Abstract
Identifying cross-species similarities and differences in immune development and function is critical for maximizing the translational potential of animal models. Co-expression of CD21 and CD24 distinguishes transitional and mature B cell subsets in mice. Here, we validate these markers for identifying analogous subsets in humans and use them to compare the non-memory B cell pools in mice and humans, across tissues, during fetal/neonatal and adult life. Among human CD19+IgM+ B cells, the CD21/CD24 schema identifies distinct populations that correspond to T1 (transitional 1), T2 (transitional 2), FM (follicular mature), and MZ (marginal zone) subsets identified in mice. Markers specific to human B cell development validate the identity of MZ cells and the maturation status of human CD21/CD24 non-memory B cell subsets. A comparison of the non-memory B cell pools in bone marrow (BM), blood, and spleen in mice and humans shows that transitional B cells comprise a much smaller fraction in adult humans than mice. T1 cells are a major contributor to the non-memory B cell pool in mouse BM where their frequency is more than twice that in humans. Conversely, in spleen the T1:T2 ratio shows that T2 cells are proportionally ∼8 fold higher in humans than mouse. Despite the relatively small contribution of transitional B cells to the human non-memory pool, the number of naïve FM cells produced per transitional B cell is 3-6 fold higher across tissues than in mouse. These data suggest differing dynamics or mechanisms produce the non-memory B cell compartments in mice and humans.
Introduction
The mouse and other animal models provide important insights into human B cell development and disease (1, 2). Murine data show that B lineage committed progenitors arise from hematopoietic stem cells in the bone marrow (BM) and transit a series of developmentally sequential stages to produce immature B cells expressing surface IgM (3, 4). Immature B cells pass through the transitional 1 (T1) and transitional 2 (T2) stages and then develop into naïve follicular mature (FM) or marginal zone (MZ) B cells as they leave the BM, travel through the periphery, and move into the spleen and other secondary lymphoid tissues (5-7). Differentiation from T1 to T2 and subsequently to FM and MZ B cells in the mouse is believed to occur mostly in the spleen. Developing B cells that are autoreactive undergo negative selection following B cell receptor (BCR) stimulation in the BM or the periphery (3, 6). Survival of transitional B cells during negative selection depends on interplay between signals mediated by the BCR and the receptor for B cell activating factor (BAFF) (8-12). Mature B cells that are activated by BCR stimulation, together with appropriate co-stimulatory signals, differentiate into antibody-producing plasma cells, as well as memory B cells, that together with non-memory B cells form the B cell pool (13, 14)
Comparative studies of mouse and human B cell development have focused on B cell precursor populations and activated B cells, while cross-species comparisons of the non-memory B cell pools are lacking (15). Identifying differences in the non-memory B cell pools are important for understanding the differences in mechanisms that contribute to B cell homeostasis in the two species and in translating information obtained from mouse models to studies of human disease. Murine disease models remain our major source of mechanistic data for human disease processes that arise due to defects in negative selection and B cell homeostasis (3, 16, 17). However, the clinical application of murine data is limited because multiple schema are used to identify transitional and mature B cells in mice (5, 8, 16, 18-20) and humans (21-26) and many of these are based on species-specific markers (Supplemental Table I). A system of common markers that can be used to identify transitional and mature B cell subsets across tissues in mice and humans has yet to be developed.
Here, we show that co-expression of CD21 and CD24 can be used to identify analogous subsets of CD19+IgM+ B cells in mice and humans. These markers allow the identification of T1, T2, and FM B cells in multiple hematopoietic tissues during fetal/neonatal and adult life in both species. Unlike other schema that are used to distinguish human transitional and FM B cells, these markers also allow MZ B cells in the human spleen to be identified. Using the CD21/CD24 schema and strict gating criteria to exclude memory B cells, we compared the contribution of transitional and naïve mature cells to the B cell pools in adult humans and mice. When compared to mice, our data show that human transitional B cells are reduced in the non-memory B cell pool across tissues. Despite the relatively small contribution of transitional B cells to the non-memory B cell pool, they give rise to a proportionally much larger naïve FM B cell compartment (3-6 fold increased across tissues) than those in the mouse. These data suggest that differences in the dynamics or mechanisms involved in B cell production are required to produce the proportionally larger FM compartment observed in humans as compared to mice.
Materials and Methods
Sample Procurement and Cell Preparation
Mouse Tissues
Male BALB/c mice, between 3 to 6 months of age (Charles River Laboratories, Inc., Boston, MA) and adult female C3H/HeN (Harlan, Inc) non-pregnant mice, were used for adult mouse studies. Spleens from fetuses of gestational age 18 days were isolated from pregnant female C3H/HeN mice. The use of animals was approved by the Institutional Animal Care Use Committee at Loma Linda University. BM cells were flushed from the femurs with 1 ml of sterile PBS (Cellgro, Manassas, VA). PB from BALB/c mice was obtained from the hepatic portal vein. A citrate-phosphate-dextrose solution (Sigma-Aldrich, St. Louis, MO) anticoagulant was added to blood in a 1.4 to 10 ratio. PBMCs were isolated using RBC lysis buffer method. Splenocytes from BALB/c mice were isolated by straining spleens through a 70 μM cell strainer (BD Falcon, Franklin Lakes, NJ) to create a single cell suspension in PBS. Fetal mouse spleens were processed using the same method as adult spleens.
Human Tissues
CB was collected from the umbilical cord of full-term neonates following caesarian section. Fetal spleens, pregnancy age between 17 and 23 weeks, were obtained from Advanced Bioscience Resources, Inc (Alameda, CA) or Novogenix Laboratories, LLC (Los Angeles, CA). PB from adult donors was obtained from Leuko-pak leukocyte filters (Fenwal Laboratories, Lake Zurich, IL), and donated by the Blood Processing and Quality Control Lifestream in San Bernardino, CA. BM from adult male donors aged 20-35 was purchased from All Cells (Emeryville, CA) or Lonza (Walkersville, MD). Adult spleens between the ages of 30 to 55 years of age were acquired from pathology specimens.
A citrate-phosphate-dextrose solution (Sigma-Aldrich) anticoagulant was added to blood in a 1.4 to 10 ratio. CBMCs were isolated using RBC lysis buffer density gradient centrifugation. Ficoll-Hypaque (GE Healthcare, Pittsburgh, PA) and/or RBC lysis density gradient centrifugation was used to isolate PBMCs from blood collected from filters (27). Spleens were processed using gentle MACS Dissociator (Miltenyi Biotec, Auburn, CA) and then pressed through a 70 μM cell strainer (BD Falcon) to create a single cell suspension in PBS. Fetal spleens were processed as described above for adult spleen tissues. All human tissues were acquired and handled according to protocols approved by the Institutional Review Board at Loma Linda University.
