Abstract
Episodic memory shows substantial declines with advancing age, but research on longitudinal trajectories of spoken discourse memory (SDM) in older adulthood is limited. Using parallel process latent growth curve models, we examined 10 years of longitudinal data from the no-contact control group (N = 698) of the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) randomized controlled trial in order to test (a) the degree to which SDM declines with advancing age, (b) predictors of these age-related declines, and (c) the within-person relationship between longitudinal changes in SDM and longitudinal changes in fluid reasoning and verbal ability over 10 years, independent of age. Individuals who were younger, White, had more years of formal education, were male, and had better global cognitive function and episodic memory performance at baseline demonstrated greater levels of SDM on average. However, only age at baseline uniquely predicted longitudinal changes in SDM, such that declines accelerated with greater age. Independent of age, within-person decline in reasoning ability over the 10-year study period was substantially correlated with decline in SDM (r = .87). An analogous association with SDM did not hold for verbal ability. The findings suggest that longitudinal declines in fluid cognition are associated with reduced spoken language comprehension. Unlike findings from memory for written prose, preserved verbal ability may not protect against developmental declines in memory for speech.
Keywords: Discourse memory, Aging, Language comprehension, Individual differences, Latent growth curve modeling
Most of the inferences about developmental trajectories of change in memory for spoken discourse come from cross-sectional studies comparing groups of individuals of different ages and ability levels (Schneider et al., 2002; Tun, McCoy, & Wingfield, 2009; Wingfield & Stine-Morrow, 2000). Although these studies are informative in describing age differences, we are aware of no research examining within-person change in memory for spoken discourse over time, or individual differences in change in spoken discourse memory (SDM) among older adults. Critically, cross-sectional and longitudinal estimates of age-related change differ for a number of cognitive abilities (Lindenberger, Timo von Oertzen, Ghisletta, & Hertzog, 2011; Sliwinski & Herman, 1999), including language production (Kemper & Sumner, 2001). Inferences about developmental change based on cross-sectional analysis alone are problematic (Baltes, Reese, & Nesselroade, 1988; Hofer & Sliwinski, 2001; Lindenberger et al., 2011). Because longitudinal data analysis involves a direct investigation of within-person developmental change and individual differences in rates of change, such analyses may yield more accurate estimates of age-related declines in memory for spoken discourse. The current study aims to fill this major gap in the research by investigating long-term changes in SDM over ten years among a group of healthy older adults.
Although memory for language is often treated as a component of episodic memory (Hultsch, Hertzog, Dixon, & Small, 1998), comprehension of discourse involves processes that are distinct from episodic memory, including decoding and integrating phonological and lexical representations, parsing incoming strings into syntactic constituents, abstracting and retaining message-level semantics separate from the verbatim form, and integrating message-level propositional information across sentences (Frazier & Rayner, 1982; Kintsch & van Dijk, 1978; Kintsch, 1998). Not surprisingly, then, memory for text may be supported by cognitive underpinnings that are distinct from those that underlie episodic memory, as such memory for word lists (Lewis & Zelinski, 2010). Maintaining a propositional representation from text is cognitively demanding and shows substantial declines in older adulthood (Payne & Stine-Morrow, 2012; Radvansky, 1999; Radvansky, Zwaan, Curiel, & Copeland, 2001; Johnson, 2003). Estimates from a recent meta-analysis have shown that, on average, older adults perform at about the 22nd percentile of the distribution of younger adults in memory for discourse (Johnson, 2003). In reading, Stine-Morrow and colleagues (see 2006 for a review) have shown that older adults may compensate for age-related declines in propositional memory in part by regulating how they allocate attention to text during encoding. However, given that auditory presentation diminishes the ability to self-regulate input (Stine, Wingfield, & Poon, 1989), age differences in memory may well be amplified in auditory modalities. In fact, a meta-analysis (Johnson, 2003) found that, across a wide range of testing conditions, older adults performed significantly better when discourse memory tasks involved written presentation (d for young-old comparison = .76) compared to aural presentation via a tape-recorder (d = .92).
Two divergent patterns of age-related change characterize findings in cognitive aging (Baltes, 1997; Schaie, 1994). For fluid cognitive abilities (Cattell, 1971), also called cognitive mechanics (Baltes, 1997) or cognitive processes (Salthouse, 2010), aging is associated with monotonic declines that are dependent upon the processing efficiency of the cognitive system. In contrast, crystallized abilities (Cattell, 1971), also called cognitive pragmatics (Baltes, 1997) or cognitive products (Salthouse, 2010), are based on the accumulation of knowledge and experience and are often stable or show selective growth into adulthood. Research in the cognitive neuroscience of aging has found support for this dichotomy in the neural substrates that subserve these functions (Hedden & Gabriele, 2004; Raz & Rodrigue, 2006). For example, tasks that tax processing speed and executive control are correlated with cortical grey matter and white matter structure and function (e.g., lower pre-frontal cortical volume; greater white matter hyperintensities), which are more vulnerable to biological senescence in normal aging (Gunning-Dixon & Raz, 2003).
