Abstract
Targeted nanomedicine holds promise to find clinical use in many medical areas. Endothelial cells that line the luminal surface of blood vessels represent a key target for treatment of inflammation, ischemia, thrombosis, stroke, and other neurological, cardiovascular, pulmonary, and oncological conditions. In other cases, the endothelium is a barrier for tissue penetration or a victim of adverse effects. Several endothelial surface markers including peptidases (e.g., ACE, APP, and APN) and adhesion molecules (e.g., ICAM-1 and PECAM) have been identified as key targets. Binding of nanocarriers to these molecules enables drug targeting and subsequent penetration into or across the endothelium, offering therapeutic effects that are unattainable by their nontargeted counterparts. We analyze diverse aspects of endothelial nanomedicine including (i) circulation and targeting of carriers with diverse geometries, (ii) multivalent interactions of carrier with endothelium, (iii) anchoring to multiple determinants, (iv) accessibility of binding sites and cellular response to their engagement, (v) role of cell phenotype and microenvironment in targeting, (vi) optimization of targeting by lowering carrier avidity, (vii) endocytosis of multivalent carriers via molecules not implicated in internalization of their ligands, and (viii) modulation of cellular uptake and trafficking by selection of specific epitopes on the target determinant, carrier geometry, and hydrodynamic factors. Refinement of these aspects and improving our understanding of vascular biology and pathology is likely to enable the clinical translation of vascular endothelial targeting of nanocarriers.
Keywords: drug targeting, intracellular delivery, nanoparticles, nanomedicine, nanocarriers, endothelium, cell adhesion molecules, ICAM-1, ACE, PECAM
Objectives and Challenges of Vascular Targeting of Nanocarriers
Drug Delivery Systems (DDS)
DDS promise to improve pharmacotherapy by optimizing: (i) drug solubility, (ii) its isolation from the body en route to the target site, (iii) its pharmacokinetics (PK) and biodistribution (BD), (iv) control of activity, (v) permeation through biological barriers, and (vi) targeting (Figure 1).1−5 Nanocarriers for drug delivery have diverse chemical contents,6−10 morphologies, surface features, geometries, and physical properties.1,11−20 Intricacies of their design have been reviewed elsewhere;21−24 this article instead focuses on the biological aspects of carrier interactions with target cells.
Figure 1.
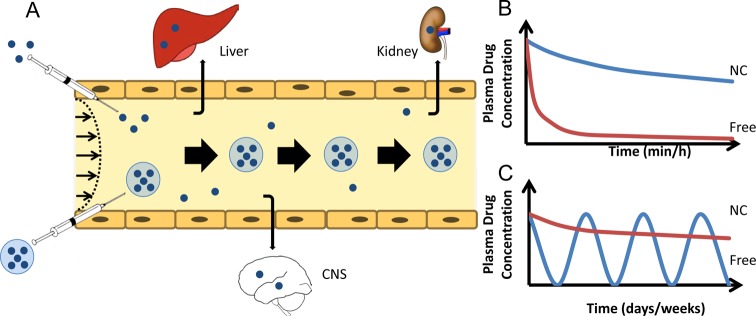
Drug delivery by nanocarriers: optimization of drug pharmacokinetics and biodistribution (PK and BD). (A) PK and BD of drugs (blue dots) vs drugs encapsulated in nanocarriers. Free drugs are eliminated from blood by clearing organs, including the reticuloendothelial system (RES, including liver, spleen, and lymphatic nodes) and excretory organs such as kidneys, lungs, and the bile tract (including hepatic uptake and renal filtration), and diffusion in nontarget tissues including the brain (CNS), where drugs may cause adverse effects. Long-circulating nanocarriers avoiding clearance alter PK (large arrows depict sustained drug circulation) and reduce diffusion in nontarget tissues, thereby improving BD and inhibiting adverse effects. Relative dimensions in this and other cartoons and schemas are not to scale. (B and C) Panels represent, respectively, short-term and long-term model graphs of blood level of free vs nanocarrier-bound drugs (NC). After a single injection in acute and subacute conditions, long-circulating NCs enhance area under the curve, thus reducing the effective dose (B). Hypothetically, using NCs with extended lifetime in circulation will help to maintain a stable therapeutic dose without the need for repeated injections (C).
DDS and drugs are eliminated by the reticuloendothelial system (RES, including liver, spleen, and lymphatic nodes) and other tissues—kidneys, lungs, and the bile tract.25,26 A carrier can “passively” accumulate in a desired sites. For example, particles in the size range ∼10–200 nm tend to accumulate in tumors and inflammation foci due to enhanced permeability of pathological vasculature25,27 (Enhanced Permeation and Retention, EPR, Figure 2B). Nonspecific retention of carriers in the microvasculature is another example of “passive targeting”28 (Figure 2C). It is exemplified by perfusion imaging using mechanical entrapment of particles with diameter 20–50 μm29 and delivery of plasmid DNA using cationic liposomes that bind to negatively charged vascular cells.30 Delivery is enriched in areas downstream from the site of injection, where released drug is removed by blood. “Passive targeting” provides little, if any, guidance in cellular delivery.
Figure 2.
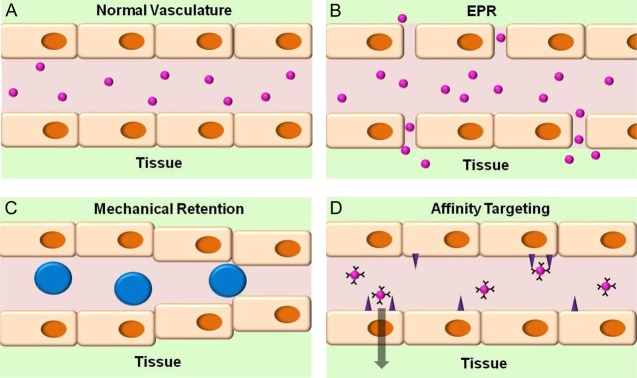
Passive uptake vs active targeting: differences in mechanism and potential biomedical utility. (A) Untargeted nanocarriers with size ranging from a few to a few hundred nanometers do not normally accumulate in healthy tissue with blood vessels lined by the continuous endothelial layer lacking large fenestrae typical of the reticuloendothelial system (RES). (B) Enhanced Permeation and Retention (EPR) effect. In this scenario, carriers accumulate in tissues with abnormally permeable vessels, such as in tumors fed by leaky vasculature as well as in sites of inflammation and angiogenesis (for example, wound healing). In tumors, deficient lymphatic drainage also favors the EPR effect, while high interstitial pressure opposes it (not shown). (C) Large particles, such as rigid spheres with diameter 10–50 μm (bigger than that of capillaries and precapillary arterioles), are mechanically retained downstream of the site of arterial injection in the microvasculature of an organ or tissue fed by this conduit artery. (D) Active targeting of nanocarriers coated by affinity ligands of specific determinants favors binding to endothelial cells exposing these determinants. In contrast with passive uptake (B and C), this mode guides subcellular delivery: binding to noninternalizable molecules vs those involved in cellular uptake and trafficking, respectively, favors retention on cell surface vs intracellular or transcellular delivery.
“Active targeting” is a more precise approach (Figure 2D). It uses ligands that bind to molecules uniquely present or enriched in a cell, tissue, or pathological structure of interest (target determinants). Antibodies and their derivatives including single chain antigen binding fragments (scFv), nutrients, hormones, receptor ligands, peptides, aptamers, and nucleic acids have been explored as targeting ligands.31−35 Targeting involves DDS delivery to the target site, initial physical contact, anchoring, residence on the cell surface or internalization, and excretion or storage.36−41
Vascular Endothelium: Drug Delivery Barrier and Destination
Intravascular injection, notwithstanding its downsides, is a preferable route for drug carriers, and their encounters with endothelial cells lining the vessels are involved in practically every conceivable drug delivery paradigm31,42,43 (Figure 3). Carriers designed for prolonged circulation must avoid binding to endothelial cells to minimize carrier’s elimination, danger of impeding blood flow and adverse effects via perturbation of these cells44,45 (Figure 3A), which exert important functions.46−48
Figure 3.
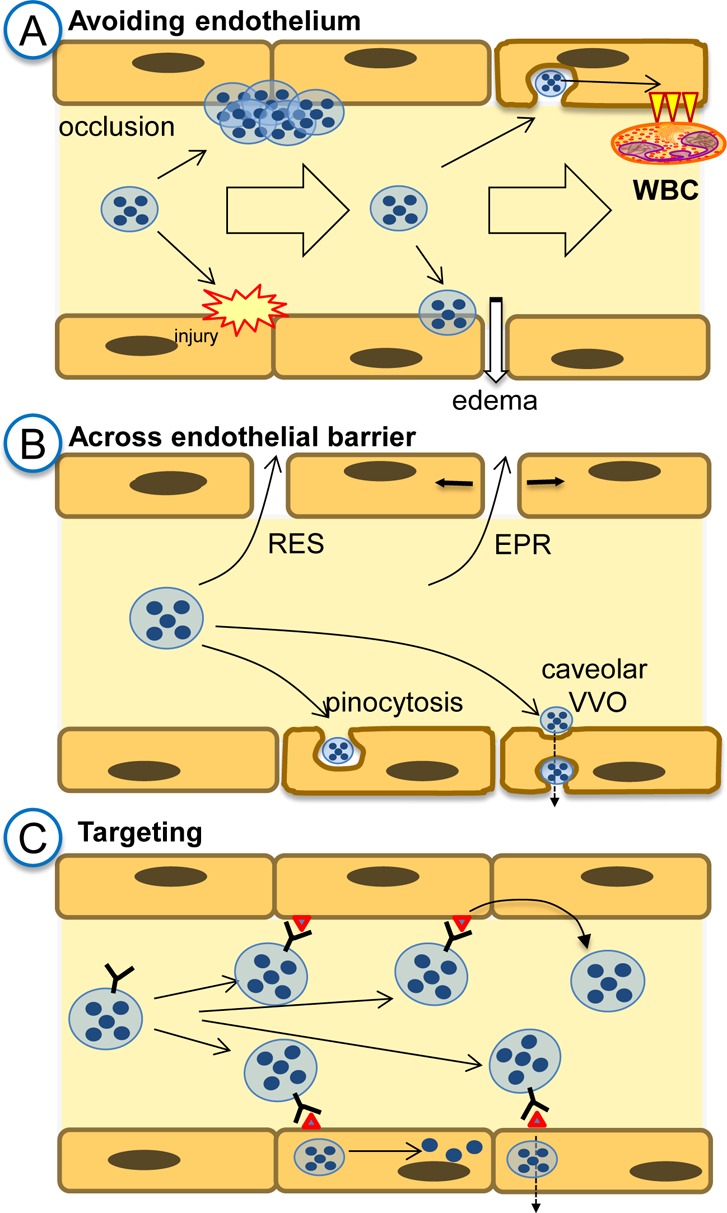
The vascular endothelium: a victim, barrier, and target of drug delivery. (A) In drug delivery strategies requiring cargoes to be released or act in the bloodstream, such as long-circulating reactors or slow release systems, respectively, carrier interaction with endothelium must be avoided. Otherwise, carriers adherence to endothelium may lead to vascular occlusion, endothelial damage, or pathological activation. (B) In strategies delivering drugs to the extravascular sites, endothelium is a barrier. Carriers may cross it by concentration gradient via large opening between endothelial cells in the RES (e.g., fenestrae) and intercellular openings in abnormally leaky vessels in tumors and sites of inflammation and angiogenesis (EPR). Vesicular transendothelial pathways include fluid phase transport via pinocytosis and transendothelial vacuolar–vesicular organelle (VVO) that opens from the caveolae. (C) In strategies targeting drug carriers to the endothelial surface determinants, ligand-mediated anchoring may result in surface retention or internalization. Depending on the nature of anchoring molecule, choice of epitopes, and carrier design, internalization may lead to recycling to the vascular lumen, delivery to the intracellular compartments, or transfer across the endothelium.
The endothelium controls vascular permeability.49 In the vascular sinuses of the RES organs, interendothelial openings are patent for micrometer-size objects. In lungs, heart, skin, mesentery, muscles, and most of other vascular areas, the endothelium transports particles in the range of ∼50–500 nm via dynamic intercellular gaps and transcellular fenestrae and vacuolar pathways initiated in endocytic vesicles.50,51 These pathways are restricted in cerebral vessels (the blood–brain barrier, BBB),52 where transport occurs via specific receptors and transporters. In conditions such as inflammation, thrombosis and ischemia, vascular permeability increases, mostly due to endothelial contraction widening pericellular gaps. Veins (especially venules53) and capillaries are more permeable than arteries.39,54−57 Pathological vascular leakiness in tumors and inflammation foci favors DDS transport to these sites, while transcellular pathways support passive extravascular delivery in normal vasculature54,58,59 (Figure 3B).
The endothelium controls the following: (i) blood fluidity and hemostasis; (ii) vascular tone, signaling, and angiogenesis; and (iii) trafficking blood cells.47,48,60 Its abnormalities are implicated in the pathogenesis of ischemia, thrombosis, inflammation, tumor growth and metastases, diabetes, hypertension, stroke, atherosclerosis and other maladies. In these conditions, endothelial cells represent therapeutic targets (Figure 3C).38,43,61,62 Yet, drugs and DDS have no natural affinity to endothelial cells. Conjugation with endothelial ligands enables delivery to, into, or across these cells (e.g., “vascular immunotargeting”, Figure 3C).63−68 Since the late 1980’s, numerous groups have pursued this strategy.68−77
Endothelium is more accessible to circulating agents than many other targets such as extravascular tumor cells. Yet, endothelial targeting has its own challenges. For example, concerns about side effects are more serious in endothelial relative to tumor targeting. Diverse medical goals require distinct and precise subcellular addressing of cargoes delivered using endothelial targeting. For example, fibrinolytics need to be anchored to the luminal surface, enzyme replacement therapies for storage disorders need to be delivered into the lysosomes and antitumor agents need to go across the endothelial monolayer (Figure 3C).33,63,68,78
Many chronic conditions involving the endothelium (e.g., hypertension, atherosclerosis, diabetes, and arthritis) do have pharmacological options, tempering the enthusiasm for development of complex targeted DDS that may cause problems when used repeatedly. Acute, life-threatening conditions lacking effective therapeutic options (e.g., sepsis, acute lung injury, ischemia, infarction, thrombosis and stroke) provide a more attractive clinical context for endothelial nanomedicine. We focus on circulation, cellular binding and transport of carriers targeted to endothelial cells, envisioned for use in these acute conditions (and, perhaps, beyond). For the sake of cohesiveness, we present here a discussion of drug delivery, not imaging, applications–despite this being a tremendously important and extensively developed area.79−89
Nanocarrier Behavior in the Circulation
To deliver drugs to endothelial cells, carriers must circulate in the bloodstream for a period of time sufficient for distribution in the vasculature. This time is less than a minute in animals with a high heart rate and small blood volume, but varies from several minutes to tens of minutes in humans, depending on their health status. Characteristics of carrier design and biological factors control its pharmacokinetics and biodistribution (PK and BD) and influence targeting.
The pace of binding correlates with carrier avidity, concentration, perfusion rate, and the density of binding sites. Avidin-coated particles bind to biotinylated surfaces almost instantaneously due to their extraordinarily high avidity,90 while antibody-coated carriers perfused over endothelial cells require minutes to hours to achieve sufficient interactions with target cells that allow for firm adherence to the cell.91−94 Further, endothelial targets that appear in the pathological sites may require more time for binding due to inefficient perfusion. Conversely, a carrier’s ability to circulate for a prolonged time, find the intended vascular network and bind to newly exposed molecular signatures of vascular pathology would be of great diagnostic and therapeutic utility.
Carrier Circulation in the Vascular System
The intravenous routes bypassing the liver direct the first pass of blood to the lungs.95 The pulmonary vasculature represents ∼25% of the total endothelial surface in the body and collects the entire cardiac output of venous blood from the right ventricle, whereas all other organs share the arterial output of equal volume. A local intra-arterial infusion via a catheter advanced to the conduit vessel favors first-pass carrier interaction with vascular cells in an organ or a vascular area downstream the vessel.95,96
Microvasculature (arterioles, capillaries, and venules) is the preferable target for endothelial nanomedicine. Extended luminal surface area, micrometer-scale vessel caliber, and low flow rate favor interaction of particles with endothelium in this vascular segment. Hydrodynamic conditions in arteries (high shear stress and pulsatile flow) are less favorable for particle interactions with endothelium than in veins. A significant (if not predominant) fraction of transport from blood to arterial walls occurs from the vasa vasorum, i.e., the microvascular network in the external adventitia layer.84,97−102
The nature of the flow and perfusion govern particle behavior in the bloodstream. Laminar flow carries particles with minimal frequency of collisions with vessel walls. At sites of disturbed flow and turbulence, either physiological (vessel bifurcations, heart chambers) or pathological (atherosclerotic plaque, aneurism, coarctation), carriers are more likely to collide with the endothelium. Therefore, carrier delivery to sites involved in or predisposed to pathology is naturally favored. For example, micrometer-sized carriers composed of polymeric nanoparticles that dissociate under high shear stress accumulate in site of vascular stenosis.103 However, perfusion insufficiency downstream of an obstruction caused by vasospasm, thrombus, or surgical ligatures diminishes carrier delivery to ischemic zones. In addition, blood stasis inhibits delivery to vasculature above the occlusion site.
Carrier Longevity in Circulation: Surface Properties, Stealth Coating, and Ligand Attachment
Particles, similar to dead cells and their fragments, are marked for elimination from the circulation by opsonization, i.e., adsorption of immunoglobulin, complement, and other plasma components that stimulate uptake by phagocytes in the RES and other tissues.104 Unless protected (see below), carriers are eliminated quickly following their injection.104,105 Generally, hydrophobic and charged carriers are opsonized and eliminated more rapidly than their hydrophilic and neutral counterparts.106
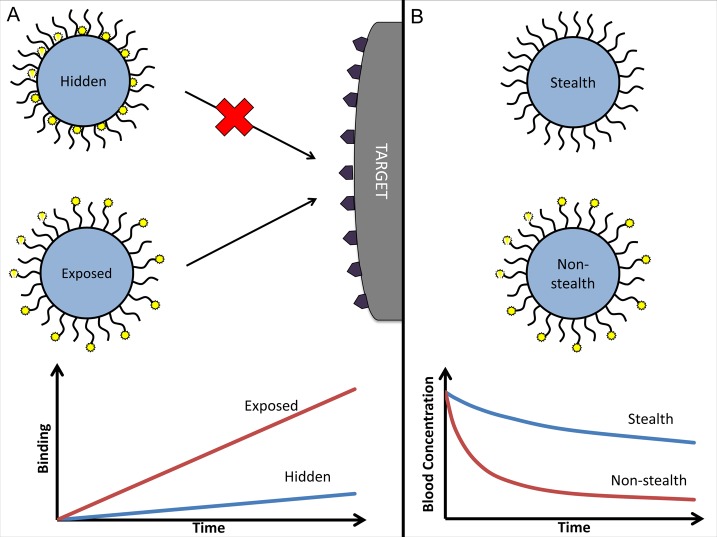
Coating with hydrophilic molecules (e.g., poly(ethylene glycol), PEG) that form a hydrated shell decelerates opsonization, inhibits a carrier’s interaction with phagocytes and other cells,107 and extends circulation lifetime.108 However, PEG chains may inhibit interactions of ligand molecules immobilized on the carrier with the target (Figure 4A). Both the masking and stealth effects are proportional to PEG chain length109 and surface density.110 Ligand conjugation via PEG alleviates the masking effect.109−111 Furthermore, ligand conjugation via PEG provides a flexible spacer that may improve the ligand steric freedom for interactions with target components (unless the spacer is so extended that it can “fold in” hiding the ligand).112 Conversely, conjugated ligand molecules diminish the stealth features of PEG coat (Figure 4B).
Figure 4.
Balancing the stealth and avidity features of carriers: effect on targeting and PK. (A) Surface modification by hydrophilic polymeric chains (e.g., PEG) provides a carrier with stealth features but affects targeting via masking affinity ligands. Conjugation of ligand molecules to the end groups of polymeric chains instead of the carrier surface helps to avoid this negative interference and provides additional steric freedom for ligand–target interaction, thereby boosting binding. (B) Ligand molecules conjugated to PEG diminish its stealth effect via nonspecific interactions (e.g., mediated by altered charge or hydrophilic features) and ligand-mediated interactions (e.g., via Fc-fragment of antibodies conjugated to PEG). Ensuing acceleration of blood clearance may affect drug delivery, if a carrier’s circulation time is insufficient for targeting.
A carrier’s stealth and targeting features may be optimized by conjugating corresponding moieties using linkers responsive to local stimuli of the target environment, such as temperature, pH, or proteolytic activity. For example, PEG conjugated via a cross-linker stable at physiological plasma pH 7.4 but labile at acidic pH will shed from a fully stealth carrier once it arrives at the acidic target site, therefore exposing ligands for binding.113 Practical implementation of “environmentally” sensitive nanocarriers depends on fine-tuning the dynamic range of their response. For example, unrelated proteases may impede the tissue selectivity of proteolytic transformation of carriers by enzymes preferentially active at target sites, such as a specific metalloproteinase. It is also difficult to fine-tune a carrier’s response to smaller pH changes, such as pH 6.0–6.5 vs <4.0, typical of ischemic and inflamed vasculature vs lysosomal vesicles, respectively.114
PEG is generally viewed as a biocompatible compound. Yet, the human immune response to PEG needs to be carefully assessed. PEG antibodies have been shown to form upon repeated administration of PEGylated compounds in animals.115,116 This phenomenon has been shown for a variety of carriers, ranging from proteins to liposomes.117−120 One possible mechanism for the accelerated blood clearance of PEGylated carriers upon repeated administration is the initial generation of anti-PEG antibodies (IgM) in the host,115 which persist in serum and bind to reinjected PEGylated carriers, reducing the circulation time. This initial anti-PEG IgM generation occurs in the spleen121 and likely involves B-cells,122 as B-cell deficient mice do not exert accelerated clearance of PEG-coated carriers.123
Coating carriers with molecules inhibiting activation of complement, opsonization, and phagocytosis such as sialic acid-containing glycolipids has been also pursued.124−126 More recently, specific biologically inspired means have been used to endow natural stealth properties to particulate carriers. For example, cells contain a membrane glycoprotein CD47, which interacts with receptors of macrophages eliciting signals that inhibit phagocytosis.127 Conjugation of CD47 and CD47-derived peptides to carriers helps to evade clearance via this natural “self-recognition” mechanism.127−130 Another approach to increase blood persistence of carriers is coating with fragments of cell membrane. Model PLGA carriers coated with mouse red blood cell (RBC) membranes circulated twice as long as PEGylated PLGA particles.131 In a similarly inspired study, leukocyte membrane coating inhibited carrier association with phagocytes and reduced hepatic uptake at short time points (<60 min) in vivo.132
Carrier Geometry and Plasticity
Neither too small nor too large particles circulate well. Renal filtration eliminates particles smaller than 10 nm.133 In addition, they extravasate via transendothelial fluid phase transport pathways,134,135 leading to elimination in skin, lungs, mesentery, muscles, and other tissues with extended surface of fenestrated microvasculature. On the other hand, particles larger than ∼500 nm are mechanically entrapped in capillaries.136 Agglomeration mediated by plasma components likely further enhances the latter outcome.
Carrier shape is an important factor of its behavior in the circulatory system and targeting.137 This is an area of research of astonishing complexity in every aspect: synthesis and quality control of carriers, rheological modeling and analysis, and appraisal of the biological relevance.138−141 The fundamental finding, confirmed in animal studies, is that elongated carriers of an appropriate size align with blood flow and have prolonged circulation in the bloodstream.142 For example, polystyrene elliptical disks with a maximal dimension of up to a few micrometers display a higher level in blood and lower nonspecific uptake in organs than spherical counterparts of a smaller size.143
One reason for the prolonged circulation of elongated objects is that they elude uptake by phagocytes.144 Macrophages internalize opsonized disk-shaped particles contacting the cell by the rounded end of the particle as effectively as spherical particles but cannot completely internalize disk-shaped particles contacting on their flat face (Figure 5).145 In support of the notion that nonspherical geometry may inhibit phagocytosis, filamentous particles coated with phagocytosis-promoting ligands were able to avoid phagocytosis by macrophages in cell culture.144
Figure 5.
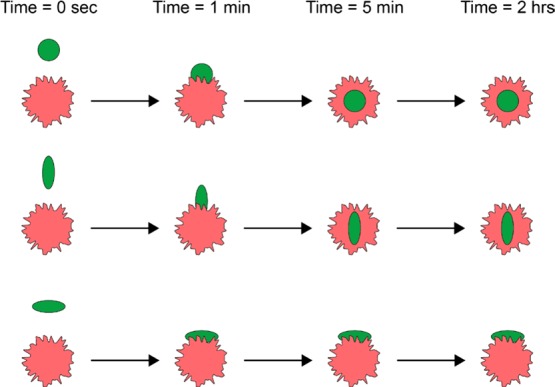
Carrier geometry modulates cellular uptake. Rate of intracellular uptake of elongated particles by phagocytic and other cells is markedly modulated by the axis of the particle binding to cell surface: it is faster than uptake of spherical particles of comparative maximal dimension in cases of binding via pointed vs flat aspects, respectively.