Flow cytometry
For flow cytometry, cells were stained with mAbs in PBS with 1% BSA for 20 minutes in the dark at 4°C. Cells were subsequently washed and fixed with a 1% paraformaldehyde/PBS solution before analysis on BD FACSCalibur flow cytometer (BD Immunocytometry Systems, [BDIS] San Jose, CA) or MACSQuant® Analyzer (Miltenyi Biotec). The anti-human antibodies used were CD19 Pacific Blue, CD24 PE-Cy7, CD38 APC, CD27 APC-Cy7, CD21 FITC, CD1c PE (all from Biolegend, San Diego, CA), CD10 PE, CD5 PE, CD23 PE, IgD PE, IgM PE-Cy5 (all from BD Bioscience), BAFF-R PE (eBioscience Inc., San Diego, CA). The following anti-mouse antibodies were used: CD24 Pacific Blue, CD21 FITC, CD23 PE, BAFF-R PE, CD21 APC, SA-APC-Cy7 (secondary antibody for IgD biotin staining), IgM PE-Cy7 (all from Biolegend), and IgD Biotin (eBioscience). Living cells were identified by forward scatter and side scatter gating and/or exclusion of 7-aminoactinomycin-D (eBioscience) added immediately prior to data acquisition or fixation. Flow cytometry data analysis was performed using Flowjo data analysis software (TreeStar, Ashland, OR).
Statistical analysis
The number of patient or mouse samples assayed (biological replicates or sample size) are indicated by n values in Figure legends. Statistical significance was evaluated using a Mann-Whitney U test for comparison of the mean values taken from B cell subsets from mice and human tissues. Differences in BAFF-R expression between B cell subsets were determined by paired-t test. Values were considered significant if p ≤ .05. Graphs and statistics of subset populations were derived using GraphPad Prism and Instat software (GraphPad Software, La Jolla, CA).
Results
CD21 and CD24 co-expression identifies distinct B cell populations in human hematopoietic tissues
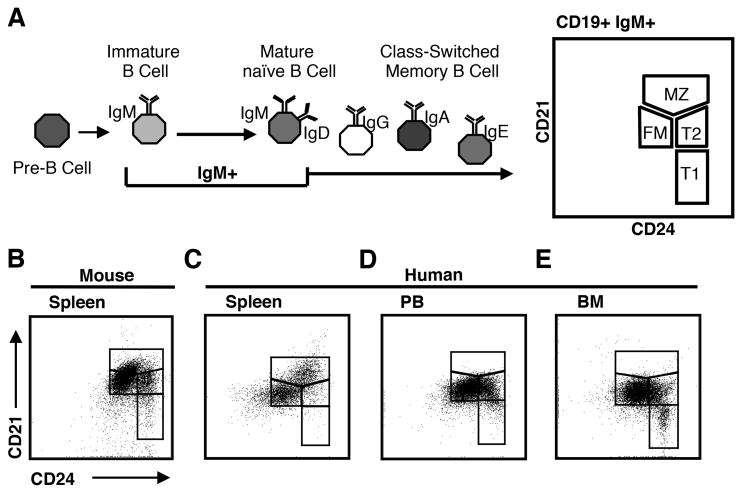
Our first goal was to identify markers that define analogous populations of transitional and naïve mature B cells in mice and humans. We evaluated multiple markers used to define these subsets in mice (Supplemental Table I (5, 8, 16, 18-26), but focused our studies on CD21 and CD24 (18) because these markers are expressed on B cells in both species. In the mouse, CD21 and CD24 have been used to discriminate between transitional and mature splenic B cell subsets (Fig. 1A). These include transitional 1 (T1), transitional 2-intermediate (T2), and follicular mature (FM) B cells, as well as a subset that contains marginal zone B cells together with marginal zone precursors (MZ) B cells (16, 18). To discriminate transitional B cells and the naïve FM B cells from other B lineage cells, we gated CD19+ B cells that were IgM+ (Supplemental Fig. 1). This excludes B cell precursors in BM and class-switched B cells in all hematopoietic tissues (3, 7). Based on the reported phenotypes of the follicular Type I and follicular Type II B cell subsets described in the mouse (28, 29), the mouse FM cells gated using this strategy would include both of these populations.
Figure 1. CD21 and CD24 co-expression identifies distinct subsets of B cells in mouse and human tissues.
A, Diagram of gating strategy to exclude B cell precursors and class-switched memory B cells prior to B cell subset identification using co-expression of CD21 and CD24. Identified are transitional 1 (T1), transitional 2 (T2), naive follicular mature (FM), and marginal zone plus marginal zone precursor (MZ) subsets. B-E, Mononuclear cells from indicated BALB/c mouse and human tissues (spleen, peripheral blood [PB], and bone marrow [BM]) were stained for flow cytometry to detect CD19, IgM, CD21, and CD24. CD19+IgM+ cells falling in lymphocyte light scatter were gated and B cell subsets as diagramed in A were gated. Data shown are representative of mouse spleen: n=4, human spleen: n=4, human PB: n=33, human BM: n=10.
First, we assessed mouse splenic CD19+IgM+ B cells for CD21/CD24 co-expression (Fig. 1B) and observed all the transitional and mature subsets described in previous studies (16, 18). We then evaluated whether distinct subsets of human CD19+IgM+ B cells could be identified by CD21 and CD24 co-expression. A distinct population of human B cells with the FM CD21/CD24 phenotype (CD24INTCD21INT) was observed in all tissues (Fig. 1C-E). CD21/CD24 subsets with the T1 (CD24HICD21LO) or T2 (CD24HICD21INT) phenotypes were easily detectable in peripheral blood (PB) and BM (Fig. 1D-E). Human CD19+IgM+ B cells with the CD21/CD24 MZ phenotype (CD24HICD21HI) were only observed in the spleen (Fig. 1C).
CD21/CD24 gating identifies MZ cells in human spleen
Our assessment of human hematopoietic tissues showed the varying levels of CD21 expression (low, intermediate, and high) that have been used to distinguish B cells subsets in the mouse. Since previous studies indicate that high levels of CD21 are characteristic of murine MZ B cells (16, 19, 30), we wanted to confirm the identity of the splenic IgM+ human B cells in the CD21/CD24 MZ gate.
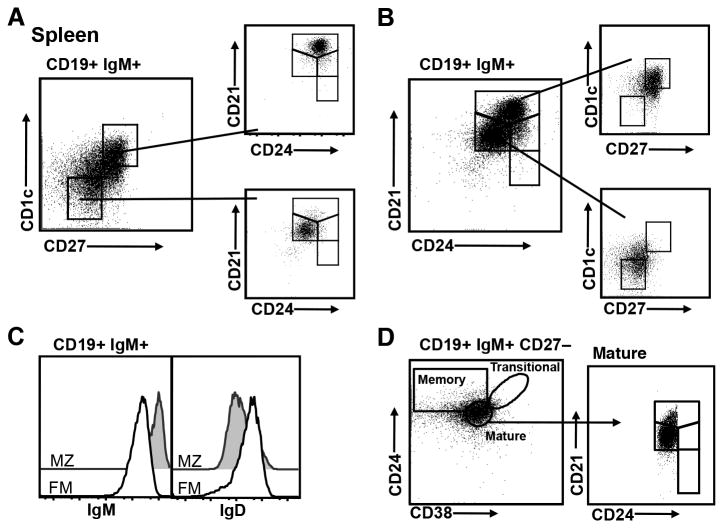
Human MZ B cells have been identified as CD1cHICD27HI (21, 31-34). As shown in Fig. 2A, IgM+ splenic B cells that are CD1cHICD27HI fall within the CD21/CD24 MZ gate while the CD1c–CD27– cells display a phenotype consistent with FM B cells. Converse gating in Fig. 2B shows that human splenic B cells in the CD21/CD24 MZ gate are predominantly CD1cHICD27HI, while cells in the FM gate show reduced co-expression of these molecules. Thus, the expression of CD1c in combination with CD27 on IgM+ B cells in the CD21/CD24 MZ gate provides evidence that CD21 and CD24 can be used to discriminate MZ lineage B cells in the human spleen. As an additional means of verifying the identity of human B cells in the CD21/CD24 MZ and FM gates, we evaluated expression of IgM and IgD. Human MZ B cells are IgMHIIgDL0, while FM B cells show lower levels of IgM and higher levels of IgD (31, 34). As shown in Fig. 2C, CD19+IgM+ human splenic B cells in the MZ gate express higher levels of IgM and lower levels of IgD, as compared to those in the FM gate.
