Summary
SHetA2 is a small molecule flexible heteroarotinoid (Flex-Het) with promising cancer prevention and therapeutic activity. Extensive preclinical testing documented lack of SHetA2 toxicity at doses 25 to 150 fold above effective doses. Knowledge of the SHetA2 molecular target(s) that mediate(s) the mechanism of SHetA2 action is critical to appropriate design of clinical trials and improved analogs. The aim of this study was to develop a method to identify SHetA2 binding proteins in cancer cells. A known metabolite of SHetA2 that has a hydroxyl group available for attachment was synthesized and conjugated to a linker for attachment to a magnetic microsphere. SHetA2-conjugated magnetic microspheres and unconjugated magnetic microspheres were separately incubated with aliquots of a whole cell protein extract from the A2780 human ovarian cancer cell line. After washing away non-specifically bound proteins with the protein extraction buffer, SHetA2-binding proteins were eluted with an excess of free SHetA2. In two independent experiments, an SDS gel band of about 72 kDa was present at differential levels in wells of eluent from SHetA2-microspheres in comparison to wells of eluent from unconjugated microspheres. Mass spectrometry analysis of the bands (QStar) and straight eluents (Orbitrap) identified mortalin (HSPA9) to be present in the eluent from SHetA2-microspheres and not in eluent from unconjugated microspheres. Co-immunoprecipitation experiments demonstrated that SHetA2 interfered with mortalin binding to p53 and p66 Src homologous-collagen homologue (p66shc) inside cancer cells. Mortalin and SHetA2 conflictingly regulate the same molecules involved in mitochondria-mediated intrinsic apoptosis. The results validate the power of this protocol for revealing drug targets.
Electronic supplementary material
The online version of this article (doi:10.1007/s10637-013-0041-x) contains supplementary material, which is available to authorized users.
Keywords: Mortalin, HSPA9, Heteroarotinoids, Ovarian cancer, p66shc, p53
Introduction
Advancement of investigational new drugs to clinical trials requires detailed knowledge of the pharmacokinetics, metabolism and toxicity to document that the compound has sufficient pharmaceutical qualities for clinical application. Knowledge of the mechanism can be used to select the appropriate patient population and to design improved analogs. Pre-clinical testing for our lead compound, a flexible heteroarotinoid (Flex-Het) called Sulfur Het A2 (SHetA2, NSC721689) demonstrated reasonable pharmacokinetics and lack of mutagenicity, carcinogenicity, teratogenicity and toxicity [1–5]. SHetA2 has a wide therapeutic window as indicated by the No Observed Adverse Effect Level (NOAEL) of >1,500 mg/kg/day identified in the 28 day dog toxicity model in comparison to the ability of 10 to 60 mg/kg/day to inhibit xenograft tumor growth [5–8]. This lack of in vivo toxicity, along with oral bioavailability and documented inhibition of colorectal tumorigenesis in the APC min/+ mouse model at 30 mg/kg given 5 days per week, make SHetA2 an ideal candidate for a cancer prevention drug [9]. SHetA2 sensitization of resistant cells to death receptor activating ligands offers promise for advancement of SHetA2 toward clinical trials as combination therapy with death receptor activating antibodies that are currently in clinical trials [10, 11]. Mechanistic studies identified that SHetA2 induces G1 cell cycle arrest through reduction of cyclin D1; induces intrinsic apoptosis through direct effects on mitochondria associated with reduction of Bcl-2; enhances death receptor activation of the extrinsic apoptosis pathway through repression of nuclear factor κB (NF-κB) and upregulation of the CAAT/enhancer binding protein homologous protein (CHOP) transcription factor; and induces autophagy and endoplasmic reticulum stress, however the direct mediators of these events remain to be elucidated [7, 12–14].
The mechanism of SHetA2 is independent of the nuclear retinoid receptors and retinoid toxicities, despite the emergence of this compound from a series of structure activity relationship (SAR) studies of retinoic acid receptor-active Hets [3, 6, 15, 16]. SHetA2 was derived from a Het backbone that was shown to have 1000-fold decreased in vivo toxicity in comparison to the parent arotinoid structure while retaining the ability to induce retinoid biological effects [6, 17]. The increased flexibility of the Flex-Hets was conferred by substituting the more-rigid two-atom linker with a more-flexible, three-atom urea or thiourea linker [16]. The Flex-Hets differ from the more conformationally-restricted heteroarotinoids in that they act independently of the retinoic acid receptors and are potent inducers of apoptosis in cancer cells while not harming normal cells [3, 7, 10, 13, 16, 18]. The potent and differential apoptosis-inducing activity and lack of retinoid and other toxicities of SHetA2 offer a dramatic improvement in the therapeutic ratio over receptor active Hets. The purpose of this project was to identify SHetA2-binding proteins that may be responsible for mediating the mechanism by which SHetA2 kills ovarian cancer cells.
A variety of approaches have been used for target identification including direct biochemical methods, genetic interactions, and computational inference. Mass spectrometry analysis of drug binding proteins isolated by affinity chromatography is currently the most powerful and frequently-used technology; however, a generic workflow for this procedure has not been established due to the wide variation in the types of drugs and the affinity and expression levels of their targets [19]. The primary limitation of affinity chromatography is the need to derivatize the drug in order to attach it to a scaffold, because the derivative has high risk for losing the bioactivity of the parent compound [20]. Linkers between the drug and scaffold may be needed to avoid interference of the scaffold with protein binding to the attached drug. In this study, a known metabolite of SHetA2 was synthesized, which permitted attachment to a linker designed in such a way that allowed conjugation to a magnetic microsphere with a physical separation between the drug and microsphere. Identification of a SHetA2-binding protein using this affinity chromatography approach was validated by identification of the same protein in two types of mass spectrometry analyses and demonstration that treatment of cells with SHetA2 interferes with target protein binding to client proteins.