Carrier flexibility also has an important influence on circulation and targeting. Rigid elongated particles (rods, disks, tubes) larger than a few micrometers quickly become entrapped in the microvasculature in vivo. However, carriers with sufficient plasticity to reversibly change shape in response to hydrodynamic factors and vessel narrowing, can pass through the microcirculation, thereby imitating erythrocytes: ∼7 μm discs that repeatedly squeeze through capillaries with 1–2 μm lumen and effectively avoid clearance by immune cells.146 Indeed, red blood cell mimetic particles were shown to have extended in vivo circulation times.147 Flexibility was tuned via the extent of cross-linking and resulted in plasticity similar to that of red blood cells.148 Enhancing carrier’s flexibility prolongs its circulation.147 Follow-up studies with similarly flexible particles varying in size have shown that particles closer in diameter to red blood cells have prolonged circulation half-lives.149
Elongated flexible carriers have also been developed. Filomicelles are a distinctive subset of micelles made of amphiphilic block copolymers that assemble in flexible flow-responsive filaments.150,151 Filomicelles are able to persist in blood for extended periods of time by taking advantage of both their worm-like shape and cell-like flexibility. Their ability to align with blood flow and avoid immune system clearance enables them to persist in circulation for days, which is up to 10 times longer than their spherical counterparts.151 Pristine filomicelles exhibit little to no cellular binding, an attractive feature for a long-circulating DDS.152
Challenges and Perplexing Issues
Studies of carrier behavior in vivo are challenging and prone to artifacts. Often, researchers bypass them and test in vivo effects of drugs loaded in DDS characterized only with a variable degree of scrutiny in vitro, betting that observation of a desired effect exceeding that of “untargeted drug” provides a mandate to publish “proof of concept” papers.153
However, effects in animals are of limited value if the mechanism of delivery and effect of the DDS is not known. PK and BD directly influence targeting, and vice versa. It is essential to compare the PK and BD of targeted vs untargeted carriers formulated identically and coated by scrambled or inactive ligand (control IgG of corresponding type is a reasonable control for targeting antibody). Pristine carriers are not a proper control (different size, charge, surface features, etc.). Carriers with ligands conjugated to PEG have shorter PK and different BD vs pristine PEGylated carriers (Figure 4). All these factors may alter the therapeutic/side effects of a ligand-coated vs uncoated carrier by mechanisms distinct from targeting.
Preferential uptake in the target site may originate from mechanisms irrelevant to the ligand, e.g., passive retention of aggregated particles or interactions with blood components that may serve as “endogenous targeting agents” (lipoproteins, transferrin, immunoglobulin, blood cells, etc.). Competitive inhibition of targeting by injected free ligand may be complicated by different PK/BD and avidity of free ligand vs those of ligand-coated carriers, or simply because an effective competitive concentration in blood is above a realistically achievable one.
Thus, a quantitative analysis of PK/BD is essential; however, its implementation is not always feasible. Optical methods reveal nanoparticles in tissues at the microscopic level at post-mortem and at macroscopic level in real time (in sufficiently transparent sites)154 but do not provide accurate quantitative measurements in the body. Isotope imaging including PET is not limited by tissue penetration and affords real-time longitudinal analysis,155−158 but quantitative measurement of signals in organs is convoluted by factors including overshadowing by sites with high basal uptake. The resolution of isotope imaging is insufficient to analyze cellular distribution.
Another issue that is associated with this field is that labeling of labile components of a nanoparticle can lead to artifacts of their dissociation in the body. Labeling ligand moieties is prone to artifacts of tracing detached ligand leading to overestimating targeting. Noncovalent intercalation of hydrophobic labels in carriers is marred by artifacts of their redistribution in cellular membranes and other biological sinks, such as lipoproteins. Ideally, both the cargo and carrier should be stably traced by conjugated labels. A direct stable conjugation of isotopes to polymeric matrix allows tracing of the carrier within a reasonable time frame from a few hours to a few days, usually sufficient for vascular targeting.159
A direct measurement of the isotope level in drawn blood samples is arguably the best method for PK studies.74,76,90,160−162 Admittedly, extensive experimentation is needed to characterize the kinetics of targeting. Nevertheless, this simple approach affords the most accurate quantitative analysis of preclinical PK/BD. It yields the key parameters of targeting and BD: percent of injected dose (%ID) in tissues, localization ratio (LR, or ratio of %ID per gram of tissue to that in blood), and immunospecificity index (ISI, or ratio of LR for targeted vs untargeted formulations normalized to their blood levels).76,163 Injecting in the same animal a mix of targeted and untargeted formulations labeled by different isotopes is a useful maneuver to account for individual variability and significantly reduce the necessary efforts. Carrier PK/BD must be normalized to the injected dose. DDS heterogeneity is a serious problem. A fraction of large particles in a formulation may be eliminated within seconds by mechanical retention and not accounted for by normalizing data to “initial” level detected in blood post-injection.
Further complication in studying the interactions of DDS is that the behavior of nanoparticles in vivo can be drastically different than that in vitro. Nanoparticles bind blood components, aggregate, and dissociate.164 Even dense PEG-coating of polymeric carriers does not prevent eventual absorption of plasma components.165 Generally, carrier interactions with body components166 influence desired as well as adverse effects, including accumulation in the target and uptake by off-target cells.167,168 Yet, these processes remain poorly understood: there are limited studies on carrier behavior in blood even in vitro (e.g., DLS measurements in plasma are not informative). The methodology of studying nanoparticles in vivo also continues to evolve: a recent study employed a new high-throughput approach to study deposition of plasma proteins on model polymeric particles.169 Better analytical techniques are necessary to understand these interactions. For example, the use of citrate anticoagulated plasma in such studies is questionable because many mechanisms are not active in such a specimen, as opposed to serum.170−172
Generally, advanced approaches for characterization of DDS behavior in vivo are needed to provide objective and accurate information on carrier PK/BD. Such data will provide valuable insight about targeting efficacy, kinetics, and specificity. It will also shed light on the mechanisms of cell-specific interactions.
Carrier Binding to Endothelium
Anchoring to specific cells is a key step in targeting. It is controlled by ligand affinity and configuration (surface density, interactive freedom, etc.) as well as other features of the nanoparticle (size, charge, PK/BD, geometry, plasticity, etc.). Equally important are target features: surface density, clustering and accessibility of binding sites, phenotypic characteristics, and the microenvironment of target cells (perfusion, pH, protease activity, etc.). Carrier interactions with target determinants govern the specificity, selectivity, efficacy, fate, and safety of the DDS.
Endothelial Determinants for Nanocarrier Targeting
A target determinant should ideally meet the following criteria: (i) it anchors a carrier to endothelium in the area of interest; (ii) it provides desirable subcellular addressing; and (iii) it is not adversely affected by carrier binding in the context of disease treatment.
Selective proteomics of the endothelial plasmalemma64,173 and in vivo phage display73 are advantageous for finding new targets, as they identify binding sites accessible from circulation in normal174 and pathological vasculature.175 The list of endothelial determinants theoretically useful for vascular drug targeting continues to grow.75,176−178Table 1 briefly outlines the main features of the most intensely studied and promising candidates; more details are provided in this section.
Table 1. Endothelial Determinants: Selected Candidate Targets for Drug Deliverya.
| target | vascular localization | cellular localization | functions | regulation |
|---|---|---|---|---|
| Constitutive | ||||
| ACE (CD143) | Ubiquitous, enriched in the lung capillaries | Cell surface, single pass type I membrane protein | Plays a key role in rennin-angiotensin system, regulates blood pressure. Converts Ang I into Ang II and degrades bradykinin. | Catalytic activity is increased by chloride. |
| TM (CD141) | Ubiquitous, specific for endothelial cells | Cell surface, single pass type I membrane protein | Receptor for thrombin, participates in the generation of activated protein C. | Oxidative stress, hypoxia, oxidized LDL, C-reactive protein, phorbolesters, cAMP, and TNF tend to downregulate TM expression. Thrombin, VEGF, retinoic acid, and heat shock upregulate TM. TM level reflects a balance between those opposite forces. |
| Cell Adhesion Molecules | ||||
| PECAM-1 (CD31) | Ubiquitous | Cell junction, lateral border recycling compartment | Leukocyte transendothelial migration; antiapoptotic repulsion signaling from phagocyte | Stable expression |
| ICAM-1 (CD54) | Ubiquitous | Tetraspanin microdomains on cell surface, single pass type I membrane protein | Leukocyte firm arrest and transendothelial migration; immunological synapse formation. Ligand of LFA-1, MAC-1 | Up-regulated in inflammation |
| VCAM-1 (CD106) | Inflamed vascular endothelium | Tetraspanin microdomains on cell surface, single pass type I membrane protein | Leukocyte firm arrest and transendothelial migration. Ligand of VLA-4 | Up-regulated in inflammation |
| E-selectin (CD62E) | Inflamed vascular endothelium | Cell surface, single pass type I membrane protein | Leukocyte rolling | Up-regulated in inflammation |
| P-selectin (CD62P) | Inflamed vascular endothelium | Cytosol: Weibel-Palade bodies of endothelial cells, alpha-granules of platelets | Leukocyte rolling | Upon activation by agonists transported rapidly to cell surface |
| Caveolar | ||||
| APP | Enriched in pulmonary vascular endothelium. Expressed in heart, liver, kidney | Enriched in caveolae on the luminal surface of endothelial cells; lipid-anchored to cell membrane | Metalloprotase that plays a role in inflammatory process; cleaves and inactivates circulating polypeptides such as bradykinin | Unknown |
| PV1 (PLVAP) | Expressed in majority of tissues | Endothelial fenestrations, caveolar membrane | Formation of stomatal and fenestral diaphragms of caveolae, regulation of microvasculature permeability | Up-regulated by VEGF in vitro |
| Angiogenesis Tumor-Related | ||||
| APN (CD13) | Vasculature of tissues that undergo angiogenesis and in multiple tumor types | Cell surface, single pass type II membrane protein; cytosol | Metabolism of regulatory peptides, processing of peptide hormones (angiotensin III and IV, neuropeptides, and chemokines) | Estradiol and interleukin-8 decrease APN activity in vitro |
| Integrins αvβ3, αvβ5, α5β1 | Enriched in tumor vessels and other types of angiogenesis | Cell surface | angiogenesis, tumor neovascularization, and tumor metastasis | αvβ3 is up-regulated by bFGF, TNF |
| αvβ5 is up-regulated by VEGF, TGF-a | ||||
| TEM1 (CD248) | Tumor endothelial cells | Cell surface, single pass type I membrane protein | Tumor angiogenesis | unknown |
EC,endothelial cells; ACE, angiotensin-converting enzyme; TM, thrombomodulin; PECAM, platelet-endothelial cell adhesion molecule; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; APN, aminopeptidase N; APP, membrane-bound aminopeptidase P; TEM-1, tumor endothelial marker-1; PV-1, plasmalemma vesicle-associated protein.
Target Molecule Expression, Localization, And Accessibility
Not all endothelial target determinants are “endothelial-specific markers”, such as E-selectin. The utility for vascular targeting depends on relative levels in endothelial vs other cells accessible to blood. For example, ICAM-1 is expressed by fibroblasts, epithelial, and muscle cells at levels comparable with those in endothelium. Nevertheless, these extravascular cells are not accessible to circulating macromolecules and carriers and hence do not compete with endothelial targeting. In contrast, cytokine and transporter receptors are abundantly expressed on cells in blood, RES, lymphoid tissues, hepatocytes, and other compartments accessible to circulating DDS and, therefore provide very modest (if any) endothelial delivery.
Constitutive determinants (e.g., PECAM, ACE, and APP) are suited for prophylactic drug delivery. Some, including APP and ACE,27,28 disappear from the endothelium in pathological conditions, which inhibits targeting.179,180 Constitutive molecules stably exposed in the lumen, such as PECAM, can be theoretically used for prophylactic and/or therapeutic effects.161 In contrast to constitutive molecules, inducible counterparts expressed or exposed to the lumen in pathological sites (e.g., APN, TEM-1, VCAM-1, and selectins) are less likely to find prophylactic utility but seem preferable for diagnostic imaging and therapeutic interventions.39,65,68,71,181
With respect to localization in the vascular system, endothelial surface molecules fulfill the continuum ranging from pan-endothelial to domain-specific determinants (i.e., expressed throughout the vasculature or in certain vascular areas). Pan-endothelial targets (e.g., PECAM) can be used for systemic delivery to treat generalized conditions: sepsis, disseminated intravascular coagulation, or hypertension. The level of expression and surface density of many pan-endothelial determinants vary between organs and types of vessels. For example, ACE and thrombomodulin are expressed in the pulmonary capillaries at several fold higher level than in other organs, and antibodies to these molecules (anti-ACE and anti-TM) preferentially accumulate in the lungs.179,182,183 Domain-specific molecules, preferentially expressed in certain vascular areas, are attractive for local delivery. For example, inducible molecules APN and TEM-1 are upregulated in endothelium involved in angiogenesis in tumors and inflammation,184,185 whereas adhesion molecules VCAM-1 and selectins are expressed predominantly in sites of inflammation.186
Determinants differ with respect to preferential localization in domains in the endothelial plasmalemma.187,188 For example, PECAM and VE-cadherin are localized predominantly in cell–cell borders.189,190 VCAM-1 and ICAM-1 are found in tetraspanin microdomains of the cellular apical surface,191−193 special types of membrane “rafts” of distinct protein and lipid composition with a diverse array of function, including adhesion, proliferation, and immune cell signaling.194 Glycoprotein GP85 localizes in the luminal surface of the plasmalemma that belongs to a thin organelle-free part of endothelial cell separating alveoli from blood,195,196 and APP and PV-1 are located within caveoli.135 The location in the plasmalemma dictates the target accessibility, interference in cellular functions, and the fate of cell-bound DDS.
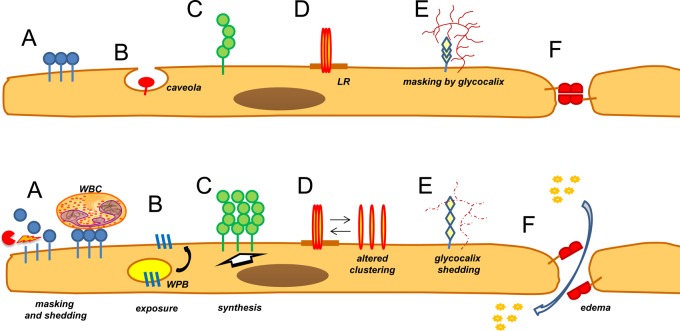
The endothelium is normally accessible to blood cells, lipoproteins, and other particles circulating in the bloodstream.43 Yet, accessibility to circulating particles varies dramatically among the endothelial surface molecules, depending on their localization in plasmalemma, location of the binding site epitope in the molecule, endothelial phenotype, and vascular conditions. Accessibility limitations are more stringent for carriers relying on multivalent binding and are proportional to the size of carriers. Epitopes located more proximally to the plasmalemma within the same target molecule are less suitable for harboring carriers than distal epitopes.197 Epitopes masked by the glycocalyx, buried in the intercellular junctions or in plasmalemma invaginations, are less accessible to affinity ligands and ligand-targeted DDS (Figure 6). Of note, shedding of endothelial glycocalyx induced by pathological mediators is implicated in enhanced accessibility of ICAM-1 to targeted carriers and activated leukocytes.198 On the other hand, adherent leukocytes may mask endothelial surface from blood.161
Figure 6.
Endothelial determinants in normal and pathological vasculature: accessibility, regulation, and localization in specific domains of the plasmalemma. (Upper panel) Normal endothelium exposes determinants that are differently accessible to circulating carriers. They include molecules clustered in apical plasmalemma, such as ACE (A); located in the caveoli, such as APP and PV1 (B); molecules, such as ICAM-1, expressed as monomers and oligomers at basal level throughout the plasmalemma (C); molecules preferentially localized in membrane domains (e.g., lipid rafts, LR) (D); molecules partially masked by glycocalyx (E); and molecules concentrated in cellular junctions, such as PECAM and VE-cadherin (F). (Lower panel) Under pathological conditions, determinants may be masked by adherent white blood cells (WBC) and/or shed from the cells (A) (both mechanisms would inhibit targeting), whereas inducible adhesion molecules may exteriorize from intracellular stores (B), such as P-selectin from Weibel-Palade bodies (WPB), or be synthesized de novo, such as E-selectin, ICAM-1, and VCAM-1 (C). Pathological mediators also induce rearrangement of natural clusters (D); shedding of glycocalyx, thereby exposing normally masked determinants (E); and cause endothelial contraction, thereby increasing pericellular permeability and accessibility of target determinants in the junctions (F), such as VE-cadherin and PECAM.
Target Function and Safety
Functions of target determinants are important in the context of drug delivery. Molecules involved in transport from the bloodstream seem attractive to deliver drugs in the corresponding addresses. On the other hand, interference with functions of target molecules must be considered in the context of pathological condition(s) to be treated. For example, ACE and APP are peptidases that cleave important peptide mediators (e.g., bradykinin);199 their unintended inhibition may cause harmful side effects, including vascular edema. Conversely, inhibition of angiotensin II production by ACE may be beneficial in management of hypertension and inflammation.
The issue of adverse inhibition of the target is illustrated by thrombomodulin, an endothelial surface glycoprotein that has been studied for targeting of diverse cargoes to the pulmonary endothelium in mice and rats.67,200,201 However, thrombomodulin controls thrombin,202 and its inhibition poses danger of thrombosis.201,203,204 This complication prohibits clinical use of this target, restricting its utility to model animal studies.180,201,205,206
Ligand-coated carriers may inflict more potent effects than ligands alone. Cross-linking of target molecules by a multivalent carrier may induce ill-understood or adverse signaling in the endothelial cells, shedding and/or internalization of target determinants, changes in their functionality, or other disturbances of the endothelium. With the exception of targeting anticancer agents to tumor endothelium, endothelial drug delivery should not damage or disturb target cells.
A more general aspect of this issue that should be considered is that biocompatibility of a single component does not guarantee safety of the nanocarrier.107 Loading a benign agent into a carrier composed of biocompatible materials decorated by innocuous ligands may yield a combination with pro-inflammatory or adjuvant features. Systemic effects, such as activation of complement, coagulation or platelets, and toxicity toward the clearing tissues (liver, kidney, lungs, etc.,), must be meticulously appraised in vivo.
Endothelial Cell Adhesion Molecules
Endothelial cell adhesion molecules (CAMs) represent arguably one of the most well-studied and versatile groups of endothelial determinants in the context of targeting nanocarriers.38,90,162,207 Inhibition of CAM-mediated leukocyte adhesion and signaling is generally viewed as safe in the context of many pathological conditions, except certain infections. Further, targeting to these molecules achieves localization of drugs to diverse endothelial compartments.
Inducible Endothelial Cell Adhesion Molecules
Vascular Cell Adhesion Molecule-1 (VCAM-1), P-selectin, and E-selectin are exposed on the surface of activated endothelial cells and facilitate the rolling phase of the vascular adhesion of leukocytes.208 Pathological factors including cytokines, oxidants, and abnormal flow cause surface exposure of P-selectin from intracellular storage within 10–30 min209 and induce de novo synthesis and surface expression of E-selectin210 and VCAM-1.211 E-selectin and VCAM-1 seem to be expressed by the activated endothelium in arteries and skin microvasculature to a higher extent than in the pulmonary vasculature.212
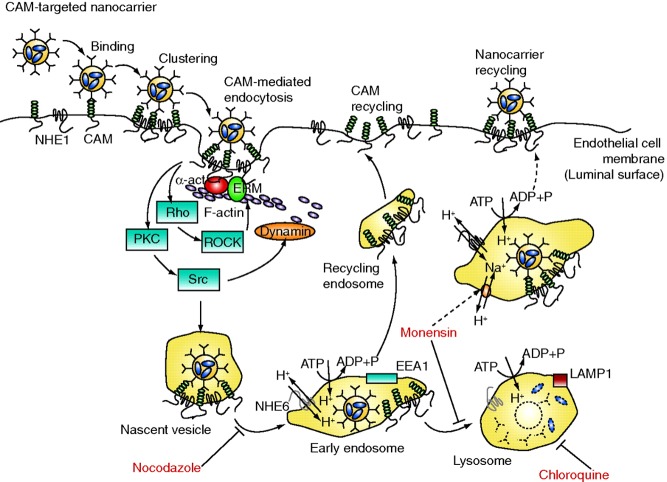
Carriers conjugated with antibodies to these molecules bind to activated endothelium.66,71,212−215 Endothelial cells internalize selectins via clathrin-coated pits,216−218 which favors intracellular delivery into endothelial cells of E-selectin targeted liposomes,219 drugs,219,220 and genetic materials.221 Inducible adhesion molecules represent excellent determinants for visualization of activated endothelium in inflammation foci by delivery of conjugated isotopes181 or ultrasound contrast agents.222,223
Constitutive Cell Adhesion Molecules PECAM and ICAM-1
PECAM and ICAM (CAMs) are glycoproteins composed of a large extracellular region containing several Ig-like domains, a transmembrane segment, and a cytoplasmic tail mediating signaling.224,225 CAMs are present in cell types including platelets (PECAM), epithelial and smooth muscle cells (ICAM), and leukocytes (both), but among the cell types accessible to blood, surface levels of PECAM and ICAM are the highest in endothelial cells.
PECAM and ICAM are expressed on the endothelium throughout the vasculature. PECAM is stably expressed at a level of (0.2–2) × 106 copies per cell,211 predominantly in the interendothelial borders.226,227 ICAM tends to localize in lipid rafts in the luminal membrane and may exist in either a monomer or oligomer form.193 Quiescent confluent endothelial cells in static culture express ICAM at very low levels, and treatment with cytokines or thrombin leads to 50- to 100-fold up-regulation.228 In contrast to such a dramatic difference in vitro, ICAM is expressed in blood vessels constitutively by quiescent endothelium in vivo at levels of ∼(0.2–1) × 105 binding sites for anti-ICAM per cell, while cytokines and other pathological mediators induce its additional synthesis and upregulate ICAM-1 surface expression roughly twice to the level of (0.5–3) × 105 sites per cell in endothelium48 and other cell types.229
CAMs are involved in adhesion and trafficking of leukocytes and in vascular signaling.230 Their clustering by multivalent ligands initiates signal transduction via their cytosolic domains.231 Adhesion via these molecules supports leukocyte mobilization in sites of inflammation.230,232Via its extracellular domain, endothelial PECAM engages in heterophilic binding to heparin-containing proteoglycans and integrins of leukocytes, and in homophilic PECAM–PECAM interactions.231,233,234 The extracellular region of ICAM binds ligands including fibrin, certain pathogens, and integrins of activated leukocytes, mediating their firm adhesion to endothelial cells.235,236 Therefore, PECAM and ICAM are involved in cellular recognition, adhesion, and migration of leukocytes.237 Inhibition of these functions may be beneficial in treatment of inflammation.238
Affinity Ligands for CAM Targeting
PECAM and ICAM antibodies (anti-ICAM and anti-PECAM) are popular ligands for experimental vascular targeting. These antibodies and antibody-carrying carriers and drug conjugates bind to endothelial cells and accumulate in vascularized organs after intravascular injection.78,95,160,201,205,239−241 Intravenous administration favors their uptake in the pulmonary vasculature, while local arterial infusions enrich accumulation in the downstream cardiac,242 cerebral,96 and mesentery243,244 vasculature.
Many groups have devised nanocarriers targeted to CAMs by conjugated antibodies.245 “Designer” affinity ligands for CAMs have also been devised,40 including scFv fragments95,206,246 and affinity peptides selected using a phage display library.247 Advantages of using scFv include lack of Fc-fragment mediated effects and feasibility of scaled-up GMP production of recombinant constructs. A humanized monoclonal antibody binding to human ICAM with 50-times higher affinity than paternal mouse anti-ICAM has been produced248 as well as multivalent Fab fragments of a monoclonal antibody to human ICAM.249
Some of these recombinant proteins have been clinically tested as anti-inflammatory agents and showed generally acceptable safety.250,251 More recently, a short 17-mer linear peptide derived from one of ICAM’s natural ligands, fibrin, provided endothelial targeting of nanoparticles on par with ICAM antibodies in animal studies.252
This type of ligand offers the advantages of a reduced risk of immune reactions and utility in diverse animal species.
Carrier Binding to Endothelium
Targeting is controlled by carrier avidity, defined by ligand affinity, density, and freedom to interact with target.253 The carrier geometry and plasticity modulate the latter parameter and carrier ability to engage in multivalent binding.160,179 The binding is also controlled by target features (surface density, accessibility, and organization of epitopes) and hydrodynamics, which all may change under pathological conditions.
Hydrodynamics
Reviewing the extensive literature on rheological control of interactions of ligand-coated particles with the vessel wall, pertinent to both adhesion of leukocytes and carrier targeting, is beyond the scope of this paper. The majority of these studies involved particles of micrometer size and surfaces coated with immobilized molecules or endothelial cells in vitro.94,254−259 These models allow quantitative analysis under controlled conditions, but revealed trends need in vivo validation in the types of vessels260 and vascular areas261,262 of interest.
