Abstract
To evaluate hematopoietic niche cell populations isolated from human embryonic stem cells (hESCs), we tested the ability of hESC-derived stromal lines to support CD34+ umbilical cord blood (UCB)- and hESC-derived CD34+45+ cells in long-term culture initiating cell (LTC-IC) assays. Specifically, these hematopoietic populations were cocultured with hESC-derived mesenchymal stromal cells (hESC-MSCs) and hESC-derived endothelial cells (hESC-ECs), and then assessed for their LTC-IC potential in comparison to coculture with bone marrow (BM)-derived MSCs and the mouse stromal line M2-10B4. We found that the hESC-derived stromal lines supported LTC-ICs from UCB similar to M2-10B4 cells and better than BM-MSCs. However, none of the stromal populations supported LTC-IC from hESC-derived CD34+45+ cells. Engraftment data using the output from LTC-IC assays showed long-term repopulation (12 weeks) of NSG mice to correlate with LTC-IC support on a given stromal layer. Therefore, hESC-derived stromal lines can be used to efficiently evaluate putative hematopoietic stem/progenitor cells derived from hESCs or other cell sources.
Introduction
Quantification of putative hematopoietic stem cells (HSCs) in human bone marrow (BM) or cord blood is typically assessed by its potential to mediate long-term, multilineage engraftment when transplanted into immunodeficient murine recipients [1,2]. However, in vitro surrogate assays are extremely attractive due to their relative ease of implementation, lower cost, and improved throughput of results. The long-term culture initiating cell (LTC-IC) assay serves this purpose by quantifying the ability of putative HSCs in a given population to be cultured for an extended period, typically 5 weeks. LTC-IC readout is then quantitatively assessed by both proliferation and the ability to produce hematopoietic colony forming cells (CFC), with the CFC output at the end of the assay being proportional to the number and survival of LTC-ICs in the starting population [3]. Distinct supporting stromal layers and the addition of hematopoietic cytokines can have varying effects on the maintenance of LTC-ICs [4–8].
Along with the ability to assess putative HSCs in hematopoietic populations, the LTC-IC assay is also a valuable tool for investigating cell types and factors that play a role in HSC maintenance. Numerous in vivo studies have examined aspects of the mouse hematopoietic niche, typically in the BM or fetal liver [9–12]. The generation of knockout and transgenic mice has been extremely useful in these experiments, as it allows researchers to selectively and systematically evaluate the functional importance of individual niche factors [12,13]. However, study of the human hematopoietic niche is largely limited to the analysis of BM biopsy specimens or culture methods that attempt to recapitulate aspects of the human hematopoietic microenvironment, such as the LTC-IC assay [14–16]. As such, genetic modification of primary human BM cell populations for use in LTC-IC has been a valuable tool in dissecting niche-related gene expression that modulates HSC function, although manipulation and continued culture of these cells is often difficult [17,18]. Human embryonic stem cells (hESCs) are capable of differentiating into any adult tissue, can be cultured long term, and are easy to genetically modify [19,20]. Therefore, the prospect of using hESC-derived niche populations represents an effective, versatile way to study human HSC maintenance in vitro.
Previously, our group and others have derived both mesenchymal stromal cells (MSCs) and endothelial cells (ECs) from hESCs [21–26]. MSCs and ECs are important components of the osteogenic and vascular hematopoietic niches and hESC-derived stromal cells can provide autologous supporting cell populations for putative hESC-derived HSCs. In this study, we test the capacity of these hESC-derived stromal layers to support LTC-IC from CD34+ umbilical cord blood (UCB)- and hESC-derived CD34+CD45+ cells. Cell proliferation and LTC-IC quantification of input populations were measured over a 5-week period and compared with the use of bone marrow-derived mesenchymal stem cells (BM-MSCs) and the murine stromal line M2-10B4. Finally, we subjected the surviving LTC-IC populations to repopulation assays to determine a relationship between LTC-IC support and the ability for long-term engraftment.
Materials and Methods
Cell culture
H9 hESCs were adapted to single cell passage with TrypLE Select (Invitrogen Corp., Carlsbad, CA) as previously described and were maintained as undifferentiated cells through coculture with irradiated mouse embryonic fibroblasts as previously described [27–29]. Karyotype of TrypLE-adapted H9 was found to be normal. M2-10B4 murine BM stromal cells, mesenchymal stromal cells derived from H9 hESCs (hESC-MSCs), bone marrow (BM-MSCs), and endothelial cells derived from H9 hESCs (hESC-ECs) were derived and cultured as previously described [21,22]. For LTC-IC assays, M2-10B4, hESC-MSCs, hESC-ECs, and BM-MSCs were incubated with the appropriate cell culture media containing 10 μg/mL of mitomycin C (Accord Healthcare, Durham, NC) before attachment to gelatin-coated 24-well plates or flat-bottom 96-well plates. The use of all human tissue was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota.