These divergent developmental patterns of change in cognitive ability have substantial effects on language comprehension (Burke & Shafto, 2008; Stine-Morrow et al., 2006). Older adults score upwards of one standard deviation higher on vocabulary measures compared to younger adults (Verhaeghen, 2003) and, even into old age, verbal and literacy skills appear to benefit multiple language comprehension mechanisms (Payne et al., 2012; Stine-Morrow et al., 2008). For example, high-verbal older adults show evidence for facilitation in visual word recognition (Lien et al., 2006; Ruthruff et al., 2008), and greater verbal skills appear to compensate for the negative effects of aging on text memory, in part, through the implementation of on-line encoding strategies (Payne et al., 2012; Stine-Morrow et al., 2008). At the same time, older adults with lower fluid cognitive abilities (e.g., working memory, reasoning, inhibitory control) show substantially worse memory for language (DeDe et al., 2004; Lustig, May, & Hasher, 2001; Payne et al., 2012; Stine-Morrow et al., 2008; van der Linden et al., 1999; Zelinski & Stewart, 1998).
Most studies assessing individual differences in cognition and memory for language are cross-sectional, however, with few studies examining how these processes relate over time. One exception to this is a longitudinal study by Zelinski and Stewart (1998). This study examined whether individual differences in baseline age and longitudinal changes in inductive reasoning and verbal ability predicted longitudinal changes in memory for written discourse over 16 years, in a sample of adults aged 55–81 years. In addition to finding substantial declines in text recall over the study period that were predicted by baseline age, this study also found that longitudinal changes in both reasoning and verbal ability were correlated with changes in text recall. In the current study, we aimed to extend the findings from Zelinski and Stewart (1998) to examine longitudinal changes in memory for spoken discourse over time. We examined individual differences in longitudinal change in SDM over 10 years, using a sample of older adults from the Advanced Cognitive Training in Independent and Vital Elderly (ACTIVE) trial (Ball et al., 2002; Jobe et al., 2001; Willis et al., 2006).
Although individual differences in text memory can be partially explained by differences in fluid and verbal ability, it is unclear whether these same effects hold for auditory memory. We hypothesized that baseline educational level, health status, and baseline cognitive function may partially account for the relationship between discourse memory, verbal ability, and reasoning ability. Therefore, we simultaneously examined the associations of these variables with SDM. Further, we hypothesized that trajectories of change in reasoning, a common proxy measure of fluid ability, and SDM would be coupled over 10 years, even after accounting for other possible variables that may explain these intercorrelations. Moreover, because verbal ability is protective of episodic memory generally (Manly et al., 2005; Stern, 2009) and text memory in particular (Payne et al., 2012; Stine-Morrow et al., 2008), we hypothesized that older adults with greater verbal ability would show relative preservation of SDM. However, given that speech occurs at a pace uncontrolled by the listener so that it does not afford the use of many of the self-regulatory strategies available to skilled readers, we also considered the possibility that verbal ability may be relatively less protective in the auditory domain, so that individual differences in verbal ability would show an attenuated relationship with changes in SDM.
METHOD
Participants
Participants were 698 older adults between the ages of 65 and 94 (M = 74.05, SD = 6.05), from the Advanced Cognitive Training in Independent and Vital Elderly study (Ball et al., 2002; Jobe et al., 2001, Willis et al., 2005). In ACTIVE, 2,802 older participants were randomized to a no-contact control group or to one of three training interventions focusing on processing speed, inductive reasoning, or episodic memory. For the current study, our sample only included individuals from the no-contact control group because we were interested in examining normative change in memory for spoken discourse, absent the effects of the cognitive training interventions (see Sisco et al., 2012 for effects of memory training on SDM).
Measures
Spoken Discourse Memory (SDM)
Memory for spoken discourse was measured with the Paragraph Recall task from the Rivermead Behavioral Memory Test, version 2 (Wilson, Cockburn, & Baddeley, 1985, 2003). Alternate passages were administered at different assessments. The Rivermead paragraph recall task was administered in small groups of 2–4 participants at each measurement occasion. Participants listened to an audiotape recording of a passage, recorded by a professional narrator. The passage was spoken with normal prosody at a comfortable and constant rate. Immediately after hearing the passages, participants were asked to write down everything they could remember. Passages were scored for the total number of propositions correctly recalled (Brown et al., 2008; Turner & Greene, 1978; Wilson et al., 1985). Scores were equated to remove form artifacts, using an equipercentile equating procedure (Gross et al., 2012; Kolen & Brennan, 1995) and were then standardized to a T-score (M = 50, SD = 10) based on the baseline mean and standard deviation of the sample. SDM was assessed across all testing sites at seven waves: baseline, post-test (.2 years), and longitudinal follow-ups at 1, 2, 3, 5, and 10 years.
Inductive Reasoning
Reasoning was measured with the Letter Sets, Letter Series, and Word Series tasks from the Schaie-Thurstone Adult Mental Ability test (Schaie, 1985). These tests require participants to identify patterns in a series of items and either generate the next item in the series, or decide which item does not adhere to the pattern. These measures have been used in previous studies to assess components of fluid ability in cross-sectional (Kyllonen & Christal, 1990; Salthouse, 2003; Süß et al., 2002), longitudinal (Baltes, 1997; Schaie, 1994), and intervention (Ball et al., 2002; Payne et al., 2012; Willis et al., 1990) research. Scores were standardized to a T-score (M = 50, SD = 10) based on the pre-test mean and standard deviation and a composite average of the three variables was formed. Reasoning was assessed across all testing sites at the seven waves of data collection. Alternate forms were administered at different assessments. Test materials were visually presented.
Verbal Ability
Verbal ability was measured with a recognition vocabulary test from the Kit of Factor-Referenced Cognitive Tests (Ekstrom et al., 1976). Participants were presented with a series of target words and were asked to choose a correct synonym from among four alternate options. Vocabulary was assessed across all testing sites at four waves: baseline, 3, 5, and 10 years. This measure was standardized to a T-score (M = 50, SD = 10), based on the baseline mean and standard deviation of the sample. Alternate forms were administered at different assessments. Test materials were visually presented.