For example, several studies have indicated that binding is inversely correlated with shear stress.255,263 Computational analysis showed that the shear stress influence diminishes for nanocarriers coated with antibodies at a surface density above a threshold level.253 Modeling studies considering the contribution of buoyancy, hemodynamic forces, van der Waals, electrostatic, and steric interactions between circulating particles and the endothelium identified a critical diameter of 200 nm for which the margination time is maximal. It was proposed that carriers either larger or smaller than this size may have an advantage anchoring on the endothelium.264 Studies in a parallel plate flow chamber indicated that particles with a diameter greater than 200 nm undergo the most effective margination due to sedimentation in horizontal capillaries and lateral drift in vertical capillaries with downward flow, enhancing the likelihood of adhesion to the endothelium.265
Similar experiments in a flow chamber model evaluated binding of spherical particles ranging from 100 nm to 10 μm coated with endothelial ligand. Binding increased with an increase in diameter from 0.5 to 10 μm at a shear rate of 200 s–1 typical of arteries, whereas a further increase in shear favored binding of 2–5 μm particles.254 One explanation is that the dragging force of the highest shear stress applied to larger particles prevails over the adhesive force. Carriers experience the hydrodynamic dragging force of blood proportional to their size; hence, the larger the DDS is the more target molecules need to be engaged to anchor DDS on the cell.253 Spherical carriers (1 μm) coated with ICAM antibody displayed a typical pattern of binding to endothelium in vitro dependent on the antibody surface density: move in the flow at high speed without visible interactions with cells, roll continuously over cells, roll first and then bind firmly to cells, or roll first and then detach thereafter traveling along the cell surface.94
In some studies carriers were perfused in the presence of blood components.253 For example, RBCs are known to occupy the mainstream in high-shear vessels, expelling smaller particles to the marginal level, thereby directing their flow in the glycocalyx-protected layer and interaction with endothelial surface.266 Indeed, it was shown that addition of RBCs to the perfusion buffer stimulates binding of ligand-coated particles to the walls of target-coated model vessel.267 Recently, studies focused on the effect of circulating RBCs on carrier margination have shed light on an area known as the cell-free layer, which separates bulk RBC flow and the endothelial wall.268,269 Simulated binding, simplified as contact between carrier and endothelium, was performed in both large and small vessels in the presence and absence of RBCs. Due to the interaction of particle and cell, smaller particles are physically pushed by RBCs via volume exclusion. Likewise, an increase in particle dispersion coefficient is attributed to the increased rotation and tumbling of RBCs under increasing shear. These two contributions, increased particle dispersion and volume exclusion, act together to increase the carrier gradient along the cell-free-layer at the vessel walls. In smaller vessels, similar simulations show that carrier accumulation is enhanced further, likely due to the larger role volume exclusion plays since RBCs may be forced to physically contact the vessel wall.269
Carrier Geometry and Plasticity
As mentioned earlier, the carrier’s physical characteristics (e.g., shape) play a critical role not only in its circulation profile, but also in its binding to target cells. Studies with targeted DDS in vivo have shown that while endothelial localization may increase with size into the micrometer range, targeting specificity tends to have a maximum in the hundreds of nanometers range. A study comparing vascular targeting (through measurements of pulmonary uptake) of anti-ICAM coated polystyrene spheres with sizes of 0.1, 1, 5, or 10 μm showed that, while the overall %ID found in the lung increased with size, the targeting specificity decreased about 5 times (the ISI was ∼10 vs ∼2 for 100 nm and 1000 nm particles, respectively), due to more pronounced mechanical retention of large untargeted carriers coated with control IgG.143 Similarly, PECAM-targeted protein carriers showed increased pulmonary uptake with an increase in size from 200 to 800 nm, but nonspecific uptake of control nontargeted counterparts increased when the size was >300 nm.136
Carrier enlargement aggravates accessibility issues. For example, determinants located in caveoli, invaginations with neck diameter <50 nm, are accessible to relatively small ligands such as antibodies but not to submicrometer carriers.197,270 In a similar example, 100 nm spheres targeted by antibodies to well-mapped epitopes of endothelial PECAM failed to bind to the cells when the antibody to the most distal of the plasmalemma epitopes was used, while the antibody itself showed excellent binding.197
At a nanometer scale, it has been shown that elongated carriers bind to endothelium more effectively than spherical ones (Figure 7A). Specifically, polystyrene nanorods (aspect ratio ∼3) functionalized with anti-ICAM showed upward of 3-fold attachment to brain endothelial cells in static cell culture.271 Higher association of rod-shaped particles with endothelial cells was also exhibited in vivo as anti-ICAM coated rods exhibited ∼2-fold higher accumulation in target tissue (lungs) than that of spheres of identical volume. This was further investigated by coating nanorods and spheres with anti-transferrin-receptor monoclonal antibody. Rods exhibited almost an order of magnitude fold enhancement in attachment to brain endothelium.271
Figure 7.
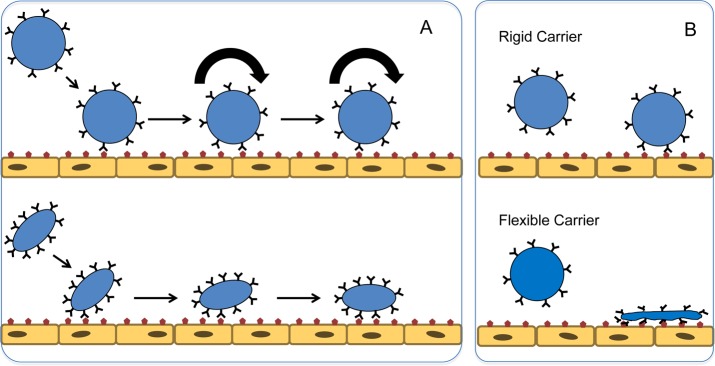
Carrier shape and plasticity modulate ligand-mediated anchoring on endothelium. (A) Spherical carriers bind to the endothelium in a fashion reminiscent of the phases of leukocyte adhesion (rolling, initial tethering, and firm adhesion), whereas elongated and discoid carriers “zip up” on the target molecules. (B) Endothelial binding of flexible carriers is advantageous over that of rigid carriers, because shape change and lateral diffusion of ligands afford better congruency between molecules of a ligand/receptor pair and a higher number of productive anchoring engagements.
In a computational model comparing spheres and nanorods, the binding probability of a nanorod under a shear rate of 8 s–1 was found to be 3 times higher than that of its spherical counterpart. Anti-ICAM coated polystyrene disks showed enhanced targeting specificity over spheres (immunospecificity index, ISI, of 35.7).143 Previously discussed long-circulating filomicelles display a unique interaction with their target surfaces: biotinylated worm micelles were shown to “zip up” on avidin-coated surfaces due to their multivalent high affinity interactions.152 In comparing the in vivo targeting of ICAM-coated filomicelles and spheres, both resulted in similar specificity though the overall targeting was lower for filomicelles.90
Flow-induced interactions play an important role in binding of nanoparticles to endothelium. Several devices have been synthesized and mathematical models have been developed to investigate and explain these phenomena.264,271−276 Mathematical modeling has shown that rod-shaped particles are more likely to adhere to endothelium than their spherical counterparts under the same flow conditions due to the tumbling and rolling motions that rods will undergo near endothelial walls.277 Numerous studies supported by mathematical models by Decuzzi and Ferrari detail various advantages of nonspherical particles in cellular adhesion under flow: (i) oblate particles adhere more strongly to surfaces under flow273 and (ii) particles with radii under 100 nm, as is typically the case with elongated particles, are ideal for margination and interaction with the endothelium.264
These interesting mathematical predictions are supported by in vitro flow studies designed to mimic in vivo flow. Notably, discoid particles have been shown to marginate to the wall under flow better than their spherical counterparts.278 BSA coated rod- and disc-shaped particles have been shown to adhere up to 5 times more than BSA coated spheres to anti-BSA microchannels (bifurcation geometry),275 and anti-ICAM coated rods were shown to adhere twice as well to ICAM-expressing endothelial cells over identically coated spheres under flow.271
Carrier plasticity is likely to contribute significantly to targeting despite the lack of studies directly confirming this. While circulating in the fluid stream, rigid and flexible carriers may retain similar morphologies. However, the latter carriers may flatten on the surface of the target (Figure 7B), thereby reducing the drag force of perfusion that leads to detachment. The same phenomenon will enhance binding via spreading over and engaging a higher number of binding sites. Additionally, lateral movement of ligand molecules in the flexible carriers favors congruency of ligand molecules interaction with multiple binding sites and their clusters.279 These advantages of more flexible carriers are beginning to be reported in literature where ligand presenting carriers of the same shape and identical coating are superior in terms of binding and binding strength over their rigid counterparts.280 Perhaps these findings will be best exploited by high-avidity multivalent binding to target sites. In addition, as noted in section Nanocarrier Behavior in the Circulation, flexible particles circulate for a longer time and have a better chance of achieving target than rigid particles. Further, rigid particles have shown to be greater than 5-fold more likely to be internalized by immune cells when compared directly to their flexible counterparts in vitro.281 However, rigid particles also seem to preferentially accumulate in lung tissue much more than flexible counterparts, likely due to passive mechanical retention in lung capillaries.
The Optimal Carrier Avidity and Ligand Density
The mode of ligand conjugation modulates carrier’s avidity. For example, antimyosin Fab conjugates produced with three different cross-linkers (SMCC, SPDP, and BrAc-NHS) showed different targeting, likely due to different stability of the bond and reduction of Fab affinity, among other potential factors.282
The transformational shift of DDS binding, which usually boosts targeting, is from a monovalent or bivalent ligand to a multivalent carrier. For example, an effective avidity of binding to endothelium of anti-ICAM coated carriers is markedly higher than that of free anti-ICAM.243,283 Multivalent binding helps retain large particles that experience more powerful detaching forces than a ligand molecule. For example, computational analysis revealed that at least three ICAM antibodies coupled to a spherical carrier with diameter 100 nm should engage simultaneously in binding to an endothelial cell in order to anchor the carrier.94,253,284 The key issue, therefore, is to find an optimal ligand configuration on the carrier that provides multivalent interactions with target molecules.
Generally, higher affinity/avidity is viewed as an advantage for targeting.285 For example, an increase in ligand density providing higher avidity results in an increase of vascular targeting.253,284 Conjugation of a number of ligand molecules per carrier less than a certain minimum leads to a failure of the carrier to bind to the target.284 Yet, an excessive ligand density beyond one that saturates binding sites may be undesirable due to costs and benefit/risk ratio.286 Additionally, studies of nanocarrier-based vaccines imply that carriers with high ligand density are likely to cause immune reactions.287−289
Furthermore, in some cases, ligands with higher affinity yield inferior targeting. One scenario where this is the case is when high-affinity nanoparticles fail to penetrate into the target due to their retention at the surface of the target mass, such as tumors290 or thrombi.291 Although such a scenario is less likely in endothelial targeting, there are additional complicating considerations. Unlike free ligands, ligand–drug or drug–carrier conjugates require congruency with target molecules for multivalent binding, which does not necessarily correspond to the maximal ligand density. In some cases, as illustrated in Figure 8, an excessive ligand density may inhibit the binding of NCs to target cells. In this phenomenon, “ligand overcrowding” on the surface of a NC could inhibit individual ligand molecules from achieving the optimal orientation or congruency with clustered target molecules favorable for binding.292,293 This phenomenon was also observed using nanocarriers targeted with aptamers intended for tumor delivery. Beyond a certain threshold, further elevation of ligand density resulted in a significant decrease in tumor localization.292,294
Figure 8.
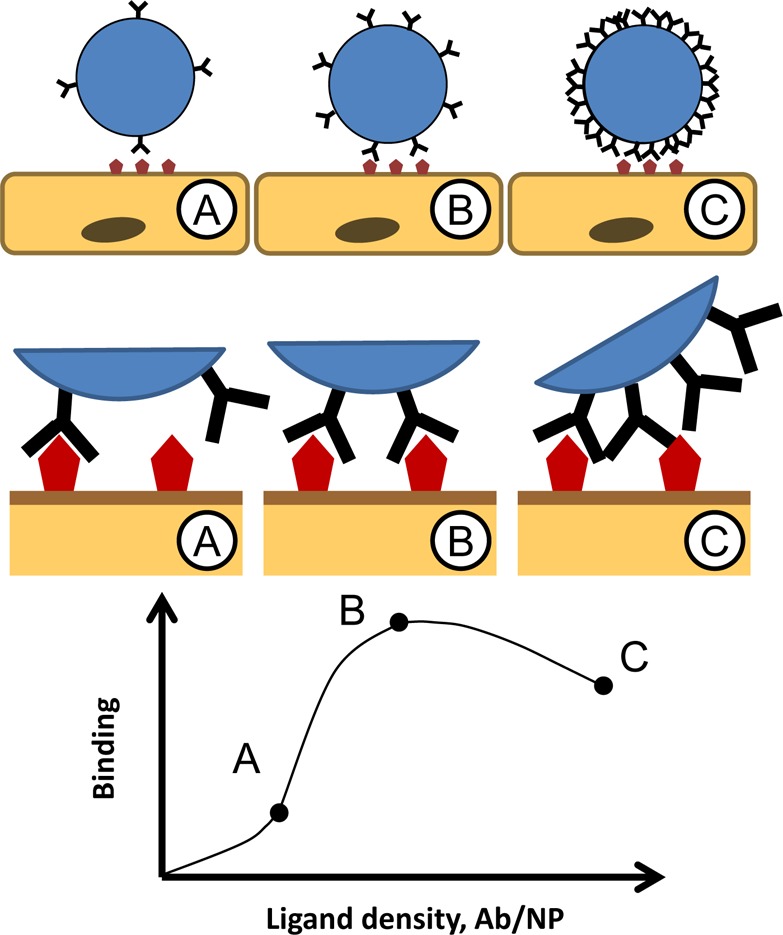
Maximal ligand surface density does not necessarily provide optimal carrier targeting. (A) Suboptimally low ligand density negatively impacts a carrier’s ability to engage in multivalent binding, hence suboptimal targeting as depicted in the model graph. (B) Surface density of ligand copies at which they engage multiple binding sites and achieve firm binding is sufficient and optimal for targeting. (C) Excessively high surface density of ligand molecules may lead to “a ligand overcrowding” phenomenon, i.e., inhibition of multivalent engagement due to steric limitations and competition of adjacent ligand molecules for the binding sites.
Defining the optimal surface density of a ligand, which may vary depending on the features of a carrier, ligand, target, and application, is a daunting and important task. Several factors could contribute to the outcome including carrier and ligand properties (size, orientation, density, etc.) and characteristics of the target molecule’s environment (membrane localization, glycocalyx, clustering, etc.). Quantitative parameters of ligand affinity and surface density on a carrier have to be determined empirically because surface density and clustering of target determinants in vivo remain to be characterized.
Varying ligand surface density also may help enhance targeting selectivity. Often, so-called “specific” markers expressed on pathologically altered target cells are also expressed at lower levels in other areas of the body. Increasing avidity of a carrier beyond a certain threshold may result in an increase in “off-target” binding to normal cells expressing target determinants at a low basal level. Specifically, in the application of imaging and detection of disease, off-target uptake should be minimized to enhance the signal-to-noise ratio of the target tissue.
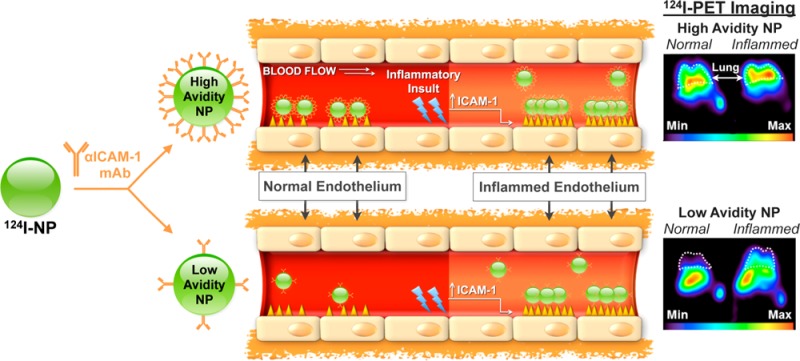
This point was validated in a study on molecular imaging of pulmonary inflammation by isotope-labeled anti-ICAM nanocarriers. Admittedly, ICAM-1 is not an ideal target for this goal, since it is expressed at a relatively high basal level among the normal endothelium, while its level roughly doubles in inflamed counterpart. In vitro studies in static and flow-exposed cell cultures, as well as computational simulation, showed that high-avidity nanoparticles anchor effectively to both naïve and activated endothelium, whereas low-avidity (i.e., low anti-ICAM density) carriers effectively engage in multivalent anchoring preferentially to inflamed endothelium.94,253Figure 9 illustrates the study that affirmed the in vivo effects of tissue selectivity. Specifically, lowering the carrier avidity using controlled reduction of anti-ICAM surface density resulted in a marked 2-fold increase in selectivity of uptake of anti-ICAM/carriers in the lungs of animals with endotoxin-induced acute pulmonary inflammation vs lungs of healthy control mice and improved detection of pathology using PET imaging.156
Figure 9.
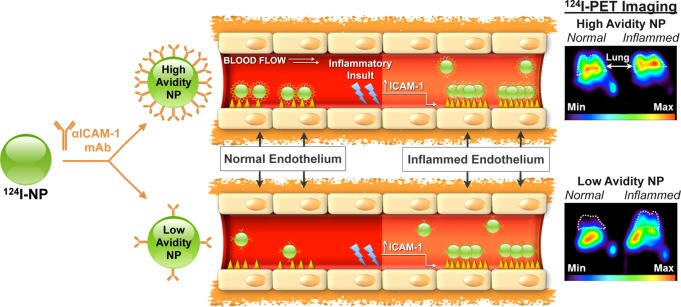
Controlled reduction of avidity (ligand surface density) enhances selectivity of carrier targeting to pathological endothelium. ICAM-1 is exposed on quiescent and pathological endothelium at modest basal and elevated levels, respectively. Carrier avidity to ICAM-1 is controlled by antibody surface density. High-avidity particles (approximately 150 nm diameter) carrying 200 molecules of anti-ICAM effectively bind to both quiescent and inflamed endothelial cells and show high pulmonary uptake after intravascular injection in both naïve and endotoxin-challenged mice (upper cartoons). In contrast, low avidity particles carrying 50 anti-ICAM molecules show negligible binding to quiescent cells, whereas elevation of surface density of ICAM-1 typical of pathological endothelium allows their multivalent anchoring and binding to cytokine activated endothelium (lower cartoons). This phenomenon helps to explain the several-fold higher selectivity of targeting to pathological vs normal endothelium demonstrated by low-avidity vs high-avidity carriers in mouse model of lung inflammation (middle panels). As result, low-avidity anti-ICAM coated carriers provided PET images of the pulmonary inflammation in endotoxin-challenged mice that were more discernible from naïve animals than images provided by high-avidity carriers (far-right panels). PET imaging at 1 h after IV injection of ICAM-targeted [124I] carriers carrying either 200 or 50 antibody molecules per particle (upper vs lower images, respectively) in naïve vs endotoxin-treated mice (left vs right). White dashed-line corresponds to lung space. (Adapted with permission from ref (156). Copyright 2013 American Chemical Society.)
Targeting Vascular Damage
Damaged vascular wall exposes specific markers including normally hidden intracellular endothelial molecules (e.g., von Willebrand Factor) and components of subendothelial layers including collagens, tissue factor, and proteoglycans. These compounds activate coagulation and platelets to form a hemostatic plug preventing bleeding. To compensate for deficiency of natural hemostatic mechanisms in patients with bleeding disorders, development of synthetic hemostats has been investigated.295−297
These synthetic hemostats must be specific and not induce clotting at off-target endothelial sites.298 Hemostatic-promoting carriers must (i) circulate in blood without interacting with off target (i.e., nondamaged) endothelium, (ii) marginate to the endothelial wall at the wound site, and (iii) specifically interact with the damaged endothelium and circulating platelets to form a suitable hemostatic plug. Carrier design parameters become increasingly important if carriers are to perform such specific interactions in terms of circulation and endothelial interaction considering the exposure of multiple binding sites at injured vasculature and the dynamic nature of blood flow. To address the former, a number of spherical carriers have been proposed presenting either fibrinogen295 or fibrinogen-derived peptides,296,299 which bind to markers exclusively present on activated platelets at the wound site. These single-ligand carriers have been successful in providing an ∼2-fold increase in survival compared to saline control mice that were subjected to liver blunt trauma wounds.300 Recently, the complexity of multiple ligand presentation following physical vascular damage has been investigated by combining collagen and von Willebrand Factor binding ligands, in addition to fibrinogen-derived peptides, onto a single carrier.297
In case of the latter, the size, shape, and flexibility must be finely tuned in order to perform on-demand hemostasis at a vascular injury site which is undergoing dramatic changes in blood flow and loss. Extensive efforts have focused on mimicking the size, shape, and flexibility of RBCs to better navigate carriers in the vasculature. Likewise, platelet mimetics are receiving much attention. Recently, Doshi et al. have shown the utility of cell-like flexible particles in targeting surfaces coated with markers for damaged endothelium.280 Synthetic platelets, which mimic the size, shape, and flexibility of real platelets, were shown to adhere over 2-fold more strongly than spherical counterparts. This enhanced effect was attributed to a combination of features as similar spherical particles were unable to perform as well. Indeed, it has yet to be shown whether or not these synthetic cells will be able to marginate in blood flow or perform hemostasis as well as real platelets. Yet engineering of the shape and flexibility of carriers that has been demonstrated by synthetic RBCs,147 synthetic platelets,280 and filomicelles90,151 is required to perform complicated functions that spherical particles may not be able to address.
Multiligand Targeting
Selective targeting to pathological endothelium is an important task. One intriguing idea explored by several laboratories is the strategy of combining on the surface of the carrier affinity ligands that bind to different determinants. This has the potential to boost the selectivity and efficacy of drug delivery. Figure 10 illustrates this principle: one ligand binding to an inducible determinant provides the selectivity of recognition of altered cells, while the second ligand binding to a pan-endothelial determinant supports the anchoring. The idea, in essence, comes from the biology of leukocyte vascular homing and infiltration of the endothelium at sites of inflammation. Leukocytes bind to two different types of adhesion molecules, low-affinity selectins (E/P-selectin) and high affinity Ig-type cell adhesion molecules (PECAM and ICAM), in order to attach, roll, and come to firm adhesion on the endothelium.
Figure 10.
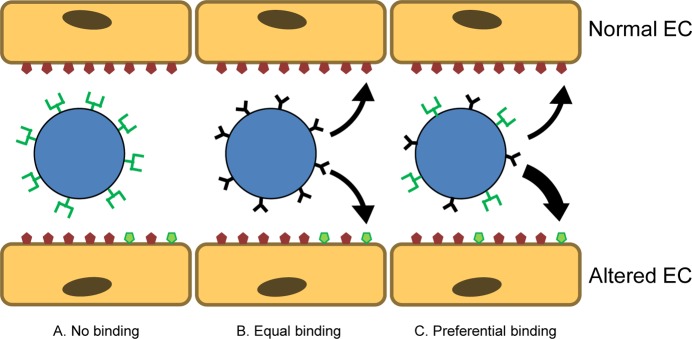
Carrier targeting using multiple ligands. (A) Carriers coated with ligands binding to endothelial determinants that are expressed in the area of interest selectively but scarcely (green) may have insufficient avidity for anchoring in this area. (B) Carriers coated with ligands binding to abundant endothelial determinants (red) anchor indiscriminately throughout the vasculature. (C) Carriers coated with a combination of two ligands may exert elevated basal avidity to endothelium, insufficient to provide a firm adhesion on its own but enabling binding in the case of simultaneous engagement of the scarce site-specific determinant.
In this vein, carriers coated by combinations of antibodies with relatively nonspecific high-density determinants (ICAM, PECAM) and antibodies to inducible adhesion molecules (selectin, VCAM-1, ELAM) have been tested in vitro in models that employ co-immobilized antigens257 or cytokine-activated cells.279 In studies with particles targeted to inflamed vasculature using ICAM-1 and P-selectin, greater binding was achieved with dually targeted particles relative to particles targeted to P-selectin or ICAM-1 alone.257,301,302 Targeting liposomes to E-selectin and either VCAM-1 or ICAM-1 on cultured endothelial cells has also been reported.279 Maximal binding was observed with equimolar ratios of both ligands.279,303,304 The biological significance of these findings to real situations in vivo remains to be defined. In addition to typical limitations associated with cell cultures and immobilized antigens, expression of binding data in relative scale further convolutes interpreting results of these studies.
However, a dual targeting strategy employing spherical 100–200 nm carriers carrying antibodies to both ICAM and transferrin receptors has recently been tested in vivo and showed promising results: each of the ligands apparently promoted targeting to the vascular area of its destination, i.e., nanocarriers could be directed to the inflamed pulmonary vasculature via ICAM and to cerebral vasculature via transferrin receptor.305
The dual targeting paradigm has also been used for molecular imaging: MRI-based NCs were targeted to P-selectin and VCAM to image atherosclerosis in mice. Dual targeted NCs demonstrated approximately a 6-fold higher binding relative to single targeted NCs.306 Another group has used dually targeted particles for imaging of inflammation via microbubble contrast agents for ultrasound. Here microbubbles were targeted to ICAM-1 and selectins and demonstrated that dually targeted bubbles had significantly better adhesion strength to activated endothelial cells relative to single target bubbles.307
Competitive and Collaborative Targeting
Monomolecular ligands interact with target determinants either in bivalent (e.g., antibodies themselves) or monovalent fashion (e.g., Fab-fragment conjugates and scFv-fragment fusion proteins). Bivalent binding of an antibody to glycoprotein(s) on the cell surface offers higher affinity yet requires more freedom and congruency of carrier-target interaction. Ligands binding to distinct epitopes on the same target molecule may influence each other, for example, inhibiting binding to adjacent epitopes. The competitive inhibition of binding to overlapping epitopes has been described for antibodies to ACE.308−310
Recently, it has been found, however, that distinct monoclonal antibodies directed to adjacent epitopes in the distal domain of the extracellular moiety of PECAM, stimulate binding of each other, both in cell culture and in vivo.311 The endothelial binding of PECAM-directed mAbs is increased by co-administration of a paired mAb directed to adjacent, yet distinct, PECAM epitopes. The “collaborative enhancement” of mAb binding was affirmed in mice, manifested by enhanced pulmonary accumulation of intravenously administered radiolabeled PECAM mAb when co-injected with an unlabeled paired mAb.
This highly unusual empirical finding can be interpreted as an increase in accessibility of an epitope to its ligand due to conformational changes in the target determinant molecule induced by binding of a paired “stimulatory” ligand to its adjacent epitope. In theory, it may find utility in targeting strategies. The premise of this strategy, “collaborative enhancement”, is that the initial binding event can lead to an increased binding event of the secondary targeting agent. With this strategy, a therapeutic effect was realized with increased activity of a scFv targeted to PECAM on endothelial cells.311 This phenomenon offers a new paradigm for optimizing the endothelial-targeted delivery of diagnostic agents and therapeutics. It will be interesting to see if this strategy of enhanced targeting can be applied to NC platforms for drug delivery and imaging of disease.
Challenges and Perplexing Issues
The assessment of carrier fate within the target tissue of live animals is such a challenging endeavor that PK-related issues pale in comparison. No method affords accurate data of binding per cell at a given location in vivo. The best data sets are provided by combining isotope tracing demonstrating uptake in an organ and microscopy imaging cellular distribution. Such a level of rigor is needed to characterize new, targeted DDS. Otherwise, studies may look enigmatic, such as vascular pulmonary targeting by an antibody to surfactant protein SP-A,312 the alveolar component inaccessible for liposomes in blood (which is difficult to comprehend except as an artifact of passive retention in the pulmonary capillaries).