Materials and methods
Chemistry
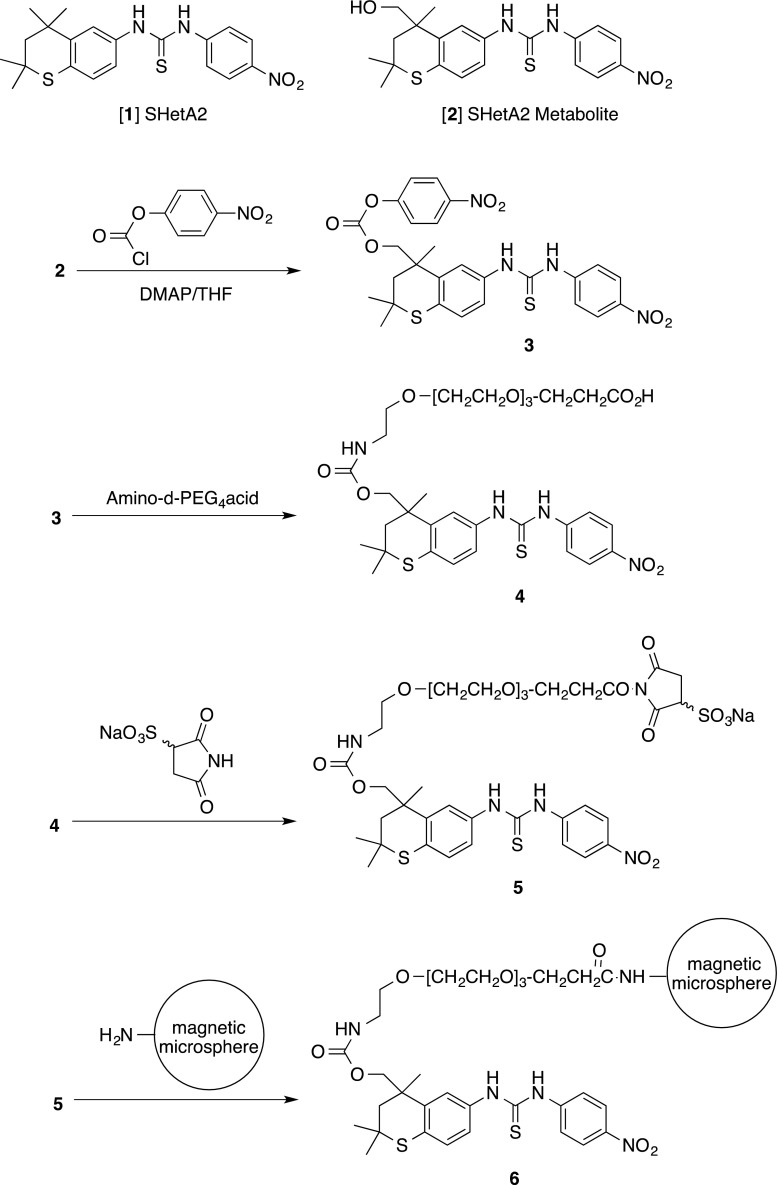
In order to attach compound SHetA2 [1] to a solid support while retaining the majority of the structure intact for protein binding, the known metabolite 2, which has a hydroxyl group available for attachment, was synthesized as described [21] (Structures of 1 and 2 are shown in Fig. 1). The SHetA2 metabolite 2 was then further modified as shown in Fig. 1. Amino-dPEG4 acid was purchased from Quanta Biodesign Limited, Powell, OH 43065, and used as received. Tetrahydrofuran was dried over potassium hydroxide pellets and distilled from lithium aluminium hydride prior to use. Dichloromethane was used from a freshly opened bottle. All reactions were run under dry nitrogen in oven-dried glassware. The reaction was monitored by thin layer chromatography (TLC) on silica gel GF plates (Analtech No 21521). Purification was performed using preparative thin layer chromatography (PTLC) on a 20-cm × 20-cm silica gel GF plate (Analtech No 02015). Band elution for both methods was monitored using a hand-held UV lamp. The infrared (IR) spectrum was run as a thin film on sodium chloride disks. 1H and 13C nuclear magnetic resonance (NMR) spectra were measured in deuteriochloroform at 300 MHz and 75 MHz, respectively, and were referenced to internal tetramethylsilane; coupling constants (J) were reported in Hz. The ultraviolet–visible (UV–vis) spectrum was collected for the sample using a Varian Cary 5000 spectrophotometer. The mass spectrum was collected for the sample using a Shimadzu LC-MS instrument.
Fig. 1.
Synthesis scheme for SHetA2 conjugated magnetic microspheres
A 25-mL, three-necked, round-bottomed flask, equipped with a reflux condenser and a magnetic stirrer was charged with 100 mg (0.24 mmol) of metabolite 2 and 5 mL of tetrahydrofuran. The solution was cooled to −40 °C, 29 mg (0.24 mmol) of 4-(dimethylamino)pyridine was added. The mixture was stirred for 5 min to dissolve the solid. To the resulting yellow solution was added dropwise a solution of 48 mg (0.239 mmol) of 4-nitrophenyl chloroformate in 1 mL of tetrahydrofuran, and the reaction mixture was allowed to stir for 30 min. At this time, TLC eluted with ether:hexane (4:1) confirmed that the reaction was complete (and formed 3).
A solution of exactly 63 mg (0.24 mmol) of amino-dPEG 4-acid in 3 mL of dichloromethane was carefully added to the above reaction mixture containing 3 at −40 °C over a period of 10 min. The reaction mixture was then slowly allowed to warm to room temperature over a period of 1 h. The reaction mixture then was stirred for 20 min, and concentrated under vacuum. Purification was achieved by PTLC eluted with 98:2 chloroform:methanol. After one elution, the product was washed from the silica gel using ethyl acetate followed by chloroform:methanol (95:5) to give 33 mg (20 %) of conjugate 4 as a pale yellow liquid. LCMS: (M+) 732 (Na+ adduct), 418; IR: 3375, 1740, 1640, 1592, 1515, 1475, 1335, 1110 cm−1; 1H NMR: 8.94 (s, 1 H), 8.76 (s, 1 H), 8.19 (d, J = 9.0 Hz, 2 H), 7.85 (obscured m, 1 H), 7.84 (d, J = 9.0 Hz, 2 H), 7.45 (s, 1 H), 7.13 (d, J = 8.2 Hz, 1 H), 5.46 (brt, J = ca 6.2 Hz, l H), 4.53 and 4.42 (2 AB patterns, J = 11.0 Hz, 1 H), 3.76 (t, J = 5.5 Hz, 2 H), 3.63 (m, 13 H), 3.40 (m, 2 H), 3.22 (m, 2 H), 2.60 (t, J = 5.5 Hz, 2 H), 2.36 (d, J = 14.2 Hz, 1 H), 1.83 (d, J = 14.2 Hz, 1 H), 1.43 (s, 6 H), 1.40 (s, 3 H); UV–vis (H3COH): ƛmax = 285 nm (ε = 1752) and 335 nm (ε = 1456). The 13C NMR experiment could not be carried out due to the instability of the product. The product decomposed rapidly at 20 °C. Thus, the polyether was attached to the microspheres without further purification.
Only a small quantity of conjugate 4 was obtained, and it was transferred in dry ice to SoluLink (San Diego, CA). Compound 4 was converted to compound 5 by a proprietary method of SoluLink who provided a mass spectrum of the compound as MW - Na + 984, the primary m/z occurring at 884.20. Compound 5 was reacted with the magnetic microspheres through an amide linkage. This led to conjugate 6 attached to the NanoLink Amino-Magnetic Microspheres which were utilized to identify SHetA2 binding proteins.
Isolation of SHetA2 binding proteins
Protein extracts of the A2780 human ovarian cancer cell line grown to 90 % confluency in 10 cm plates were isolated using m-PER-PPI (m-PER solution [Thermo Scientific] containing a protease inhibitor cocktail [Active Motif] and a phosphatase inhibitor cocktail [Active Motif]) and stored at −80 °C until use. The protein concentration of the extracts was measured with the BCA Protein Assay (Thermo Scientific). SHetA2-conjugated NanoLink Amino-Magnetic Microspheres 6 were pelleted with a magnet for 2 min, and the supernatant was removed. The pellet was washed once in 100 μL PBS and re-suspended in 100 μL of m-PER-PPI. Unconjugated NanoLink Amino-Magnetic Microspheres were manipulated under the same conditions in parallel as a negative control. The general experimental procedure is as follows with specific modifications detailed in the subsequent paragraphs and Table 1. Equal volumes of the SHetA2- and unconjugated-microsphere suspensions were pelleted and re-suspended in A2780 protein extract and incubated. The microspheres were pelleted with a magnet and washed one to three times with m-PER-PPI at a volume equal to the incubation volume. Bound proteins were then eluted with excess free SHetA2 in m-PER-PPI, followed by removal of the microspheres with a magnet. Aliquots of the washes and eluents of the SHetA2- and unconjugated microspheres were electrophoresed into 2-D SDS-PAGE gels. Four repeats of the experiment were performed in efforts to optimize differential bands observed in the SDS gel wells corresponding to SHetA2-microspheres in comparison to unconjugated microspheres.
Table 1.