Hematopoietic differentiation of hESCs as spin EBs
H9 were differentiated using the previously described spin EB method [27]. Briefly, TrypLE-adapted H9 were dissociated to a single cell suspension through a 5-min incubation with TrypLE Select. The undifferentiated H9 were plated at 3000 cells per well into untreated, round-bottom, 96-well plates in BPEL media [27] containing 40 ng/mL human stem cell factor (Peprotech, Rockey Hill, NJ), 20 ng/mL human vascular endothelial growth factor (R&D Systems, Minneapolis, MN), and 20 ng/mL human bone morphogenic protein 4 (R&D Systems). After 12 days in spin EB culture, the differentiated hESC spin EBs were harvested and made into a single cell suspension using 0.05% trypsin/EDTA (Invitrogen) supplemented with 2% chicken serum (Sigma, St. Louis, MO). The differentiated cells were analyzed through flow cytometry and hematopoietic colony-forming cell (CFC) assay for the presence of hematopoietic progenitor cells [30].
Positive selection of CD34+ and CD34+CD45+ cells through magnetic sorting
Single cell suspensions of 11–12-day H9 spin EB cultures were obtained as described above. Cells were resuspended in Dulbecco's phosphate buffered saline (ThermoFisher Hyclone, Logan, UT) containing 2% fetal bovine serum (FBS) and 1 mM EDTA (Invitrogen) before magnetic sorting. EasySep Human CD34 Positive Selection kit (StemCell Technologies, Vancouver, BC) was used to isolate CD34+ cells from differentiated hESCs. For enrichment of the CD34+CD45+ cell population, the EasySep PE Selection kit (StemCell Technologies) was used on CD34+ cells labeled with anti-human CD45-PE. CD34+ cells were positively selected through magnetic sorting from UCB mononuclear cells using the Miltenyi CD34 microbead kit (Miltenyi Biotec, Boston, MA) and AutoMACS Pro Separator (Miltenyi). CD34+ UCB were frozen down in 90% FBS+10% DMSO and stored in liquid nitrogen until use. Enrichment for CD34+/CD34+CD45+ cells was assessed through staining with mouse anti-human CD34-APC, CD45-PE, CD43-PE, CD41a-PE, CD31-PE, and the corresponding isotype controls (all from BD Biosciences, Sparks, MD). Additional analysis of stromal populations was conducted using CD73-PE, Stro-1-PE (Novus Biologicals, Littleton, CO), VEcadheren-PE, CD146-PE, CD31-APC, CD44-APC, and CD105-APC.
Bulk culture LTC-IC
M2-10B4, hESC-MSCs, hESC-ECs, and BM-MSCs were treated with mitomycin C before plating on fibronectin-coated or gelatin-coated 24-well plates at 105 to 2×105 cells/well. For a combined hESC-MSC+hESC-EC condition, 5×104 hESC-MSCs and 5×104 hESC-ECs were added to each well. Cells were allowed to adhere overnight before addition of UCB- or hESC-derived hematopoietic progenitor cells.
About 5×103 CD34+ UCB-derived or 2.5×104 to 105 CD34+CD45+ hESC-derived cells were plated per well of mitotically inactivated cells in 1 mL/well Myelocult H5100 media (StemCell Technologies) containing 10−6 M hydrocortisone (Sigma). Cultures were incubated at 37°C, 5% CO2. Cells were fed with a fresh medium by half medium changes every 7 days. At the indicated time points, cells were harvested through collection of nonadherent cells and dissociation of the adherent cell layer with 0.05% trypsin containing 2% chicken serum for 5 min. The nonadherent and adherent cells were combined and clumps removed by a 70-μm filter (BD Biosciences, Sparks, MD) for counting and transfer to hematopoietic CFC assay. Photos were taken on an Olympus CKX41 microscope attached to a Nikon D90 camera.
Hematopoietic CFC assays
CD34+ UCB- or CD34+CD45+ hESC-derived cells were cultured for the indicated number of days on M2-10B4 or the human-derived cell layers before harvest to a single cell suspension. Colony forming assays were performed by culturing these cells in semisolid Methocult H4435 Enriched (StemCell Technologies). About 2.5×104 to 5×104 UCB-derived cells or 5×104 to 105 hESC-derived cells were cultured per 35-mm culture dish (Greiner, Monroe, NC). After 14 days, the plates were scored for colony-forming units according to standard criteria [30]. For each adherent cell population, CFC yield per 103 CD34+ UCB or 105 CD34+CD45+ hESCs initially placed into the LTC-IC culture was determined by dividing the total number of cells harvested per well by the number of cells put into CFC assay, and using this factor to multiply the total number of colonies yielded by the CFC assay.
Limiting dilution CFC assay
For comparison of the LTC-IC frequency from CD34+ UCB cultured on specific feeder layers, CD34+ UCB were plated in limiting dilutions (semilog dilution from 300 to 3 cells/well) in flat-bottom 96-well plates with a confluent monolayer of M2-10B4, hESC-MSC, hESC-EC, or BM-MSC. Cells were cultured in the same media/incubation conditions as described above for bulk LTC-IC culture and were similarly provided with fresh media every 7 days. After 5 weeks of culture, cells were placed in hematopoietic CFC assay conditions by removing all but 20 μL of media from each well and replacing with 100 μL of Methocult H4435 Enriched per well. After 14 days in Methocult, wells were scored for the presence of hematopoietic colonies. The frequency of LTC-ICs from each feeder condition was calculated by Poisson distribution (L-Calc software; StemCell Technologies) based on the number of wells at each cell dose with one or more hematopoietic colonies after 5 weeks of culture.