Even at the current limited level of understanding of target biology and processes to intervene, many targeting paradigms are incredibly simplistic. For example, tens of thousands of publications characterize ACE, PECAM and ICAM-1: structure, functions, tissue distribution, and role in a plethora of human maladies. Yet, data on their distribution in the vascular lumen throughout blood vessels and in the endothelial cell plasmalemma are fragmentary, at best. Immunostaining, in situ hybridization, Western-blotting, and PCR do not distinguish surface and intracellular proteins. Isotope-labeled antibodies detect surface target, but offer no insight into the protein organization in oligomers or/and clusters and their distribution in the plasmalemma. Systematic information on normal and pathological distribution of any of the endothelial target molecules in the in the vasculature is still missing. An immunochemistry-based atlas of the vascular distribution of ACE, the target of a widely used inhibitor therapy, is arguably the best attempt so far, but ACE organization in the endothelial plasmalemma in the vessels is still enigmatic.179,182
In vitro experiments and computational studies complement expensive in vivo studies but must be interpreted carefully. In the context of targeting, issues of cell culture include (i) degeneration of the cell phenotype; (ii) conditions irrelevant to in vivo (high dose, incubation without washing, prolonged exposure); and (iii) lack of a proper cell environment (for example, tissue components, flow, blood). In particular, endothelial cells grown in cell culture are known to lose phenotypic features–caveolae, glycocalyx, ACE and other marker proteins, basal level of ICAM-1, etc.(64,313−315) Studies using immobilized molecules seem even less relevant. For example, studies of dual ligand targeting using densely co-immobilized target molecules hardly represent the in vivo situation accurately, especially in the case that one of the targets is scarce in the vasculature. Most likely, this strategy may only be useful for a few determinants as both must coexist in close proximity to each other in order to permit simultaneous anchoring of both ligands.
Cellular Uptake and Trafficking of Targeted Carriers
Intracellular delivery and addressing to proper cellular compartments are needed for the desirable effects of many drugs, especially biotherapeutics.114,316,317 Endothelial cells, like other cell types in the body, except professional phagocytes, exert fairly selective mechanisms for the uptake and transport of macromolecules and nanoparticles.59,318 Without targeting, such compounds poorly enter cells unless exposed at high concentration for a prolonged time.
Anchoring on selected surface determinants offers mechanisms to boost the uptake and control the rate and pathway of internalization, subsequent trafficking, and fate of nanocarriers. The conventional wisdom is that anchoring of a drug or carrier to a molecule involved in uptake of its natural ligand(s) provides the intracellular delivery. Targeting nanocarriers to endothelial cells provides ample examples illustrating this principle but also examples of less intuitive and somewhat paradoxical pathways for intracellular drug delivery.
Entering through Open Doors: Targeting Carriers to Determinants Involved in Natural Endocytic Pathways
The endothelium exerts active fluid phase uptake via vesicular micro- and macropinocytosis pathways.39 They transport compounds present in the blood at high concentrations, but efficacy and specificity of these pathways seem unlikely to meet the requirements for drug targeting.
Uptake of ligands that bind to internalizing receptors is more effective, specific, and controlled than passive fluid phase uptake. Like other cells, endothelial cells internalize ligands by phagocytosis and receptor-mediated endocytosis via caveoli39,319,320 and clathrin-coated321 and uncoated vesicles,322−324 as well as noncanonical pathways (see below). Endocytic, phagocytic, and pinocytic pathways have selectivity to certain ligands and pharmacological or genetic methods of inhibition (to a degree) and deliver cargoes to distinct destinations.38
Diverse cell types and phenotypes internalize and traffic carriers differently. For example, phagocytes internalize ligands of Fc-receptors, whereas nonphagocytic cells internalize transferrin via its receptor into cellular vesicles.322 Further, the lysosomal traffic of IgG-opsonized particles in phagocytes increases proportionally to their size in the range of 100–10 000 nm in diameter,325 whereas endothelial cells display an opposite trend.326 Phagocytosis is viewed as a relatively minor contributor to endothelial uptake (this assumption may not necessarily reflect the phagocytic capacity of endothelium in vivo). Caveolar- and clathrin-mediated endocytosis are conventionally viewed as the predominant endothelial vesicular pathways.47
Another conventional notion is that anchoring to a receptor leads to carrier uptake via the pathway naturally serving this molecule.39 For example, antibodies against gp90, a 90 kDa glycoprotein located in the caveoli, and compounds conjugated to these antibodies enter vesicular organelles from caveoli (see below).68 In contrast, liposomes targeted to the E-selectin, a transmembrane glycoprotein that is taken up by clathrin-mediated endocytosis,217,218 enter cells via this pathway and traffic to lysosomes.219,327 Similarly, antibodies to vascular cell adhesion molecule-1 (VCAM-1) are internalized by endothelial cells and addressed in the lysosomes via clathrin-mediated endocytosis327,328 and anti-VCAM conjugated compounds generally follow this fate.212,215,262 Antibodies to transferrin receptor (TfR) and compounds conjugated with TfR ligands also enter cells via this pathway, similarly to the endogenous ligand transferrin.329
Ligands binding to distinct epitopes of anchoring molecules may enter cells differently. Selection of ligands facilitating cellular uptake is an important and so far mostly empirical task.330,331 With the use of phage-display library, the series of peptides binding to diverse VCAM-1 epitopes was identified, some of which have shown enhanced uptake.212 Labeled VCAM-1 binding peptides undergoing enhanced endocytosis provided improved imaging of vascular inflammation in animal models.212,215 Phage display and other high-throughput methods facilitate selection of internalizable antibodies and their fragments (keeping in mind that multivalent interaction of the ligand-exposing phages is different from that of ligand).332−334
Ligand modification affects internalization; obviously, loss of affinity cancels specific uptake mechanism. Modifications of antibodies that change their charge or enable hydrophobic interactions with phospholipids in cellular membranes stimulate their internalization.56,335 Yet, such modifications reduce targeting specificity. This concern is relevant to use of relatively promiscuous secondary interactions, such as those mediated by charge or hydrophobic interactions.
It has been recognized for a long time that conversion of monovalent or bivalent ligands into multivalent carriers that can engage numerous copies of the receptors enhances internalization.40 An extensive clustering of receptors eliciting strong endocytic signaling, as well as dissociation of pre-existing clusters and “unnatural” signaling, among other factors, may be involved in the enhanced uptake of multivalent carriers. Generally, this high carrier avidity is viewed as favorable for intracellular delivery. However, excessive avidity may be detrimental for the subsequent dissociation of the carrier from the receptors during intracellular trafficking. This intriguing scenario has been illustrated in a study of transferrin-mediated transcytosis across the blood–brain barrier.336 High avidity particles got stuck in the endothelium, whereas particles coated with a lower affinity antibody were able to dissociate from the receptor on the basal surface of endothelium, thereby enhancing delivery to the brain (Figure 11).
Figure 11.
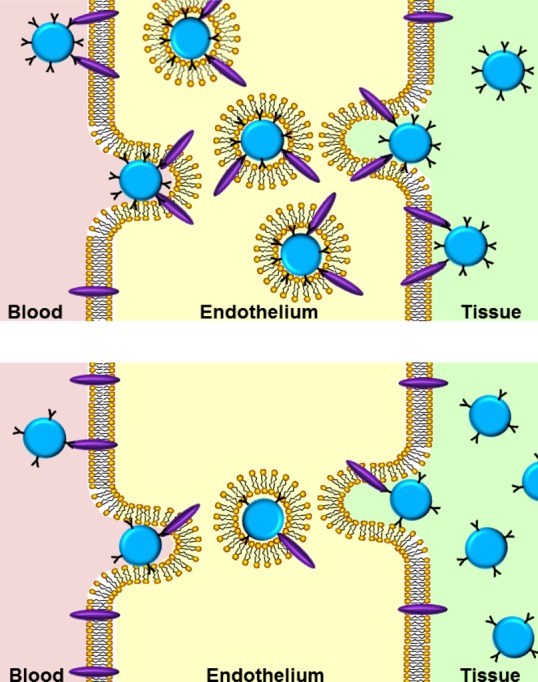
Optimization of transcellular transport via controlled reduction of carrier avidity. (Upper panel) Carriers with high avidity to a receptor involved in endocytosis and transcellular transport more effectively bind to and enter endothelium than carriers with low avidity (lower panel). However, high-avidity carriers less effectively dissociate from the receptor after the internalization, which may impede transfer to the tissue. In this simplified cartoon, the high and low avidity of carriers is depicted as proportional surface density of a ligand, whereas avidity may also be regulated by different affinity of the ligands coated at similar density.
However, coating a nanocarrier with internalizable molecules does not necessarily result in internalization. For example, the uptake of a large, micrometer-size carrier may exceed cellular endocytic capacity; formation of such a large vacuole would require prohibitively extensive mobilization of cell membrane and cytoskeleton. Further, coupling ligands to carriers impedes their interaction with receptors and epitopes localized in invaginations and domains of the plasmalemma inaccessible to the particles of such size. For example, conjugation of ligands of caveolar epitopes to carriers larger than the diameter of the neck of caveoli (70 nm) abolishes endothelial targeting and transfer.54
Walking through the Walls: Intracellular Utpake via “Noninternalizable” Cellular Determinants, PECAM and ICAM
PECAM and ICAM exert low level of uptake of their antibodies.38,337 For example, the rate of internalization of labeled anti-PECAM and anti-ICAM by endothelial cells in culture rather marginally (by 5–10%) exceeds the background uptake level on ice.160,338 However, endothelial cells internalize multivalent anti-ICAM and anti-PECAM conjugates and carriers coated with multiple copies of anti-CAM with a t1/2 of ∼10–20 min and uptake levels achieving 85–90% of the total amount of particles bound to the cells160,201,241,338 (Figure 12).
Figure 12.
CAM-mediated endocytosis. Clustering cellular CAMs by multivalent anti-CAM conjugates activates a specific set of signaling kinases and the sodium/proton exchanger, NHE1, which leads to formation of actin stress fibers needed for the uptake of endocytic vesicles. Internalized carriers traffic to endosomes, where engaged CAM molecules dissociate from the carriers and recycle to the plasma membrane. Several hours after internalization, carriers arrive at lysosomes where proteolysis-sensitive carriers and cargoes are degraded. Nocodazole (which disrupts the cell microtubule network), chloroquine (which inhibits lysosomal acidification), and monensin (which enhances Na+/H+ exchange in endosomes and induces recycling of anti-CAM nanoconjugates to the plasma membrane) modulate intracellular traffic and effects of CAM-targeted DDS. (Reproduced with permission from B. Ding et al., Advanced Drug Delivery Systems That Target the Vascular Endothelium, Mol. Interv.2006, 6, 98–112.)
Multivalent ligands of PECAM and ICAM trigger a unique pathway, named CAM-mediated endocytosis,39,338 distinct from phagocytosis, pinocytosis, and the canonical endocytic pathways.228 Molecular signaling in CAM-mediated endocytosis involves sodium-proton exchanger NHE-1, release of Ca2+, and a series of kinases and messengers mediating reorganization of the cytoskeleton that drives the uptake of CAM-anchored conjugates.228,338
Internalized anti-CAM conjugates initially reside in the nascent vesicles negative for endosomal markers (for <60 min), subsequently traffic to EEA-1 positive sorting endosomes (1 to 2 h post internalization), and reach lysosomes about 3 h after uptake in endothelial cells.228 Therefore, most (not all) anti-CAM conjugates arrive in lysosomes several hours after uptake.39,228,239,338,339 This pace of vesicular traffic is fairly slow compared with the classical endocytic pathways that deliver their ligands to lysosomes 10–60 min after internalization in endothelial cells.228
Co-internalized ICAM dissociates from the anti-ICAM coated carriers in the endosomes and recycles to the cell surface, while the carriers traffic further to lysosomes.239 This mechanism allows multiple cycles of intracellular delivery in vitro and in vivo.239 Using pharmacological agents interfering with vesicular transport and the cytoskeleton permits the deflection of vesicular transport from the lysosomal destination and facilitates recycling of the internalized carriers to the cell surface. A body of evidence accumulated in vitro and in animal models indicates that PECAM and ICAM represent highly unusual endothelial targets providing either surface anchoring or internalization with the choice controlled by the parameters of the DDS design, i.e., valence of binding. This feature permits targeting of drugs that either need to be retained on the cell surface such as monovalent antithrombotic fusion proteins340 or delivered inside the cell.
Intracellular Delivery of Targeted Carriers: Geometry Does Matter
Carrier size, shape, and plasticity modulate cellular uptake and trafficking. Depending on cell type, their functional states, and selection of anchoring molecules, the effects and mechanisms of the modulation vary. Size-mediated restriction of accessibility to the binding site or the endocytic entry (e.g., in caveolae341) and exceeding of the capacity of endocytic machinery represent well-known phenomena. Endothelial cells internalize polymorphous anti-CAM conjugates with size <500 nm,326 but internalize anti-CAM coated spherical particles with diameter of up to a few micrometers,143 revealing the endocytic potency typical of phagocytes.
Our current understanding of the geometrical control of targeted DDS uptake is empirical and fragmentary. For example, in one prototype study, ICAM-targeted disks entered endothelial cells more slowly than spherical carriers of a similar size, whereas the pace of traffic through the vesicular compartments was controlled by size: smaller particles arrived at the lysosomes faster, regardless of their shape.143 In a follow-up study, co-delivery of differently shaped, or sized, carriers was investigated for preferential perinuclear distribution. Larger particles (either spheres or rods) were more likely to localize in this region as compared to smaller particles. In the same study, rods were far less likely to accumulate in perinuclear regions as compared to spheres of the same volume.342
In extreme cases, high aspect ratio carriers (aspect ratio close to 10, i.e., needle-shaped particles) can breach the membrane of cells delivering cargo into the cytoplasm.343 Nontargeted filomicelles showed 100% internalization in human derived lung endothelial cells.151 While internalization studies with endothelial cells have been limited, internalization of different shaped carriers by cancer cells has been widely explored and in some cases contrast what is reported for endothelial cells in terms of internalization. For instance, in the HeLa cancer cell line, higher aspect ratio particles exhibited higher internalization rates than those exhibited by spherical carriers.344 In this study, clathrin-mediated, caveolae-mediated, and to a lesser extent macropinocytosis were involved in both the nanocarrier and microcarrier uptake; however, it is believed that they play a more important role for the internalization of nanocarriers.
Biological Modulation of Carrier Intracellular Delivery
Binding to distinct epitopes on the same anchoring molecule may lead to different outcomes. For example, spherical nanocarriers (diameter ∼150 nm) directed to adjacent PECAM epitopes are internalized and trafficked by endothelial cells differently. Testing of a series of monoclonal antibodies directed to distinct epitopes of PECAM revealed marked differences in the rate of internalization of nanoparticles coated by different clones of anti-PECAM: one anti-PECAM coated nanocarrier failed to enter the endothelium, whereas three other types of anti-PECAM nanocarriers exhibited markedly different kinetics of trafficking from early endosomes to lysosomes despite the fact that they all entered cells with a similar rate (T1/2 was close to 20–30 min and max uptake reached 80–90%).197
The functional status of target cells and their microenvironment modulate endocytosis. Thus, activated endothelium internalizes ICAM-targeted nanocarriers faster than the quiescent endothelium does both in vitro and in vivo.345 The effects of hydrodynamic factors are more enigmatic and less well-studied. The majority of publications on cellular uptake and trafficking of nanocarriers have employed static cell lines. A few studies have attempted to define the role of flow in endothelial uptake of nanoparticles using flow chambers and microfluidics; most dealt with nontargeted particles.346,347 However, recent studies revealed interesting effects of chronic and acute flow on endothelial uptake of CAM-targeted particles (Figure 13). Experiments in flow chambers revealed that prolonged exposure to flow leads to partial, yet significant, inhibition of endocytosis of nanocarriers targeted to ICAM and PECAM.159,160 These results correlated with in vivo data showing less effective internalization of anti-ICAM nanocarriers in arterioles relative to capillaries, i.e., vascular areas in which endothelial cells do and do not adapt to flow, respectively.345 This effect is attributed to organization of the actin cytoskeleton into stress fibers in the course of endothelial adaptation to flow, which impedes actin involvement in the endocytosis.345,348 In contrast, exposure to acute shear stress (which happens in reperfusion and in physiological hyperperfusion in exertion) accelerated endocytosis of PECAM-targeted nanocarriers.348 Results of pharmacological studies suggest that this effect is due to mechanical stimulation of the signaling mechanisms including caveoli (Figure 13). The rheological regulation of intracellular delivery represents an intriguing area of bioengineering and biomedicine.
Figure 13.
Flow-mediated modulation of CAM endocytosis. Endothelium in vivo is constantly or intermittently exposed to blood flow and chronic and acute exposure to flow differentially regulates endocytosis of CAM-targeted carriers. (A) Endothelial adaptation to chronic flow, manifested by cellular alignment with flow direction and formation of actin stress fibers, inhibits anti-CAM/carrier endocytosis, likely via impaired recruitment of actin in the stress fibers needed for endocytosis. (B) In contrast, acute exposure of endothelium to flow (imitating reperfusion of exertion perfusion) stimulates endocytosis of carriers, likely through mechanisms involving signaling via cholesterol-rich domains of plasmalemma such as caveoli (cav).
Very recently, a novel mode of biological modulation of internalization of targeted nanocarriers has been described. It is mediated by activation of the enzyme(s) metabolizing sphingomyelin in the plasmalemma and induced by binding of nanocarriers to ICAM-1 in endothelial and other cell types. Studies in cell culture and in genetically modified mice revealed that clustering of ICAM-1 leads to activation of sphingomyelinase and subsequent cleavage of its substrate, stimulating endocytosis of anti-ICAM coated carriers ranging in size from <100 to >1000 nm in diameter.349 This mechanism does not operate in sphingomyelinase-deficient cells and animals; however, carriers coated with both anti-ICAM and the enzyme, devised to deliver enzyme replacement therapy in Neumann-Pick syndrome, do compensate for the genetic defect and get internalized by cells.244 Furthermore, co-immobilization of sphingomyelinase with ligands anchoring carriers to transferrin receptor permitted internalization of carriers larger than those normally permitted by clathrin vesicles (<200 nm).350 This very unusual, serendipitously discovered mechanism further extends the versatility of targeted nanocarriers for intracellular delivery.
Transendothelial Delivery
Adhesion of carriers to endothelium may favor their transport across the cells via diverse mechanisms. For example, elevation of the local concentration of a carrier in close proximity to the vessel wall may facilitate its passive entrance into transcellular and pericellular pathways, on the condition that binding is reversible and the gradient direction supports extravasation.
However, anchoring to some endothelial molecules provides more specific and effective mechanisms. Some ligands of receptors involved in endocytosis via clathrin-coated pits, such as transferrin receptor,351 and caveoli, such as APP,352−354 are capable of crossing the endothelial barrier. These pathways provide an opportunity for transendothelial transport of DDS with size suitable for these endocytic vesicles (<100 nm). For example, antibodies to caveolar APP undergo fast transport across the endothelium, but particles >100 nm do not enter this pathway.135 Some data has promoted the idea that caveoli merge into “caveolosomes” supporting uptake of large particles, but it is unclear to what extent data obtained in static cell cultures reflect this aspect of endothelial physiology in vivo.135,355,356 However, the potential side effects of engaging caveolar determinants must be more fully understood in order to define the biomedical utility of this transcellular pathway.72,314,357 In addition, many disease conditions, including inflammation, may affect this pathway.39,54−57,358
In this context, it is intriguing to explore endothelial transport opportunities offered by CAM-mediated endocytosis, which can allow entrance of objects up to several micrometers. It has recently been reported that gastrointestinal epithelial cells, which normally express ICAM, take up anti-ICAM coated nanocarriers (∼100 nm diameter spheres) via CAM-mediated endocytosis and transport the carriers across the cellular monolayer without cell damage or disruption of intercellular junctions.359 Further, this team reported that orally administered anti-ICAM coated nanocarriers enter endocytic pathway(s) in the epithelial cells in the gastroenteral tract and that this process may be differentially modulated by auxiliary drugs that regulate intestinal digestion and peristalsis, opening an opportunity for oral delivery of nanocarriers into the vascular compartment.360 It is quite plausible that this transcellular transport pathway operates in the vascular endothelium as well and can be modulated by rational carrier design.
Challenges and Perplexing Issues
All the issues discussed above in this rubric are pertinent here, including optimization of carrier labeling and tracing.361 Data of PK/BD and targeting are vital for understanding of cellular traffic. Thus, enhanced transendothelial transport of low avidity TfR-targeted carriers (attributed to facilitated intracellular dissociation from the receptor, Figure 12) can be also explained by elevation of the blood/tissue gradient via two mechanisms. First, carriers with lower ligand density retain better stealth features (Figure 4). Second, the majority of TfR-mediated uptake occurs in liver; inhibition of hepatic uptake via reducing TfR avidity may also result in elevated blood level (Figure 14).
Figure 14.
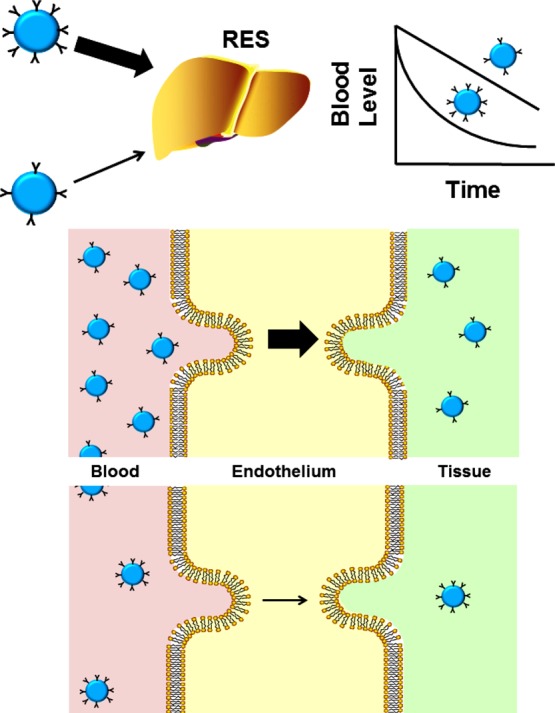
Enhanced blood level of low-avidity carriers as an additional factor facilitating cellular transport. PEGylated nanocarriers coated with a ligand at high density are more rapidly eliminated from blood by the clearing organs (e.g., liver) than carriers coated by a ligand at lower density (and thus retaining more preserved stealth features). This inequity in the PK may help to explain more effective cellular uptake and transport of low-avidity carriers due to elevated concentration in blood favoring binding to the target determinants.
In vitro models do allow quantitative assessment of internalization339,361,362 and probing cellular pathways by pharmacological and genetic inhibitors.338,363 Yet, keeping in mind limited specificity and efficacy and considerable toxicity of these agents, their effects need validation using classical ligands of the pathway of interest. Lack of systemic and microenvironmental factors is a common shortcoming of cell culture studies.356,364−369 Endothelial cells rapidly lose their phenotype in static culture.64,315,370−372 Use of in vitro flow models is more informative but is challenging and adds just one factor of vascular physiology.345,348,373 Current in vitro models employ a limited range of flow parameters and “vessel geometries” from those observed in the body (turbulent, laminar, pulsating, etc.).271,374−378
Prolonged incubations of serum-starved static cells with high doses of particles and use of acidic or proteolytic elution are prone to false-positive results. “Metabolism-independent” internalization of TAT-conjugates that turned out to be endocytosis serves as an example.379 Background “uptake” level on ice should not exceed 5–10%; higher levels are indicative of an artifact. Even meticulously performed, common microscopy-based methods are not truly quantitative, while more precise methods are low-throughput.380,381 Electron microscopy remains the gold standard for evaluating cellular localization of carriers and cargoes.382 No doubt, much needed new approaches to inquire into a carrier’s fate in the body at the subcellular level are evolving.
Vascular Targeting: Perspectives
In our opinion, successful endothelial nanomedicine requires both adequate understanding of biomedical factors pertinent the medical objectives and optimal nanocarrier design. The latter includes quantitative real-time tracing of both carrier and cargo, high-resolution localization in tissues, balanced combination of stealth and targeting features (e.g., attained by responsiveness to local microenvironment), and adequate (not necessarily the highest) avidity. Carrier elongated geometry and plasticity allowing flow alignment and lateral diffusivity of ligands are likely to improve circulation, targeting and subcellular delivery. Targeting these new DDS to the determinants optimally serving given biomedical context will yield the winning combination.
Endothelial targeting is developing at a vigorous pace. Since first reports two decades ago, hundreds of studies have elaborated the strategy, which is on the verge of translation into the clinical domain. This objective represents a set of paramount challenges. Animal studies only partially reflect therapy of patients with unique individual profiles. Mice provide high-throughput models including mutants lacking or expressing genes of interest, but interspecies differences are difficult to overestimate. Clinical relevance is inversely proportional to species availability. Many industrial and healthcare counterparts hesitate to embrace complex and costly DDS. Undoubtedly, well-defined targeted DDS will find additional utility as tools for probing of physiological pathways in animals. And, of course, the targeted endothelial nanomedicine formulations enabling novel therapeutic mechanisms and qualitatively improving the outcome of dangerous human maladies will eventually be translated into the clinical domain.