Experimental Variables Used in Optimization
| Protein/SHetA2 ratio | Protein/Beads Ratio | Incubation Conditions | W | Eluent Conditions | Result | |
|---|---|---|---|---|---|---|
| 1 |
1360:1 6.8 mg: 5 μg |
1:1 100 μL:100 μL |
rt, 30 min | 3 |
10 μM SHetA2 rt, 10 min |
No differential bands |
| 2 |
720:1 1.8 mg: 2.5 μg |
1:1 50 μL:50 μL |
37 °C, 30 min → rt, 5 min | 1 |
1 mM SHetA2 37 °C, 30 min |
72 kDa specific banda |
| 3 |
104:1 2.6 mg: 25 μg |
1:100 50 μL:500 μL |
37 °C, rt, 30 min → rt, 5 min | 1 |
1 mM SHetA2 rt, 30 min |
No Differential bands |
| 4 |
72.7:1 10 mg: 13.75 μg |
1:1.375 200 μL:275 μL |
rt, 5 min → 37 °C, 25 min | 2 |
1 mM SHetA2 37 °C, 30 min |
Differential 72 kDa band b |
aProteins in silver stain-detected band could not be detected with Coomassie Blue or QStar, but proteins in free eluent could be detected with Orbitrap
bBand and eluent were successfully evaluated by Mass Spec using QStar and Orbitrap, respectively. Specific band was present at a higher level than the non-specific band in Coomassie stained SDS-gel
W = Number of washes, Beads = Microspheres
Bolded rows were used in Mass Spectrometry Analyses
In experiment 1, 100 μL of A2780 protein extract (6.8 mg total) was mixed with a volume of 100 μL of SHetA2 microspheres (containing approximately 5 μg or 12.45 nM SHetA2) from the starting solution and incubated at room temperature in the dark with agitation for 30 min. After 3 washes, bound proteins were eluted by incubating the microspheres in 10 μM SHetA2 for 10 min with agitation in the dark. Differential bands in the lanes corresponding to SHetA2 eluents from the SHetA2- and unconjugated microspheres could not be visualized upon staining the SDS-PAGE gel with Coomassie Blue.
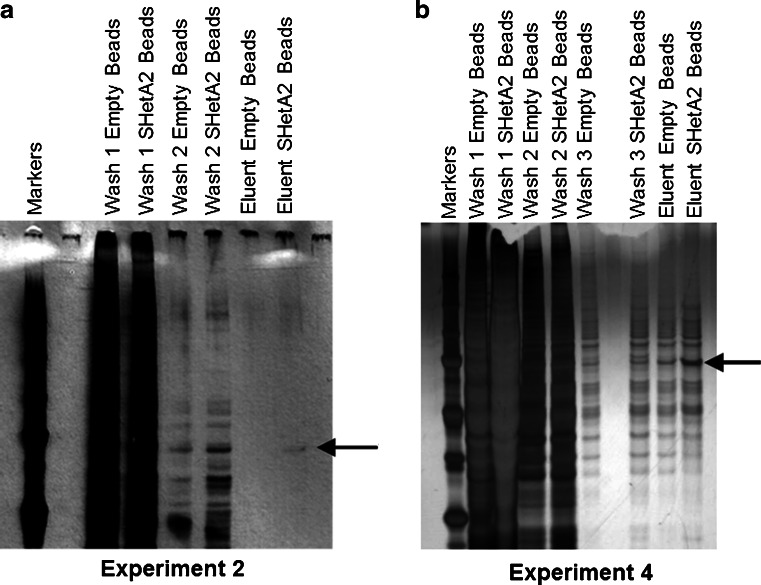
Experiment 2 was designed to reduce the non-specific binding in SDS-gel bands corresponding to unconjugated microspheres by decreasing the amount of protein added and to increase the specific binding in SDS-gel bands corresponding to SHetA2-microspheres by increasing the incubation temperature. Because a limited amount of microspheres was available, both parameters were altered simultaneously. In experiment 2, 50 μL of A2780 protein extract (1.8 mg) was incubated with the SHetA2- or unconjugated magnetic microspheres at 37 °C for 30 min in the dark without agitation, followed by 5 min of slow agitation at room temperature in the dark. After one wash, bound proteins were eluted by incubating the microspheres with 1 mM SHetA2 in 20 μL of m-PER-PPI for 30 min at 37 °C in the dark. Again no differential bands were observed between the lanes corresponding to the SHetA2- and unconjugated microspheres eluents when the gel was stained with Coomassi blue; however, a differential band was observed upon staining of a repeat gel with the Plus One DNA Silver Staining Kit (Amersham Biosciences) (Fig. 2a).
Fig. 2.
SDS Gels. a Silver-stained SDS-gel of aliquots of the indicated washes and eluents from experiment 2. b Coomassie Blue-stained SDS-gel of SDS-G of aliquots of the indicated washes and eluents from experiment 4. The arrow indicates the location in the dried gel where bands were excised from the last two lanes for QStar Mass Spectrometry Analysis. Beads = Microspheres
Experiment 3 was designed to increase the yield of this specific band to levels that could be evaluated by mass spectrometry. Based on the presumption that the SHetA2 attached to the microspheres was the limiting factor, the ratio of protein to microspheres was altered to 1:100 by using a volume of 50 μL of A2780 protein extract containing 2.6 mg of protein and incubating with 500 μL of unconjugated microspheres or SHetA2-microspheres (25 μg or 62.25 nM SHetA2). The extract was incubated and eluted as described for experiment 2; however, the levels of proteins present in the SDS-PAGE gel lanes corresponding to SHetA2- and unconjugated microspheres were too filled with non-specific binding proteins to reveal a differential band.
Experiment 4 was designed to increase the yield by increasing the amount of protein added and to reduce the non-specific background binding by increasing the number of washes. Thus, 275 μL of SHetA2-microspheres (13.75 μg or 34.24 nM SHetA2) or unconjugated-microspheres were mixed with 200 μL of A2780 protein extract (approximately 10 μg), agitated for 5 min in the dark at room temperature and then incubated without agitation at 37 °C for 25 min in the dark. Two washes with 300 μL of m-PER-PPI were performed, followed by an elution step with 30 μL of 1 mM SHetA2 in m-PER-PPI for 5 min at 37 °C. A differential band was discerned between the lanes corresponding to the SHetA2- and unconjugated microspheres upon Coomassie blue staining of the SDS-PAGE gel; however, the non-specific binding was very high (Fig. 2b).
Efforts to regenerate and re-use the SHetA2-microspheres were unsuccessful. The specific bands in experiments 2 and 4 were evaluated by QStar Mass spectrometry and frozen aliquots of the eluents were thawed and evaluated by Orbitrap Mass spectrometry.