Figure 2. CD1c and CD27 co-expression validate the identity of the CD21/CD24 MZ subset in human spleen.
Human splenic cells were co-stained for flow cytometry to detect CD19, IgM, CD24, CD21, and indicated markers. CD19+IgM+ cells falling in lymphocyte light scatter were gated. A, Co-expression of CD1c and CD27 was plotted (left panel). CD1c–CD27– and CD1c+CD27+ populations were gated and their distribution with respect to CD21/CD24 subsets was plotted (right panels). B, CD19+IgM+ cells were gated into CD21/CD24 subsets (left panel). Co-expression of CD1c and CD27 in the CD21CD24 MZ (top right panel) and FM (bottom right panel) subsets is shown. C, CD19+IgM+ cells were gated into CD21/CD24 subsets and expression of IgM and IgD in the MZ and FM subsets is shown in histograms. D, CD24 and CD38 co-expression in gated CD19+IgM+CD27− cells was plotted. Gates for memory, transitional and mature B cell subsets based on the CD24/CD38 identification schema are shown (left panel). Cells in the mature subset were gated and their distribution with respect to CD21/CD24 subsets is plotted (right panels). Data shown are representative of n=4 adult human spleens.
The co-expression of CD24 and CD38 has been used to distinguish mature B cells from transitional and memory B cells in human studies (22, 23, 25, 26, 35, 36). We compared the ability of CD24/CD38 and the CD21/CD24 schema to discriminate human FM and MZ cells in combination with CD27. First, to eliminate the majority of memory B cells (including MZ B cells), we gated on CD19+IgM+CD27− human splenic B cells. The remaining B cells in the spleen form a homogeneous population that falls within the mature CD24/CD38 B cell gate (Fig. 2D, left panel). When the CD24/CD38 “mature” population was gated and evaluated for CD21/CD24 co-expression, a distinct FM population was observed, as well as B cells expressing the high level of CD21 that is characteristic of MZ cells. Thus, co-expression of CD21 and CD24, as compared to CD24 and CD38, allows splenic MZ B cells to be more stringently distinguished from FM B cells. Taken together, the above data demonstrate that markers commonly used to distinguish human MZ and FM B cells support the use of CD21/CD24 co-expression to identify FM and MZ cells in human spleen.
Human-specific developmental markers confirm the maturation status of CD21/CD24 subsets
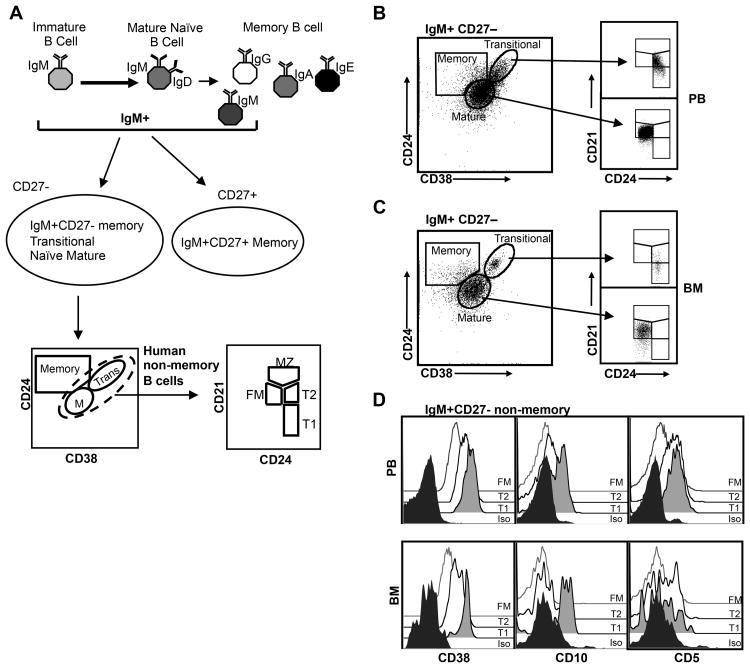
Memory B cells make up approximately 40% of the B cell pool in adult humans, but less than 5% in adult mice (14, 37). Given the large percentage of memory B cells in humans, as compared to mice (14, 37), we developed a stringent gating strategy to exclude human memory B cells prior to assessing the developmental status of immature and naïve B cells identified using the CD21/24 schema (14, 38, 39). In PB and BM, human memory B cells have classically been identified based on their expression of CD27. However, recent reports suggest that not all IgM+ memory B cells express CD27 (38, 39). Therefore, we used expression of CD27 together with strategies based on CD24/CD38 co-expression to exclude memory B cells. PB and BM cells were co-stained with CD27, CD38, CD24, CD21, IgM and CD19. To exclude CD27+ memory B cells, IgM+CD27− cells were gated (Fig. 3A, top panel). Then, co-expression of CD24 and CD38 was plotted and gates were drawn to exclude the CD24/CD38 “memory” B cells (Fig. 3A bottom panel). The application of this gating strategy allowed us to identify non-memory human B cells so that we could evaluate the maturation status of CD21/CD24 subsets using a panel of human-specific developmental markers.
Figure 3. Human markers validate the developmental status of B cell subsets identified by CD21 and CD24 co-expression.
A, Diagram of gating strategy to exclude both CD27+ and CD27− IgM+ memory B cells in human tissues. B-C, PB and BM cells were co-stained for flow cytometry to detect IgM, CD27, CD38, CD24, and CD21. IgM+CD27− cells in lymphocyte light scatter were gated as shown to identify memory, transitional and mature B cell subsets based CD24/CD38 expression as shown (left panel). Cells in the mature and transitional subsets were gated and their distribution with respect to CD21/CD24 subsets is shown (right panels). D, IgM+CD27− cells in the CD24/CD38 non-memory gate (dashed oval in panel A) were gated into CD21/CD24 subsets and evaluated for CD38, CD10 and CD5 expression. Data shown are representative of n=27 adult human PB and n=10 adult human BM.
In human B cells, CD24 and CD38 are developmentally regulated with increased levels of these markers correlating with immaturity (22, 25, 26, 35). To verify the maturation status of human CD21/CD24 B cell subsets we evaluated CD24/CD38 co-expression in human hematopoietic tissues. Human PB and BM were co-stained for flow cytometry and gated as shown in Fig. 3A. To determine whether B cell subsets identified based on co-expression of CD24 and CD38 supported the developmental status predicted by the mouse markers (CD21/CD24), each of the gated CD24/CD38 transitional and mature subsets (Fig. 3B-C, left panel) were evaluated for CD21/CD24 co-expression (Fig. 3B-C, right panels). Cells in the CD24/CD38 “transitional” B cell gate are distributed within the CD21/CD24 T1 and T2 gates (Fig. 3B-C, top right panels) while cells in the CD24/CD38 “mature” gate fall exclusively in the CD21/CD24 FM gate (Fig. 3B-C, bottom right panel). This distribution supports the developmentally immature status of T1 and T2 phenotypes based on co-expression of CD21 and CD24.