Demographics and Covariates
The final model adjusted for: 1) demographics including age, sex, race, and years of education; 2) self-reported health status on a 5-point scale (1 = excellent; 5 = poor); 3) global cognitive function as measured by the MMSE (cutoff score for participation in ACTIVE = 23) (Folstein, Folstein, & McHugh, 1975); 4) baseline psychomotor speed, as measured by a composite of the the Digit Symbol Substitution test, Digit Symbols Copy, and Useful Field of View (UFOV) (Wechsler, 1981; Ball & Owsley, 1993) tasks; and 5) baseline auditory episodic memory, as measured by the Auditory Verbal Learning Test (Schmidt, 2004).
Analysis Plan
The major goal of this study was to examine whether within-person trajectories of change in reasoning and verbal ability over 10 years are related to within-person changes in SDM, independent of age and other demographic characteristics. To address this aim, we used parallel process latent growth curve models (Cheong, McKinnon, & Khoo, 2003; Muthen, 2008; Preacher et al., 2008). In parallel process models, multiple latent growth processes are estimated simultaneously (in this case, for SDM, reasoning, and verbal ability). This approach allows us to explicitly test whether random intercepts (initial level) and slopes (longitudinal changes) for one process are correlated with intercepts and slopes for other processes. Absolute model goodness of fit was assessed using the Comparative Fit Index (CFI), the Non-Normed Fit Index (NNFI), and the Root Mean Square Error of Approximation (RMSEA). The latent slope factors were specified by fixing factor loadings to the number of years from baseline, so that the mean slope of these models could be interpreted as annual changes in SDM, reasoning, and verbal ability.
Figure 1 presents the structural equation model diagram for the parallel process latent growth model of SDM, verbal ability, and reasoning. Preliminary analyses showed that the best growth trajectories for SDM and reasoning included a fixed and random intercept, fixed and random linear term, a fixed second intercept to adjust for practice or retest, and a fixed quadratic effect, indicating a non-linear trajectory. The best fitting model for verbal ability was similar, but did not include a quadratic term. Models were adjusted for individual differences in age at baseline, sex, race, education, health status, global cognitive functioning, episodic memory, and psychomotor speed. These models were conducted using the structural equation modeling program AMOS version 16 (Arbuckle, 2006), with Full Information Maximum Likelihood estimation.
Figure 1.
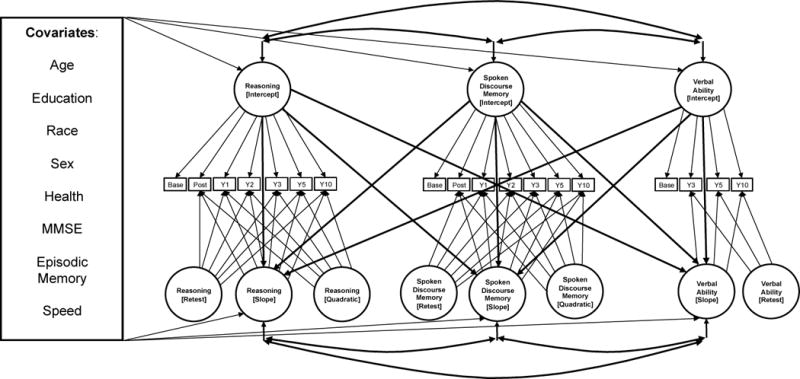
Structural Equation Model Diagram for Parallel Process Latent Growth Model of Spoken Discourse Memory, Reasoning, and Vocabulary Score.
RESULTS
Table 1 includes sample characteristics for each variable of interest at the baseline measurement. Participants were mostly White (72%), female (74%), on average 74 years old, and had, on average, 13 years of education.
Table 1.
Sample Characteristics at Baseline (N = 698).
|
|
|||
|---|---|---|---|
| Variable | Mean or N | Standard deviation or Percent | Observed range |
| Age | 74.05 | 6.05 | 65, 94 |
| Years of Education | 13.37 | 2.71 | 6, 20 |
| Female | 514 | 74% | – |
| Health Status | 2.64 | .87 | 1, 5 |
| Mini-Mental State Examination (MMSE) | 27.27 | 1.99 | 23, 30 |
| White | 503 | 72% | – |
| Spoken Discourse Memory (SDM) | 49.83 | 9.68 | 20.73, 80.18 |
| Reasoning | 49.38 | 9.13 | 20.78, 80.07 |
| Verbal ability | 49.55 | 10.03 | 17.54, 64.65 |
Note. We derived T-scores for SDM, reasoning, and verbal ability standardized to baseline in the full sample (N = 2,802).
Estimated means, variances, and regressions from the parallel process growth curve model are presented in Table 2. This model showed good fit to the data (RMSEA = .03; CFI = .98). The retest effect for SDM was not statistically significant, but for verbal ability and reasoning, retest effects were equivalent to approximately 6.3 years and 15.3 years of age, respectively (Table 2)1. Although there was significant heterogeneity in the random slopes for SDM, verbal ability, and reasoning, annual decline in these variables was not statistically significant after adjustment for covariates (Table 2). In the following subsection, we first present the longitudinal relationships between changes in SDM, verbal ability, and reasoning. We then turn to the findings for the effects of the covariates on individual differences in level and rates of change in SDM, verbal ability, and reasoning.
