Abstract
Background/aim
Age-related metabolic diseases are often associated with low-grade inflammation. The aim of the present study was to investigate the role of the transcriptional co-activator PGC-1α in the potential beneficial effects of exercise training and/or resveratrol in the prevention of age-associated low-grade inflammation. To address this, a long-term voluntary exercise training and resveratrol supplementation study was conducted.
Experimental setup
Three month old whole body PGC-1α KO and WT mice were randomly assigned to four groups: untrained chow-fed, untrained chow-fed supplemented with resveratrol, chow-fed voluntarily exercise trained and chow-fed supplemented with resveratrol and voluntarily exercise trained. The intervention lasted 12 months and three month old untrained chow-fed mice served as young controls.
Results
Voluntary exercise training prevented an age-associated increase (p<0.05) in systemic IL-6 and adiposity in WT mice. PGC-1α expression was required for a training-induced prevention of an age-associated increase (p<0.05) in skeletal muscle TNFα protein. Independently of PGC-1α, both exercise training and resveratrol prevented an age-associated increase (p<0.05) in skeletal muscle protein carbonylation.
Conclusion
The present findings highlight that exercise training is a more effective intervention than resveratrol supplementation in reducing age-associated inflammation and that PGC-1α in part is required for the exercise training-induced anti-inflammatory effects.
Keywords: Aging, low-grade inflammation, exercise training, resveratrol, PGC-1α
1. Introduction
Aging is associated with a broad range of metabolic complications including increased adiposity (Schaap et al., 2012; Wu et al., 2007), loss of muscle mass and strength (Brooks & Faulkner, 1994; Doherty et al., 1993) and oxidative stress (HARMAN, 1956). These unfavorable complications may be caused by aging per se or lifestyle such as decreased physical activity with increasing age.
Many factors are likely involved in the initiation and/or progression of age-related diseases, but reports showing that the majority of lifestyle-related diseases are associated with chronic low-grade inflammation (Handschin & Spiegelman, 2008; Wellen & Hotamisligil, 2005) support the possibility that low-grade inflammation is an important component in the pathogenesis of these diseases (Pedersen et al., 2000; Woods et al., 2012). In addition, low-grade inflammation is observed in elderly subjects even in the absence of chronic diseases (Wei et al., 1992), but whether low-grade inflammation is cause or consequence of age-related metabolic dysfunction is still debated (Woods et al., 2012).
Chronic low-grade inflammation is defined as sustained 2–4 fold elevations in systemic levels of pro-inflammatory cytokines like tumor necrosis factor (TNF)α and interleukin (IL)-6 (Bruunsgaard & Pedersen, 2003; Woods et al., 2012). The immune system is thought to contribute to low-grade inflammation (Woods et al., 2012), but the ability of several tissues like liver, adipose tissue and skeletal muscle (SkM) to express and secrete cytokines (Borge et al., 2009; Frost et al., 2002; Hotamisligil et al., 1993; Pedersen & Febbraio, 2012) raises the possibility that these highly metabolically active tissues also contribute to the systemic levels of inflammatory cytokines during low-grade inflammation. Circulating TNFα is increased in type 2 diabetes patients (Hotamisligil et al., 1995; Plomgaard et al., 2007) and TNFα has been shown to induce insulin resistance in humans (Plomgaard et al., 2005) and rodents (Hotamisligil et al., 1994), indicating that TNFα could be involved in the pathogenesis of type 2 diabetes. Thus, suppression of systemic TNFα may be expected to prevent the development and progression of lifestyle-related diseases.
Exercise training elicits a broad range of adaptations including increased skeletal muscle mass (Frontera et al., 1988) as well as increased skeletal muscle oxidative (Holloszy, 1967) and anti-oxidant capacity (Oh-ishi et al., 1997) and previous studies indicate that exercise training may have anti-inflammatory effects (Starkie et al., 2003; Woods et al., 2012). Intriguingly, the natural anti-oxidant resveratrol (RSV), primarily found in the skin of dark grapes, has been reported to exert effects almost similar to exercise training. Hence, RSV has been shown to possess anti-inflammatory effects in rodents and humans (Olholm et al., 2010; Pearson et al., 2008) as well as to protect rodents from high fat diet-induced obesity and insulin resistance (Baur et al., 2006; Lagouge et al., 2006). Moreover, RSV has been shown to increase longevity in several lower species (Howitz et al., 2003; Wood et al., 2004). Both RSV and exercise training have been shown to activate the energy sensors AMP-activated protein kinase (AMPK) (Baur et al., 2006; Um et al., 2010) and sirtuin (SIRT)1 (Lagouge et al., 2006), which both are believed to converge on the transcriptional co-activator peroxisome proliferator activated receptor γ co-activator (PGC)-1α (Canto et al., 2010; Jager et al., 2007).
PGC-1α is known as a master regulator of mitochondrial biogenesis and anti-oxidant defense (Leick et al., 2010; Lin et al., 2002; St-Pierre et al., 2006; Wu et al., 1999). The finding that PGC-1α expression is transiently increased in recovery from a single exercise bout (Baar et al., 2002; Pilegaard et al., 2003) suggests that PGC-1α is a likely mediator of exercise training-induced adaptations in oxidative and anti-oxidant proteins in SkM. Supportive of this, previous findings have highlighted the importance of PGC-1α in exercise training-induced prevention of age-associated reductions in oxidative and anti-oxidant proteins (Leick et al., 2010). Moreover, a positive correlation between physical activity level and SkM PGC-1α mRNA content exists in humans (Alibegovic et al., 2010). Accordingly, an inverse correlation between increasing age and SkM PGC-1α mRNA level (Ling et al., 2004) may suggest that lack of exercise training-induced induction of PGC-1α with aging contributes to age-associated deteriorations in skeletal muscle. Recent studies in mice also indicate that PGC-1α has anti-inflammatory effects (Handschin et al., 2007b; Handschin et al., 2007a; Wenz et al., 2009). However, whether the potential anti-inflammatory effects of exercise training and RSV require PGC-1α and whether combining RSV and exercise training elicits additive effects via PGC-1α are still unresolved.
The aim of the present study was to test the hypothesis that PGC-1α is required for the beneficial effects of long-term exercise training and RSV supplementation in the prevention of age-associated low-grade inflammation. To address this, metabolic and inflammatory markers were determined in adipose tissue, liver, skeletal muscle and blood from whole body PGC-1α knockout (KO) and littermate wild type (WT) mice after long-term voluntary exercise training and/or RSV supplementation.
