Background: Adiponectin has vascular and metabolic protective actions; however, its in vivo receptor-mediated signaling pathways are incompletely understood.
Results: Adiponectin receptor 1 (AdipoR1)-deficiency exacerbates diet-induced metabolic dysfunction, and AdipoR2 deficiency results in impaired revascularization.
Conclusion: AdipoR2, but not AdipoR1, is essential for the revascularization actions of adiponectin.
Significance: Adiponectin receptors, AdipoR1 and AdipoR2, confer different functions in vivo.
Keywords: Adiponectin, Angiogenesis, Metabolism, Receptor, Vascular Biology, AdipoR1, AdipoR2
Abstract
Adiponectin is a well described anti-inflammatory adipokine that is highly abundant in serum. Previous reports have found that adiponectin deficiency promotes cardiovascular and metabolic dysfunction in murine models, whereas its overexpression is protective. Two candidate adiponectin receptors, AdipoR1 and AdipoR2, are uncharacterized with regard to cardiovascular tissue homeostasis, and their in vivo metabolic functions remain controversial. Here we subjected AdipoR1- and AdipoR2-deficient mice to chronic hind limb ischemic surgery. Blood flow recovery in AdipoR1-deficient mice was similar to wild-type; however, revascularization in AdipoR2-deficient mice was severely attenuated. Treatment with adiponectin enhanced the recovery of wild-type mice but failed to rescue the impairment observed in AdipoR2-deficient mice. In view of this divergent receptor function in the hind limb ischemia model, AdipoR1- and AdipoR2-deficient mice were also evaluated in a model of diet-induced obesity. Strikingly, AdipoR1-deficient mice developed severe metabolic dysfunction compared with wild type, whereas AdipoR2-deficient mice were protected from diet-induced weight gain and metabolic perturbations. These data show that AdipoR2, but not AdipoR1, is functionally important in an in vivo model of ischemia-induced revascularization and that its expression is essential for the revascularization actions of adiponectin. These data also show that, in contrast to revascularization responses, AdipoR1, but not AdipoR2 deficiency, leads to diet-induced metabolic dysfunction, revealing that these receptors have highly divergent roles in vascular and metabolic homeostasis.
Introduction
Factors secreted from adipose tissue, termed adipokines, can directly and indirectly affect vascular function and metabolic disease (1). Most adipokines are proinflammatory, and their up-regulation under conditions of obesity contributes to the development of a chronic inflammatory state. In contrast, adiponectin is an adipokine with anti-inflammatory and insulin-sensitizing properties (2). Clinically, circulating levels of adiponectin are reduced in obesity and type II diabetes, whereas weight loss elevates serum adiponectin (3). Adiponectin deficiency in mice is reported to exacerbate metabolic dysfunction under conditions of diet-induced obesity in some (4–6) but not all studies (7). Conversely, the overexpression of adiponectin alleviates metabolic dysfunction independent of weight loss in both genetic and diet-induced models of obesity (8, 9). Thiazolidinediones (TZDs),3 peroxisome proliferator-activated receptor γ activators, elevate circulating adiponectin levels in animal models as well as the patient population (6, 10). Furthermore, the beneficial metabolic actions of TZDs appear to be dependent on expression of adiponectin because these drugs have been shown to be ineffective in normalizing blood glucose levels in ob/ob mice that are also deficient for adiponectin (6).
Candidate adiponectin receptors, AdipoR1 and AdipoR2, were initially identified by expression cloning and reported to mediate the insulin-sensitizing actions of adiponectin (11). These proteins are predicted to have seven transmembrane domains with the opposite topology of G protein-coupled receptors. Both AdipoR1 and AdipoR2 are reported to be ubiquitously expressed with the highest expression in skeletal muscle and liver, respectively (11). Although there are many studies on AdipoR1 and AdipoR2 function and regulation in cell culture models (for example, see Refs. 12–17), relatively few studies have explored their roles in mouse genetic models. Yamauchi et al. (18) reported that deficiency of AdipoR1, AdipoR2, or both adiponectin receptors resulted in glucose intolerance in a mouse model of diet-induced obesity. It has also been reported that both whole body and muscle-specific AdipoR1-KO mice display metabolic dysfunction (19). Consistent with these observations, AdipoR1 overexpression in rat skeletal muscle ameliorates insulin resistance and promotes glucose uptake (20), and overexpression of AdipoR1 in murine macrophages attenuates weight gain and improves glucose metabolism models of metabolic dysfunction (21). However, controversy about the metabolic functions of AdipoR1 and AdipoR2 also exists. Bjursell et al. (22) independently constructed AdipoR1 and AdipoR2 gene knock-out mice and reported that they display opposing effects on glucose metabolism. In this study, AdipoR1-KO mice showed increased adiposity and decreased glucose clearance, whereas AdipoR2-KO mice were lean and displayed improved glucose clearance. Furthermore, a third strain of AdipoR2-KO mouse was constructed by Liu et al. (23), who reported biphasic effects of a high fat diet. In this study AdipoR2-KO mice were initially resistant to metabolic dysfunction, but glucose homeostasis deteriorated as the high fat feeding was continued.
In addition to its metabolic role, adiponectin has been shown to be associated with various clinical cardiovascular disorders including myocardial infarction (24), peripheral artery disease (25), and endothelial dysfunction (26). Because low levels of adiponectin are found in individuals that are prone to vascular diseases, the association between obesity and vascular disease could be partly attributed to hypoadiponectinemia. Consistent with these findings, our laboratory has previously identified adiponectin as a cardiovascular-protective factor in mouse models of myocardial infarction (27) and cardiac hypertrophy (28) under dietary conditions where systemic metabolic properties are normal. Adiponectin also has the property of promoting angiogenesis in the ischemic tissue in mouse models of peripheral artery disease (17, 29–31). In this model adiponectin-deficient mice have impaired revascularization compared with wild-type mice, whereas the addition of exogenous adiponectin rescues the impairment in adiponectin-deficient mice and enhances the angiogenic response in wild-type mice. Adiponectin has also been shown to be protective in a model of retinal neovascularization (32, 33). The ability of adiponectin to promote revascularization and angiogenesis in these models may be due in part to its ability to stimulate protective signaling pathways within vascular endothelial cells and promote endothelial progenitor cell mobilization (for example, see Refs. 34–36). Furthermore, TZDs upregulate adiponectin expression, and this is one mechanism for their vascular-protective actions in mice (33, 37, 38).
Although the cardiovascular-protective activities of adiponectin are well documented, the roles of AdipoR1 and AdipoR2 have never been evaluated in an in vivo angiogenesis model using strains of mice that are genetically engineered to be void of these receptors. In contrast, in vitro studies with cultured endothelial cells have reported that both AdipoR1 and AdipoR2 are expressed by endothelial cells and that both are required to mediate various actions of adiponectin (for example, see Refs. 13, 14, and 17). Thus, the aim of this study was to determine if expression of AdipoR1 or AdipoR2 is required for the revascularizing actions of adiponectin in a model of peripheral artery disease. First, we subjected AdipoR1-KO mice, AdipoR2-KO mice, and wild-type littermate controls to hind limb ischemia to determine if the candidate adiponectin receptor-KO mice have a similar impairment in blood flow recovery as reported for adiponectin-deficient mice. We also tested whether the administration of exogenous adiponectin could rescue any impaired revascularization in adiponectin receptor-deficient mice. Finally, using the same mouse strains employed for the vascular measurements, we analyzed the respective roles of AdipoR1 and AdipoR2 in metabolic dysfunction.
EXPERIMENTAL PROCEDURES
Mice
AdipoR1-KO and AdipoR2-KO mice (C57BL/6 background) were originally created by Deltagen and ordered from Mutant Mouse Regional Resource Centers (MMRRC) and The Jackson Laboratory (#005775), respectively. Mice were maintained as heterozygous breeding pairs, and wild-type littermates were used as controls. The Institutional Animal Care and Use Committees of Boston University approved all study procedures. Mice were maintained in a 12-h light/dark schedule and given chow diet (Teklad Global 18% protein rodent diet, #2018) or high fat/high sucrose diet (Bioserv #1850) and water ad libitum. The composition of the high fat/high sucrose diet is as follows: 36.0% fat (primarily lard), 36.2% carbohydrates (primarily sucrose), and 20.5% protein.
Hind Limb Ischemia Model
Blood flow was unilaterally restricted in the femoral artery of 9–11-week-old male mice. AdipoR1-KO, AdipoR2-KO, or wild-type mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Under sterile conditions, an incision was made in the upper thigh. