Abstract
Background
Formation of compression (CW) and opposite wood (OW) in branches and bent trunks is an adaptive feature of conifer trees in response to various displacement forces, such as gravity, wind, snow and artificial bending. Several previous studies have characterized tracheids, wood and gene transcription in artificially or naturally bent conifer trunks. These studies have provided molecular basis of reaction wood formation in response to bending forces and gravity stimulus. However, little is known about reaction wood formation and gene transcription in conifer branches under gravity stress. In this study SilviScan® technology was used to characterize tracheid and wood traits in radiate pine (Pinus radiata D. Don) branches and genes differentially transcribed in CW and OW were investigated using cDNA microarrays.
Results
CW drastically differed from OW in tracheids and wood traits with increased growth, thicker tracheid walls, larger microfibril angle (MFA), higher density and lower stiffness. However, CW and OW tracheids had similar diameters in either radial or tangential direction. Thus, gravity stress largely influenced wood growth, secondary wall deposition, cellulose microfibril orientation and wood properties, but had little impact on primary wall expansion. Microarray gene transcription revealed about 29% of the xylem transcriptomes were significantly altered in CW and OW sampled in both spring and autumn, providing molecular evidence for the drastic variation in tracheid and wood traits. Genes involved in cell division, cellulose biosynthesis, lignin deposition, and microtubules were mostly up-regulated in CW, conferring its greater growth, thicker tracheid walls, higher density, larger MFA and lower stiffness. However, genes with roles in cell expansion and primary wall formation were differentially transcribed in CW and OW, respectively, implicating their similar diameters of tracheid walls and different tracheid lengths. Interestingly, many genes related to hormone and calcium signalling as well as various environmental stresses were exclusively up-regulated in CW, providing important clues for earlier molecular signatures of reaction wood formation under gravity stimulus.
Conclusions
The first comprehensive investigation of tracheid characteristics, wood properties and gene transcription in branches of a conifer species revealed more accurate and new insights into reaction wood formation in response to gravity stress. The identified differentially transcribed genes with diverse functions conferred or implicated drastic CW and OW variation observed in radiata pine branches. These genes are excellent candidates for further researches on the molecular mechanisms of reaction wood formation with a view to plant gravitropism.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2164-14-768) contains supplementary material, which is available to authorized users.
Keywords: Compression wood, Tracheid, Conifers, Transcriptome, Microarray, Plant gravitropism, Microfibril angle (MFA), Wood stiffness
Background
Trees can change their growth orientation in response to various environmental stresses (ie, wind, snow, light, gravity, artificial bending) [1], and during this process reaction wood with modified characteristics and properties is formed. In gymnosperms, compression wood (CW) is produced on the lower side of inclined (bent) trunks or naturally growing branches [2]. This reaction wood helps conifer trees maintain stem straightness and branches at certain angles. Characterization of CW formed in bent trunks has been extensively investigated in many conifer species [1, 3–10]. Compared to its opposite wood (OW) formed on the upper side of branches and bent trunks, CW has shorter tracheids, larger microfibril angle (MFA), greater shrinkage, higher density, lower stiffness, more lignin and less cellulose [1, 3–10]. Thus, it is considered undesirable for both solid wood and pulp products [10, 11].
Molecular basis of CW formation has been previously studied in the bent trunks of several conifer species. Using the semi-quantitative RT-PCR technique a few cell wall structural protein genes and several lignin-related genes were identified with differential transcription in the bent trunks of loblolly pine [12] and Japanese cypress [9, 13], respectively. Based on suppression subtractive hybridization (SSH) and qRT-PCR, several genes involved in cell wall modification, lignin biosynthesis and transcription regulation had differential transcription in the inclined stems of radiata pine and maritime pine [14]. Furthermore, genomic approaches such as cDNA microarrays revealed differential gene transcription in the bent trunks of loblolly pine [15] and maritime pine [16]. These studies have provided valuable insights into the molecular basis of CW formed in bunt trunks of conifers, particularly with regard to its higher lignin content.
To our knowledge all published researches on the properties and gene transcription of reaction wood in conifer species (CW) and angiosperms (tension wood, TW) were based on the bent trunks under artificial bending or natural inclining forces. It should be noted that these trees had experienced both external forces and gravity stimulus. Particularly, the bending force in young trees and seedlings could be much larger than gravity stress. Thus, wood properties and gene transcription observed in bent trunks may not accurately reflect gravity stress. In contrast, naturally grown branches are mostly under gravity stress, providing excellent materials for the study of wood formation in response to gravity stimulus. Although one previous study with eucalyptus branches identified a few cell wall genes in response to gravity stress in angiosperms [17], wood property variation and the xylem transcriptome changes between the upper and lower sides of branches remains largely unknown in any conifer species.
Radiata pine (Pinus radiata Don.) is the most important conifer species for commercial forestry in Australia, New Zealand and Chile. CW properties of radiata pine have been previously characterized using inclined trees [3, 6, 8]. In the present study radiata pine branches were used to study CW formation. Firstly, tracheid characteristics and wood properties were measured using the SilviScan® technology [18, 19]. Then, differential gene transcription between the upper (OW) and lower sides of branches (CW) was investigated using radiata pine cDNA microarrays. The aim of this study is to reveal insights into the molecular mechanisms of reaction wood formation in conifer branches with a view to plant gravitropism.
Results
Characterization of tracheid and wood traits in CW and OW of branches
In the cross-section of the six branch discs, average radius of the lower side xylem (CW) was 4.6 cm, significantly longer than that of total OW formed on the upper side of branches (3.1 cm, P-value = 0.0002). The six branch discs had 10 growth rings in the cross-section. Within a ring formed in a growing circle CW was significant wider than OW (P-values ≤ 0.01) except for the first four rings from pith. These results indicated that gravity stress significantly increased wood formation on the lower side of branches. The larger wood growth in CW could generate compression force to maintain branches at certain orientation.
SilviScan measurement of the six branch discs showed significant differences between CW and OW in wood growth, tracheid characteristics and wood properties in terms of average ring values (Figure 1). Significant CW and OW variation was also observed in ring 10 (Figure 1), which represented developing xylem tissues sampled for the two microarray experiments. In both comparisons CW had greater growth, thicker tracheid walls, larger MFA, greater coarseness, lower specific surface, higher density and lower stiffness compared to OW. Interestingly, MFA was drastically altered during CW formation. Average MFA in CW (lower side) of branches was 33.2 degrees, significantly larger than that of OW (26.2 degrees). Surprisingly, diameters of CW tracheids were similar to that of OW in both radial and tangential directions, respectively (Figure 1). Thus, gravity stress appeared to have little influence on tracheid dimensions in the two directions. Moreover, radial dimension of CW and OW tracheids (24.2-24.3 μm) was slightly larger than their tangential dimension (23.1-23.5 μm), resulting in nearly round or square shapes of tracheids in the cross-section.
Figure 1.
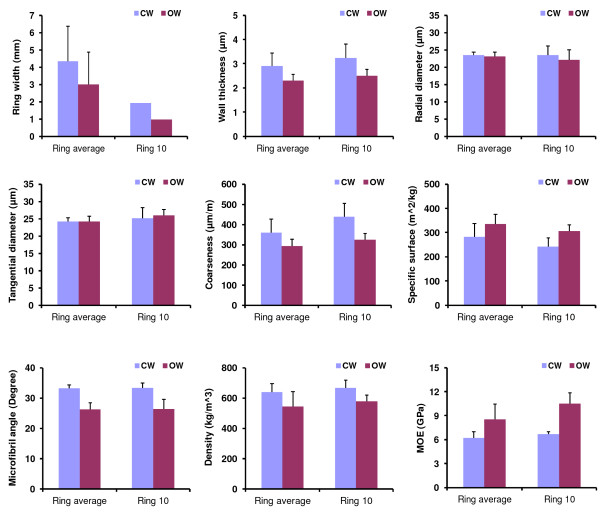
Variation in tracheid characteristics and wood properties between compression (CW) and opposite wood (OW) of branches. Ring width, tracheid wall thickness, radial diameter, tangential diameter, coarseness, specific surface, microfibril angle (MFA), wood density and stiffness (modulus of elasticity, MOE) were measured in six wood strips of radiata pine branches using SilviScan 2. Average ring values of each trait were compared between the lower side (CW) and upper side (OW) of the six branches. Tracheid and wood traits in ring 10 representing developing xylem tissues collected for microarray experiments were also compared between CW and OW. Error bars represent the standard deviation of the mean value of each trait. CW and OW variation is statistically significant (P-values ≤ 0.05) except for the two tracheid diameters.
Transcriptome comparison between CW and OW formed in branches
The xylem transcriptomes of CW and OW sampled in spring (called earlywood, EW) and autumn (latewood, LW) were compared respectively using radiata pine cDNA microarrays. In the first comparison between CW and OW sampled in spring, 944 out of 3,320 xylem unigenes (28.4%) on the microarrays had differential transcription, including 781 and 163 unigenes preferentially transcribed in CW and OW, respectively (Figure 2a). Using samples collected in autumn slightly more unigenes (970, 29.2%) were identified with differential transcription (552 and 418 unigenes for CW and OW, respectively) (Figure 2b). Thus, different growing seasons may only have little impact on the proportion of the xylem transcriptomes differentially transcribed in CW and OW of radiata pine branches. However, genes up-regulated in CW in spring (781) were five times more than that in OW (163); while in autumn genes preferentially transcribed in CW were slightly more than that in OW. Nearly half of the identified genes (46.4% for CW and 40.5% for OW) had similar transcription patterns in the two seasons during reaction wood formation (Figure 2c).