2. Methods
2.1 Mice
Generation, phenotype and genotyping of whole body PGC-1α KO mice have previously been described in detail (Leick et al., 2008; Lin et al., 2004). Briefly, whole body PGC-1α KO and littermate WT mice were obtained by crossbreeding of heterozygous whole body PGC-1α KO parents. The genotype was assessed on DNA extracted from a small piece of the tail tip by the phenol-chloroform:isoamyl method. DNA fragments were amplified by PCR using specific primers against the WT and the KO alleles (Lin et al., 2004) and subsequently separated on an agarose gel. Whole body PGC-1α KO mice have a CNS-linked neurological disorder resembling Huntingtons disease making them more anxious with sudden movements (Lin et al., 2004). They have reduced oxidative capacity in skeletal muscle (Leick et al., 2008; Leick et al., 2010; Lin et al., 2004), but normal glucose tolerance and do not develop diet-induced obesity (Lin et al., 2004). PGC-1α KO mice run voluntarily less than WT mice when offered a running wheel, (Leick et al., 2008; Leick et al., 2010).
Mice were kept on a 12:12 hour light/dark cycle and had access to water and food ad libitum. The experiments was approved by the “Animal Experiment Inspectorate” in Denmark (#2009/561-1689) and complied with the European convention for the protection of vertebrate animals used for experiments and other scientific purposes (Council of Europe, no. 123, Strasbourg, France, 1985).
2.2 Experimental Setup
Whole body PGC-1α KO and littermate WT female mice were randomly assigned to a young group and four intervention groups with mice housed individually. The intervention groups were divided into: untrained mice receiving rodent chow (Altromin no. 1324, Brogården, Lynge, Denmark) (UT-C), untrained mice receiving chow supplemented with RSV (UT-R), voluntary exercise trained mice having access to a running wheel (Minimitter, Italy) (T-C), and voluntary exercise trained mice having access to a running wheel and receiving chow supplemented with RSV (T-R). The interventions lasted from 3 months to 15 months of age, where the mice were euthanized. The young mice receiving chow and serving as young UT-C were euthanized at 3 months of age. Each of the groups consisted of 8–10 mice. Based on the observation that mice of the PGC-1α KO strain (6 WT mice and 6 PGC-1α KO mice), in our animal facility, on average reach ~18 months of age with no apparent difference between WT and PGC-1α KO mice, we chose to examine 15 month old mice as these mice would be in their last quartile of their lifespan.
Based on previous studies from our group (Leick et al., 2008; Leick et al., 2010) we expected the PGC-1α KO mice to run less voluntarily than WT mice. Running distance and duration was monitored by a regular cycle computer and differences between WT and PGC-1α KO mice were daily adjusted by wheel blocking of WT for shorter periods to ensure similar exercise distance between the different genotypes and interventions. The running distance was on average 5.9±1.9 (WT, T-C), 6.0±2.4 (KO, T-C), 6.0±1.2 (WT, T-R) and 5.8±1.7 (KO, T-R) km per week. Pure RSV (Orchid chemicals, India) was mixed with chow to a concentration of 4 g RSV/kg chow as previously used (Lagouge et al., 2006; Um et al., 2010). The concentration was subsequently confirmed by liquid chromatography-mass spectrometry (Eurofins, Denmark). Body weight and food intake were monitored throughout the experiment. Running wheels were blocked 24 hours before the animals were euthanized. All mice were euthanized by cervical dislocation followed by decapitation to collect trunk blood. Quadriceps muscles, perigonadal visceral adipose tissue (VAT), inguinal subcutaneous adipose tissue (S-AT) and liver were quickly removed, quick frozen in liquid nitrogen and stored at −80° C until analyses. These tissues were chosen based on previous reports showing marked inflammatory responses as well as adaptations to metabolic challenges (Gollisch et al., 2009; Handschin et al., 2007b; Hotamisligil et al., 1993; Wenz et al., 2009).
A separate manuscript covers oxidative adaptations in skeletal muscle from the current experimental setup (Ringholm et al. – In pending review, Exp Gerontology).
2.3 Analyses
2.3.1 Echo MRI scanning
Body composition was determined by MRI scanning (EchoMRI, Echo Medical Systems, Houston, TX, USA). A reduced number of animals were measured (n=3–8) due to limited access to the Echo MRI scanner.
2.3.2 Plasma
Plasma cytokines were analyzed using an ultra-sensitive customized MSD multi-spot assay system pre-coated with antibodies against TNFα and IL-6 (MesoScaleDiscovery, Gaithersburg, Maryland, USA) according to manufacturer’s protocol. The lower limit of detection (LLOD) was 1.0 pg/ml for TNFα and 4.5 pg/ml for IL-6.
2.3.3 RNA isolation and Reverse Transcription
Total RNA was isolated from crushed quadriceps muscle (20–25 mg), liver (20–25 mg) and V-AT (40–45 mg) by a modified guanidinium thiocyanate-phenol-chloroform extraction method (Chomczynski & Sacchi, 1987) as previously described (Pilegaard et al., 2000), except that the tissue was homogenized for 2 min at 30 s−1 in a tissue lyser (TissueLyser II; QIAGEN, Germany).
Reverse transcription (RT) was performed using Superscript II RNase H− and Oligo dT system (Invitrogen, Carlsbad, CA, USA) as previously described (Pilegaard et al., 2000), and the cDNA samples were diluted in nuclease-free H2O.
2.3.4 Real-time PCR
Real-time PCR was performed using an ABI 7900 sequence-detection system (Applied Biosystems, Foster City, CA, USA) as previously described (Lundby et al., 2005). Primers and Taqman probes were obtained from TAG Copenhagen (Copenhagen, Denmark) (table 1). Real-time PCR was performed in triplicates in a total reaction volume of 10 μl using Universal Mastermix (Applied Biosystems). Cycle threshold was converted to a relative amount by use of a standard curve constructed from a serial dilution of a pooled RT sample run together with the samples. Target gene mRNA content was for each sample normalized to single-stranded cDNA content determined by OliGreen reagent (Molecular Probes, Leiden, The Netherlands) as previously described (Lundby et al., 2005).
Table 1.
