Abstract
Background
Healthcare providers have little population-based evidence about health-related quality of life (HRQOL) changes, from the pre- to post-diagnosis period, and treatment-related recovery time for women ages 65 and older diagnosed with breast cancer.
Methods
Older women with and without breast cancer completed self-reports of HRQOL at baseline and 2 years later as part of annual Medicare Health Outcomes Surveys (MHOS). MHOS was linked to Surveillance, Epidemiology, and End Results registries, which were used to categorize women with breast cancer by treatment type (breast-conserving surgery, breast-conserving+radiation, mastectomy) and time since diagnosis at follow-up. Each cancer case diagnosed in 1998-2007 (N=542) was matched to five women without cancer (N=2,710) using propensity score matching. Analysis of covariance models examined changes in HRQOL, adjusting for demographics and initial functioning.
Results
Older women within 6 months of diagnosis had greater declines than women without cancer in SF-36 Physical (-5.8 vs. -1.8) and Mental (-3.6 vs. -0.7) Component Summary scores, General Health (-12.3 vs. -4.6), Vitality (-11.0 vs. -2.2), Bodily Pain (-8.5 vs. -2.1), Social Functioning (-15.1 vs. -3.3), Role-Physical (-26.5 vs. -3.9), and Role-Emotional (-13.1 vs. -3.1) scores (all p<.05). By approximately 1 year, women with and without breast cancer had similar HRQOL. Comparable declines in Physical Component Summary and Role-Physical occurred across treatment types.
Conclusion
Women ages 65 and older diagnosed with breast cancer should be counseled that survivors within six months of diagnosis are vulnerable to HRQOL declines, compared to women without breast cancer, but that decrements generally wane after 12 months.
Keywords: quality of life, recovery of function, geriatrics, breast cancer, treatment-related recovery time
Introduction
There is growing emphasis on post-treatment health-related quality of life (HRQOL) for women ages 65 and older with breast cancer. However, population-based evidence is limited because older women are systematically underrepresented in breast cancer clinical trials, and observational and cohort studies focusing exclusively on older breast cancer survivors are rare.1 Older women have a 6-fold higher incidence rate for breast cancer than younger women,2 yet little is known about how HRQOL changes from before to after a breast cancer diagnosis and the duration of treatment-related HRQOL recovery time in this older population. Projections for the years 2020 and 2030 indicate that the proportion of women diagnosed at ages 65 and older will increase by 33% and 56%, respectively,3 which underscores the need for prospective HRQOL studies.
At diagnosis, women are presented with a range of treatment options. Important considerations in the choice of treatment(s) include HRQOL, comorbid conditions, and life expectancy (e.g., a 65-year-old woman has an average life expectancy of 20 years). Older women with breast cancer (OWBC) are a heterogeneous population with varying levels of functioning and, thus, treatment choice cannot be made on the basis of age alone.4,5 However, healthcare providers have little population-based evidence to counsel OWBC about changes to expect in HRQOL and how long it will take to recover from different treatment options, beyond overall survival and recurrence rates. This leaves OWBC in an uncertain position for making an informed decision about treatment(s) to pursue.
To our knowledge, two population-based studies have focused exclusively on OWBC to examine prospective HRQOL changes over the first year after diagnosis for OWBC receiving different treatments.6-7 Ganz et al. conducted a prospective study with 691 OWBC in four U.S. regions to examine HRQOL at 3, 6 (mental health only), and 15 months post-diagnosis.6 Treatment categories included breast-conserving surgery (BCS), breast-conserving surgery+radiation therapy (BCS+RT), mastectomy, and adjuvant chemotherapy and endocrine therapy.6 Significant predictors of a decline in physical function were greater comorbid conditions and Medicaid insurance.6 The proportion of OWBC recovering in HRQOL scores by the 15-month assessment was not reported, and thus, treatment-related recovery time could not be examined.6
Prescott et al. randomized 255 OWBC at 53 centers in the U.K. to BCS or BCS+RT.7 HRQOL was a primary endpoint assessed at baseline (post-surgery), 2 weeks, 9 months, and 15 months; and similar HRQOL changes were observed for BCS and BCS+RT groups. Physical functioning was significantly lower at 9 and 15 months for both groups.7 Social functioning and breast symptoms improved for both groups by 9 months.7 Mobility and home maintenance improved by 15 months for both groups and other domains did not change significantly.7 Both the Ganz and Prescott studies6-7 did not have HRQOL assessments available prior to cancer diagnosis, which limits the ability to predict the OWBC who should be targeted for rehabilitation or other supportive care services. Both studies6-7 also did not have a control group of older women without breast cancer that would allow assessment of the impact of cancer against age- or comorbidity-related changes in HRQOL.
