log-transformed data.
Statistics by Kruskal–Wallis test.
Numbers with different capital letters (A-C) across columns are significantly different, P < 0.05.
Results
Whey Feeding Reduced Adipose Tissue Mass and Hepatic Lipid Accumulation
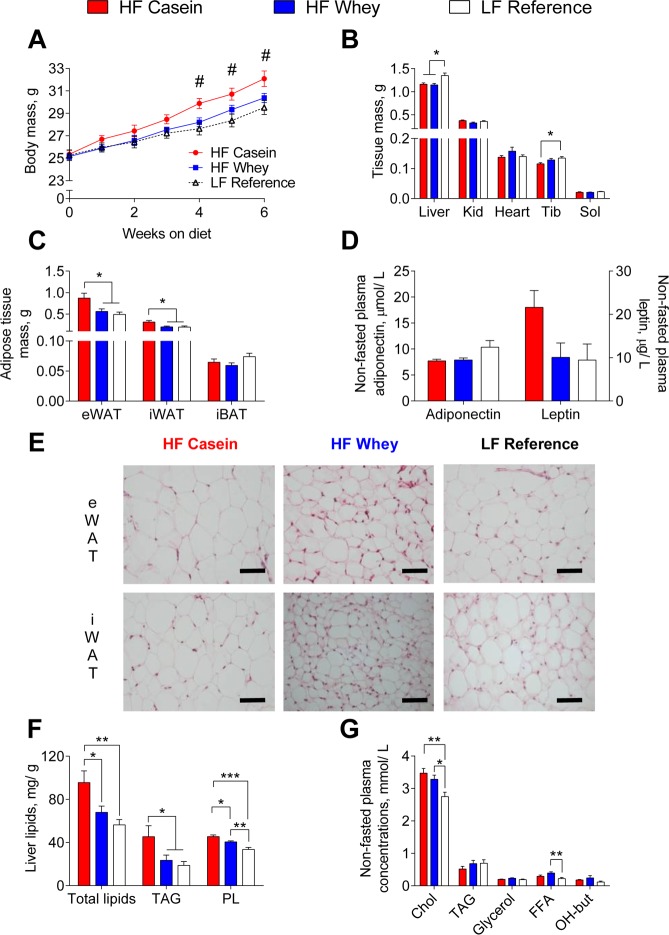
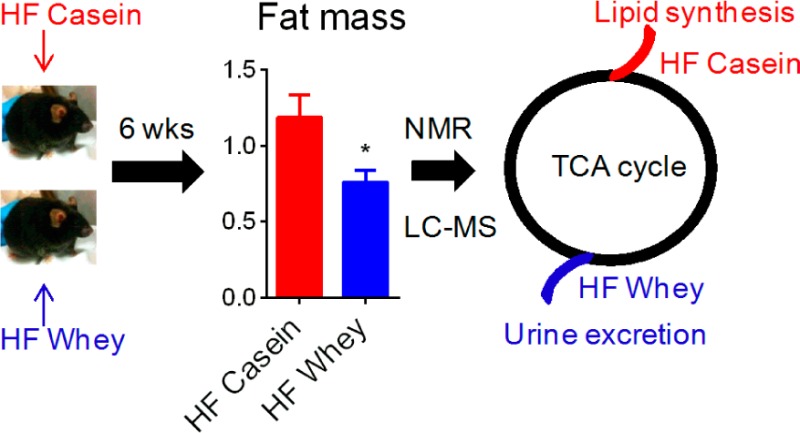
In accordance with previous studies in C57BL/6J mice,10−12 we found reduced body mass gain in HF-whey-fed compared with HF-casein-fed mice, and the difference was significant from week 4 to week 6 (Figure 1A). Moreover, the cumulative energy intake was equal between the HF-casein-fed and HF-whey-fed mice (both 2.60 MJ/42days), and also the apparent fat digestibility (96.5 ± 0.3 and 96.2 ± 0.2%, respectively) and apparent nitrogen digestibility (94.9 ± 0.2 and 95.3 ± 0.1%, respectively) were equal. Thus, at equal energy intake, the HF-whey-fed mice gained less body mass. The reduced body mass could be ascribed to lower white adipose tissue masses (Figure 1C), whereas no difference was observed in iBAT (Figure 1C) or lean tissue masses (Figure 1B) between the high fat fed mice. However, the HF-casein-fed mice had significantly lower tibialis anterior muscle mass, relative to the LF reference fed mice, whereas HF-whey-fed mice did not show this reduction in muscle mass (Figure 1B). Plasma leptin concentration (Figure 1D), which is generally proportional to adipose tissue mass,24,25 was borderline different (ANOVA univariate test, P = 0.059). No difference was found in plasma concentration of adiponectin between the two high-fat-diet-fed groups (Figure 1D). In line with the lower white adipose tissue masses, the mice fed the HF whey diet appeared to have smaller eWAT and iWAT adipocytes compared with mice fed HF casein (Figure 1E).
Figure 1.
Reduced energy deposition as adipose tissue mass and hepatic lipids in mice fed HF whey diets. From weeks 4–6 of the feeding trial, HF casein had higher body mass than HF whey and LF reference fed mice (A). Both HF-fed groups had lower liver tissue mass, whereas only HF-casein-fed mice had reduced tibialis anterior (skeletal muscle) mass compared with LF reference fed mice (B). Both HF whey and LF reference fed mice had reduced white adipose masses, relative to HF-casein-fed mice (C). No difference was observed in nonfasted plasma concentrations of adiponectin or leptin (D). Adipocyte size in eWAT and iWAT seemed reduced in HF whey, relative to HF-casein-fed mice. Scale bar represents 50 μm (E). Reduced liver total lipids and TAG concentration in HF whey and LF reference compared with HF-casein-fed mice (F). Both HF groups have higher nonfasted plasma total cholesterol, and HF-whey-fed mice also had higher free fatty acid (FFA) concentration, relative to LF reference fed mice (G). Abbreviations: Kid, kidneys; Tib, tibialis anterior; Sol, soleus; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue; iBAT, interscapular brown adipose tissue; TAG, triacylglycerol; PL, phospholipids; FFA, free fatty acids, OH-but, hydroxybutyrate. Values are given as mean ± SEM (n = 12–14: A, Liver, Tib in B, C; n = 5–8: Kid, Heart, Sol in B, D, F, G). Significant differences marked by # (P < 0.02 HFC vs LF and HFW); * (P < 0.05); ** (P < 0.01); *** (P < 0.001).