Mass spectrometry analysis of bands excised from SDS-PAGE gels
Identification of proteins that bind to the SHetA2 affinity resin was determined by a standard proteomics analysis as briefly described below. The protein bands were excised and cut into small pieces and destained with 5 mM sodium thiosulfate/15 mM potassium ferricyanide in water. After rinsing with 25 mM ammonium bicarbonate in 50 % acetonitrile, the proteins in the gel were reduced in 55 mM tris[2-carboxylethyl]phosphine in 25 mM ammonium bicarbonate at 60 °C for 10 min, followed by alkylation in 100 mM iodoacetoamide/25 mM ammonium bicarbonate at room temperature for 60 min. After washing the alkylation buffer, the gel pieces were washed in 50 % acetonitrile, followed by 100 % acetonitrile. In-gel tryptic digestion was conducted by adding 100 ng of trypsin (Sequencing Grade Modified Trypsin; Promega, Madison, WI) in 10 μL of 25 mM ammonium bicarbonate. The gel pieces were allowed to swell, and an additional 25 μL of 25 mM ammonium bicarbonate was added to submerge the gel pieces. The digestion reaction was incubated at 30 °C for 16 hrs. Twenty-five microliters of 1 % trifluoroacetic acid was added to stop the digestion reaction, and the tryptic peptides were extracted into 50 % acetonitrile/0.5 % trifluoroacetic acid. The extract was evaporated by a SpeedVac concentrator (Thermo Electron Corporation, Waltham, MA). All water used was an ultra-pure grade. A Dionex UltiMate 3000 HPLC interfaced to an ABI Sciex QStar Elite Hybrid Quadrupole TOF mass spectrometer was used to analyze the tryptic digests by peptide mass fingerprinting. MASCOT (Matrix Science, Boston, MA) analysis of the MS/MS data against NCBInr 20100405 identified three candidates that show Probability Based MOWSE Score higher than 41 (p < 0.05); gi|6470150 (Homo sapiens BiP protein; MOWSE Score, 1202), gi|5729877 (Homo sapiens heat shock cognate 71 kDa protein isoform 1; MOWSE Score, 1030), and gi|292059 (Homo sapiens MTHSP75; MOWSE Score, 423).
Shotgun-mass spectrometry analysis
Aliquots of eluents from the SHetA2-microspheres and unconjugated microspheres were submitted for mass spectrometry analysis without electrophoresing them into gels. MS-grade solvents were from Burdick and Jackson, or Baker. Sequencing grade trypsin was from Promega. Other solutions were the highest grade available from Sigma-Aldrich. Samples were dissolved in 8 M urea, 100 mM Tris–HCl pH = 8.5 at RT, 5 mM tris(2-carboxyethyl)phosphine, and reduced at room temperature for 20 min. After incubation, 1/20th volume of 200 mM iodoacetamide was added. The alkylation was allowed to proceed for 15 min in the dark at room temperature, after which the samples were diluted with four volumes of 100 mM TrisHCl pH 8.5 and digested with 4 μg/mL trypsin overnight at 37 °C. Digested samples were acidified with 1 % formic acid, and purified by tip-based C18 chromatography (OMIX tips from Agilent). Samples were analyzed on a hybrid LTQ-OrbitrapXL mass spectrometer (Thermo Fisher Scientific) coupled to a New Objectives PV-550 nanoelectrospray ion source and an Eksigent NanoLC-2D chromatography system.
Peptides were analyzed by trapping on a 2.5 cm pre-column, followed by analytical separation on a 15–20 cm 75 μm ID fused silica column, both packed with Magic C18 AQ (Bruker). Columns were terminated with an integral fused silica emitter prepared in house. Peptides were eluted using a 5–40 % ACN/0.1 % formic acid gradient performed over 116 min at a flow rate of 250–300 nL/min. For each full-range Fourier transform mass spectrometry (FT-MS) scan (nominal resolution of 60,000), the six most intense ions were analyzed via data-dependent MS/MS in the linear ion trap using dynamic exclusion for 150 % of the observed chromatographic peak width. MS/MS settings used a trigger threshold of 8,000 counts, monoisotopic precursor selection (MIPS), and rejection of parent ions that had unassigned charge states or were previously identified as contaminants. Centroided ion masses were extracted using the extract_msn.exe utility from Bioworks 3.3.1 and were used for database searching with MASCOT v2.2.04 (Matrix Science) and X Tandem v2007.01.01.1 (www.thegpm.org).
Searches utilized a local database of human sequences, as well as sequences for 114 common adventitious laboratory contaminants. Trypsinolytic parent ions were searched with a parent ion tolerance of 10 ppm, and fragment ion masses were searched with a mass tolerance of 0.8 Da. Variable modifications included modification of cysteine by iodoacetamide or acrylamide, oxidation of methionine, N-terminal peptide cyclization via pyroglutamate or S-carbamoylmethylcysteine, and N-terminal protein modification by formylation or acetylation.
Scaffold (Version 3, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95 % probability as specified by the Peptide Prophet algorithm [22]. Protein identifications were accepted if they could be established at greater than 99.0 % probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm [23]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. A t-test was performed to determine significant differences in proteins identified in the eluents from SHetA2-microspheres in comparison to the eluents from unconjugated microspheres. P values of less than 0.05 were considered to be statistically significant.
Cell culture and co-immunoprecipation experiments
The A2780 human ovarian cancer cell line (gift of Michael Birrer, Harvard Medical School, Boston, MA) was cultured in RPMI 1640 tissue culture medium supplemented with 10 % fetal bovine serum (FBS), antibiotic/antimycotic, 1 nM sodium pyruvate and 1 mM HEPES buffer. Whole cell protein extracts were prepared from cultures treated with SHetA2 or DMSO for various times using M-PER Mammalian Protein Extraction Reagent (Thermo Scientific) or Triton × 100 lysis buffer consisting of 1 % Triton × 100, 10 mM Tris pH 7.4, 5 mM EDTA pH 8.0, and 50 mM NaCl. Cells were incubated and agitated by pipette or vortex every 5 min for 30 min. After incubation, samples were spun down for 3 min at 10,000 g to remove debris. Cell lysates were centrifuged to remove debris for 5 min at 3,000 g using a 1:100 ratio of anti-p66shc antibody (Santa Cruz, Cat # sc-967), anti-p53 antibody (Santa Cruz, Cat # sc-126) or anti-mortalin antibody (Cell signaling Cat # 3593) to lysate was added to each lysate immediately and incubated at 4 °C overnight. The next day, cells were incubated with Protein G-PLUS Agarose microspheres (Santa Cruz) for 1 hr. Microspheres were then washed 3 times with 1 mL of 10 % NP-40/Tris pH 8.0 buffer and centrifuged at 3,000 ×g for 4 min. Twenty microliters of SDS loading buffer was then added to each sample, and the solution was boiled for 5 min before being electrophoresed into a 12–15 % sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane, blocked in 5 % milk for 1 hr at room temperature, and then immunoblotted with the desired primary antibody (anti-mortalin antibody (Cell signaling Cat # 3593), anti-p53 antibody (Santa Cruz, Cat # sc-126) or anti-p66shc antibody (Santa Cruz, Cat # sc-967) and then incubated overnight at 4 °C. The membranes were then washed 3 times with 10 mL of PBS-Tween 20 (0.1 %), incubated with the appropriate HRP-conjugated secondary antibody for 30 min at room temperature and twashed 3 times with 10 mL of PBS-Tween 20 (0.1 %). Finally, specifically bound antibody was detected using Western Blotting Luminol Reagent (Santa Cruz Biotechnology) and exposure to X-Ray negative film.
