Abstract
The human intestinal tract is comprised of a rich and complex microbial ecosystem. This intestinal microbota provides a large reservoir of potentially toxic molecules, including bacterial endotoxin (i.e., lipopolysaccharide). This potent inflammatory molecule is detectable in the circulation of healthy individuals and levels transiently increase following ingestion of energy rich meals. Chronic exposure to circulating endotoxin has been associated with obesity, diabetes, and cardiovascular disease. Western-style meals augment LPS translocation and by this mechanism may contribute to the pathogenesis of these diseases. By contrast, the gut and other organs have evolved mechanisms to detoxify endotoxin and to neutralize the potentially inflammatory qualities of circulating endotoxin. Of specific interest to clinicians is evidence that acute postprandial elevation of circulating endotoxin is dependent on meal composition. In this review we present an overview of the biochemical and cellular mechanisms that lead to endotoxemia, with emphasis on the interplay between microbial and nutritional determinants of this condition. The link between endotoxemia, diet, and changes in the intestinal microbiota raise the possibility that dietary interventions can, at least in part, ameliorate the detrimental outcomes of endotoxemia.
Keywords: gut, microbiota, microbiome, inflammation, postprandial, endotoxin, endotoxemia, lipopolysaccharide, obesity, diabetes
Introduction
The gastrointestinal tracts of humans and other mammals are complex bioreactors that have evolved to extract nutrients from diverse natural products. Critical to the functions of these systems are dynamic communities of microorganisms that likely have co-evolved with their hosts to provide myriad beneficial services, including nutrient provision.1 The high-carbohydrate diets ingested by omnivores and herbivores typically contain plant polysaccharides that cannot be directly hydrolyzed by the mammalian gut. Instead, the intestinal microbiota, which in adults is dominated by diverse members of the bacterial phyla Firmicutes (e.g., clostridia) and Bacteroidetes,2–4 encodes a variety of hydrolytic enzymes that can convert otherwise indigestible polysaccharides into relatively simple compounds such as short-chain fatty acids (SCFAs), which are more readily absorbed by the mammalian intestines.1 In this way, the microbiota collectively provides genomic coding capacity -- the “microbiome”5,6 -- that supplements the function of the host genome. That mammalian breast-milk is composed of many types of oligosaccharide that both direct the early development of the intestinal microbiota and are fermented by this microbiota into compounds that nourish the developing infant is testament to the deeply interlinked relationships between host and microbiota.7,8
The intimate association of numerically rich and diverse microbial communities with the human host potentially comes at a price. Commensal microorganisms are, in many instances, separated from the interior of the body by a single layer of epithelial cells, which in the case of the distal gut must form a barrier to entry of as many as 1012 microbial cells per gram of luminal content.9 To prevent potentially lethal microbial infections, the immune system is tuned to detect and respond to microbes that breach the epithelium. A variety of chemical moieties that are conserved across broad ranges of microbes – termed microbe-associated molecular patterns (MAMPs) – trigger signaling cascades in diverse host cells that result in recruitment and activation of innate and adaptive immune effector cells to sites of infection. Once the threat of infection is mitigated, the immune system must be down-regulated in order to prevent prolonged, destructive inflammation. For example, an inability to quell localized inflammatory responses to the intestinal microbiota is a hallmark of the inflammatory bowel diseases.10
Despite diligent immune surveillance, small amounts of gut-derived bacterial MAMPs, such as endotoxin (i.e., lipopolysaccharide, LPS), enter the circulation of healthy mammals,11 possibly as the result of particular diets. Systemic exposure to MAMPs may incite low-level, chronic inflammation even in the absence of viable microbial cells in the bloodstream. The deleterious effects of such persistent exposure to inflammatory inducers have been proposed to be causal factors in the development of metabolic syndrome.12 The purpose of this review is to provide clinicians and healthcare professionals with an overview of the biochemical and cellular mechanisms that lead to endotoxemia, with emphasis on the interplay between microbial and nutritional determinants of this condition.
Postprandial Endotoxemia
Recent human clinical trials13–17 demonstrate a transient increase of circulating LPS following consumption of an energy rich meal and suggest a mechanistic link between diet, postprandial inflammation, and disease. Great interest has surrounded these trials because circulating LPS is associated with inflammatory mediators,18 and obesity,18,19 diabetes,20,21 steatohepatitis,22 renal,19,23 and cardiovascular disease.24 LPS is the primary structural component of the outer membrane of Gram-negative bacteria. It is composed of carbohydrate containing domains and the highly-immunogenic lipid-A domain. Extensive species-specific variation exists between the carbohydrate containing domains whereas the lipid-A domain is highly conserved. This conserved domain provides MAMPs for host immune recognition by the LPS receptor, Toll-like receptor 4 (TLR4).25 Several events must take place before LPS can bind and signal through TLR4. First, LPS must bind the secreted LPS-binding protein (LBP), an acute phase protein synthesized in the liver and lung.26,27 The LBP-LPS complex is then capable of binding CD14, which exists in soluble and membrane bound forms. Finally, the LPS/LBP/CD14 complex is capable of signaling through TLR4, leading to downstream inflammatory responses.28
In 2007, Erridge, et al17 published results of a feeding trial in humans that demonstrated postprandial endotoxemia following ingestion of a single high fat meal. Twelve healthy men were fed a 900 kcal meal (3 slices of toast with 50 grams of butter). Median plasma LPS concentration increased by 50% (8.2 pg/mL baseline; 12.3 pg/mL postprandial). Elevated plasma LPS also was observed following the feeding of healthy subjects egg and sausage muffin sandwiches and hash browns in a meal that contained similar energy and fat content.16 More recently, Laugerette, et al reported a similar outcome following ingestion of a moderate fat meal (882 kcal, 33% fat).13 Only one study has attempted to identify specific macronutrients responsible for postprandial endotoxemia. In this study,15 healthy participants were given only 300 kcal of either: glucose drink (100% carbohydrate), orange juice (92% carbohydrate), or cream (100% fat). Only the cream caused elevation of plasma LPS (41% increase). In a cross sectional study, Amar, et al29 measured circulating LPS in 201 French adults randomly selected from polling lists. A dietitian-reviewed 3-day food record enabled correlation of circulating LPS with macronutrient intake. Plasma LPS concentration was independently associated with total energy intake, but not fat intake. Taken together, current evidence indicates that endotoxemia can result from a variety of diets that range from 300 kcal of pure cream,15 to a more typical 882 kcal (33% fat),13 to the relatively large 1,200 kcal (38% fat).14 The demonstration that 300 kcal of cream is sufficient to produce endotoxemia raises the important question of whether other dietary lipids, protein, and non-glucose carbohydrates may also induce endotoxemia. As the role of endotoxemia in the pathogenesis of common diseases becomes better established, making these determinations will become increasingly important.