Previously, we have observed in mice14,26−28 and in rats29,30 that reduced adiposity was associated with increased adipose tissue expression of genes involved in energy metabolism and dissipation of energy in the form of heat. Therefore, we measured mRNA expression of Ucp1 (uncoupling protein 1), Ppargc1a (peroxisome proliferator-activated receptor gamma coactivator 1 alpha), Dio2 (deiodinase, iodothyronine, type II), and Cpt1b (carnitine palmitoyltransferase 1b) in iWAT and iBAT. We also measured Ucp3 (uncoupling protein 3) and Cpt1b mRNA expression in fat-oxidizing slow fiber-type skeletal muscle soleus. No differences in the expression of these genes were found between mice fed the HF casein and the HF whey diets (Figure S2A–C, Supporting Information). Thus, our data did not indicate that the reduced adipose tissue mass in the HF-whey-fed mice was due to increased dissipation of energy in the form of heat as compared with the HF-casein-fed mice.
Visceral adipose tissue mass is correlated with intrahepatic triglyceride content, and hepatic lipid accumulation might be a good marker of the metabolic derangements associated with obesity.31 In the present study, the HF-whey-fed mice had significantly lower liver total lipids and triacylglycerol (TAG) concentrations than the mice fed the HF casein diet, indicating less ectopic fat accumulation in these mice (Figure 1F). Despite the lower hepatic TAG level in HF-whey-fed mice, we observed differences in neither hepatic expression of genes involved in de novo fatty acid synthesis (Figure S3A, Supporting Information) nor ex vivo hepatic fatty acid oxidation capacity compared with the HF-casein-fed mice (Figure S3B, Supporting Information). Gene expression analyses have previously indicated that hepatic oxidative phosphorylation was up-regulated in obese diabetic subjects.32 Similarly, proteomic studies in diabetic rats pointed in the same direction,33 and increased hepatic oxidative phosphorylation was reported for high-fat-fed mice.34 In the present study, the HF-casein-fed mice had significantly higher hepatic gene expression of Hmgcs2 (3-hydroxy-3-methylglutaryl-coenzyme A synthase 2) relative to the LF-fed mice (Figure S3C, Supporting Information). Moreover, hepatic expression of Acadm (medium-chain acyl-CoA dehydrogenase) was borderline (ANOVA Univariate Test, P = 0.051) different (Figure S3C, Supporting Information). In contrast, the HF-whey-fed mice did not show significant different hepatic expression of genes involved in fatty acid oxidation relative to the LF fed mice (Figure S3C, Supporting Information).
Because hepatic steatosis is associated with adverse alterations in glucose, fatty acid, and lipoprotein metabolism,35 we measured parameters related to plasma lipids and glucose metabolism. Nonfasted plasma concentration of total cholesterol was higher in both of the high-fat-fed groups relative to the LF-fed mice (Figure 1G). No difference was observed in nonfasted plasma concentrations of TAG or glycerol or in the ketone body β-hydroxybutyrate (OH-butyrate) (Figure 1G). The nonfasted plasma concentration of free fatty acids (FFAs) was higher in the HF-whey-fed mice compared with the LF fed mice and tended (P = 0.06) to be higher than in the HF-casein-fed mice (Figure 1G). No difference in nonfasted plasma glucose, lactate, insulin, or glucagon concentrations was found (Figure S4A,B, Supporting Information). However, hepatic gene expression of the rate-limiting enzyme in gluconeogenesis Pck1 (phosphoenolpyruvate carboxykinase 1) was significantly reduced in the HF-whey-fed and LF-fed relative to the HF-casein-fed mice (Figure S4C, Supporting Information).
Thus, at equal energy intake and uptake and with no indications of different loss of energy through dissipation of energy in the form of heat, the HF-whey-fed mice had reduced energy storages, evident as lower white adipose tissue masses and lower hepatic lipid accumulation compared with HF-casein-fed mice.
Urinary 1H NMR Analyzes Revealed Different Urine Excretion Pattern in Mice Fed HF Casein and HF Whey Diet
Metabolites present in urine reflect whole body metabolism, and NMR-based metabolomics on urine has previously been used in nutritional studies to explore both exposure and endogenous markers related to diet.36−39 To further elucidate how HF whey feeding could induce a phenotype with less energy accretion as body fat, we utilized an explorative analysis of the 48 h urine by 1H NMR spectroscopy using supervised OPLS-DA between the HF casein and LF reference (Figure 2A) between the HF-whey-fed and the LF-reference-fed (Figure 2B) and between the HF-whey-fed and HF-casein-fed (Figure 2C) mice. The urine from LF-reference-fed mice contained larger amounts of sucrose, glucose, and molecules within the sugar region relative to the urine from the HF-fed mice (Figure 2 A,B), probably due to the higher carbohydrate content in the LF reference diet (Table S1 in the Supporting Information). The urinary content of trimethylamine appeared to be associated with the consumption of casein, as it was higher in urine from both LF reference and HF-casein-fed, relative to that in urine from HF-whey-fed mice (Figure 2A–C). Urinary taurine excretion (Figure 2A–C) appeared to follow the dietary content of its precursor molecule cysteine (Table S3 in the Supporting Information). When comparing the two groups of HF-fed mice, the urine of HF-whey-fed mice contained higher levels of metabolites such as the simple carboxylate anion formate (singlet, 8.46 ppm), the purine degradation product allantoin (singlet, 5.40 ppm), the sulfur amino acid metabolite taurine (triplet, 3.27 ppm; triplet, 3.44 ppm), and the TCA cycle intermediates citrate (doublet, 2.59 ppm; doublet, 2.73 ppm) and succinate (singlet, 2.45 ppm). In the HF-casein-fed mice, the urinary concentration of the metabolites sucrose (major peaks: singlet, 3.67 ppm; multiplet, 3.83 ppm), creatinine (singlet, 3.06 ppm; singlet, 4.10 ppm), the tertiary amine trimethylamine (singlet, 3.90 ppm), and the glucose metabolite lactate (doublet, 1.33 ppm; the other proton signal was heavily overlapped by sucrose signal at 4.04 ppm) were present at higher concentrations (Figure 2C).