Figure 2.
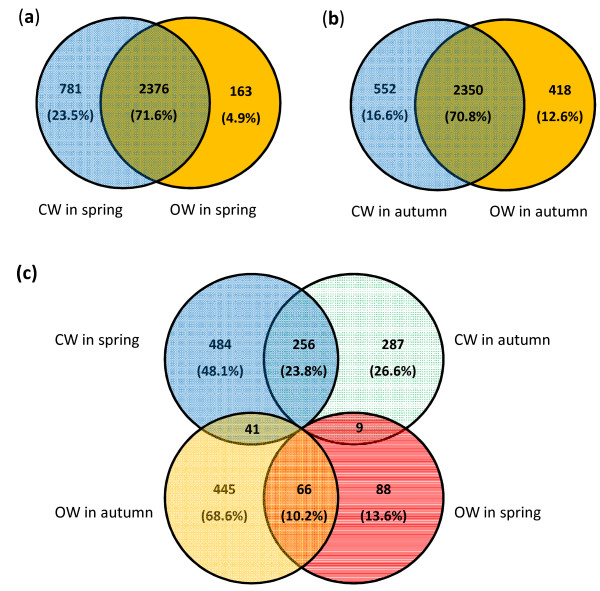
Transcriptome comparisons between compression (CW) and opposite wood formed in branches. Genes differentially transcribed in CW and OW sampled in spring and autumn were identified using radiata pine cDNA microarrays, respectively. Numbers of preferentially transcribed genes identified from developing xylem sampled in spring (a) and autumn (b) were present. Differentially transcribed genes were further compared between the two seasons. A number of genes showed consistently differential transcription in the two wood tissues across the two seasons (c).
The two microarray experiments identified a total of 1,204 and 514 genes differentially transcribed in CW and OW, respectively (Additional file 1). Almost all these genes (98.0% for CW and 98.6% for OW) had close matches in the UniProt known proteins and TIGR gene indices databases (tblastx, E-value ≤ 1e-5). However, about 35% of the matches did not have a clear function. This is because CW and OW formation has been poorly characterized at the molecular level. Of 1,204 genes identified in CW, 588 genes were annotated with gene ontology (GO) terms, and majority (90.6%) showed molecular functions, 75.5% had roles in biological processes and less than half (48.6%) might be cellular components. Similarly, in the 514 genes preferentially transcribed in OW 276 transcripts were annotated with GO terms, including molecular functions (85.5%), biological process (74.3%) and cellular components (47.8%).
Microarray results of seven selected genes with differential transcription in CW and OW sampled in autumn were validated using the RT-MLPA method. The magnitudes of differential gene transcription measured by RT-MLPA had no significant differences compared to that in the microarray experiment (P-values ≤ 0.05) (Figure 3). This result indicated that the microarray experiments conducted in this study were sufficiently reliable for the identification of genes differentially transcribed in lower and upper sides of radiata pine branches under gravity stress.
Figure 3.
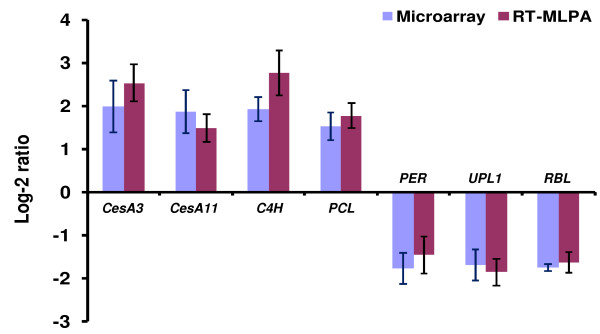
Validation of microarray transcription of selected differentially transcribed genes. A total of seven differentially transcribed genes were selected in the validation using reverse transcriptase-multiplex ligation dependent probe amplification (RT-MLPA). These genes include four genes up-regulated in CW: cellulose synthase 3 (PrCesA3), PrCesA11, cinnamic acid 4-hydroxylase (C4H) and plastocyanin-like (PCL); three genes more highly transcribed in OW: peroxidase (PER), E3 ubiquitin protein ligase (UPL1) and retinoblastoma-like protein (RBL). Developing xylem (CW and OW) sampled in autumn for the microarray experiment was used in the validation, including three biological and four technical replicates. Mean log-2 ratios (CW/OW) of the 12 replicates were calculated for the selected genes and compared with their microarray transcription results. The mean log-2 ratio values > 0 and < 0 indicate genes preferentially transcribed in CW and OW, respectively. Error bars represent the standard deviation of the mean log-2 ratio.
Differential transcription of cytoskeleton-related genes
From a total of 1,728 genes with differential transcription identified in this study, 28 genes were involved in actin filaments and microtubules (Table 1). In the development of actin filaments, genes encoding different actins, actin bundling proteins and actin related proteins were preferentially transcribed in either CW or OW. However, actin depolymerizing factor (ADF) and ADF-like genes were exclusively up-regulated in CW of branches. In the microtubule development, different members of the same gene families encoding tubulin folding cofactors, microtubule-associated proteins (MAPs) and MAP kinases were differentially transcribed in CW and OW, respectively. Interestingly, seven tubulins (two alpha- and five beta-tubulins) were exclusively up-regulated in CW sampled in the two seasons (three tubulins) or a single season (four).
Table 1.
Cytoskeleton-related genes differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes* | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-spring | Actin | TWL22A2 | FE523923 | 1.532 | 0.048 |
| CW-spring | Actin 3 | MWL23B3 | GO269503 | 0.905 | 0.003 |
| CW-autumn | Actin 3 | JWLxF8 | FE521083 | 0.574 | 0.033 |
| CW-autumn | Actin 7 | JWEeC7 | FE518833 | 2.150 | 0.048 |
| CW-spring | Actin 7 | TWE23D12 | FE523186 | 1.794 | 0.050 |
| CW-spring | Actin bundling protein ABP135 | TWE5D1 | FE523228 | 0.800 | 0.010 |
| CW-autumn | Actin depolymerizing factor | MWE24D11 | FE521861 | 2.167 | 0.034 |
| CW-spring | Actin depolymerizing factor | MWE5G10 | FE521693 | 1.772 | 0.047 |
| CW-autumn | Actin depolymerizing factor-like | JWEqB11 | FE518555 | 0.664 | 0.011 |
| CW-spring | Actin filament bundling protein P-115 | JWL19E9 | FE521315 | 1.676 | 0.048 |
| CW-autumn | Actin filament bundling protein P-115 | JWL19E9 | FE521315 | 1.539 | 0.016 |
| CW-autumn | Actin related protein 2 | JWLfF5 | FE519755 | 1.159 | 0.035 |
| CW-autumn | Actin related protein 3 | JWEmB5 | FE518964 | 1.211 | 0.040 |
| CW-autumn | Tubulin alpha | JWEbH8 | FE519649 | 1.942 | 0.049 |
| CW-spring | Tubulin alpha 1 | JWEsG6 | FE519115 | 1.580 | 0.033 |
| CW-autumn | Tubulin alpha 1 | TWL9D10 | FE524256 | 1.830 | 0.050 |
| CW-spring | Tubulin beta | JWLlA9 | FE519966 | 1.944 | 0.037 |
| CW-autumn | Tubulin beta 1 | JWLwC2 | FE520255 | 1.694 | 0.044 |
| CW-spring | Tubulin beta 1 | JWLwC2 | FE520255 | 1.706 | 0.010 |
| CW-spring | Tubulin beta 3 | MWE31B9 | FE521866 | 1.571 | 0.042 |
| CW-spring | Tubulin beta 6 | MWL30D8 | FE522772 | 1.340 | 0.047 |
| CW-autumn | Tubulin beta 6 | JWLrC8 | FE520019 | 1.557 | 0.032 |
| CW-spring | Tubulin beta 7 | JWLhG4 | FE520687 | 1.993 | 0.024 |
| CW-spring | Tubulin folding cofactor B | TWL22B11 | FE524340 | 2.023 | 0.050 |
| CW-autumn | Microtubial binding protein | MWE30H10 | FE521906 | 0.650 | 0.026 |
| CW-autumn | MAP-like | MWE9A10 | FE521409 | 1.479 | 0.009 |
| CW-spring | MAP kinase-like | MWE31C11 | FE521885 | 1.888 | 0.015 |
| OW-spring | Actin 2 | TWE16C11 | FE523366 | -1.470 | 0.049 |
| OW-autumn | Actin 2 | MWL29C3 | FE522532 | -2.139 | 0.049 |
| OW-autumn | Actin bundling protein ABP135 | TWE5D1 | FE523228 | -1.162 | 0.019 |
| OW-autumn | Actin related protein 6 | TWE4H5 | FE523100 | -2.163 | 0.042 |
| OW-autumn | Tubulin folding cofactor A | MWL33E1 | FE522629 | -1.448 | 0.000 |
| OW-spring | MAP | JWLlA3 | FE520828 | -1.777 | 0.043 |
| OW-autumn | MAP | JWL11A8 | FE521181 | -1.328 | 0.043 |
| OW-autumn | MAP kinase | JWEiD6 | FE518418 | -1.342 | 0.010 |
| OW-autumn | MAP kinase phosphatase | MWE1B4 | FE521481 | -1.621 | 0.007 |
| OW-spring | MAPKK | TWE5D10 | FE523280 | -1.982 | 0.014 |
| OW-autumn | MAPKK | TWE5D10 | FE523280 | -1.914 | 0.034 |
*MAP, microtubule-associated protein; MAPKK, mitogen-activated protein kinase kinase.