Acknowledgments
Authors are grateful to current and former members of the Muzykantov’s laboratory, Drs. Silvia Muro, Carmen Garnacho, Thomas Dziubla, Eric Simone, Ann-Marie Chacko, Elizabeth Hood, and Jingyan Han for their invaluable contributions to the studies analyzed in this review. This work is supported by NIH Grants HL087036 and HL091950. A.C.A. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1144085. This publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
§ M.H. and B.J.Z. equally contributed to this paper.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Farokhzad O. C.; Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [DOI] [PubMed] [Google Scholar]
- Langer R. Drug Delivery and Targeting. Nature 1998, 392, 5–10. [PubMed] [Google Scholar]
- Wood K. C.; Azarin S. M.; Arap W.; Pasqualini R.; Langer R.; Hammond P. T. Tumor-Targeted Gene Delivery Using Molecularly Engineered Hybrid Polymers Functionalized with a Tumor-Homing Peptide. Bioconjugate Chem. 2008, 19, 403–405. [DOI] [PubMed] [Google Scholar]
- Davis M. E. The First Targeted Delivery of Sirna in Humans via a Self-Assembling, Cyclodextrin Polymer-Based Nanoparticle: From Concept to Clinic. Mol. Pharmaceutics 2009, 6, 659–668. [DOI] [PubMed] [Google Scholar]
- Lee J. B.; Zhang K.; Tam Y. Y. C.; Tam Y. K.; Belliveau N. M.; Sung V. Y. C.; Lin P. J. C.; LeBlanc E.; Ciufolini M. A.; Rennie P. S.; et al. Lipid Nanoparticle Sirna Systems for Silencing the Androgen Receptor in Human Prostate Cancer in Vivo. Int. J. Cancer 2012, 131, E781–E790. [DOI] [PubMed] [Google Scholar]
- Pagano R. E.; Weinstein J. N. Interactions of Liposomes with Mammalian Cells. Annu. Rev. Biophys. Bioeng. 1978, 7, 435–468. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Liposomes in Therapeutic and Preventive Medicine: The Development of the Drug-Carrier Concept. Ann. N.Y. Acad. Sci. 1978, 308, 343–370. [DOI] [PubMed] [Google Scholar]
- Ryman B. E.; Tyrrell D. A. Liposomes—Methodology and Applications. Front. Biol. 1979, 48, 549–574. [PubMed] [Google Scholar]
- Juliano R. L. Drug Delivery Systems: A Brief Review. Can. J. Physiol. Pharmacol. 1978, 56, 683–690. [DOI] [PubMed] [Google Scholar]
- Langer R. S.; Peppas N. A. Present and Future Applications of Biomaterials in Controlled Drug Delivery Systems. Biomaterials 1981, 2, 201–214. [DOI] [PubMed] [Google Scholar]
- Elsabahy M.; Wooley K. L. Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L.; Herlihy K. P.; Napier M. E.; Desimone J. M. Print: A Novel Platform toward Shape and Size Specific Nanoparticle Theranostics. Acc. Chem. Res. 2011, 44, 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant R. R.; Torchilin V. P. Multifunctionality of Lipid-Core Micelles for Drug Delivery and Tumour Targeting. Mol. Membr. Biol. 2010, 27, 232–246. [DOI] [PubMed] [Google Scholar]
- Venkataraman S.; Hedrick J. L.; Ong Z. Y.; Yang C.; Ee P. L.; Hammond P. T.; Yang Y. Y. The Effects of Polymeric Nanostructure Shape on Drug Delivery. Adv. Drug Delivery Rev. 2011, 63, 1228–1246. [DOI] [PubMed] [Google Scholar]
- van der Poll D. G.; Kieler-Ferguson H. M.; Floyd W. C.; Guillaudeu S. J.; Jerger K.; Szoka F. C.; Frechet J. M. Design, Synthesis, and Biological Evaluation of a Robust, Biodegradable Dendrimer. Bioconjugate Chem. 2010, 21, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F. Jr.; Papahadjopoulos D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [DOI] [PubMed] [Google Scholar]
- Percec V.; Wilson D. A.; Leowanawat P.; Wilson C. J.; Hughes A. D.; Kaucher M. S.; Hammer D. A.; Levine D. H.; Kim A. J.; Bates F. S.; et al. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science 2010, 328, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Eniola A. O.; Hammer D. A. Characterization of Biodegradable Drug Delivery Vehicles with the Adhesive Properties of Leukocytes II: Effect of Degradation on Targeting Activity. Biomaterials 2005, 26, 661–670. [DOI] [PubMed] [Google Scholar]
- Discher B. M.; Won Y. Y.; Ege D. S.; Lee J. C.; Bates F. S.; Discher D. E.; Hammer D. A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. [DOI] [PubMed] [Google Scholar]
- Liong M.; Lu J.; Kovochich M.; Xia T.; Ruehm S. G.; Nel A. E.; Tamanoi F.; Zink J. I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Mirkin C. A.; Park S. J. Nanofabrication Beyond Electronics. ACS Nano 2009, 3, 1049–1056. [DOI] [PubMed] [Google Scholar]
- Bayer C. L.; Peppas N. A. Advances in Recognitive, Conductive and Responsive Delivery Systems. J. Controlled Release 2008, 132, 216–221. [DOI] [PubMed] [Google Scholar]
- Kieler-Ferguson H. M.; Frechet J. M.; Szoka F. C. Jr. Clinical Developments of Chemotherapeutic Nanomedicines: Polymers and Liposomes for Delivery of Camptothecins and Platinum (II) Drugs. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2013, 5, 130–138. [DOI] [PubMed] [Google Scholar]
- Batrakova E. V.; Kabanov A. V. Pluronic Block Copolymers: Evolution of Drug Delivery Concept from Inert Nanocarriers to Biological Response Modifiers. J. Controlled Release 2008, 130, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucuta S. E. Systemic and Biophase Bioavailability and Pharmacokinetics of Nanoparticulate Drug Delivery Systems. Curr. Drug Delivery 2013, 10, 208–240. [DOI] [PubMed] [Google Scholar]
- Fraley R.; Papahadjopoulos D. Liposomes: The Development of a New Carrier System for Introducing Nucleic Acid into Plant and Animal Cells. Curr. Top. Microbiol. Immunol. 1982, 96, 171–191. [DOI] [PubMed] [Google Scholar]
- Maeda H. Macromolecular Therapeutics in Cancer Treatment: The Epr Effect and Beyond. J. Controlled Release 2012, 164, 138–144. [DOI] [PubMed] [Google Scholar]
- Uliel L.; Royal H. D.; Darcy M. D.; Zuckerman D. A.; Sharma A.; Saad N. E. From the Angio Suite to the Gamma-Camera: Vascular Mapping and 99mTc-MAA Hepatic Perfusion Imaging before Liver Radioembolization—A Comprehensive Pictorial Review. J. Nucl. Med. 2012, 53, 1736–1747. [DOI] [PubMed] [Google Scholar]
- Huo D.; Deng S.; Li L.; Ji J. Studies on the Poly(lactic-co-glycolic) Acid Microspheres of Cisplatin for Lung-Targeting. Int. J. Pharm. 2005, 289, 63–67. [DOI] [PubMed] [Google Scholar]
- Muller D. W.; Gordon D.; San H.; Yang Z.; Pompili V. J.; Nabel G. J.; Nabel E. G. Catheter-Mediated Pulmonary Vascular Gene Transfer and Expression. Circ. Res. 1994, 75, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Huang R. B.; Mocherla S.; Heslinga M. J.; Charoenphol P.; Eniola-Adefeso O. Dynamic and Cellular Interactions of Nanoparticles in Vascular-Targeted Drug Delivery. Mol. Membr. Biol. 2010, 27, 312–327. [DOI] [PubMed] [Google Scholar]
- Corti A.; Pastorino F.; Curnis F.; Arap W.; Ponzoni M.; Pasqualini R. Targeted Drug Delivery and Penetration into Solid Tumors. Med. Res. Rev. 2012, 32, 1078–1091. [DOI] [PubMed] [Google Scholar]
- Wickline S. A.; Neubauer A. M.; Winter P. M.; Caruthers S. D.; Lanza G. M. Molecular Imaging and Therapy of Atherosclerosis with Targeted Nanoparticles. J. Magn. Reson. Imaging 2007, 25, 667–680. [DOI] [PubMed] [Google Scholar]
- Noble C. O.; Kirpotin D. B.; Hayes M. E.; Mamot C.; Hong K.; Park J. W.; Benz C. C.; Marks J. D.; Drummond D. C. Development of Ligand-Targeted Liposomes for Cancer Therapy. Expert. Opin. Ther. Targets 2004, 8, 335–353. [DOI] [PubMed] [Google Scholar]
- Xia W.; Low P. S. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010, 53, 6811–6824. [DOI] [PubMed] [Google Scholar]
- Saravanakumar G.; Kim K.; Park J. H.; Rhee K.; Kwon I. C. Current Status of Nanoparticle-Based Imaging Agents for Early Diagnosis of Cancer and Atherosclerosis. J. Biomed. Nanotechnol. 2009, 5, 20–35. [DOI] [PubMed] [Google Scholar]
- Simone E.; Ding B. S.; Muzykantov V. Targeted Delivery of Therapeutics to Endothelium. Cell Tissue Res. 2009, 335, 283–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S.; Muzykantov V. R. Targeting of Antioxidant and Anti-Thrombotic Drugs to Endothelial Cell Adhesion Molecules. Curr. Pharm. Des. 2005, 11, 2383–2401. [DOI] [PubMed] [Google Scholar]
- Muro S.; Koval M.; Muzykantov V. Endothelial Endocytic Pathways: Gates for Vascular Drug Delivery. Curr. Vasc. Pharmacol. 2004, 2, 281–299. [DOI] [PubMed] [Google Scholar]
- Muro S. Challenges in Design and Characterization of Ligand-Targeted Drug Delivery Systems. J. Controlled Release 2012, 164, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R.; Radhakrishnan R.; Eckmann D. M. Dynamic Factors Controlling Targeting Nanocarriers to Vascular Endothelium. Curr. Drug Metab. 2012, 13, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R. Targeted Therapeutics and Nanodevices for Vascular Drug Delivery: Quo Vadis?. IUBMB Life 2011, 63, 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R. Biomedical Aspects of Targeted Delivery of Drugs to Pulmonary Endothelium. Expert Opin. Drug Delivery 2005, 2, 909–926. [DOI] [PubMed] [Google Scholar]
- Igarashi E. Factors Affecting Toxicity and Efficacy of Polymeric Nanomedicines. Toxicol. Appl. Pharmacol. 2008, 229, 121–134. [DOI] [PubMed] [Google Scholar]
- Wischke C.; Kruger A.; Roch T.; Pierce B. F.; Li W.; Jung F.; Lendlein A. Endothelial Cell Response to (Co)Polymer Nanoparticles Depending on the Inflammatory Environment and Comonomer Ratio. Eur. J. Pharm. Biopharm. 2013, 84, 288–296. [DOI] [PubMed] [Google Scholar]
- Garcia A. N.; Vogel S. M.; Komarova Y. A.; Malik A. B. Permeability of Endothelial Barrier: Cell Culture and in Vivo Models. Methods Mol. Biol. (N.Y., NY, U.S.) 2011, 763, 333–354. [DOI] [PubMed] [Google Scholar]
- Aird W. C. Endothelium in Health and Disease. Pharmacol. Rep. 2008, 60, 139–143. [PubMed] [Google Scholar]
- Thomas S. R.; Witting P. K.; Drummond G. R. Redox Control of Endothelial Function and Dysfunction: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signaling 2008, 10, 1713–1765. [DOI] [PubMed] [Google Scholar]
- Stan R. V.; Tse D.; Deharvengt S. J.; Smits N. C.; Xu Y.; Luciano M. R.; McGarry C. L.; Buitendijk M.; Nemani K. V.; Elgueta R.; et al. The Diaphragms of Fenestrated Endothelia: Gatekeepers of Vascular Permeability and Blood Composition. Dev. Cell 2011, 23, 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan R. V. Endothelial Stomatal and Fenestral Diaphragms in Normal Vessels and Angiogenesis. J. Cell. Mol. Med. 2007, 11, 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D.; Nagy J. A.; Pyne K.; Hammel I.; Dvorak H. F.; Dvorak A. M. Pathways of Macromolecular Extravasation across Microvascular Endothelium in Response to Vpf/Vegf and Other Vasoactive Mediators. Microcirculation 1999, 6, 23–44. [PubMed] [Google Scholar]
- Thomsen L. B.; Lichota J.; Eskehave T. N.; Linemann T.; Mortensen J. H.; du Jardin K. G.; Moos T. Brain Delivery Systems via Mechanism Independent of Receptor-Mediated Endocytosis and Adsorptive-Mediated Endocytosis. Curr. Pharm. Biotechnol. 2012, 13, 2349–2354. [DOI] [PubMed] [Google Scholar]
- Feng D.; Nagy J. A.; Dvorak H. F.; Dvorak A. M. Ultrastructural Studies Define Soluble Macromolecular, Particulate, and Cellular Transendothelial Cell Pathways in Venules, Lymphatic Vessels, and Tumor-Associated Microvessels in Man and Animals. Microsc. Res. Technol. 2002, 57, 289–326. [DOI] [PubMed] [Google Scholar]
- Carver L. A.; Schnitzer J. E. Caveolae: Mining Little Caves for New Cancer Targets. Nat. Rev. Cancer 2003, 3, 571–581. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial Adherens Junctions: Implications in the Control of Vascular Permeability and Angiogenesis. J. Clin. Invest. 1996, 98, 1949–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M.; Buciak J.; Yang J.; Wu D. Enhanced Endocytosis in Cultured Human Breast Carcinoma Cells and in Vivo Biodistribution in Rats of a Humanized Monoclonal Antibody after Cationization of the Protein. J. Pharmacol. Exp. Ther. 1998, 286, 548–554. [PubMed] [Google Scholar]
- Predescu D.; Vogel S. M.; Malik A. B. Functional and Morphological Studies of Protein Transcytosis in Continuous Endothelia. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2004, 287, L895–901. [DOI] [PubMed] [Google Scholar]
- Simionescu N. Cellular Aspects of Transcapillary Exchange. Physiol. Rev. 1983, 63, 1536–1579. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Tiruppathi C.; Cho J.; Minshall R. D.; Malik A. B. Delivery of Nanoparticle: Complexed Drugs across the Vascular Endothelial Barrier via Caveolae. IUBMB Life 2011, 63, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F. Endothelial Mechanisms of Flow-Mediated Athero-Protection and Susceptibility. Circ. Res. 2007, 101, 10–12. [DOI] [PubMed] [Google Scholar]
- Kowalski P. S.; Leus N. G.; Scherphof G. L.; Ruiters M. H.; Kamps J. A.; Molema G. Targeted Sirna Delivery to Diseased Microvascular Endothelial Cells: Cellular and Molecular Concepts. IUBMB Life 2011, 63, 648–658. [DOI] [PubMed] [Google Scholar]
- Hood E.; Simone E.; Wattamwar P.; Dziubla T.; Muzykantov V. Nanocarriers for Vascular Delivery of Antioxidants. Nanomedicine (London, U.K.) 2011, 6, 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R. Immunotargeting of Drugs to the Pulmonary Vascular Endothelium as a Therapeutic Strategy. Pathophysiology 1998, 5, 15–33. [Google Scholar]
- Oh P.; Li Y.; Yu J.; Durr E.; Krasinska K. M.; Carver L. A.; Testa J. E.; Schnitzer J. E. Subtractive Proteomic Mapping of the Endothelial Surface in Lung and Solid Tumours for Tissue-Specific Therapy. Nature 2004, 429, 629–635. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E. Vascular Targeting as a Strategy for Cancer Therapy. N. Engl. J. Med. 1998, 339, 472–474. [DOI] [PubMed] [Google Scholar]
- Spragg D. D.; Alford D. R.; Greferath R.; Larsen C. E.; Lee K. D.; Gurtner G. C.; Cybulsky M. I.; Tosi P. F.; Nicolau C.; Gimbrone M. A. Jr. Immunotargeting of Liposomes to Activated Vascular Endothelial Cells: A Strategy for Site-Selective Delivery in the Cardiovascular System. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 8795–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel S. J.; Lee R.; Bultman S.; Kabalka G. Rat Monoclonal Antibody Distribution in Mice: An Epitope inside the Lung Vascular Space Mediates Very Efficient Localization. Int. J. Radiat. Appl. Instrum., Part B 1990, 17, 193–200. [DOI] [PubMed] [Google Scholar]
- McIntosh D. P.; Tan X. Y.; Oh P.; Schnitzer J. E. Targeting Endothelium and Its Dynamic Caveolae for Tissue-Specific Transcytosis in Vivo: A Pathway to Overcome Cell Barriers to Drug and Gene Delivery. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R., Danilov S. M.. Targeting of Radiolabeled Monoclonal Antibody against Ace to the Pulmonary Endothelium. In Targeted Delivery of Imaging Agents; Torchilin V., Ed. CRC Press: Roca Baton, FL, 1995; pp 465–485. [Google Scholar]
- Goetz D. J.; el-Sabban M. E.; Hammer D. A.; Pauli B. U. Lu-Ecam-1-Mediated Adhesion of Melanoma Cells to Endothelium under Conditions of Flow. Int. J. Cancer 1996, 65, 192–199. [DOI] [PubMed] [Google Scholar]
- Huang X.; Molema G.; King S.; Watkins L.; Edgington T. S.; Thorpe P. E. Tumor Infarction in Mice by Antibody-Directed Targeting of Tissue Factor to Tumor Vasculature. Science 1997, 275, 547–550. [DOI] [PubMed] [Google Scholar]
- Stan R. V.; Ghitescu L.; Jacobson B. S.; Palade G. E. Isolation, Cloning, and Localization of Rat Pv-1, a Novel Endothelial Caveolar Protein. J. Cell Biol. 1999, 145, 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajotte D.; Arap W.; Hagedorn M.; Koivunen E.; Pasqualini R.; Ruoslahti E. Molecular Heterogeneity of the Vascular Endothelium Revealed by in Vivo Phage Display. J. Clin. Invest. 1998, 102, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov S. M.; Muzykantov V. R.; Martynov A. V.; Atochina E. N.; Sakharov I.; Trakht I. N.; Smirnov V. N. Lung Is the Target Organ for a Monoclonal Antibody to Angiotensin-Converting Enzyme. Lab. Invest. 1991, 64, 118–124. [PubMed] [Google Scholar]
- Pasqualini R.; McDonald D. M.; Arap W. Vascular Targeting and Antigen Presentation. Nat. Immunol. 2001, 2, 567–568. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R.; Atochina E. N.; Ischiropoulos H.; Danilov S. M.; Fisher A. B. Immunotargeting of Antioxidant Enzyme to the Pulmonary Endothelium. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. N.; Nicklin S. A.; Kaliberova L.; Boatman B. G.; Grizzle W. E.; Balyasnikova I. V.; Baker A. H.; Danilov S. M.; Curiel D. T. Combined Transductional and Transcriptional Targeting Improves the Specificity of Transgene Expression in Vivo. Nat. Biotechnol. 2001, 19, 838–842. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J.; Huang S. L.; Warnick D.; Rabbat M.; Kane B.; Nagaraj A.; Klegerman M.; McPherson D. D. Intravascular Ultrasound Molecular Imaging of Atheroma Components in Vivo. J. Am. Coll. Cardiol. 2004, 43, 453–460. [DOI] [PubMed] [Google Scholar]
- Kastrup C. J.; Nahrendorf M.; Figueiredo J. L.; Lee H.; Kambhampati S.; Lee T.; Cho S.-W.; Gorbatov R.; Iwamoto Y.; Dang T. T. Painting Blood Vessels and Atherosclerotic Plaques with an Adhesive Drug Depot. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 21444–21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmudar M. D.; Yoo J.; Keliher E. J.; Truelove J. J.; Iwamoto Y.; Sena B.; Dutta P.; Borodovsky A.; Fitzgerald K.; Di Carli M. F. Polymeric Nanoparticle Pet/Mr Imaging Allows Macrophage Detection in Atherosclerotic Plaques. Circ. Res. 2013, 112, 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder A. H.; Wang K.; Zhang H.; Senpan A.; Pan D.; Keupp J.; Caruthers S. D.; Wickline S. A.; Shen B.; Wagner E. M. Characterization of Early Neovascular Response to Acute Lung Ischemia Using Simultaneous 19f/1h Mr Molecular Imaging. Angiogenesis 2014, 17, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D.; Pramanik M.; Senpan A.; Allen J. S.; Zhang H.; Wickline S. A.; Wang L. V.; Lanza G. M. Molecular Photoacoustic Imaging of Angiogenesis with Integrin-Targeted Gold Nanobeacons. FASEB J. 2011, 25, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G.; Winter P.; Caruthers S.; Hughes M.; Hu G.; Schmieder A.; Wickline S. Theragnostics for Tumor and Plaque Angiogenesis with Perfluorocarbon Nanoemulsions. Angiogenesis 2010, 13, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M. M.; Glover D. K.; Lanza G. M.; Fayad Z. A.; Johnson L. L. Imaging Atherosclerosis and Vulnerable Plaque. J. Nucl. Med. 2010, 51, 51S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Lobatto M. E.; Kawahara T.; Chung B. L.; Mieszawska A. J.; Sanchez-Gaytan B. L.; Fay F.; Senders M. L.; Calcagno C.; Becraft J.; et al. Probing Nanoparticle Translocation across the Permeable Endothelium in Experimental Atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara N.; Mukherjee J.; de Haas H. J.; Petrov A. D.; Tawakol A.; Haider N.; Tahara A.; Constantinescu C. C.; Zhou J.; Boersma H. H. 2-Deoxy-2-[18F] Fluoro-d-Mannose Positron Emission Tomography Imaging in Atherosclerosis. Nat. Med. 2014, 20, 215–219. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Fay F.; Cormode D. P.; Sanchez-Gaytan B. L.; Tang J.; Hennessy E. J.; Ma M.; Moore K.; Farokhzad O. C.; Fisher E. A. Single Step Reconstitution of Multifunctional High-Density Lipoprotein-Derived Nanomaterials Using Microfluidics. ACS Nano 2013, 7, 9975–9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieszawska A. J.; Kim Y.; Gianella A.; van Rooy I.; Priem B.; Labarre M. P.; Ozcan C.; Cormode D. P.; Petrov A.; Langer R. Synthesis of Polymer–Lipid Nanoparticles for Image-Guided Delivery of Dual Modality Therapy. Bioconjugate Chem. 2013, 24, 1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T.; Bhayana B.; Thompson B.; Kessinger C. W.; Khatri A.; McCarthy J. R.; Weissleder R.; Lin C. P.; Tearney G. J.; Jaffer F. A. Molecular Imaging of Fibrin Deposition in Deep Vein Thrombosis Using Fibrin-Targeted near-Infrared Fluorescence. JACC 2012, 5, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev V. V.; Ilies M. A.; Simone E.; Zaitsev S.; Kim Y.; Cai S.; Mahmud A.; Dziubla T.; Muro S.; Discher D. E.; et al. Endothelial Targeting of Antibody-Decorated Polymeric Filomicelles. ACS Nano 2011, 5, 6991–6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun J. B.; Pepper L. R.; Boder E. T.; Hammer D. A. Using Engineered Single-Chain Antibodies to Correlate Molecular Binding Properties and Nanoparticle Adhesion Dynamics. Langmuir 2011, 27, 13701–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun J. B.; Hammer D. A. Quantifying Nanoparticle Adhesion Mediated by Specific Molecular Interactions. Langmuir 2008, 24, 8821–8832. [DOI] [PubMed] [Google Scholar]
- Eniola A. O.; Krasik E. F.; Smith L. A.; Song G.; Hammer D. A. I-Domain of Lymphocyte Function-Associated Antigen-1 Mediates Rolling of Polystyrene Particles on ICAM-1 under Flow. Biophys. J. 2005, 89, 3577–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon A. J.; Muzykantov V.; Muro S.; Eckmann D. M. Flow Dynamics, Binding and Detachment of Spherical Carriers Targeted to ICAM-1 on Endothelial Cells. Biorheology 2009, 46, 323–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpereel A.; Rome J. J.; Wiewrodt R.; Watkins S. C.; Harshaw D. W.; Alder S.; Christofidou-Solomidou M.; Haut E.; Murciano J. C.; Nakada M.; et al. Platelet-Endothelial Cell Adhesion Molecule-1-Directed Immunotargeting to Cardiopulmonary Vasculature. J. Pharmacol. Exp. Ther. 2002, 300, 777–786. [DOI] [PubMed] [Google Scholar]
- Danielyan K.; Ding B.-S.; Gottstein C.; Cines D. B.; Muzykantov V. R. Delivery of Anti-Platelet-Endothelial Cell Adhesion Molecule Single-Chain Variable Fragment-Urokinase Fusion Protein to the Cerebral Vasculature Lyses Arterial Clots and Attenuates Postischemic Brain Edema. J. Pharmacol. Exp. Ther. 2007, 321, 947–952. [DOI] [PubMed] [Google Scholar]
- Matter C. M.; Schuler P. K.; Alessi P.; Meier P.; Ricci R.; Zhang D.; Halin C.; Castellani P.; Zardi L.; Hofer C. K. Molecular Imaging of Atherosclerotic Plaques Using a Human Antibody against the Extra-Domain B of Fibronectin. Circ. Res. 2004, 95, 1225–1233. [DOI] [PubMed] [Google Scholar]
- Virmani R.; Kolodgie F. D.; Burke A. P.; Finn A. V.; Gold H. K.; Tulenko T. N.; Wrenn S. P.; Narula J. Atherosclerotic Plaque Progression and Vulnerability to Rupture Angiogenesis as a Source of Intraplaque Hemorrhage. Arterioscler., Thromb., Vasc. Biol. 2005, 25, 2054–2061. [DOI] [PubMed] [Google Scholar]
- Winter P. M.; Neubauer A. M.; Caruthers S. D.; Harris T. D.; Robertson J. D.; Williams T. A.; Schmieder A. H.; Hu G.; Allen J. S.; Lacy E. K. Endothelial Aνβ3 Integrin–Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. Arterioscler., Thromb., Vasc. Biol. 2006, 26, 2103–2109. [DOI] [PubMed] [Google Scholar]
- Moreno P. R.; Purushothaman K.-R.; Sirol M.; Levy A. P.; Fuster V. Neovascularization in Human Atherosclerosis. Circulation 2006, 113, 2245–2252. [DOI] [PubMed] [Google Scholar]