Figure 2.
Explorative 1H NMR analyses of urine from mice fed LF reference, HF casein, or HF whey diets. During week 4 of the study, urine was collected for 48 h (LF n = 6 and HF n = 8/ diet). Loadings are shown for OPLS-DA models of mice fed HF casein and LF control (A), HF whey and LF control (B), and HF casein and HF whey diets (C). All OPLS-DA models have one predicted and one orthogonal component. Quality parameters for OPLS-DA models: HF casein and LF control (A), (R2X = 0.69, R2Y = 0.92, Q2 = 0.59, CV-ANOVA p value = 0.068); HF whey and LF control (B) (R2X = 0.71, R2Y = 0.90, Q2 = 0.70, CV-ANOVA p value = 0.019), and HF casein and HF whey (C) (R2X = 0.29, R2Y = 0.93, Q2 = 0.63, CV-ANOVA p value = 0.03). The metabolites are colored according to high urinary concentration in mice fed HF casein (red), HF whey (blue), and LF control (black), as indicated by the loadings of the predictive component.
Urinary Metabolomes Differ with Regard to TCA Cycle Intermediates and Amino Acid Metabolites
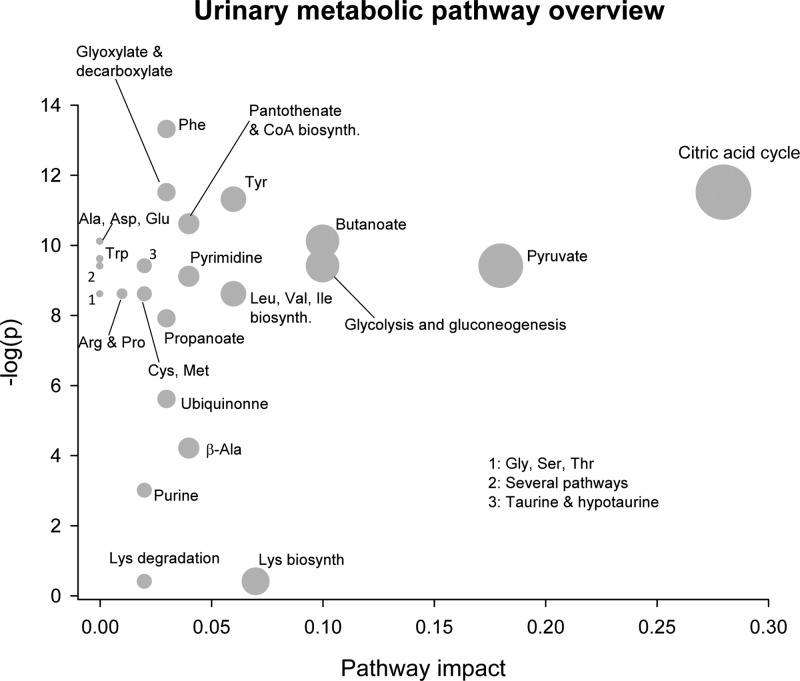
To further explore the differences in the urinary metabolome, we performed an explorative LC–MS metabolomic analysis on the 48 h urine, which provided a higher sensitivity than the applied 1H NMR analyses. For identification of metabolic pathways differently regulated in the HF whey and HF-casein-fed mice, the obtained LC–MS data were analyzed by the Metaboanalyst software. This approach unveiled that in particular TCA cycle metabolism but also metabolic pathways involved in amino acid, carbohydrate, purine, pyrimidine, glycolysis, and gluconeogenesis were differently regulated in the HF-whey-fed and HF-casein-fed mice (Figure 3 and Table 1). Confirming our findings from 1H NMR spectroscopy, our LC–MS analyses showed that the mice fed HF whey diet had significantly higher urinary intensities of the TCA-cycle intermediates citric acid and succinic acid, relative to HF-casein-fed mice (Table 1). In addition, the LC–MS analyses also identified higher urinary intensities of the TCA cycle intermediates cis-aconitic acid and isocitric acid and of the TCA-precursor pyruvate in the HF whey, relative to in the HF-casein-fed mice (Table 1). Moreover, the HF-whey-fed mice had higher urinary ion count of citramalic acid, a methylated analogue of malic acid, relative to the HF-casein-fed mice (Table 1). Interestingly, the HF-whey-fed mice had higher urinary ion counts of N-acetylglutamine and N-acetyl-l-glutamic acid, metabolites from glutamine and glutamate metabolism, respectively, relative to the HF-casein-fed mice (Table 1). The dietary amount of glutamate and glutamine was lower in the HF whey, relative to the HF casein diet (Table S3 Supporting Information). This finding might indicate differences in glutamate and glutamine metabolism between the two high-fat-fed groups.
Figure 3.
Urinary pathway overview of the explorative LC–MC analysis from mice fed HF casein or HF whey diets. Explorative LC–MS analysis was performed on urine sampled for 48 h from mice fed HF casein or HF whey diets (n = 8/diet). By use of the software Metaboanalyst 2.0, the urinary metabolome was matched with metabolic pathways. The metabolome view above shows matched pathways according to p values (y axis) from pathway enrichment analysis and pathway impact values (x axis) from pathway topology analysis. The TCA cycle pathway identified in the upper right corner is of greater importance for the separation of the urine metabolome between treatments.