In vivo engraftment
All animals were housed, treated, and handled in accordance with the guidelines set forth by the University of Minnesota Institutional Animal Care and Use Committee and by the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The day before engraftment, 6–8-week-old NOD-scid IL2rγnull (NSG) mice were irradiated. On the day of engraftment, LTC-ICs were harvested as described in bulk culture LTC-ICs. Cells were then enumerated, resuspended in Iscove's modified Dulbecco's medium, and injected through the tail vein at a dose equivalent of 7.5–10×103 initially seeded CD34+ UCB cells per mouse. At both 4 and 8 weeks, mice were bled through the facial vein for analysis of peripheral blood. At 12 weeks, each mouse was bled and then sacrificed for the collection of spleen and BM. All tissue samples were treated with ammonium chloride for the lysis or red blood cells, blocked with human serum, then stained with conjugated antibodies, and analyzed in a flow cytometer as described.
Results
hESC-derived stromal cells support LTC-IC from CD34+ UCB
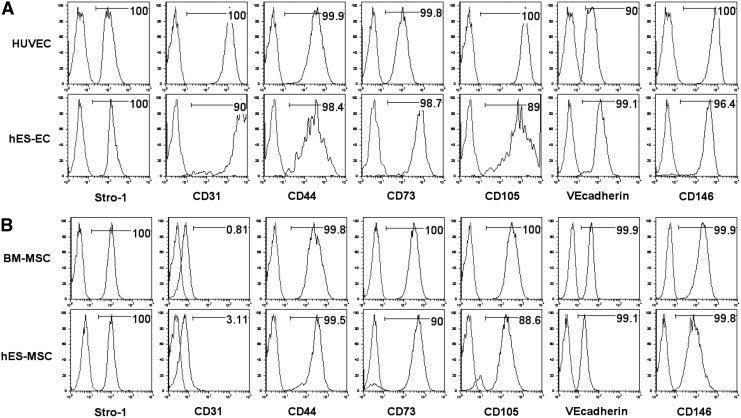
Previous studies by our group and others have derived both MSCs and ECs from hESCs [21–26]. Phenotypic analysis demonstrates our hESC-derived populations to express several common endothelial and MSC extracellular markers (Fig. 1A, B). In this study, we evaluated the ability of hESC-MSCs and hESC-ECs to support LTC-ICs using UCB- and hESC-derived hematopoietic cells compared with BM-MSCs and M2-10B4. All human cell populations (hESC-ECs, hESC-MSCs, and BM-MSCs) as well as a combined layer of hESC-MSCs and hESC-ECs, supported short-term expansion of CFCs from CD34+ cells isolated from UCB (CD34+ UCB). After 3 weeks, UCB cells cultured on hESC-derived stromal layers for three weeks produced a similar number of CFCs as compared to culture with M2-10B4, and more than with BM-MSCs (Fig. 2A). Over this time, the UCB CD34+ cell population expanded most rapidly on hESC-MSCs, hESC-ECs, and the combined hESC-MSC/EC conditions (Fig. 2B; also see Fig. 2E for photographs of representative cultures).
FIG. 1.
Phenotype analysis of hESC-derived stromal layers as compared to their primary-derived counterparts by flow cytometry. (A) Analysis of human umbilical vascular endothelial cells (HUVECs) and hESC-derived ECs. (B) Analysis of bone marrow-derived MSCs (BM-MSCs) and hESC-derived MSCs. ECs, endothelial cells; hESC, human embryonic stem cell; MSCs, mesenchymal stromal cells.