Differential transcription of cell wall-related genes
Genes related to cell wall formation at various developmental stages were identified with differential transcription in CW and OW of radiata pine branches. Interestingly, these genes were more frequently up-regulated in CW than in OW (Table 2 and Table 3). In cell division, four genes (cell cycle switch protein, cell division cycle protein 48, cyclin, profilin-1) were up-regulated in CW; while only one gene (cyclin-like F-box domain) was preferentially transcribed in OW. In cell expansion, three expansin and extensin genes were up-regulated in CW (expansin beta 1, expansin ripening related and extensin-like); while only one up-regulated in OW (expansin alpha 3). In pectin biosynthesis, three genes (pectin lyase 2, pectinesterase-like and pectin-glucuronyltransferase) were up-regulated in OW; while only a pectin lyase-like had higher transcription in CW. In primary wall modification, two xyloglucan endotransglucosylase/hydrolases (XET8 and 32) were up-regulated in CW of branches, and a gene encoding ovule/fiber cell elongation proteins was more highly transcribed in OW.
Table 2.
Cell division and primary wall modification genes differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes* | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-spring | Cell cycle switch protein | MWL10H5 | FE522899 | 2.570 | 0.048 |
| CW-autumn | Cell cycle switch protein | MWL10H5 | FE522899 | 0.893 | 0.041 |
| CW-spring | Cell division cycle protein 48 | TWE20C6 | FE523511 | 1.064 | 0.041 |
| CW-spring | Cyclin | JWL24C10 | FE520640 | 2.214 | 0.031 |
| CW-spring | Profilin-1 | MWE1E2 | FE521468 | 1.310 | 0.037 |
| CW-autumn | Profilin-1 | MWE6H12 | FE521797 | 2.001 | 0.040 |
| CW-spring | Expansin-B1 precursor | JWLsE7 | FE520098 | 2.584 | 0.037 |
| CW-autumn | Expansin-B1 precursor | JWLsE7 | FE520098 | 1.527 | 0.041 |
| CW-spring | Expansin, Ripening-related | JWLlF11 | FE520876 | 1.973 | 0.040 |
| CW-autumn | Expansin, Ripening-related | MWL18G4 | FE522961 | 1.753 | 0.040 |
| CW-spring | Extensin-like protein | JWEuG12 | FE519300 | 1.831 | 0.003 |
| CW-autumn | XET8 | JWEqD8 | FE519429 | 1.656 | 0.047 |
| CW-autumn | XET32 | JWLvE1 | FE520165 | 0.976 | 0.017 |
| CW-spring | Pectate lyase-like | MWL25G12 | FE522408 | 1.347 | 0.047 |
| CW-spring | Cellulase 24 | MWL15E11 | FE522326 | 1.455 | 0.036 |
| OW-autumn | Cyclin-like F-box | JWL11H8 | FE520391 | -2.085 | 0.034 |
| OW-spring | Expansin alpha- 3 | TWE13E12 | FE523142 | -1.650 | 0.016 |
| OW-autumn | Expansin alpha- 3 | TWE33F8 | FE523674 | -2.028 | 0.042 |
| OW-spring | Pectate lyase 2 | TWE8C12 | FE523428 | -1.453 | 0.019 |
| OW-autumn | Pectate lyase 2 | TWE8C12 | FE523428 | -1.674 | 0.046 |
| OW-spring | Pectinesterase-like | JWLjC8 | FE521362 | -1.460 | 0.032 |
| OW-autumn | Pectin-glucuronyltransferase | TWE16C7 | FE523338 | -1.898 | 0.042 |
| OW-autumn | Ovule/fiber cell elongation protein | TWE15H7 | GO269508 | -0.628 | 0.049 |
*XET, xyloglucan endotransglucosylase/hydrolase.
Table 3.
Genes related to cellulose and lignin biosynthesis differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes* | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-spring | PrCesA3 | TWL7C2 | FE524088 | 1.316 | 0.045 |
| CW-autumn | PrCesA3 | TWL1D5 | FE524207 | 1.984 | 0.046 |
| CW-autumn | PrCesA7 | JWE3D1 | FE518578 | 1.464 | 0.041 |
| CW-spring | PrCesA11 | TWL21B5 | FE523881 | 1.336 | 0.041 |
| CW-autumn | PrCesA11 | TWL28A3 | FE523830 | 1.867 | 0.038 |
| CW-spring | PrCesA-like | JWLvB12 | FE520241 | 1.661 | 0.016 |
| CW-autumn | PrCesA-like | JWLvB12 | FE520241 | 1.417 | 0.012 |
| CW-spring | Sucrose synthase | TWL8B9 | FE524247 | 1.467 | 0.038 |
| CW-autumn | Sucrose synthase | JWEtG3 | FE519171 | 2.153 | 0.045 |
| CW-spring | Sucrose synthase 1 | JWE5H8 | FE519487 | 1.377 | 0.033 |
| CW-spring | Callose synthase-like | TWL14D7 | FE524183 | 0.933 | 0.028 |
| CW-spring | Glycosyl transferase 48 | JWLxB9 | FE521086 | 1.444 | 0.037 |
| CW-spring | Chorismate synthase | TWL12H4 | FE524274 | 2.155 | 0.046 |
| CW-autumn | Chorismate synthase | TWL12H4 | FE524274 | 1.336 | 0.177 |
| CW-spring | Chorismate mutase | MWE4C8 | FE521447 | 1.308 | 0.010 |
| CW-spring | Shikimate kinase 2 | JWL17G12 | FE520492 | 2.685 | 0.046 |
| CW-spring | EPSPS | JWLbD8 | FE519700 | 2.111 | 0.018 |
| CW-autumn | EPSPS | JWLbD8 | FE519700 | 1.906 | 0.040 |
| CW-spring | DAHPS | MWE11H6 | FE521953 | 1.295 | 0.048 |
| CW-autumn | DAHPS | MWE11H6 | FE521953 | 1.966 | 0.041 |
| CW-spring | 4CL | JWE8F8 | FE519348 | 1.289 | 0.046 |
| CW-autumn | 4CL | JWEnE8 | FE519597 | 1.830 | 0.046 |
| CW-spring | C4H | TWL23G12 | FE523933 | 1.547 | 0.047 |
| CW-autumn | C4H | MWE8E8 | FE522027 | 1.921 | 0.048 |
| CW-spring | CCR-like | JWE3H12 | FE518650 | 1.180 | 0.043 |
| CW-spring | COMT | MWL29C12 | FE522592 | 1.449 | 0.049 |
| CW-autumn | COMT | MWE7C3 | FE522592 | 1.571 | 0.014 |
| CW-autumn | PAL2 | JWL4F3 | FE521295 | 1.635 | 0.050 |
| CW-spring | Laccase 2 | JWL22G2 | FE520504 | 1.810 | 0.049 |
| CW-autumn | Laccase 2 | JWL22G2 | FE520504 | 1.238 | 0.039 |
| CW-spring | Laccase 4 | TWL13F12 | FE524173 | 1.616 | 0.070 |
| CW-autumn | Methionine synthase | JWLuE3 | FE520972 | 1.558 | 0.031 |
| CW-autumn | Methionine synthase 2 | JWEuE1 | FE519234 | 1.663 | 0.049 |
| CW-autumn | MetE | MWE9E1 | GO269413 | 1.749 | 0.045 |
| CW-spring | SAMS | TWL18G9 | FE523865 | 1.552 | 0.044 |
| CW-autumn | SAMDC | JWE8E2 | FE519501 | 1.881 | 0.048 |
| CW-spring | Dirigent-like protein pDIR3 | JWLf10D9 | FE519781 | 1.493 | 0.035 |
| CW-autumn | Dirigent-like protein pDIR3 | TWL26F6 | FE524371 | 1.226 | 0.043 |
| CW-autumn | Dirigent-like protein pDIR4 | JWLwD4 | FE520269 | 1.807 | 0.042 |
| CW-autumn | Dirigent-like protein pDIR14 | JWEdE1 | FE518247 | 2.298 | 0.042 |
| OW-autumn | PrCesA10 | TWE21G7 | FE523538 | -2.497 | 0.041 |
| OW-autumn | Glycosyl transferase 8-like | TWE16F7 | FE523341 | -1.532 | 0.048 |
| OW-autumn | Glycosyl transferase NTGT5a | TWE13G1 | FE523678 | -1.638 | 0.047 |
| OW-spring | COMT-like | JWLkA8 | FE520785 | -2.112 | 0.035 |
| OW-spring | Peroxidase precursor | TWE24D10 | FE523740 | -1.376 | 0.030 |
| OW-autumn | Peroxidase precursor | TWE27F5 | FE523746 | -1.774 | 0.032 |
| OW-spring | Peroxidase PSYP1,Class III | MWL21F8 | GO269502 | -1.999 | 0.039 |
| OW-autumn | Peroxidase PSYP1,Class III | MWL21F8 | GO269502 | -1.474 | 0.045 |
| OW-autumn | Dirigent protein pDIR18 | TWE21H12 | FE523546 | -0.636 | 0.021 |
*PrCesA, Pinus radiata cellulose synthase; 4CL, 4-coumarate:CoA ligase; C4H, cinnamic acid 4-hydroxylase; CCR, cinnamoyl CoA reductase; COMT, caffeic acid ortho-methyltransferase; PAL, phenylalanine ammonia-lyase; DAHPS, 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase; EPSPS, 5-enolpyruvylshikimate 3-phosphate synthase; SAMS, S-adenosylmethionine synthetase; SAMDC, S-adenosylmethionine decarboxylase; MetE, cobalamin-independent methionine synthase.