The LC–MS urinary metabolome analyses also indicated differences in metabolites associated with phenylalanine and tyrosine catabolism, that is, hippuric acid, 4-hydroxybenzoic acid, p-hydroxyphenylacetic acid, and 4-hydroxyphenyllactic acid between the HF-whey-fed and the HF-casein-fed mice (Table 1), which are in accordance with the amino acid composition in the diets (Table S3 in the Supporting Information). Likewise, the urinary differences in metabolites associated with tryptophan or lysine catabolism, that is, kynurenic acid, xanthurenic acid, and glutaconic acid (Table 1), reflected the dietary content of these amino acids (Table S3 Supporting Information). The HF-whey-fed mice had higher urinary ion counts of orotic acid and ureidopropionic acid than the HF-casein-fed mice (Table 1). Interestingly, urinary excretion of several metabolites related to TCA cycle, glutamine/glutamate metabolism, and purine/pyrimidine metabolism was intermediate in the LF reference fed, relative to mice fed HF whey or HF casein diets (Table 1).
Our data from the urine metabolome indicated that in particular TCA metabolism was altered between mice fed HF whey or HF casein diets. To explore whether changes in TCA intermediate concentrations were also evident in the liver and plasma, we performed targeted LC–MS analyses. In the fed state plasma but not liver significantly elevated ion counts of citric acid and cis-aconitic acid were found in the HF whey relative to HF-casein-fed mice (Table 2). Thus, changes in TCA cycle metabolism were evident both from fed state plasma and from 48 h urine metabolome analyses, which might be an underlying mechanism to the reduced body lipid accretion in HF whey compared with HF-casein-fed mice.
Table 2. Targeted LC–MS Analysis in Plasma and Livers Collected from Non-Fasted Mice Fed HF Casein or HF Whey Dietsa.
| ion count |
||||||
|---|---|---|---|---|---|---|
| metabolite | m/z | RT | HF casein | HF whey | P value | change (%)b |
| Plasma | ||||||
| citric acid | 133.014 | 5.3 | 150934 ± 15691 | 211928 ± 16135 | 0.019 | 40 |
| cis-aconitic acid | 173.009 | 5.7 | 13917 ± 1 982 | 20319 ± 1239 | 0.018 | 46 |
| l-malic acid | 133.014 | 5.3 | 3348 ± 836 | 4852 ± 1213 | 0.31 | 45 |
| succinic acid | 117.02 | 4.3 | 60003 ± 4265 | 55579 ± 7374 | 0.61 | –7 |
| fumaric acidc | 115.004 | 5.3 | 3876 ± 592 | 3496 ± 322 | 0.69 | –10 |
| Liver | ||||||
| citric acid | 133.014 | 5.3 | 120730 ± 11342 | 117688 ± 12096 | 0.86 | –3 |
| cis-aconitic acid | 173.009 | 5.7 | 103527 ± 15497 | 95481 ± 13699 | 0.70 | –8 |
| l-malic acid | 133.014 | 5.3 | 380520 ± 51786 | 411773 ± 45487 | 0.65 | 8 |
| fumaric acid | 115.004 | 5.3 | 178735 ± 19996 | 183413 ± 22171 | 0.87 | 3 |
LC–MS data were obtained in negative mode, and mass to charge ratio (m/z) and retention times (RTs) for the given metabolites are given. Ion counts are reported as mean ± SEM (n = 6–8).
Change represents percent change in the HF whey group relative to the HF casein group, with negative values for decrease and positive values for increase.
Student’s t test performed on log-transformed data.
Discussion
The present study showed that at equal energy intake and apparent nitrogen and -fat digestibility, HF-whey-fed mice stored less energy as lipids, evident both as lower white adipose tissue mass and as reduced liver lipids compared with HF-casein-fed mice. Our data are in agreement with other studies feeding high fat casein or whey diets to mice.10−12 Gene expression analyses of white and brown adipose tissues as well as skeletal muscle did not support the fact that the reduced lipid deposition was associated with increased dissipation of energy in the form of heat. Moreover, hepatic gene expression analyses and ex vivo hepatic fatty acid oxidation measurements did not support the fact that lipids were differently catabolized in livers of the HF whey and HF-casein-fed mice. Our data are supported by the findings of McAllan et al., who did not observe different heat production between mice on HF casein and HF whey diets.11
Our 48 h urinary metabolome analyses provided evidence as to how HF whey feeding might reduce body energy deposition relative to HF casein feeding in mice, namely, through altered TCA cycle metabolism, verified by both 1H NMR-based and LC–MS metabolomics. Enhanced urinary excretion of TCA cycle intermediates by HF whey feeding represents a direct loss of energy through drainage of carbon-containing molecules from the body. In addition to the direct loss of energy, removal of substrates from the TCA cycle might also have contributed to reduced lipid accretion because TCA cycle intermediates and citrate, in particular, have been shown to stimulate fatty acid de novo synthesis.40 The stimulation of lipogenesis by citrate is multifaceted; first, citrate is the major carrier in the transfer of acetyl groups from the mitochondria to the cytosol, where the fatty acid synthesis occurs.41 Second, citrate is an allosteric activator of acetyl-CoA carboxylase (ACC), the rate-limiting enzyme in fatty acid synthesis.42 Third, in the cytosol, citrate can be metabolized by the action of ATP-citrate lyase to yield oxaloacetate and acetyl-CoA, the latter being the substrate for ACC. Finally, cytosolic oxaloacetate is metabolized to malate, which by the action of malic enzyme can be further metabolized to pyruvate, a reaction that generates reduced nicotinamide adenine dinucleotide phosphate (NADPH), the reducing equivalent needed for fatty acid synthesis. Thus, in the present study, the higher urinary intensities of the TCA cycle intermediates in the HF-whey-fed mice relative to the HF-casein-fed mice suggest that fewer TCA cycle intermediates were available for lipid synthesis. This is likely to be important for the reduced body lipid accretion in general and for the lower hepatic lipid levels in the HF-whey-fed mice observed in the present study as well as by others.10