Human transitional cells identified by CD24 and CD38 co-expression fall within both the CD21/CD24 T1 and T2 gates. Further analysis was required to determine whether cells in the CD21/CD24 T2 gate represent a more mature B cell population than those in the T1 gate. Expression of the human-specific developmental markers, CD10 and CD5, as well CD38 has been used to distinguish between transitional and mature subsets in human CD19+ B cells (22-26, 35, 40, 41). Each of these markers is expressed at higher levels in the most immature IgM+ cells and their expression progressively declines during maturation of B cells in adults (36, 41). We evaluated expression levels of these markers in CD21/CD24 subsets to confirm maturation status.
PB and BM cells were stained for co-expression of CD21, CD24, CD38, CD27, CD10, CD5, IgM and CD19 and gated into CD21/CD24 subsets, excluding memory B cells as shown in Fig. 3A. Histograms of developmental marker expression in CD21/CD24 subsets are shown in Fig. 3D. The marked decrease in CD10 and CD38 expression between T1 and T2 subsets provides evidence that the T1 subset is more immature than the T2 subset. This is also supported by a similar decrease in CD5 expression that is most clearly observed in PB. The lack of CD10 and CD5 expression in cells within the CD21/CD24 FM gate in human PB and BM (Fig. 3D) as well as spleen (data not shown) provides additional evidence that CD21/CD24 co-staining can be used to distinguish transitional and mature naïve human B cells. Taken together, these data indicate that in humans, as in mice, CD21/CD24 co-staining can be used to identify developmentally sequential populations of transitional and mature naïve B cells, as well as a population that contains MZ cells in the spleen.
Patterns of differential BAFF-R expression are similar in mouse and human CD21/CD24 subsets
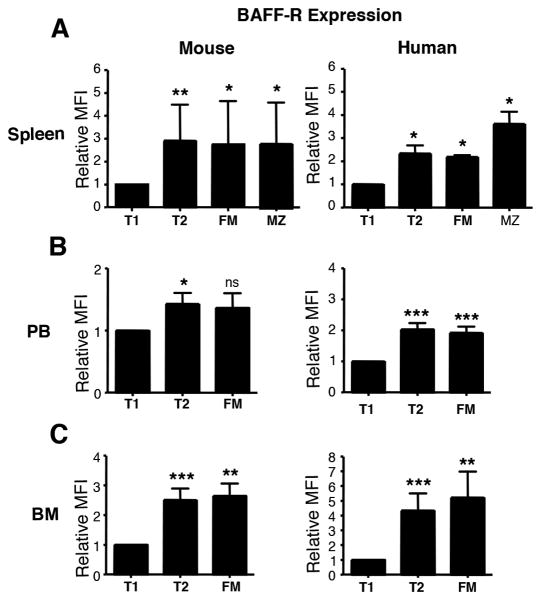
BAFF provides survival, differentiation, and growth signals that play a major role in the maintenance of the normal B cell pool (42-44). Differences in the levels of BAFF-R expression in different B cell subsets are believed to provide one mechanism for differential BAFF responses during the negative selection process (44). Studies in the mouse have shown that BAFF-R is reduced in T1 cells as compared to later stages of B cell development (12, 45). Data obtained using human-specific markers to identify developmental subsets suggests that this is also the case in humans (23, 25, 26, 36). Here we compared BAFF-R expression in analogous B cell subsets in mouse and human tissues identified using the CD21/CD24 schema.
Non-memory B cells from mouse and human spleen, PB, and BM were gated into CD21/CD24 subsets as described in Fig. 3A. For the evaluation of MZ cells in human spleen, IgM+CD27+ cells were included. Median fluorescence intensity (MFI) of BAFF-R staining is shown in Fig. 4. These data show that the pattern of BAFF-R expression is similar in mouse and human B cell subsets, identified based on CD21/CD24 co-expression Consistent with previous reports in mouse and human (12, 23, 25, 26, 36, 45), we found that BAFF-R expression is lower in T1 cells and increases with maturation. These data establish that conclusions drawn from the previously reported patterns of differential levels of BAFF-R expression are applicable to B cell populations compared across species in mouse and humans when markers common to both species are used to identify developmental subsets of B cells.
Figure 4. Patterns of differential BAFF-R expression are similar in mouse and human CD21/CD24 B cell subsets.
A-C, Mononuclear cells isolated from adult mouse and human spleen, PB, and BM were stained for IgM, CD21, CD24, BAFF-R and in human samples for CD27, CD38 and CD19 as well. IgM+ B cells falling in lymphocyte light scatter in mouse tissues were gated into CD21/CD24 subsets. Human PB, BM, and splenic T1, T2, and FM cells were gated as described in Fig. 3. For MZ cells in human spleen, CD19+IgM+ cells were assessed for CD21/CD24 MZ phenotype and this included both CD27+ and CD27− cells. For each tissue, graphed are the means + SEM of relative BAFF-R expression in each CD21/CD24 subset (normalized to BAFF-R levels in the T1 subset in that tissue). Data are from n=9 mouse spleen, n=7 mouse PB; n=9 mouse BM, n=4 human spleen, n= 15 human PB and n=9 human BM. Statistical differences are shown as *p < .05; **p< .01; *** and p < .001.
CD21/CD24 co-staining identifies developmental subsets of human B cells in fetal/neonatal tissues
In the experiments described above, we established the validity of CD21 and CD24 co-staining for the identification of developmental subsets of immature B cells and for distinguishing FM and MZ B cells in adult human tissues. Next, we wanted to determine whether CD21/CD24 co-staining can be used to identify developmental subsets at early points in life and thus provides a tool for comparing the timeline for emergence of mature B cells in mice and humans.
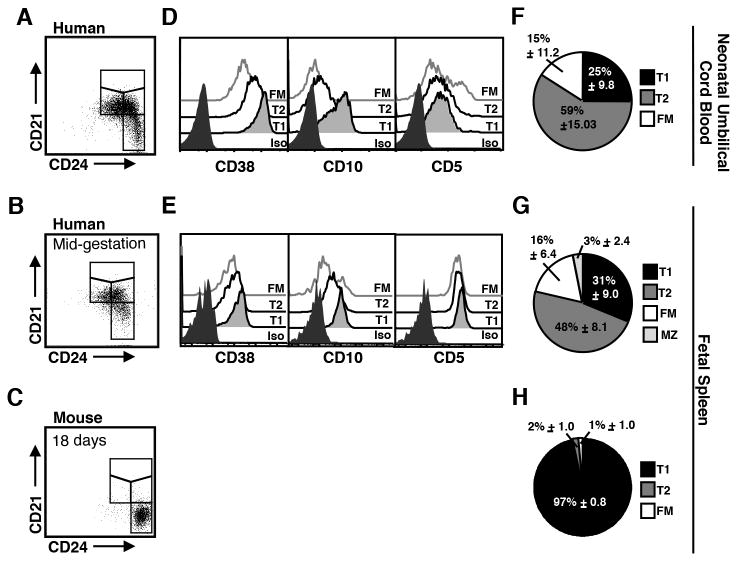
We first determined whether CD21/CD24 could be used to identify distinct B cell subsets present in human neonatal blood and fetal spleen. Populations corresponding to T1, T2, and FM were observable in both neonatal blood (umbilical cord blood, CB) (Fig. 5A) and fetal spleen (Fig. 5B).