Mechanisms of Translocation
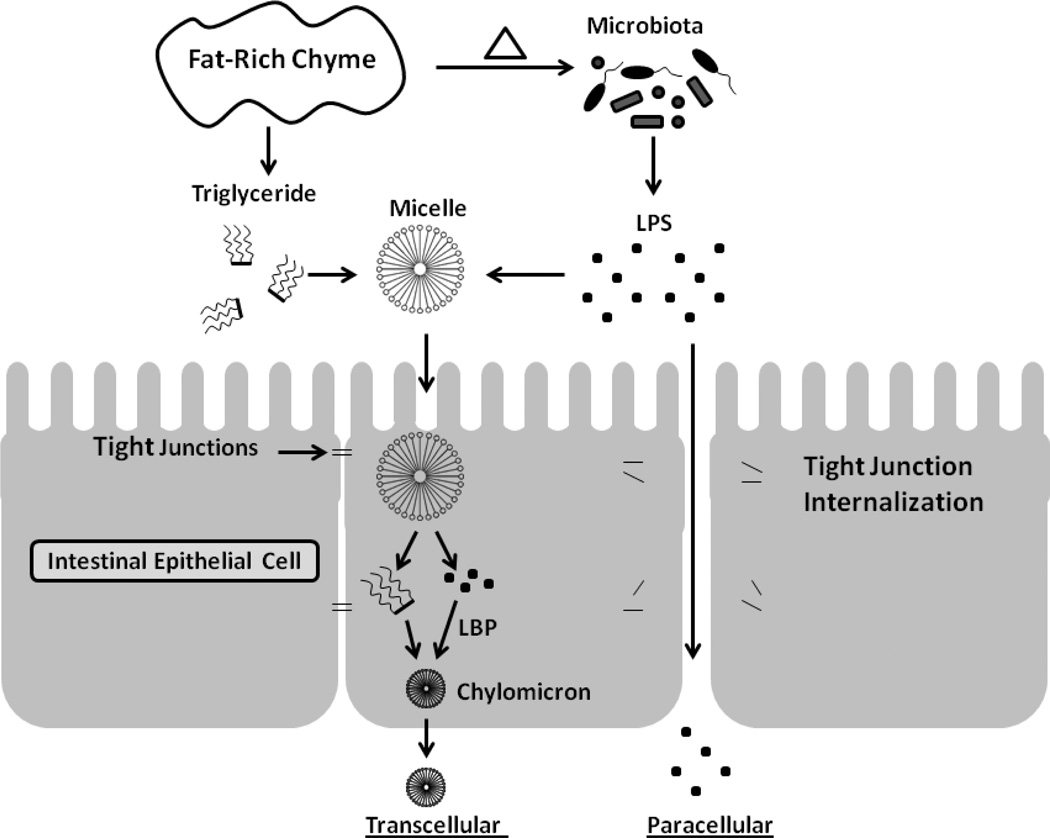
Multiple theories seek to explain how gut-derived LPS navigates into the circulation.30 One hypothesis proposes that impaired epithelial resistance associated with a high energy intake permits paracellular (between epithelial cell) movement (figure 1). Evidence supporting this model comes from Cani, et al31 who demonstrated increased intestinal permeability to an inert fluorescent molecule in mice fed a high fat (72% kcal) diet, whereas permeability was not detected in mice fed a standard diet. Furthermore, these animals had reduced expression of the tight junction proteins, ZO-1 and occludin. Barrier integrity was also studied by Brun, et al32 who utilized mice deficient in leptin (ob/ob mice) and the leptin receptor (db/db mice). Loss of leptin, a hormone that suppresses appetite and energy intake caused both hyperphagia and obesity in these animals. In this study, less electrical resistance and greater permeability to horseradish peroxidase was observed in small intestine tissue from ob/ob and db/db mice compared with wild type mice. Immunofluorescence staining demonstrated redistribution of tight junction proteins ZO-1 and occludin away from the cellular border. Importantly, these mice exhibited elevated LPS in the portal circulation even when consuming a standard diet.
Figure 1.
Transcellular and paracellular transport represent non-exclusive pathways for LPS movement from the enteric lumen into circulation. In the transcellular model, lipid absorption serves as a vehicle for LPS, which is included in micelles and later incorporated into chylomicrons through interaction with LPS-binding protein (LBP). In the paracellular model, fat-rich chyme results in internalization of tight junction proteins by mechanisms that remain unclear. The impaired epithelial barrier then permits LPS to pass between epithelial cells.
Another hypothesis that could explain impaired barrier integrity following high energy intake comes from work by Kvietys, et al33 who reported epithelial injury in response to normal digestion in the small intestine. They perfused physiologic quantities of glucose, hydrolyzed casein, and bile emulsified oleic acid into the jejunum of anesthetized rats. In contrast to the benign effects of the carbohydrate and protein treatment, the oleic acid emulsion induced a transient epithelial injury at the villous tips and increased intestinal permeability (similar results were also found in pigs34,35 and dogs).36
Alternatively to paracellular LPS transport, a transcellular pathway (through epithelial cells) is another potential route for LPS entry (figure 1). Cellular uptake of LPS occurs in cultured intestinal epithelial cells.37 In a mouse model, Ghoshal, et al30 identified increased LPS in plasma chylomicron remnants following gavage with 200ul of long-chain triolein, a triglyceride of the long-chain oleic acid, which enters the circulation through the lymphatics in chylomicrons. In contrast, gavage with butyrin, a triglyceride of butyric acid, enters directly into portal circulation, but does not raise circulatory LPS levels. In this model system, treatment with an inhibitor of chylomicron formation prevented the increase of LPS in the circulation and mesenteric lymph nodes. An in vitro system, utilizing intestinal epithelial monolayers, confirmed these results and in contrast to the previously mentioned effect of oleic acid on tight junctions, no change in permeability to fluorescent dextran was observed. The transcellular model in which lipid digestion and micelle transport facilitate LPS absorption is further strengthened by evidence that LBP increases transport of LPS from micelles to lipoproteins,38 including chylomicrons,30,39–41 HDL,42,43 and LDL.44 In addition to lipid facilitated LPS transport, nutrient independent uptake might explain baseline fasting levels of LPS in circulation. In support of this idea, Drewe, et al45 employed in situ injection of fluorescently labeled LPS into ligated jejunal loops of fasted rats. They identified LPS absorption into jejunal brush border membrane vesicles. Further studies indicated that this process was disrupted by a metabolic (dinitrophenol) and microtubule inhibitor (colcemid) indicating active LPS uptake. Taken together, these studies implicate multiple, non-exclusive pathways for LPS translocation into the circulation, though transport in micelles may best account for post-prandial endotoxemia.