Tumor necrosis factor (TNF)α, interleukin (IL)-6 and F4/80 primer and TaqMan probe sequences used for real time PCR.
| Gene | Forward primer | Reverse primer | probe |
|---|---|---|---|
| TNFα | 5′ ATGGCCCAGACCCTCACA 3′ | 5′ TTGCTACGACGTGGGCTACA 3′ | 5′ TCAGATCATCTTCTCAAAATTCGAGTGACAAGC 3′ |
| IL-6 | 5′ GCTTAATTACACATGTTCTCTGGGAAA′3 | 5′ CAAGTGCATCATCGTTGTTCATAC′3 | 5′ ATCAGAATTGCCATTGCACAACTCTTTTCTCAT′3 |
| F4/80 | 5′ GGCTGCCTCCCTGACTTTC 3′ | 5′ TGCACTGCTTGGCATTGC 3′ | 5′ TCCTTTTGCAGTTGAAGTTTCCATATCCTTGG 3′ |
| TACE | 5′ TGCAAGGCTGGGAAATGC 3′ | 5′ TTG CACGAGTTGTCAGTGTCAA 3′ | 5′ AGCAGGAGCTGGAGTCCTGCGC 3′ |
| SOD2 | 5′ GTGGTGGAGAACCCAAAGGA 3′ | 5′ AACCTTGGACTCCCACAGACA 3′ | 5′ AGTTGCTGGAGGCTATCAAGCGTGACTTT 3′ |
| Catalase | 5′ CTGGACGTTTTACATCCAGGTCA 3′ | 5′ TCCTTG TGAGGCCAAACCTT 3′ | 5′ AGGCAGAAACTTTCCCATTTAATCCATTTGATC 3′ |
| GPX1 | 5′ GACTGGTGGTGCTCGGTTTC 3′ | 5′ TTGAGGGAATTCAGAATCTCTTCA 3′ | 5′ AATCAGTTCGGACACCAGGAGAATGGC 3′ |
2.3.5 Lysate
Crushed quadriceps muscles (~20–25 mg), liver (~20–25 mg) and adipose tissue (~25–35 mg) were homogenized for 2 min at 30 s−1 in a tissue lyser (TissueLyser II; QIAGEN) in an ice-cold buffer as previously described (Birk & Wojtaszewski, 2006). Protein content in lysates was measured by the bicinchoninic acid method (Thermo Scientific, Rockford, IL, USA).
2.3.6 SDS-PAGE and western blotting
Protein content and phosphorylation of various proteins were measured in lysates by SDS-PAGE and western blotting as previously described (Birk & Wojtaszewski, 2006). Band intensity was quantified using Carestream IS 4000 MM (Fisher Scientific, ThermoFisher Scientific, Waltman, MA, USA) and Carestream health molecular imaging software. Protein content and phosphorylation were expressed as arbitrary units relative to control samples loaded on each site of each gel and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein. Commercially available antibodies were used to detect TNFα (#3707), nitric oxide synthase (iNOS) (#2977), nuclear factor of kappa light polypeptide gene enhancer in B-cells (NFκB) inhibitor (IκB)-α (#9242), IκB-β (#9248), p65 (#4764) and GAPDH (#2118) protein as well as p65ser536 (#3033), c-Jun N-terminal kinases (JNK) (#9252), JNKThr183, Tyr185 (#9251), p38 mitogen-activated protein kinase (p38) (#9212) and p38Thr180, Tyr182 (# 4511) phosphorylation all from Cell Signaling and superoxide dismutase (SOD)2 (#06-984, Millipore), Catalase (#SC-50508) and glutathione peroxidase (GPX)1 (#SC-30147) protein from Santa Cruz Biotechnology inc.
2.3.7 Protein Carbonylation
Protein carbonyl content was determined in quadriceps muscle samples homogenized in phosphate-buffer using an OxiSelect™ ELISA-kit (Cell Biolabs, San Diego, USA) according to manufacturer’s protocol. Absorbance was measured at 450 nm and an oxidized/reduced BSA standard curve was generated to determine the concentration of protein carbonyl in each sample.
2.4 Statistics
Results are presented as means ± S.E. Unless otherwise noted, two-way analysis of variance (ANOVA) was applied to test the main effects of genotype and interventions and one-way ANOVA was used to test for differences between the interventions separately within each genotype. If either the equal variance test or the normality test failed, the data were logarithmically transformed before applying the ANOVA test. Student Newman Keuls post hoc test was used to locate differences when applicable. The non-parametric Mann-Whitney U test was applied when the equal variance test or the normality test failed even after logarithmically transformation. A p<0.05 was considered significant and a tendency is reported for 0.05 p≤0.1.
3. Results
3.1 Body weight, food intake and body composition
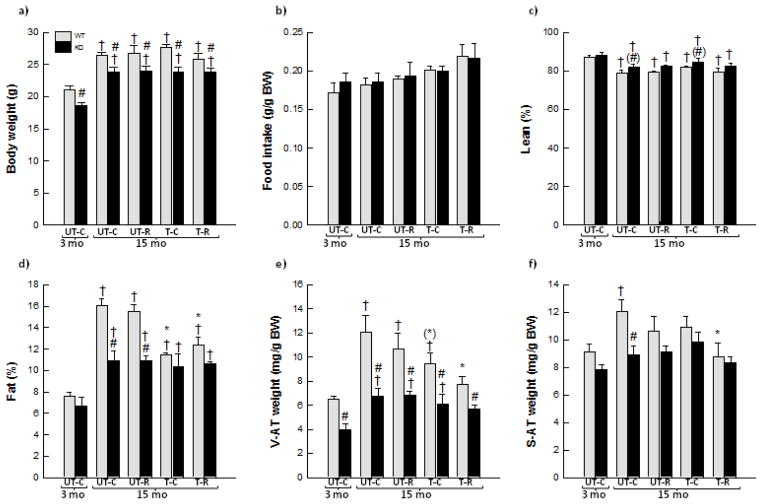
Body weights were initially similar within the WT groups and within the PGC-1α KO groups (data not shown). Total body weight increased (p<0.05) ~20% with age in WT and PGC-1α KO mice (Fig. 1a). There was no effect of exercise training and/or RSV on body weight in either WT or PGC-1α KO mice. PGC-1α KO mice weighed ~10% less (p<0.05) than WT mice in all groups. There was no difference in food intake (given pr. gram of mice) between any of the groups (Fig. 1b).
Figure 1.
Body weight (a), food intake (b), lean body mass in % (c), fat % (d), perigonadal visceral adipose tissue (V-AT) weight (e) and inguinal subcutaneous adipose tissue (S-AT) weight from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10 except in (c) and (d) where n=3–8. †: Significantly different from 3 month old UT-C within genotype, p<0.05. *: Significantly different from 15 month old UT-C within genotype, p<0.05. (*): Tends to be significantly different from 15 month old UT-C within genotype, 0.05≤p<0.1. #: Significantly different from WT within group, p<0.05. (#) Tends to be significantly different from WT within group, 0.05≤p<0.1.
Lean body mass (given as %) decreased (p<0.05) 5–10% with age in all groups (Fig. 1c). There was no effect of exercise training and/or RSV on percentage lean body mass in either WT or PGC-1α KO mice. Fifteen month old untrained and exercise trained PGC-1α KO mice tended to have 3–4% higher (p=0.053 and p=0.051, respectively) percentage lean body mass than WT mice, while no genotype differences were observed in the other groups.