Secondary cell wall genes were mostly up-regulated in CW of radiata pine branches (Table 3). Of genes involved in cellulose biosynthesis, cellulose synthases (PrCesA3, 7, 11), PrCesA-like and sucrose synthases (SuSy and SuSy1) were preferentially transcribed in CW. Four of these genes (PrCesA3, 11, PrCesA-like and SuSy) were up-regulated in CW sampled in both spring and autumn. In the lignin-related genes, 4-coumarate:CoA ligase (4CL), cinnamic acid 4-hydroxylase (C4H), cinnamoyl CoA reductase-like (CCR-like), caffeic acid ortho-methyltransferase (COMT), phenylalanine ammonia-lyase 2 (PAL2), methionine synthases, S-adenosylmethionine synthetase (SAMS), S-adenosylmethionine decarboxylase (SAMDC), laccases (LAC2 and 4) and dirigent-like were up-regulated in CW. A wide range of cell wall structural protein genes were up-regulated in CW, such as arabinogalactan proteins (AGP4, 5, 6 and AGP-like), fasciclin-like arabinogalactan proteins (FLA1 and 8), glycine-rich protein, proline-rich protein and so on (Table 4). In contrast, only three FLAs (FLA10, 17 and 26) were preferentially transcribed in OW of branches.
Table 4.
Cell wall structural protein genes differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes* | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-spring | Arabinogalactan/proline-rich protein, AGP4 | MWE13B9 | FE521826 | 1.559 | 0.050 |
| CW-spring | Arabinogalactan protein 5, AGP5 | TWL29C3 | FE524468 | 1.593 | 0.037 |
| CW-autumn | Arabinogalactan protein 5, AGP5 | TWL1A1 | FE524552 | 2.018 | 0.049 |
| CW-spring | Arabinogalactan protein 6, AGP6 | JWEmA4 | FE518957 | 1.330 | 0.038 |
| CW-autumn | Arabinogalactan protein 6, AGP6 | JWLtE9 | FE520147 | 2.009 | 0.035 |
| CW-spring | Arabinogalactan-like | MWE12B5 | FE518750 | 1.826 | 0.031 |
| CW-autumn | Arabinogalactan-like | MWE12B5 | FE518750 | 1.466 | 0.046 |
| CW-spring | FLA1 | MWL28F5 | FE522423 | 0.421 | 0.004 |
| CW-spring | FLA8 | JWLxF4 | FE521059 | 2.546 | 0.029 |
| CW-autumn | FLA8 | JWLxF4 | FE521059 | 1.911 | 0.037 |
| CW-spring | Glycine-rich protein 1 | MWE6G10 | FE521816 | 2.044 | 0.017 |
| CW-autumn | Glycine-rich protein 1 | MWE6G10 | FE521816 | 1.469 | 0.016 |
| CW-spring | Glycine-rich protein 2 | JWEgF6 | FE519572 | 1.577 | 0.039 |
| CW-autumn | Glycine-rich protein 2 | JWErH8 | FE519061 | 1.519 | 0.031 |
| CW-spring | Proline-rich protein | MWE5D11 | FE521995 | 1.521 | 0.047 |
| CW-spring | Plastocyanin-like | TWL16D4 | FE523902 | 1.464 | 0.044 |
| CW-autumn | Plastocyanin-like | TWL26B4 | FE524367 | 1.525 | 0.049 |
| CW-autumn | Plantacyanin | TWL22G11 | GO269524 | 1.513 | 0.043 |
| CW-spring | Uclacyanin 3 | TWL19C11 | FE524322 | 1.104 | 0.020 |
| CW-autumn | Uclacyanin 3 | TWL19C11 | FE524322 | 1.433 | 0.028 |
| CW-spring | Blue copper protein | JWLbE4 | FE519677 | 1.944 | 0.042 |
| CW-autumn | Blue copper protein | TWL19E3 | GO269525 | 2.235 | 0.047 |
| OW-autumn | FLA10 | MWE16A4 | FE522128 | -1.654 | 0.003 |
| OW-autumn | FLA17 | TWL29A10 | FE524499 | -1.736 | 0.050 |
| OW-autumn | FLA26 | TWE27C11 | FE523610 | -2.270 | 0.049 |
*FLA, fasciclin-like arabinogalactan protein.
Hormone and calcium signalling genes differentially transcribed in CW and OW formation
Similar to genes involved in cell wall formation, differentially transcribed genes related to hormone and calcium signalling were mostly up-regulated in CW of branches; while only a few of these genes were preferentially transcribed in OW (Table 5). These hormone signalling genes were related to five major hormones (auxin, gibberellin, cytokinin, ethylene and abscisic acid). For example, three auxin-related genes were up-regulated in CW, including auxin-induced protein, auxin-regulated protein (ARP) and ARP-like. Other hormone-related genes up-regulated in CW included genes encoding gibberellins-induced receptor-like kinase, cytokinin-binding protein, ethylene response factor-like, ethylene responsive element binding factor and ethylene-forming enzyme. In addition, six calcium-related genes (calcium dependent protein kinase, calcium/calmodulin-dependent protein kinase CaMK3, calcium-binding EF-hand, calcium-binding protein, and calcium-binding protein-like) were up-regulated in CW of branches in both spring and autumn (Table 5).
Table 5.
Genes related to hormone and calcium signalling were differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-autumn | Auxin-induced protein | JWLwB8 | FE520292 | 1.587 | 0.043 |
| CW-spring | Auxin-regulated protein | JWElD4 | FE519586 | 1.206 | 0.026 |
| CW-spring | Auxin-regulated protein-like | MWL10D2 | FE522886 | 1.964 | 0.049 |
| CW-autumn | Auxin-regulated protein-like | MWL5D1 | FE522833 | 2.045 | 0.044 |
| CW-autumn | Cytokinin-binding protein | JWLuD5 | FE520986 | 1.839 | 0.049 |
| CW-spring | Ethylene reponse factor-like | MWL23D7 | FE522376 | 2.893 | 0.051 |
| CW-spring | Ethylene responsive element binding factor | JWEmG12 | FE519013 | 1.103 | 0.032 |
| CW-spring | Ethylene-forming enzyme | JWEhA9 | FE518368 | 1.392 | 0.038 |
| CW-autumn | Ethylene-forming enzyme | JWEhC9 | FE518368 | 1.514 | 0.041 |
| CW-autumn | Gibberellin-induced receptor-like kinase | TWL24D10 | FE523925 | 1.959 | 0.006 |
| CW-spring | Abscisic acid-induced protein | TWE30F7 | FE523634 | 0.983 | 0.021 |
| CW-spring | Calcium dependent protein kinase | JWLbH5 | FE519684 | 2.196 | 0.049 |
| CW-autumn | Calcium dependent protein kinase | JWLbH5 | FE519684 | 1.691 | 0.050 |
| CW-spring | Calcium/calmodulin-dependent protein kinase | TWL20F6 | FE523844 | 1.832 | 0.005 |
| CW-autumn | Calcium/calmodulin-dependent protein kinase | TWL20F6 | FE523844 | 1.660 | 0.039 |
| CW-spring | Calcium-binding EF-hand | JWLxA12 | FE521106 | 1.077 | 0.018 |
| CW-autumn | Calcium-binding EF-hand | JWLrA7 | FE520012 | 2.045 | 0.048 |
| CW-spring | Calcium-binding protein | MWL14A2 | FE523065 | 0.086 | 0.036 |
| CW-autumn | Calcium-binding protein | MWL14A2 | FE523065 | 1.853 | 0.067 |
| CW-spring | Calcium-binding protein-lik | MWL21C7 | FE522357 | 1.053 | 0.0002 |
| CW-autumn | Calcium-binding protein-lik | MWL21C7 | FE522357 | 1.248 | 0.016 |
| OW-autumn | Abscisic acid-induced protein | TWE30F7 | FE523634 | -0.912 | 0.033 |
| OW-autumn | Calcium-transporting ATPase 2 | MWL33C2 | FE522634 | -1.896 | 0.048 |
CW and OW formation involves extensive transcription regulation
Most hormone signalling genes up-regulated in CW (Table 5) had functions in transcription regulation. Besides, many other transcription factor (TF) genes were also identified with differential transcription in CW and OW (Additional files 2). Different members of homeodomain, LIM and Zinc finger gene families were up-regulated in CW and OW, respectively. In contrast, several other TFs were preferentially transcribed in either CW or OW formation. For example, BHLH, BTF, HD-ZIP and MYB were exclusively up-regulated in CW; while WRKY and transcriptional corepressor were only highly transcribed in OW.
Differential gene transcription related to divergent environmental stresses
Fifteen genes involved in various environmental stresses (i.e., water, light, diseases and salt) were up-regulated in CW of branches; while only two genes related to environmental stresses were preferentially transcribed in OW (Table 6). Of genes related to water stress, aquaporin, water deficit inducible protein, dehydrin, dehydrin 1 and dehydration-responsive protein-like were up-regulated in CW. Several genes responding to salt stress (salt tolerance protein 1, 2 and salt-induced AAA-type ATPase) and disease resistance (disease resistance gene, nucleotide-binding site (NBS) protein and TIR/P-loop/LRR) were exclusively up-regulated in CW formation. Surprisingly, light-inducible protein ATLS1, light-induced protein-like and phytochrome were exclusively up-regulated in CW, suggesting CW formation may be affected by light signals.
