Abstract
Objective
To evaluate the association between baseline measurements of iris thickness at three positions and change in anterior segment biometric parameters after prophylactic laser peripheral iridotomy (LPI).
Design
Prospective clinical cohort study.
Participants
Fifty-two eyes of 52 nonglaucomatous subjects with anatomically narrow angles.
Methods
Anterior segment optical coherence tomography images captured before and after LPI were analyzed using customized software, the Zhongshan Angle Assessment Program. Differences in preoperative and postoperative measurements for anterior segment biometric parameters were compared by paired Student’s t-tests. Multivariate linear regression models, adjusted for age, sex, ethnicity, and preoperative pupil diameter, were used to examine the association between the baseline measurements of iris thickness at three positions and the change in anterior segment biometric parameters after LPI.
Main Outcome Measures
Baseline iris thickness measured at 750μm (IT750) and 2000μm (IT2000) from the scleral spur and maximal iris thickness (ITM). Changes in iris curvature (ICURV) and trabecular–iris space area at 500μm (TISA500) and 750μm (TISA750) from the scleral spur after LPI.
Results
ICURV significantly decreased, while TISA500 and TISA750 significantly increased following LPI (all P<0.0001). Lower baseline IT750 was significantly associated with greater postoperative increases in TISA500 and TISA750 (both P<0.05). Lower baseline IT2000 and ITM were significantly associated with greater postoperative decrease in ICURV (both P<0.05).
Conclusion
Our results showed that lower baseline measurements of iris thickness are associated with greater decrease in ICURV and increases in TISA500 and TISA750 after LPI. This suggests that eyes with thinner irides undergoing LPI were more likely to exhibit greater magnitude of change in terms of flattening of the iris convexity (i.e., ICURV) and widening of the anterior chamber angle (i.e., TISA500 and TISA750).
Keywords: laser peripheral iridotomy, anterior segment optical coherence tomography, iris thickness, iris curvature, trabecular-iris space area
Introduction
Primary angle-closure glaucoma (PACG) is a major cause of irreversible blindness worldwide.1 Since an anterior chamber angle judged to be narrow is one of the most predictive risk factors for the development of primary angle closure (PAC) and subsequent progression to PACG, evaluation of the anterior chamber angle is important in characterizing and distinguishing open configuration from narrow configuration. Although static and dynamic indentation gonioscopy are considered the reference standard for assessing the anterior chamber angle, its assessment is subjective. Clinical suspicion of angle-closure-related disease could be corroborated by diagnostic imaging modalities such as anterior segment optical coherence tomography (ASOCT).2 Analysis of ASOCT images with customized software, the Zhongshan Angle Assessment Program (ZAAP, Zhongshan Ophthalmic Centre, Guangzhou, China), allows objective and reproducible quantification of anterior segment biometric parameters.3 Recent studies using ASOCT to examine anterior chamber dimensions demonstrated that greater iris thickness is independently associated with narrow angles and angle closure.4–7 Their findings suggest that thicker irides play a role in the pathogenesis of angle closure because the thickened irides would be in closer proximity to the drainage angle.
Currently, the prevailing practice is to offer laser peripheral iridotomy (LPI) as a preventative intervention that induces changes in anterior segment morphology in order to prevent the onset of angle closure and the development of optic nerve injury.8 Early LPI has been shown to favorably alter the natural history of primary angle closure suspects (PACS), PAC, and PACG.9 Since iris thickness has been identified as a significant risk factor for angle closure, a better understanding of how iris thickness influences outcomes of LPI may hold implications for managing patients with, or at risk for developing, angle closure. This prospective study aimed to assess whether baseline measurements of iris thickness are predictive of anterior segment morphological changes following LPI in a cohort of PACS eyes.
Methods
Approval for this prospective study was obtained from the University of California, San Francisco (UCSF) Committee on Human Research. The study was carried out in accordance with the tenets of the Declaration of Helsinki. Caucasian and Chinese subjects were consecutively recruited from the UCSF general ophthalmology and glaucoma clinics between April 2008 and December 2012. All enrolled subjects provided written informed consent. Inclusion criteria for subject enrollment included: (1) adult patients (age >18 years) with narrow angles as defined by the Shaffer gonioscopic classification (described below) in at least one eye; (2) patients with confirmed normal perimetry and normal appearing optic discs; (3) patients who consented to receiving LPI; (4) patients who consented to undergo standardized preoperative and postoperative ophthalmic examination and ASOCT imaging; and (5) self-reported Caucasian or Chinese ancestry in both parents (the term “Caucasian” for the purposes of this study included only European-derived whites). Exclusion criteria for enrollment included the following: (1) eyes with PAC, PACG, or glaucomatous optic neuropathy; (2) eyes with corneal abnormalities such as edema, abrasion or dystrophy, pterygium, and other degenerative changes; (3) eyes with irido-ciliary cysts; (4) eyes with incomplete LPI perforation; and (5) patients who could not return for follow-up in the specified time period. The International Society of Geographic and Epidemiologic Ophthalmology (ISGEO) created a classification scheme for diagnosing glaucoma in cross-sectional population based research. 10 In this study, we utilized the diagnostic criteria developed by the ISGEO as the basis for diagnosis of PAC, PACG, and glaucomatous optic neuropathy. PAC was defined as eyes graded as occludable on gonioscopy with either the presence of peripheral anterior synechiae or intraocular pressure (IOP) > 21 mm Hg or both. PACG was defined as eyes with PAC and evidence of glaucomatous optic nerve damage. Glaucomatous optic nerve damage was confirmed by a glaucomatous optic nerve appearance (vertical cup:disc ratio > 0.6) and a corresponding reliable visual field defect. A glaucomatous visual field defect was considered to be present when the hemifield test was graded outside normal limits (with a probability of less than 5% based upon comparison with age-matched controls in the pattern deviation plot) and showed a cluster of three or more non–edge contiguous points that did not cross the horizontal meridian. Visual fields were defined as reliable if they fulfilled the following criteria: fixation losses < 33%, false positives < 20%, and false negatives < 20%.