We examined the duration of treatment-related HRQOL recovery time and the extent to which treatment modality affected changes in HRQOL, from the pre- to post-diagnosis period, among women ages 65 years and older. We also compared older survivors' HRQOL changes over a 2-year interval to matched older women without cancer.
Methods
Dataset
Clinical data from Surveillance, Epidemiology, and End Results (SEER) cancer registries were linked to HRQOL data from the Medicare Health Outcomes Survey (MHOS).8-9 The MHOS is administered annually to approximately 1,000-1,200 randomly selected beneficiaries from participating managed care organizations in the Medicare Advantage program (including institutionalized and disabled beneficiaries). Each year (starting in 1998), a baseline survey is administered along with a follow-up survey two years later if the beneficiary remains in the same managed care organization.9 Eight SEER-MHOS cohorts are included in this study (1998-2007).
Participants
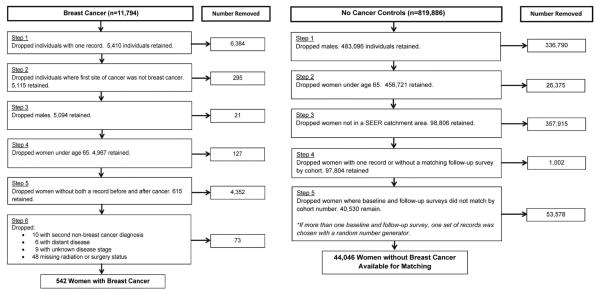
The SEER-MHOS dataset includes 11,794 beneficiaries with breast cancer and 819,886 without cancer. Individuals with the following characteristics were excluded: men; <65 years old; living outside a SEER catchment area; and did not complete a follow-up MHOS (Figure 1). For OWBC, further exclusions included: cancer was not diagnosed between at least one baseline and follow-up MHOS; prior cancer other than breast; missing radiation or surgery status; second non-breast cancer diagnosis between baseline and follow-up; unknown disease stage status; and metastatic disease. Propensity score matching methods10 were used to match each breast cancer case (N=542) to 5 women without cancer (N=2,710) based on demographics (age, race/ethnicity, education, marital status, smoking status), whether a proxy filled out the baseline survey, type of pre-existing comorbid conditions (e.g., heart disease, diabetes, etc.), SEER catchment area, and cohort year (Table 1). Propensity score matching variables were chosen based on literature with OWBC showing correlations between these variables and HRQOL outcomes2,11 and clinical expertise. For women without breast cancer who may have participated in more than one MHOS cohort (thus having multiple baseline and follow-up surveys), we used a random number generator to pick which records would be retained.
Figure 1. Selection of Older Women with and without Breast Cancer.
Table 1. Comparison of Covariates for Older Women With and Without Breast Cancera.