Table 2.
Estimated Means, Variances, and Regressions from Parallel Process Latent Growth Curve Model of Spoken Discourse Memory, Reasoning, and Verbal Ability over 10 years. (N = 698)
| Spoken Discourse Memory (SDM) |
Verbal ability | Reasoning | |||||
|---|---|---|---|---|---|---|---|
| Parameter Estimate |
|
||||||
| Estimate | SE | Estimate | SE | Estimate | SE | ||
| Latent Variable Means | |||||||
| Baseline Intercept | 50.21*** | .29 | 49.85*** | .30 | 49.84*** | .24 | |
| Trajectory | −.54 | .64 | .36 | .47 | .38 | .42 | |
| Retest | .47 | .32 | 1.69*** | .35 | 2.30*** | .16 | |
| Quadratic | .08*** | .01 | @0 | – | −.05*** | .01 | |
| Latent Residual Variances | |||||||
| Baseline Intercept | 23.24*** | 1.98 | 46.60*** | 3.22 | 32.39*** | 1.91 | |
| Trajectory | .14** | .06 | .08* | .04 | .20*** | .03 | |
| Regressions on Baseline Intercept | |||||||
| Age | −.13*** | .04 | .27** | .06 | −.15** | .05 | |
| Female sex | −1.55** | .57 | .67 | .73 | .13 | .57 | |
| Education | .67*** | .09 | 1.26*** | .12 | .72*** | .09 | |
| MMSE | .86*** | .13 | 1.09*** | .17 | .72*** | .09 | |
| White | 3.80*** | .54 | 4.92*** | .68 | 3.53*** | .53 | |
| Health | −.27 | .28 | −.57 | .36 | −1.22*** | .28 | |
| Episodic Memory | 2.94*** | .28 | .74* | .35 | 1.26*** | .27 | |
| Processing Speed | −.54 | .27 | −.67 | .35 | −2.58*** | .30 | |
| Regressions on Trajectory | |||||||
| Age | −.03** | .01 | .01 | <.01 | −.03*** | <.01 | |
| Sex | −.13 | .12 | −.03 | .09 | .06 | .08 | |
| Education | <.01 | .02 | −.01 | .02 | −.03 | .02 | |
| MMSE | .03 | .03 | −.05* | .02 | −.03 | .02 | |
| White | .05 | .12 | .06 | .09 | .05 | .08 | |
| Health | <.01 | .06 | .03 | .04 | −.01 | .04 | |
| Episodic Memory | .09 | .07 | .02 | .05 | .12** | .05 | |
| Processing Speed | −.08 | .06 | <.01 | .05 | −.02 | .04 | |
| Baseline SDM | −.01 | .01 | <.01 | <.01 | <.01 | <.01 | |
| Baseline Vocab | <.01 | <.01 | −.02* | <.01 | <.01 | <.01 | |
| Baseline Reasoning | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 | |
|
| |||||||
| Model Fit Statistics | |||||||
|
| |||||||
| χ2 / df | 1.69 | ||||||
| RMSEA | 0.03 | ||||||
| CFI | 0.98 | ||||||
| NNFI | 0.96 | ||||||
Note. MMSE: Mini-Mental State Examination. @0: parameter constrained to 0 in model. Sex is parameterized as Female =1; White is parameterized as White =1.
p <.05;
p <.01;
p <.001. MMSE: Mini-Mental State Examination; SE: standard error; RMSEA: root mean squared error of approximation; CFI: comparative fit index; NNFI: Non-Normed Fit Index.
Longitudinal Changes in Spoken Discourse Memory, Reasoning, and Verbal Ability
Towards our primary goal of examining the longitudinal relationships between SDM, reasoning ability, and verbal ability, the main results of interest from the parallel process growth curve model are the residual correlations between the latent intercept parameters and latent slope parameters (Table 3).
Table 3.
Estimated Latent Residual Correlations from Parallel Process Latent Growth Curve Model of Spoken Discourse Memory, Reasoning and Vocabulary Score over 10 years. (N = 698) *p <.05; **p <.01; ***p <.001.
| Latent Residual Correlations | ||
|---|---|---|
|
| ||
| Initial Levels | Trajectories | |
|
|
||
| r (SDM, Verbal ability) | .34*** | .43 |
| r (SDM, Reasoning) | .38*** | .87*** |
| r (Verbal ability, Reasoning) | .35*** | .22 |
Note. SDM: Spoken Discourse Memory
At baseline, SDM, verbal ability, and reasoning were all significantly intercorrelated, suggesting substantial shared variance between initial performance on SDM, verbal ability, and reasoning even after adjusting for baseline covariates. Importantly, the pattern of correlations among longitudinal trajectories was different. The residual correlation between change in reasoning and change in SDM (r = .87), was considerably larger than the correlation at baseline, indicating that approximately 76% of the variance in change in SDM is shared with changes in reasoning over a 10-year period. Although verbal ability and SDM were strongly related at baseline, the correlation between changes in SDM and changes in verbal ability failed to reach statistical significance (r = .43), with the growth processes only sharing approximately 18% of variance in within-person trajectories over 10 years. The same pattern was found between changes in verbal ability and reasoning, as the correlation between changes in these two processes also failed to reach statistical significance (r = .22).