Pre-operative and Post-operative Evaluation
All enrolled subjects received a standardized ophthalmic examination that included visual field testing using the 24-2 Swedish Interactive Threshold Algorithm (SITA) standard strategy with the Humphrey Visual Field Analyzer (model 750i, Carl Zeiss Meditec, Inc., Dublin, CA), IOP measurement by Goldmann applanation tonometry (model AT900, Haag-Streit AG, Koeniz, Switzerland), slit lamp examination (model BM900, Haag-Streit AG, Koeniz, Switzerland), and gonioscopy with a Zeiss-style 4-mirror lens (model OPDSG, Ocular Instruments, Inc., Bellevue, WA). A single trained ophthalmologist (SCL) performed gonioscopy at x16 magnification with slit-lamp biomicroscopy in a darkroom setting. The Shaffer gonioscopic classification was used to determine the anterior chamber angle grading in all four quadrants: an angle between the iris and the trabecular meshwork surface of 35° to 45° was classified as grade 4, between 20° and 35° was classified as grade 3, between 10° to 20° was classified as grade 2, and less than 10° was classified as grade 1. Grade 0 was assigned if angle structures were not observed.11 For this study, eyes with narrow angles were defined as those with Shaffer grades of 2 or less in 3 or more quadrants. This definition of narrow angle has previously been used by other studies.12–18 To improve accuracy in IOP measurements, the same ophthalmologist (SCL) measured IOP twice for each subject during a designated time period between 1 and 3 PM. From the two IOP measurements, an average was derived for statistical analysis. If the two IOP values differed by more than 2 mmHg, then a third measurement was performed and the median value was used.
Laser Peripheral Iridotomy
All LPIs were performed by the same ophthalmologist (SCL). After pharmacological pupil constriction with topical 2% pilocarpine, topical proparacaine hydrochloride ophthalmic solution 0.5% (Alcaine, Alcon Pharmaceuticals, Fort Worth, TX) was applied to the surgical eye for anesthesia. An Abraham lens filled with Hypromellose 2.5% ophthalmic lubricant solution (Goniovisc™, HUB pharmaceuticals, Rancho Cucamonga, CA) was placed on the cornea and a neodymium:yttrium aluminum garnet (Nd:YAG) laser was used to create a peripheral iridotomy approximately 1 mm from the limbus in the supero-temporal region between 10–11 and 1–2 o’clock positions on the right and left irides, respectively. Triple bursts of 7 mJ per laser shot were used initially to achieve patency, and then single bursts of 6 mJ per laser shots were used to enlarge the iridotomy. An average of 25 shots were used. At the end of every procedure, the performing physician confirmed the achievement of full-thickness perforation. The size of the iridotomy was approximately 1.5 mm in diameter. Immediately following the procedure, topical 0.1% apraclonidine (Iopidine, Alcon Pharmaceuticals, Fort Worth, TX) and 1% prednisolone acetate (Pred Forte, Allergan Pharmaceuticals, Irvine, CA) were applied. IOP was measured approximately 1 hour after the procedure. Patients with significantly elevated IOP received appropriate topical ocular hypotensives for IOP control prior to leaving the clinic. All patients were instructed to use 1% prednisolone acetate 4 times a day for 3 days. Post-LPI assessment was performed 2–3 weeks after the procedure. The post-LPI ASOCT image was obtained at this visit, gonioscopic examination was performed, and all iridotomies were examined for patency and confirmed to be of adequate size. LPI was repeated if patency could not be confirmed.
Anterior Segment Optical Coherence Tomography
All qualified study subjects received imaging in the dark with ASOCT (Visante OCT, Carl Zeiss Meditec, Inc., Dublin, CA), a non-contact optical coherence tomographic system using 1310-nm wavelength light to capture high-resolution cross-sectional images of the anterior segment of the eye. ASOCT imaging was performed under standardized dark conditions with illuminations below 1 lux as measured by EasyView Digital Light Meter (model EA30, Extech Instruments, Inc., Waltham, MA). Patients were allowed 5 minutes for dark adaptation before image acquisition. Each ASOCT scan captured both the temporal and nasal quadrants (nasal-temporal 0–180°) in a single image while the patient looked straight ahead. An experienced operator, masked to the standardized ophthalmic examination findings, performed all the ASOCT scans. Three to five images were acquired for the one randomly selected eye of each subject and the image with the best quality was selected for analysis using the ZAAP software. Image quality was evaluated on the basis of a steady central fixation as judged by a clear corneal reflection, good visibility of the scleral spurs, the presence of continuity in anterior segment structures, and the absence of motion artifacts.
The ZAAP software contained algorithms that automatically defined the borders and curvatures of anterior segment structures after the scleral spurs were localized manually on the ASOCT images. Measurements for both the nasal and temporal angles were simultaneously produced. Since the peripheral iridotomies were created closer to the temporal angles, only the nasal angles were analyzed in this study. The following parameters were derived from the ZAAP software: pupil diameter (PD), iris thickness measured at 750 μm from the scleral spur (IT750), iris thickness measured at 2000 μm from the scleral spur (IT2000), maximal iris thickness (ITM), iris curvature (ICURV), trabecular–iris space area at 500 μm from the scleral spur (TISA500), trabecular–iris space area at 750 μm from the scleral spur (TISA750), and central corneal thickness (CCT).