Whole body fat percentage as well as V-AT and S-AT mass increased (p<0.05) 1.6–2.1 fold with age in both WT and PGC-1α KO mice (Fig. 1d, 1e, 1f). While no effect was observed on adiposity by RSV supplementation alone, exercise training decreased (p<0.05) fat percentage ~30% and tended to decrease (p=0.082) V-AT mass in WT mice. Combined exercise training and RSV also decreased (p<0.05) fat percentage (23%) as well as V-AT (37%) and S-AT (27%) mass in WT mice. Fat percentage was ~30% lower (p<0.05) in PGC-1α KO than WT within 15 month old untrained and RSV supplemented mice, and SAT mass was 30 % lower (p<0.05) in PGC-1α KO mice than WT within 15 month old untrained mice. In addition, PGC-1α KO mice had in all groups 25–40% less (p<0.05) V-AT than WT mice.
3.2 Plasma
Selected plasma cytokines were analyzed to evaluate the general systemic inflammatory status associated with the different interventions.
Plasma TNFα
There was an overall 1.7–2.0 fold increase (p<0.05) in plasma TNFα with age in WT and PGC-1α KO mice (Fig. 2a). No significant effect of RSV, exercise training alone or exercise training in combination with RSV was observed on plasma TNFα. The plasma TNFα level was 1.7–2.7 fold higher (p<0.05) in PGC-1α KO than WT in young and 15 month old untrained mice.
Figure 2.
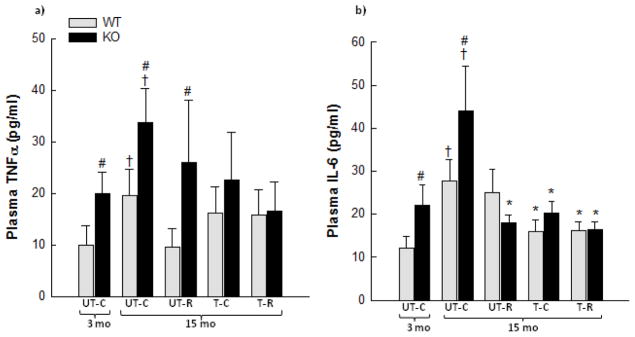
Plasma tumor necrosis factor (TNF)α (a) and plasma interleukin (IL)-6 (b) from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. A two-way ANOVA and one-way ANOVA test was applied on logarithmically transformed data when appropriate. †: Significantly different from 3 month old UT-C within genotype, p<0.05. *: Significantly different from 15 month old UT-C within genotype, p<0.05. #: Significantly different from WT within group, p<0.05.
Plasma IL-6
Plasma IL-6 increased 2–2.3 fold (p<0.05) with age in WT and PGC-1α KO mice (Fig. 2b). Exercise training alone as well as in combination with RSV reduced (p<0.05) the plasma IL-6 level 40–60% in WT and PGC-1α KO mice compared with 15 month old untrained mice. In PGC-1α KO mice, RSV supplementation reduced (p<0.05) the plasma IL-6 level ~60% compared with 15 month old untrained mice. In young and 15 month old untrained mice, the plasma IL-6 level was 1.6–1.8 fold higher (p<0.05) in PGC-1α KO mice than WT mice.
3.3 Inflammatory mRNA markers in V-AT, liver and SkM
The TNFα and IL-6 mRNA content was determined in V-AT, liver and SkM in order to evaluate the tissue specific inflammatory status. Additionally, the mRNA content of the macrophage specific F4/80 (Khazen et al., 2005) was analyzed as a marker of macrophage infiltration in these tissues.
V-AT
In WT mice, V-AT TNFα mRNA increased (p<0.05) 4.4 fold with age, but mice supplemented with RSV, exercise trained as well as exercise trained combined with RSV supplementation did not differ from young control mice in V-AT TNFα mRNA content. In PGC-1α KO mice, RSV increased (p<0.05) V-AT TNFα mRNA 1.8 fold compared with 15 month old untrained mice (table 2).
Table 2. mRNA content in visceral adipose tissue, liver and skeletal muscle.
Tumor necrosis factor (TNF)α, interleukin (IL)-6 and F4/80 mRNA in perigonadal visceral adipose tissue (V-AT), liver and skeletal muscle (SkM) from 3 months old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. Two-way ANOVA and one-way ANOVA tests were applied on logarithmically transformed data when appropriate. Mann-Whitney U nonparametric test was applied when appropriate
| 3 mo UT-C | 15 mo UT-C | 15 mo UT-R | 15 mo T-C | 15 mo T-R | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| gene | WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | |
| V-AT | TNFα | 0.5 ± 0.1 | 0.8 ± 0.2 | 2.3 ± 0.7† | 0.7 ± 0.2# | 0.9 ± 0.2 | 1.3 ± 0.3* | 1.0 ± 0.3 | 0.7 ± 0.2 | 0.9 ± 0.1 | 1.4 ± 0.4 |
| IL-6 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.9 ± 0.1* | 0.8 ± 0.1* | 1.2 ± 0.1* | 0.7 ± 0.2# | 0.7 ± 0.1 | 0.5 ± 0.1 | |
| F4/80 | 0.8 ± 0.2 | 0.5 ± 0.2 | 1.2 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.7 ± 0.1 | 1.3 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.9 ± 0.3 | |
| Liver | TNFα | 0.3 ± 0.03 | 0.3 ± 0.04 | 0.6 ± 0.1† | 0.3 ± 0.03# | 0.5 ± 0.1 | 0.5 ± 0.1* | 0.5 ± 0.1 | 0.4 ± 0.04 | 0.5 ± 0.04 | 0.5 ± 0.1* |
| IL-6 | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.2 ± 0.03 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.03 | |
| F4/80 | 0.6 ± 0.04 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1# | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.03 | 0.7 ± 0.1 | |
| SkM | TNFα | 1.1 ± 0.1 | 0.7 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.3† | 1.2 ± 0.3 | 1.6 ± 0.2 | 2.6 ± 0.6* | 1.9 ± 0.2 | 1.9 ± 0.3 | 2.4 ± 0.4 |
| IL-6 | 0.7 ± 0.1 | 0.5 ± 0.1 | 1.0 ± 0.1(†) | 1.0 ± 0.1† | 0.7 ± 0.2 | 1.0 ± 0.1 | 0.7 ± 0.1* | 0.7 ± 0.1* | 0.9 ± 0.1* | 0.7 ± 0.1* | |
| F4/80 | 2.4 ± 0.3 | 2.3 ± 0.3 | 1.2 ± 0.2† | 1.6 ± 0.3 | 0.8 ± 0.2 | 1.6 ± 0.3# | 3.7 ± 1.1* | 3.0 ± 0.4* | 2.0 ± 0.3* | 3.5 ± 0.4#* | |
Significantly different from 3 month old UT-C within genotype, p<0.05.