Microbiota and Endotoxemia
Acute changes in gut bacteria following ingestion of high energy meals could also contribute to endotoxemia by shifting the balance of LPS producing and non-producing microorganisms in the gut. Recent advances in sequencing and genomics have facilitated study of these collective organisms (the microbiota) and their collective genome (the microbiome) at a new level of resolution. An association of endotoxemia with obesity18 makes it possible that properties unique to the obese microbiota contribute to endotoxemia. Conflicting data support supposed differences in the microbiota when obese are compared to lean individuals. Although multiple studies have reported greater abundance of Firmicutes and concomitantly reduced abundance of Bacteroidetes in obese subjects,3,5 others have produced seemingly contradictory results.46–50 The results of interventional trials raise the possibility that this discrepancy might be explained by the rapid adaptation of the microbiota to ingested nutrients such that the effect of recent energy intake (weight loss vs. maintenance vs. gain) is dominant. For example, no baseline differences in Firmicutes or Bacteroidetes were detected comparing lean and obese humans in a controlled environment while they consumed a weight maintenance diet.46 However, the composition of the microbiota distinctly changed when subjects were switched between 2,400 and 3,400 kcal diets with Firmicutes and Bacteroidetes positively and negatively associated with energy intake respectively. A similar response in Firmicutes and Bacteroidetes was observed in individuals who lost weight with48,51 or without3,52,53 gastric bypass surgery. It is also evident that high fat diets alter the microbiota31,54 and that this can occur independent of the obese state.54,55
The influence of the gut microbiota, the dominant source of LPS among commensal microbiota, on postprandial endotoxemia was demonstrated through antibiotic treatment that reduced the gut bacterial load and caused a decrease of plasma LPS in mice fed a high fat diet. In contrast, plasma LPS did not decrease in control animals that received antibiotics.31 In animals consuming the high fat diet, antibiotic treatment also restored expression of ZO-1 and ameliorated the adverse effects of diet on intestinal permeability. Interestingly, manipulation of the microbiota with an oligofructose prebiotic dietary supplement normalized plasma LPS. Several mechanisms may underlie the ability of the microbiota to influence endotoxemia. First, the luminal concentration of LPS may be important. The ratio of total enteric LPS (>1 gram)56 to the large surface area of the gut (>300m2) suggest that the law of mass action may contribute to LPS translocation. However, animals subjected to high energy diets have decreased bacterial loads per gram of cecal material.31,54,57 In addition, Gram-negative members of the prominent Bacteroidetes phylum decrease in abundance on such diets,55,58 whereas Gram-positive clostridiales, a prominent class of the phylum Firmicutes, expand in abundance.54,55 Despite several recent studies linking energy intake and the microbiota, it is not clear that high energy meals induce endotoxemia by increasing the luminal LPS concentration. Perhaps as important as considering which bacteria proliferate in response to high energy diets is identification of bacterial populations that contract and may thereby transiently stimulate endotoxemia through release of LPS or other factors.
Endotoxemia and Inflammation
Four of the five human feeding trials that documented postprandial endotoxemia also sought evidence of concurrent inflammation. A meal consisting of egg and sausage muffin sandwiches with hash browns (900 kcal, 51 g fat) caused activation of circulating mononuclear and polymorphonuclear cells.14 The immune response included increased generation of reactive oxygen species (ROS), as well as increased expression of peptidoglycan-sensing TLR2, and the LPS-sensing TLR4.16 The moderate fat meal (882 kcal, 33 g fat) used to induce endotoxemia by Laugerette, et al13 increased circulating IL-6 and the TLR4 co-receptor, CD14. These findings are in line with other studies describing acute postprandial elevation of IL-6 (Table 1). Finally, Deopurkar, et al15 compared 300 kcal of cream, glucose drink, and orange juice. Only the cream caused a rise in plasma LPS and mononuclear cell TLR4, yet the cream and the glucose drink increased several inflammatory markers, including mononuclear cell NF-κB binding activity, TNF-α, and IL-1β, indicating that not all aspects of postprandial inflammation are dependent on endotoxemia. In fact, it remains difficult to attribute postprandial inflammation directly to translocation of gut-derived LPS. Although an acute elevation of circulating IL-6 is typically induced by both high-fat meals and direct LPS infusion in healthy participants (Tables 1, 2), other mediators do not correlate. For example, the effect of a high fat meal on circulating TNF-α and CRP is highly variable and often at odds with the consistent elevation that follows direct LPS infusion (Tables 1, 2). Some evidence indicates that circulating LPS following a meal is less “toxic” than native LPS. For example, Erridge, et al17 report median postprandial LPS at 12.3 pg/mL and that human plasma supplemented with commercially available LPS to 10 pg/mL induced adhesion molecule expression in human primary aortic endothelial cells and TNF-α in freshly collected human monocytes. Surprisingly, plasma samples from their clinical study were not able to produce these effects despite containing even higher concentrations of LPS. This may reflect a weakness in the predominant method used to quantify LPS (Limulus Amebocyte Lysate assay), which does not differentiate between the “toxic” (diphosphoryl) and “nontoxic” (monophosphoryl) LPS59 (see later). Moreover, LPS-independent mechanisms may account for postprandial inflammation. For example, free fatty acids alone can induce expression of TNF-α and IL-6 in macrophages, adipocytes, and adipose tissue in a TLR4 dependent pathway.60 Thus it remains difficult to appraise the contribution of gut-derived LPS to postprandial inflammation in humans. Another area of uncertainty surrounds the phenomenon of LPS tolerance, in which prior exposure to LPS renders an individual less responsive to subsequent challenge61. Little is known about induction of LPS tolerance in the context of postprandial endotoxemia, but such a scenario is imaginable.
Table 1.
Acute effects of a single high-fat meal on inflammatory indices in plasma/serum from healthy subjects
| Test Meal | LPS | IL-6 | TNF-α | CRP | Reference |
|---|---|---|---|---|---|
| 910 kcal (50% fat, 15% protein, 39% CHO) | ↑ | ↔ | ↔ | Ghanim16 | |
| 882 kcal (33% fat, protein/CHO not reported) | ↑ | ↑ | Laugerette13 | ||
| 900 kcal (50% fat, protein/CHO not reported) | ↑ | ↔ | Erridge17 | ||
| 923–970 kcal (42–45% fat, 6–12% protein, 40–46% CHO) | ↓ | ↓ | ↓ | Nestel95 | |
| 778 kcal (60% fat, 12% protein, 28% CHO) | Esposito96 | ||||
| 1800–2200 kcal (64% fat, 18% protein, 18% fat) | ↑ | ↓ | ↔ | Blackburn97 | |
| 1076 kcal (67% fat, 4% protein, 30% CHO) | ↑ | ↔ | Orban98 | ||
| 760 kcal (59% fat, 12% protein, 29% CHO) | ↑ | ↑ | Nappo99 | ||
| Kcal not reported: 50 g fat and 3.75 g glucose/m2 body surface area (97% fat, 3% CHO) | ↑ | ↔ | van Wijk100 | ||
| 658–869 kcal (68–77% fat, 9–12% protein, 16–22% CHO) | ↓ | ↔ | Manning86 | ||
| 485–543 kcal (50 E% fat, 20 E% protein, 30 E% CHO) | ↑ | El-Khoury101 | |||
| 910 kcal (50% fat, 14% protein, 36% CHO) | ↑* | Aljada102 | |||
| 415 kcal (100% fat) | ↔ or ↓ | Papageorgiou103 | |||
| 621 kcal (76% fat, 3% protein, 21% CHO) | ↓ | ↔ | Tholstrup104 | ||
| 748 kcal (71% fat, 5% protein, 23% CHO) | ↑ | ↓ | ↔ | Poppitt105 | |
| 553 (51% fat, 11% protein, 38% CHO) | ↔ | Igarashi106 | |||
| 879 kcal (51% fat, protein/CHO not reported) | ↔ | Tsai107 | |||
| 675 kcal (100% fat) | ↔ | Zahedi108 | |||
| 1000 kcal (60% fat, 13% protein, 27% CHO) | ↑ | Lundman109 | |||
| Kcal not reported. 1 g fat/kg (100% fat) | ↑ | ↔ | Dekker110 | ||
| 1600–2200 kcal (64% fat, 18% protein, 18% CHO) | ↑ | ↔ or ↓ | ↔ | Payette111 |
Table 2.