The above listed ASOCT parameters were defined previously in other studies. PD was calculated by measuring the distance between the pupil edges on the cross-sectional images.7 For parameters associated with iris thickness, a circle centered at the scleral spur was drawn with a radius of 750 μm, and the point of intersection between the circle and the anterior surface of the iris was identified. The shortest distance from this point to the posterior surface of the iris was calculated as IT750. The same method was used for IT2000. ITM was defined as the highest value of iris thickness along the entire iris.6 To calculate ICURV, a line was drawn from the most peripheral to the most central point of the iris pigmented epithelium, and a perpendicular line was extended from this line to the iris pigmented epithelium at the point of greatest convexity.7 An illustration of the iris parameters is provided in Figure 1. TISA500 was defined as the trapezoidal area with the following boundaries: anteriorly, a perpendicular line between the inner corneoscleral wall and the iris surface at 500 μm anterior to the scleral spur; posteriorly, a line perpendicular to the inner corneoscleral wall extending from the scleral spur to the iris surface; superiorly, the inner corneoscleral wall; and inferiorly, the iris surface. The same method was used for TISA750.19,20 An illustration of TISA500 and TISA750 is provided in Figure 2. CCT was measured from the anterior to the posterior surface of the cornea at the center of the ASOCT image.21
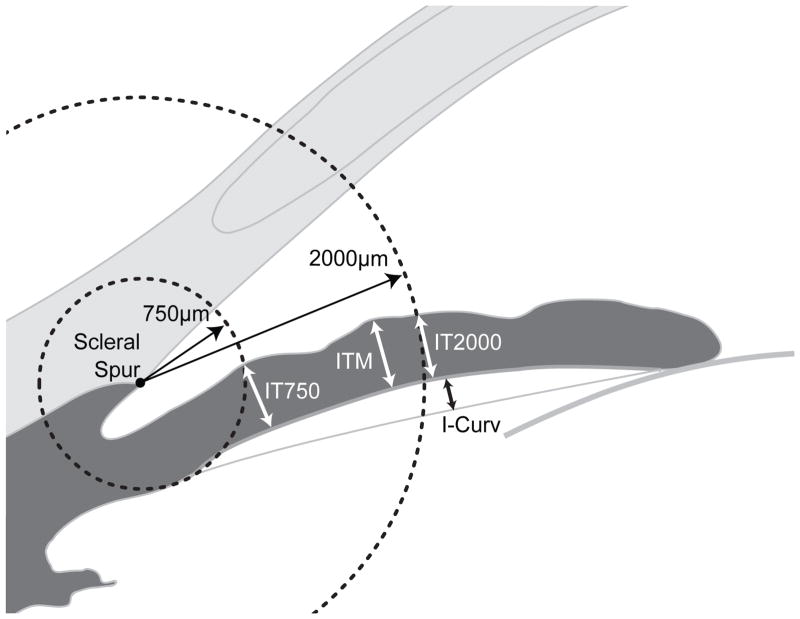
Figure 1.
An illustration of iris curvature (ICURV), iris thickness measured at 750 μm from the scleral spur (IT750), iris thickness measured at 2000 μm from the scleral spur (IT2000), and maximal iris thickness (ITM). For parameters associated with iris thickness, a circle centered at the scleral spur was drawn with a radius of 750 μm, and the point of intersection between the circle and the anterior surface of the iris was identified. The shortest distance from this point to the posterior surface of the iris was calculated for IT750. The same method was used for IT2000. ITM was defined as the highest value of iris thickness along the entire iris. To calculate ICURV, a line was drawn from the most peripheral to the most central point of the iris pigmented epithelium, and a perpendicular line was extended from this line to the iris pigmented epithelium at the point of greatest convexity.
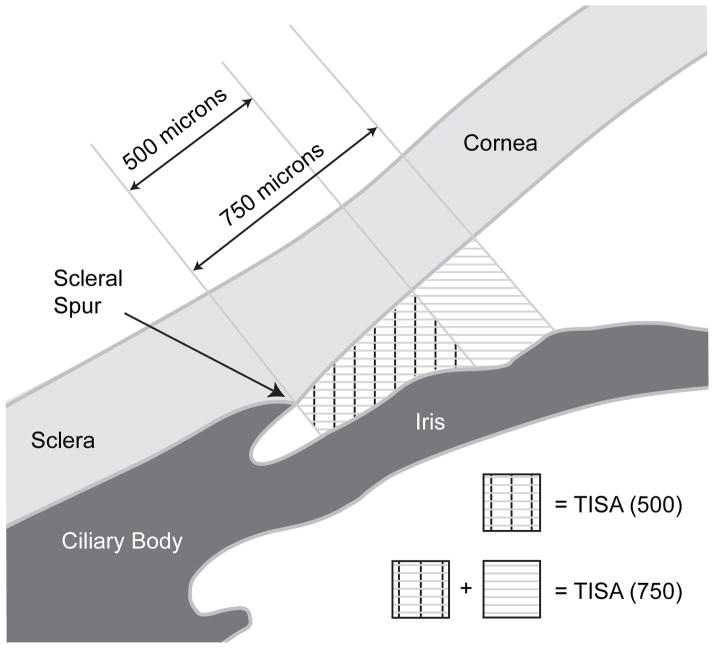
Figure 2.
An illustration of trabecular–iris space area measured at 500 μm from the scleral spur (TISA500) and trabecular–iris space area measured at 750 μm from the scleral spur (TISA750). TISA500 was defined as the trapezoidal area with the following boundaries: anteriorly, a perpendicular line between the inner corneoscleral wall and the iris surface at 500 μm anterior to the scleral spur; posteriorly, a line perpendicular to the inner corneoscleral wall extending from the scleral spur to the iris surface; superiorly, the inner corneoscleral wall; and inferiorly, the iris surface. The same method was used for TISA750.
Statistical Analysis
All statistical analyses were conducted with R-statistics (Ver. 2.15.1 software for Macintosh, R Foundation for Statistical Computing, Vienna, Austria) and P values <0.05 were considered to indicate statistical significance. One eye per subject was randomly selected for inclusion in the statistical analysis. Mean and standard deviation were calculated for all variables. Differences in preoperative and postoperative measurements for PD, ICURV, TISA500, and TISA750 were compared by paired Student’s t-tests. Differences in preoperative measurements for IT750, IT2000, and ITM between Caucasian and Chinese subjects were compared by unpaired Student’s t-tests. Univariate and multivariate linear regression models, the latter adjusted for age, sex, ethnicity, and preoperative PD, were used to investigate the association between the baseline measurements of iris thickness at the three positions (IT750, IT2000, and ITM) and the change in ICURV, TISA500, and TISA750 after LPI in the entire study population. The same statistical models, without adjusting for ethnicity, were then used to separately examine Caucasian and Chinese subjects. Change was calculated as (postoperative mean – preoperative mean). Because manual scleral spur localization is subjective in nature, to ensure consistency, a single residency-trained ophthalmologist (TK), who has had post-residency glaucoma training in Japan and the United States, manually located the scleral spurs on all ASOCT images while masked to the patient’s standardized ophthalmic examination results. To evaluate intraobserver variance, 20 eyes were randomly selected and the anterior segment biometric parameters were re-measured by the same observer (TK) at a separate session. The intraobserver reproducibility was assessed with the intraclass correlation coefficient.