Tends to be significantly different from 3 month old UT-C within genotype, 0.05≤p<0.1.
Significantly different from 15 month old UT-C within genotype, p<0.05.
Significantly different from WT within group, p<0.05.
In WT mice, both RSV and exercise training increased (p<0.05) V-AT IL-6 mRNA 1.5–1.9 fold compared with 15 month old untrained mice. In PGC-1α KO mice, RSV increased (p<0.05) V-AT IL-6 mRNA content 1.5 fold compared with 15 month old untrained mice (table 2).
No differences were observed in V-AT F4/80 mRNA between genotypes or any of the interventions (table 2).
Liver
Liver TNFα mRNA increased 1.6 fold (p<0.05) with age only in WT mice. Liver TNFα mRNA increased (p<0.05) in PGC-1α KO mice with RSV and combined exercise training and RSV, while no change occurred in WT mice. Fifteen month old PGC-1α KO mice had lower (p<0.05) liver TNFα mRNA content than WT, whereas no difference was observed between genotypes in the other groups (table 2).
No differences were observed in liver IL-6 mRNA between genotypes or any of the interventions (table 2.)
No effect of age or any of the interventions was observed in F4/80 mRNA content in the liver. However, 15 month old untrained PGC-1α KO mice had 47% lower (p<0.05) F4/80 mRNA content in the liver than WT mice (table 2).
SkM
TNFα mRNA increased (p<0.05) ~2.1 fold in SkM with age in PGC-1α KO mice. TNFα mRNA content was 1.8 fold higher (p<0.05) in exercise trained WT mice than 15 month old untrained WT mice, whereas no effect was observed with RSV and combined exercise training and RSV.
IL-6 mRNA increased ~2.3 fold (p<0.05) with age in PGC-1α KO mice and tended to increase 1.4 fold (p=0.06) with age in WT mice. Exercise training combined with RSV decreased (p<0.05) SkM IL-6 mRNA content 10–37% in WT and PGC-1α KO mice, while no effect was observed in SkM IL-6 mRNA with RSV alone or exercise training alone (table 2).
SkM F4/80 mRNA content decreased (p<0.05) 50% with age in WT mice, whereas exercise training alone (2.5 fold) or in combination with RSV (1.9 fold) increased (p<0.05) the F4/80 mRNA content in SkM of both WT and KO mice compared with 15 month old untrained mice (table 2). In the RSV group and in the combined exercise trained and RSV group, PGC-1α KO mice had 1.8–2.1 fold higher (p<0.05) F4/80 mRNA level in SkM than WT mice, whereas no differences were present between genotypes in the other groups (table 2).
3.4 TNFα protein in V-AT, liver and SkM
TNFα protein content was further determined in V-AT, liver and SkM. No differences were observed in VAT TNFα protein content between any of the interventions or genotypes (Fig. 3a). An overall tendency (p=0.079) for a 1.2–1.3 fold increase in liver TNFα protein was observed with age in WT and PGC-1α KO mice (Fig. 3b). No effect of exercise training and/or RSV was observed in liver TNFα protein, but these groups did not differ from young mice either. SkM TNFα protein content increased 1.4–1.5 fold (p<0.05) with age in WT and PGC-1α KO mice. Both exercise training alone and exercise training in combination with RSV prevented this age-associated increase in TNFα protein in WT mice, but this response was blunted in PGC-1α KO mice (Fig. 3c). PGC-1α KO mice had 1.5–2.2 fold higher (p<0.05) SkM TNFα protein content than WT mice in RSV, exercise trained as well as combined exercise trained and RSV groups (Fig. 3c).
Figure 3.
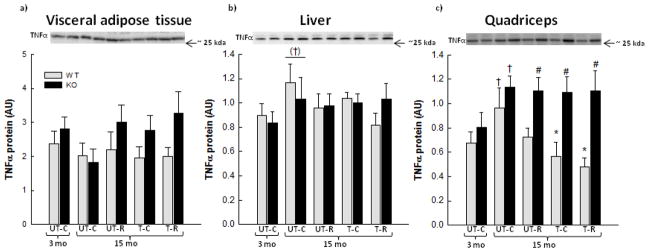
Tumor necrosis factor (TNF)α protein in perigonadal visceral adipose tissue (V-AT) (a), liver (b) and quadriceps muscle (c) from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. †: Significantly different from 3 month old UT-C within genotype, p<0.05. (†): Tends to be significantly different from 3 month old UT-C, 0.05≤p<0.1. *: Significantly different from 15 month old UT-C within genotype, p<0.05. #: Significantly different from WT within group, p<0.05. A horizontal line indicates a main effect. Representative blots are shown on each figure with samples loaded in the same order as depicted on the graph.
3.5 Inflammatory signaling in skeletal muscle
The 3 major inflammatory signaling pathways, IKK/NfκB, JNK and p38 were analyzed in SkM in order to investigate the underlying mechanisms behind the observed differences in SkM TNFα protein levels.
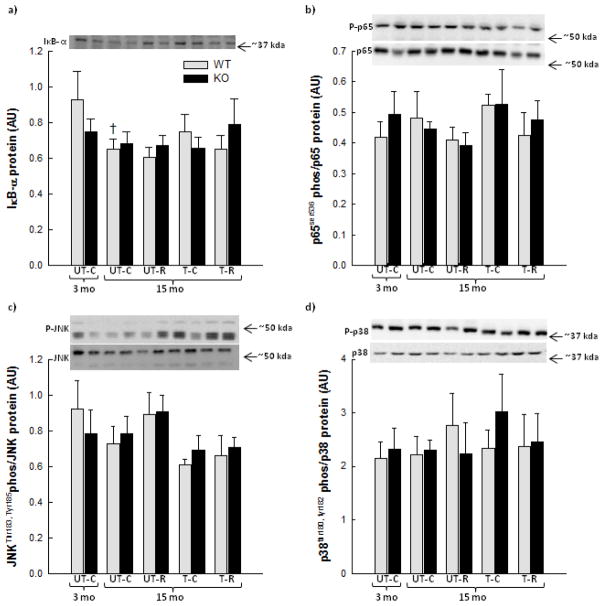
No difference was observed in total p65, JNK and p38 protein content between genotypes or interventions (data not shown). In WT mice, IκB-α protein decreased 30% (p<0.05) in SkM with age (Fig. 4a). No differences were observed in SkM IκB-α protein content with exercise training and/or RSV supplementation in any of the genotypes. SkM p65 phosphorylation did not differ between the interventions or the genotypes (Fig. 4b). There were no differences in JNK (Fig. 4c) or p38 (Fig. 4d) signaling in SkM in any of the interventions or between the genotypes.