Acute Effects of LPS infusion on inflammatory indices in plasma/serum from healthy subjects
| LPS Dose | IL-6 | TNF-α | CRP | Reference |
|---|---|---|---|---|
| 2 ng/kg | ↑ | ↑ | ↑ | van der Meer112 |
| 2 ng/kg | ↑ | ↑ | ↑ | Dorresteijn113 |
| 2 ng/kg | ↑ | ↑ | Sauermann114 | |
| 2 ng/kg | ↑ | ↑ | van Eijk115 | |
| 2 ng/kg | ↑ | ↑ | Soop116 | |
| 2 ng/kg | ↑ | ↑ | Mayr117 | |
| 20 IU/kg | ↑ | Steiner118 | ||
| 20 IU/kg | ↑ | ↑ | ↑ | van Zee119 |
| 4 ng/kg | ↑ | ↑ | Pajkrt120 | |
| 4 ng/kg | ↑ | ↑ | Pajkrt121 | |
| 4 ng/kg | ↑ | Dekkers122 | ||
| 4 ng/kg | ↑ | ↑ | de Jonge123 | |
| 4 ng/kg | Lauw124 | |||
| 4 ng/kg | ↑ | ↑ | ↑ | Bunnell125 |
| 0.8 ng/kg | ↑ | ↑ | Reichenberg126 | |
| 4 ng/kg | ↑ | ↑ | ↑ | Branger127 |
| 4 ng/kg | ↑ | ↑ | ↑ | Lynn128 |
| 4 ng/kg | ↑ | ↑ | van Bockel129 | |
| 4 ng/kg | ↑ | ↑ | de Kruif130 |
In contrast to uncertainty surrounding human data, a direct link between postprandial endotoxemia, inflammation, and morbid sequelae was thoroughly demonstrated in animal experiments by Cani, et al.31,58 In these studies, mice fed a high fat diet (72% fat, 28% protein) consumed twice the energy as controls, exhibited elevated plasma LPS, and acquired features of metabolic disease that could be reproduced by a 4-week infusion of low-dose LPS through osmotic pumps. Both oral antibiotics that prevented endotoxemia and the CD14−/− genotype with defective LPS signaling ameliorated nearly every inflammatory, oxidative, and metabolic derangement in both high-fat-fed and leptin-deficient mice. Work by de La Serre, et al54 offers insight that might reconcile the apparent conflict between human and animal data. They took advantage of an observation that some Sprague-Dawley rats are susceptible, while others resist, diet induced obesity. Obesity-resistant animals avoided excess energy intake on the high fat diet and maintained expression of intestinal alkaline phosphatase (ALPI), an enzyme that detoxifies LPS (see later). By contrast, obesity-prone rats displayed reduced ALPI activity presumably accounting for the observed increased intestinal permeability and TLR4 activation. Thus, the capacity of the gut to detoxify LPS might account for variation between animal and human data.
LPS Detoxification
It remains unclear how much of post-prandial circulating LPS is available to activate the classical TLR4-dependent inflammatory response. There are, for example, endogenous mechanisms at the various mucosal surfaces to detoxify/inactivate LPS. Standard assays used to measure LPS in serum (e.g. limulus lysate assay) are not able to distinguish LPS which is active or inactive on mammalian cells (see below).
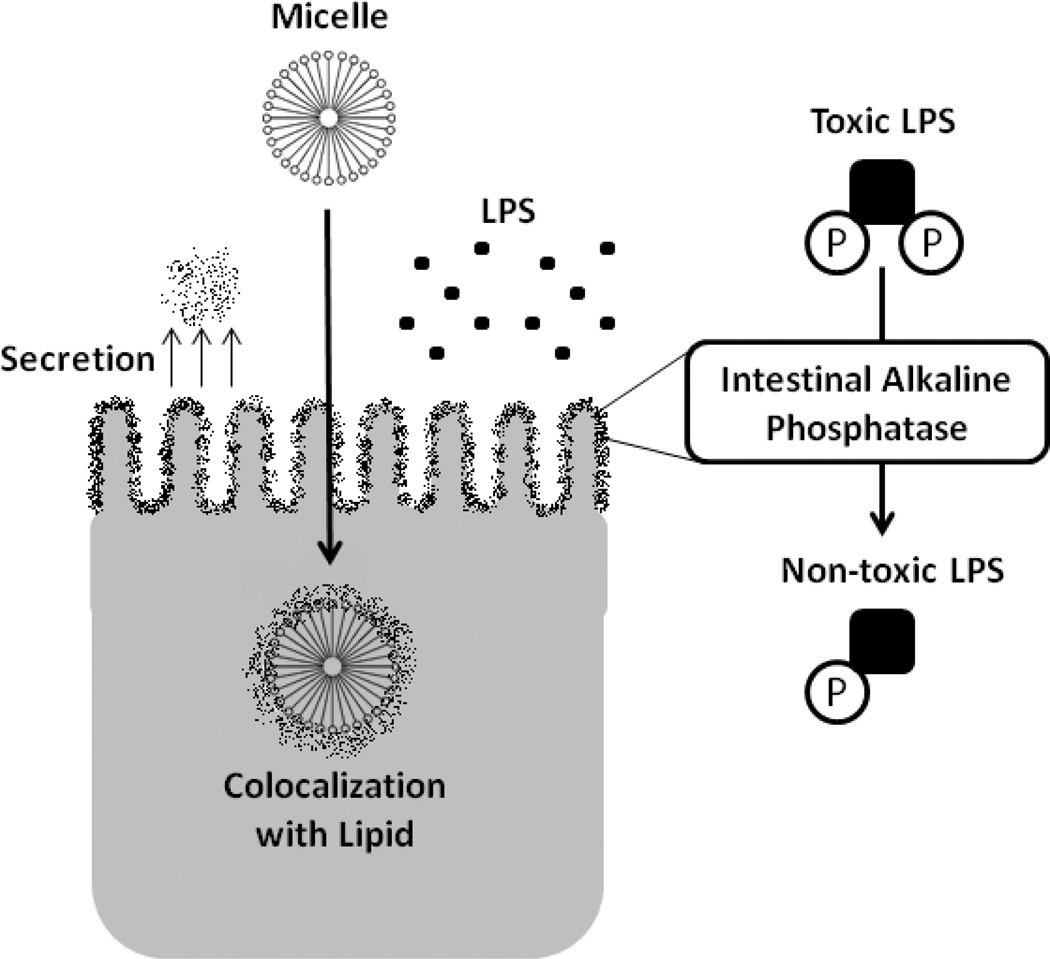
There is much recent interest in intestinal alkaline phosphatase (ALPI), a 70 kDa GPI-anchored protein expressed on the apical (luminal) aspect of intestinal epithelial cell62, in the detoxification of LPS. In the past, ALPI had been viewed as one of the better epithelial differentiation markers, with little understanding of the true function of this molecule within the mucosa. More recent studies have identified ALPI as a central player in microbial homeostasis.63–65 Surface expressed ALPI has been shown to retard Gram negative bacterial growth and to potently neutralize LPS through a mechanism involving dephosphorylation of 1,4’-bisphosphorylated glucosamine disaccharide of LPS lipid A (figure 2).64,65 The resulting monophosphoryl lipid-A is unable to initiate the classical LPS-dependent inflammatory response. ALPI is active beyond the epithelial surface. It was shown to be secreted into the lumen and impart a LPS-dephosphorylating property to the stool.63 Furthermore, intracellular colocalization with absorbed lipid droplets provide further opportunity for LPS detoxification (figure 2).66 In a striking demonstration, administration of calf ALPI prevented death of mice injected with a lethal dose of E. coli.67 ALPI appears to affect live bacteria as well. In an animal model, oral administration prevented translocation of live bacteria to the mesenteric lymph nodes following intestinal injury.63 A role for ALPI in shaping the gut microbiota has been recently identified wherein it maintains an environment favorable to commensal organisms and inhibitory to pathogenic Salmonella typhimurium.68 Relevant for this review, ALPI was recently shown to be highly induced by resolvin E1 (RvE1), an omega-3 fatty acid-derived lipid mediator which promotes the resolution of inflammation.69 In this study, Cambell et al screened epithelial cells expressing the RvE1 receptor by microarray and revealed a prominent and specific induction of ALPI by RvE1. Surface expressed ALPI was shown to detoxify extracellular LPS and to retard the growth of E. coli. Likewise, administration of RvE1 to mice in an in vivo colitis model revealed that decreased disease activity strongly paralleled tissue ALPI levels and that inhibition of ALPI reversed such protection. These data provide a previously unappreciated role for ALPI in omega-3 fatty acid-mediated inflammatory resolution.
Figure 2.