Results
Identification of mortalin as an SHetA2-binding protein
To identify SHetA2 binding proteins, whole cell protein extracts isolated from the human A2780 ovarian cancer cell line were incubated with NanoLink Amino-Magnetic Microspheres conjugated to SHetA2. Unconjugated NanoLink Amino-Magnetic Microspheres microspheres were used as a control for non-specific binding. Solutions obtained through washing the microspheres and eluting with excess SHetA2 were evaluated on SDS-PAGE gels. Conditions were modified in 4 independent experiments to optimize the specificity and yield of proteins that bound to the SHetA2 microspheres (Table 1). In the second experiment, a band of approximately 75 kDa was observed in the lane corresponding to the SHetA2 microsphere eluent and not in the lane corresponding to the unconjugated microsphere eluent in a silver-stained SDS-PAGE gel (Fig. 2a). The amount of protein present in the gel however was insufficient for detection by the less-sensitive Coomassie blue stain or QTrap mass spectrometry. By increasing the microsphere to protein extract ratio and the number of washing steps, conditions were optimized to enable a sufficient amount of protein differentially bound to the SHetA2-microspheres compared to the unconjugated microspheres that could be detected in an SDS-gel stained with Coomassie blue (Fig. 2b). The areas of the dried gels for experiments 2 and 4 corresponding to the differential 75 kDa band in the lanes corresponding to the SHetA2- and unconjugated microsphere eluents were excised and subjected to QStar mass spectrometry analysis. Although no proteins could be detected in the bands from experiment 2, three related heat shock protein A (HSPA) family members, HSPA5, HSPA8 and HSPA9/mortalin, were identified to be present in the band from the SHetA2-microspheres and not in the band from the unconjugated-microspheres from experiment 4 (Table 2).
Table 2.
QStar Analysis of isolated 72 kDa band of SHetA2 Binding Proteins
| Hit | Accession Number | MOWSE Score | Mass | Avgerage Intensity | Name: alias’ |
|---|---|---|---|---|---|
| 2 | gi|5729877 | 1030 | 71082.81 | 628.0486 | HSPA8: Hsc70, 2410008N15Rik, Heat Shock Cognate 71-KDA, Heat Shock Cognate 8, HS7C, HSC54, HSC71, HSC73, HSP/C70, HSP70, HSP71, HSP73, Hsp 8, HSPA10, LAP1, MGC102007, MGC106514, MGC114311, MGC118485, MGC128130, MGC131511, MGC29929, NIP71 |
| 3 | gi|292059 | 423 | 74019.46 | 409.2786 | mortalin: Mortalin, Mitochondrial stress-70, Glucose Regulated Protein 75, GRP-75, 74 kDa, CSA, Hsc74, Hsp74, Hsp74a, Mortalina, mortalinB, MGC4500,, MOT, Mot1, MOT2, Mthsp70, MTHSP75, PBP74, Stress-70 |
| 1 | gi|6470150 | 1202 | 71002.07 | 312.8802 | HSPA 5:Glucose Regulated Protein 78, GRP78, BiP, 78 kDa, AL022860, AU019543, BIP, D2Wsu141e, D2Wsu17e, FLJ26106,, HEAT SHOCK 70KDA PROTEIN5, Hsce70, HSP70-5, Immunoglobulin heavy chain binding, mBiP, MIF2, SEZ-7 |
To further validate the identification of these proteins, aliquots of the eluents from the SHetA2- and unconjugated microspheres from experiments 2 and 4 were evaluated by “Shotgun” Orbitrap Mass spectrometry analysis. Twenty-five individual proteins were identified to be present at significantly different levels in the SHetA2-microsphere eluent compared to the unconjugated-microsphere eluent in experiment 2 as determined by a t-test with p values below 0.05 being considered significant (Table 3). Among these proteins, all three HSPA5, HSPA8 and mortalin proteins, which were identified in the previous QStar analysis, also were found to be differentially present in the eluent from the SHetA2-microspheres in comparison to the eluent from the unconjugated microspheres. Because experiment 4 had a much higher background of non-specifically bound proteins, it was not surprising that a much higher number (224) of individual proteins, were identified to be differentially present by using the identical analysis procedure (Supplemental Table 1). Among these proteins, mortalin, (with a p value of 0.00031 in Supplemental Table 1) was among the significant proteins in both experiments 2 and 4, while HSPA5 and HSPA8 were found to be significant in experiment 2, but had p-values slightly above 0.05 in experiment 4 (listed at the bottom of Supplemental Table 1). Thus, in two independent experiments evaluated by two different proteomics approaches, mortalin was identified to be specifically bound by SHetA2.
Table 3.
Orbitrap analysis of SHetA2-microsphere eluent from experiment 2
| Identified Protein | Accession # | MS | p-value | |
|---|---|---|---|---|
| 1 | Heterogeneous nuclear ribonucleoprotein G | IPI00304692 | 42 kDa | 0.00007 |
| 2 | Uncharacterized protein | IPI01021093 (+4) | 33 kDa | 0.00015 |
| 3 | Endoplasmin | IPI00027230 | 92 kDa | 0.00015 |
| 4 | ATP synthase subunit beta, mitochondrial | IPI00303476 (+1) | 57 kDa | 0.00063 |
| 5 | Insulin-like growth factor 2 mRNA-binding protein 1 | IPI00008557 | 63 kDa | 0.00078 |
| 6 | 30 kDa protein | IPI00964648 (+8) | 30 kDa | 0.0015 |
| 7 | Keratin, type I cytoskeletal 17 | IPI00450768 | 48 kDa | 0.0033 |
| 8 | Nucleolin | IPI00604620 | 77 kDa | 0.0043 |
| 9 | ATP synthase subunit alpha, mitochondrial | IPI00440493 | 60 kDa | 0.0052 |
| 10 | Isoform B1 of Heterogeneous nuclear ribonucleoproteins A2/B1 | IPI00396378 | 37 kDa | 0.0061 |
| 11 | cDNA FLJ55072, highly similar to Succinate dehydrogenase (ubiquinone) flavoprotein subunit, mitochondrial | IPI00964764 | 67 kDa | 0.0065 |
| 12 | Isoform 3 of Heterogeneous nuclear ribonucleoprotein K | IPI00807545 (+2) | 49 kDa | 0.0072 |
| 13 | Heat shock protein HSP 90-beta | IPI00414676 | 83 kDa | 0.008 |
| 14 | cDNA FLJ57283, highly similar to Actin, cytoplasmic 2 | IPI00930226 (+3) | 40 kDa | 0.0084 |
| 15 | Keratin, type I cytoskeletal 16 | IPI00217963 | 51 kDa | 0.0092 |
| 16 | Peroxiredoxin-1 | IPI00000874 (+1) | 22 kDa | 0.016 |
| 17 | X-ray repair cross-complementing protein 6 | IPI00644712 (+2) | 70 kDa | 0.016 |
| 18 | Glyceraldehyde-3-phosphate dehydrogenase | IPI00219018 (+2) | 36 kDa | 0.016 |
| 19 | Isoform 1 of Polyadenylate-binding protein 1 | IPI00008524 (+2) | 71 kDa | 0.019 |
| 20 | Putative cytochrome b-c1 complex subunit Rieske-like protein 1 | IPI00889196 (+1) | 31 kDa | 0.019 |
| 21 | Vimentin | IPI00418471 | 54 kDa | 0.021 |
| 22 | HSPA5/78 kDa glucose-regulated protein | IPI00003362 | 72 kDa | 0.023 |
| 23 | Isoform 2 of Heterogeneous nuclear ribonucleoprotein A/B | IPI00334587 (+4) | 36 kDa | 0.025 |
| 24 | mortalin/Stress-70 protein, mitochondrial | IPI00007765 (+1) | 74 kDa | 0.034 |
| 25 | HSPA8/Isoform 1 of Heat shock cognate 71 kDa protein | IPI00003865 (+1) | 71 kDa | 0.042 |
Validation of SHetA2 effects on mortalin
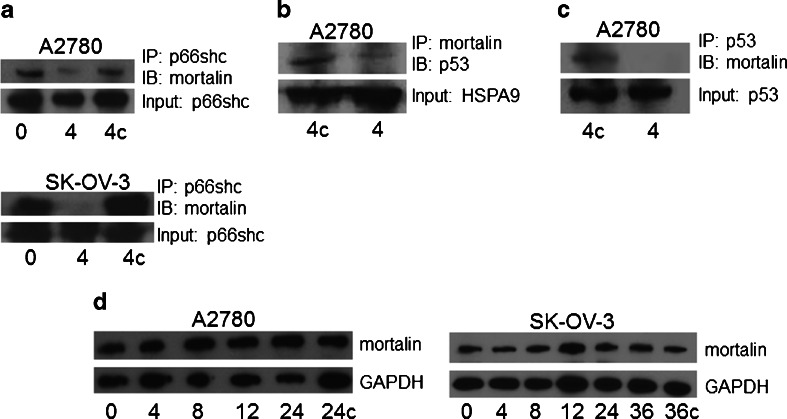