Table 6.
Genes responding to environmental stresses differentially transcribed in compression (CW) and opposite wood (OW) formed in branches
| Xylem tissues | Differentially transcribed genes | Representing ESTs | GenBank accession | Log-2 ratios | P-values |
|---|---|---|---|---|---|
| CW-autumn | Aquaporin | MWL5E11 | FE522873 | 2.002 | 0.045 |
| CW-spring | Water deficit inducible protein | TWE12B12 | FE523997 | 2.102 | 0.008 |
| CW-autumn | Water deficit inducible protein | TWE12B12 | FE523997 | 1.059 | 0.008 |
| CW-spring | Dehydrin | TWL14B4 | FE524283 | 1.415 | 0.036 |
| CW-autumn | Dehydrin | TWL36E7 | FE524023 | 2.125 | 0.045 |
| CW-spring | Dehydrin 1 | JWLwA1 | FE520245 | 2.175 | 0.019 |
| CW-spring | Dehydration-responsive protein-like | TWL17D5 | FE523858 | 1.534 | 0.038 |
| CW-spring | Light-inducible protein ATLS1 | MWE5D1 | FE521627 | 1.785 | 0.039 |
| CW-autumn | Light-inducible protein ATLS1 | TWL27A8 | GO269536 | 1.648 | 0.039 |
| CW-spring | Light-induced protein like | JWEcC9 | FE518768 | 0.932 | 0.051 |
| CW-spring | Phytochrome | TWL37H8 | FE524199 | 1.347 | 0.044 |
| CW-autumn | Phytochrome | TWL5H7 | FE524072 | 1.945 | 0.046 |
| CW-spring | Disease resistance gene | JWLlA4 | FE520833 | 1.733 | 0.043 |
| CW-autumn | Disease resistance gene | TWL36H4 | FE523993 | 1.454 | 0.044 |
| CW-autumn | nucleotide-binding site (NBS) protein | JWLjF7 | FE519913 | 0.969 | 0.041 |
| CW-spring | TIR/P-loop/LRR | TWL27G11 | FE524445 | 1.159 | 0.032 |
| CW-spring | Multidrug resistance associated protein 1 | JWLwF8 | FE520296 | 1.350 | 0.034 |
| CW-spring | Multidrug resistance associated protein 6 | MWE4G8 | FE521986 | 1.228 | 0.002 |
| CW-spring | Salt tolerance protein 1 | JWLxA5 | FE521062 | 1.569 | 0.047 |
| CW-autumn | Salt tolerance protein 1 | JWLxA5 | FE521062 | 1.515 | 0.039 |
| CW-spring | Salt tolerance protein 4 | MWE29C5 | FE521898 | 1.798 | 0.048 |
| CW-autumn | Salt-induced AAA-Type ATPase | JWE1D5 | FE519463 | 0.998 | 0.024 |
| OW-autumn | NBS/LRR | MWL21G9 | FE523021 | -1.441 | 0.042 |
| OW-spring | Aluminium induced protein | TWL29A2 | FE524459 | -1.615 | 0.044 |
Discussion
Extensive transcriptome remodelling underlies drastic CW and OW variation
Drastic variation between CW and OW of radiata pine branches indicated that gravity stimulus affects cell division, secondary wall deposition, cellulose microfibril orientation and overall wood properties. The larger MFA in CW greatly contributes to its lower wood stiffness despite of its higher density. This is because MFA rather than density has a predominant and adverse effect on wood stiffness [20]. The greater growth of CW on the lower side branches helps to push the branches up; while the lower MFA and higher stiffness in OW could contribute to pull up branches against gravitational force. Since TW formed on the upper side of angiosperm branches also had a drastically declined MFA and larger stiffness [17, 21], this pull-push mechanism appears to be conserved in gymnosperms and angiosperms. CW and OW variation observed in the radiata pine branches were mostly in agreement with previous data derived from bent trunks of conifer species [2]. Although gravity stress has little impact on tracheid wall expansion in radial and tangential directions (Figure 1), it does affect longitudinal growth of tracheids as CW has longer tracheids than OW [2].
Differential gene transcription could provide molecular evidence for the drastic variation between CW and OW. The xylem transcriptome changes in CW and OW of radiata pine branches (28-29%) are among the highest in a number of microarray comparisons with regard to radiata pine wood development, including earlywood vs. latewood (11-30%) [22], juvenile wood vs. mature wood (9.2-19.3%) [23], high stiffness vs. low stiffness wood (3.4-14.5%) [24], high density vs. low density wood (10-19%) [25]. Genes differentially transcribed in CW and OW had various functions in cell division, cell expansion, primary wall synthesis, secondary wall deposition, hormone and calcium signallings, transcription and environmental stresses. The extensive transcriptome remodelling and divergent functions of differentially transcribed genes could underlie drastic CW and OW variation observed in radiata pine branches. Genes involved in cell wall formation, hormone and calcium signallings, and various environmental stresses were mostly up-regulated in CW. Thus, CW experienced more transcriptome remodelling than OW, resulting in greater phenotypic variation between CW and wood formed in normal conditions (NW) compared to that between OW and NW [2].
Cytoskeleton-related genes affect cellulose microfibril orientation
The cytoskeleton is made up of microtubules, actin filaments, and intermediate filaments [26]. There is growing evidence that cortical microtubules play a key role during the crystallization of cellulose microfibrils [27] by directing their orientation in the wall [28]. Up-regulation of alpha- and beta-tubulins in CW (with larger MFA) of radiata pine branches is in agreement with previous study using bent trunks of maritime pine [16]. Association of allelic variation in an alpha-tubulin with MFA was observed in secondary xylem of loblolly pine [29]. In angiosperms, several alpha- and beta-tubulins were highly transcribed in TW (with reduced MFA) formed in bent poplar trunks [30] and eucalypt branches [17]. Over-transcription of an eucalypt beta-tubulin gene in transgenic xylem directly influenced MFA [31]. Taken together, the functions of tubulin genes involved in cellulose microfibril orientation of secondary xylem have been conserved in both gymnosperms and angiosperms.
Actin filaments are much less rigid compared to microtubules [32]. Interaction of actin filaments with cortical microtubules altered the orientation of cellulose microfibrils in cultured cotton fiber cells [33]. Our study identified two ADFs (ADF and ADF-like) that were exclusively up-regulated in CW of branches. ADF plays an important role in regulating the optimum balance between unpolymerised actin molecules and assembled actin filaments [34]. Genes involved in actin filaments showed different transcription patterns in reaction wood between branches (this study) and bent trunks [16] in conifers. For example, actin polymerizing factors up-regulated in CW of bent trunks in spring were not identified in CW of branches in either spring or autumn, suggesting their responses exclusive to bending forces rather than gravity stimulus. In contrast, genes (i.e., four actins, two ADFs and two actin bundling proteins) with differential transcription in branches were not identified in bent trunks, highlighting their possible roles in response to gravity stress.
Secondary cell wall genes confer tracheid wall thickness and wood density
This study identified many secondary cell wall genes with preferential transcription in CW of radiata pine branches (Table 3 and 4). Three PrCesA genes (PrCesA3, 7, 11) up-regulated in CW were previously clustered as secondary wall genes and PrCesA10 preferentially transcribed in OW is a primary wall gene [35]. Several CesAs were also up-regulated in CW of bent maritime pine [16], TW of bent eucalypts [36, 37] and poplars [38, 39]. Besides, CesA-like and SuSy genes were up-regulated in CW of both branches (this study) and bent trunks of conifers [16]. In Scots pine SuSy activity was observed to peak in the zone of maturing tracheids where the secondary wall is formed, and its transcription was lower in primary wall tissues [40]. Over-transcription of a SuSy gene increased cellulose content, secondary wall thickness and wood density in poplars [41].
Lignin biosynthesis consists of three major steps: shikimate pathway, monolignol pathway and monolignol polymerization [42]. Phenylalanine is an end product of the shikimate pathway with seven enzymes involved [43]. Five of these genes were up-regulated in CW of radiata pine branches, including shikimate kinase 2, chorismate synthase, chorismate mutase, 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DAHPS) and 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS) (Additional file 1). Phenylalanine and other precursors for monolignol biosynthesis are extensively methylated in the S-adenosyl methionine (SAM) dependent reaction [44]. Genes related to SAM metabolism (SAMS, SAMDC, MetE and methionine synthases) were up-regulated in CW of radiata pine branches. Preferential transcription of genes related to shikimate pathway and SAM metabolism in CW may result in more production of monolignol precursors. Finally, monolignol synthesis could be also enhanced in CW due to up-regulation of several key genes involved in monolignol pathway (e.g., PAL, C4H, COMT, 4CL and CCR-like). Most of these lignin-related genes were also up-regulated in CW of bent trunks in maritime pine [16] and TW of bent eucalypts [36]. Besides, several other lignin-related proteins were highly presented in CW of bent trunks in maritime pine (COMT, caffeoyl CoA-O-methyltransferase and SAMS) [45] and in Japanese cypress at the transcript level (laccases, COMT and methionine synthase) [13]. In summary, up-regulation of lignin-related genes in CW provided the molecular basis for its higher lignin content and thicker tracheid walls compared to that in OW.