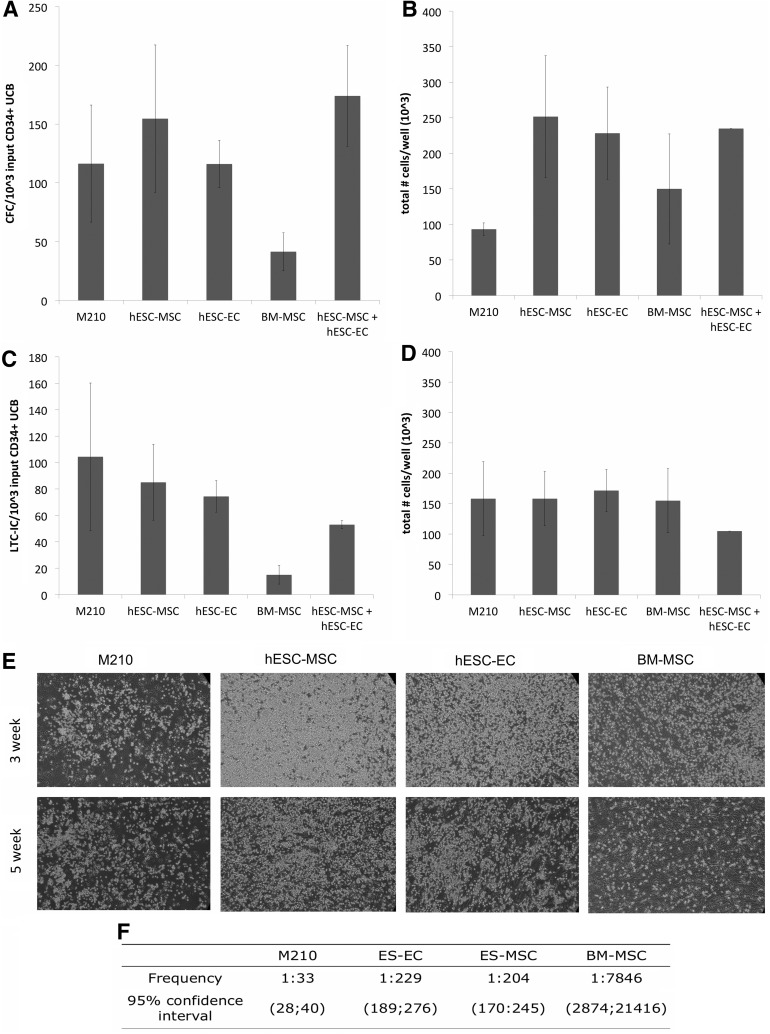
FIG. 2.
hESC-derived MSCs and ECs support CFCs and LTC-ICs from CD34+ UCB hematopoietic progenitors. For LTC-IC assays, 3–5×103 CD34+ UCB/well were cocultured with mitotically inactivated M2-10B4, hESC-MSCs, hESC-ECs, a 1:1 mix of hESC-MSCs and hESC-ECs, or human BM-MSCs, as indicated. Wells were harvested from each cell condition after 3 weeks to quantify hematopoietic CFC yield and total cell number. (A) CFC yield/well quantified based on the input of 103 CD34+ UCB cells in LTC-IC culture and (B) total cell number per well (output) after 3 weeks, as this also accounts for cell proliferation over this time course. (C, D) Studies to quantify LTC-ICs at 5 weeks were assessed based on LTC-IC yield and total cell number, similar to (A) and (B). All values shown for (A–D) are the mean±SEM of three trials, except for the combined hESC-MSC/hESC-EC condition (mean±SEM of two trials). (E) Photos of representative wells at 100×final magnification after 3 weeks (top row) and 5 weeks (bottom row) of coculture in Myelocult H5100 media. (F) Additional studies using limiting dilution LTC-IC analysis with CD34+ UCB were conducted to compare LTC-IC frequency between M210, hESC-MSCs, hESC-ECs, and BM-MSCs. CFC, colony-forming cell; CFU, colony forming units; LTC-IC, long-term culture initiating cell; UCB, umbilical cord blood; SEM, standard error of the mean.
After 5 weeks of LTC-IC conditions, both hESC-MSCs and hESC-ECs supported LTC-ICs from UCB similar to M2-10B4, yielding on average 85±29 and 74±12 LTC-IC-derived CFCs per 103 input UCB, respectively. This result compares with 104±56 CFCs per 103 input UCB cocultured with M2-10B4 cells (Fig. 2C). Likewise, UCB CD34+ cells cocultured with hESC-MSCs or hESC-ECs continued to maintain a population of nonadherent cells (Fig. 2E, bottom row) with total cell numbers of 158±78×103 and 172±60×103, respectively, which were similar to the use of M2-10B4 cells (Fig. 2D). These LTC-IC assays demonstrated the highest frequency when UCB CD34+ cells were cultured on M2-10B4 cells (1:33), although hESC-MSCs and hESC-ECs supported a far higher frequency of LTC-ICs than BM-MSCs (1:204, 1:229, and. 1:7846, respectively; Fig. 2F). These data demonstrate that the hESC-derived ECs and MSCs are able to support LTC-ICs from UCB over a 5-week period similar to M2-10B4 cells and better than BM-MSCs. Interestingly, the combination of hESC-MSCs and hESC-ECs was no better than the use of single supporting cell populations in these studies.