Mortalin is a molecular chaperone that binds client proteins and supports their functional configuration and intracellular localization [24]. To determine if SHetA2 interferes with mortalin binding to client proteins in situ, the ability of mortalin protein to be co-immunoprecipitated with its client proteins was evaluated in protein extracts from the A2780 and SK-OV-3 human ovarian cancer cell lines treated with SHetA2 or control solvent for 4 hrs. Western blot analysis of co-immunoprecipitates of protein extracts from both cell lines demonstrated that an antibody to the mortalin client protein called p66shc can pull down mortalin protein in the untreated controls, but not in the SHetA2-treated cultures, indicating that SHetA2 disrupted the interaction of these two proteins inside the cell (Fig. 3a). Another mortalin client protein, p53, was found to co-immunoprecipitate with the anti-mortalin specific antibody in untreated control A2780 cultures, but not in the SHetA2-treated cultures indicating that SHetA2 disrupts the interaction of these two proteins inside cells also (Fig. 3b). To further confirm SHetA2 disruption of this interaction, the A2780 protein extracts were co-immunoprecipitated with an anti-p53 specific antibody. Western blot analysis confirmed that mortalin was co-immunoprecipitated with p53 in the control cultures, but not in the SHetA2-treated cultures (Fig. 3c). The SK-OV-3 cell line was not evaluated for mortalin/p53 interactions, because this cell line does not express p53. To verify that this effect was not due to a decrease in mortalin levels potentially caused by SHetA2 treatment, protein extracts from A2780 and SK-OV-3 cultures treated with SHetA2 or control solvent over a range of treatment times were evaluated by Western blot, which demonstrated similar levels of mortalin regardless of the treatment time (Fig. 3d).
Fig. 3.
SHetA2 disrupts mortalin binding to client proteins p66shc and p53. a. Protein extracts from cultures of the A2780 and SK-OV-3 human ovarian cancer cell lines that were either untreated (0), treated with 10 μM SHetA2 for 4 hrs (4) or treated with the same volume of DMSO solvent used to administer the SHetA2 for 4 hrs (4c) were incubated with an anti- p66shc antibody overnight. The next day, antibody/protein complexes were immunoprecipated with protein G microspheres and non-specific binding was washed away with a Tris buffer containing NP-40 detergent. The microspheres were boiled to remove the antibody and the samples were electrophoresed into an SDS-gel and transferred to a Western blot membrane which was probed with an antibody to mortalin to detect co-immunoprecipitation and with an antibody to p66shc to detect input. b The experiment was performed as for A, except that the immunoprecipitation antibody used was against mortalin and the Western blot antibody used recognized p53. c, The experiment was performed as for A, except that the immunoprecipitation antibody use was against p53 and the Western blot antibody used recognized mortalin. d Western blot of protein extracts from A2780 and SK-OV-3 cells treated with 10 μM SHetA2 for the number of hours indicated at the bottom of the gel or treated with the same volume of DMS0 solvent for 24 hrs (24c) or 36 hrs (36c). The blots were stripped and re-probed with an antibody that recognizes GAPDH as a protein loading control. These results are representative of at least three separate experiments. IP = immunoprecipitating antibody, IB = Immunoblotting antibody
Discussion
This work has demonstrated that SHetA2 conjugated magnetic microspheres in concert with mass spectrometry approaches can be used to identify SHetA2 binding proteins. In our review of the literature to this date, there is no other report of using drug conjugated microspheres to identify a drug binding protein. The validity of the identified mortalin protein as a SHetA2-binding protein was supported by the identification of mortalin in two different approaches: QStar analysis of excised SDS-Gel bands and Orbitrap analysis of aliquots of the whole microsphere eluents. Although QStar analysis could not detect proteins in SDS-gel bands that were too low for visualization by Coomassie blue staining, the higher level of a non-specific unconjugated microsphere band present when the experiment was scaled up to a Coomassie blue-detectable level did not interfere with the ability of mortalin to be identified as being differentially present in the specific over the non-specific bands. Biological validation of mortalin was demonstrated by SHetA2 interference with mortalin binding to client proteins in human ovarian cancer cell lines.
Inhibition of mortalin is a likely mechanism of the SHetA2 effects on mitochondria and apoptosis in cancer cells. Within 30 min of treating cancer cells, SHetA2 induces mitochondrial swelling and loss of mitochondrial membrane potential, leading to release of cytochrome c, generation of reactive oxygen species (ROS) and activation of the intrinsic apoptosis pathway [7, 13, 15, 16, 18, 25, 26]. Although mortalin can be found in the endoplasmic reticulum (ER), cytoplasmic vesicles and cytosol, the majority of the protein present in the cell is located within mitochondria [24]. Maintenance of mitochondrial membrane potential needed for electron transport chain function and ATP generation is dependent on mortalin interaction with a protein called p66shc [27]. Release of p66shc from mortalin has been shown to cause opening of mitochondrial pores, release of cytochrome c and ROS generation [28]. Overexpression of mortalin can reduce ROS and protect against ischemic injury in vitro and in vivo [29]. Thus, we hypothesize that release of p66shc from mortalin repression mediates the mechanism by which SHetA2 induces intrinsic apoptosis. Current experiments are testing the validity of this hypothesis. Many of the other proteins identified in the two mass spectrometry approaches are known to be localized to the mitochondria and involved in metabolism. The fact that such proteins were identified in both approaches suggests that they may have attached indirectly to the magnetic microspheres through their affinity to mortalin attached to SHetA2.
SHetA2 disruption of mortalin binding to p53 is hypothesized to contribute to the mechanism by which SHetA2 regulates transcription factors and apoptosis. SHetA2 induces expression of the CHOP protein leading to enhancement of the death receptor extrinsic apoptosis pathway [10, 11, 30]. Mortalin binds to and sequesters p53 from translocating to the nucleus where it acts as a transcription factor to induce multiple genes that mediate apoptosis [31–33]. One of these p53-induced transcription factors is the SHetA2-induced CHOP [34, 35]. CHOP expression could also be altered as a result of indirect effects of SHetA2 releasing p66shc, thereby allowing it to interact with signal transducer and activator of transcription 3 (STAT3) and Forkhead Box O3A (FOXO3) [36–40], both of which regulate CHOP expression [41, 42]. Thus, we hypothesize that SHetA2-induced release of p53 from mortalin repression could augment SHetA2 mediated apoptosis. The ability of SHetA2 to induce apoptosis in p53 null cell lines, such as SK-OV-3, suggests that SHetA2-induced release of p53 from mortalin repression can augment but is not required for SHetA2 apoptosis. The observation that mortalin binds and inhibits p53 induction of apoptosis in stressed cells, but not in weakly stressed or unstressed cells, could explain the differential induction of apoptosis by SHetA2 in cancer over normal cells [43].