Results
A total of 74 consecutively enrolled Caucasian and Chinese patients received LPI and agreed to participate in the study during the specified time period. Among them, 22 subjects were excluded from the study due to one or more of the following reasons: (1) five subjects could not or did not complete the ASOCT examination, (2) thirteen subjects had poor ASOCT imaging quality (evaluated on the basis of corneal reflection, continuity of anterior segment structures, motion artifacts, and indeterminate scleral spurs), and (3) four subjects had incomplete data in at least 1 of the analysis variables. Following exclusion, one eye in each of the remaining 52 subjects was randomly selected for inclusion in the statistical analysis. There were no significant differences in age (P = 0.733, unpaired Student’s t-test), sex (P = 0.317, Fisher’s Exact test), ethnicity (P = 1.000, Fisher’s Exact test), and residual narrow angle (P = 0.775, Fisher’s Exact test) between the included and excluded subjects. At the time of ASOCT imaging, none of the subjects were on pilocarpine, other muscarinic agonists, or agents that could affect the iris thickness or anterior chamber. Table 1 provides the demographics and baseline clinical characteristics of the included study subjects. Comparison of preoperative measurements for IT750, IT2000, and ITM between Caucasian and Chinese subjects using unpaired Student’s t-tests showed that Chinese subjects had significantly greater preoperative IT2000 than Caucasian subjects (P = 0.036). However, Chinese subjects did not differ from Caucasian subjects for preoperative IT750 (P = 0.197) and ITM (P = 0.291).
Table 1.
Demographics and Baseline Clinical Characteristics of the Study Population
| No. Patients | 52 |
| Sex (Male:Female) | 26:26 |
| Ethnicity (Caucasian:Chinese) | 28:24 |
| Age (years) | 65.3 ± 11.5a |
| Preoperative Shaffer Gonioscopy Grade of All Four Quadrantsb | 1.3 ± 0.5a |
| Preoperative CCT (ìm) | 548.7 ± 32.3a |
| Preoperative IT750 (mm) | 0.469 ± 0.088a |
| Preoperative IT2000 (mm) | 0.462 ± 0.088a |
| Preoperative ITM (mm) | 0.588 ± 0.086a |
Data are expressed as mean value ± standard deviation.
Evaluation of the anterior chamber angle was based on the Shaffer gonioscopic grading classification: an angle between the iris and the trabecular meshwork surface of 35° to 45° was classified as grade 4, between 20° and 35° was classified as grade 3, between 10° to 20° was classified as grade 2, and less than 10° was classified as grade 1. Grade 0 was assigned if angle structures were not observed.
CCT = central corneal thickness, IT750 = iris thickness measured at 750 μm from the scleral spur, IT2000 = iris thickness measured at 2000 μm from the scleral spur, ITM = maximal iris thickness.
Table 2 lists the preoperative and postoperative measurements of PD, ICURV, TISA500, and TISA750, as well as the differences between the two sets of measurements by paired Student’s t-tests. PD decreased from a pre-LPI average of 4.00 ± 0.88 mm to a post-LPI average of 3.90 ± 0.99 mm, a 2.50% reduction. However, this change in PD was not statistically significant (P = 0.413). ICURV significantly decreased, while TISA500 and TISA750 significantly increased following LPI (all P < 0.0001). ICURV significantly decreased from a pre-LPI average of 0.33 ± 0.10 mm to a post-LPI average of 0.15 ± 0.10 mm, a 54.4% reduction. TISA500 and TISA750 both showed approximately 50% enlargement after LPI, significantly increasing from pre-LPI averages of 0.057 ± 0.031 and 0.106 ± 0.046 mm2 to post-LPI averages of 0.086 ± 0.035 and 0.159 ± 0.055 mm2, respectively.
Table 2.
Comparisons Between Preoperative and Postoperative Anterior Segment Biometric Parameters Related to Laser Peripheral Iridotomy in Primary Angle Closure Suspects
| Parameter | Preoperative Measurements a | Postoperative Measurements a | Outcome Difference (%) | P Valueb |
|---|---|---|---|---|
| PD (mm) | 4.00 ± 0.88 (3.75, 4.24) | 3.90 ± 0.99 (3.61, 4.07) | 0.10 (2.50% decrease) | 0.413 |
| ICURV (mm) | 0.33 ± 0.10 (0.306, 0.360) | 0.15 ± 0.10 (0.124, 0.179) | 0.18 (54.4% decrease) | <0.0001 |
| TISA500 (mm2) | 0.057 ± 0.031 (0.049, 0.066) | 0.086 ± 0.035 (0.076, 0.096) | 0.029 (50.9% increase) | <0.0001 |
| TISA750 (mm2) | 0.106 ± 0.046 (0.094, 0.120) | 0.159 ± 0.055 (0.144, 0.175) | 0.053 (49.4% increase) | <0.0001 |
Data are expressed as mean value ± standard deviation (95% confidence interval).
P values by paired Student’s t-tests.
PD = pupil diameter, ICURV = iris curvature, TISA500 = trabecular–iris space area at 500 μm from the scleral spur, TISA750 = trabecular–iris space area at 750 μm from the scleral spur.
Table 3 summarizes the results of univariate and multivariate linear regression analyses of the association between the baseline measurements of iris thickness at the three positions (IT750, IT2000, and ITM) and the change in ICURV, TISA500, and TISA750 after LPI in the entire study population. The multivariate linear regression models adjusted for age, sex, ethnicity, and preoperative PD. In both univariate and multivariate linear regression models, lower baseline IT750 was found to be significantly associated with greater postoperative increases in TISA500 and TISA750 (all P < 0.05). Lower baseline IT2000 and lower baseline ITM were found to be significantly associated with greater postoperative decrease in ICURV in both univariate and multivariate linear regression models (all P < 0.05). Overall, these results demonstrated that lower iris thickness measurements prior to LPI are associated with greater increases in TISA500 and TISA750 and decrease in ICURV after LPI.
Table 3.