Our 1H NMR metabolome analyses of 48 h urine also revealed that the HF-casein-fed mice had higher signal intensity of lactate than the HF-whey-fed mice. Elevated urinary lactate concentration might indicate an enhanced whole body glycolysis rate. High rates of glucose catabolism and lactate production, even in the presence of oxygen (aerobic glycolysis), were first described in cancer cells43 and are known as the “Warburg effect”. Beyond transformed cells, many other cell types use aerobic glycolysis during proliferation, and it has been suggested that the major function of aerobic glycolysis is to provide glycolytic intermediates to support anabolic reactions in cells.44 In glioblastoma cells exhibiting the Warburg effect, the exit of TCA intermediates (cataplerosis) was shown to support fatty acid synthesis, and 60% of the fatty acyl carbons were estimated to be glucose-derived.45 Moreover, glutaminolysis (i.e., the conversion of glutamine to lactate) was sufficiently high to both provide necessary NADPH to support fatty acid synthesis and to supply anaplerotically oxaloacetate to the TCA cycle.45 Recently, glutaminolysis was shown to activate mammalian target of rapamycin complex 1,46 further linking glutamine metabolism to anabolic processes in cells. In the present study, despite ∼13% higher dietary content of glutamine and glutamate in the HF casein diet than in the HF whey diet, the HF-casein-fed mice had about two-fold lower urinary signals from the glutamine and glutamate metabolites N-acetyl glutamine and N-acetyl-l-glutamic acid, respectively, compared with the HF-whey-fed mice. This notion, together with the elevated urinary lactate (which may originate from glutaminolysis) in the HF-casein-fed rats, indicated that the glutaminolysis was elevated in these animals, providing a carbon source that facilitated glucose conversion to lipids in the HF-casein-fed mice relative to in the HF-whey-fed mice.
It has been shown that rats fed an HF casein diet had increased hepatic and reduced urinary concentrations of TCA intermediates (in particular, citrate) relative to rats on a low fat casein diet.47 Several factors are known to stimulate urinary citrate excretion (metabolic alkalosis, organic acids, TCA cycle inhibitors), whereas metabolic acidosis is known to reduce urinary citrate excretion.48 In the present study, urinary lactate concentration was higher in urine from the HF-casein-fed mice compared with the HF-whey-fed mice, and it is possible that the higher lactate level might have contributed to a slight metabolic acidosis suppressing TCA cycle intermediate excretion. Furthermore, plasma from HF-whey-fed mice contained higher levels of the organic acids citrate and cis-aconitic acid that might have contributed to enhanced urinary excretion of TCA cycle intermediates. In addition, urine from HF-whey-fed mice contained higher levels of citramalic acid, which possibly can act as a fumarase inhibitor,49 thereby promoting increased urinary TCA cycle excretion.
Despite higher 48 h urine TCA cycle intermediate excretion in HF-whey-fed mice that could be an underlying factor to the reduced fat accretion in these mice, we did not observe differences in hepatic expression of genes related to lipogenesis. It is possible that the lipogenic flux was different without altering gene expression. Alternatively, lipogenesis was unaltered in the liver but was altered in extrahepatic tissues. Previously, a strong correlation has been demonstrated between TCA cycle flux and Pck-1 flux (gluconeogenesis) both in humans50 and in mice.51 Moreover, endogenous glucose production has been demonstrated to be elevated in HF casein, relative to in LF reference fed mice.52 In the present study, hepatic Pck-1 gene expression was higher in the HF-casein-fed mice, which could indicate higher hepatic gluconeogenesis. Furthermore, as long as the mice remained insulin-sensitive, it could be speculated that the glucose was effectively taken up by peripheral tissues, such as skeletal muscle and white adipose tissue. In adipose tissue, the glucose could be used in de novo lipogenesis.53 Such a scenario is in keeping with the observation of increased concentration of TCA cycle intermediates in plasma but not in liver, potentially reflecting differences in extrahepatic TCA cycle metabolism. Moreover, the higher 48 h urine lactate levels observed in the HF-casein-fed mice could also be caused by higher muscle glucose oxidation.
The different amino acid composition in the HF casein and HF whey diets is likely to have contributed to the diverging phenotypes of mice in the present study. Amino acid metabolism is linked to both carbohydrate and fat metabolism through the TCA cycle. The HF whey diet contained particularly high amounts of alanine (+81%), aspartate/aspargine (+74%), threonine (+82%), and trytophane (+54%) relative to the HF casein diet, and because these amino acids are linked to the TCA cycle54 it is likely that the higher urinary TCA cycle intermediate excretion reflected differences in amino acid metabolism, which would have direct influence on anabolic processes in the mice. Moreover, it has been shown that supplementing casein-based low-protein diets with threonine + lysine prevented hepatic lipid accumulation in young rats,55 whereas partial exchange of casein by a mixture of leucine, isoleucine, valine, threonine, and lysine in high-fat diets prevented hepatic steatosis, body fat accretion, and insulin resistance in mice, at least partially by reducing de novo lipogenesis fluxes.56 Caution should be exerted in translating the findings from the previously mentioned studies into ours due to different experimental settings. However, the HF whey diet in the present study had ∼46% higher threonine + lysine content and ∼26% higher leucine + isoleucine + valine + threonine + lysine level compared with the HF casein diet. The higher level of these specific amino acids in the HF whey diet might be related to the reduced lipid deposition observed in the HF-whey-fed mice.
Even though we are unable to identify the specific factor(s) or the specific tissue(s) responsible for the observed difference in urine metabolome in the present study, it is evident that HF casein and HF whey feeding induced metabolic changes that altered urinary TCA intermediate excretion, thereby providing different amounts of TCA cycle intermediates for sustained anabolic processes that could lead to differences in body lipid deposition.