Mortalin and SHetA2 also conflictingly regulate the Bcl-2 family of proteins. Upon activation, the pro-apoptotic Bax protein of this family undergoes a conformational change and migrates to the mitochondria where it forms a pore that allows release of cytochrome c and other pro-apoptotic factors. The anti-apoptotic Bcl-2 protein binds to Bax and prevents this pore formation. The ratio of Bax to Bcl-2 has been shown to be a key determinant that can drive the initiation of apoptosis. SHetA2 reduces the expression of Bcl-2, but not Bax in cancer cells in vitro [7, 13, 26] and tumors in vivo[9] leading to induction of the intrinsic apoptosis pathway, while mortalin can prevent reduction of Bcl-2 and conformational changes in Bax leading to inhibition of the intrinsic apoptosis pathway [44–46].
Conclusions
SHetA2 binding proteins, such as mortalin, can be identified by SHetA2-affinity chromatography combined with mass spectroscopic analysis leading to testable hypotheses regarding the SHetA2 molecular mechanism of action. SHetA2 interferes with mortalin binding to client proteins in ovarian cancer cells. Inhibition of mortalin interaction with client proteins represents a logical mechanism for SHetA2-induced apoptosis, a theory that will be tested in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(XLS 66.5 kb)
Acknowledgments
The research was supported by an Oklahoma State University grant for Technology Business Assessment Group Grant (KDB/RAB), a University of Oklahoma Health Sciences Center Vice President for Research Bridge Grant (DMB, AL), a Stephenson Cancer Center Gynecologic Cancer Program Grant (DMB, AL) and by the NIH EY017888 Grant (HM, AS). The QStar mass spectrometry analysis was performed by the Mass Spectrometry and Proteomics Facility at the University of Oklahoma Health Sciences Center. The Orbitrap mass spectrometry analyses were performed in the DNA/Protein Resource Facility at Oklahoma State University, using resources supported by the NSF MRI and EPSCoR programs (DBI/0722494). We thank Elangovan Thavathiru (University of Oklahoma Health Sciences Center) for technical assistance in working out the parameters for co-immunoprecipitation analysis.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
- CHOP
CAAT/enhancer binding protein homologous protein
- FBS
fetal bovine serum
- Flex-Het
flexible heteroarotinoid
- FOXO3A
forkhead box O3A
- FT-MS
Fourier transform mass spectrometry
- HSPA
heat shock protein A
- IR
infrared
- MIPS
monoisotopic precursor selection
- NF-κB
nuclear factor-κB
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- p66shc
p66 Src homologous-collagen homologue
- PTLC
preparative thin layer chromatography
- PVDS
polyvinylidene difluoride
- SAR
structure activity relationship
- SDS
sodium dodecyl sulfate-polyacrylamide
- SHetA2
sulfur heteroarotinoid A2
- STAT3
signal transducer and activator of transcription 3
- TLC
thin layer chromatograpy
- UV–vis
ultraviolet–visible
References
- 1.Zhang Y, Hua Y, Benbrook DM, Covey JM, Dai G, Liu Z, Chan KK. High performance liquid chromatographic analysis and preclinical pharmacokinetics of the heteroarotinoid antitumor agent, SHetA2. Cancer Chemother Pharmacol. 2006;58:561–569. doi: 10.1007/s00280-006-0211-z. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Zhang Y, Hua YF, Covey JM, Benbrook DM, Chan KK. Metabolism of a sulfur-containing heteroarotionoid antitumor agent, SHetA2, using liquid chromatography/tandem mass spectrometry. Rap Comm Mass Spec. 2008;22:3371–3381. doi: 10.1002/rcm.3744. [DOI] [PubMed] [Google Scholar]
- 3.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. PNAS USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doppalapudi RS, Riccio ES, Davis Z, Menda S, Wang A, Du N, Green C, Kopelovich L, Rao CV, Benbrook DM, Kapetanovic IM. Genotoxicity of the cancer chemopreventive drug candidates CP-31398, SHetA2, and phospho-ibuprofen. Mutat Res. 2012;746:78–88. doi: 10.1016/j.mrgentox.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabirov KK, Kapetanovic IM, Benbrook DM, Dinger N, Mankovskaya I, Zakharov A, Detrisac C, Pereira M, Martín-Jiménez T, Onua E, Banerjee A, van Breemen RB, Nikolić D, Chen L, Lyubimov AV. Oral toxicity and pharmacokinetic studies of SHetA2, a new chemopreventive agent, in rats and dogs. Drug Chem Toxicol. 2012;36:284–295. doi: 10.3109/01480545.2012.710632. [DOI] [PubMed] [Google Scholar]
- 6.Benbrook DM, Kamelle S, Guruswamy S, Lightfoot S, Rutledge T, Gould N, Hannafon B, Dunn ST, Berlin KD (2005) Flexible heteroarotinoids (Flex-Hets) exhibit improved therapeutic ratios as anti-cancer agents over retinoic acid receptor agonists. Inv New Drugs 23:417–428. doi:10.1007/s10637-005-2901-5 [DOI] [PubMed]
- 7.Liu T, Masamha CP, Chengedza S, Berlin KD, Lightfoot S, He F, Benbrook DM. Development of flexible-heteroarotinoids for kidney cancer. Mol Cancer Ther. 2009;8(5):1227–1238. doi: 10.1158/1535-7163.MCT-08-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naylor M, Thompson DM, Lightfoot S, Benbrook DM. Anti-cancer activities and interaction of imiquimod and Flex-Het, SHetA2, in melanoma and ovarian cancer. J Cancer Ther. 2013;4:7–19. doi: 10.4236/jct.2013.46A1002. [DOI] [Google Scholar]
- 9.Benbrook DM, Guruswamy S, Wang Y, Sun Z, Mohammed A, Zhang Y, Li Q, Rao CV. Chemoprevention of colon and small intestinal tumorigenesis in APCmin/+ Mice By SHetA2 (NSC721689) without toxicity. Cancer Prev Res. 2013;6:908–916. doi: 10.1158/1940-6207.CAPR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moxley KC, Chengedza S, Benbrook DM (2009) Induction of death receptor ligand-mediated apoptosis in epithelial ovarian carcinoma: the search for sensitizing agents. Gyn Onc 115:438–442. doi:10.1016/j.ygyno.2009.09.007 [DOI] [PMC free article] [PubMed]
- 11.Chengedza S, Benbrook DM. NF-kB is involved in SHetA2 circumvention of TNF-a resistance, but not induction of intrinsic apoptosis. Anti-Cancer Drugs. 2010;21:297–305. doi: 10.1097/CAD.0b013e3283350e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69:6565–6572. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 13.Liu T-Z, Hannafon B, Gill L, Kelly B, Benbrook DM. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol Cancer Ther. 2007;6:1814–1822. doi: 10.1158/1535-7163.MCT-06-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long A, Masamha CP, Zhao D, Benbrook DM (2013) Differential roles of autophagy in ovarian cancer and healthy cells in response to SHetA2 Drug. Cell Death Dis:publication pending review of revision
- 15.Chun K-H, Benbrook DM, Berlin KD, Hong WK, Lotan R. Induction of apoptosis in head and neck squamous cell carcinoma (HNSCC) cell lines by heteroarotinoids through a mitochondrial dependent pathway. Cancer Res. 2003;63:3826–3832. [PubMed] [Google Scholar]
- 16.Liu S, Brown CW, Berlin KD, Dhar A, Guruswamy S, Brown D, Gardner GJ, Birrer MJ, Benbrook DM. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J Med Chem. 2004;47:999–1007. doi: 10.1021/jm030346v. [DOI] [PubMed] [Google Scholar]