Intestinal alkalkine phosphatase (ALPI) is an important enzyme in LPS detoxification. This highly expressed brush border enzyme is secreted into the lumen and co-localizes with intracellular lipid, maximizing contact with LPS. Under physiologic conditions, this enzyme converts the “toxic” LPS moiety (diphosphoryl lipid-A) to a less inflammatory form (monophosphoryl lipid-A). Of clinical importance, expression of ALPI decreases during fasting, but is maintained by enteral nutrition.
LPS sequestration may also prevent an inflammatory response during postprandial endotoxemia. Possible mechanisms include anti-LPS antibodies70 and LBP mediated uptake by chylomicrons,30,39–41 LDL,44 and HDL.42,43 LBP itself has a dual role; at low concentrations it can facilitate LPS-TLR4 signaling, whereas at high concentrations LBP paradoxically blocks the inflammatory effects of LPS.71
Other antimicrobial peptides exist which can inactivate or detoxify LPS. For example, bactericidal/permeability increasing protein (BPI) shares structurally similarity with LBP and is capable of binding and neutralizing LPS. BPI is a 55–60 kDa protein originally found in neutrophil azurophilic granules, on the neutrophil cell surface, and to a lesser extent, in specific granules of eosinophils.72,73 Subsequently, BPI was found to be widely expressed in various epithelial cells.74 As its name infers, BPI selectively exerts multiple antimicrobial actions against Gram-negative bacteria, including cytotoxicity through damage to bacterial inner / outer membranes, neutralization of bacterial lipopolysaccharide (endotoxin), as well as serving as an opsonin for phagocytosis of gram-negative bacteria by neutrophils.75–77 The high affinity of BPI for the lipid A region of LPS78 targets its cytotoxic activity to Gram-negative bacteria. Binding of BPI to the Gram-negative bacterial outer membrane is followed by a time-dependent penetration of the molecule to the bacterial inner membrane where damage results in loss of membrane integrity, dissipation of electrochemical gradients, and bacterial death79. BPI binds the lipid A region of LPS with high affinity80,81 and thereby prevents its interaction with other (pro-inflammatory) LPS-binding molecules, including LBP and CD14.82 Since BPI binds the lipid A region common to all LPS, it is able to neutralize endotoxin from a broad array of Gram-negative pathogens.75 The selective and potent action of BPI against Gram-negative bacteria and their LPS is fully manifest in biologic fluids, including plasma, serum, and whole blood.83 In multiple animal models of Gram-negative sepsis and/or endotoxemia, administration of BPI congeners is associated with improved outcome.84,85
Implications and Future Directions for Clinical Practice
Postprandial endotoxemia is dependent on dietary selection. Ghanim, et al16 found that a 900-kcal “American Heart Association” (AHA) meal of oatmeal, milk, orange juice, raisins, peanut butter, and English muffin prevented postprandial endotoxemia, whereas an isocaloric meal of egg and sausage muffin sandwiches with hashbrowns could not. The former meal also prevented rise in various markers of oxidative stress, NF-κB activity, TLR2 and TLR4expression. They later reported that addition of 300 kcal of orange juice to an endotoxemia-producing meal prevented any rise in circulating LPS.14 Despite ingesting 1,200 kcal, the addition of orange juice also ameliorated indicators of inflammation and oxidative stress compared to a glucose drink and water only control. An assortment of foods and dietary components can reduce markers of inflammation including wheat bran,86 olive oil,87–89 walnuts,87,90 and strawberry anthocyanin.91 Additional studies are merited to determine wither the anti-inflammatory properties of these foods are related to an effect on LPS mediated inflammation. For example, parenteral administration of dietary components such as quercetin92 and curcumin93 can attenuate the effects of LPS infusion, although such a response from normal consumption of these food components remains to be demonstrated. An important point for those practicing clinical nutrition is that expression of ALPI decreases during fasting, and is restored upon refeeding.94 This may underlie the benefit that patients receive from trophic feeding in acute illness.63 More practical knowledge in this area is likely to emerge. For example, ALPI expression was augmented by an omega-3 fatty acid derived compound, Resolvin E1, setting a precedent that specific dietary components might influence expression of this protective enzyme.69
Much remains to be learned about the phenomenon of postprandial endotoxemia and how diet and gut microbiota mediate chronic inflammation. Despite results from animal experiments that convincingly demonstrate a role in pathology, whether postprandial endotoxemia mediates postprandial inflammation and pathology in humans remains to be determined. It is tempting to speculate that interpersonal variability in LPS detoxification renders some individuals susceptible and others resistant to the outcomes of endotoxemia. To date, feeding trials have relied on healthy individuals who may have a higher capacity for LPS detoxification compared with morbid individuals. Nevertheless, the dramatic ability of specific foods and meals to prevent endotoxemia predicts that postprandial endotoxemia may become an important target for nutritional intervention.