- Gerrit L.; Sijbrands E. J.; Valkema R.; Folkert J.; Feinstein S. B.; Van der Steen A. F.; Daemen M. J.; Schinkel A. F. Molecular Imaging of Inflammation and Intraplaque Vasa Vasorum: A Step Forward to Identification of Vulnerable Plaques?. J. Nucl. Cardiol 2010, 17, 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A. F.; Krueger C. G.; Tellez A.; Granada J. F.; Reed J. D.; Hall A.; Zang W.; Owens C.; Kaluza G. L.; Staub D. Contrast-Enhanced Ultrasound for Imaging Vasa Vasorum: Comparison with Histopathology in a Swine Model of Atherosclerosis. Eur. J. Echocardiogr. 2010, 11, 659–664. [DOI] [PubMed] [Google Scholar]
- Korin N.; Kanapathipillai M.; Matthews B. D.; Crescente M.; Brill A.; Mammoto T.; Ghosh K.; Jurek S.; Bencherif S. A.; Bhatta D.; et al. Shear-Activated Nanotherapeutics for Drug Targeting to Obstructed Blood Vessels. Science 2012, 337, 738–742. [DOI] [PubMed] [Google Scholar]
- Owens D. E. III; Peppas N. A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [DOI] [PubMed] [Google Scholar]
- Roser M.; Fischer D.; Kissel T. Surface-Modified Biodegradable Albumin Nano- and Microspheres. II: Effect of Surface Charges on in Vitro Phagocytosis and Biodistribution in Rats. Eur. J. Pharm. Biopharm. 1998, 46, 255–63. [DOI] [PubMed] [Google Scholar]
- Carrstensen H.; Muller R. H.; Muller B. W. Particle Size, Surface Hydrophobicity and Interaction with Serum of Parenteral Fat Emulsions and Model Drug Carriers as Parameters Related to Res Uptake. Clin. Nutr. 1992, 11, 289–97. [DOI] [PubMed] [Google Scholar]
- Howard M. D.; Jay M.; Dziubla T. D.; Lu X. Pegylation of Nanocarrier Drug Delivery Systems: State of the Art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar]
- Gref R.; Minamitake Y.; Peracchia M. T.; Trubetskoy V.; Torchilin V.; Langer R. Biodegradable Long-Circulating Polymeric Nanospheres. Science 1994, 263, 1600–1603. [DOI] [PubMed] [Google Scholar]
- Mori A.; Klibanov A. L.; Torchilin V. P.; Huang L. Influence of the Steric Barrier Activity of Amphipathic Poly(Ethyleneglycol) and Ganglioside Gm1 on the Circulation Time of Liposomes and on the Target Binding of Immunoliposomes in Vivo. FEBS Lett. 1991, 284, 263–266. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P.; Klibanov A. L.; Huang L.; O’Donnell S.; Nossiff N. D.; Khaw B. A. Targeted Accumulation of Polyethylene Glycol-Coated Immunoliposomes in Infarcted Rabbit Myocardium. FASEB J. 1992, 6, 2716–2719. [DOI] [PubMed] [Google Scholar]
- Klibanov A. L.; Maruyama K.; Beckerleg A. M.; Torchilin V. P.; Huang L. Activity of Amphipathic Poly(Ethylene Glycol) 5000 to Prolong the Circulation Time of Liposomes Depends on the Liposome Size and Is Unfavorable for Immunoliposome Binding to Target. Biochim. Biophys. Acta 1991, 1062, 142–148. [DOI] [PubMed] [Google Scholar]
- Sawant R. R.; Sawant R. M.; Kale A. A.; Torchilin V. P. The Architecture of Ligand Attachment to Nanocarriers Controls Their Specific Interaction with Target Cells. J. Drug Targeting 2008, 16, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.; Dodwadkar N. S.; Sawant R. R.; Torchilin V. P. Development of the Novel Peg-Pe-Based Polymer for the Reversible Attachment of Specific Ligands to Liposomes: Synthesis and in Vitro Characterization. Bioconjugate Chem. 2011, 22, 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Huang Y.; Kumar A.; Tan A.; Jin S.; Mozhi A.; Liang X. J. Ph-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv. 2013, 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Maeda R.; Ichihara M.; Mukai Y.; Motoki Y.; Manabe Y.; Irimura K.; Kiwada H. The Accelerated Clearance on Repeated Injection of Pegylated Liposomes in Rats: Laboratory and Histopathological Study. Cell. Mol. Biol. Lett. 2002, 7, 286. [PubMed] [Google Scholar]
- Ishida T.; Maeda R.; Ichihara M.; Irimura K.; Kiwada H. Accelerated Clearance of Pegylated Liposomes in Rats after Repeated Injections. J. Controlled Release 2003, 88, 35–42. [DOI] [PubMed] [Google Scholar]
- Lu W.; Wan J.; She Z.; Jiang X. Brain Delivery Property and Accelerated Blood Clearance of Cationic Albumin Conjugated Pegylated Nanoparticle. J. Controlled Release 2007, 118, 38–53. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Ichihara M.; Wang X.; Yamamoto K.; Kimura J.; Majima E.; Kiwada H. Injection of Pegylated Liposomes in Rats Elicits Peg-Specific Igm, Which Is Responsible for Rapid Elimination of a Second Dose of Pegylated Liposomes. J. Controlled Release 2006, 112, 15–25. [DOI] [PubMed] [Google Scholar]
- Dams E. T.; Laverman P.; Oyen W. J.; Storm G.; Scherphof G. L.; van Der Meer J. W.; Corstens F. H.; Boerman O. C. Accelerated Blood Clearance and Altered Biodistribution of Repeated Injections of Sterically Stabilized Liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [PubMed] [Google Scholar]
- Laverman P.; Carstens M. G.; Boerman O. C.; Dams E. T.; Oyen W. J.; van Rooijen N.; Corstens F. H.; Storm G. Factors Affecting the Accelerated Blood Clearance of Polyethylene Glycol-Liposomes Upon Repeated Injection. J. Pharmacol. Exp. Ther. 2001, 298, 607–612. [PubMed] [Google Scholar]
- Ishida T.; Ichihara M.; Wang X.; Kiwada H. Spleen Plays an Important Role in the Induction of Accelerated Blood Clearance of Pegylated Liposomes. J. Controlled Release 2006, 115, 243–250. [DOI] [PubMed] [Google Scholar]
- Zandvoort A.; Timens W. The Dual Function of the Splenic Marginal Zone: Essential for Initiation of Anti-Ti-2 Responses but Also Vital in the General First-Line Defense against Blood-Borne Antigens. Clin. Exp. Immunol. 2002, 130, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara M.; Shimizu T.; Imoto A.; Hashiguchi Y.; Uehara Y.; Ishida T.; Kiwada H. Anti-Peg Igm Response against Pegylated Liposomes in Mice and Rats. Pharmaceutics 2010, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. S.; Huang L. Effect of Chemically Modified Gm1 and Neoglycolipid Analogs of Gm1 on Liposome Circulation Time: Evidence Supporting the Dysopsonin Hypothesis. Biochim. Biophys. Acta 1993, 1166, 105–114. [DOI] [PubMed] [Google Scholar]
- Michalek M. T.; Mold C.; Bremer E. G. Inhibition of the Alternative Pathway of Human Complement by Structural Analogues of Sialic Acid. J. Immunol. 1988, 140, 1588–1594. [PubMed] [Google Scholar]
- Shichijo S.; Alving C. R. Inhibitory Effects of Gangliosides on Immune Reactions of Antibodies to Neutral Glycolipids in Liposomes. Biochim. Biophys. Acta 1986, 858, 118–124. [DOI] [PubMed] [Google Scholar]
- Rodriguez P. L.; Harada T.; Christian D. A.; Pantano D. A.; Tsai R. K.; Discher D. E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachelek S. J.; Finley M. J.; Alferiev I. S.; Wang F.; Tsai R. K.; Eckells E. C.; Tomczyk N.; Connolly J. M.; Discher D. E.; Eckmann D. M.; et al. The Effect of Cd47 Modified Polymer Surfaces on Inflammatory Cell Attachment and Activation. Biomaterials 2011, 32, 4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R. K.; Rodriguez P. L.; Discher D. E. Self Inhibition of Phagocytosis: The Affinity of ‘Marker of Self’ Cd47 for Sirpalpha Dictates Potency of Inhibition but Only at Low Expression Levels. Blood Cells Mol., Dis. 2010, 45, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S.; Parthasarathy R.; Sen S.; Boder E. T.; Discher D. E. Species- and Cell Type-Specific Interactions between Cd47 and Human Sirpalpha. Blood 2006, 107, 2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. M.; Zhang L.; Aryal S.; Cheung C.; Fang R. H.; Zhang L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A.; Quattrocchi N.; van de Ven A. L.; Chiappini C.; Evangelopoulos M.; Martinez J. O.; Brown B. S.; Khaled S. Z.; Yazdi I. K.; Enzo M. V.; et al. Synthetic Nanoparticles Functionalized with Biomimetic Leukocyte Membranes Possess Cell-Like Functions. Nat. Nanotechnol. 2013, 8, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S.; Liu W.; Misra P.; Tanaka E.; Zimmer J. P.; Ipe B. I.; Bawendi M. G.; Frangioni J. V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu S. A.; Predescu D. N.; Malik A. B. Molecular Determinants of Endothelial Transcytosis and Their Role in Endothelial Permeability. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2007, 293, L823–L842. [DOI] [PubMed] [Google Scholar]
- Oh P.; Borgstrom P.; Witkiewicz H.; Li Y.; Borgstrom B. J.; Chrastina A.; Iwata K.; Zinn K. R.; Baldwin R.; Testa J. E.; et al. Live Dynamic Imaging of Caveolae Pumping Targeted Antibody Rapidly and Specifically across Endothelium in the Lung. Nat. Biotechnol. 2007, 25, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvaev V. V.; Tliba S.; Pick J.; Arguiri E.; Christofidou-Solomidou M.; Albelda S. M.; Muzykantov V. R. Modulation of Endothelial Targeting by Size of Antibody-Antioxidant Enzyme Conjugates. J. Controlled Release 2011, 149, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum N.; Tscheka C.; Neumeyer A.; Schneider M. Novel Approaches for Drug Delivery Systems in Nanomedicine: Effects of Particle Design and Shape. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2012, 4, 52–65. [DOI] [PubMed] [Google Scholar]
- Simone E. A.; Dziubla T. D.; Muzykantov V. R. Polymeric Carriers: Role of Geometry in Drug Delivery. Expert Opin. Drug Delivery 2008, 5, 1283–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Tan J.; Thomas A.; Ou-Yang D.; Muzykantov V. R. The Shape of Things to Come: Importance of Design in Nanotechnology for Drug Delivery. Ther. Delivery 2012, 3, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion J. A.; Katare Y. K.; Mitragotri S. Particle Shape: A New Design Parameter for Micro- and Nanoscale Drug Delivery Carriers. J. Controlled Release 2007, 121, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S. In Drug Delivery, Shape Does Matter. Pharm. Res. 2009, 26, 232–234. [DOI] [PubMed] [Google Scholar]
- Herd H.; Daum N.; Jones A. T.; Huwer H.; Ghandehari H.; Lehr C. M. Nanoparticle Geometry and Surface Orientation Influence Mode of Cellular Uptake. ACS Nano 2013, 7, 1961–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro S.; Garnacho C.; Champion J. A.; Leferovich J.; Gajewski C.; Schuchman E. H.; Mitragotri S.; Muzykantov V. R. Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of ICAM-1-Targeted Carriers. Mol. Ther. 2008, 16, 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion J. A.; Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009, 26, 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion J.; Mitragotri S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R. Drug Delivery by Red Blood Cells: Vascular Carriers Designed by Mother Nature. Expert Opin. Drug Delivery 2010, 7, 403–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel T. J.; Jones S. W.; Herlihy K. P.; Kersey F. R.; Shields A. R.; Napier M.; Luft J. C.; Wu H.; Zamboni W. C.; Wang A. Z.; et al. Using Mechanobiological Mimicry of Red Blood Cells to Extend Circulation Times of Hydrogel Microparticles. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y.-c.Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, 1993. [Google Scholar]
- Merkel T. J.; Chen K.; Jones S. W.; Pandya A. A.; Tian S.; Napier M. E.; Zamboni W. E.; DeDimone J. M. The Effect of Particle Size on the Biodistribution of Low-Modulus Hydrogel Print Particles. J. Controlled Release 2012, 162, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone E. A.; Dziubla T. D.; Colon-Gonzalez F.; Discher D. E.; Muzykantov V. R. Effect of Polymer Amphiphilicity on Loading of a Therapeutic Enzyme into Protective Filamentous and Spherical Polymer Nanocarriers. Biomacromolecules 2007, 8, 3914–3921. [DOI] [PubMed] [Google Scholar]
- Geng Y.; Dalhaimer P.; Cai S.; Tsai R.; Tewari M.; Minko T.; Discher D. E. Shape Effects of Filaments versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol. 2007, 2, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhaimer P.; Engler A. J.; Parthasarathy R.; Discher D. E. Targeted Worm Micelles. Biomacromolecules 2004, 5, 1714–1719. [DOI] [PubMed] [Google Scholar]
- Venditto V. J.; Szoka F. C. Jr. Cancer Nanomedicines: So Many Papers and So Few Drugs!. Adv. Drug Delivery Rev. 2013, 65, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollinger K.; Hennig R.; Ohlmann A.; Fuchshofer R.; Wenzel R.; Breunig M.; Tessmar J.; Tamm E. R.; Goepferich A. Ligand-Functionalized Nanoparticles Target Endothelial Cells in Retinal Capillaries after Systemic Application. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhuis R. P.; Stojanov K.; Laverman P.; Eilander J.; Zuhorn I. S.; Rutjes F. P.; van Hest J. C. Size Dependent Biodistribution and Spect Imaging of (111) in-Labeled Polymersomes. Bioconjugate Chem. 2012, 26, 958–965. [DOI] [PubMed] [Google Scholar]
- Zern B. J.; Chacko A.-M.; Liu J.; Greineder C. F.; Blankemeyer E. R.; Radhakrishnan R.; Muzykantov V. Reduction of Nanoparticle Avidity Enhances the Selectivity of Vascular Targeting and Pet Detection of Pulmonary Inflammation. ACS Nano 2013, 7, 2461–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin R.; Muro S.; Welch M. J.; Muzykantov V. R.; Schuster D. P. In Vivo Imaging of 64cu-Labeled Polymer Nanoparticles Targeted to the Lung Endothelium. J. Nucl. Med. 2008, 49, 103–111. [DOI] [PubMed] [Google Scholar]
- Danilov S. M.; Martynov A. V.; Klibanov A. L.; Slinkin M. A.; Sakharov I.; Malov A. G.; Sergienko V. B.; Vedernikov A.; Muzykantov V. R.; Torchilin V. P. Radioimmunoimaging of Lung Vessels: An Approach Using Indium-111-Labeled Monoclonal Antibody to Angiotensin-Converting Enzyme. J. Nucl. Med. 1989, 30, 1686–1692. [PubMed] [Google Scholar]
- Simone E. A.; Zern B. J.; Chacko A. M.; Mikitsh J. L.; Blankemeyer E. R.; Muro S.; Stan R. V.; Muzykantov V. R. Endothelial Targeting of Polymeric Nanoparticles Stably Labeled with the Pet Imaging Radioisotope Iodine-124. Biomaterials 2012, 33, 5406–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R.; Christofidou-Solomidou M.; Balyasnikova I.; Harshaw D. W.; Schultz L.; Fisher A. B.; Albelda S. M. Streptavidin Facilitates Internalization and Pulmonary Targeting of an Anti-Endothelial Cell Antibody (Platelet-Endothelial Cell Adhesion Molecule 1): A Strategy for Vascular Immunotargeting of Drugs. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R.; Puchnina E. A.; Atochina E. N.; Hiemish H.; Slinkin M. A.; Meertsuk F. E.; Danilov S. M. Endotoxin Reduces Specific Pulmonary Uptake of Radiolabeled Monoclonal Antibody to Angiotensin-Converting Enzyme. J. Nucl. Med. 1991, 32, 453–460. [PubMed] [Google Scholar]
- Pan H.; Myerson J. W.; Hu L.; Marsh J. N.; Hou K.; Scott M. J.; Allen J. S.; Hu G.; San Roman S.; Lanza G. M.; et al. A. Programmable Nanoparticle Functionalization for in Vivo Targeting. FASEB J. 2013, 27, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V. R.; Gavriluk V. D.; Reinecke A.; Atochina E. N.; Kuo A.; Barnathan E. S.; Fisher A. B. The Functional Effects of Biotinylation of Anti-Angiotensin-Converting Enzyme Monoclonal Antibody in Terms of Targeting in Vivo. Anal. Biochem. 1995, 226, 279–287. [DOI] [PubMed] [Google Scholar]
- Tenzer S.; Docter D.; Rosfa S.; Wlodarski A.; Kuharev J.; Rekik A.; Knauer S. K.; Bantz C.; Nawroth T.; Bier C.; et al. Nanoparticle Size Is a Critical Physicochemical Determinant of the Human Blood Plasma Corona: A Comprehensive Quantitative Proteomic Analysis. ACS Nano 2011, 5, 7155–7167. [DOI] [PubMed] [Google Scholar]
- Monopoli M. P.; Aberg C.; Salvati A.; Dawson K. A. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat. Nanotechnol. 2012, 7, 779–786. [DOI] [PubMed] [Google Scholar]
- Andersen A. J.; Hashemi S. H.; Andresen T. L.; Hunter A. C.; Moghimi S. M. Complement: Alive and Kicking Nanomedicines. J. Biomed. Nanotechnol. 2009, 5, 364–372. [DOI] [PubMed] [Google Scholar]
- Salvati A.; Pitek A. S.; Monopoli M. P.; Prapainop K.; Bombelli F. B.; Hristov D. R.; Kelly P. M.; Aberg C.; Mahon E.; Dawson K. A. Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat. Nanotechnol. 2013, 8, 137–143. [DOI] [PubMed] [Google Scholar]
- Lesniak A.; Fenaroli F.; Monopoli M. P.; Aberg C.; Dawson K. A.; Salvati A. Effects of the Presence or Absence of a Protein Corona on Silica Nanoparticle Uptake and Impact on Cells. ACS Nano 2012, 6, 5845–5857. [DOI] [PubMed] [Google Scholar]
- Tenzer S.; Docter D.; Kuharev J.; Musyanovych A.; Fetz V.; Hecht R.; Schlenk F.; Fischer D.; Kiouptsi K.; Reinhardt C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R.; Smirnov M. D.; Samokhin G. P. Avidin Attachment to Biotinylated Erythrocytes Induces Homologous Lysis via the Alternative Pathway of Complement. Blood 1991, 78, 2611–2618. [PubMed] [Google Scholar]
- Lesniak A.; Campbell A.; Monopoli M. P.; Lynch I.; Salvati A.; Dawson K. A. Serum Heat Inactivation Affects Protein Corona Composition and Nanoparticle Uptake. Biomaterials 2010, 31, 9511–9518. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R.; Smirnov M. D.; Samokhin G. P. Streptavidin-Induced Lysis of Homologous Biotinylated Erythrocytes. Evidence against the Key Role of the Avidin Charge in Complement Activation via the Alternative Pathway. FEBS Lett. 1991, 280, 112–114. [DOI] [PubMed] [Google Scholar]
- Durr E.; Yu J.; Krasinska K. M.; Carver L. A.; Yates J. R.; Testa J. E.; Oh P.; Schnitzer J. E. Direct Proteomic Mapping of the Lung Microvascular Endothelial Cell Surface in Vivo and in Cell Culture. Nat. Biotechnol. 2004, 22, 985–992. [DOI] [PubMed] [Google Scholar]
- Hajitou A.; Trepel M.; Lilley C. E.; Soghomonyan S.; Alauddin M. M.; Marini F. C. III; Restel B. H.; Ozawa M. G.; Moya C. A.; Rangel R.; et al. A Hybrid Vector for Ligand-Directed Tumor Targeting and Molecular Imaging. Cell 2006, 125, 385–398. [DOI] [PubMed] [Google Scholar]
- Nicklin S. A.; White S. J.; Watkins S. J.; Hawkins R. E.; Baker A. H. Selective Targeting of Gene Transfer to Vascular Endothelial Cells by Use of Peptides Isolated by Phage Display. Circulation 2000, 102, 231–237. [DOI] [PubMed] [Google Scholar]
- Christofidou-Solomidou M.; Muzykantov V. R. Antioxidant Strategies in Respiratory Medicine. Treat. Respir. Med. 2006, 5, 47–78. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R. Targeting of Superoxide Dismutase and Catalase to Vascular Endothelium. J. Controlled Release 2001, 71, 1–21. [DOI] [PubMed] [Google Scholar]
- Pasqualini R.; Arap W.; McDonald D. M. Probing the Structural and Molecular Diversity of Tumor Vasculature. Trends Mol. Med. 2002, 8, 563–571. [DOI] [PubMed] [Google Scholar]
- Danilov S. M.; Gavrilyuk V. D.; Franke F. E.; Pauls K.; Harshaw D. W.; McDonald T. D.; Miletich D. J.; Muzykantov V. R. Lung Uptake of Antibodies to Endothelial Antigens: Key Determinants of Vascular Immunotargeting. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2001, 280, L1335–13347. [DOI] [PubMed] [Google Scholar]
- Shuvaev V. V.; Christofidou-Solomidou M.; Scherpereel A.; Simone E.; Arguiri E.; Tliba S.; Pick J.; Kennel S.; Albelda S. M.; Muzykantov V. R. Factors Modulating the Delivery and Effect of Enzymatic Cargo Conjugated with Antibodies Targeted to the Pulmonary Endothelium. J. Controlled Release 2007, 118, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan E. T.; Harrison A. A.; Chapman P. T.; Binns R. M.; Peters A. M.; Haskard D. O. Imaging Vascular Endothelial Activation: An Approach Using Radiolabeled Monoclonal Antibodies against the Endothelial Cell Adhesion Molecule E-Selectin. J. Nucl. Med. 1994, 35, 276–281. [PubMed] [Google Scholar]
- Metzger R.; Franke F. E.; Bohle R. M.; Alhenc-Gelas F.; Danilov S. M. Heterogeneous Distribution of Angiotensin I-Converting Enzyme (Cd143) in the Human and Rat Vascular Systems: Vessel, Organ and Species Specificity. Microvasc. Res. 2011, 81, 206–215. [DOI] [PubMed] [Google Scholar]
- Ford V. A.; Stringer C.; Kennel S. J. Thrombomodulin Is Preferentially Expressed in Balb/C Lung Microvessels. J. Biol. Chem. 1992, 267, 5446–5450. [PubMed] [Google Scholar]
- Carson-Walter E. B.; Winans B. N.; Whiteman M. C.; Liu Y.; Jarvela S.; Haapasalo H.; Tyler B. M.; Huso D. L.; Johnson M. D.; Walter K. A. Characterization of Tem1/Endosialin in Human and Murine Brain Tumors. BMC Cancer 2009, 9, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R.; Koivunen E.; Kain R.; Lahdenranta J.; Sakamoto M.; Stryhn A.; Ashmun R. A.; Shapiro L. H.; Arap W.; Ruoslahti E. Aminopeptidase N Is a Receptor for Tumor-Homing Peptides and a Target for Inhibiting Angiogenesis. Cancer Res. 2000, 60, 722–727. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S.; Sessa W. C. Evolving Functions of Endothelial Cells in Inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [DOI] [PubMed] [Google Scholar]
- Aird W. C.; Edelberg J. M.; Weiler-Guettler H.; Simmons W. W.; Smith T. W.; Rosenberg R. D. Vascular Bed-Specific Expression of an Endothelial Cell Gene Is Programmed by the Tissue Microenvironment. J. Cell Bio. 1997, 138, 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird W. C. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ. Res. 2007, 100, 158–173. [DOI] [PubMed] [Google Scholar]
- Newman P. J.; Albelda S. M. Cellular and Molecular Aspects of PECAM-1. Nouv. Rev. Fr. Hematol. 1992, 34Suppl.S9–13. [PubMed] [Google Scholar]
- Giannotta M.; Trani M.; Dejana E. Ve-Cadherin and Endothelial Adherens Junctions: Active Guardians of Vascular Integrity. Dev. Cell 2013, 26, 441–454. [DOI] [PubMed] [Google Scholar]
- Barreiro O.; Yanez-Mo M.; Sala-Valdes M.; Gutierrez-Lopez M. D.; Ovalle S.; Higginbottom A.; Monk P. N.; Cabanas C.; Sanchez-Madrid F. Endothelial Tetraspanin Microdomains Regulate Leukocyte Firm Adhesion During Extravasation. Blood 2005, 105, 2852–2861. [DOI] [PubMed] [Google Scholar]
- Cook-Mills J. M.; Marchese M. E.; Abdala-Valencia H. Vascular Cell Adhesion Molecule-1 Expression and Signaling During Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid. Redox Signaling 2011, 15, 1607–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almenar-Queralt A.; Duperray A.; Miles L. A.; Felez J.; Altieri D. C. Apical Topography and Modulation of ICAM-1 Expression on Activated Endothelium. Am. J. Pathol. 1995, 147, 1278–1288. [PMC free article] [PubMed] [Google Scholar]
- Wright M. D.; Moseley G. W.; van Spriel A. B. Tetraspanin Microdomains in Immune Cell Signalling and Malignant Disease. Tissue Antigens 2004, 64, 533–542. [DOI] [PubMed] [Google Scholar]
- Ghitescu L.; Jacobson B. S.; Crine P. A Novel, 85 kDa Endothelial Antigen Differentiates Plasma Membrane Macrodomains in Lung Alveolar Capillaries. Endothelium 1999, 6, 241–250. [DOI] [PubMed] [Google Scholar]
- Murciano J. C.; Harshaw D. W.; Ghitescu L.; Danilov S. M.; Muzykantov V. R. Vascular Immunotargeting to Endothelial Surface in a Specific Macrodomain in Alveolar Capillaries. Am. J. Respir. Crit. Care Med. 2001, 164, 1295–1302. [DOI] [PubMed] [Google Scholar]