Figure 5. Use of CD21/CD24 to compare emerging B cell pools during fetal/neonatal development in mice and humans.
Mononuclear cells were isolated from human cord blood (CB), human fetal spleen and pooled mouse fetal spleens. Cells were stained for flow cytometry to detect CD19, IgM, CD21, CD24, and in human samples for CD38, CD10, and CD5 as well. A-C, Cells falling in lymphocyte light scatter and that were CD19+IgM+ or IgM+ were gated into CD21/CD24 subsets. D-E, Subsets in human CB and human fetal spleens were evaluated for CD38, CD10 and CD5 expression. F-H, Graphed are the frequencies ±SD that each of the CD21/CD24 subsets contribute to the composition of the B cell pool. The mean ±SD for subsets in each tissue was derived from: n=10 human CB; n=6 human fetal spleens obtained at 18–23 weeks, (pregnancy date); and pooled mouse fetal spleen from C3H/HeN mice, day 18 of 20 day gestation, assayed in 3 independent experiments. Flow cytometry histogram and dot plots are representative data from indicated tissues.
To verify the maturation status of these subsets, we evaluated the expression of the human-specific developmental markers CD38, CD10 and CD5. B cells in CD21/CD24 subsets present in CB (Fig. 5D) and fetal spleen (Fig. 5E) show a progressive decline in the expression of CD38 and CD10 in the T1, T2 and FM subsets, respectively, similar to that observed in adult PB and BM. All the CD21/CD24 B cell subsets in human CB and spleen show at least low levels of CD5, regardless of maturation status (Fig. 5D, E). This is consistent with previous reports of CD5 expression on human fetal/neonatal B cells (23, 24, 46, 47). These results provide evidence that the CD21/CD24 schema can be used to identify developmental subsets of human transitional and mature B cells in fetal/neonatal tissue, as well as adult tissue.
By mid gestation, the B cell pool in humans, but not mouse, includes mature B cell subsets
The period of fetal development is considerably longer in humans than in mice (∼266 days versus 20 days) (48, 49). To determine whether this disparity impacts the composition of the B cell pool prior to birth we used the CD21/24 schema to identify the B cell subsets present in human and mouse fetal spleens (Fig. 5B-C). The IgM+ cells in human fetal spleens obtained at approximately mid-gestation (18-23 weeks, pregnancy age) included substantial populations of T1, T2, and FM B cells (Fig. 5G). In contrast, virtually all of the IgM+ cells in mouse fetal spleen (harvested 2 days prior to end of gestation which occurs at 20 days) have a T1 phenotype (Fig. 5C, H). Consistent with the emergence of mature B cells in human fetal spleen prior to birth, the B cell pool in the circulating blood of human newborns (umbilical cord blood) shows a B cell pool comprised of primarily of T2 and FM (Fig. 5F). In contrast, in the mouse at a point ∼90% of the way through gestation T1 cells make up ∼97% of splenic B cells (Fig. 5 D, H). This is consistent with reports of murine neonatal B cell development which show that the splenic B cell pool consists primarily of T1 cells as late as one week after birth (5). Thus, using the CD21/CD24 schema to identify analogous developmental subsets we show that the human non-memory B cell pool is comprised of T1, T2 and FM B cells well before birth, while the mouse B cell pool includes only T1 cells late in gestation.
Transitional B cells are reduced in the non-memory B cell pool in humans as compared to mice
Comparisons of the non-memory B cell pools in mice and human have been limited, in part because obtaining splenic tissue from human patients is challenging and PB has rarely been evaluated in the mouse. Here, we compare the contribution of B cell subsets to the IgM+ non-memory B cell pool in multiple tissues across species, using the CD21/CD24 schema to identify B cell subsets in spleen, PB, and BM from mice and humans (Fig. 6).
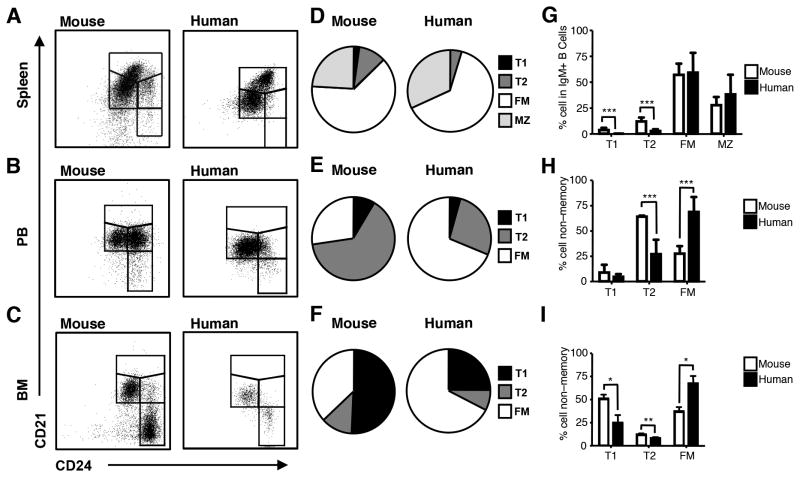
Figure 6. Transitional B cells are reduced in the non-memory B cell pool in humans as compared to mice.
Mononuclear cells were isolated from adult mouse and human spleen, PB, and BM. Cells were stained for IgM, CD21, CD24, and in human samples for CD27, CD38 and CD19 as well. A-C, IgM+ B cells falling in lymphocyte light scatter were gated in mouse tissues. For human spleen, CD19+IgM+ cells falling in lymphocyte light scatter were gated. Human PB and BM were gated as described in Fig. 3. CD21/CD24 gates are as shown. D, of the IgM+ B cell pool with respect CD21/CD24 subsets gated as described in results to include CD27+ and CD27− MZ cells. E-F. Pie graphs showing the composition of the non-memory B cell pool with respect CD21/CD24 subsets. G-I, Graphed are the mean ± SD of the percentages that each CD21/CD24 subset contributes to the IgM+ B cell pool in spleen and the non-memory B cell pool in PB and BM. Graphed are data from n=16 adult mouse spleen; n=6 adult mouse PB; n=9 adult mouse BM; n=4 adult human spleen; n= 33 adult human PB; and n= 11 adult human BM; Mice were adult male BALB/c and non-pregnant female C3H/HeN mice. Statistical differences between analogous subsets are shown as *p < .05; **p< .01; *** p < .001; and **** p< .0001.
First, we compared B cell pools in adult spleen in mice and humans. To include MZ cells in our census of the splenic B cell pool in humans, CD21/CD24 gates were set on CD19+IgM+ cells gated without regard to CD27 expression (Fig. 6A). For the identification of human transitional and FM cells, memory B cells were excluded using the strategy diagramed in Fig. 3. Data from the analyses of human MZ and non-memory subsets were combined for comparison to CD21/CD24 subsets in the IgM+ splenic B cell pool in the mouse (Fig. 6D, G).
In both mice and humans, the splenic IgM+ B cell pool is comprised primarily of FM and MZ cells (Fig. 6D, G). Mouse spleen shows clear populations of T1 and T2 cells, although 5% of total IgM+ cells are T1 cells. In contrast, T1 cells are barely detectable in human spleen, while the contribution of T2 cells is less than half that observed in mouse (Fig. 6D, G). Thus, the major difference in the splenic non-memory B cell pool in mice and humans is the reduced contribution of transitional B cells, primarily T1 cells, in humans.