Predictors of Change in Spoken Discourse Memory, Reasoning, and Verbal Ability
At baseline, participants who were younger, were White, had more years of formal education, and had better episodic memory and global cognitive function demonstrated better baseline performance in SDM. In contrast to these relationships with initial status, predictors of rates of change were less robust. The only covariate that reliably predicted individual differences in longitudinal trajectories of change in SDM was age at baseline, such that older adults had a steeper annual rate of decline. Figure 2 illustrates the age-related differences in rates of change in SDM over 10 years. This plot shows the model-estimated 10-year slopes at three different levels of age at baseline: 66 years (the 10th percentile of age at baseline), 73 years (the 50th percentile of age at baseline), and 83 years (the 90th percentile of age at baseline). As can be seen, the 10-year change in SDM is accelerated among adults who were older at baseline.
Figure 2.
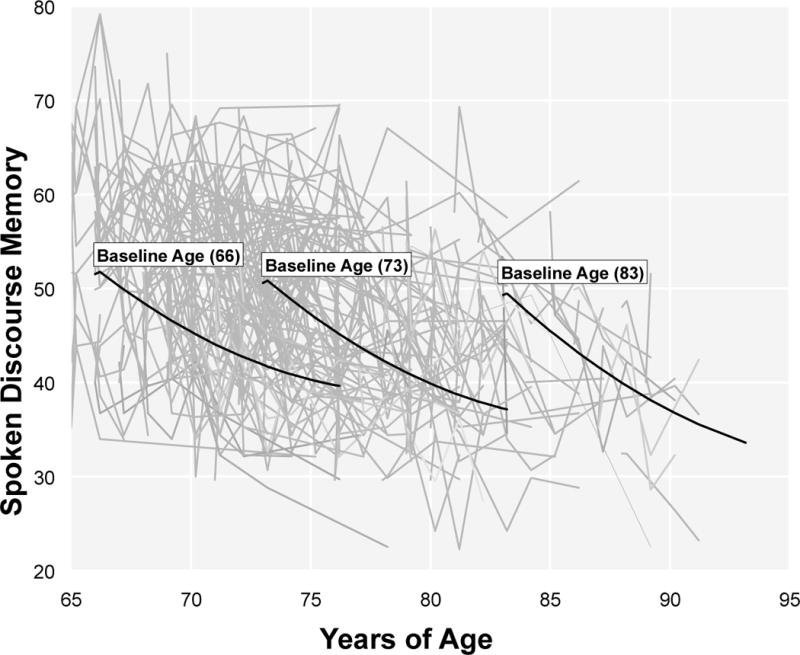
Model Estimated 10-Year Trajectories of Change in Spoken Discourse Memory as a Function of Age at Baseline, Adjusted for Covariates in Table 2 (N = 698).
(Note. Overlaid with Random 20% Sample of Raw Individual Scores in Gray). Model-implied trajectories shown for ages 66 (M -1SD), 73 (M), and 83 (M + 1SD).
At baseline, individuals who were younger, were White, had more years of formal education, and had better episodic memory and overall cognitive function had better baseline verbal ability. However, only global cognitive function and initial verbal ability emerged as significant predictors of change in verbal ability over 10 years.
For reasoning, the overall pattern of findings was similar to the SDM findings. At baseline, participants who were younger, were White, had more years of formal education, had better episodic memory, psychomotor speed, and global cognitive function, and were higher in health status had better baseline reasoning. However, only baseline age and baseline episodic memory performance were reliable predictors of change in reasoning over time. Older adults showed a steeper annual rate of decline in the reasoning composite over 10 year study period, and those with better initial episodic memory at baseline showed attenuated declines in reasoning.
DISCUSSION
The findings from the present study contribute to the literature on aging and memory for language (Light, 1991; Johnson, 2003) by demonstrating the long-term longitudinal relationships between memory for spoken discourse and markers of fluid reasoning and verbal cognition. As far as we know, this paper provides the first longitudinal investigation of individual differences in developmental changes in memory for spoken discourse among older adults. Our findings indicated that there was substantial longitudinal coupling between SDM and reasoning ability over up to 10 years, but that this relationship did not hold for SDM and verbal ability. Moreover, of all the measures at baseline, age was the only reliable predictor of change in both reasoning and SDM. This finding is particularly interesting, given that age remained a unique predictor after adjusting for several covariates known to influence discourse memory and non-verbal reasoning.
Our findings are largely consistent with those from Zelinski and Stewart (1998) in showing longitudinal declines in discourse memory that could be predicted by age at baseline. At the same time, our findings provide important extensions to their study. First, our longitudinal assessment is based on a larger sample, which is especially important given power issues for assessing longitudinal intercorrelations (Hertzog et al., 2008). The use of structural equation modeling techniques for examining correlated change is strength. By modeling correlated change in all three processes simultaneously, our analyses could be adjusted for initial levels of each process, as well as health status, global and specific cognitive function (MMSE, psychomotor speed, episodic memory), and demographic characteristics, including age. Lastly and most importantly, examining memory for discourse in the auditory modality complements existing longitudinal investigations into discourse memory that have predominantly used written text material (Dixon et al., 2004; Hultsch et al., 1998; Small et al., 1999; Zelinski & Stewart, 1998).