| Breast Cancer Cases by Treatment Type | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic | Women Without Breast Cancer (N = 2710) | Women With Breast Cancer (N = 542) | Breast-Conserving Surgery (N = 96) | Breast-Conserving Surgery + RT (N = 258) | Mastectomy (±RT)(N = 188) |
| Education, N (%) | |||||
| ≤High school | 1614 (60%) | 335 (62%) | 69 (72%) | 143 (55%) | 123 (65%) |
| Some college or 2-year degree | 676 (25%) | 127 (23%) | 18 (19%) | 68 (26%) | 41 (22%) |
| College graduate or higher | 420 (15%) | 80 (15%) | 9 (9%) | 47 (18%) | 24 (13%) |
| Mean age at baseline survey (SD), years | 74.76 (6.36) | 74.33 (5.76) | 76.21 (7.16) | 73.48 (5.01) | 74.54 (5.72) |
| Race, N (%) | |||||
| White | 2156 (80%) | 418 (77%) | 80 (83%) | 193 (75%) | 145 (77%) |
| Asian/Pacific Islander | 209 (8%) | 48 (9%) | 5 (5%) | 25 (10%) | 18 (9%) |
| Hispanic | 176 (6%) | 33 (6%) | 4 (4%) | 18 (7%) | 11 (6%) |
| Black/African American | 128 (5%) | 33 (6%) | 6 (6%) | 16 (6%) | 11 (6%) |
| Another race or multirace | 41 (1%) | 10 (2%) | 1(1%) | 6(2%) | 3 (2%) |
| Marital status (baseline), N (%) | |||||
| Married | 1330 (49%) | 259 (48%) | 45 (47%) | 136 (53%) | 78 (41%) |
| Widowed | 960 (35%) | 192 (35%) | 36 (38%) | 77 (30%) | 79 (42%) |
| Divorced/separated | 312 (12%) | 64 (12%) | 10 (10%) | 28 (11%) | 26 (14%) |
| Never married | 35 (1%) | 9 (2%) | 2 (2%) | 7 (3%) | 0 (0%) |
| Smoking status, N (%) | |||||
| Never | 1739 (64%) | 345 (64%) | 69 (72%) | 170 (66%) | 106 (56%) |
| Former | 694 (26%) | 132 (24%) | 21 (22%) | 51 (20%) | 60 (32%) |
| Current | 160 (6%) | 34 (6%) | 2 (2%) | 18 (7%) | 14 (7%) |
| Proxy (baseline), N (%) | |||||
| Yes | 135 (5%) | 33 (6%) | 10 (10%) | 10 (4%) | 13 (7%) |
| Preexisting conditions, N (%) | |||||
| Hypertension/high blood pressure | 1611 (60%) | 339 (62%) | 61 (63%) | 157 (61%) | 121 (64%) |
| Angina pectoris/coronary artery disease | 275 (10%) | 55 (10%) | 14 (14%) | 25 (10%) | 16 (9%) |
| Arthritis of hip or knee | 1157 (43%) | 241 (44%) | 52 (54%) | 99 (38%) | 90 (48%) |
| Sciatica | 644 (24%) | 135 (25%) | 22 (23%) | 62 (24%) | 50 (27%) |
| Other heart conditions | 549 (20%) | 122 (22%) | 21 (22%) | 48 (19%) | 53 (28%) |
| Diabetes | 369 (14%) | 75 (14%) | 10 (10%) | 31 (12%) | 34 (18%) |
| Emphysema, asthma, or COPD | 287 (11%) | 62 (11%) | 13 (13%) | 25 (10%) | 24 (13%) |
| Stroke | 153 (6%) | 36 (7%) | 7 (7%) | 16 (6%) | 13 (7%) |
| Myocardial infarction/heart attack | 135 (5%) | 23 (4%) | 5 (5%) | 9 (3%) | 9 (5%) |
| Congestive heart failure | 87 (3%) | 21 (4%) | 3 (3%) | 9 (3%) | 9 (5%) |
| Crohn's, ulcerative colitis, or IBD | 135 (5%) | 29 (5%) | 4 (4%) | 11 (4%) | 14 (7%) |
All baseline patient characteristics and preexisting conditions were matched between women with and without cancer.
Abbreviations: COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; RT, radiation therapy; SD, standard deviation.
Measures
HRQOL was measured using the RAND Short Form-36 (SF-36)12 for all MHOS through 2005. The SF-36 has been used extensively in individuals with and without cancer.12 The 8 scales of the SF-36 (Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Mental Health, Role-Emotional, Social Functioning) along with two summary scores, Physical Component Summary (PCS) and Mental Component Summary (MCS), were evaluated.