- Garnacho C.; Albelda S. M.; Muzykantov V. R.; Muro S. Differential Intra-Endothelial Delivery of Polymer Nanocarriers Targeted to Distinct PECAM-1 Epitopes. J. Controlled Release 2008, 130, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor A. W.; Lipowsky H. H. Role of Glycocalyx in Leukocyte-Endothelial Cell Adhesion. Am. J. Physiol.: Heart Circ. Physiol. 2002, 283, H1282–1291. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A. Bradykinin-Degrading Enzymes: Structure, Function, Distribution, and Potential Roles in Cardiovascular Pharmacology. J. Cardiovasc. Pharmacol. 1992, 20Suppl 9S4–9. [DOI] [PubMed] [Google Scholar]
- Maruyama K.; Kennel S. J.; Huang L. Lipid Composition Is Important for Highly Efficient Target Binding and Retention of Immunoliposomes. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 5744–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofidou-Solomidou M.; Kennel S.; Scherpereel A.; Wiewrodt R.; Solomides C. C.; Pietra G. G.; Murciano J. C.; Shah S. A.; Ischiropoulos H.; Albelda S. M.; et al. Vascular Immunotargeting of Glucose Oxidase to the Endothelial Antigens Induces Distinct Forms of Oxidant Acute Lung Injury: Targeting to Thrombomodulin, but Not to PECAM-1, Causes Pulmonary Thrombosis and Neutrophil Transmigration. Am. J. Pathol. 2002, 160, 1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. Thrombomodulin as a Model of Molecular Mechanisms That Modulate Protease Specificity and Function at the Vessel Surface. FASEB J. 1995, 9, 946–955. [DOI] [PubMed] [Google Scholar]
- Brisson C.; Archipoff G.; Hartmann M. L.; Hanau D.; Beretz A.; Freyssinet J. M.; Cazenave J. P. Antibodies to Thrombomodulin Induce Receptor-Mediated Endocytosis in Human Saphenous Vein Endothelial Cells. Thromb. Haemost. 1992, 68, 737–743. [PubMed] [Google Scholar]
- Guermazi S.; Mellouli F.; Trabelsi S.; Bejaoui M.; Dellagi K. Anti-Thrombomodulin Antibodies and Venous Thrombosis. Blood Coagulation Fibrinolysis 2004, 15, 553–558. [DOI] [PubMed] [Google Scholar]
- Christofidou-Solomidou M.; Scherpereel A.; Wiewrodt R.; Ng K.; Sweitzer T.; Arguiri E.; Shuvaev V.; Solomides C. C.; Albelda S. M.; Muzykantov V. R. PECAM-Directed Delivery of Catalase to Endothelium Protects against Pulmonary Vascular Oxidative Stress. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2003, 285, L283–292. [DOI] [PubMed] [Google Scholar]
- Gow A. J.; Branco F.; Christofidou-Solomidou M.; Black-Schultz L.; Albelda S. M.; Muzykantov V. R. Immunotargeting of Glucose Oxidase: Intracellular Production of H2O2 and Endothelial Oxidative Stress. Am. J. Physiol. 1999, 277, L271–281. [DOI] [PubMed] [Google Scholar]
- Chacko A. M.; Hood E. D.; Zern B. J.; Muzykantov V. R. Targeted Nanocarriers for Imaging and Therapy of Vascular Inflammation. Curr. Opin. Colloid Interface Sci. 2011, 16, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion Receptors of the Immune System. Nature 1990, 346, 425–434. [DOI] [PubMed] [Google Scholar]
- Kumasaka T.; Quinlan W. M.; Doyle N. A.; Condon T. P.; Sligh J.; Takei F.; Beaudet A.; Bennett C. F.; Doerschuk C. M. Role of the Intercellular Adhesion Molecule-1(ICAM-1) in Endotoxin-Induced Pneumonia Evaluated Using ICAM-1 Antisense Oligonucleotides, Anti-ICAM-1 Monoclonal Antibodies, and ICAM-1 Mutant Mice. J. Clin. Invest. 1996, 97, 2362–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K.; Rothlein R. Integrins, ICAMs, and Selectins: Role and Regulation of Adhesion Molecules in Neutrophil Recruitment to Inflammatory Sites. Adv. Pharmacol. 1994, 25, 117–169. [DOI] [PubMed] [Google Scholar]
- Albelda S. M. Endothelial and Epithelial Cell Adhesion Molecules. Am. J. Respir. Cell Mol. Biol. 1991, 4, 195–203. [DOI] [PubMed] [Google Scholar]
- Kelly K. A.; Allport J. R.; Tsourkas A.; Shinde-Patil V. R.; Josephson L.; Weissleder R. Detection of Vascular Adhesion Molecule-1 Expression Using a Novel Multimodal Nanoparticle. Circ. Res. 2005, 96, 327–336. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I.; Gimbrone M. A. Jr. Endothelial Expression of a Mononuclear Leukocyte Adhesion Molecule during Atherogenesis. Science 1991, 251, 788–791. [DOI] [PubMed] [Google Scholar]
- Taichman D. B.; Cybulsky M. I.; Djaffar I.; Longenecker B. M.; Teixido J.; Rice G. E.; Aruffo A.; Bevilacqua M. P. Tumor Cell Surface Alpha 4 Beta 1 Integrin Mediates Adhesion to Vascular Endothelium: Demonstration of an Interaction with the N-Terminal Domains of Incam-110/Vcam-1. Cell Regul. 1991, 2, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourkas A.; Shinde-Patil V. R.; Kelly K. A.; Patel P.; Wolley A.; Allport J. R.; Weissleder R. In Vivo Imaging of Activated Endothelium Using an Anti-Vcam-1 Magnetooptical Probe. Bioconjugate Chem. 2005, 16, 576–581. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W.; Raleigh M.; Kavanagh T.; Janssen H.; Calafat J.; Roos D.; Harlan J. M. Cytokine-Activated Endothelial Cells Internalize E-Selectin into a Lysosomal Compartment of Vesiculotubular Shape. A Tubulin-Driven Process. J. Immunol. 1994, 152, 5060–5069. [PubMed] [Google Scholar]
- Straley K. S.; Green S. A. Rapid Transport of Internalized P-Selectin to Late Endosomes and the Tgn: Roles in Regulating Cell Surface Expression and Recycling to Secretory Granules. J. Cell Biol. 2000, 151, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Asmuth E. J.; Smeets E. F.; Ginsel L. A.; Onderwater J. J.; Leeuwenberg J. F.; Buurman W. A. Evidence for Endocytosis of E-Selectin in Human Endothelial Cells. Eur. J. Immunol. 1992, 22, 2519–2526. [DOI] [PubMed] [Google Scholar]
- Kessner S.; Krause A.; Rothe U.; Bendas G. Investigation of the Cellular Uptake of E-Selectin-Targeted Immunoliposomes by Activated Human Endothelial Cells. Biochim. Biophys. Acta 2001, 1514, 177–190. [DOI] [PubMed] [Google Scholar]
- Kok R. J.; Everts M.; Asgeirsdottir S. A.; Meijer D. K.; Molema G. Cellular Handling of a Dexamethasone-Anti-E-Selectin Immunoconjugate by Activated Endothelial Cells: Comparison with Free Dexamethasone. Pharm. Res. 2002, 19, 1730–1735. [DOI] [PubMed] [Google Scholar]
- Harari O. A.; Wickham T. J.; Stocker C. J.; Kovesdi I.; Segal D. M.; Huehns T. Y.; Sarraf C.; Haskard D. O. Targeting an Adenoviral Gene Vector to Cytokine-Activated Vascular Endothelium via E-Selectin. Gene Ther. 1999, 6, 801–807. [DOI] [PubMed] [Google Scholar]
- Lindner J. R.; Song J.; Christiansen J.; Klibanov A. L.; Xu F.; Ley K. Ultrasound Assessment of Inflammation and Renal Tissue Injury with Microbubbles Targeted to P-Selectin. Circulation 2001, 104, 2107–2112. [DOI] [PubMed] [Google Scholar]
- Lindner J. R.; Klibanov A. L.; Ley K.. Targeting Inflammation. In Biomedical Aspects of Drug Targeting; Muzykantov V. R., Torchilin V. P., Eds.; Kluwer Academic Publishers: Boston, MA, 2003; pp 149–172. [Google Scholar]
- Carpen O.; Pallai P.; Staunton D. E.; Springer T. A. Association of Intercellular Adhesion Molecule-1 (ICAM-1) with Actin-Containing Cytoskeleton and Alpha-Actinin. J. Cell Biol. 1992, 118, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutiger C. J.; Heddini A.; Fernandez V.; Muller W. A.; Wahlgren M. PECAM-1/Cd31, an Endothelial Receptor for Binding Plasmodium Falciparum-Infected Erythrocytes. Nat. Med. 1997, 3, 1405–1408. [DOI] [PubMed] [Google Scholar]
- Romer L. H.; McLean N. V.; Yan H. C.; Daise M.; Sun J.; DeLisser H. M. Ifn-Gamma and Tnf-Alpha Induce Redistribution of PECAM-1 (Cd31) on Human Endothelial Cells. J. Immunol. 1995, 154, 6582–6592. [PubMed] [Google Scholar]
- Scalia R.; Lefer A. M. In Vivo Regulation of PECAM-1 Activity During Acute Endothelial Dysfunction in the Rat Mesenteric Microvasculature. J. Leukocyte Biol. 1998, 64, 163–169. [DOI] [PubMed] [Google Scholar]
- Muro S.; Cui X.; Gajewski C.; Murciano J. C.; Muzykantov V. R.; Koval M. Slow Intracellular Trafficking of Catalase Nanoparticles Targeted to ICAM-1 Protects Endothelial Cells from Oxidative Stress. Am. J. Physiol.: Cell Physiol. 2003, 285, C1339–1347. [DOI] [PubMed] [Google Scholar]
- Rothlein R.; Wegner C. Role of Intercellular Adhesion Molecule-1 in the Inflammatory Response. Kidney Int. 1992, 41, 617–619. [DOI] [PubMed] [Google Scholar]
- Hubbard A. K.; Rothlein R. Intercellular Adhesion Molecule-1 (ICAM-1) Expression and Cell Signaling Cascades. Free Radical Biol. Med. 2000, 28, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Cao M. Y.; Huber M.; Beauchemin N.; Famiglietti J.; Albelda S. M.; Veillette A. Regulation of Mouse PECAM-1 Tyrosine Phosphorylation by the Src and Csk Families of Protein-Tyrosine Kinases. J. Biol. Chem. 1998, 273, 15765–15772. [DOI] [PubMed] [Google Scholar]
- Cao G.; O’Brien C. D.; Zhou Z.; Sanders S. M.; Greenbaum J. N.; Makrigiannakis A.; DeLisser H. M. Involvement of Human PECAM-1 in Angiogenesis and in Vitro Endothelial Cell Migration. Am. J. Physiol.: Cell Physiol. 2002, 282, C1181–1190. [DOI] [PubMed] [Google Scholar]
- DeLisser H. M.; Christofidou-Solomidou M.; Strieter R. M.; Burdick M. D.; Robinson C. S.; Wexler R. S.; Kerr J. S.; Garlanda C.; Merwin J. R.; Madri J. A.; et al. M. Involvement of Endothelial PECAM-1/Cd31 in Angiogenesis. Am. J. Pathol. 1997, 151, 671–677. [PMC free article] [PubMed] [Google Scholar]
- DeLisser H. M.; Yan H. C.; Newman P. J.; Muller W. A.; Buck C. A.; Albelda S. M. Platelet/Endothelial Cell Adhesion Molecule-1 (Cd31)-Mediated Cellular Aggregation Involves Cell Surface Glycosaminoglycans. J. Biol. Chem. 1993, 268, 16037–16046. [PubMed] [Google Scholar]
- Diamond M. S.; Staunton D. E.; de Fougerolles A. R.; Stacker S. A.; Garcia-Aguilar J.; Hibbs M. L.; Springer T. A. ICAM-1 (Cd54): A Counter-Receptor for Mac-1 (Cd11b/Cd18). J. Cell Biol. 1990, 111, 3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun C. D.; Shimaoka M.; Carman C. V.; Takagi J.; Springer T. A. Dimerization and the Effectiveness of ICAM-1 in Mediating Lfa-1-Dependent Adhesion. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 6830–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M. T.; Amin K.; Christofidou-Solomidou M.; O’Brien C. D.; Sun J.; Gurubhagavatula I.; Heavner G. A.; Taylor A. H.; Paddock C.; Sun Q. H.; et al. Antibodies against the First Ig-Like Domain of Human Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) That Inhibit PECAM-1-Dependent Homophilic Adhesion Block in Vivo Neutrophil Recruitment. J. Immunol. 2000, 164, 452–462. [DOI] [PubMed] [Google Scholar]
- Murohara T.; Delyani J. A.; Albelda S. M.; Lefer A. M. Blockade of Platelet Endothelial Cell Adhesion Molecule-1 Protects against Myocardial Ischemia and Reperfusion Injury in Cats. J. Immunol. 1996, 156, 3550–3557. [PubMed] [Google Scholar]
- Muro S.; Gajewski C.; Koval M.; Muzykantov V. R. ICAM-1 Recycling in Endothelial Cells: A Novel Pathway for Sustained Intracellular Delivery and Prolonged Effects of Drugs. Blood 2005, 105, 650–658. [DOI] [PubMed] [Google Scholar]
- Panes J.; Perry M. A.; Anderson D. C.; Muzykantov V. R.; Carden D. L.; Miyasaka M.; Granger D. N. Portal Hypertension Enhances Endotoxin-Induced Intercellular Adhesion Molecule 1 Up-Regulation in the Rat. Gastroenterology 1996, 110, 866–874. [DOI] [PubMed] [Google Scholar]
- Murciano J. C.; Muro S.; Koniaris L.; Christofidou-Solomidou M.; Harshaw D. W.; Albelda S. M.; Granger D. N.; Cines D. B.; Muzykantov V. R. ICAM-Directed Vascular Immunotargeting of Antithrombotic Agents to the Endothelial Luminal Surface. Blood 2003, 101, 3977–3984. [DOI] [PubMed] [Google Scholar]
- Scherpereel A.; Wiewrodt R.; Christofidou-Solomidou M.; Gervais R.; Murciano J. C.; Albelda S. M.; Muzykantov V. R. Cell-Selective Intracellular Delivery of a Foreign Enzyme to Endothelium in Vivo Using Vascular Immunotargeting. FASEB J. 2001, 15, 416–426. [DOI] [PubMed] [Google Scholar]
- Muro S.; Dziubla T.; Qiu W.; Leferovich J.; Cui X.; Berk E.; Muzykantov V. R. Endothelial Targeting of High-Affinity Multivalent Polymer Nanocarriers Directed to Intercellular Adhesion Molecule 1. J. Pharmacol. Exp. Ther. 2006, 317, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Garnacho C.; Dhami R.; Simone E.; Dziubla T.; Leferovich J.; Schuchman E. H.; Muzykantov V.; Muro S. Delivery of Acid Sphingomyelinase in Normal and Niemann-Pick Disease Mice Using Intercellular Adhesion Molecule-1-Targeted Polymer Nanocarriers. J. Pharmacol. Exp. Ther. 2008, 325, 400–408. [DOI] [PubMed] [Google Scholar]
- Hua S.; Chang H. I.; Davies N. M.; Cabot P. J. Targeting of ICAM-1-Directed Immunoliposomes Specifically to Activated Endothelial Cells with Low Cellular Uptake: Use of an Optimized Procedure for the Coupling of Low Concentrations of Antibody to Liposomes. J. Liposome Res. 2011, 21, 95–105. [DOI] [PubMed] [Google Scholar]
- Ding B. S.; Gottstein C.; Grunow A.; Kuo A.; Ganguly K.; Albelda S. M.; Cines D. B.; Muzykantov V. R. Endothelial Targeting of a Recombinant Construct Fusing a PECAM-1 Single-Chain Variable Antibody Fragment (Scfv) with Prourokinase Facilitates Prophylactic Thrombolysis in the Pulmonary Vasculature. Blood 2005, 106, 4191–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizaire A. K.; Tchistiakova L.; St-Pierre Y.; Alakhov V. Identification of a Murine ICAM-1-Specific Peptide by Subtractive Phage Library Selection on Cells. Biochem. Biophys. Res. Commun. 2003, 309, 625–630. [DOI] [PubMed] [Google Scholar]
- Luo G. X.; Kohlstaedt L. A.; Charles C. H.; Gorfain E.; Morantte I.; Williams J. H.; Fang F. Humanization of an Anti-ICAM-1 Antibody with over 50-fold Affinity and Functional Improvement. J. Immunol. Methods 2003, 275, 31–40. [DOI] [PubMed] [Google Scholar]
- Charles C. H.; Luo G. X.; Kohlstaedt L. A.; Morantte I. G.; Gorfain E.; Cao L.; Williams J. H.; Fang F. Prevention of Human Rhinovirus Infection by Multivalent Fab Molecules Directed against ICAM-1. Antimicrob. Agents Chemother. 2003, 47, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K.; Takeda H.; Azhar S.; McCarron R. M.; Chen Y.; Ruetzler C. A.; Wolcott K. M.; DeGraba T. J.; Rothlein R.; Hugli T. E.; et al. Examination of Several Potential Mechanisms for the Negative Outcome in a Clinical Stroke Trial of Enlimomab, a Murine Anti-Human Intercellular Adhesion Molecule-1 Antibody: A Bedside-to-Bench Study. Stroke 2001, 32, 2665–2674. [DOI] [PubMed] [Google Scholar]
- Rothlein R.; Mainolfi E. A.; Kishimoto T. K. Treatment of Inflammation with Anti-ICAM-1. Res. Immunol. 1993, 144, 735–739. [DOI] [PubMed] [Google Scholar]; discussion754 - 762.
- Garnacho C.; Serrano D.; Muro S. A Fibrinogen-Derived Peptide Provides Intercellular Adhesion Molecule-1-Specific Targeting and Intraendothelial Transport of Polymer Nanocarriers in Human Cell Cultures and Mice. J. Pharmacol. Exp. Ther. 2012, 340, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Weller G. E.; Zern B.; Ayyaswamy P. S.; Eckmann D. M.; Muzykantov V. R.; Radhakrishnan R. Computational Model for Nanocarrier Binding to Endothelium Validated Using in Vivo, in Vitro, and Atomic Force Microscopy Experiments. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 16530–16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenphol P.; Huang R. B.; Eniola-Adefeso O. Potential Role of Size and Hemodynamics in the Efficacy of Vascular-Targeted Spherical Drug Carriers. Biomaterials 2010, 31, 1392–1402. [DOI] [PubMed] [Google Scholar]
- Blackwell J. E.; Dagia N. M.; Dickerson J. B.; Berg E. L.; Goetz D. J. Ligand Coated Nanosphere Adhesion to E- and P-Selectin under Static and Flow Conditions. Ann. Biomed. Eng. 2001, 29, 523–533. [DOI] [PubMed] [Google Scholar]
- Swaminathan T. N.; Liu J.; Balakrishnan U.; Ayyaswamy P. S.; Radhakrishnan R.; Eckmann D. M. Dynamic Factors Controlling Carrier Anchoring on Vascular Cells. IUBMB Life 2011, 63, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omolola Eniola A.; Hammer D. A. In Vitro Characterization of Leukocyte Mimetic for Targeting Therapeutics to the Endothelium Using Two Receptors. Biomaterials 2005, 26, 7136–7144. [DOI] [PubMed] [Google Scholar]
- Charoenphol P.; Onyskiw P. J.; Carrasco-Teja M.; Eniola-Adefeso O. Particle-Cell Dynamics in Human Blood Flow: Implications for Vascular-Targeted Drug Delivery. J. Biomechanics 2012, 45, 2822–2828. [DOI] [PubMed] [Google Scholar]
- Namdee K.; Thompson A. J.; Charoenphol P.; Eniola-Adefeso O. Margination Propensity of Vascular-Targeted Spheres from Blood Flow in a Microfluidic Model of Human Microvessels. Langmuir 2013, 29, 2530–2535. [DOI] [PubMed] [Google Scholar]
- Kiani M. F.; Yuan H.; Chen X.; Smith L.; Gaber M. W.; Goetz D. J. Targeting Microparticles to Select Tissue via Radiation-Induced Upregulation of Endothelial Cell Adhesion Molecules. Pharm. Res. 2002, 19, 1317–1322. [DOI] [PubMed] [Google Scholar]
- Sakhalkar H. S.; Hanes J.; Fu J.; Benavides U.; Malgor R.; Borruso C. L.; Kohn L. D.; Kurjiaka D. T.; Goetz D. J. Enhanced Adhesion of Ligand-Conjugated Biodegradable Particles to Colitic Venules. FASEB J. 2005, 19, 792–794. [DOI] [PubMed] [Google Scholar]
- Sakhalkar H. S.; Dalal M. K.; Salem A. K.; Ansari R.; Fu J.; Kiani M. F.; Kurjiaka D. T.; Hanes J.; Shakesheff K. M.; Goetz D. J. Leukocyte-Inspired Biodegradable Particles That Selectively and Avidly Adhere to Inflamed Endothelium in Vitro and in Vivo. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 15895–15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.; Sabnis A.; Kona S.; Nattama S.; Patel H.; Dong J.-F.; Nguyen K. T. Shear-Regulated Uptake of Nanoparticles by Endothelial Cells and Development of Endothelial-Targeting Nanoparticles. J. Biomed. Mater. Res., Part A 2010, 93A, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuzzi P.; Lee S.; Bhushan B.; Ferrari M. A Theoretical Model for the Margination of Particles within Blood Vessels. Ann. Biomed. Eng. 2005, 33, 179–190. [DOI] [PubMed] [Google Scholar]
- Gentile F.; Curcio A.; Indolfi C.; Ferrari M.; Decuzzi P. The Margination Propensity of Spherical Particles for Vascular Targeting in the Microcirculation. J. Nanobiotechnol. 2008, 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins C.; Duling B. R. Heparinase Treatment Suggests a Role for the Endothelial Cell Glycocalyx in Regulation of Capillary Hematocrit. Am. J. Physiol. 1990, 258, H647–654. [DOI] [PubMed] [Google Scholar]
- Samokhin G. P.; Smirnov M. D.; Muzykantov V. R.; Domogatsky S. P.; Smirnov V. N. Red Blood Cell Targeting to Collagen-Coated Surfaces. FEBS Lett. 1983, 154, 257–261. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Johnson P. C.; Popel A. S. Effects of Erythrocyte Deformability and Aggregation on the Cell Free Layer and Apparent Viscosity of Microscopic Blood Flows. Microvasc. Res. 2009, 77, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.; Thomas A.; Liu Y. Influence of Red Blood Cells on Nanoparticle Targeted Delivery in Microcirculation. Soft Matter 2011, 8, 1934–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastina A.; Valadon P.; Massey K. A.; Schnitzer J. E. Lung Vascular Targeting Using Antibody to Aminopeptidase P: Ct-Spect Imaging, Biodistribution and Pharmacokinetic Analysis. J. Vasc. Res. 2010, 47, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhar P.; Anselmo A. C.; Gupta V.; Pant K.; Prabhakarpandian B.; Ruoslahti E.; Mitragotri S. Using Shape Effects to Target Antibody-Coated Nanoparticles to Lung and Brain Endothelium. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakarpandian B.; Pant K.; Scott R. C.; Patillo C. B.; Irimia D.; Kiani M. F.; Sundaram S. Synthetic Microvascular Networks for Quantitative Analysis of Particle Adhesion. Biomed. Microdevices 2008, 10, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuzzi P.; Ferrari M. The Adhesive Strength of Non-Spherical Particles Mediated by Specific Interactions. Biomaterials 2006, 27, 5307–5314. [DOI] [PubMed] [Google Scholar]
- Decuzzi P.; Ferrari M. The Role of Specific and Non-Specific Interactions in Receptor-Mediated Endocytosis of Nanoparticles. Biomaterials 2007, 28, 2915–2922. [DOI] [PubMed] [Google Scholar]
- Doshi N.; Prabhakarpandian B.; Rea-Ramsey A.; Pant K.; Sundaram S.; Mitragotri S. Flow and Adhesion of Drug Carriers in Blood Vessels Depend on Their Shape: A Study Using Model Synthetic Microvascular Networks. J. Controlled Release 2010, 146, 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano J. M.; Tousi N.; Scott R. C.; Krynska B.; Rizzo V.; Prabhakarpandian B.; Pant K.; Sundaram S.; Kiani M. F. A Physiologically Realistic in Vitro Model of Microvascular Networks. Biomed. Microdevices 2009, 11, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.; Liu Y.; Hu W.; Gao J. Modeling Particle Shape-Dependent Dynamics in Nanomedicine. J. Nanosci. Nanotechnol. 2011, 11, 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile F.; Chiappini C.; Fine D.; Bhavane R. C.; Peluccio M. S.; Cheng M. M.; Liu X.; Ferrari M.; Decuzzi P. The Effect of Shape on the Margination Dynamics of Non-Neutrally Buoyant Particles in Two-Dimensional Shear Flows. J. Biomechanics 2008, 41, 2312–2318. [DOI] [PubMed] [Google Scholar]
- Gunawan R. C.; Auguste D. T. The Role of Antibody Synergy and Membrane Fluidity in the Vascular Targeting of Immunoliposomes. Biomaterials 2010, 31, 900–907. [DOI] [PubMed] [Google Scholar]
- Doshi N.; Orje J. N.; Molins B.; Smith J. W.; Mitragotri S.; Ruggeri Z. M. Platelet Mimetic Particles for Targeting Thrombi in Flowing Blood. Adv. Mater. 2012, 24, 3864–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo K. A.; Wang Y.-l. Fc-Receptor-Mediated Phagocytosis Is Regulated by Mechanical Properties of the Target. J. Cell Sci. 2002, 115, 849–856. [DOI] [PubMed] [Google Scholar]
- Trubetskoy V. S.; Narula J.; Khaw B. A.; Torchilin V. P. Chemically Optimized Antimyosin Fab Conjugates with Chelating Polymers: Importance of the Nature of the Protein-Polymer Single Site Covalent Bond for Biodistribution and Infarction Localization. Bioconjugate Chem. 1993, 4, 251–255. [DOI] [PubMed] [Google Scholar]
- Muro S. New Biotechnological and Nanomedicine Strategies for Treatment of Lysosomal Storage Disorders. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2010, 2, 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon A. J.; Bhowmick T.; Leferovich J.; Burman B.; Pichette B.; Muzykantov V.; Eckmann D. M.; Muro S. Optimizing Endothelial Targeting by Modulating the Antibody Density and Particle Concentration of Anti-ICAM Coated Carriers. J. Controlled Release 2011, 150, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams G. P.; Tai M. S.; McCartney J. E.; Marks J. D.; Stafford W. F. III; Houston L. L.; Huston J. S.; Weiner L. M. Avidity-Mediated Enhancement of in Vivo Tumor Targeting by Single-Chain Fv Dimers. Clin. Cancer Res. 2006, 12, 1599–1605. [DOI] [PubMed] [Google Scholar]