Acknowledgments
Financial support: This work was supported in part by NIH grant HG005964 (DNF), TL1 RR025778 (CJK).
Footnotes
Financial disclosures: none
Conflicts of Interest: none
References
- 1.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual review of nutrition. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 2.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007 Aug 21;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 4.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005 Jun 10;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Relman DA. New technologies, human-microbe interactions, and the search for previously unrecognized pathogens. The Journal of infectious diseases. 2002 Dec 1;186(Suppl 2):S254–S258. doi: 10.1086/344935. [DOI] [PubMed] [Google Scholar]
- 7.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends in microbiology. 2010 Jul;18(7):298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. The American journal of clinical nutrition. 1999 May;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 9.Savage DC. Microbial ecology of the gastrointestinal tract. Annual review of microbiology. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 10.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008 Feb;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Ravin HA, Rowley D, Jenkins C, Fine J. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. The Journal of experimental medicine. 1960 Nov 1;112:783–792. doi: 10.1084/jem.112.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annual review of nutrition. 2011 Aug 21;31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 13.Laugerette F, Vors C, Geloen A, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. The Journal of nutritional biochemistry. 2011 Jan;22(1):53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. The American journal of clinical nutrition. 2010 Apr;91(4):940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes care. 2010 May;33(5):991–997. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes care. 2009 Dec;32(12):2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. The American journal of clinical nutrition. 2007 Nov;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Haghiac M, Surace P, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011 Mar;19(3):476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassenius MI, Pietilainen KH, Kaartinen K, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes care. 2011 Aug;34(8):1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes care. 2011 Feb;34(2):392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Yu Z, Ye X, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes care. 2010 Sep;33(9):1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alisi A, Manco M, Devito R, Piemonte F, Nobili V. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. Journal of pediatric gastroenterology and nutrition. 2010 Jun;50(6):645–649. doi: 10.1097/MPG.0b013e3181c7bdf1. [DOI] [PubMed] [Google Scholar]
- 23.Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop PH, Lehto M. Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes care. 2009 Sep;32(9):1689–1693. doi: 10.2337/dc09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arteriosclerosis, thrombosis, and vascular biology. 2007 Jun;27(6):1433–1439. doi: 10.1161/ATVBAHA.106.138743. [DOI] [PubMed] [Google Scholar]
- 25.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998 Sep 17;395(6699):284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 26.Gamble L, Bagby GJ, Quinton LJ, et al. The systemic and pulmonary LPS binding protein response to intratracheal lipopolysaccharide. Shock. 2009 Feb;31(2):212–217. doi: 10.1097/SHK.0b013e31817c0d7d. [DOI] [PubMed] [Google Scholar]
- 27.Grube BJ, Cochane CG, Ye RD, et al. Lipopolysaccharide binding protein expression in primary human hepatocytes and HepG2 hepatoma cells. The Journal of biological chemistry. 1994 Mar 18;269(11):8477–8482. [PubMed] [Google Scholar]
- 28.Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. Journal of dental research. 2005 Jul;84(7):584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 29.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. The American journal of clinical nutrition. 2008 May;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. Journal of lipid research. 2009 Jan;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008 Jun;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 32.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. American journal of physiology. Gastrointestinal and liver physiology. 2007 Feb;292(2):G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 33.Kvietys PR, Specian RD, Grisham MB, Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. The American journal of physiology. 1991 Sep;261(3 Pt 1):G384–G391. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- 34.Velasquez OR, Place AR, Tso P, Crissinger KD. Developing intestine is injured during absorption of oleic acid but not its ethyl ester. The Journal of clinical investigation. 1994 Feb;93(2):479–485. doi: 10.1172/JCI116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velasquez OR, Henninger K, Fowler M, Tso P, Crissinger KD. Oleic acid-induced mucosal injury in developing piglet intestine. The American journal of physiology. 1993 Mar;264(3 Pt 1):G576–G582. doi: 10.1152/ajpgi.1993.264.3.G576. [DOI] [PubMed] [Google Scholar]
- 36.Kvietys PR, Wilborn WH, Granger DN. Effect of atropine on bile-oleic acid-induced alterations in dog jejunal hemodynamics, oxygenation, and net transmucosal water movement. Gastroenterology. 1981 Jan;80(1):31–38. [PubMed] [Google Scholar]
- 37.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. The Journal of experimental medicine. 2002 Mar 4;195(5):559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurfel MM, Wright SD. Lipopolysaccharide-binding protein and soluble CD14 transfer lipopolysaccharide to phospholipid bilayers: preferential interaction with particular classes of lipid. J Immunol. 1997 Apr 15;158(8):3925–3934. [PubMed] [Google Scholar]
- 39.Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van 't Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003 Feb 1;170(3):1399–1405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 40.Read TE, Grunfeld C, Kumwenda ZL, et al. Triglyceride-rich lipoproteins prevent septic death in rats. The Journal of experimental medicine. 1995 Jul 1;182(1):267–272. doi: 10.1084/jem.182.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris HW, Grunfeld C, Feingold KR, et al. Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. The Journal of clinical investigation. 1993 Mar;91(3):1028–1034. doi: 10.1172/JCI116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. The Journal of experimental medicine. 1994 Sep 1;180(3):1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulevitch RJ, Johnston AR, Weinstein DB. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. The Journal of clinical investigation. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navab M, Hough GP, Van Lenten BJ, Berliner JA, Fogelman AM. Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. The Journal of clinical investigation. 1988 Feb;81(2):601–605. doi: 10.1172/JCI113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drewe J, Beglinger C, Fricker G. Effect of ischemia on intestinal permeability of lipopolysaccharides. European journal of clinical investigation. 2001 Feb;31(2):138–144. doi: 10.1046/j.1365-2362.2001.00792.x. [DOI] [PubMed] [Google Scholar]