Figure 4.
Nuclear factor of kappa light polypeptide gene enhancer in B-cells (NFκB) inhibitor (IκB)-α protein (a), p65 phosphorylation (phos) (b), c-Jun N-terminal kinase (JNK) phosphorylation (c) and p38 mitogen-activated protein kinase (p38) phosphorylation (d) in quadriceps muscle from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. †: Significantly different from 3 month old UT-C within genotype, p<0.05. Representative blots are shown on each figure with samples loaded in the same order as depicted on the graph.
3.6 Protein carbonylation and iNOS protein in skeletal muscle
As oxidative stress has been suggested to be a stimulus for inducing inflammation, SkM protein carbonylation (a marker of oxidative stress) was determined. SkM protein carbonylation increased 1.6–2.1 fold (p<0.05) with age in both WT and PGC-1α KO mice (Fig. 5a). RSV, exercise training and combined exercise training and RSV prevented (p<0.05) this age-associated increase in SkM protein carbonylation in both WT and PGC-1α KO mice. Furthermore, young PGC-1α KO mice had 1.8 fold higher (p<0.05) protein carbonylation in SkM than young WT mice (Fig. 5a).
Figure 5.
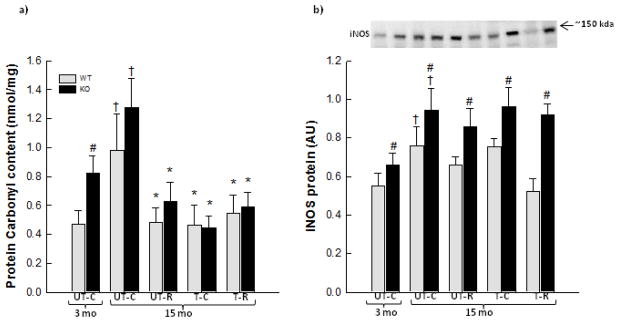
Protein carbonylation (a) and iNOS protein (b) in quadriceps muscle from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. †: Significantly different from 3 month old UT-C within genotype, p<0.05. *: Significantly different from 15 month old UT-C within genotype, p<0.05. #: Significantly different from WT within group, p<0.05. Representative blots are shown on each figure with samples loaded in the same order as depicted on the graph.
In accordance, the protein content of iNOS increased (p<0.05) ~1.4 fold with age in SkM of WT and PGC-1α KO mice (Fig. 5b). Exercise training and/or RSV supplementation did not change iNOS protein content relative to 15 month old untrained mice, but iNOS protein content was in these groups not different from young mice. Interestingly, PGC-1α KO mice had in all groups 1.2–1.8 fold higher (p<0.05) iNOS protein content than WT mice (Fig. 5b).
3.7 Anti-oxidant enzymes in skeletal muscle
The anti-oxidant enzymes SOD2, catalase and GPX1 were analyzed in SkM to indirectly examine whether the observed indications of oxidative stress in SkM were related to ROS neutralization capacity. Catalase protein content decreased (p<0.05) ~50% with age in SkM of WT mice (Fig. 6b). RSV increased (p<0.05) SkM GPX1 protein 1.8 fold, whereas no effect of RSV was observed in SOD2 or catalase protein. Exercise training increased (p<0.05) GPX1 protein 1.8 fold (Fig. 6c) and tended to increase SOD2 protein (p=0.051) and catalase protein (p=0.075) 1.3–2.2 fold in SkM of WT mice. In WT mice, combined exercise training and RSV increased (p<0.05) catalase protein and GPX1 protein 2.2–2.5 fold compared with 15 month old untrained mice, but with no difference between the exercise trained group and the combined exercise trained and RSVgroup. No effect of RSV, exercise training or combined exercise training and RSV were observed in SOD2, catalase or GPX1 protein in PGC-1α KO mice.
Figure 6.
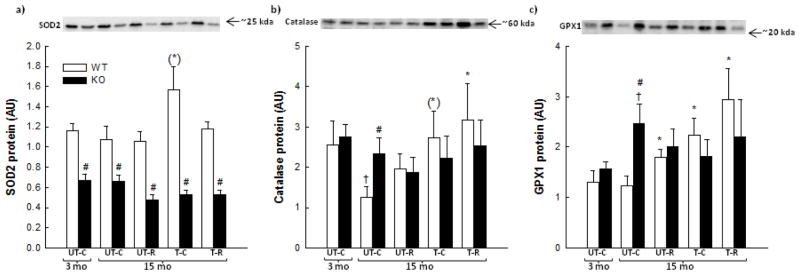
Superoxide dismustase (SOD)2 protein (a), catalase protein (b) and glutathione peroxidase (GPX)1 protein (c) in quadriceps muscle from 3 month old (mo) untrained chow mice (UT-C), 15 month old untrained chow mice (UT-C), 15 month old untrained resveratrol (RSV) supplemented mice (UT-R), 15 month old exercise trained (T-C) and 15 month old exercise trained and RSV supplemented (T-R) whole body PGC-1α knockout (KO) and littermate wild type (WT) mice. Values are presented as means ± S.E.; n=8–10. †: Significantly different from 3 month old UT-C within genotype, p<0.05. *: Significantly different from 15 month old UT-C within genotype, p<0.05. (*): Tends to be significantly different from 15 month old UT-C within genotype, 0.05≤p<0.1. #: Significantly different from WT within group, p<0.05. Representative blots are shown on each figure with samples loaded in the same order as depicted on the graph.
PGC-1α KO mice had in all groups 25–50% lower (p<0.05) SkM SOD2 protein content than WT mice. In contrast, 15 month old untrained PGC-1α KO mice had ~2.0 fold higher (p<0.05) GPX1 and catalase protein content than WT and GPX1 protein increased (p<0.05) 1.6 fold with age in SkM of PGC-1α KO mice.
4. Discussion
The main findings of the present study were that long-term exercise training prevented an age-associated increase in TNFα protein in SkM in a PGC-1α dependent manner. Independently of PGC-1α, long-term exercise training prevented an age-associated increase in systemic IL-6 levels and oxidative stress in SkM. Long-term RSV supplementation also prevented an age-associated increase in systemic IL-6 and oxidative stress in SkM independently of PGC-1α. Taken together, the beneficial effects of long-term RSV supplementation seemed minor compared with previous reports on short-term RSV supplementation and compared with the present effects of long-term exercise training.