Conclusions
This study has demonstrated that at equal energy intake HF-whey-fed mice have reduced white adipose tissue masses and lower hepatic lipid concentrations. These phenotypic alterations are unlikely to be due to different fat catabolism. Rather, 1H NMR-based and LC–MS metabolomics analyses unveiled a higher urinary excretion of TCA cycle precursor and intermediates in the HF whey relative to the HF-casein-fed mice. We suggest that this loss of TCA cycle compounds through urine removes available substrate for lipogenesis, thereby leading to reduced lipid accretion in the HF-whey-fed compared with the HF-casein-fed mice. Further studies on using isotope labeling and flux analysis to quantify the effects on the TCA metabolism would be of great interest.
Acknowledgments
This work was supported by grants from The Danish Dairy Research Foundation and The Danish Council for Strategic Research, Grant no. 2101-08-0053. This work was also supported by the EU FP7 project DIABAT, HEALTH-F2-2011-278373 and by the National Institute of Nutrition and Seafood Research.
Supporting Information Available
Table S1. Composition of the low fat reference and the experimental high fat diets. Table S2. Primer sequences used for real-time qPCR determinations. Table S3. Amino acid composition in the LF reference and HF diets. Figure S1. Study design used in the present study. Figure S2. No difference in expression of genes involved in energy metabolism and uncoupling in mice fed HF casein or HF whey diets. Figure S3. No difference in expression of genes involved in fatty acid metabolism or ex vivo fatty acid oxidation in livers from mice fed HF casein or HF whey diets. Figure S4. No difference in fed plasma glucose related parameters but reduced hepatic gluconeogenic gene expression in HF whey relative to HF-casein-fed mice. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Finucane M. M.; Stevens G. A.; Cowan M. J.; Danaei G.; Lin J. K.; Paciorek C. J.; Singh G. M.; Gutierrez H. R.; Lu Y.; Bahalim A. N.; Farzadfar F.; Riley L. M.; Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011, 3779765557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran P.; Esmaillzadeh A.; Azizi F. Dairy consumption and body mass index: an inverse relationship. Int. J. Obes. 2005, 291115–121. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P.; Goncalves A.; Dias C. M. Milk intake is inversely related to obesity in men and in young women: data from the Portuguese Health Interview Survey 1998–1999. Int. J. Obes. 2006, 30188–93. [DOI] [PubMed] [Google Scholar]
- Abargouei A. S.; Janghorbani M.; Salehi-Marzijarani M.; Esmaillzadeh A. Effect of dairy consumption on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Int. J. Obes. 2012, 36121485–1493. [DOI] [PubMed] [Google Scholar]
- Chen M.; Pan A.; Malik V. S.; Hu F. B. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 964735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougkas A.; Reynolds C. K.; Givens I. D.; Elwood P. C.; Minihane A. M. Associations between dairy consumption and body weight: a review of the evidence and underlying mechanisms. Nutr. Res. Rev. 2011, 24172–95. [DOI] [PubMed] [Google Scholar]
- Takahira M.; Noda K.; Fukushima M.; Zhang B.; Mitsutake R.; Uehara Y.; Ogawa M.; Kakuma T.; Saku K. Randomized, Double-Blind, Controlled, Comparative Trial of Formula Food Containing Soy Protein vs. Milk Protein in Visceral Fat Obesity - FLAVO Study. Circ. J. 2011, 7592235–2243. [DOI] [PubMed] [Google Scholar]
- Acheson K. J.; Blondel-Lubrano A.; Oguey-Araymon S.; Beaumont M.; Emady-Azar S.; Ammon-Zufferey C.; Monnard I.; Pinaud S.; Nielsen-Moennoz C.; Bovetto L. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 933525–534. [DOI] [PubMed] [Google Scholar]
- Baer D. J.; Stote K. S.; Paul D. R.; Harris G. K.; Rumpler W. V.; Clevidence B. A. Whey Protein but Not Soy Protein Supplementation Alters Body Weight and Composition in Free-Living Overweight and Obese Adults. J. Nutr. 2011, 14181489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Tauriainen E.; Martonen E.; Finckenberg P.; Ahlroos-Lehmus A.; Tuomainen A.; Pilvi T. K.; Korpela R.; Mervaala E. M. Whey protein isolate protects against diet-induced obesity and fatty liver formation. Int. Dairy J. 2011, 218513–522. [Google Scholar]
- McAllan L.; Keane D.; Schellekens H.; Roche H. M.; Korpela R.; Cryan J. F.; Nilaweera K. N. Whey protein isolate counteracts the effects of a high-fat diet on energy intake and hypothalamic and adipose tissue expression of energy balance-related genes. Br. J. Nutr. 2013, 110, 2114–2126. [DOI] [PubMed] [Google Scholar]
- Tranberg B.; Hellgren L. I.; Lykkesfeldt J.; Sejrsen K.; Jeamet A.; Rune I.; Ellekilde M.; Nielsen D. S.; Hansen A. K. Whey Protein Reduces Early Life Weight Gain in Mice Fed a High-Fat Diet. PLoS One 2013, 88e71439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastesen H. S.; Keenan A. H.; Madsen L.; Kristiansen K.; Liaset B. Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids 2014, 1–13 10.1007/s00726-014-1715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillefosse H. H.; Tastesen H. S.; Du Z.-Y.; Ditlev D. B.; Thorsen F. A.; Madsen L.; Kristiansen K.; Liaset B. Hydrolyzed Casein Reduces Diet-Induced Obesity in Male C57BL/6J Mice. J. Nutr. 2013, 14391367–1375. [DOI] [PubMed] [Google Scholar]