Our findings that both baseline age and change in reasoning were related to changes in SDM are entirely consistent with Zelinski and Stewart (1998). However, in contrast to their findings in the text domain, neither level nor change in verbal ability significantly influenced individual differences in rates of change in SDM. It may be that preserved verbal ability does not provide the same degree of protective benefits for discourse memory in the auditory domain. One reason for this was suggested earlier: perhaps individual differences in attentional allocation during encoding serve as a mediating mechanism underlying the relationship between verbal ability and memory for written text. That is, there may be differences in attentional allocation strategies during encoding between high and low verbal individuals that differentially impact text memory. Findings by Stine-Morrow, Miller, Gagne, and Hertzog (2008) are consistent with this interpretation. In this study, participants read a series of sentences and larger discourses for recall in a self-paced reading paradigm. Stine-Morrow et al. examined changes in reading time as a function of a number of indices of linguistic difficulty and found that highly verbal individuals showed facilitated word-level processing, but also allocated more time to encoding propositional information online, leading to improved memory (see also Payne et al., 2012).
Because the rate of auditory input is fixed by an external source (the speaker), there is no clear way to regulate the pacing and timing of input at encoding. Thus, preserved verbal abilities may have little effect on memory for spoken discourse. Such ecological constraints on strategy use in the auditory domain may be another reason why reasoning was such a strong predictor of rate of change in SDM, given that there is a low likelihood that individual differences in encoding strategies may affect memory performance in this domain (cf. Friedman & Miyake, 2004). These findings are also consistent with a larger number of studies suggesting that control over input during encoding across a variety of tasks is essential to learning and memory (Chin, Payne, Fu, Morrow & Stine-Morrow, 2013; Voss, Gonsalves, Federmeier, Tranel, & Cohen, 2011).
Note that it is not necessarily true that listeners cannot modulate speech input in all cases. For example, speakers and listeners rely on a number of mechanisms to regulate comprehension during conversations (Garrod & Pickering, 2004; Tanenhaus & Brown-Schmidt, 2008). When speech is received at a pre-determined rate (e.g., radio and television; Stine, Wingfield, & Myers, 1990) however, age differences in memory may be more robust (Johnson, 2003). A recent study by Piquado, Benichov, Brownell, and Wingfield (2012) showed that self-regulatory control over speech input serves as a compensatory mechanism among older adults, especially those with central hearing acuity deficits. These findings warrant further research, including direct comparisons between auditory and visual text memory over time.
To the extent that non-verbal reasoning is a proxy for general fluid cognitive ability and executive control abilities (Kyllonen & Christal, 1990; Salthouse et al., 2003; Süß et al., 2002), these findings are consistent with the claim that preserved fluid reasoning ability may be an important domain-general cognitive component underlying effective language comprehension in older adulthood (Zelinski & Stewart, 1998). Goh, An, and Resnick (2011) recently examined cross-sectional and longitudinal relationships between a number of measures of executive control and episodic memory among middle-aged and older adults. They found substantial baseline correlations between most executive control and memory variables, but at the same time, heterogeneous trajectories of change over time among most cognitive abilities. While our findings are globally consistent with these results, given that we found very few predictors of within-person change in reasoning, discourse memory, or verbal ability, our results stand in contrast to those of Goh et al. (2011) in one major way: the substantial longitudinal correlation found in the current study between change in reasoning and change in SDM, which was actually larger longitudinally than cross-sectionally.
Important caveats and limitations in the current study need to be addressed. First, ACTIVE included no measures of sensory ability, including auditory function. This is problematic for the current study, given the substantial literature indicating that age-related declines in audition are a critical aspect of speech comprehension and memory (Schneider, Pichora-Fuller, & Daneman, 2010; Tun Benichchov, & Wingfield, 2010; Tun et al., 2009; Wingfield Tun, & McCoy, 2005). Age-related declines in auditory processing have broad influences on cognitive performance and may result in permanent alternations of how auditory information is processed in the aging brain (Lindenberger & Baltes, 1994). Although presentation of the passages in the Rivermead task were presented at a volume that was comfortable for the participants, it is possible that misperceptions of the auditory signal were at least partially responsible for age-related memory errors. As a result, this likely leads to an overestimated effect of age-related decline and the possibility of an overestimated correlation between reasoning and SDM at baseline, along with change in reasoning and SDM over time. Note, however, that modality specific sensory deficits have been shown to share high correlations with a number of other biomarkers of physical and cognitive health (Antsey, 2011), suggesting that acuity measures may serve as a proxy for more general function. Given that it is likely that audition is comorbid with at least some of the individual difference indicators included as covariates in the current study, we believe it is unlikely that the substantial relationship found between change in reasoning (a non-verbal visual task) and change in SDM could be completely mediated by auditory sensory processing. Nevertheless, future research should aim to examine how longitudinal declines in SDM are influenced by both cognitive and sensory factors simultaneously.
Lastly the recognition vocabulary measure used in the current study may have been less demanding on participants than other commonly used verbal ability tasks (e.g., WAIS, WRAT; Strauss, Sherman, & Spreen, 2006). Indeed, there was some evidence for non-normality in the distribution of this measure at each test occasion, with a greater degree of clustering of scores at the higher range. At the same time, such recognition vocabulary tasks have shown to be reliable and valid predictors of language comprehension in prior research (Stine-Morrow et al., 2008; Lewis & Zelinski, 2010). Indeed, in the current study, vocabulary scores did show an adequate range at baseline compared to our other measures (see Table 1), and showed good external validity, relating to a number of covariates at baseline (see regression estimates for covariates in Table 2).