- Cheng Z.; Al Zaki A.; Hui J. Z.; Muzykantov V. R.; Tsourkas A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine D. J.; Swartz M. A.; Szeto G. L. Engineering Synthetic Vaccines Using Cues from Natural Immunity. Nat. Mater. 2013, 12, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Moynihan K. D.; Zheng Y.; Szeto G. L.; Li A. V.; Huang B.; Van Egeren D. S.; Park C.; Irvine D. J. Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature 2014, 507, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe S. M.; Fahmy T. M. Targeted Nanotherapy for Induction of Therapeutic Immune Responses. Trends Mol. Med. 2012, 18, 72–80. [DOI] [PubMed] [Google Scholar]
- Adams G. P.; Schier R.; McCall A. M.; Simmons H. H.; Horak E. M.; Alpaugh R. K.; Marks J. D.; Weiner L. M. High Affinity Restricts the Localization and Tumor Penetration of Single-Chain Fv Antibody Molecules. Cancer Res. 2001, 61, 4750–4755. [PubMed] [Google Scholar]
- Sakharov D. V.; Rijken D. C. Superficial Accumulation of Plasminogen During Plasma Clot Lysis. Circulation 1995, 92, 1883–1890. [DOI] [PubMed] [Google Scholar]
- Elias D. R.; Poloukhtine A.; Popik V.; Tsourkas A. Effect of Ligand Density, Receptor Density, and Nanoparticle Size on Cell Targeting. Nanomedicine 2013, 9, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhari A.; Baoum A.; Siahaan T. J.; Le K. B.; Berkland C. Controlling Ligand Surface Density Optimizes Nanoparticle Binding to ICAM-1. J. Pharm. Sci. 2011, 100, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F.; Zhang L.; Teply B. A.; Mann N.; Wang A.; Radovic-Moreno A. F.; Langer R.; Farokhzad O. C. Precise Engineering of Targeted Nanoparticles by Using Self-Assembled Biointegrated Block Copolymers. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 2586–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M.; Friederich P. W.; Middleton S.; de Groot P. G.; Wu Y. P.; Harris R.; Biemond B. J.; Heijnen H. F.; Levin J.; ten Cate J. W. Fibrinogen-Coated Albumin Microcapsules Reduce Bleeding in Severely Thrombocytopenic Rabbits. Nat. Med. 1999, 5, 107–111. [DOI] [PubMed] [Google Scholar]
- Bertram J. P.; Williams C. A.; Robinson R.; Segal S. S.; Flynn N. T.; Lavik E. B. Intravenous Hemostat: Nanotechnology to Halt Bleeding. Sci. Transl. Med. 2009, 1, 11ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modery-Pawlowski C. L.; Tian L. L.; Ravikumar M.; Wong T. L.; Sen Gupta A. In Vitro and in Vivo Hemostatic Capabilities of a Functionally Integrated Platelet-Mimetic Liposomal Nanoconstruct. Biomaterials 2013, 34, 3031–3041. [DOI] [PubMed] [Google Scholar]
- Modery-Pawlowski C. L.; Tian L. L.; Pan V.; McCrae K. R.; Mitragotri S.; Sen Gupta A. Approaches to Synthetic Platelet Analogs. Biomaterials 2012, 34, 526–541. [DOI] [PubMed] [Google Scholar]
- Lashof-Sullivan M.; Shoffstall A.; Lavik E. Intravenous Hemostats: Challenges in Translation to Patients. Nanoscale 2013, 5, 10719–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffstall A. J.; Everhart L. M.; Varley M. E.; Soehnlen E. S.; Shick A. M.; Ustin J. S.; Lavik E. B. Tuning Ligand Density on Intravenous Hemostatic Nanoparticles Dramatically Increases Survival Following Blunt Trauma. Biomacromolecules 2013, 14, 2790–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.; Nakao S.; Xie F.; Zandi S.; Schering A.; Hafezi-Moghadam A. Superior Sensitivity of Novel Molecular Imaging Probe: Simultaneously Targeting Two Types of Endothelial Injury Markers. FASEB J. 2010, 24, 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eniola A. O.; Willcox P. J.; Hammer D. A. Interplay between Rolling and Firm Adhesion Elucidated with a Cell-Free System Engineered with Two Distinct Receptor-Ligand Pairs. Biophys. J. 2003, 85, 2720–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan R. C.; Auguste D. T. Immunoliposomes That Target Endothelium in Vitro Are Dependent on Lipid Raft Formation. Mol. Pharmaceutics 2010, 7, 1569–1575. [DOI] [PubMed] [Google Scholar]
- Gunawan R. C.; Almeda D.; Auguste D. T. Complementary Targeting of Liposomes to Il-1alpha and Tnf-Alpha Activated Endothelial Cells via the Transient Expression of Vcam1 and E-Selectin. Biomaterials 2011, 32, 9848–9853. [DOI] [PubMed] [Google Scholar]
- Papademetriou I. T.; Garnacho C.; Schuchman E. H.; Muro S. In Vivo Performance of Polymer Nanocarriers Dually-Targeted to Epitopes of the Same or Different Receptors. Biomaterials 2013, 34, 3459–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer M. A.; Schneider J. E.; Ali Z. A.; Warrick N.; Bursill C. A.; von zur Muhlen C.; Greaves D. R.; Neubauer S.; Channon K. M.; Choudhury R. P. Magnetic Resonance Imaging of Endothelial Adhesion Molecules in Mouse Atherosclerosis Using Dual-Targeted Microparticles of Iron Oxide. Arterioscler., Thromb., Vasc. Biol. 2008, 28, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller G. E.; Villanueva F. S.; Tom E. M.; Wagner W. R. Targeted Ultrasound Contrast Agents: In Vitro Assessment of Endothelial Dysfunction and Multi-Targeting to ICAM-1 and Sialyl Lewisx. Biotechnol. Bioeng. 2005, 92, 780–788. [DOI] [PubMed] [Google Scholar]
- Danilov S.; Jaspard E.; Churakova T.; Towbin H.; Savoie F.; Wei L.; Alhenc-Gelas F. Structure-Function Analysis of Angiotensin I-Converting Enzyme Using Monoclonal Antibodies. Selective Inhibition of the Amino-Terminal Active Site. J. Biol. Chem. 1994, 269, 26806–26814. [PubMed] [Google Scholar]
- Balyasnikova I. V.; Karran E. H.; Albrecht R. F. II; Danilov S. M. Epitope-Specific Antibody-Induced Cleavage of Angiotensin-Converting Enzyme from the Cell Surface. Biochem. J. 2002, 362, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K.; Balyasnikova I. V.; Nesterovitch A. B.; Schwartz D. E.; Sturrock E. D.; Danilov S. M. Fine Epitope Mapping of Monoclonal Antibodies 9b9 and 3g8 to the N Domain of Angiotensin-Converting Enzyme (Cd143) Defines a Region Involved in Regulating Angiotensin-Converting Enzyme Dimerization and Shedding. Tissue Antigens 2010, 75, 136–150. [DOI] [PubMed] [Google Scholar]
- Chacko A. M.; Nayak M.; Greineder C. F.; Delisser H. M.; Muzykantov V. R. Collaborative Enhancement of Antibody Binding to Distinct PECAM-1 Epitopes Modulates Endothelial Targeting. PLoS One 2012, 7, e34958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Y.; Wang S. M.; Li N.; Hu Y.; Zhang Y.; Xu J. F.; Li X.; Ren J.; Su B.; Yuan W. Z.; Teng X. R.; Zhang R. X.; Jiang D. H.; Mulet X.; Li H. P. Creation of Lung-Targeted Dexamethasone Immunoliposome and Its Therapeutic Effect on Bleomycin-Induced Lung Injury in Rats. PLoS One 2013, 8, e58275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird W. C.; Kwaan H. C. Under-Recognized Significance of Endothelial Heterogeneity: Hemostasis, Thrombosis, and Beyond. Semin. Thromb. Hemostasis 2010, 36, 225–226. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E. Caveolae: From Basic Trafficking Mechanisms to Targeting Transcytosis for Tissue-Specific Drug and Gene Delivery in Vivo. Adv. Drug Delivery Rev. 2001, 49, 265–280. [DOI] [PubMed] [Google Scholar]
- Balyasnikova I. V.; Danilov S. M.; Muzykantov V. R.; Fisher A. B. Modulation of Angiotensin-Converting Enzyme in Cultured Human Vascular Endothelial Cells. In Vitro Cell. Dev. Biol.: Anim. 1998, 34, 545–554. [DOI] [PubMed] [Google Scholar]
- Biswas S.; Torchilin V. P. Nanopreparations for Organelle-Specific Delivery in Cancer. Adv. Drug Delivery Rev. 2013, 66, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R.; Roy K. Intracellular Delivery of Polymeric Nanocarriers: A Matter of Size, Shape, Charge, Elasticity and Surface Composition. Ther. Delivery 2013, 4, 705–723. [DOI] [PubMed] [Google Scholar]
- Predescu S. A.; Predescu D. N.; Malik A. B. Molecular Determinants of Endothelial Transcytosis and Their Role in Endothelial Permeability. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2007, 293, L823–842. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Ghosh R. N.; Maxfield F. R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [DOI] [PubMed] [Google Scholar]
- Caron E.; Hall A.. Phagocytosis; Oxford University Press: Oxford, U.K., 2001; p 58–77. [Google Scholar]
- Popova N. V.; Deyev I. E.; Petrenko A. G. Clathrin-Mediated Endocytosis and Adaptor Proteins. Acta Nat. 2013, 5, 62–73. [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and Molecular Sorting. Annu. Rev. Cell Dev. Biol. 1996, 12, 575–625. [DOI] [PubMed] [Google Scholar]
- Minshall R. D.; Tiruppathi C.; Vogel S. M.; Niles W. D.; Gilchrist A.; Hamm H. E.; Malik A. B. Endothelial Cell-Surface Gp60 Activates Vesicle Formation and Trafficking via G(I)-Coupled Src Kinase Signaling Pathway. J. Cell Biol. 2000, 150, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D.; Predescu S.; Malik A. B. Transport of Nitrated Albumin across Continuous Vascular Endothelium. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 13932–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M.; Preiter K.; Adles C.; Stahl P. D.; Steinberg T. H. Size of Igg-Opsonized Particles Determines Macrophage Response During Internalization. Exp. Cell Res. 1998, 242, 265–273. [DOI] [PubMed] [Google Scholar]
- Wiewrodt R.; Thomas A. P.; Cipelletti L.; Christofidou-Solomidou M.; Weitz D. A.; Feinstein S. I.; Schaffer D.; Albelda S. M.; Koval M.; Muzykantov V. R. Size-Dependent Intracellular Immunotargeting of Therapeutic Cargoes into Endothelial Cells. Blood 2002, 99, 912–922. [DOI] [PubMed] [Google Scholar]
- Everts M.; Kok R. J.; Asgeirsdottir S. A.; Melgert B. N.; Moolenaar T. J.; Koning G. A.; van Luyn M. J.; Meijer D. K.; Molema G. Selective Intracellular Delivery of Dexamethasone into Activated Endothelial Cells Using an E-Selectin-Directed Immunoconjugate. J. Immunol. 2002, 168, 883–889. [DOI] [PubMed] [Google Scholar]
- Ricard I.; Payet M. D.; Dupuis G. Vcam-1 Is Internalized by a Clathrin-Related Pathway in Human Endothelial Cells but Its Alpha 4 Beta 1 Integrin Counter-Receptor Remains Associated with the Plasma Membrane in Human T Lymphocytes. Eur. J. Immunol. 1998, 28, 1708–1718. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Blood-Brain Barrier Drug Targeting: The Future of Brain Drug Development. Mol. Interventions 2003, 3, 90–105. [DOI] [PubMed] [Google Scholar]
- Tagliabue E.; Centis F.; Campiglio M.; Mastroianni A.; Martignone S.; Pellegrini R.; Casalini P.; Lanzi C.; Menard S.; Colnaghi M. I. Selection of Monoclonal Antibodies Which Induce Internalization and Phosphorylation of P185her2 and Growth Inhibition of Cells with Her2/Neu Gene Amplification. Int. J. Cancer 1991, 47, 933–937. [DOI] [PubMed] [Google Scholar]
- Feero W. G.; Li S.; Rosenblatt J. D.; Sirianni N.; Morgan J. E.; Partridge T. A.; Huang L.; Hoffman E. P. Selection and Use of Ligands for Receptor-Mediated Gene Delivery to Myogenic Cells. Gene Ther. 1997, 4, 664–674. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Zhao L.; Marks J. D. Selection and Characterization of Cell Binding and Internalizing Phage Antibodies. Arch. Biochem. Biophys. 2012, 526, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Zou H.; Zhang S.; Marks J. D. Internalizing Cancer Antibodies from Phage Libraries Selected on Tumor Cells and Yeast-Displayed Tumor Antigens. J. Mol. Biol. 2010, 404, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitner T.; Moor A.; Garrison J. L.; Marks C.; Hasan T.; Marks J. D. Selection of Cell Binding and Internalizing Epidermal Growth Factor Receptor Antibodies from a Phage Display Library. J. Immunol. Methods 2001, 248, 17–30. [DOI] [PubMed] [Google Scholar]
- Anderson D. C.; Manger R.; Schroeder J.; Woodle D.; Barry M.; Morgan A. C.; Fritzberg A. R. Enhanced in Vitro Tumor Cell Retention and Internalization of Antibody Derivatized with Synthetic Peptides. Bioconjugate Chem. 1993, 4, 10–18. [DOI] [PubMed] [Google Scholar]
- Couch J. A.; Yu Y. J.; Zhang Y.; Tarrant J. M.; Fuji R. N.; Meilandt W. J.; Solanoy H.; Tong R. K.; Hoyte K.; Luk W.; et al. Addressing Safety Liabilities of Tfr Bispecific Antibodies That Cross the Blood-Brain Barrier. Sci. Transl. Med. 2013, 5, 183ra57. [DOI] [PubMed] [Google Scholar]
- Ilan N.; Mohsenin A.; Cheung L.; Madri J. A. PECAM-1 Shedding During Apoptosis Generates a Membrane-Anchored Truncated Molecule with Unique Signaling Characteristics. FASEB J. 2001, 15, 362–372. [DOI] [PubMed] [Google Scholar]
- Muro S.; Wiewrodt R.; Thomas A.; Koniaris L.; Albelda S. M.; Muzykantov V. R.; Koval M. A Novel Endocytic Pathway Induced by Clustering Endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003, 116, 1599–1609. [DOI] [PubMed] [Google Scholar]
- Muro S.; Muzykantov V. R.; Murciano J. C. Characterization of Endothelial Internalization and Targeting of Antibody-Enzyme Conjugates in Cell Cultures and in Laboratory Animals. Methods Mol. Biol. 2004, 283, 21–36. [DOI] [PubMed] [Google Scholar]
- Ding B. S.; Hong N.; Christofidou-Solomidou M.; Gottstein C.; Albelda S. M.; Cines D. B.; Fisher A. B.; Muzykantov V. R. Anchoring Fusion Thrombomodulin to the Endothelial Lumen Protects against Injury-Induced Lung Thrombosis and Inflammation. Am. J. Respir. Crit. Care Med. 2009, 180, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L.; Helenius A. Endocytosis via Caveolae. Traffic 2002, 3, 311–320. [DOI] [PubMed] [Google Scholar]
- Kolhar P.; Mitragotri S. Polymer Microparticles Exhibit Size and Shape Dependent Accumulation around the Nucleus after Endocytosis. Adv. Funct. Mater. 2012, 22, 3759–3764. [Google Scholar]
- Kolhar P.; Doshi N.; Mitragotri S. Polymer Nanoneedle-Mediated Intracellular Drug Delivery. Small 2011, 7, 2094–2100. [DOI] [PubMed] [Google Scholar]
- Gratton S. E.; Ropp P. A.; Pohlhaus P. D.; Luft J. C.; Madden V. J.; Napier M. E.; DeSimone J. M. The Effect of Particle Design on Cellular Internalization Pathways. Proc. Natl. Acad.Sci. U.S.A. 2008, 105, 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick T.; Berk E.; Cui X.; Muzykantov V. R.; Muro S. Effect of Flow on Endothelial Endocytosis of Nanocarriers Targeted to ICAM-1. J. Controlled Release 2012, 157, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S. P.; Jain N.; O’Dowd F.; Paul T.; Kashanin D.; Gerard V. A.; Gun’ko Y. K.; Prina-Mello A.; Volkov Y. Multifactorial Determinants That Govern Nanoparticle Uptake by Human Endothelial Cells under Flow. Int. J. Nanomed. 2012, 7, 2943–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrisse A. D.; Puech N.; Allart S.; Gourdy P.; Xuereb J. M.; Payrastre B.; Sie P. Internalization of Microparticles by Endothelial Cells Promotes Platelet/Endothelial Cell Interaction under Flow. J. Thromb. Haemost. 2010, 8, 2810–2819. [DOI] [PubMed] [Google Scholar]
- Han J.; Zern B. J.; Shuvaev V. V.; Davies P. F.; Muro S.; Muzykantov V. Acute and Chronic Shear Stress Differently Regulate Endothelial Internalization of Nanocarriers Targeted to Platelet-Endothelial Cell Adhesion Molecule-1. ACS Nano 2012, 6, 8824–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano D.; Bhowmick T.; Chadha R.; Garnacho C.; Muro S. Intercellular Adhesion Molecule 1 Engagement Modulates Sphingomyelinase and Ceramide, Supporting Uptake of Drug Carriers by the Vascular Endothelium. Arterioscler., Thromb., Vasc. Biol. 2012, 32, 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar M.; Serrano D.; Papademetriou I.; Bhowmick T. K.; Muro S. Biological Functionalization of Drug Delivery Carriers to Bypass Size Restrictions of Receptor-Mediated Endocytosis Independently from Receptor Targeting. ACS Nano 2013, 7, 10597–10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Schlachetzki F.; Pardridge W. M. Global Non-Viral Gene Transfer to the Primate Brain Following Intravenous Administration. Mol. Ther. 2003, 7, 11–18. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E.; Oh P.; McIntosh D. P. Role of Gtp Hydrolysis in Fission of Caveolae Directly from Plasma Membranes. Science 1996, 274, 239–242. [DOI] [PubMed] [Google Scholar]
- Vogel S. M.; Easington C. R.; Minshall R. D.; Niles W. D.; Tiruppathi C.; Hollenberg S. M.; Parrillo J. E.; Malik A. B. Evidence of Transcellular Permeability Pathway in Microvessels. Microvasc. Res. 2001, 61, 87–101. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M.; Feng D. The Vesiculo-Vacuolar Organelle (Vvo). A New Endothelial Cell Permeability Organelle. J. Histochem. Cytochem. 2001, 49, 419–432. [DOI] [PubMed] [Google Scholar]
- Iversen T. G.; Frerker N.; Sandvig K. Uptake of Ricinb-Quantum Dot Nanoparticles by a Macropinocytosis-Like Mechanism. J. Nanobiotechnol. 2012, 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Tiruppathi C.; Minshall R. D.; Malik A. B. Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells. ACS Nano 2009, 3, 4110–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E.; Liu J.; Oh P. Endothelial Caveolae Have the Molecular Transport Machinery for Vesicle Budding, Docking, and Fusion Including Vamp, Nsf, Snap, Annexins, and Gtpases. J. Biol. Chem. 1995, 270, 14399–14404. [DOI] [PubMed] [Google Scholar]
- Mehta D.; Bhattacharya J.; Matthay M. A.; Malik A. B. Integrated Control of Lung Fluid Balance. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2004, 287, L1081–1090. [DOI] [PubMed] [Google Scholar]
- Ghaffarian R.; Bhowmick T.; Muro S. Transport of Nanocarriers across Gastrointestinal Epithelial Cells by a New Transcellular Route Induced by Targeting ICAM-1. J. Controlled Release 2012, 163, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mane V.; Muro S. Biodistribution and Endocytosis of ICAM-1-Targeting Antibodies versus Nanocarriers in the Gastrointestinal Tract in Mice. Int. J. Nanomed. 2012, 7, 4223–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer A.; Bukowski M.; Veith M.; Lehr C. M.; Daum N. Propidium Iodide Labeling of Nanoparticles as a Novel Tool for the Quantification of Cellular Binding and Uptake. Nanomedicine 2011, 7, 410–419. [DOI] [PubMed] [Google Scholar]
- Shuvaev V. V.; Dziubla T.; Wiewrodt R.; Muzykantov V. R. Streptavidin-Biotin Crosslinking of Therapeutic Enzymes with Carrier Antibodies: Nanoconjugates for Protection against Endothelial Oxidative Stress. Methods Mol. Biol. 2004, 283, 3–19. [DOI] [PubMed] [Google Scholar]
- Garnacho C.; Shuvaev V.; Thomas A.; McKenna L.; Sun J.; Koval M.; Albelda S.; Muzykantov V.; Muro S. Rhoa Activation and Actin Reorganization Involved in Endothelial Cam-Mediated Endocytosis of Anti-PECAM Carriers: Critical Role for Tyrosine 686 in the Cytoplasmic Tail of PECAM-1. Blood 2008, 111, 3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. A.; Aberg C.; Salvati A.; Dawson K. A. Role of Cell Cycle on the Cellular Uptake and Dilution of Nanoparticles in a Cell Population. Nat. Nanotechnol. 2012, 7, 62–68. [DOI] [PubMed] [Google Scholar]
- Georgieva J. V.; Kalicharan D.; Couraud P. O.; Romero I. A.; Weksler B.; Hoekstra D.; Zuhorn I. S. Surface Characteristics of Nanoparticles Determine Their Intracellular Fate in and Processing by Human Blood-Brain Barrier Endothelial Cells in Vitro. Mol. Ther. 2011, 19, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastl L.; Sasse D.; Wulf V.; Hartmann R.; Mircheski J.; Ranke C.; Carregal-Romero S.; Martinez-Lopez J. A.; Fernandez-Chacon R.; Parak W. J.; et al. Multiple Internalization Pathways of Polyelectrolyte Multilayer Capsules into Mammalian Cells. ACS Nano 2013, 7, 6605–6618. [DOI] [PubMed] [Google Scholar]
- Vasdekis A. E.; Scott E. A.; O’Neil C. P.; Psaltis D.; Hubbell J. A. Precision Intracellular Delivery Based on Optofluidic Polymersome Rupture. ACS Nano 2012, 6, 7850–7857. [DOI] [PubMed] [Google Scholar]
- Kardara M.; Hatziantoniou S.; Sfika A.; Vassiliou A. G.; Mourelatou E.; Muagkou C.; Armaganidis A.; Roussos C.; Orfanos S. E.; Kotanidou A.; et al. Caveolar Uptake and Endothelial-Protective Effects of Nanostructured Lipid Carriers in Acid Aspiration Murine Acute Lung Injury. Pharm. Res. 2013, 30, 1836–1847. [DOI] [PubMed] [Google Scholar]
- Rejman J.; Oberle V.; Zuhorn I. S.; Hoekstra D. Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T.; Sugiyama A.; Wu S. Q.; Abid R.; Kodama T.; Aird W. C. Thrombin and Phenotypic Modulation of the Endothelium. Arterioscler., Thromb., Vasc. Biol. 2004, 24, 41–53. [DOI] [PubMed] [Google Scholar]
- Comhair S. A.; Xu W.; Mavrakis L.; Aldred M. A.; Asosingh K.; Erzurum S. C. Human Primary Lung Endothelial Cells in Culture. Am. J. Respir. Cell Mol. Biol. 2012, 46, 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S.; Zensi A.; Wien S. L.; Tschickardt S. E.; Maier W.; Vogel T.; Worek F.; Pietrzik C. U.; Kreuter J.; von Briesen H. Uptake Mechanism of Apoe-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood-Brain Barrier Model. PLoS One 2012, 7, e32568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Zhao F.; Li Y.; Xu M.; Li L.; Wu C.; Miyoshi H.; Liu Y. Vcam-1-Targeted Core/Shell Nanoparticles for Selective Adhesion and Delivery to Endothelial Cells with Lipopolysaccharide-Induced Inflammation under Shear Flow and Cellular Magnetic Resonance Imaging in Vitro. Int. J. Nanomed. 2013, 8, 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F. Hemodynamic Shear Stress and the Endothelium in Cardiovascular Pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. M.; Trappmann B.; Stapleton S. C.; Toro E.; Chen C. S. Microfluidics Embedded within Extracellular Matrix to Define Vascular Architectures and Pattern Diffusive Gradients. Lab Chip 2013, 13, 3246–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose J.; Zhang H.; Gagnon M. K.; Pan T.; Simon S. I.; Ferrara K. W. Microfluidic System for Facilitated Quantification of Nanoparticle Accumulation to Cells under Laminar Flow. Ann. Biomed. Eng. 2013, 41, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O. F.; Chamberlain M. D.; Sefton M. V. Toward an in Vitro Vasculature: Differentiation of Mesenchymal Stromal Cells within an Endothelial Cell-Seeded Modular Construct in a Microfluidic Flow Chamber. Tissue Eng., Part A 2012, 18, 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. W.; Simmons C. A. Macro- and Microscale Fluid Flow Systems for Endothelial Cell Biology. Lab Chip 2010, 10, 143–160. [DOI] [PubMed] [Google Scholar]
- Wadia J. S.; Stan R. V.; Dowdy S. F. Transducible Tat-Ha Fusogenic Peptide Enhances Escape of Tat-Fusion Proteins after Lipid Raft Macropinocytosis. Nat. Med. 2004, 10, 310–315. [DOI] [PubMed] [Google Scholar]
- Elsaesser A.; Barnes C. A.; McKerr G.; Salvati A.; Lynch I.; Dawson K. A.; Howard C. V. Quantification of Nanoparticle Uptake by Cells Using an Unbiased Sampling Method and Electron Microscopy. Nanomedicine (London, U.K.) 2011, 6, 1189–1198. [DOI] [PubMed] [Google Scholar]
- ur Rehman Z.; Hoekstra D.; Zuhorn I. S. Mechanism of Polyplex- and Lipoplex-Mediated Delivery of Nucleic Acids: Real-Time Visualization of Transient Membrane Destabilization without Endosomal Lysis. ACS Nano 2013, 7, 3767–3777. [DOI] [PubMed] [Google Scholar]
- Fiandra L.; Mazzucchelli S.; De Palma C.; Colombo M.; Allevi R.; Sommaruga S.; Clementi E.; Bellini M.; Prosperi D.; Corsi F. Assessing the in Vivo Targeting Efficiency of Multifunctional Nanoconstructs Bearing Antibody-Derived Ligands. ACS Nano 2013, 7, 6092–6102. [DOI] [PubMed] [Google Scholar]