- 46.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. The American journal of clinical nutrition. 2011 Jul;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010 Jan;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proceedings of the National Academy of Sciences of the United States of America. 2009 Feb 17;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. The American journal of clinical nutrition. 2008 Oct;88(4):894–899. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 50.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008 Nov;32(11):1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 51.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010 Dec;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santacruz A, Marcos A, Warnberg J, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009 Oct;17(10):1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 53.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2009 Jul;33(7):758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 54.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2010 Aug;299(2):G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009 Nov;137(5):1716–1724. e1711–1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berg RD. The indigenous gastrointestinal microflora. Trends in microbiology. 1996 Nov;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007 Nov;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 58.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007 Jul;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 59.Takayama K, Qureshi N, Raetz CR, et al. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infection and immunity. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006 Nov;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kox M, de Kleijn S, Pompe JC, et al. Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Critical care medicine. 2011 Aug;39(8):1866–1870. doi: 10.1097/CCM.0b013e3182190d5d. [DOI] [PubMed] [Google Scholar]
- 62.Vaishnava S, Hooper LV. Alkaline phosphatase: keeping the peace at the gut epithelial surface. Cell host & microbe. 2007 Dec 13;2(6):365–367. doi: 10.1016/j.chom.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg RF, Austen WG, Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proceedings of the National Academy of Sciences of the United States of America. 2008 Mar 4;105(9):3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moyle PM, Toth I. Self-adjuvanting lipopeptide vaccines. Current medicinal chemistry. 2008;15(5):506–516. doi: 10.2174/092986708783503249. [DOI] [PubMed] [Google Scholar]
- 65.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007 Jun 15;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 66.Mahmood A, Shao JS, Alpers DH. Rat enterocytes secrete SLPs containing alkaline phosphatase and cubilin in response to corn oil feeding. American journal of physiology. Gastrointestinal and liver physiology. 2003 Aug;285(2):G433–G441. doi: 10.1152/ajpgi.00466.2002. [DOI] [PubMed] [Google Scholar]
- 67.Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. The Journal of pharmacology and experimental therapeutics. 2003 Nov;307(2):737–744. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 68.Malo MS, Alam SN, Mostafa G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010 Nov;59(11):1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 69.Campbell EL, MacManus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proceedings of the National Academy of Sciences of the United States of America. 2010 Aug 10;107(32):14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buttenschoen K, Berger D, Strecker W, et al. Association of endotoxemia and production of antibodies against endotoxins after multiple injuries. The Journal of trauma. 2000 May;48(5):918–923. doi: 10.1097/00005373-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 71.Schumann RR. Recognition of Bacterial Endotoxin and Modulation of the Inflammatory Response: The LBP/CD14 Pathway During the Acute Phase. Sepsis. 1998;2:149–155. [Google Scholar]
- 72.Canny G, Levy O. Bactericidal/permeability-increasing protein (BPI) and BPI homologs at mucosal sites. Trends in immunology. 2008 Nov;29(11):541–547. doi: 10.1016/j.it.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Canny G, Cario E, Lennartsson A, et al. Functional and biochemical characterization of epithelial bactericidal/permeability-increasing protein. American journal of physiology. Gastrointestinal and liver physiology. 2006 Mar;290(3):G557–G567. doi: 10.1152/ajpgi.00347.2005. [DOI] [PubMed] [Google Scholar]
- 74.Canny G, Levy O, Furuta GT, et al. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2002 Mar 19;99(6):3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levy O, Sisson RB, Kenyon J, Eichenwald E, Macone AB, Goldmann D. Enhancement of neonatal innate defense: effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein on growth and tumor necrosis factor-inducing activity of gram-negative bacteria tested in neonatal cord blood ex vivo. Infection and immunity. 2000 Sep;68(9):5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elsbach P. The bactericidal/permeability-increasing protein (BPI) in antibacterial host defense. Journal of leukocyte biology. 1998 Jul;64(1):14–18. doi: 10.1002/jlb.64.1.14. [DOI] [PubMed] [Google Scholar]
- 77.Elsbach P, Weiss J. Role of the bactericidal/permeability-increasing protein in host defence. Current opinion in immunology. 1998 Feb;10(1):45–49. doi: 10.1016/s0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 78.Gazzano-Santoro H, Parent JB, Grinna L, et al. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infection and immunity. 1992 Nov;60(11):4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mannion BA, Weiss J, Elsbach P. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. The Journal of clinical investigation. 1990 Mar;85(3):853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Current opinion in immunology. 1999 Feb;11(1):19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 81.Levy O, Ooi CE, Elsbach P, Doerfler ME, Lehrer RI, Weiss J. Antibacterial proteins of granulocytes differ in interaction with endotoxin. Comparison of bactericidal/permeability-increasing protein, p15s, and defensins. J Immunol. 1995 May 15;154(10):5403–5410. [PubMed] [Google Scholar]
- 82.Gazzano-Santoro H, Meszaros K, Birr C, et al. Competition between rBPI23, a recombinant fragment of bactericidal/permeability-increasing protein, and lipopolysaccharide (LPS)-binding protein for binding to LPS and gram-negative bacteria. Infection and immunity. 1994 Apr;62(4):1185–1191. doi: 10.1128/iai.62.4.1185-1191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell EL, Serhan CN, Colgan SP. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol. 2011 Oct 1;187(7):3475–3481. doi: 10.4049/jimmunol.1100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin Y, Leach WJ, Ammons WS. Synergistic effect of a recombinant N-terminal fragment of bactericidal/permeability-increasing protein and cefamandole in treatment of rabbit gram-negative sepsis. Antimicrobial agents and chemotherapy. 1996 Jan;40(1):65–69. doi: 10.1128/aac.40.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans TJ, Carpenter A, Moyes D, Martin R, Cohen J. Protective effects of a recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in an animal model of gram-negative sepsis. The Journal of infectious diseases. 1995 Jan;171(1):153–160. doi: 10.1093/infdis/171.1.153. [DOI] [PubMed] [Google Scholar]
- 86.Manning PJ, Sutherland WH, McGrath MM, de Jong SA, Walker RJ, Williams MJ. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity (Silver Spring) 2008 Sep;16(9):2046–2052. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- 87.Jimenez-Gomez Y, Lopez-Miranda J, Blanco-Colio LM, et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009 Jun;204(2):e70–e76. doi: 10.1016/j.atherosclerosis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Pacheco YM, Lopez S, Bermudez B, Abia R, Villar J, Muriana FJ. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. The Journal of nutritional biochemistry. 2008 Mar;19(3):200–205. doi: 10.1016/j.jnutbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Bogani P, Galli C, Villa M, Visioli F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis. 2007 Jan;190(1):181–186. doi: 10.1016/j.atherosclerosis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Cortes B, Nunez I, Cofan M, et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. Journal of the American College of Cardiology. 2006 Oct 17;48(8):1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 91.Edirisinghe I, Banaszewski K, Cappozzo J, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin. The British journal of nutrition. 2011 Sep;106(6):913–922. doi: 10.1017/S0007114511001176. [DOI] [PubMed] [Google Scholar]
- 92.Tang D, Kang R, Xiao W, et al. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. American journal of respiratory cell and molecular biology. 2009 Dec;41(6):651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen HW, Kuo HT, Chai CY, Ou JL, Yang RC. Pretreatment of curcumin attenuates coagulopathy and renal injury in LPS-induced endotoxemia. Journal of endotoxin research. 2007;13(1):15–23. doi: 10.1177/0968051907078605. [DOI] [PubMed] [Google Scholar]