Next, we used the CD21/CD24 schema to compare B cell subsets in the PB and BM from adult mice and humans (Fig. 6B-C). In contrast to the spleen where mature B cells comprise most of the non-memory pool, in mouse PB and BM, transitional B cells predominate (Fig. 6E, F, H, I). This was strikingly different from what we observed in human tissues where transitional B cells comprised 30% or less of the non-memory B cell pool in PB and BM (Fig. 6E, F, H, I). Thus, while transitional B cells formed the majority of cells in the non-memory B cell pool in mouse PB and BM, in humans, transitional B cells comprised a much smaller fraction of the non-memory B cell pool in these tissues, as they did in the spleen.
Production of FM B cells, per transitional B cell, is greater in humans than mice
To gain insights into differences in the dynamics of B cell production in mice and humans, we examined the relative proportions of T1, T2 and FM subsets in the non-memory B cell pool across tissues in mice and humans (Table I). To facilitate comparisons across tissues, the MZ compartment was excluded from the IgM+ B cell pool in the spleen.
Table I.
Ratios of FM to transitional B cells in the non-memory B cell pool across tissues in mice and humans.
| Mouse | Human | |
|---|---|---|
|
|
|
|
| Subset Ratio | FM : T2 : T1 | FM : T2 : T1 |
| Spleen | 15.9 : 3.3 : 1 | 577 : 25.4 : 1 |
| PB | 3.2 : 7.4 : 1 | 15.3: 6.0 : 1 |
| BM | 0.7: 0.2 : 1 | 2.7 : 0.3 : 1 |
| Subset Ratio | FM:Transitional | FM:Transitional |
| Spleen | 3.7 : 1 | 21.9 : 1 |
| PB | 0.4 : 1 | 2.2 : 1 |
| BM | 0.6 : 1 | 2.1 : 1 |
| Mouse:Human Ratio | Mouse (FM:Transitional) : |
Human (FM:Transitional) |
| Spleen | 1 : 6.0 | |
| PB | 1 : 5.8 | |
| BM | 1 : 3.5 | |
We used the ratio of FM to transitional B cells (T1+T2 cells) as an indicator of the capacity for transitional B cells to give rise to naïve FM B cells (Table I). In the mouse spleen, the ratio of FM B cells to transitional B cells was 3.7 to 1 while in human spleen it was 21.9 to 1. In mouse PB and BM, the ratio of FM to transitional B cells was ≤0.6. to 1. In human PB and BM, this ratio is much higher, 2.2 to 1, and 2.1 to 1, respectively. Taken together, these data show that across tissues the ratio of FM to transitional B cells is ∼3-6 fold higher in humans than mouse (Table I). Our human data is consistent with previous reports that used human-specific markers to evaluate transitional B cell frequencies. Work by Cuss et al. showed that the frequency of transitional B cells is much lower than that of naive mature cells and is reduced in the periphery and secondary lymphoid tissues as compared to BM (24). Thus, if the FM to transitional B cell ratio is used as an indicator of the capacity for transitional B cells to give rise to FM B cells, our data suggest that this capacity is much higher for human than mouse transitional B cells.
Differences in transitional subset predominance in mice and humans show tissue specificity
To gain further insights into the dynamics of naïve B cell production in mice and humans, we used data obtained with the CD21/CD24 schema to compare the relative contribution of T1 and T2 cells to the non-memory B cell pool and to the transitional B cell compartment across tissues in mice and humans (Fig. 6D-I). A comparison of T1 and T2 cell frequencies in the non-memory B cell pool shows that in the mouse, the contribution of T1 cells was the greatest in the BM. Here they comprised ∼50% of the non-memory B cell pool with progressively smaller contributions in PB and spleen (Fig. 6G-I). While the frequency of human T1 cells was greater in BM than PB or spleen, T1 cells made up only ∼25% of the BM non-memory B cell pool (Fig. 6F, I) in humans. Murine T2 cells contribute most to the non-memory B cell pool in PB where they comprise a majority of the cells (Fig. 6E, H). In human tissues, T2 cells are also most abundant in the PB non-memory B cell pool, however, their frequency is about one third of that observed in mouse. Our human data is consistent with a previous study which found that T2 cells, identified using human-specific markers, are most abundant in PB (26).
A comparison of the T1 to T2 ratios among transitional B cells in BM shows that T1 cells outnumbered T2 cells by 5 to 1 in the mouse, but only by 3 to 1 in humans (Table I). In PB, the ratio of T1 to T2 cells is similar in both species (∼1:7). However, when mouse and human spleen are compared we find that the ratio of T1 to T2 cells is 1:3.3 in mice versus 1:25.4 in humans (Table I). Thus, PB has the highest frequency of T2 cells in both mice and humans, but the relative proportion of T2 cells, as compared to T1 cells, is highest in the spleen, particularly in humans where this ratio is ∼8 fold greater than in the mouse. Taken together these data show a disparity between T1 to T2 cell ratios in mice and humans. While ratios in the periphery are similar, the proportion of T1 cells is larger in mouse BM while the proportion of T2 cells in the transitional B cell compartment is larger in human spleen.
Discussion
To facilitate translational studies of human B cell maturation, our first goal was to identify, from among the multiple murine and human schema, a set of common markers that could be used to identify analogous populations of immature and mature B cells across tissues and at different points in life in mice and humans. Such a tool could be used by basic science and clinical researchers to translate data from animal models to studies of human B cells and for developing and testing new hypotheses. To provide a foundation for such studies, our second goal was to use the CD21/CD24 schema to compare the composition of the non-memory B cell pool across tissues in fetal/neonatal and adult B cell production in mice and humans.
We evaluated several candidate markers by flow cytometry for their conserved ability to identify analogous B cell subsets in mice and humans. CD21 emerged as a promising candidate based on its ability to identify functionally distinct subsets in both species. In human studies, CD21 expression was used by Suriyani et al. (26) to subdivide immature B cells into two transitional populations. During B cell reconstitution in patients receiving hematopoietic stem cell transplants, they noted that B cells expressing lower levels of CD21 precede those expressing higher levels (26). In the mouse, CD21 together with CD24 had been used to identify T1 B cells, as well as a T2-intermediate population (designated T2 here) that gives rise to both FM and MZ precursors/MZ B cells–all identified based on CD21/CD24 co-expression (16, 18). In addition, rabbit T1 and T2 subsets had recently been identified based on CD21/CD24 co-expression patterns similar to those observed in mice (1) suggesting that CD21 expression in context of CD24 might be broadly conserved across species in B cell development. The above data suggested that CD21 had the potential to distinguish between developmentally sequential subsets of immature B cells, as well as FM and MZ B cells in humans. However, CD21 expression had not been evaluated in context of CD24 co-expression in humans, and its ability to identify analogous B cell subsets in mice and humans was unknown. Here, we validate the use of C21/CD24 co-expression to identify analogous B cell subsets in human hematopoietic tissues. Among human CD19+IgM+ B cells, we show that co-expression of CD21 and CD24 identifies distinct populations that correspond to the T1, T2, FM, and MZ subsets identified in the mouse (18).