In 2006, the MHOS switched from the SF-36 to the Veterans Rand-12 (VR-12), which affected the follow-up data for cohorts 7-8. The follow-up scores for 77 women with breast cancer (14%) and 362 women without breast cancer (13%) were affected by the VR-12 switch. The VR-12 mirrors the same 8 subscales from the SF-36 but with fewer items. Subscale scores from the SF-36/VR-12 were rescaled so that every response option had a value in the range of 0-100 (i.e., the lowest score possible is 0 and the highest possible score is 100) and then averaging items within each subscale.13 This allows items with different response options to be directly comparable. In order to harmonize across questionnaire versions, published algorithms were used to derive VR-12 PCS and MCS scores for all cohorts.14 Population norms for the PCS and MCS scores are reported using 1990 U.S. norms14 and scored on a T-score metric (mean=50, SD=10). No population-based reference norms are available for the VR-12 subscale scores.14 Higher SF-36/VR-12 scores reflect better HRQOL.12
Surgery type was categorized as breast-conserving surgery or mastectomy, according to SEER guidelines.15 Radiation therapy was dichotomized as yes/no. We created a combined variable capturing surgery type and radiation status: breast-conserving surgery alone (BCS), breast-conserving surgery+radiation therapy (BCS+RT), or mastectomy to reflect categories used by Ganz6 and Prescott.7 Most mastectomy cases (165/188 or 88%) did not receive radiation. Given the small sample size that would have resulted for a mastectomy+RT category (n=23), we created one category for mastectomy with or without radiation. We did a sensitivity analysis excluding the 23 women from the mastectomy group who received RT and all results remained the same (data not shown).
Analysis Strategy
Analysis of covariance models were used to examine changes in HRQOL controlling for initial HRQOL and covariates (age, ethnicity/race, marital status, education, smoking status, SEER catchment area, baseline comorbid conditions, and comorbidities diagnosed between baseline and follow-up MHOS). SEER variables were also included as covariates in analyses involving only survivors: breast cancer stage and age at diagnosis.
We first compared changes in HRQOL, from before to after diagnosis, stratified by treatment type and compared OWBC's changes to women without cancer. We then categorized OWBC as being 0-6, 7-12, 13-18, or 19+ months post-diagnosis at the time of MHOS follow-up (because breast cancer was required to be diagnosed between baseline and follow-up). HRQOL changes were compared between OWBC categorized by time since diagnosis (regardless of treatment type) and women without breast cancer.
Analyses were performed in SAS (version 9.3) with two-sided statistical tests. IRB exemption was granted from the University of North Carolina at Chapel Hill.
Results
Participant Characteristics
Demographic characteristics are provided in Table 1 and clinical characteristics for OWBC are provided in Table 2. Half of the OWBC were ages 65-74 at the time of diagnosis (52%) with the remainder diagnosed at ≥75 years. Most OWBC were early stage (79%), Caucasian (77%), and completed ≤ high school (62%). Matched women without cancer were selected to reflect similar characteristics.
Table 2. SEER-Reported Variables for Older Women With Breast Cancer.
| Breast Cancer Cases by Treatment Type | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | Women With Breast Cancer(N = 542) | Breast-Conserving Surgery(N = 96) | Breast-Conserving Surgery + RT(N = 258) | Mastectomy (±RT)(N = 188) |
| Age at diagnosis, N (%) | ||||
| 65-69 y | 116 (21%) | 15 (16%) | 60 (23%) | 41 (22%) |
| 70-74 y | 168 (31%) | 33 (34%) | 81 (31%) | 54 (29%) |
| 75-79 y | 137 (25%) | 16 (16%) | 74 (29%) | 47 (25%) |
| 80-84 y | 88 (16%) | 15 (16%) | 39 (15%) | 34 (18%) |
| 85+ y | 33 (6%) | 17 (18%) | 4 (1%) | 12 (6%) |
| Time from cancer diagnosis to follow-up survey, mean (SD), months | 13.11 (8.45) | 13.33 (8.49) | 12.59 (8.66) | 13.71 (8.14) |
| Time from cancer diagnosis to survey, N (%) | ||||
| 0-6 mo | 126 (23%) | 20 (21%) | 67 (26%) | 39 (21%) |
| 7-12 mo | 147 (27%) | 30 (31%) | 61 (24%) | 56 (30%) |
| 13-18 mo | 134 (25%) | 23 (24%) | 70 (27%) | 41 (22%) |
| 19+ mo | 135 (25%) | 23 (24%) | 60 (23%) | 51 (28%) |
| Cancer stage, N (%) | ||||
| In situ | 94 (17%) | 34 (35%) | 44 (17%) | 16 (9%) |
| Localized | 339 (62%) | 50 (52%) | 178 (69%) | 111 (59%) |
| Regional | 109 (20%) | 12 (13%) | 36 (14%) | 61 (32%) |
| Estrogen receptor–positive (ER+) | 411 (76%) | 71 (75%) | 187 (72%) | 153 (81%) |
Abbreviations: RT, radiation therapy; SD, standard deviation.