A number of cell wall structural protein genes (AGPs, AGP-like, glycine-rich proteins and proline-rich proteins) were exclusively up-regulated in CW of radiata pine branches. In loblolly pine six PtaAGPs were predominantly transcribed in secondary xylem development [46]. Our study revealed that FLAs were differentially transcribed in either CW (FLA1 and 8) or OW (FLA10, 17 and 26) of radiata pine branches. In angiosperms FLAs were up-regulated in TW of bent poplar trees [47] and eucalypt branches [17]. Gene function studies further confirmed that FLAs affect MFA and tensile stiffness in transgenic eucalypts and Arabidopsis by altering cellulose deposition and the integrity of the cell wall matrix [48].
Tracheid wall thickness and wood density are determined by secondary cell wall synthesis and deposition. Up-regulation of genes related to cellulose and lignin biosynthesis and cell wall structure in CW of radiata pine branches coincided with its drastically increased tracheid walls and wood density. These results were generally in agreement with previous studies in the bent trunks of maritime pine [16] and eucalypts [36] as well as in radiata pine juvenile wood with higher density [25]. However, it has been well documented that CW has lower cellulose content [2, 11]. This is because lignin synthesis is also greatly increased in CW as suggested in this study and demonstrated elsewhere [2]. Thus, CW has relatively lower cellulose content.
Genes involved in cell division and primary wall modification implicate wood growth and tracheid dimensions
The quantity of wood formation is largely related to cell division and expansion during primary cell wall development. Four cell division-related genes were identified with up-regulation in CW of radiata pine branches. Rapid cell division in CW could be an earlier response of reaction wood formation in conifer branches under gravity stress. It can partly explain the greater wood growth on the lower side of branches.
Tracheids are structures of three dimensions (radial, tangential and longitudinal directions) which are determined in cell expansion during primary wall formation. Several expansins and XETs were differentially transcribed in CW and OW of radiata pine branches (this study) and bent trunks of maritime pine [16]. XETs can cut and rejoin xyloglucan (XG) chains, and are believed to be important regulators of primary wall expansion [49]. Different genes involved in pectin biosynthesis were up-regulated in CW (pectin lyase-like) or OW (pectin lyase 2, pectinesterase-like and pectin-glucuronyltransferase) of radiata pine branches. Differential transcription of these genes in CW and OW could provide molecular evidence for their similar tracheid diameters (either radial or tangential directions). On the other hand, a gene encoding ovule/fiber cell elongation protein with up-regulation in OW could suggest its possible function in the longitudinal growth of tracheids.
Gravity stress triggers hormone, calcium and other environmental signals
Plant gravitropism is a complex process including three major stages: gravity perception, signal transduction, and growth response. In this study many genes related to hormone and calcium signalling as well as environmental stresses were up-regulated in CW of branches; while only a few genes in these categories were preferentially transcribed in OW (Table 5 and 6). These results provided valuable clues for the understanding of reaction wood formation in response to gravity stimulus during earlier perception and signal transduction.
Some hormone signalling genes with up-regulation in CW of radiata pine branches (Table 5) had preferential transcription in CW of inclined pines [14, 45] and TW of bent eucalypts [36]. Auxin is widely believed to be the primary effector of gravitropism since its asymmetric distribution drives the gravitropic growth [50]. Gravity stimulus also induces other hormones, such as ethylene (on the lower side of branches [51] and bent trunks [52]) and gibberellins (TW in tilted Acacia mangium seedlings [53]). Cytokinin increased secondary xylem formation with higher lignification and thicker cell walls [54]. The identified hormone signalling genes (Table 5) and other TF genes (Additional file 2) could provide additional candidates of gravity preceptors or signal transduction.
Calcium (Ca2+) signalling has a strong relationship with plant gravitropism [55]. It has functions in all steps of the signal transduction pathway by acting as a second messenger to mediate auxin redistribution [55]. A calcium/calmodulin-dependent protein kinase from maize showed light-regulated gravitropism [56]. Calcium has been proven a role in secondary xylem development and CW formation [57]. In the present study several calcium signalling genes were consistently up-regulated in CW of branches in both spring and autumn (Table 5), providing further evidence for calcium signals with roles in reaction wood formation. The identified calcium signalling genes could be important candidates of earlier signals of conifer reaction wood formation in response to gravity stress.
The majority of differentially transcribed genes involved in various environmental stresses (e.g., water, light, diseases and salt) were up-regulated in CW of branches (Table 6). This is because rapid CW formation requires more resources for cell division, primary wall formation and secondary wall deposition that trigger different environmental stresses. Up-regulation of three light signalling genes (light-inducible protein ATLS1, light-induced protein-like and phytochrome) in CW of radiata pine branches could be a result of reduced light radiation on the lower side of branches. Phytochromes are red and far-red light photoreceptors, and they regulate a large number of genes involved in hormone signalling or enzymes involved in cell wall modification [58]. In hypocotyls gravitropism phytochromes inhibited four phytochrome-interacting factors [59]. Thus, plant gravitropism may be regulated by the interaction between light, hormone and calcium signallings [60, 61].
Conclusions
Compression wood formed in radiata pine branches showed greater radial growth, thicker tracheid walls, larger microfibril angle (MFA), higher density and lower stiffness, but similar tracheid diameters compared to its opposite wood. Extensive remodelling of the xylem transcriptomes (29%) observed in compression and opposite wood could provide molecular evidence for their drastic variation in tracheid and wood traits. Many genes involved in cell division, cellulose biosynthesis, lignin pathway and microtubules were exclusively up-regulated in compression wood, conferring its greater radial growth, thicker tracheid walls, higher density, larger MFA and lower stiffness. In contrast, genes related to cell expansion and primary wall modification were differentially transcribed in either compression or opposite wood, implicating their similar tracheid diameters but different tracheid lengths. Of particular interest, a broad range of genes related to hormone and calcium signalling and various environmental stresses were exclusively up-regulated in compression wood, suggesting possible earlier molecular signatures of plant gravitropism during reaction wood formation in conifers. The first transcriptome profiling of radiate pine branches provides more accurate insights into the molecular basis of reaction wood formation in response to gravity stimulus without external bending forces.
Methods
Plant materials and sampling
Six trees with well-developed branches were selected from a radiata pine commercial plantation located at Bondo, NSW, Australia (35º 16' 44.04" S, 148º 26' 54.66" E). These trees were originated from seedlings with different genotypes and they were 13 years old at the time of sampling. The largest branch from each tree was further selected for study, including three branches sampled in autumn and three sampled in spring. Bark was removed from the base part (about 10 cm in length) of each branch. Developing xylem tissues were scraped from the exposed upper and lower side surface respectively with a sharp chisel. Samples were immediately placed into 50 ml BD Falcon™ tubes filled with liquid nitrogen. One branch disc (approximately 5 cm in length) was then cut off from the larger end of each branch adjacent to the base part used for developing xylem sampling. Location of upper and lower side zones was immediately marked on all discs collected from branches.
Measurements of tracheid and wood traits
After removing the bark from each branch disc a block of wood (about 2 cm in length in both tangential and longitudinal directions) was cut from the top of upper side to the bottom of lower side through pith. A twin-blade saw was used to trim the wood blocks to produce strips (containing pith) of 2 mm in the tangential direction and 7 mm in the longitudinal direction. The wood strips were characterized using the SilviScan® instrument [18, 19]. A total of eight tracheid and wood traits were measured, including tracheid wall thickness, radial diameter, tangential diameter, coarseness (tracheid mass per unit length), specific surface (tracheid surface area per unit mass), cellulose microfibril angle (MFA, the angle of cellulose fibrils in wood cell walls versus the longitudinal cell axis), as well as wood density (the dry weight per unit volume of wood) and stiffness or modulus of elasticity (MOE) (the degree of wood deflected when a load is applied perpendicular to the grain). All eight traits were analyzed at 25 μm interval across the wood strips.
Microarray experiments and data analysis
Total RNA was extracted from developing xylem tissues using a modified CTAB method [62]. Transcript abundance on the upper and lower side of branches sampled in spring and autumn was compared, respectively, using radiata pine cDNA microarrays containing 18,432 clones derived from six developing xylem libraries [22, 35]. Of these cDNAs, 6,169 were randomly sequenced and assembled into 3,320 xylem unigenes (986 contigs and 2,334 singletons) [22, 35]. A dye swap was performed for each biological replicate, resulting in a total of six replicates in each of the two microarray experiments.
Construction of cDNA microarrays, synthesis of probes and microarray hybridization were performed in methods described previously [22–24]. Hybridized microarrays were scanned using a GenePix Personal 4100A scanner (Axon Instruments, CA). Images were pre-processed using GenePix® Pro 6.0 (Axon Instruments, CA). Median values of fluorescence intensity of the red and green colours were used to generate a ratio representing the difference of gene transcription in the two tissues being compared. Differential gene transcription in the six microarrays of each experiment were jointly normalized at both print-tip and slide scale levels using GEPAS v3.1 [63]. The raw dataset of all 12 microarrays was registered in the NCBI GEO database with accession number GSE47167. Mean fold changes of gene transcription in CW compared to OW ≥ 1.5 times (or log-2 ratio ≥ 0.584 and ≤ -0.584) and P-values ≤ 0.05 calculated with Cyber-T [64] were used as thresholds for the selection of differentially transcribed unigenes. Putative candidate genes were further shortlisted after removing redundant unigenes showing identical accession numbers in the UniProt known proteins and TIGR gene indices databases.