- 17.Benbrook DM, Madler MM, Spruce LW, Birckbichler PJ, Nelson EC, Subramanian S, Weerasekare GM, Gale JB, Patterson MK, Jr, Wang B, Wang W, Lu S, Rowland TC, DiSilvestro P, Lindamood C, III, Hill DL, Berlin KD. Biologically active heteroarotinoids exhibit anticancer activity and decreased toxicity. J Med Chem. 1997;40:3567–3583. doi: 10.1021/jm970196m. [DOI] [PubMed] [Google Scholar]
- 18.Guruswamy S, Lightfoot S, Gold M, Hassan R, Berlin KD, Ivey RT, Benbrook DM. Effects of retinoids on cancerous phenotype and apoptosis in organotypic culture of ovarian carcinoma. J Nat Cancer Inst. 2001;93:516–525. doi: 10.1093/jnci/93.7.516. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler S, Pries V, Hedberg C, Waldmann H. Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chemie Int Ed. 2013;52:2744–2792. doi: 10.1002/anie.201208749. [DOI] [PubMed] [Google Scholar]
- 20.Lomenick B, Olsen RW, Huang J. Identification of direct protein targets of small molecules. ACS Chem Biol. 2010;6(1):34–46. doi: 10.1021/cb100294v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nammalwar B, Bunce RA, Benbrook DM, Lu T, Li H-F, Chen Y-D, Berlin KD. Synthesis of N-[3,4-dihydro-4-(acetoxymethyl)-2,2,4-trimethyl-2H-1-benzothiopyran-6-yl]-N'-(4-nitrophenyl)thiourea and N-[3,4-dihydro-4-(hydroxymethyl)-2,2,4-trimethyl-2H-1-benzothiopyran-6-yl]-N'-(4-nitrophenyl)thiourea, a major metabolite of N-(3,4-Dihydro-2,2,4,4-tetramethyl-2H-1-benzothiopyran-6-yl)-N'-(4-nitrophenyl)thiourea. Phosphorus Sulfur Silicon Rel Elem. 2011;186:189–204. doi: 10.1080/10426507.2010.534521. [DOI] [Google Scholar]
- 22.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 23.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 24.Londono C, Osorio C, Gama V, Alzate O. Mortalin, apoptosis, and neurodegeneration. Biomol. 2012;2:143–164. doi: 10.3390/biom2010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benbrook DM. Refining retinoids with heteroatoms. Minirev Med Chem. 2002;2:271–277. doi: 10.2174/1389557023406160. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Liu X, Yue P, Benbrook DM, Berlin KD, Khuri FR, Sun S-Y. Involvement of c-FLIP and survivin down-regulation in flexible heteroarotinoid-induced apoptosis and enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol Cancer Ther. 2008;7:3556–3565. doi: 10.1158/1535-7163.MCT-08-0648. [DOI] [PubMed] [Google Scholar]
- 27.Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chap. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2008;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y-D, Chen S, Yue P, Zou W, Benbrook DM, Liu S, Le TC, Berlin KD, Khuri FR, Sun S-Y. CAAT/enhancer binding protein homologous protein-dependent death receptor 5 induction is a major component of SHetA2-induced apoptosis in lung cancer cells. Cancer Res. 2008;68(13):5335–5344. doi: 10.1158/0008-5472.CAN-07-6209. [DOI] [PubMed] [Google Scholar]
- 31.Gestl EE, Anne Bottger S. Cytoplasmic sequestration of the tumor suppressor p53 by a heat shock protein 70 family member, mortalin, in human colorectal adenocarcinoma cell lines. Biochem Biophys Res Comm. 2012;423:411–416. doi: 10.1016/j.bbrc.2012.05.139. [DOI] [PubMed] [Google Scholar]
- 32.Grover A, Priyandoko D, Gao R, Shandilya A, Widodo N, Bisaria VS, Kaul SC, Wadhwa R, Sundar D. Withanone binds to mortalin and abrogates mortalin-p53 complex: computational and experimental evidence. Int J Biochem Cell Biol. 2012;44:496–504. doi: 10.1016/j.biocel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Lu WJ, Lee NP, Kaul SC, Lan F, Poon RT, Wadhwa R, Luk JM. Induction of mutant p53-dependent apoptosis in human hepatocellular carcinoma by targeting stress protein mortalin. Int J Cancer. 2011;129:1806–1814. doi: 10.1002/ijc.25857. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Laurell C, Selivanova G, Lundeberg J, Nilsson P, Wiman K. Hypoxia induces p53-dependent transactivation and Fas/CD95-dependent apoptosis. Cell Death Differ. 2007;14:411–421. doi: 10.1038/sj.cdd.4402022. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Lebedeva I, Sarka RD, Gopalkrishnan R, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher P. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 36.Skolnik E, Lee C, Batzer A, Vicentini L, Zhou M, Daly R, Myers M, Backer J, Ullrich A, White M. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderKuur J, Allevato G, Billestrup N, Norstedt G, Carter-Su C. Growth hormone-promoted tyrosyl phosphorylation of SHC proteins and SHC association with Grb2. J Biol Chem. 1995;270:7587–7593. doi: 10.1074/jbc.270.37.21738. [DOI] [PubMed] [Google Scholar]
- 38.Smit L, de Vries-Smits A, Bos J, Borst J. B cell antigen receptor stimulation induces formation of a Shc-Grb2 complex containing multiple tyrosine-phosphorylated proteins. J Biol Chem. 1994;269:20209–20212. [PubMed] [Google Scholar]
- 39.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Wang X, Zeng L, Cai D, Sabapathy K, Goff S, Firpo E, Li B. ERK phosphorylates p66shcA on Ser36 and subsequently regulates p27kip1 expression via the Akt-FOXO3a pathway: implication of p27kip1 in cell response to oxidative stress. Mol Biol Cell. 2005;16:3705–3718. doi: 10.1091/mbc.E05-04-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timofeeva O, Tarasova N, Zhang X, Chasovskikh S, Cheema A, Wang H, Brown M, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. PNAS USA. 2013;110:1267–1272. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greer E, Oskoui P, Banko M, Maniar J, Gygi M, Gygi S, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 43.Lu WJ, Lee NP, Kaul SC, Lan F, Poon RTP, Wadhwa R, Luk JM. Mortalin-p53 interaction in cancer cells is stress dependent and constitutes a selective target for cancer therapy. Cell Death Differ. 2011;18(6):1046–1056. doi: 10.1038/cdd.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Liu X, Hao J, Yang Y, Zhao M, Zuo J, Liu W. Glucose-regulated protein 75 suppresses apoptosis induced by glucose deprivation in PC12 cells through inhibition of Bax conformational change. Acta Biochimet Biophys Sin. 2008;40:339–348. doi: 10.1111/j.1745-7270.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 45.Qu M, Zhou Z, Chen C, Li M, Pei L, Yang J, Wang Y, Li L, Liu C, Zhang G, Yu Z, Wang D. Inhibition of mitochondrial permeability transition pore opening is involved in the protective effects of mortalin overexpression against beta-amyloid-induced apoptosis in SH-SY5Y cells. Neurosci Res. 2012;72:94–102. doi: 10.1016/j.neures.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Guo W, Zhang Q, Li H, Liu X, Yang Y, Zuo J, Liu W. Crosstalk between Raf/MEK/ERK and PI3K/AKT in suppression of Bax conformational change by Grp75 under glucose deprivation conditions. J Mol Biol. 2011;414:654–666. doi: 10.1016/j.jmb.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS 66.5 kb)