- 94.Hodin RA, Graham JR, Meng S, Upton MP. Temporal pattern of rat small intestinal gene expression with refeeding. The American journal of physiology. 1994 Jan;266(1 Pt 1):G83–G89. doi: 10.1152/ajpgi.1994.266.1.G83. [DOI] [PubMed] [Google Scholar]
- 95.Nestel PJ, Pally S, Macintosh GL, et al. Circulating inflammatory and atherogenic biomarkers are not increased following single meals of dairy foods. European journal of clinical nutrition. 2011 Aug 3; doi: 10.1038/ejcn.2011.134. [DOI] [PubMed] [Google Scholar]
- 96.Esposito K, Nappo F, Giugliano F, et al. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. The American journal of clinical nutrition. 2003 Dec;78(6):1135–1140. doi: 10.1093/ajcn/78.6.1135. [DOI] [PubMed] [Google Scholar]
- 97.Blackburn P, Despres JP, Lamarche B, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006 Oct;14(10):1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 98.Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. The Journal of clinical endocrinology and metabolism. 1999 Jun;84(6):2126–2133. doi: 10.1210/jcem.84.6.5747. [DOI] [PubMed] [Google Scholar]
- 99.Nappo F, Esposito K, Cioffi M, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. Journal of the American College of Cardiology. 2002 Apr 3;39(7):1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 100.van Wijk JP, Cabezas MC, Coll B, Joven J, Rabelink TJ, de Koning EJ. Effects of rosiglitazone on postprandial leukocytes and cytokines in type 2 diabetes. Atherosclerosis. 2006 May;186(1):152–159. doi: 10.1016/j.atherosclerosis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Khoury DE, Hwalla N, Frochot V, Lacorte JM, Chabert M, Kalopissis AD. Postprandial metabolic and hormonal responses of obese dyslipidemic subjects with metabolic syndrome to test meals, rich in carbohydrate, fat or protein. Atherosclerosis. 2010 May;210(1):307–313. doi: 10.1016/j.atherosclerosis.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. The American journal of clinical nutrition. 2004 Apr;79(4):682–690. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- 103.Papageorgiou N, Tousoulis D, Psaltopoulou T, et al. Divergent anti-inflammatory effects of different oil acute consumption on healthy individuals. European journal of clinical nutrition. 2011 Apr;65(4):514–519. doi: 10.1038/ejcn.2011.8. [DOI] [PubMed] [Google Scholar]
- 104.Tholstrup T, Teng KT, Raff M. Dietary cocoa butter or refined olive oil does not alter postprandial hsCRP and IL-6 concentrations in healthy women. Lipids. 2011 Apr;46(4):365–370. doi: 10.1007/s11745-011-3526-4. [DOI] [PubMed] [Google Scholar]
- 105.Poppitt SD, Keogh GF, Lithander FE, et al. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition. 2008 Apr;24(4):322–329. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Igarashi M, Hirata A, Yamauchi T, et al. Clinical utility and approach to estimate postprandial hypertriglycemia by a newly designed oral fat-loading test. Journal of atherosclerosis and thrombosis. 2003;10(5):314–320. doi: 10.5551/jat.10.314. [DOI] [PubMed] [Google Scholar]
- 107.Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci (Lond) 2004 Mar;106(3):315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- 108.Zahedi RG, Summers LK, Lumb P, Chik G, Crook MA. The response of serum sialic acid and other acute phase reactants to an oral fat load in healthy humans. European journal of internal medicine. 2001 Dec;12(6):510–514. doi: 10.1016/s0953-6205(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 109.Lundman P, Boquist S, Samnegard A, et al. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2007 Mar;17(3):195–202. doi: 10.1016/j.numecd.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 110.Dekker MJ, Wright AJ, Mazurak VC, et al. Fasting triacylglycerol status, but not polyunsaturated/saturated fatty acid ratio, influences the postprandial response to a series of oral fat tolerance tests. The Journal of nutritional biochemistry. 2009 Sep;20(9):694–704. doi: 10.1016/j.jnutbio.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 111.Payette C, Blackburn P, Lamarche B, et al. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations. Metabolism: clinical and experimental. 2009 Nov;58(11):1593–1601. doi: 10.1016/j.metabol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 112.van der Meer W, Pickkers P, Scott CS, van der Hoeven JG, Gunnewiek JK. Hematological indices, inflammatory markers and neutrophil CD64 expression: comparative trends during experimental human endotoxemia. Journal of endotoxin research. 2007;13(2):94–100. doi: 10.1177/0968051907079101. [DOI] [PubMed] [Google Scholar]
- 113.Dorresteijn MJ, van Eijk LT, Netea MG, Smits P, van der Hoeven JG, Pickkers P. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. Journal of endotoxin research. 2005;11(5):287–293. doi: 10.1179/096805105X58715. [DOI] [PubMed] [Google Scholar]
- 114.Sauermann R, Marsik C, Steiner I, et al. Immunomodulatory effects of fosfomycin in experimental human endotoxemia. Antimicrobial agents and chemotherapy. 2007 May;51(5):1879–1881. doi: 10.1128/AAC.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Critical care medicine. 2007 Jun;35(6):1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 116.Soop A, Albert J, Weitzberg E, Bengtsson A, Nilsson CG, Sollevi A. Nicotinamide does not influence cytokines or exhaled NO in human experimental endotoxaemia. Clinical and experimental immunology. 2004 Jan;135(1):114–118. doi: 10.1111/j.1365-2249.2004.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mayr FB, Spiel A, Leitner J, et al. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. American journal of respiratory and critical care medicine. 2005 Feb 15;171(4):354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 118.Steiner S, Speidl WS, Pleiner J, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 2005 Apr 12;111(14):1841–1846. doi: 10.1161/01.CIR.0000158665.27783.0C. [DOI] [PubMed] [Google Scholar]
- 119.Van Zee KJ, Coyle SM, Calvano SE, et al. Influence of IL-1 receptor blockade on the human response to endotoxemia. J Immunol. 1995 Feb 1;154(3):1499–1507. [PubMed] [Google Scholar]
- 120.Pajkrt D, Doran JE, Koster F, et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. The Journal of experimental medicine. 1996 Nov 1;184(5):1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pajkrt D, Camoglio L, Tiel-van Buul MC, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J Immunol. 1997 Apr 15;158(8):3971–3977. [PubMed] [Google Scholar]
- 122.Dekkers PE, Lauw FN, ten Hove T, et al. The effect of a metalloproteinase inhibitor (GI5402) on tumor necrosis factor-alpha (TNF-alpha) and TNF-alpha receptors during human endotoxemia. Blood. 1999 Oct 1;94(7):2252–2258. [PubMed] [Google Scholar]
- 123.de Jonge E, Dekkers PE, Creasey AA, et al. Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood. 2000 Feb 15;95(4):1124–1129. [PubMed] [Google Scholar]
- 124.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000 Sep 1;165(5):2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 125.Bunnell E, Lynn M, Habet K, et al. A lipid A analog, E5531, blocks the endotoxin response in human volunteers with experimental endotoxemia. Critical care medicine. 2000 Aug;28(8):2713–2720. doi: 10.1097/00003246-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 126.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001 May;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 127.Branger J, van den Blink B, Weijer S, et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002 Apr 15;168(8):4070–4077. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 128.Lynn M, Rossignol DP, Wheeler JL, et al. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. The Journal of infectious diseases. 2003 Feb 15;187(4):631–639. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 129.van Bockel EA, Tulleken JE, Muller Kobold AC, et al. Cardiac troponin I release and cytokine response during experimental human endotoxaemia. Intensive care medicine. 2003 Sep;29(9):1598–1600. doi: 10.1007/s00134-003-1893-x. [DOI] [PubMed] [Google Scholar]
- 130.de Kruif MD, Lemaire LC, Giebelen IA, et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J Immunol. 2007 Feb 1;178(3):1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]