In conclusion, a growing number of studies examining individual differences in cognition have suggested that there are substantial individual differences in language comprehension among older adults (see Burke & Shafto, 2008 for a review). The findings from the current study contribute to this literature by showing that analyses of longitudinal change can provide powerful insights into developmental changes in memory for language. Further, our study extends this literature to the auditory domain. Despite a growing number of studies recently suggesting rather heterogeneous longitudinal relationships between changes in cognition over time (Goh et al., 2011; Tucker-Drob, 2011; McArdle et al., 2009), our findings suggest strong relationships between declines in memory for spoken discourse and non-verbal executive reasoning abilities. Collectively, our findings suggest that longitudinal declines in cognitive ability are strongly associated with declines in memory for speech, but in contrast to the text memory domain, preserved verbal ability may not be as protective against developmental declines.
Acknowledgments
This research was supported by a series of grants awarded from the National Institutes of Health to the six field sites and the coordinating center for the ACTIVE study, including the Hebrew Rehabilitation Center for the Aged (R01 NR04507), the Indiana University School of Medicine (R01 NR04508), The Johns Hopkins University (R01 AG14260), the New England Research Institutes (R01 AG14282), The Pennsylvania State University (R01 AG14263), the University Alabama at Birmingham (R01 AG14289), and Wayne State University (R01 AG014276). The first author was supported by an NIH training grant (T32-HD055272) and by NIH R01 AG13935 during the preparation of this manuscript.
Footnotes
These estimates were calculated as the ratio of the model-estimated retest effect and the model-estimated cross-sectional age-related effect. The ratio describes the magnitude of the retest effect relative to the effect of age on cognition.
References
- Anstey KJ. Biomarkers and memory aging: A life-course perspective. In: Naveh-Benjamin Moshe, Ohta Nobuo., editors. Memory and Aging: Current Issues and Future Directions. Psychology Press, Taylor & Francis; UK: 2011. pp. 349–372. [Google Scholar]
- Arbuckle JL. Amos (Version 16.0) [Computer Program] Chicago: SPSS; 2006. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults. Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Brown C, Snodgrass T, Kemper S, Herman R, Covington MA. Automatic measurement of propositional idea density from part-of-speech tagging. Behavior Research Methods. 2008;40:540–545. doi: 10.3758/brm.40.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3. New York: 2008. pp. 373–443. [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. New York: Houghton Mifflin; 1971. [Google Scholar]
- Cheong J, MacKinnon DP, Khoo S-T. Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling. 2003;10:238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Payne BR, Fu WT, Morrow DG, Stine-Morrow EAL. Information foraging across the lifespan: Search and switch in unknown patches. Topics in Cognitive Science. 2013 doi: 10.1111/tops.12147. in press. [DOI] [PubMed] [Google Scholar]
- DeDe G, Caplan D, Kemtes K, Waters G. The relationship between age, verbal working memory, and language comprehension. Psychology and Aging. 2004;19:601–616. doi: 10.1037/0882-7974.19.4.601. [DOI] [PubMed] [Google Scholar]
- Folstein MR, Folstein SE, McHugh PR. Mini-mental state. A practice method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frazier L, Rayner K. Making and correcting errors during sentence comprehension: Eye movements in the analysis of structurally ambiguous sentences. Cognitive Psychology. 1982;14:178–210. [Google Scholar]
- Friedman NP, Miyake A. The reading span test and its predictive power for reading comprehension ability. Journal of Memory and Language. 2004;51:136–158. [Google Scholar]
- Goh JOS, An Y, Resnick SM. Differential trajectories of age-related changes in components of executive and memory processes. Psychology and Aging. 2012;27:707–719. doi: 10.1037/a0026715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Inouye SK, Rebok GW, Brandt J, Crane PK, Jones RN. Parallel but not equivalent: Challenges and solutions for repeated assessment of cognition over time. Journal of Clinical and Experimental Neuropsychology. 2012;34:758–772. doi: 10.1080/13803395.2012.681628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabriele JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hertzog C, von Oertzen T, Ghisletta P, Lindenberger U. Evaluating the power of latent growth curve models to detect individual differences in change. Structural Equation Modeling. 2008;15:541–563. [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. New York: Cambridge University Press; 1998. [Google Scholar]
- Jefferies E, Lambon-Ralph MA, Baddeley AD. Automatic and controlled processing in sentence recall: The role of long-term and working memory. Journal of Memory and Language. 2004;51:623–643. [Google Scholar]
- Jobe JB, Smith D, Ball K, Tennstedt S, Marsiske M, Willis SL, et al. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE. Aging and the remembering of text. Developmental Review. 2003;23:261–346. [Google Scholar]
- Kintsch W. Comprehension: A paradigm for cognition. New York: Cambridge University Press; 1998. [Google Scholar]
- Kintsch W, van Dijk TA. Toward a model of text comprehension and production. Psychological Review. 1978;85:363–394. [Google Scholar]
- Kolen MJ, Brennan RL. Test equating. New York: Spring; 1995. [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Lewis KL, Zelinski EM. List and text recall differ in their predictors: Replication over samples and time. Journal of Gerontology: Psychological Sciences. 2010;65B:449–458. doi: 10.1093/geronb/gbq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Schupf N, Tank MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. Journal of Geriatric Psychiatry and Neurology. 2005;18:213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Grimm KJ, Hamagami F, Bowles RP, Meredith W. Modeling lifespan growth curves of cognition using longitudinal data with multiple samples and changing scales of measurement. Psychological Methods. 2009;14:126–149. doi: 10.1037/a0015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B. Latent variable hybrids: Overview of old and new models. In: Hancock GR, Samuelsen KM, editors. Advances in latent variable mixture models. Charlotte, NC: Information Age Publishing, Inc; 2008. pp. 1–24. [Google Scholar]