Association Between Preoperative Iris Thickness and Changes in Anterior Segment Biometric Parameters After Laser Peripheral Iridotomy in Primary Angle Closure Suspects (All Subjects)
| Outcome | Predictor | P Values of Univariate Regression Model | Regression Coefficients (95% CI) | P Values of Multivariate Linear Regression Modela | Regression Coefficients (95% CI) |
|---|---|---|---|---|---|
| Δ ICURV (mm) | Preoperative IT750 (mm) | 0.104 | 0.296 (−0.055, 0.647) | 0.085 | 0.335 (−0.037, 0.707) |
| Preoperative IT2000 (mm) | <0.0001 | 0.844 (0.563, 1.125) | <0.0001 | 0.941 (0.640, 1.242) | |
| Preoperative ITM (mm) | 0.022 | 0.425 (0.071, 0.778) | 0.012 | 0.491 (0.119, 0.863) | |
| Δ TISA500 (mm2) | Preoperative IT750 (mm) | 0.028 | −0.096 (−0.180, −0.012) | 0.011 | −0.118 (−0.205, −0.031) |
| Preoperative IT2000 (mm) | 0.491 | −0.031 (−0.120, 0.057) | 0.273 | −0.055 (−0.152, 0.042) | |
| Preoperative ITM (mm) | 0.654 | −0.020 (−0.111, 0.069) | 0.474 | −0.035 (−0.131, 0.609) | |
| Δ TISA750 (mm2) | Preoperative IT750 (mm) | 0.030 | −0.164 (−0.309, −0.019) | 0.012 | −0.197 (−0.347, −0.048) |
| Preoperative IT2000 (mm) | 0.263 | −0.087 (−0.240, 0.064) | 0.107 | −0.137 (−0.301, 0.026) | |
| Preoperative ITM (mm) | 0.640 | −0.037 (−0.194, 0.119) | 0.466 | −0.061 (−0.227, 0.103) |
Adjusted for age, sex, ethnicity, and preoperative pupil diameter.
ICURV = iris curvature, TISA500 = trabecular–iris space area at 500 μm from the scleral spur, TISA750 = trabecular–iris space area at 750 μm from the scleral spur, IT750 = iris thickness measured at 750 μm from the scleral spur, IT2000 = iris thickness measured at 2000 μm from the scleral spur, ITM = maximal iris thickness, CI = confidence interval
Table 4 outlines the results of univariate and multivariate linear regression analyses in only Caucasian subjects. In both univariate and multivariate linear regression models, lower baseline IT750 was found to be significantly associated with greater postoperative increases in TISA500 and TISA750 (all P < 0.05), while lower baseline IT2000 was found to be significantly associated with greater postoperative increases in TISA750 (both P < 0.05). The results of examining only Caucasian subjects mirrored the results of the examination of the entire study population (both Caucasian and Chinese subjects combined), except with the addition of the significant negative association between baseline IT2000 and postoperative change in TISA750.
Table 4.
Association Between Preoperative Iris Thickness and Changes in Anterior Segment Biometric Parameters After Laser Peripheral Iridotomy in Primary Angle Closure Suspects (Caucasian Subjects)
| Outcome | Predictor | P Values of Univariate Regression Model | Regression Coefficients (95% CI) | P Values of Multivariate Linear Regression Modela | Regression Coefficients (95% CI) |
|---|---|---|---|---|---|
| Δ ICURV (mm) | Preoperative IT750 (mm) | 0.142 | −0.355 (−0.113, 0.880) | 0.157 | 0.385 (−0.131, 0.901) |
| Preoperative IT2000 (mm) | <0.0001 | 0.928 (0.566, 1.290) | <0.0001 | 0.887 (0.518, 1.256) | |
| Preoperative ITM (mm) | 0.009 | 0.668 (0.199, 1.137) | 0.012 | 0.682 (0.185, 1.179) | |
| Δ TISA500 (mm2) | Preoperative IT750 (mm) | 0.005 | −0.186 (−0.308, −0.064) | 0.013 | −0.179 (−0.310, −0.048) |
| Preoperative IT2000 (mm) | 0.085 | −0.119 (−0.250, 0.011) | 0.075 | −0.127 (−0.261, 0.006) | |
| Preoperative ITM (mm) | 0.128 | −0.111 (−0.251, 0.027) | 0.155 | −0.113 (−0.265, 0.038) | |
| Δ TISA750 (mm2) | Preoperative IT750 (mm) | 0.017 | −0.302 (−0.536, −0.069) | 0.031 | −0.285 (−0.528, −0.042) |
| Preoperative IT2000 (mm) | 0.047 | −0.251 (−0.488, −0.014) | 0.038 | −0.263 (−0.498, −0.028) | |
| Preoperative ITM (mm) | 0.158 | −0.191 (−0.450, 0.067) | 0.163 | −0.199 (−0.472, 0.072) |
Adjusted for age, sex, and preoperative pupil diameter.
ICURV = iris curvature, TISA500 = trabecular–iris space area at 500 μm from the scleral spur, TISA750 = trabecular–iris space area at 750 μm from the scleral spur, IT750 = iris thickness measured at 750 μm from the scleral spur, IT2000 = iris thickness measured at 2000 μm from the scleral spur, ITM = maximal iris thickness, CI = confidence interval
Table 5 presents the results of univariate and multivariate linear regression analyses in only Chinese subjects. The results in examining only Chinese subjects were similar to the examinations of the entire study population and only Caucasian subjects in that lower baseline IT2000 was significantly associated with greater postoperative decrease in ICURV. The significant association between lower baseline IT750 and greater postoperative decrease in ICURV is a new outcome. However, lower baseline IT750 was not found to be significantly associated with greater postoperative increases in TISA500 and TISA750.
Table 5.