Validation of microarray gene transcription
Microarray results of selected genes were validated using the reverse transcriptase-multiplex ligation dependent probe amplification (RT-MLPA) method [65]. A total of seven genes consistently up-regulated in CW or OW in both spring and autumn were selected for validation, including four genes for CW: cellulose synthase 3 (PrCesA3), PrCesA11, cinnamic acid 4-hydroxylase (C4H) and plastocyanin-like (PCL); and three genes for OW: peroxidase (PER), E3 ubiquitin protein ligase (UPL1) and retinoblastoma-like protein (RBL) (Additional file 1). Developing xylem (CW and OW) sampled in autumn for the microarray experiment was used in the validation, including three biological and four technical replicates. Mean log-2 ratios of the 12 replicates were calculated for selected genes and then compared with microarray results.
Approximately 400 ng of purified total RNA was reverse transcribed into first strand cDNA using the ImProm-II Reverse Transcription System (Promega, WI). The cDNA was hybridized at 60°C overnight with bulked RPO (right probe oligo) and LPO (left probe oligo) probes designed for the selected genes (Additional file 3). Ligation and PCR amplification were performed with SALSA D4 primer. Individual gene fragments were separated from the mixed PCR products using a CEQ™ 8000 Genetic Analysis System (Beckman Coulter, CA) and relative gene transcription levels were determined using the built-in software.
Electronic supplementary material
Additional file 1: Genes differentially transcribed in compression (CW) and opposite wood (OW) of radiata pine branches sampled in spring and autumn. In total, 846 genes were identified with differential transcription in CW (693) and OW (153) of radiata pine branches sampled in spring; while 872 genes were preferentially transcribed in CW (511) and OW (361) sampled in autumn. (XLS 506 KB)
Additional file 2: Transcription factors differentially transcribed in compression (CW) and opposite wood (OW) of radiata pine branches. (XLS 25 KB)
Additional file 3: LPO (left probe oligo) and RPO (right probe oligo) of selected differentially transcribed genes involved in the validation. A total of seven differentially transcribed genes identified in the microarray experiments were selected in the validation by reverse transcriptase-multiplex ligation dependent probe amplification (RT-MLPA). Their LPO and RPO sequences were listed in the table. (XLS 24 KB)
Acknowledgments
The authors thank Iain Wilson for assistance with the microarray printing and scanning facilities, Simon Southerton and Aljoy Abarquez for participation in field sampling, and Robert Evans for SilviScan analysis. We also thank Iain Wilson and Colleen MacMillan for valuable comments to improve the manuscript. This work was part of the Juvenile Wood Initiative (JWI) (PNC050-0304), a project funded by Forest and Wood Products Australia (FWPA), ArborGen LLC, the Southern Tree Breeding Association (STBA), Queensland Department of Primary Industry (QDPI) and the Commonwealth Scientific and Industrial Research Organization (CSIRO).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XL designed the whole experiment, constructed cDNA microarrays, conducted microarray experiments, analysed SilviScan and gene transcription data, and wrote the manuscript. XY analysed part of the gene transcription data. HW proposed the research project and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xinguo Li, Email: xinguo.li@csiro.au.
Xiaohui Yang, Email: yangxiaohui82122@163.com.
Harry X Wu, Email: harry.wu@csiro.au.
References
- 1.Yamashita S, Yoshida M, Takayama S, Okuyama T. Stem-righting mechanism in gymnosperm trees deduced from limitations in compression wood development. Ann Bot. 2007;99:487–493. doi: 10.1093/aob/mcl270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timell TE. Compression wood in gymnosperms. Berlin: Springer-Verlag; 1986. [Google Scholar]
- 3.Donaldson LA, Grace J, Downes GM. Within-tree variation in anatomical properties of compression wood in radiata pine. IAWA J. 2004;25:253–271. doi: 10.1163/22941932-90000364. [DOI] [Google Scholar]
- 4.Kibblewhite RP, Evans R, Grace JC, Riddell MJC. Fibre length, microfibril angle and wood colour variation and interrelationships for two radiata pine trees with mild and severe compression wood. Appita Journal. 2005;58:316–322. [Google Scholar]
- 5.Tarmian A, Azadfallah M. Variation of cell features and chemical composition in spruce consisting of opposite, normal, and compression wood. Bioresources. 2009;4:194–204. [Google Scholar]
- 6.Diaz-Vaz JE, Ananias RA, Rodriguez S, Torres M, Fernandez A, Poblete H. Compression wood in Pinus radiata II: density and chemical composion. Maderas-Ciencia Y Tecnologia. 2009;11:139–151. [Google Scholar]
- 7.Yeh TF, Braun JL, Goldfarb B, Chang HM, Kadla JF. Morphological and chemical variations between juvenile wood, mature wood, and compression wood of loblolly pine (Pinus taeda L.) Holzforschung. 2006;60:1–8. doi: 10.1515/HF.2006.001. [DOI] [Google Scholar]
- 8.Lachenbruch B, Droppelmann F, Balocchi C, Peredo M, Perez E. Stem form and compression wood formation in young Pinus radiata trees. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 2010;40:26–36. doi: 10.1139/X09-169. [DOI] [Google Scholar]
- 9.Yamashita S, Yoshida M, Yamamoto H. Relationship between development of compression wood and gene expression. Plant Sci. 2009;176:729–735. doi: 10.1016/j.plantsci.2009.02.017. [DOI] [Google Scholar]
- 10.Yeh TF, Goldfarb B, Chang HM, Peszlen I, Braun JL, Kadla JF. Comparison of morphological and chemical properties between juvenile wood and compression wood of loblolly pine. Holzforschung. 2005;59:669–674. doi: 10.1515/HF.2005.107. [DOI] [Google Scholar]
- 11.Lohrasebi H, Mabee WE, Roy DN. Chemistry and pulping feasibility of compression wood in black spruce. Journal of Wood Chemistry and Technology. 1999;19:13–25. doi: 10.1080/02773819909349597. [DOI] [Google Scholar]
- 12.Zhang Y, Sederoff RR, Allona I. Differential expression of genes encoding cell wall proteins in vascular tissues from vertical and bent loblolly pine trees. Tree Physiol. 2000;20:457–466. doi: 10.1093/treephys/20.7.457. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita S, Yoshida M, Yamamoto H, Okuyama T. Screening genes that change expression during compression wood formation in Chamaecyparis obtusa. Tree Physiol. 2008;28:1331–1340. doi: 10.1093/treephys/28.9.1331. [DOI] [PubMed] [Google Scholar]
- 14.Ramos P, Le Provost G, Gantz C, Plomion C, Herrera R. Transcriptional analysis of differentially expressed genes in response to stem inclination in young seedlings of pine. Plant Biol. 2012;14:923–933. doi: 10.1111/j.1438-8677.2012.00572.x. [DOI] [PubMed] [Google Scholar]
- 15.Whetten R, Sun YH, Zhang Y, Sederoff R. Functional genomics and cell wall biosynthesis in loblolly pine. Plant Mol Biol. 2001;47:275–291. doi: 10.1023/A:1010652003395. [DOI] [PubMed] [Google Scholar]
- 16.Villalobos DP, Diaz-Moreno SM, Said ESS, Canas RA, Osuna D, Van Kerckhoven SHE, Bautista R, Claros MG, Canovas FM, Canton FR. Reprogramming of gene expression during compression wood formation in pine: Coordinated modulation of S-adenosylmethionine, lignin and lignan related genes. BMC Plant Biol. 2012;12:100. doi: 10.1186/1471-2229-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu D, Wilson IW, Gan S, Washusen R, Moran GF, Southerton SG. Gene expression in Eucalyptus branch wood with marked variation in cellulose microfibril orientation and lacking G-layers. New Phytol. 2008;179:94–103. doi: 10.1111/j.1469-8137.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- 18.Evans R. Rapid measurement of the transverse dimensions of tracheids in radial wood sections from Pinus radiata. Holzforschung. 1994;48:168–172. doi: 10.1515/hfsg.1994.48.2.168. [DOI] [Google Scholar]
- 19.Evans R, Stringer S, Kibblewhite RP. Variation of microfibril angle, density and fibre orientation in twenty-nine Eucalyptus nitens trees. Appita Journal. 2000;53:450–457. [Google Scholar]
- 20.Evans R, Ilic J. Rapid prediction of wood stiffness from microfibril, angle and density. For Prod J. 2001;51:53–57. [Google Scholar]
- 21.Washusen R, Evans R, Southerton S. A study of Eucalyptus grandis and Eucalyptus globulus branch wood microstructure. IAWA J. 2005;26:203–210. doi: 10.1163/22941932-90000112. [DOI] [Google Scholar]
- 22.Li XG, Wu HX, Southerton SG. Seasonal reorganization of the xylem transcriptome at different tree ages reveals novel insights into wood formation in Pinus radiata. New Phytol. 2010;187:764–776. doi: 10.1111/j.1469-8137.2010.03333.x. [DOI] [PubMed] [Google Scholar]
- 23.Li XG, Wu HX, Southerton SG. Transcriptome profiling of wood maturation in Pinus radiata identifies differentially expressed genes with implications in juvenile and mature wood variation. Gene. 2011;487:62–71. doi: 10.1016/j.gene.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Li XG, Wu HX, Southerton SG. Transcriptome profiling of Pinus radiata juvenile wood with contrasting stiffness identifies putative candidate genes involved in microfibril orientation and cell wall mechanics. BMC Genomics. 2011;12:480. doi: 10.1186/1471-2164-12-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XG, Wu HX, Southerton SG. Identification of putative candidate genes for juvenile wood density in Pinus radiata. Tree Physiol. 2012;32:1046–1057. doi: 10.1093/treephys/tps060. [DOI] [PubMed] [Google Scholar]