hESC-derived hematopoietic progenitors do not contain LTC-ICs
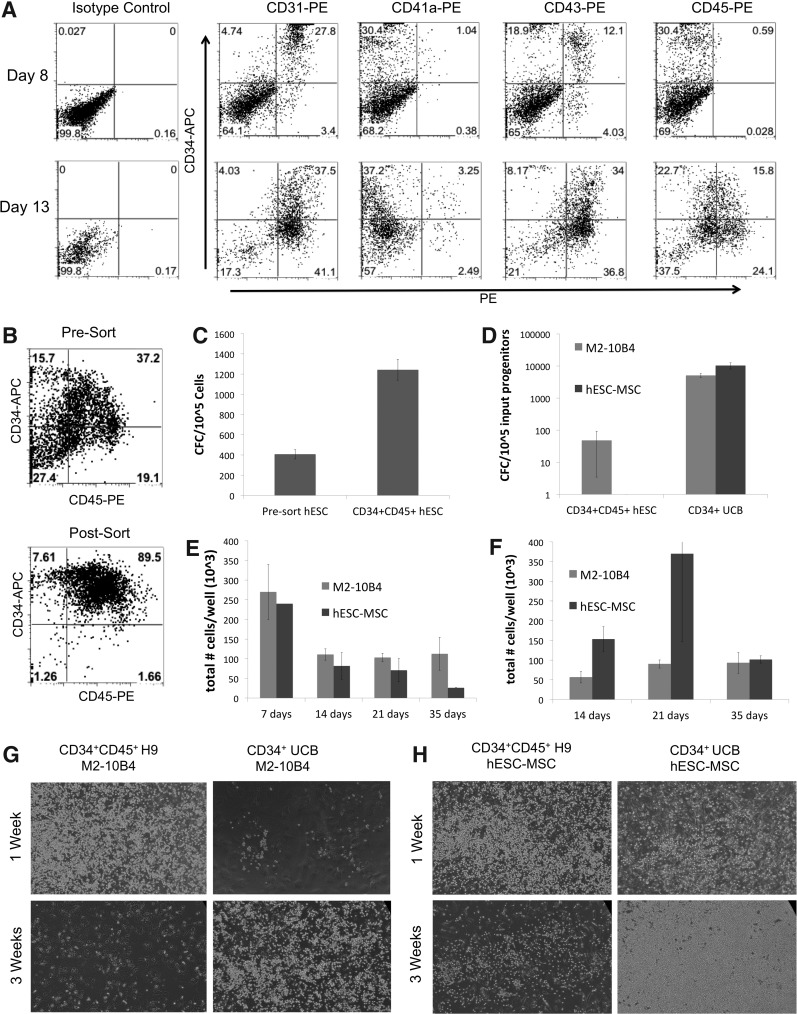
We next evaluated the ability for hESC-derived stromal cells and M2-10B4 cells to support LTC-ICs from hESC-derived CD34+CD45+ hematopoietic progenitors (Fig. 3A). With H9 hESCs, we routinely obtain over 20% CD34+ and CD34+CD31+ cells after 8 days of differentiation using the spin-EB method [27,31,32] with subsequent emergence of CD34+CD43+ and CD34+CD41a+ populations previously shown to indicate early hematopoietic development [33] (Fig. 3A, top row). Between 11–13 days of differentiation, CD34+ cells can reach over 50% of total live cells with populations of CD34+CD31+, CD34+CD43+, and CD34+CD45+ ranging from 20% to 50% of total live cells (Fig. 3A, bottom row).
FIG. 3.
CD34+CD45+ cells derived from hESCs demonstrate only a short-term hematopoietic progenitor potential. Hematopoietic differentiation of hESCs was done through spin EB culture to derive CD34+CD45+ hematopoietic progenitor cells. (A) Phenotypic analyses of hESC-derived cells at day 8 (top row) and day 13 (bottom row) of differentiation. (B) hESCs differentiated in the spin EB culture system for 12 days were magnetically sorted to enrich for CD34+CD45+ hematopoietic progenitors. Representative flow cytometry data comparing the presort and postsort (CD34+CD45+) populations are shown. (C) hESCs differentiated for 12 days produced colonies in hematopoietic CFU assay, with enrichment for CD34+CD45+ cells showing a corresponding increase in the frequency of CFCs. (D) Short-term analysis using 5–10×104 CD34+CD45+ hESCs/well and 5×103 CD34+ UCB/well cocultured in parallel on M2-10B4 cells and hESC-MSCs. After 2 weeks of culture, the CFC yield per 105 UCB- or hESC-derived cells initially plated in M2-10B4 cell or hESC-MSC coculture was quantified, with values shown as the average of three trials±SEM. (E) CD34+CD45+ hESCs were harvested at regular intervals beginning at day 7 of coculture to assess the total cell numbers. (F) CD34+ UCB cultured in parallel with hESC-derived cells were harvested at regular intervals beginning at day 14 of coculture for assessment of the total cell number. Values shown are the mean±SEM of three trials. (G, H) Photographs of representative wells of (G) M2-10B4 cell coculture and (H) hESC-MSC coculture were taken after 1 week (top row) and 3 weeks (bottom row) at 100×magnification.
hESCs allowed to differentiate for 14 days were sorted for CD34+CD45+ cells (Fig. 3B). As expected, the hESC-derived CD34+CD45+ population was enriched for hematopoietic progenitor cells, as quantified by CFC assay (Fig. 3C). However, after 2 weeks of coculture with either the hESC-MSCs or M2-10B4 cells, they were markedly reduced for CFCs. Whereas coculture of CD34+ UCB yielded 5125±725 CFCs on M2-10B4 cells after 2 weeks, progenitors from hESCs cultured on M2-10B4 cells yielded relatively few CFCs (averaging 48±45 CFCs per 105 initial CD34+CD45+ hESCs; Fig. 3D). After more than 2 weeks of M2-10B4 cell coculture, hematopoietic progenitor cells (CFCs) were absent from the hESC-derived cell population. Using hESC-MSCs as supportive stromal cells, CD34+ UCB yielded 12767±5525 CFCs, with hESC-derived progenitors yielding no hematsopoietic colonies (Fig. 3D). CD34+CD45+ hESC-derived cells did not maintain a nonadherent population in prolonged (5 week) culture on M210 or hESC-MSCs, with the total cell number declining markedly after 1 week of culture, unlike CD34+ UCB cells (Fig. 3F–H).