Association Between Preoperative Iris Thickness and Changes in Anterior Segment Biometric Parameters After Laser Peripheral Iridotomy in Primary Angle Closure Suspects (Chinese Subjects)
| Outcome | Predictor | P Values of Univariate Regression Model | Regression Coefficients (95% CI) | P Values of Multivariate Linear Regression Modela | Regression Coefficients (95% CI) |
|---|---|---|---|---|---|
| Δ ICURV (mm) | Preoperative IT750 (mm) | 0.035 | 0.251 (−0.284, 0.787) | 0.236 | 0.262 (−0.158, 0.683) |
| Preoperative IT2000 (mm) | 0.001 | 0.920 (0.463, 1.377) | 0.009 | 0.715 (0.233, 1.198) | |
| Preoperative ITM (mm) | 0.339 | 0.273 (−0.274, 0.820) | 0.104 | 0.360 (−0.054, 0.775) | |
| Δ TISA500 (mm2) | Preoperative IT750 (mm) | 0.455 | −0.045 (−0.164, 0.072) | 0.234 | −0.071 (−0.185, 0.042) |
| Preoperative IT2000 (mm) | 0.679 | 0.028 (−0.102, 0.158) | 0.632 | 0.038 (−0.117, 0.195) | |
| Preoperative ITM (mm) | 0.570 | 0.035 (−0.086, 0.157) | 0.582 | 0.034 (−0.085, 0.154) | |
| Δ TISA750 (mm2) | Preoperative IT750 (mm) | 0.394 | −0.082 (−0.270, 0.104) | 0.210 | −0.120 (−0.303, 0.061) |
| Preoperative IT2000 (mm) | 0.781 | 0.029 (−0.178, 0.237) | 0.641 | 0.060 (−0.271, 0.260) | |
| Preoperative ITM (mm) | 0.528 | 0.063 (−0.130, 0.256) | 0.542 | 0.060 (−0.131, 0.252) |
Adjusted for age, sex, and preoperative pupil diameter.
ICURV = iris curvature, TISA500 = trabecular–iris space area at 500 μm from the scleral spur, TISA750 = trabecular–iris space area at 750 μm from the scleral spur, IT750 = iris thickness measured at 750 μm from the scleral spur, IT2000 = iris thickness measured at 2000 μm from the scleral spur, ITM = maximal iris thickness, CI = confidence interval
Table 6 displays the intraobserver reproducibility of anterior segment biometric parameters measurements in a randomly selected subset of 20 eyes. All parameters demonstrated fair to excellent reproducibility with intraclass correlations.
Table 6.
Reproducibility of Anterior Segment Biometric Parameter Measurements in a Random Subset of 20 Eyes
| Parameters | ICC | Mean | Difference | 95% CI |
|---|---|---|---|---|
| PD | 0.908 | 4.426 | 0.155 | 0.777, 0.964 |
| CCT (ìm) | 0.979 | 642.79 | 1.34 | 0.945, 0.992 |
| IT750 (mm) | 0.921 | 0.494 | 0.001 | 0.805, 0.969 |
| IT2000 (mm) | 0.980 | 0.495 | 0.005 | 0.949, 0.992 |
| ITM (mm) | 0.861 | 0.647 | 0.010 | 0.673, 0.945 |
| ICURV(mm) | 0.885 | 0.15 | 0.01 | 0.724, 0.955 |
| TISA500 (mm2) | 0.977 | 0.125 | 0.001 | 0.942, 0.991 |
| TISA750 (mm2) | 0.979 | 0.244 | 0.002 | 0.947, 0.992 |
PD = pupil diameter, CCT = central corneal thickness, IT750 = iris thickness measured at 750 μm from the scleral spur, IT2000 = iris thickness measured at 2000 μm from the scleral spur, ITM = maximal iris thickness, ICURV = iris curvature, TISA500 = trabecular–iris space area at 500 μm from the scleral spur, TISA750 = trabecular–iris space area at 750 μm from the scleral spur, ICC = intraclass correlation coefficient, CI = confidence interval
Discussion
This study evaluated the association between baseline measurements of iris thickness at three positions (IT750, IT2000, and ITM) and the change in anterior segment biometric parameters (ICURV, TISA500, and TISA750) after prophylactic LPI in nonglaucomatous subjects with anatomically narrow angles. Our results showed significant decrease in ICURV and increases in TISA500 and TISA750 following LPI. These findings are consistent with previous imaging studies using ASOCT to examine the morphology of the anterior segment before and after LPI.22,23 Furthermore, our study found that lower baseline measurements of iris thickness are associated with greater decrease in ICURV and greater increases in TISA500 and TISA750 after LPI. To our knowledge, this is the first study to demonstrate that eyes with thinner irides are more likely to exhibit greater magnitudes of change in terms of flattening of the iris convexity (i.e., ICURV) and widening of the anterior chamber angle (i.e., TISA500 and TISA750) after LPI. Baseline iris thickness measurements at the three different positions represent novel predictors of ICURV, TISA500, and TISA750 after LPI.
In the present study, lower baseline measurements of iris thickness at two positions (IT2000 and ITM) were significantly associated with greater decrease in ICURV after LPI. The convex iris configuration is due to a build up of pressure in the posterior chamber that arises from pupillary block. LPI reduces anterior iris bowing by equalizing the pressure between the two chambers through the creation of a hole in the iris that allows aqueous humor to bypass the pupillary block and flow directly from the posterior chamber to the anterior chamber. Difference in elasticity between thick and thin irides provides a possible explanation for the association between baseline iris thickness and ICURV reduction after LPI. Type I collagen in the stroma decreases the elasticity of the iris.24 Thicker irides may possess higher quantities of type I collagen in the stroma in comparison to thinner irides, causing thicker irides to be more inelastic and less malleable. Stiffening of the iris could induce loss of mechanical properties in the iris, affect iris movement compliance, and reduce the iris’ capability to flatten after LPI.25
Lower baseline measurements of iris thickness at the most peripheral position (IT750) showed significant associations with greater increases in both TISA500 and TISA750. The width of the anterior chamber angle is considered the key anatomic parameter that determines the risk for PAC. In this study, TISA500 and TISA750 were selected as surrogates for measuring the anterior chamber angle width. TISA was chosen to represent the anterior chamber angle width over the parameter of angle opening distance (AOD) because AOD is a point distance and treats the iris surface as a straight line, which may be problematic in the presence of irregularities in iris curvature and thickness.26 TISA, however, overcomes this potential source of error because it is a measurement of area and may be less influenced by localized variations in the iris, as these may average out over the area and the overall area may remain the same or only slightly altered.27 Iris thickness has been shown to influence variance in AOD but not in TISA.28 Additionally, TISA has been reported to have an excellent correlation with gonioscopy.20 Out of the three iris positions, only lower baseline measurements at the most peripheral position of the iris (IT750) showed association with greater increases in TISA500 and TISA750 after LPI. The peripheral iris thickness may play a role in determining the amount of angle opening because the iris periphery forms one of the boundaries of the anterior chamber angle and a thicker peripheral iris may directly contribute to more angle crowding as it would be positioned in closer proximity to the angle.