Human B cells within the CD21/CD24 MZ gate were found exclusively in the spleen and their identity was validated by co-expression of the human MZ lineage markers, CD1c and CD27. The ability to distinguish FM cells from cells that are MZ or MZ precursors in the human spleen (Fig. 2) is an advantage of the CD21/CD24 schema over other systems currently used in human studies (23, 50). While mouse MZ B cells are restricted to the spleen, in humans MZ-like B cells (circulating IgM+ memory B cells that have similarities to MZ B cells) have been associated with other secondary lymphoid tissue including tonsils, lymph nodes and mucosal-associated lymphoid tissues (31, 34, 51). In addition, circulating CD1c+CD27+ cells have been observed in PB and are considered MZ-like cells (31, 34, 51). We found that the CD21/CD24 schema identifies a distinct population of CD21HI MZ cells present in the human spleen, but absent from PB (Fig. 1C-D). Non-splenic MZ-like B cells have been described as CD21+ (34). However, consistent with data shown in previous reports (21, 24, 31, 34,, 52), our studies indicate that these cells express the intermediate levels of CD21 that we observed for FM B cells in the spleen and all mature IgM+ B cells in the PB. It is possible that the splenic microenvironment is required for expression of the high levels of CD21 found on splenic MZ lineage B cells (52-54). Alternatively, it has been suggested that the diverse functional and phenotypic characteristics ascribed to MZ and MZ-like cells may be due to multiple MZ and MZ-llike B cell lineages (51). The ability of the CD21/CD24 schema to identify immature transitional B cells as well as to distinguish FM and MZ B cells in mouse and human spleen will facilitate studies to define different pathways/lineages of human MZ and MZ-like B cell differentiation and the role of the spleen and other secondary lymphoid tissues in this process.
The maturation status of human CD21/CD24 non-memory B cell subsets in multiple adult and fetal/neonatal tissues was verified using markers of maturation specific to human B cell development (22, 23). Our data provide evidence that the CD21/CD24 schema can be used to identify B cell subsets in multiple tissues and at different points in ontogeny across species (Fig. 3-4). Data from clinical studies demonstrate that CD10 and CD38 are expressed at high levels on transitional B cells that first emerge in the periphery during B cell re-constitution following stem cell transplant (24, 26, 36) or B cell depletion therapy (25) and that expression of these markers decreases with differentiation to the mature B cell stage. Changes in expression of CD38 and CD10 verified the maturation status of human T1, T2, and FM cells identified based on CD21/CD24 in both adult (Fig. 3) and fetal/neonatal (Fig. 5) tissues.
Mouse studies indicate that BAFF functions as a growth stimulus, promoting the survival and proliferation of T2 but not T1 cells (6, 43). The differential effects of BAFF on murine T1 and T2 cells is due in part to differential BAFF-R expression during differentiation: T1 cells show reduced BAFF-R as compared to T2 and mature B cells (7). Here, we evaluated levels of BAFF-R expression in analogous subsets of human B cells identified using the CD21/CD24 schema. Consistent with previous mouse and human reports (12, 23, 25, 26, 36, 45), we found that BAFF-R is reduced on human T1 cells as compared to later stages in B cell development. However, unlike previous studies that used markers whose expression was mouse-specific or human specific to identify B cell subsets, we assessed BAFF-R expression using common markers between the species.
The period of fetal development and adult life span are dramatically different in mice and humans (48, 49). To gain insights into the impact of life span on human versus mouse B lymphopoiesis, we used the CD21/CD24 schema to identify analogous populations of mouse and human B cell subsets at early points in life. We compared the composition of the non-memory B cell pools during fetal/neonatal development in mice and humans. Our fetal data (Fig. 5) are consistent with previous studies showing the murine splenic B cell compartment is comprised primarily of T1 B cells as late as one week post partum (5) and that substantial populations of mature B cells are present in the human spleen midway through fetal development (33). Our data from human CB and adult mouse PB show that when humans first encounter pathogens at birth their non-memory peripheral B cell pool is populated with T1, T2 and mature B cells (Fig. 5H) and that the distribution of B cell subsets within this pool is similar to that in adult mice (Fig. 6E). An interesting question is whether the mechanisms at work in the production and maintenance of the adult mouse B cell pool most closely model those at work in humans during adult life or during the fetal/neonatal period.
A comparison of the non-memory B cell pool in adult mice and humans showed dramatic differences. In contrast to adult mice, where transitional B cells predominate in the BM and PB, humans show a non-memory B cell pool comprised of ∼70% FM B cells, across tissues in BM, PB and spleen. A comparison of T1 to T2 ratios in the transitional B cell compartment in mice and humans shows that the proportion of T1 cells in BM is greater in the mouse than in humans. Conversely, the proportion of T2 cells, within the transitional B cell compartment, is larger in spleen in humans than in mice. Taken together, these data provide evidence that transitional B cells comprise a much smaller fraction of the overall human non-memory B cell pool than in the mouse. Despite their low frequency, human transitional B cells, give rise to a proportionally much larger naïve B cell compartment than those in the mouse. These data suggest that the dynamics, and potentially the mechanisms, of new B cell production, negative selection, and homeostasis that act in concert to give rise to the non-memory B cell pool present in mice and humans, are different. The disparity in T1 to T2 ratios in mice and humans suggest potential points in B cell development where differing dynamics and/or mechanisms may be at work: 1) reduced de novo B cell production and/or increased negative selection among human T1 cells in the BM and/or spleen and 2) increased expansion at the human T2 stage in the spleen and/or periphery.
The production of new B cells declines during life (55), and given the differing life spans in mice and humans, the extent of this decline may not be the same in both species (56). Given the similarity between B cell pools in fetal/neonatal humans and adult mice, it seems plausible that the progressive declines in lymphopoiesis that occur during life and the increased life span of humans compared to mice might be a contributing factor to the reduction in T1 cells that we observed in human BM as compared to mice (Fig 6R, I).
The above findings raise the question of whether similar mechanisms are responsible for the survival and proliferation of transitional B cells to produce the FM and MZ of the non-memory B cell pool. While BAFF plays a critical role in mouse B cell homeostasis (6, 43), its role in human B cell development is less clear. BAFF levels are elevated in patients with autoreactive B cells (12), but biologics that target BAFF have been less effective in clinical trials than expected (12, 44), raising the question of whether additional mechanisms may be key players in the production of normal and autoimmune B cells in humans. It is interesting to note that normal serum BAFF levels in mouse are 10 fold higher (57) than those in humans (58), raising the question of whether factors other than BAFF may be more important in the production and maintenance of human B cells. In mouse, recent studies suggest that factors other than BAFF may contribute to these processes (59-61) raising the possibility that they play a role in human B cell development as well. It is also possible that differences in other types of cells that interact with B cells, such as follicular dendritic cells (62), influence B cell differentiation and growth leading to increased efficiency in the production of mature B cells from T1 cells in humans as compared to mouse.
The implications of our data for the differences in mouse and human B cell production and homeostasis are striking if we consider that the reduced transitional B cell compartment in humans is able to give rise to a FM B cell compartment that is 3-6 fold expanded across tissues in humans as compared to mice. Even if the fundamental mechanisms responsible for B cell production and homeostasis are similar in mouse and humans, at minimum a difference in the dynamics of these processes is needed to account for the difference in composition of the non-memory B cell pool between mice and humans.