The present increase in adiposity and total body weight with aging is in accordance with previous studies (Guo et al., 1999; Wu et al., 2007). Although a genotype specific difference in body weight and adiposity between PGC-1α KO and WT mice has previously been described (Lin et al., 2004), the current finding that exercise training as well as exercise training combined with RSV supplementation only prevented the age-associated adiposity in WT mice is of notice. The age-associated increase in plasma TNFα and IL-6 with aging in the present study is also in agreement with previous studies (Wei et al., 1992) and supports that increased adiposity is associated with systemic low-grade inflammation. The observed prevention of an age-associated elevation in plasma IL-6 with long-term exercise training is in line with previous studies indicating that exercise training has anti-inflammatory effects (Olholm et al., 2010; Woods et al., 2012). However, it should be noted that the present age-associated low-grade inflammation and improvements with exercise training were not linked to altered glucose tolerance (Ringholm et al., in pending review Exp Gerontol), which may suggest that low-grade inflammation precedes glucose intolerance at least in the present setting. A potential role of PGC-1α in the regulation of systemic inflammation has previously been suggested based on studies in both muscle-specific PGC-1α KO mice (Handschin et al., 2007b; Handschin et al., 2007a) and muscle-specific PGC-1α overexpression mice (Olesen et al., 2012; Wenz et al., 2009). The proposal that lack of PGC-1α leads to low-grade inflammation is supported by the present findings that young and aged PGC-1α KO mice had increased plasma TNFα and IL-6 compared with WT mice.
The present findings that aging was associated with increased TNFα mRNA in V-AT and TNFα mRNA and protein in liver as well as increased iNOS and TNFα protein in SkM are in line with previous studies (Lumeng et al., 2011; Wu et al., 2007) and with the present age-associated increase in plasma TNFα. Together, these findings strongly indicate that several tissues are inflamed with aging and potentially contribute to the observed increase in the systemic levels of inflammatory mediators. However, the novel finding that long-term exercise training prevented the age-associated increase in TNFα protein in SkM supports that exercise training specifically elicits anti-inflammatory responses in SkM. Furthermore, the almost similar patterns of plasma TNFα and SkM TNFα protein in response to aging and exercise training indicate that SkM can be an important contributor to the systemic plasma TNFα levels as previously suggested during acute inflammation (Borge et al., 2009; Frost et al., 2002; Olesen et al., 2012). The current demonstration that the reduction in SkM TNFα protein with exercise training was completely blunted in PGC-1α KO mice provides evidence that PGC-1α is required for the exercise training-induced anti-inflammatory effects in SkM. In addition, the novel observation that PGC-1α KO mice had higher iNOS protein levels than WT mice adds to the previously reported anti-inflammatory effect of muscle PGC-1α (Handschin et al., 2007b; Wenz et al., 2009).
To delineate the potential underlying mechanisms behind the observed TNFα protein expression pattern in SkM, several intracellular inflammatory signaling pathways were investigated in SkM. The observed age-associated decrease in SkM IκB-α protein is in accordance with previous findings (Wenz et al., 2009) and may indirectly reflect increased translocation of NFκB to the nucleus, and thus potentially explain the observed age-associated increase in TNFα protein in WT mice. In contrast, NFκB, JNK or p38 signaling neither explains the observed exercise training-induced nor genotype specific differences in SkM TNFα protein. To our knowledge no previous studies have shown PGC-1α dependent alterations in p38 or JNK signaling, but divergent results have been reported for NFκB signaling (Alvarez-Guardia et al., 2010; Eisele et al., 2013; Olesen et al., 2012; Wenz et al., 2009). Hence, previous studies have both reported that 24 month old muscle-specific PGC-1α overexpression mice had decreased SkM p65 phosphorylation (Wenz et al., 2009) and that young muscle-specific PGC-1α overexpression mice had elevated SkM p65 phosphorylation compared with WT (Olesen et al., 2012). Together with the present findings this may indicate that the interaction between PGC-1α and NFκB depends on the specific experimental setting.
As macrophages are considered an important source of TNFα and iNOS production, the mRNA content of the highly specific murine macrophage marker, F4/80 (Khazen et al., 2005), was determined to evaluate whether differences in macrophage infiltration could explain the observed differences in SkM TNFα and/or iNOS protein content. The finding that SkM F4/80 mRNA content decreased with age and increased with long-term exercise training indicates that macrophages within SkM or in the surrounding capillaries were not responsible for the observed differences in TNFα and iNOS protein in SkM. Although the present data cannot exclude the possibility that infiltrated immune cells have contributed to the observed TNFα and iNOS, these observations imply that SkM fibers may indeed be a considerable source of TNFα (Borge et al., 2009; Frost et al., 2002; Olesen et al., 2012) as well as iNOS production. Taken together, the observed signaling events in SkM in concert with the F4/80 mRNA levels are inadequate to explain the present TNFα and iNOS protein expression patterns. Although speculative this may indicate that the observed TNFα differences are not due to changes in synthesis, but rather degradation/turnover. However, future studies are needed to fully understand the underlying mechanism.
The observed age-associated increase in protein carbonylation in SkM is in accordance with previous studies and supports an association between adiposity, inflammation and oxidative stress (Berg & Scherer, 2005; Hotamisligil et al., 1995). In addition, the increase in protein carbonylation with age may potentially be explained by the observed age-associated decrease in the ROS scavenging protein catalase. Interestingly, exercise training alone and in combination with RSV prevented the age-associated increase in protein carbonylation, which may be due to the observed exercise training and combined exercise training- and RSV-induced increases in anti-oxidant enzymes. These data support that both exercise training and RSV increase the anti-oxidant capacity (Hellsten et al., 1996; Jackson et al., 2011; Leick et al., 2010). However, the observation that RSV supplementation counteracted the age-associated oxidative stress and at the same time only affected GPX1 protein levels may suggest that the RSV-mediated decrease in oxidative stress in part was due to its direct anti-oxidant properties as previously suggested (Howitz et al., 2003; Wood et al., 2004).
Notably, the present finding that young PGC-1α KO mice had increased protein carbonylation in conjunction with reduced SOD2 levels compared with WT mice indicates that PGC-1α is important for the basal ROS handling in young mice (Leick et al., 2010; St-Pierre et al., 2006). Furthermore these findings support that the increased oxidative stress in these animals in part is due to reduced ROS neutralization capacity as previously reported (St-Pierre et al., 2006). Moreover, while PGC-1α was not required for the observed exercise training and RSV-induced prevention of age-associated oxidative stress, PGC-1α was required for the exercise training-induced increase in SOD2 protein content and in part also for the exercise training- and combined exercise training and RSV-induced increase in catalase and GPX1 protein content. Together these data support that changes in the endogenous anti-oxidant system contribute to the age-associated increase in oxidative stress as well as the exercise training-induced prevention of oxidative stress. However, the precise role of PGC-1α in this is still not clarified.