A previous study by How et al. also examined the association between baseline measurements of iris thickness and change in anterior chamber angle width after LPI.23 However, their results showed a positive association, which is in contrast to the negative association found in the present study. Three potential factors may have led to this discrepancy. First, How et al.’s study utilized AOD to represent the anterior chamber angle width. As explained in the preceding paragraph, AOD may not be the optimal parameter for measuring the effects of iris thickness on LPI-induced angle opening because this anterior chamber angle width parameter is influenced by irregularities in iris curvature and thickness. Second, the statistical model employed in How AC et al’s study neither adjusted for baseline pupil size nor examined change in pupil size after LPI. It is possible that the positive association between ΔAOD and baseline iris thickness found in their study may be attributable to differences in baseline pupil size or change in pupil size after LPI. Third, How AC et al’s study was composed primarily of Chinese subjects. The present study’s population contained both Chinese and Caucasian subjects. When only Caucasian subjects were examined in the current study, the results were similar to our examination of the entire study population with both Chinese and Caucasian subjects. When only Chinese subjects were examined, the association between baseline measurements of iris thickness and change in anterior chamber angle width after LPI became statistically insignificant, perhaps due to a lack of power. There is indirect evidence in the literature to support that the true association between baseline measurements of iris thickness and change in anterior chamber angle width after LPI is negative rather than positive. Hirose F et al. investigated light-dark changes in iris thickness and anterior chamber angle width and demonstrated that when going from light to dark conditions, peripheral iris thickening induces narrowing of the anterior chamber angle.29 Specifically, they reported significant negative correlations between the differences in mean iris thickness and the differences in mean AOD and TISA under light and dark conditions.
A higher baseline measurement of iris thickness may also be associated with smaller post-LPI increases in TISA500 and TISA750 through another non-pupillary block mechanism. LPI widens the anterior chamber angle by removing the pupillary block component, but some eyes remain narrow after LPI.30 Persistent narrow angle after post-LPI indicates underlying secondary mechanisms of angle closure such as anteriorly positioned ciliary processes.31 The anteriorly positioned ciliary processes provide structural support beneath the peripheral iris, preventing the iris root from falling away from the trabecular meshwork after LPI.32 However, posterior movement of the ciliary body after LPI has been observed even in cases of residual angle closure, suggesting that eyes with anteriorly positioned ciliary processes still experience opening of the anterior chamber angle, but only to a smaller degree.33 The thicker and more rigid irides of Asians have been postulated as possible causes for their higher incidence of residual angle closure after LPI.30,31,34 A higher baseline measurement of iris thickness may be associated with smaller posterior movement of the ciliary body after LPI, which would result in smaller amount of anterior chamber angle widening. This notion remains speculative, as our study cannot examine this relationship due to a lack measurement of the position of ciliary processes before and after LPI.
This study has several potential limitations. First, the ZAAP software depended upon manual identification of the scleral spur as a measurement reference point for image processing. Manual scleral spur localization is subjective in nature, so our study-design attempted to control for this by utilizing a single ophthalmologist to read all the ASOCT images and selecting a random subset of 20 ASOCT images for reproducibility testing by the same ophthalmologist at a separate session. The random subset of 20 ASOCT images selected for reproducibility testing yielded fair to excellent intraobserver correlations. Second, the present study recruited subjects from a university-based general ophthalmology and glaucoma clinic, thus our study population may have suffered from selection bias despite consecutive subject enrollment. Consequently, the results of this study may not apply to the general population. Third, the study only recruited subjects who are PACS, so our findings may not be fully extrapolated to those with PAC and PACG. Another limitation is the lack of gonioscopic correlation and UBM data to compare with the ASOCT parameters. UBM data would also have allowed us to determine the association of plateau iris configuration with our outcomes. To help assess whether the excluded group had an excess of plateau iris configuration and thus may have biased our results, we used residual narrow angle as a surrogate marker for plateau iris and did not find a difference with our included study group.
In summary, this study showed that lower baseline measurements of iris thickness at three different positions are associated with greater increases in TISA500 and TISA750 as well as greater decrease in ICURV after LPI. These results may have implications for the clinical prevention and treatment of angle closure. Although prophylactic LPI is effective for preventing PAC in those with anatomically narrow angles, only a minority of individuals with anatomically narrow angles develops PAC. Therefore, prophylactic treatment in those with narrow angles remains controversial, as it may result in a large number of unnecessary LPIs. Incorporating measurements of iris thickness in the risk-benefit assessment of preventative LPI can provide a more quantitative approach and augment an ophthalmologist’s clinical judgment in directing therapy to individuals who would benefit the most from treatment or at least alert the clinician as to who will likely remain narrow after laser. Further studies on a larger population are warranted to confirm our findings.
Acknowledgments
Financial support:
This study was supported by NIH-NEI EY002162 – Core Grant for Vision Research, Research to Prevent Blindness, and That Man May See, Inc.