Missing Data
Given our inclusion criteria requiring both a baseline and follow-up MHOS, missing data were minimal. For demographics, missing data ranged from education (2%) to proxy filling out form (8%); ≤ 5% and <1% were missing for comorbidities and SF-36/VR-12 subscales, respectively. Consistent with standards for missing data, we did not impute data and used Complete Case Analysis.16
HRQOL Changes by Cancer Status and Time since Diagnosis
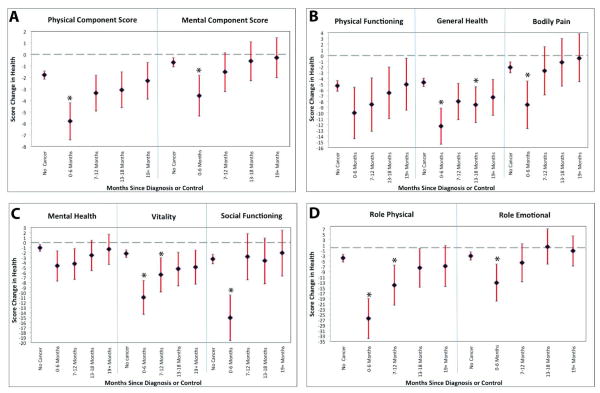
OWBC who were within 6 months of cancer diagnosis at follow-up had significantly worse decline than women without cancer in PCS (-5.8 vs. -1.8), MCS (-3.6 vs. -.07), General Health (-12.3 vs. -4.6), Bodily Pain (-8.5 vs. -2.1), Vitality (-11.0 vs. -2.2), Social Functioning (-15.1 vs. -3.3), Role-Physical (-26.5 vs. -3.9), and Role-Emotional (-13.1 vs. -3.1) scores (all p<.05) (Figure 2 a-d). OWBC and women without cancer did not show differences in subscale scores for Physical Functioning (-4.9 vs. -1.8) and Mental Health (-4.6 vs. -1.0) (Figure 2 b-c). With notable exceptions, by approximately 7-12 months after diagnosis no differences in HRQOL were observed between women with and without breast cancer.
Figure 2. VR-12 Subscale Score Changes and 95% Confidence Intervals for Older Women with and without Breast Cancer: Time Since Diagnosis.
Vitality, Role-Physical, and General Health scores for OWBC were slower to recover to the levels of women without breast cancer. At 7-12 months, OWBC remained significantly lower than women without cancer in Vitality (-6.7 vs. -2.3) and Role-Physical (-14.1 vs. -3.8) scores (both p<.05), which subsided by 13-18 months post-diagnosis (Figure 2 c-d). General Health for OWBC was significantly lower at 13-18 months post-diagnosis (-8.4 vs. -4.5, p<.05) (but not at 7-12 months) (Figure 2 b).
HRQOL Changes by Cancer Status and Treatment Modality
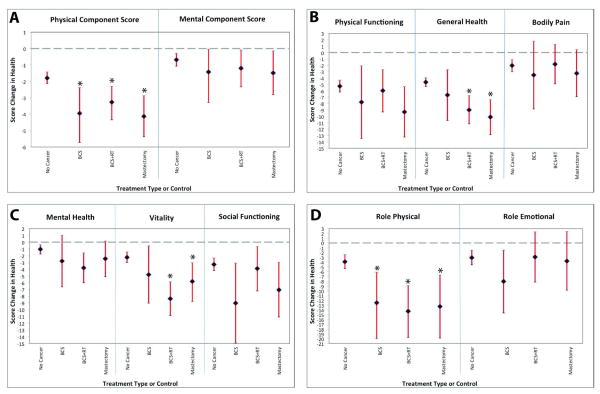
OWBC, across all treatment types, had greater declines over 2 years than women without breast cancer in PCS (-3.4 vs. -1.8) and Role-Physical (-12.6 vs. -3.8) scores (both p<.05) (Figure 3 a, d). Women with and without breast cancer did not differ in changes on the MCS (-1.5 vs. -0.7), Physical Functioning (-7.2 vs. -5.5), Mental Health (-3.0 vs. -1.0), Social Functioning (-5.6 vs. -3.3), or Role-Emotional (-4.2 vs. -3.4) scores (Figure 3 a-d). OWBC receiving BCS+RT had greater declines than women without cancer in General Health (-8.9 vs. −4.5) and Vitality (-7.5 vs. -2.3) (both p<.05) (Figure 3 b-c). OWBC receiving a mastectomy (with or without RT) also showed greater declines in General Health (-9.3 vs. -4.5) and Vitality (-6.3 vs. -2.3) than women without cancer (both p<.05; Figure 3 b-c). Head-to-head HRQOL comparisons between OWBC receiving BCS, BCS+RT, or mastectomy were not significantly different from one another in PCS (-3.2 vs. −3.3 vs. -3.5), MCS (-1.8 vs. −1.2 vs. −1.6), or subscale scores (Figure 3 a-d), indicating that similar HRQOL decrements occurred across treatment types.