- Payne BR, Stine-Morrow EAL. Aging, parafoveal preview, and semantic integration in sentence processing: Testing the cognitive workload of wrap-up. Psychology and Aging. 2012;27:638–649. doi: 10.1037/a0026540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Gao X, Noh SR, Anderson CJ, Stine-Morrow EAL. The effects of print exposure on sentence processing and memory among older adults: Evidence for efficiency and reserve. Aging, Neuropsychology, and Cognition. 2012;19:122–149. doi: 10.1080/13825585.2011.628376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Jackson JJ, Hill PL, Gao X, Roberts BW, Stine-Morrow EAL. Memory self-efficacy predicts responsiveness to inductive reasoning training in older adults. Journal of Gerontology: Psychological Sciences. 2012;67:27–35. doi: 10.1093/geronb/gbr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA. Reading ability: Lexical quality to comprehension. Scientific Studies of Reading. 2007;11:357–383. [Google Scholar]
- Piquado T, Benichov JI, Brownell H, Wingfied A. The hidden effect of hearing acuity on speech recall, and compensatory effects of self-paced listening. International Journal of Audiology. 2012;51:576–583. doi: 10.3109/14992027.2012.684403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent growth curve modeling. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- Radvansky GA. Memory retrieval and suppression: The inhibition of situation models. Journal of Experimental Psychology: General. 1999;128:563–579. doi: 10.1037//0096-3445.128.4.563. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, Zwaan RA, Curiel JM, Copeland DE. Situation models and aging. Psychology and Aging. 2001;16:145–160. doi: 10.1037/0882-7974.16.1.145. [DOI] [PubMed] [Google Scholar]
- Rawson KA. Testing the shared resource assumption in theories of text processing. Cognitive Psychology. 2007;54:155–183. doi: 10.1016/j.cogpsych.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19:532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Major issues in cognitive aging. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Schaie KW. The course of adult intellectual development. American Psychologist. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey auditory and verbal learning test: A handbook. Los Angeles: Western Psychological Services; 2004. [Google Scholar]
- Schneider BA, Daneman M, Pichora-Fuller MK. Listening in aging adults: From discourse comprehension to psychoacoustics. Canadian Journal of Experimental Psychology. 2002;56:139–152. doi: 10.1037/h0087392. [DOI] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, Hultsch DF, Hertzog C. Longitudinal changes in quantitative and qualitative indicators of word and text recall in young-old and old-old adults. Journal of Gerontology: Psychological Sciences. 1999;54B:P107–P115. doi: 10.1093/geronb/54b.2.p107. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine EAL, Wingfield A, Myers SD. Age differences in processing information from television news: The effects of bisensory augmentation. Journal of Gerontology: Psychological Sciences. 1990;45:P1–8. doi: 10.1093/geronj/45.1.p1. [DOI] [PubMed] [Google Scholar]
- Stine EAL, Wingfield A, Poon LW. Speech comprehension and memory through adulthood: The roles of time and strategy. In: Poon LW, Rubin DC, Wilson BA, editors. Everyday cognition in adult and late life. New York: Cambridge University Press; 1989. pp. 195–221. [Google Scholar]
- Stine EAL, Wingfield A, Poon LW. Aging and self-regulated language processing. Psychological Bulletin. 2006;132:582–606. doi: 10.1037/0033-2909.132.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow EAL, Miller LMS, Hertzog C. Self-regulated reading in adulthood. Psychology and Aging. 2008;23:131–153. doi: 10.1037/0882-7974.23.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss EM, Sherman S, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York, NY: 2006. [Google Scholar]
- Suß H, Oberauer K, Wittmann WW, Wilhelm O, Schulze R. Working-memory capacity explains reasoning ability—and a little bit more. Intelligence. 2002;30:261–288. [Google Scholar]
- Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Developmental Psychology. 2011;47:331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Benichov J, Wingfield A. Response latencies in auditory sentence comprehension: Effects of linguistic versus perceptual challenge. Psychology and Aging. 2010;25:730–735. doi: 10.1037/a0019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional cost of effortful listening. Psychology and Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Greene F. Construction and use of a propositional text base. JSAS: Catalog of Selected Documents in Psychology. 1978;8:1713. [Google Scholar]
- van der Linden M, Hupet M, Feyereisen P, Schelstraete M, Bestgen Y, Bruyer R, et al. Cognitive mediators of age-related differences in language comprehension and verbal memory performance. Aging, Neuropsychology, and Cognition. 1999;6:32–55. [Google Scholar]
- Verhaeghen P. Aging and vocabulary score: A meta-analysis. Psychology & Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nature Neuroscience. 2011;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Baddeley AD. The Rivermead Behavioural Memory Test. London: Pearson Assessment; 1985. [Google Scholar]
- Wilson BA, Cockburn J, Baddeley AD. The Rivermead Behavioural Memory Test – Second Edition. London: Pearson Assessment; 2003. [Google Scholar]
- Wingfield A, Stine-Morrow EAL. Language and speech. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. pp. 359–416. [Google Scholar]
- Wingfield A, Tun PA, McCoy SL. Hearing loss in older adults: What it is and how it interacts with cognitive performance. Current Directions in Psychological Science. 2005;14:144–148. [Google Scholar]
- Zelinski EM, Stewart ST. Individual differences in 16-year memory changes. Psychology and Aging. 1998;13:622–630. doi: 10.1037//0882-7974.13.4.622. [DOI] [PubMed] [Google Scholar]