Footnotes
Conflict of Interest:
The authors have no financial or other conflicts of interest concerning this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol. 2002;17:50–8. doi: 10.1076/soph.17.2.50.14718. [DOI] [PubMed] [Google Scholar]
- 2.Ramos JL, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography - a review. Clin Experiment Ophthalmol. 2009;37:81–9. doi: 10.1111/j.1442-9071.2008.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan S, See J, Smith SD, et al. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:3683–8. doi: 10.1167/iovs.06-1120. [DOI] [PubMed] [Google Scholar]
- 4.Lee RY, Huang G, Porco TC, et al. Differences in iris thickness among African Americans, Caucasian Americans, Hispanic Americans, Chinese Americans, and Filipino-Americans. J Glaucoma. 2013;22:673–8. doi: 10.1097/IJG.0b013e318264ba68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JH, Sung KR, Yun SC, et al. Factors associated with anterior chamber narrowing with age: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012;53:2607–10. doi: 10.1167/iovs.11-9359. [DOI] [PubMed] [Google Scholar]
- 6.Wang BS, Narayanaswamy A, Amerasinghe N, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95:46–50. doi: 10.1136/bjo.2009.178129. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Sakata LM, Friedman DS, et al. Quantitative iris parameters and association with narrow angles. Ophthalmology. 2010;117:11–7. doi: 10.1016/j.ophtha.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Ophthalmology Glaucoma Panel. Primary Angle Closure. San Francisco, CA: American Academy of Ophthalmology; 2010. [Accessed December 10, 2013]. Preferred Practice Pattern Guidelines. Available at: http://one.aao.org/guidelines-browse?filter=preferredpracticepatterns. [Google Scholar]
- 9.Pandav SS, Kaushik S, Jain R, et al. Laser peripheral iridotomy across the spectrum of primary angle closure. Can J Ophthalmol. 2007;42:233–7. [PubMed] [Google Scholar]
- 10.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer RN. Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:112–27. [PubMed] [Google Scholar]
- 12.Lee RY, Huang G, Cui QN, et al. Association of lens vault with narrow angles among different ethnic groups. Curr Eye Res. 2012;37:486–91. doi: 10.3109/02713683.2012.669006. [DOI] [PubMed] [Google Scholar]
- 13.Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011;129:1283–90. doi: 10.1001/archophthalmol.2011.272. [DOI] [PubMed] [Google Scholar]
- 14.Seider MI, Pekmezci M, Han Y, et al. High prevalence of narrow angles among Chinese-American glaucoma and glaucoma suspect patients. J Glaucoma. 2009;18:578–81. doi: 10.1097/IJG.0b013e3181996f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CY, Liu S, Weinreb RN, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:4040–6. doi: 10.1167/iovs.09-3941. [DOI] [PubMed] [Google Scholar]
- 16.Leung CK, Palmiero PM, Weinreb RN, et al. Comparisons of anterior segment biometry between Chinese and Caucasians using anterior segment optical coherence tomography. Br J Ophthalmol. 2010;94:1184–9. doi: 10.1136/bjo.2009.167296. [DOI] [PubMed] [Google Scholar]
- 17.Lee RY, Kasuga T, Cui QN, et al. Association between baseline angle width and induced angle opening following prophylactic laser peripheral iridotomy. Invest Ophthalmol Vis Sci. 2013;54:3763–70. doi: 10.1167/iovs.13-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RY, Kasuga T, Cui QN, et al. Comparison of anterior segment morphology following prophylactic laser peripheral iridotomy in Caucasian and Chinese eyes. Clin Experiment Ophthalmol. doi: 10.1111/ceo.12243. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foo LL, Nongpiur ME, Allen JC, et al. Determinants of angle width in Chinese Singaporeans. Ophthalmology. 2012;119:278–82. doi: 10.1016/j.ophtha.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–9. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 21.Chan JB, Yuen LH, Huang EH, et al. Reproducibility of cornea measurements in anterior segment OCT images of normal eyes and eyes with bullous keratopathy analyzed with the Zhongshan Assessment Program. Invest Ophthalmol Vis Sci. 2011;52:8884–90. doi: 10.1167/iovs.10-6411. [DOI] [PubMed] [Google Scholar]
- 22.Huang G, Gonzalez E, Lee R, et al. Anatomic predictors for anterior chamber angle opening after laser peripheral iridotomy in narrow angle eyes. Curr Eye Res. 2012;37:575–82. doi: 10.3109/02713683.2012.655396. [DOI] [PubMed] [Google Scholar]
- 23.How AC, Baskaran M, Kumar RS, et al. Changes in anterior segment morphology after laser peripheral iridotomy: an anterior segment optical coherence tomography study. Ophthalmology. 2012;119:1383–7. doi: 10.1016/j.ophtha.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Konstas AG, Marshall GE, Lee WR. Immunocytochemical localisation of collagens (I–V) in the human iris. Graefes Arch Clin Exp Ophthalmol. 1990;228:180–6. doi: 10.1007/BF00935730. [DOI] [PubMed] [Google Scholar]
- 25.He M, Lu Y, Liu X, et al. Histologic changes of the iris in the development of angle closure in Chinese eyes. J Glaucoma. 2008;17:386–92. doi: 10.1097/IJG.0b013e31815c5f69. [DOI] [PubMed] [Google Scholar]
- 26.Dorairaj S, Liebmann JM, Ritch R. Quantitative evaluation of anterior segment parameters in the era of imaging. Trans Am Ophthalmol Soc. 2007;105:99–108. [PMC free article] [PubMed] [Google Scholar]
- 27.Amerasinghe N, Foster PJ, Wong TY, et al. Variation of angle parameters in Asians: an anterior segment optical coherence tomography study in a population of Singapore Malays. Invest Ophthalmol Vis Sci. 2009;50:2626–31. doi: 10.1167/iovs.08-2582. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Yu M, Ye C, et al. Anterior chamber angle imaging with swept-source optical coherence tomography: an investigation on variability of angle measurement. Invest Ophthalmol Vis Sci. 2011;52:8598–603. doi: 10.1167/iovs.11-7507. [DOI] [PubMed] [Google Scholar]
- 29.Hirose F, Hata M, Ito SI, et al. Light-dark changes in iris thickness and anterior chamber angle width in eyes with occludable angles. Graefes Arch Clin Exp Ophthalmol. 2013;251:2395–401. doi: 10.1007/s00417-013-2378-4. [DOI] [PubMed] [Google Scholar]
- 30.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114:494–500. doi: 10.1016/j.ophtha.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 31.Yeung BY, Ng PW, Chiu TY, et al. Prevalence and mechanism of appositional angle closure in acute primary angle closure after iridotomy. Clin Experiment Ophthalmol. 2005;33:478–82. doi: 10.1111/j.1442-9071.2005.01065.x. [DOI] [PubMed] [Google Scholar]
- 32.Pavlin CJ, Foster FS. Plateau iris syndrome: changes in angle opening associated with dark, light, and pilocarpine administration. Am J Ophthalmol. 1999;128:288–91. doi: 10.1016/s0002-9394(99)00149-x. [DOI] [PubMed] [Google Scholar]
- 33.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in eyes with narrow drainage angles: ultrasound biomicroscopy outcomes. The Liwan Eye Study. Ophthalmology. 2007;114:1513–9. doi: 10.1016/j.ophtha.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Nonaka A, Kondo T, Kikuchi M, et al. Cataract surgery for residual angle closure after peripheral laser iridotomy. Ophthalmology. 2005;112:974–9. doi: 10.1016/j.ophtha.2004.12.042. [DOI] [PubMed] [Google Scholar]