- Liaset B.; Julshamn K.; Espe M. Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymic hydrolysis of salmon frames with Protamex (TM). Proc. Biochem. 2003, 38121747–1759. [Google Scholar]
- Bas S.; Giacobino J. P. Peroxisomal and Mitochondrial Palmitoyl Coenzyme-a Beta-Oxidation Activities in Rat White Adipose-Tissue and Their Regulation in Hypothyroidism. Arch. Biochem. Biophys. 1983, 2222416–423. [DOI] [PubMed] [Google Scholar]
- Du Z. Y.; Araujo P.; Stubhaug I.; Froyland L. Unbound DHA causes a high blank value in beta-oxidation assay: a concern for in vitro studies. Eur. J. Lipid Sci. Technol. 2010, 1123333–342. [Google Scholar]
- Wishart D. S.; Tzur D.; Knox C.; Eisner R.; Guo A. C.; Young N.; Cheng D.; Jewell K.; Arndt D.; Sawhney S.; Fung C.; Nikolai L.; Lewis M.; Coutouly M. A.; Forsythe I.; Tang P.; Shrivastava S.; Jeroncic K.; Stothard P.; Amegbey G.; Block D.; Hau D. D.; Wagner J.; Miniaci J.; Clements M.; Gebremedhin M.; Guo N.; Zhang Y.; Duggan G. E.; MacInnis G. D.; Weljie A. M.; Dowlatabadi R.; Bamforth F.; Clive D.; Greiner R.; Li L.; Marrie T.; Sykes B. D.; Vogel H. J.; Querengesser L. HMDB: the human metabolome database. Nucleic Acids Res. 2007, 35, D521–D526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savorani F.; Tomasi G.; Engelsen S. B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 2022190–202. [DOI] [PubMed] [Google Scholar]
- Zhang X. M.; Clausen M. R.; Zhao X. L.; Zheng H.; Bertram H. C. Enhancing the Power of Liquid Chromatography-Mass Spectrometry-Based Urine Metabolomics in Negative Ion Mode by Optimization of the Additive. Anal. Chem. 2012, 84187785–7792. [DOI] [PubMed] [Google Scholar]
- Pluskal T.; Castillo S.; Villar-Briones A.; Oresic M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 2010, 11, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A.; O’Maille G.; Want E. J.; Qin C.; Trauger S. A.; Brandon T. R.; Custodio D. E.; Abagyan R.; Siuzdak G. METLIN - A metabolite mass spectral database. Ther. Drug Monit. 2005, 276747–751. [DOI] [PubMed] [Google Scholar]
- Xia J. G.; Psychogios N.; Young N.; Wishart D. S. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine R. V.; Sinha M. K.; Heiman M. L.; Kriauciunas A.; Stephens T. W.; Nyce M. R.; Ohannesian J. P.; Marco C. C.; McKee L. J.; Bauer T. L.; Caro J. F. Serum immunoreactive leptin concentrations in normal-weight and obese humans. New Engl. J. Med. 1996, 3345292–295. [DOI] [PubMed] [Google Scholar]
- Maffei M.; Halaas J.; Ravussin E.; Pratley R. E.; Lee G. H.; Zhang Y.; Fei H.; Kim S.; Lallone R.; Ranganathan S.; Kern P. A.; Friedman J. M. Leptin Levels in Human and Rodent - Measurement of Plasma Leptin and Ob Rna in Obese and Weight-Reduced Subjects. Nat. Med. 1995, 1111155–1161. [DOI] [PubMed] [Google Scholar]
- Madsen L.; Pedersen L. M.; Liaset B.; Ma T.; Petersen R. K.; van den Berg S.; Pan J.; Muller-Decker K.; Dulsner E. D.; Kleemann R.; Kooistra T.; Doskeland S. O.; Kristiansen K. cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J. Biol. Chem. 2008, 283117196–7205. [DOI] [PubMed] [Google Scholar]
- Ma T.; Liaset B.; Hao Q.; Petersen R. K.; Fjære E.; Ngo H. T.; Lillefosse H. H.; Ringholm S.; Sonne S. B.; Treebak J. T.; Pilegaard H.; Frøyland L.; Kristiansen K.; Madsen L. Sucrose Counteracts the Anti-Inflammatory Effect of Fish Oil in Adipose Tissue and Increases Obesity Development in Mice. PLoS One 2011, 66e21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q.; Lillefosse H. H.; Fjaere E.; Myrmel L. S.; Midtbo L. K.; Jarlsby R. H.; Ma T.; Jia B. B.; Petersen R. K.; Sonne S. B.; Chwalibog A.; Froyland L.; Liaset B.; Kristiansen K.; Madsen L. High-glycemic index carbohydrates abrogate the antiobesity effect of fish oil in mice. Am. J. Physiol.: Endocrinol. Metab. 2012, 3029E1097–E1112. [DOI] [PubMed] [Google Scholar]
- Liaset B.; Madsen L.; Hao Q.; Criales G.; Mellgren G.; Marschall H.-U.; Hallenborg P.; Espe M.; Frøyland L.; Kristiansen K. Fish protein hydrolysate elevates plasma bile acids and reduces visceral adipose tissue mass in rats. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2009, 17914254–262. [DOI] [PubMed] [Google Scholar]
- Liaset B.; Hao Q.; Jørgensen H.; Hallenborg P.; Du Z.-Y.; Ma T.; Marschall H.-U.; Kruhöffer M.; Li R.; Li Q.; Yde C. C.; Criales G.; Bertram H. C.; Mellgren G.; Øfjord E. S.; Lock E.-J.; Espe M.; Frøyland L.; Madsen L.; Kristiansen K. Nutritional Regulation of Bile Acid Metabolism Is Associated with Improved Pathological Characteristics of the Metabolic Syndrome. J. Biol. Chem. 2011, 2863228382–28395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E.; Magkos F.; Mohammed B. S.; Pietka T.; Abumrad N. A.; Patterson B. W.; Okunade A.; Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. 2009, 1063615430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura T.; Misu H.; Matsuzawa-Nagata N.; Sakurai M.; Ota T.; Shimizu A.; Kurita S.; Takeshita Y.; Ando H.; Honda M.; Kaneko S. Obesity Upregulates Genes Involved in Oxidative Phosphorylation in Livers of Diabetic Patients. Obesity 2008, 16122601–2609. [DOI] [PubMed] [Google Scholar]