Other hESC-derived populations also failed to demonstrate LTC-IC potential. CD34+CD43+ cells derived from hESCs differentiated for 8 days and CD34+CD45+ cells isolated from hESCs differentiated for a longer period of time (12–15 days) both proliferated for the first 1–2 weeks of culture in LTC-IC and then started to decline (data not shown). Both cell populations failed to produce colonies in hematopoietic CFC assay after more than 2 weeks of culture (data not shown).
Stromal support of LTC-IC from CD34+ UCB predicts engraftment potential
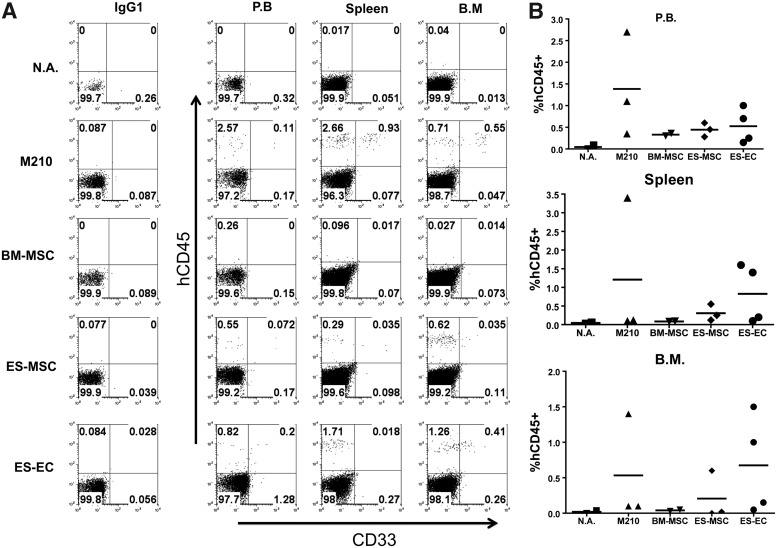
We next wanted to determine whether the ability of our hESC-derived stromal layers to support LTC-IC from CD34+ UCB correlated with the engraftment potential of the hematopoietic cells after 5 weeks in culture. To do so, we performed LTC-IC assays with CD34+ UCB using M2-10B4, BM-MSCs, hESC-MSCs, and hESC-ECs as supporting stromal layers for 5 weeks, and then injected the cells into NOD/SCID/IL-2Rγcnull (NSG) mice at doses equivalent of 7,500–10,000 starting CD34+ cells per mouse. We were careful to include both male and female mice in each group, as female NSG mice show more robust HSC engraftment than males [34]. Flow cytometric analysis of peripheral blood at 4 and 8 weeks showed little to no engraftment in each group, with exception to a single female from the hESC-EC group showing 0.4% engraftment (data not shown). For week 12 analysis, all mice were sacrificed and analyzed for engraftment in peripheral blood, spleen, and BM. By this point, all groups exhibited limited but noticeable engraftment (Fig. 4A, B). Rates of engraftment between groups were consistent in each tissue, with M2-10B4 cultured cells showing the highest levels, followed by hESC-ECs and hESC-MSCs. BM-MSC cultured cells, while showing some engraftment in peripheral blood, were not detected in the spleen or BM. These results demonstrate that the LTC-ICs maintained on our hESC-derived ECs and MSCs are capable of long-term engraftment in NSG mice. Likewise, we show that the level of LTC-IC support between these different stromal layers is predictive of engraftment potential.
FIG. 4.
Stromal cultured LTC-ICs exhibit engraftment potential in NSG mice. Bulk cell populations were harvested from each LTC-IC condition after 5 weeks of culture. Cells were then divided among two to four nonlethally irradiated NSG mice and injected through the tail vain at a dose equivalent of 7.5–10×103 CD34+ UCB initially seeded per mouse. Each group had at least one male and at least one female and was divided evenly where possible. (A) Flow cytometric analysis of peripheral blood, spleen, and bone marrow from a single mouse representing each experimental group. (B) Dot plot representing % human CD45 chimerism in each tissue for individual mice. Horizontal bars represent the group mean values. BM, bone marrow; PB, peripheral blood; N.A., nothing applied (no cells injected).