Figure 3. VR-12 Subscale Score Changes and 95% Confidence Intervals for Older Women with and without Breast Cancer: Treatment Types.
Discussion
This study quantifies the effects of breast cancer and its treatments on HRQOL for women ages 65 years and older in comparison with similar women without cancer. Our results identify the domains of HRQOL most acutely affected by breast cancer and corresponding recovery time in order to facilitate age-appropriate, early supportive efforts. Inclusion of a matched control group allowed us to assess the unique impact of breast cancer, controlling for age- and comorbidity-related changes in HRQOL.
OWBC within 6 months of diagnosis had greater declines than women without cancer in Physical and Mental Component Summary scores and six out of eight subscales (General Health, Vitality, Bodily Pain, Social Functioning, Role-Physical, and Role-Emotional). We observed recovery by 7-12 months in most domains, but slower recovery in Vitality, Role-Physical, and General Health. In other words, HRQOL declines among OWBC generally resolved and returned to the same levels as women without breast cancer around 1 year post-diagnosis.
OWBC's experiences of slower recovery time in Vitality, Role-Physical, and General Health may be due to radiation or endocrine therapy, chemotherapy, or other unmeasured variables (e.g., reduced physical activity17). Ionizing radiation may cause damage to normal and malignant cells and side effects generally occur from damage to normal tissue, which likely affects HRQOL. Loss of vitality (i.e., fatigue) is a common symptom experienced shortly after completing radiation therapy.11,17 Our study shows that OWBC continue to experience greater declines in Vitality and General Health 1-2 years after receiving radiation therapy than women without cancer. However, omission of radiation therapy is controversial for OWBC.5
Endocrine therapy may have also played a role in slower recovery times in Vitality, Role-Physical, and General Health. In a large trial with OWBC, women randomized to an aromatase inhibitor (after five years on tamoxifen) reported worse HRQOL scores in vitality, pain, and physical functioning after 12 months compared to OWBC receiving placebo.18 Endocrine therapy data are not available in SEER,8-9 but 76% of OWBC in our study had hormone-receptor-positive tumors, which are clinically indicated for adjuvant endocrine therapy.5 The proportion of hormone-receptor-positive tumors was spread evenly across treatment types (75% BCS, 72% BCS+RT, 81% mastectomy), suggesting that influence on our results would have been diffused across groups. However, this diffusion may partially explain why we did not observe HRQOL differences across treatment types. More research is warranted with OWBC to determine the effects of endocrine therapy on HRQOL changes and recovery time.
Chemotherapy may have played a part in slower recovery times but we were not able to examine this in our dataset. Chemotherapy data, although available in SEER, are believed to be unreliable due to limited follow-up and a modest correlation with chart reviews for women with breast cancer.19 OWBC are considered for chemotherapy when tumors are grade 3, hormone-receptor-negative with her2 overexpression, or ≥4 nodes are positive.5 Approximately 15-20% of our sample would have been considered for chemotherapy with these guidelines so we controlled for stage in analyses. The optimal duration of chemotherapy is not known for OWBC,5 but regimens generally last between 12-24 weeks (at least 4 cycles). Very little data exist about HRQOL declines from chemotherapy in OWBC.11,20 Twenty-one percent of the Ganz study participants received chemotherapy and no significant differences were found in comparison to surgery types, radiation, and tamoxifen.6 This suggests that the lack of chemotherapy data did not have a large impact on our results.