- Deng W.-J.; Nie S.; Dai J.; Wu J.-R.; Zeng R. Proteome, Phosphoproteome, and Hydroxyproteome of Liver Mitochondria in Diabetic Rats at Early Pathogenic Stages. Mol. Cell. Proteomics 2010, 91100–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.; Darshi M.; Ma Y.; Perkins G. A.; Shen Z.; Haushalter K. J.; Saito R.; Chen A.; Lee Y. S.; Patel H. H.; Briggs S. P.; Ellisman M. H.; Olefsky J. M.; Taylor S. S. Quantitative Proteomic and Functional Analysis of Liver Mitochondria from High Fat Diet Diabetic Mice. Mol. Cell. Proteomics 2013, 10.1074/mcp.M113.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E.; Sullivan S.; Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 512679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram H. C.; Knudsen K. E. B.; Serena A.; Malmendal A.; Nielsen N. C.; Frette X. C.; Andersen H. J. NMR-based metabonomic studies reveal changes in the biochemical profile of plasma and urine from pigs fed high-fibre rye bread. Br. J. Nutr. 2006, 955955–962. [DOI] [PubMed] [Google Scholar]
- Bertram H. C.; Hoppe C.; Petersen B. O.; Duus J. O.; Molgaard C.; Michaelsen K. F. An NMR-based metabonomic investigation on effects of milk and meat protein diets given to 8-year-old boys. Br. J. Nutr. 2007, 974758–763. [DOI] [PubMed] [Google Scholar]
- Legido-Quigley C.; Stella C.; Perez-Jimenez F.; Lopez-Miranda J.; Ordovas J.; Powell J.; Van-Der-Ouderaa F.; Ware L.; Lindon J. C.; Nicholson J. K.; Holmes E. Liquid chromatography-mass spectrometry methods for urinary biomarker detection in metabonomic studies with application to nutritional studies. Biomed. Chromatogr. 2010, 247737–743. [DOI] [PubMed] [Google Scholar]
- Jung J. Y.; Kim I. Y.; Kim Y. N.; Kim J. S.; Shin J. H.; Jang Z. H.; Lee H. S.; Hwang G. S.; Seong J. K. H-1 NMR-based metabolite profiling of diet-induced obesity in a mouse mode. BMB Rep. 2012, 457419–424. [DOI] [PubMed] [Google Scholar]
- Martin D. B.; Vagelos P. R. The Mechanism of Tricarboxylic Acid Cycle Regulation of Fatty Acid Synthesis. J. Biol. Chem. 1962, 23761787–1792. [PubMed] [Google Scholar]
- Watson J. A.; Lowenstein J. M. Citrate and the Conversion of Carbohydrate into Fat: Fatty Acid Synthesis by a Combination of Cytoplasm and Mitochondria. J. Biol. Chem. 1970, 245225993–6002. [PubMed] [Google Scholar]
- Wakil S. J.; Stoops J. K.; Joshi V. C. Fatty Acid Synthesis and its Regulation. Annu. Rev. Biochem. 1983, 521537–579. [DOI] [PubMed] [Google Scholar]
- Warburg O. Origin of Cancer Cells. Science 1956, 1233191309–314. [DOI] [PubMed] [Google Scholar]
- Lunt S. Y.; Vander Heiden M. G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 271441–464. [DOI] [PubMed] [Google Scholar]
- DeBerardinis R. J.; Mancuso A.; Daikhin E.; Nissim I.; Yudkoff M.; Wehrli S.; Thompson C. B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A. 2007, 1044919345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán R. V.; Oppliger W.; Robitaille A. M.; Heiserich L.; Skendaj R.; Gottlieb E.; Hall M. N. Glutaminolysis Activates Rag-mTORC1 Signaling. Mol. Cell 2012, 473349–358. [DOI] [PubMed] [Google Scholar]
- An Y.; Xu W.; Li H.; Lei H.; Zhang L.; Hao F.; Duan Y.; Yan X.; Zhao Y.; Wu J.; Wang Y.; Tang H. High-Fat Diet Induces Dynamic Metabolic Alterations in Multiple Biological Matrices of Rats. J. Proteome Res. 2013, 1283755–3768. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Citrate excretion: a window on renal metabolism. Am. J. Physiol.: Renal Physiol 1983, 2443F223–F234. [DOI] [PubMed] [Google Scholar]
- HMBD Showing Metabocard for Citramalic acid (HMDB00426). http://www.hmdb.ca/metabolites/HMDB00426.
- Sunny N. E.; Parks E. J.; Browning J. D.; Burgess S. C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 146804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S. C.; Leone T. C.; Wende A. R.; Croce M. A.; Chen Z. J.; Sherry A. D.; Malloy C. R.; Finck B. N. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1 alpha)-deficient mice. J. Biol. Chem. 2006, 2812819000–19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S.; Sunny N. E.; Kucejova B.; Fu X.; He T. T.; Méndez-Lucas A.; Shelton J. M.; Perales J. C.; Browning J. D.; Burgess S. C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 5361080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M. A.; Peroni O. D.; Villoria J.; Schon M. R.; Abumrad N. A.; Bluher M.; Klein S.; Kahn B. B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 4847394333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E.; Kalhan S. C.; Hanson R. W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 2773430409–30412. [DOI] [PubMed] [Google Scholar]
- Singal S. A.; Hazan S. J.; Sydenstricker V. P.; Littlejohn J. M. The Production of Fatty Livers in Rats on Threonine-Deficient and Lysine-Deficient Diets. J. Biol. Chem. 1953, 2002867–874. [PubMed] [Google Scholar]
- Noguchi Y.; Nishikata N.; Shikata N.; Kimura Y.; Aleman J. O.; Young J. D.; Koyama N.; Kelleher J. K.; Takahashi M.; Stephanopoulos G. Ketogenic Essential Amino Acids Modulate Lipid Synthetic Pathways and Prevent Hepatic Steatosis in Mice. PLoS One 2010, 58e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.