Discussion
We have shown that hESC-derived ECs and MSCs are able to support the survival and proliferation of CD34+ UCB in the LTC-IC assay similar to M2-10B4 cells and better than BM-MSCs, but that hESC-derived hematopoietic progenitors could not be maintained in LTC-IC regardless of the stromal layer used for support. Based on these data, hESC-derived MSCs and ECs are a viable alternative to M2-10B4 cells for long-term culture of multipotent hematopoietic cells. Notably, other studies have used coculture with human umbilical vein ECs as well as MSCs from human BM to expand LTC-ICs from CD34+ UCB [7,35]. However, the use of hESC- and induced pluripotent stem cell (iPSC)-derived MSCs and ECs has advantages over MSCs and ECs from other human sources. hESCs and iPSCs have the ability to derive multiple cell populations that make up the hematopoietic niche (including MSCs, ECs, osteoblasts, and osteoclasts) from a single cell line—providing a shared genetic background between these interacting cells. Moreover, the ability to genetically modify hESCs with relative ease allows a renewable source of cells that express transgenes of interest to hematopoietic development [20,36]. These benefits could provide researchers with the means to better mimic the human in vivo niche environment using several lineage types derived from a single cell line with either normal or modulated gene expression, thus allowing for a detailed assessment of factors crucial to the maintenance of human HSCs. Of course, similar studies can also be done using human iPSC-derived cells.
hESC-derived cells may differ from their postnatal-derived counterparts in terms of their ability to support hematopoietic cultures, as we observed by comparing hESC-MSCs and BM-MSCs. The ability of hESC-MSCs, but not BM-MSCs to support LTC-ICs from CD34+ UCB reflects our previous findings that demonstrate distinct gene expression patterns for these two MSC populations. For example, hESC-MSCs express higher levels of the hemato-endothelial genes, vWF and FLK-1, and the pluripotency-associated genes REX-1, hTERT, and CD133 [21].
The inability of hESC-derived hematopoietic progenitor cells to demonstrate LTC-IC potential, in contrast to progenitors from UCB, is consistent with in vivo studies of hematopoietic engraftment from hESCs. Although SCID-repopulating cells (SRCs), an in vivo surrogate for HSCs, can be isolated from UCB, adult BM, and peripheral blood, hESC-derived cells have thus far demonstrated a relatively limited potential for long-term, multilineage hematopoietic engraftment in vivo [28,37,38]. The reasons for this are not completely understood, but our results suggest that these cells may be more similar to short-term repopulating cells or multipotent progenitors than HSCs, as demonstrated by an initial expansion but quick proliferative exhaustion. Indeed, we have shown through gene array studies that, while hESC- and iPSC-derived CD34+CD45+ and CD34+CD43+ cells share expression patterns typical of hematopoietic progenitor cells, they are distinct from populations capable of long-term engraftment such as CD34+ UCB and CD34+ fetal liver (unpublished). The challenges posed by generation of hematopoietic cells with LTC-IC/engraftment potential from hESCs also highlight the need for a better understanding of the in vivo marrow microenvironment, including the role of MSCs, ECs, and related cell types. Finally, this in vitro data combined with the results of our in vivo engraftment studies using LTC-IC cultured CD34+ UCB suggest that, as previously demonstrated [39,40], LTC-IC is an accurate indicator for SRC potential, thus providing researchers with an accessible way to screen numerous putative hematopoietic progenitor populations before subjecting them to time-consuming and costly in vivo experiments.
Acknowledgments
Studies are supported by NIH grants HL067828 (D.S.K.), DE22556 (D.S.K.), DE020976 (R.A.K.), 5T32HD007480 (P.I.F.), and a University of Minnesota-Mayo Partnership grant (D.S.K.).
Author Disclosure Statement
The authors have no competing financial interests to declare.
References
- 1.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I. and Dick JE. (2011). Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333:218–221 [DOI] [PubMed] [Google Scholar]
- 2.Fibbe WE,, Noort WA, Schipper F. and Willemze R. (2001). Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice. Ann N Y Acad Sci 938:9–17 [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Miller CL. and Eaves CJ. (2013). Human long-term culture initiating cell assay. Methods Mol Biol 946:241–256 [DOI] [PubMed] [Google Scholar]
- 4.Sutherland HJ,, Eaves CJ, Lansdorp PM, Thacker JD. and Hogge DE. (1991). Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood 78:666–672 [PubMed] [Google Scholar]
- 5.Petzer AL,, Hogge DE, Landsdorp PM, Reid DS. and Eaves CJ. (1996). Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium. Proc Natl Acad Sci U S A 93:1470–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punzel M, Moore KA, Lemischka IR. and Verfaillie CM. (1999). The type of stromal feeder used in limiting dilution assays influences frequency and maintenance assessment of human long-term culture initiating cells. Leukemia 13:92–97 [DOI] [PubMed] [Google Scholar]
- 7.Yildirim S, Boehmler AM, Kanz L. and Mohle R. (2005). Expansion of cord blood CD34+ hematopoietic progenitor cells in coculture with autologous umbilical vein endothelial cells (HUVEC) is superior to cytokine-supplemented liquid culture. Bone Marrow Transplant 36:71–79 [DOI] [PubMed] [Google Scholar]
- 8.Gottschling S, Saffrich R, Seckinger A, Krause U, Horsch K, Miesala K. and Ho AD. (2007). Human mesenchymal stromal cells regulate initial self-renewing divisions of hematopoietic progenitor cells by a beta1-integrin-dependent mechanism. Stem Cells 25:798–806 [DOI] [PubMed] [Google Scholar]