A key finding of the present study is that long-term RSV supplementation only showed minor effects, which is in contrast to many previous studies in mice (Baur et al., 2006; Lagouge et al., 2006; Um et al., 2010). However, a recent study in mice (Menzies et al., 2013) and two independent human studies in obese men and non-obese women (Poulsen et al., 2012; Yoshino et al., 2012) also failed to show any major impact of RSV supplementation. A study in cultured human primary muscle cells even showed impaired glucose uptake after incubation with RSV (Skrobuk et al., 2012). This underlines the importance of additional studies to fully understand the metabolic effects of RSV. Moreover, the observed additive effect of exercise training and RSV on V-AT and S-AT mass in the present study is modest compared with previous studies in rodents examining the additive effects of exercise training and RSV (Dolinsky et al., 2012; Menzies et al., 2013). The relatively small effects of RSV in the present study do not seem to be dose related. The present dose used corresponds to ~0.7 mg RSV per gram mouse per day, which is similar to previous studies where profound metabolic effects have been observed (Lagouge et al., 2006; Um et al., 2010). The lack of RSV-mediated effects may alternatively be related to the duration of the treatment. Hence, while previous studies have focused on shorter durations (1–4 month), the 12 month treatment in the present study may have had a desensitizing effect. Another likely explanation may be that the 15 month old “control” animals, despite the observed age-associated deteriorations already discussed, were too metabolically “healthy” to obtain metabolic improvements through the RSV treatment. Hence, in contrast to several previous reports on RSV supplementation (Baur et al., 2006; Lagouge et al., 2006; Um et al., 2010), the old mice from the present study did not have impaired glucose tolerance (Ringholm et al., in pending review, Exp. Gerontology) as already mentioned. The novel finding that PGC-1α was not required for the RSV-induced improvements in systemic IL-6 and SkM oxidative stress may indicate that PGC-1α is activated by RSV as suggested (Baur et al., 2006; Lagouge et al., 2006; Timmers et al., 2011), but not mandatory for the metabolic effects of RSV.
In conclusion, the present findings demonstrate that long-term exercise training prevented an age-associated increase in adiposity, systemic low-grade inflammation as well as SkM oxidative stress. In addition, these results show that PGC-1α was required for an exercise training-induced prevention of an age-associated increase in SkM TNFα protein. Long-term RSV supplementation elicited only few effects on SkM oxidative stress and in part on low-grade inflammation and PGC-1α was not required for these effects. Together, the present findings indicate that regular physical activity is a more powerful intervention to prevent or postpone age-related inflammation than RSV supplementation.
Bullet points.
Aging increases adiposity, inflammation and oxidative stress in mice
PGC-1α is required for exercise training-induced reductions in TNFα protein in SkM
Exercise training reduces oxidative stress independently of PGC-1α
Long-term resveratrol suppl. reduces oxidative stress in SkM independently of PGC-1α
Minor effects of long-term resveratrol compared with long-term exercise training
Acknowledgments
Funding
This present study was supported by grants from The Danish Medical Research Council (#11-104199) to HP; Novo Nordisk foundation to HP; Augustinus foundation to JO; The national Institutes of Health (R01 AR42238) to LJG. The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (# 02-512-55). CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology, and Innovation. CIM is a member of DD2 - the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Professor Bruce M. Spiegelman (Harvard Medical School, Boston, Massachusetts, USA) for the kind donation of breeding pairs to the initial breeding of the whole body PGC-1α knockout and littermate wild type mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alibegovic AC, Sonne MP, Hojbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab. 2010;299:E752–E763. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- Alvarez-Guardia D, Palomer X, Coll T, Davidson MM, Chan TO, Feldman AM, Laguna JC, Vazquez-Carrera M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc Res. 2010;87:449–458. doi: 10.1093/cvr/cvq080. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borge BA, Kalland KH, Olsen S, Bletsa A, Berggreen E, Wiig H. Cytokines are produced locally by myocytes in rat skeletal muscle during endotoxemia. Am J Physiol Heart Circ Physiol. 2009;296:H735–H744. doi: 10.1152/ajpheart.01309.2008. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–439. [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JR. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590:2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C. The Peroxisome Proliferator-activated Receptor gamma Coactivator 1alpha/beta (PGC-1) Coactivators Repress the Transcriptional Activity of NF-kappaB in Skeletal Muscle Cells. J Biol Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2009;297:E495–E504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, LeBrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007a;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007b;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Apple FS, Sjodin B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Appl Physiol. 1996;81:1484–1487. doi: 10.1152/jappl.1996.81.4.1484. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazen W, M’bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579:5631–5634. doi: 10.1016/j.febslet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol. 2005;95:351–360. doi: 10.1007/s00421-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013 doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-ishi S, Kizaki T, Nagasawa J, Izawa T, Komabayashi T, Nagata N, Suzuki K, Taniguchi N, Ohno H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin Exp Pharmacol Physiol. 1997;24:326–332. doi: 10.1111/j.1440-1681.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Olesen J, Larsson S, Iversen N, Yousafzai S, Hellsten Y, Pilegaard H. Skeletal muscle PGC-1alpha is required for maintaining an acute LPS-induced TNFalpha response. PLoS One. 2012;7:e32222. doi: 10.1371/journal.pone.0032222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le CD, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de CR. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Bruunsgaard H, Ostrowski K, Krabbe K, Hansen H, Krzywkowski K, Toft A, Sondergaard SR, Petersen EW, Ibfelt T, Schjerling P. Cytokines in aging and exercise. Int J Sports Med. 2000;21(Suppl 1):S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M, Krogh-Madsen R, Erikstrup C, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Associations between insulin resistance and TNF-alpha in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia. 2007;50:2562–2571. doi: 10.1007/s00125-007-0834-6. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-Dose Resveratrol Supplementation in Obese Men: An Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes. 2012 doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholm S, Olesen J, Pedersen JT, Brandt CT, Halling JF, Hellsten Y, Prats C, Pilegaard H. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1α. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.08.012. In pending review. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Koster A, Visser M. Adiposity, Muscle Mass, and Muscle Strength in Relation to Functional Decline in Older Persons. Epidemiol Rev. 2012 doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- Skrobuk P, von KS, Semenova MM, Zitting A, Koistinen HA. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia. 2012;55:3051–3060. doi: 10.1007/s00125-012-2691-1. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3:130–140. [PMC free article] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi FF, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]