- 26.Shibaoka H, Nagai R. The plant cytoskeleton. Curr Opin Cell Biol. 1994;6:10–15. doi: 10.1016/0955-0674(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 27.Hofte H. Plant cell biology: How to pattern a wall. Curr Biol. 2010;20:R450–R452. doi: 10.1016/j.cub.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Chaffey N. Microfibril orientation in wood cells: new angles on an old topic. Trends Plant Sci. 2000;5:360–362. doi: 10.1016/S1360-1385(00)01695-2. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Martinez SC, Wheeler NC, Ersoz E, Nelson CD, Neale DB. Association genetics in Pinus taeda L I Wood property traits. Genetics. 2007;175:399–409. doi: 10.1534/genetics.106.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakley RV, Wang YS, Ramakrishna W, Harding SA, Tsai CJ. Differential expansion and expression of alpha- and beta-tubulin gene families in Populus. Plant Physiol. 2007;145:961–973. doi: 10.1104/pp.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spokevicius AV, Southerton SG, MacMillan CP, Qiu D, Gan S, Tibbits JFG, Moran GF, Bossinger G. beta-tubulin affects cellulose microfibril orientation in plant secondary fibre cell walls. Plant J. 2007;51:717–726. doi: 10.1111/j.1365-313X.2007.03176.x. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher DA, Mullins D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seagull RW. The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibers. Protoplasma. 1990;159:44–59. doi: 10.1007/BF01326634. [DOI] [Google Scholar]
- 34.Blanchoin L, Boujemaa-Paterski R, Henty JL, Khurana P, Staiger CJ. Actin dynamics in plant cells: a team effort from multiple proteins orchestrates this very fast-paced game. Curr Opin Plant Biol. 2010;13:714–723. doi: 10.1016/j.pbi.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Li XG, Wu HX, Dillon SK, Southerton SG. Generation and analysis of expressed sequence tags from six developing xylem libraries in Pinus radiata D Don. BMC Genomics. 2009;10:41. doi: 10.1186/1471-2164-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paux E, Carocha V, Marques C, de Sousa AM, Borralho N, Sivadon P, Grima-Pettenati J. Transcript profiling of Eucalyptus xylem genes during tension wood formation. New Phytol. 2005;167:89–100. doi: 10.1111/j.1469-8137.2005.01396.x. [DOI] [PubMed] [Google Scholar]
- 37.Lu SF, Li LG, Yi XP, Joshi CP, Chiang VL. Differential expression of three eucalyptus secondary cell wall-related cellulose synthase genes in response to tension stress. J Exp Bot. 2008;59:681–695. doi: 10.1093/jxb/erm350. [DOI] [PubMed] [Google Scholar]
- 38.Bhandari S, Fujino T, Thammanagowda S, Zhang DY, Xu FY, Joshi CP. Xylem-specific and tension stress-responsive coexpression of KORRIGAN endoglucanase and three secondary wall-associated cellulose synthase genes in aspen trees. Planta. 2006;224:828–837. doi: 10.1007/s00425-006-0269-1. [DOI] [PubMed] [Google Scholar]
- 39.Andersson-Gunneras S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B. Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J. 2006;45:144–165. doi: 10.1111/j.1365-313X.2005.02584.x. [DOI] [PubMed] [Google Scholar]
- 40.Uggla C, Magel E, Moritz T, Sundberg B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol. 2001;125:2029–2039. doi: 10.1104/pp.125.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman HD, Yan J, Mansfield SD. Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc Natl Acad Sci U S A. 2009;106:13118–13123. doi: 10.1073/pnas.0900188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 43.Tohge T, Watanabe M, Hoefgen R, Fernie AR. Shikimate and phenylalanine biosynthesis in the green lineage. Front Plant Science. 2013;4:66. doi: 10.3389/fpls.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye ZH, Kneusel RE, Matern U, Varner JE. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell. 1994;6:1427–1439. doi: 10.1105/tpc.6.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plomion C, Pionneau C, Brach J, Costa P, Bailleres H. Compression wood-responsive proteins in developing xylem of maritime pine (Pinus pinaster Ait.) Plant Physiol. 2000;123:959–969. doi: 10.1104/pp.123.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang SH, Wang HY, Sathyan P, Stasolla C, Loopstra CA. Real-time RT-PCR analysis of loblolly pine (Pinus taeda) arabinogalactan-protein and arabinogalactan-protein-like genes. Physiol Plant. 2005;124:91–106. doi: 10.1111/j.1399-3054.2005.00479.x. [DOI] [Google Scholar]
- 47.Lafarguette F, Leple JC, Dejardin A, Laurans F, Costa G, Lesage-Descauses MC, Pilate G. Poplar genes encoding fasciclin-like arabinogalactan proteins are highly expressed in tension wood. New Phytol. 2004;164:107–121. doi: 10.1111/j.1469-8137.2004.01175.x. [DOI] [PubMed] [Google Scholar]
- 48.MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG. Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J. 2010;62:689–703. doi: 10.1111/j.1365-313X.2010.04181.x. [DOI] [PubMed] [Google Scholar]
- 49.Darley CP, Forrester AM, McQueen-Mason SJ. The molecular basis of plant cell wall extension. Plant Mol Biol. 2001;47:179–195. doi: 10.1023/A:1010687600670. [DOI] [PubMed] [Google Scholar]
- 50.Philosoph-Hadas S, Friedman H, Meir S. Gravitropic bending and plant hormones. Plant Hormones. 2005;72:31–78. doi: 10.1016/S0083-6729(05)72002-1. [DOI] [PubMed] [Google Scholar]
- 51.Blake TJ, Pharis RP, Reid DM. Ethylene, gibberellins, auxin and the apical control of branch angle in a conifer Cupressus Arizonica. Planta. 1980;148:64–68. doi: 10.1007/BF00385443. [DOI] [PubMed] [Google Scholar]
- 52.Du S, Yamamoto F. Ethylene evolution changes in the stems of Metasequoia glyptostroboides and Aesculus turbinata seedlings in relation to gravity-induced reaction wood formation. Trees-Structure and Function. 2003;17:522–528. doi: 10.1007/s00468-003-0275-x. [DOI] [Google Scholar]
- 53.Nugroho WD, Yamagishi Y, Nakaba S, Fukuhara S, Begum S, Marsoem SN, Ko JH, Jin HO, Funada R. Gibberellin is required for the formation of tension wood and stem gravitropism in Acacia mangium seedlings. Ann Bot. 2012;110:887–895. doi: 10.1093/aob/mcs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saks Y, Feigenbaum P, Aloni R. Regulatory effect of cytokinin on secondary xylem fiber formation in an invivo system. Plant Physiol. 1984;76:638–642. doi: 10.1104/pp.76.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinclair W, Trewavas AJ. Calcium in gravitropism A re-examination. Planta. 1997;203:S85–S90. doi: 10.1007/PL00008120. [DOI] [PubMed] [Google Scholar]
- 56.Lu YT, Hidaka H, Feldman LJ. Characterization of a calcium/calmodulin-dependent protein kinase homolog from maize roots showing light-regulated gravitropism. Planta. 1996;199:18–24. doi: 10.1007/BF00196876. [DOI] [PubMed] [Google Scholar]
- 57.Du S, Yamamoto F. A study on the role of calcium in xylem development and compression wood formation in Taxodium distichum seedlings. IAWA J. 2003;24:75–85. doi: 10.1163/22941932-90000322. [DOI] [Google Scholar]
- 58.Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, Choi G. Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc Natl Acad Sci U S A. 2011;108:1729–1734. doi: 10.1073/pnas.1011066108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leopold AC, Lafavre AK. Interactions between red-light, abscisic-acid, and calcium in gravitropism. Plant Physiol. 1989;89:875–878. doi: 10.1104/pp.89.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perdue DO, Lafavre AK, Leopold AC. Calcium in the regulation of gravitropism by light. Plant Physiol. 1988;86:1276–1280. doi: 10.1104/pp.86.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang SJ, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report. 1993;11:113–116. doi: 10.1007/BF02670468. [DOI] [Google Scholar]
- 63.Tarraga J, Medina I, Carbonell J, Huerta-Cepas J, Minguez P, Alloza E, Al-Shahrour F, Vegas-Azcarate S, Goetz S, Escobar P, et al. GEPAS, a web-based tool for microarray data analysis and interpretation. Nucleic Acids Res. 2008;36:W308–W314. doi: 10.1093/nar/gkn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 65.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Genes differentially transcribed in compression (CW) and opposite wood (OW) of radiata pine branches sampled in spring and autumn. In total, 846 genes were identified with differential transcription in CW (693) and OW (153) of radiata pine branches sampled in spring; while 872 genes were preferentially transcribed in CW (511) and OW (361) sampled in autumn. (XLS 506 KB)
Additional file 2: Transcription factors differentially transcribed in compression (CW) and opposite wood (OW) of radiata pine branches. (XLS 25 KB)
Additional file 3: LPO (left probe oligo) and RPO (right probe oligo) of selected differentially transcribed genes involved in the validation. A total of seven differentially transcribed genes identified in the microarray experiments were selected in the validation by reverse transcriptase-multiplex ligation dependent probe amplification (RT-MLPA). Their LPO and RPO sequences were listed in the table. (XLS 24 KB)