- 9.Scadden DT. (2006). The stem-cell niche as an entity of action. Nature 441:1075–1079 [DOI] [PubMed] [Google Scholar]
- 10.Calvi LM,, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846 [DOI] [PubMed] [Google Scholar]
- 11.Chitteti BR,, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA. and Srour EF. (2010). Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood 115:3239–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Saunders TL, Enikolopov G. and Morrison SJ. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L. and Morrison SJ. (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495:231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A. and Bhatia M. (2013). Regional Localization within the Bone Marrow Influences the Functional Capacity of Human HSCs. Cell Stem Cell 13:175–189 [DOI] [PubMed] [Google Scholar]
- 15.Martin MA. and Bhatia M. (2005). Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev 14:493–504 [DOI] [PubMed] [Google Scholar]
- 16.Prewitz MC,, Seib FP, von Bonin M, Friedrichs J, Stissel A, Niehage C, Muller K, Anastassiadis K, Waskow C, et al. (2013). Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 10:788–794 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Ma W, Wu D, Huang Y, Li H, Zou J, Zhang Y, Feng M. and Luo J. (2013). MiR-17 Partly Promotes Hematopoietic Cell Expansion through Augmenting HIF-1alpha in Osteoblasts. PLoS One 8:e70232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duryagina R, Thieme S, Anastassiadis K, Werner C, Schneider S, Wobus M, Brenner S. and Bornhauser M. (2013). Overexpression of Jagged-1 and Its intracellular domain in human mesenchymal stromal cells differentially affect the interaction with hematopoietic stem and progenitor cells. Stem Cells Dev 22:2736–2750 [DOI] [PubMed] [Google Scholar]
- 19.Thomson JA. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 20.Wilber A, Linehan JL, Tian X, Woll PS, Morris JK, Belur LR, McIvor RS. and Kaufman DS. (2007). Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells 25:2919–2927 [DOI] [PubMed] [Google Scholar]
- 21.Kopher RA,, Penchev VR, Islam MS, Hill KL, Khosla S. and Kaufman DS. (2010). Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone 47:718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill KL,, Obrtlikova P, Alvarez DF, King JA, Keirstead SA, Allred JR. and Kaufman DS. (2010). Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp Hematol 38:246–257.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barberi T, Willis LM, Socci ND. and Studer L. (2005). Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J. and Langer R. (2002). Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 99:4391–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B. and Lim SK. (2007). Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 25:425–436 [DOI] [PubMed] [Google Scholar]
- 26.Ferreira LS,, Gerecht S, Shieh HF, Watson N, Rupnick MA, Dallabrida SM, Vunjak-Novakovic G. and Langer R. (2007). Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res 101:286–294 [DOI] [PubMed] [Google Scholar]
- 27.Ng ES,, Davis R, Stanley EG. and Elefanty AG. (2008). A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc 3:768–776 [DOI] [PubMed] [Google Scholar]
- 28.Tian X, Hexum MK, Penchev VR, Taylor RJ, Shultz LD. and Kaufman DS. (2009). Bioluminescent imaging demonstrates that transplanted human embryonic stem cell-derived CD34(+) cells preferentially develop into endothelial cells. Stem Cells 27:2675–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woll PS,, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, Moon RT. and Kaufman DS. (2008). Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood 111:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman DS,, Hanson ET, Lewis RL, Auerbach R. and Thomson JA. (2001). Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 98:10716–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng ES,, Davis RP, Azzola L, Stanley EG. and Elefanty AG. (2005). Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood 106:1601–1603 [DOI] [PubMed] [Google Scholar]
- 32.Hexum MK,, Tian X. and Kaufman DS. (2011). In vivo evaluation of putative hematopoietic stem cells derived from human pluripotent stem cells. Methods Mol Biol 767:433–447 [DOI] [PubMed] [Google Scholar]
- 33.Vodyanik MA,, Thomson JA. and Slukvin II. (2006). Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108:2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notta F, Doulatov S. and Dick JE. (2010). Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115:3704–3707 [DOI] [PubMed] [Google Scholar]
- 35.Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E, Daum-Woods K, Goetchius GW, Fu P, et al. (2002). Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells 20:573–582 [DOI] [PubMed] [Google Scholar]
- 36.Giudice A. and Trounson A. (2008). Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2:422–433 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Menendez P, Cerdan C. and Bhatia M. (2005). Hematopoietic development from human embryonic stem cell lines. Exp Hematol 33:987–996 [DOI] [PubMed] [Google Scholar]
- 38.Ledran MH,, Krassowska A, Armstrong L, Dimmick I, Renstrom J, Lang R, Yung S, Santibanez-Coref M, Dzierzak E, et al. (2008). Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3:85–98 [DOI] [PubMed] [Google Scholar]
- 39.Dick JE,, Bhatia M, Gan O, Kapp U. and Wang JC. (1997). Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells 15 Suppl 1:199–203; discussion 204–207. [DOI] [PubMed] [Google Scholar]
- 40.de Wynter EA,, Heyworth CM, Mukaida N, Matsushima K. and Testa NG. (2001). NOD/SCID repopulating cells but not LTC-IC are enriched in human CD34+ cells expressing the CCR1 chemokine receptor. Leukemia 15:1092–1101 [DOI] [PubMed] [Google Scholar]