The data presented here provide a foundation for understanding how the production and maintenance of the B cell pool in humans is different from that in mice and the CD21/CD24 schema provides an important new tool to address this question. This will be important for understanding B cell-mediated autoimmunity (63) and the reconstitution of B cells following stem cell transplant and radiation or chemotherapy. It will also be important for understanding mechanisms that regulate human B lymphopoiesis, an area of increasing importance since the use of B cell depletion therapies is becoming more prevalent in the treatment of B-cell mediated autoimmune disease and malignancy. The CD21/CD24 schema will facilitate the two-way flow of information from the bench to the clinic allowing data obtained from murine disease models, as well as transgenic and knockout mice to be directly related to analogous human B cell populations identified in patient samples. The ability to isolate B cell subsets from patient samples based on CD21/CD24 co-expression opens new opportunities for functional comparisons of human hematopoietic processes with those in mouse, rabbit and other animals. Information obtained from patient samples can now be used to refine animal models to address the clinical questions that are key to impacting patient care and improving health.
Supplementary Material
Acknowledgments
We would like to thank Shannalee Martinez and Dequina Nicholas for critical review of the manuscript.
Footnotes
This work was supported by the Department of Pathology and Human Anatomy, The Department of Medicine, the Department of Basic Sciences, and the Center for Health Disparities and Molecular Medicine, Loma Linda University. Research reported in this publication was supported by the National Institute of General Medical Sciences and the National Institute of Health Disparities and Minority Health of the National Institutes of Health (NIH) under award numbers 2 R25 GM060507 and P20MD006988, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used in this article: BAFF, B cell activating factor; BAFF-R, B cell activating factor receptor; BD, Becton Dickinson; BM, bone marrow; CB, cord blood; CBMCs, cord blood mononuclear cells; C3H/HeN, mouse strain; PB, peripheral blood; FM, follicular mature; MFI, median fluorescence intensity; MZ, marginal zone; T1, transitional 1; T2, transitional.
References
- 1.Yeramilli VA, Knight KL. Somatically diversified and proliferating transitional B cells: implications for peripheral B cell homeostasis. J Immunol. 2011;186:6437–6444. doi: 10.4049/jimmunol.1003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vossenkamper A, Spencer J. Transitional B cells: how well are the checkpoints for specificity understood? Arch Immunol Ther Exp (Warsz) 2011;59:379–384. doi: 10.1007/s00005-011-0135-0. [DOI] [PubMed] [Google Scholar]
- 3.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava B, Lindsley RC, Nikbakht N, Allman D. Models for peripheral B cell development and homeostasis. Semin Immunol. 2005;17:175–182. doi: 10.1016/j.smim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends in immunology. 2003;24:343–349. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 7.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–178. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 8.Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277:48009–48019. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- 9.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat Rev Immunol. 2009;9:657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JP, Stadanlick JE, Cancro MP. Space, selection, and surveillance: setting boundaries with BLyS. J Immunol. 2006;176:6405–6410. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- 11.Scholz JL, Cancro MP. Resolve, revise, and relax: the 3 Rs of B cell repertoire adjustment. Immunol Lett. 2012;143:2–8. doi: 10.1016/j.imlet.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunol Rev. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorner T, Radbruch A. Selecting B cells and plasma cells to memory. J Exp Med. 2005;201:497–499. doi: 10.1084/jem.20050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 17.Anolik J, Sanz I. B cells in human and murine systemic lupus erythematosus. Curr Opin Rheumatol. 2004;16:505–512. doi: 10.1097/01.bor.0000133660.52599.f6. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 21.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 23.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, Tangye SG. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 25.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, Gibson J, Williams A, Tangye SG. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 27.Tamul KR, Schmitz JL, Kane K, Folds JD. Comparison of the effects of Ficoll-Hypaque separation and whole blood lysis on results of immunophenotypic analysis of blood and bone marrow samples from patients with hematologic malignancies. Clin Diagn Lab Immunol. 1995;2:337–342. doi: 10.1128/cdli.2.3.337-342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J Immunol. 2007;179:2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 29.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 30.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001 May;14(5):603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 31.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 32.Steiniger B, Timphus EM, Jacob R, Barth PJ. CD27+ B cells in human lymphatic organs: re-evaluating the splenic marginal zone. Immunology. 2005;116:429–442. doi: 10.1111/j.1365-2567.2005.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–2042. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, Quinti I, Carsetti R. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 36.Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, Contentin N, Tilly H, Tron F, Vannier JP, Jacquot S. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127:14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Schittek B, Rajewsky K. Natural occurrence and origin of somatically mutated memory B cells in mice. J Exp Med. 1992;176:427–438. doi: 10.1084/jem.176.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 40.Ho J, Moir S, Malaspina A, Howell ML, Wang W, DiPoto AC, O’Shea MA, Roby GA, Kwan R, Mican JM, Chun TW, Fauci AS. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proc Natl Acad Sci U S A. 2006;103:19436–19441. doi: 10.1073/pnas.0609515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 42.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 43.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 45.Tussiwand R, Rauch M, Fluck LA, Rolink AG. BAFF-R expression correlates with positive selection of immature B cells. Eur J Immunol. 2012;42:206–216. doi: 10.1002/eji.201141957. [DOI] [PubMed] [Google Scholar]
- 46.Dono M, Cerruti G, Zupo S. The CD5+ B-cell. Int J Biochem Cell Biol. 2004;36:2105–2111. doi: 10.1016/j.biocel.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Richl P, Stern U, Lipsky PE, Girschick HJ. The lambda gene immunoglobulin repertoire of human neonatal B cells. Mol Immunol. 2008;45:320–327. doi: 10.1016/j.molimm.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 48.Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whiteley K, Jurisica I, Adamson SL, Rossant J, Kislinger T. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim HJ, Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest. 2010;120:1004–1015. doi: 10.1172/JCI41210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Adv Immunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 51.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dono M, Zupo S, Colombo M, Massara R, Gaidano G, Taborelli G, Ceppa P, Burgio VL, Chiorazzi N, Ferrarini M. The human marginal zone B cell. Ann N Y Acad Sci. 2003;987:117–124. doi: 10.1111/j.1749-6632.2003.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 53.Gorelik L, Cutler AH, Thill G, Miklasz SD, Shea DE, Ambrose C, Bixler SA, Su L, Scott ML, Kalled SL. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol. 2004;172:762–766. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- 54.Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Signer RA, Montecino-Rodriguez E, Dorshkind K. Aging, B lymphopoiesis, and patterns of leukemogenesis. Exp Gerontol. 2007;42:391–395. doi: 10.1016/j.exger.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon CJ, Grafton G, Wood PM, Larche M, Armitage RJ. Modelling the human immune response: can mice be trusted? Commentary. Curr Opin Pharmacol. 2001;1:431–435. doi: 10.1016/s1471-4892(01)00074-1. [DOI] [PubMed] [Google Scholar]
- 57.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121:3991–4002. doi: 10.1172/JCI45563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, Plebani A, Lougaris V, Quinti I, Thon V, Litzman J, Schlesier M, Warnatz K, Thiel J, Rolink AG, Eibel H. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188:497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 59.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 60.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. 2007;204:645–655. doi: 10.1084/jem.20060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cancro MP, Smith SH. Peripheral B cell selection and homeostasis. Immunol Res. 2003;27:141–148. doi: 10.1385/IR:27:2-3:141. [DOI] [PubMed] [Google Scholar]
- 62.Dubois B, Bridon JM, Fayette J, Barthelemy C, Banchereau J, Caux C, Briere F. Dendritic cells directly modulate B cell growth and differentiation. J Leukoc Biol. 1999;66:224–230. doi: 10.1002/jlb.66.2.224. [DOI] [PubMed] [Google Scholar]
- 63.Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.