When treating OWBC, careful attention to the management of treatment-related symptoms and comorbid conditions is warranted, especially during the first 6 months after diagnosis. In turn, enhanced symptom monitoring may shorten recovery time to usual functioning. A comprehensive geriatric assessment instrument is informative for symptom management because it estimates functional status, comorbid conditions, cognitive status, mental and social health, nutritional state, and polypharmacy.4,5 More research is needed with OWBC to identify better models for predicting slower recovery time and to determine if rehabilitation between the time of diagnosis and start of acute treatment minimizes declines.21
Similar to Ganz,6 Prescott,7 and smaller studies,11,20 we observed comparable HRQOL changes when comparing treatment types, suggesting that other variables play a greater role in OWBC's HRQOL outcomes. For instance, in Alberg and Singh's conceptual model of aging and treatment for breast cancer, comorbid conditions assume the central role.2 The length of time women have comorbid conditions may affect HRQOL in important ways but we were not able to determine this from our data. In analyses, we controlled for comorbid conditions present at baseline and conditions developed between baseline and follow-up. The interrelationships between breast cancer and comorbid conditions are not well understood in OWBC and the mechanisms by which they affect HRQOL and recovery time need to be elucidated.
Our study builds on the population-based findings of Prescott7 and Ganz6 by including assessments prior to diagnosis, more geographic sites in the U.S. (14 vs. 4 in Ganz6), and a matched-control group of older women without breast cancer. We used propensity score matching to select the most appropriate controls for each breast cancer case, which reduces bias by balancing demographic characteristics and comorbid conditions between cancer and no-cancer groups.10
Finally, our results are also consistent with research showing initial declines and then improvement in HRQOL scores from 6-18 months post-diagnosis for younger pre-menopausal and middle-aged women with early stage breast cancer.22 Unfortunately, extrapolating guidelines for treating OWBC from research conducted with younger women is not clinically indicated because OWBC have different pathology, clinical, and psychological profiles. OWBC are less likely to be node-positive, have smaller and slower growing tumors, greater comorbid conditions, more interactions with medications, and less psychological impact from cancer than younger women.2,5 It is imperative that OWBC be included in clinical trials and prospective observational studies of HRQOL (in sufficient numbers to meet a priori power requirements for sub-analyses) to better understand HRQOL outcomes and recovery time across a range of treatment situations.
Limitations
Dataset limitations are important to consider when interpreting these findings. SEER limitations may have led to misclassification of treatment types. MHOS limitations include a modified HRQOL measure administered to later cohorts and a lack of cancer-specific HRQOL variables. We consider each of these in turn.
Radiation therapy received beyond 6 months post-diagnosis is not captured well in SEER,23 which may have led to misclassification of treatment types. Radiation therapy involves treatment five days a week for 3-7 weeks. The median time from surgery to initiation of radiation is 34 days,24 suggesting that the lack of this data beyond six months after diagnosis had minimal impact on our data.
SEER reports only the most invasive surgery so it was not possible to identify OWBC who first received BCS and received a mastectomy later.25 We also were not able to form a no-surgery group because this has not been validated in SEER,25 nor groups for reconstructive surgery because the sample size would have been too low to produce reliable estimates. Given that women in our sample were enrolled in managed care plans, we also did not have access to claims information. All of these situations are important to understand for better treatment management in OWBC.
MHOS dataset limitations include different versions of the SF-36/VR-12 items being administered for follow-up in cohorts 7-8. Only 13-14% of follow-up scores were affected by this change in both cancer and no-cancer groups. We used published algorithms to create variables that were directly comparable for SF-36/VR-12.14 Cohort year was reflected in propensity scores and controlled for in analyses. The MHOS also lacks variables assessing body mass index and cancer-specific HRQOL variables such as bowel functioning, sexual functioning, cognitive functioning, and body image.9 These would be important variables to consider in future research with OWBC.
Conclusions
Women ages 65 years and older diagnosed with breast cancer should be counseled that treated survivors within 6 months of diagnosis are particularly vulnerable to declines in HRQOL, as compared to older women without breast cancer, but that these decrements generally wane after 12 months. This should be reassuring to older breast cancer patients who are frequently in good health otherwise with relatively long life expectancies. Our findings also suggest that enhanced symptom monitoring may be warranted for older women during treatment. Careful attention to the management of treatment-related symptoms may shorten recovery time to usual functioning among OWBC, especially during the first 6 months after diagnosis.
Acknowledgments
Funding sources for this work: AS: NIH (5R25-CA057726, 5T32-HS000032). DKM: AHRQ (HHSA290201200008I). SW: AHRQ (1K12HS019468). HM, JL, and BB: None.
Footnotes
Disclosures: None.
References
- 1.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Singh S. Epidemiology of breast cancer in older women: Implications for future healthcare. Drugs Aging. 2001;18:761–772. doi: 10.2165/00002512-200118100-00005. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB. Coming of age: breast cancer in seniors. Oncologist. 2011;16:79–87. doi: 10.1634/theoncologist.2011-S1-79. [DOI] [PubMed] [Google Scholar]
- 5.Baganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer. Lancet. 2012;13:e148–160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: Quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 7.Prescott RJ, Kunkler IH, Williams LJ, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population: The PRIME trial. Health Technol Assess. 2007;11:31. doi: 10.3310/hta11310. [DOI] [PubMed] [Google Scholar]
- 8.Reeve BB, Potosky AL, Wilder Smith A, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambs A, Warren JL, Bellizzi KM, et al. Overview of the SEER-Medicare Health Outcomes Survey linked dataset. Health Care Financ Rev. 2008;29:5–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Reeve BB, Smith AW, Arora NK, Hays RD. Reducing bias in cancer research: application of propensity score matching. Health Care Financ Rev. 2008;29:69–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger RS, Fallowfield LJ. Quality of life and patient-reported outcomes in the older breast cancer patient. Clin Oncol. 2009;21:140–155. doi: 10.1016/j.clon.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Turner-Bowker DM, Bartley PJ, Ware JE. SF-36® Health Survey & “SF” Bibliography: Third Edition (1988 – 2000) Lincoln, RI: QualityMetric, Inc.; 2002. [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. 2004 – 2006 Cohort 7 QIO Performance Measurement Data User's Guide. Washington, D.C.: Author; 2009. [Google Scholar]
- 14.Fleishman JA, Selim AJ, Kazis LE. Deriving SF-12v2 physical and mental health summary scores: a comparison of different scoring algorithms. Qual Life Res. 2010;19:231–41. doi: 10.1007/s11136-009-9582-z. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. SEER surgery codes for breast cancer. [accessed June, 2013]; Available from URL: http://seer.cancer.gov/manuals/2013/AppendixC/breast/surgery_codes.pdf.
- 16.Fox-Wasylyshyn SM, Maher ME. Focus on research methods: Handling missing data in self-report measures. Res Nurs Health. 2005;28:488–495. doi: 10.1002/nur.20100. [DOI] [PubMed] [Google Scholar]
- 17.Courneya KS, Mackey JR, McKenzie DC. Exercise for breast cancer survivors: Research evidence and clinical guidelines. Phys Sportsmed. 2002;30:33–42. doi: 10.3810/psm.2002.08.402. [DOI] [PubMed] [Google Scholar]
- 18.Muss HB, Dongsheng T, Ingle JN, et al. Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with Letrozole or placebo after five years of tamoxifen. J Clin Oncol. 2008;26:1956–1964. doi: 10.1200/JCO.2007.12.6334. [DOI] [PubMed] [Google Scholar]
- 19.Du XL, Key CR, Dickie L, et al. Information on chemotherapy and hormone therapy from tumor registry had moderate agreement with chart reviews. J Clin Epidemiol. 2006;59:53–60. doi: 10.1016/j.jclinepi.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelblatt J, Figueiredo M, Cullen J. Outcomes and quality of life following breast cancer treatment in older women: When, why, how much, and what do women want?: A review. Health Qual Life Outcomes. 2003;1:45. doi: 10.1186/1477-7525-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver JK, Baima J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715–727. doi: 10.1097/PHM.0b013e31829b4afe. [DOI] [PubMed] [Google Scholar]
- 22.Montazeri A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(supplement):IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 24.Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. Br Med J. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(supplement):IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]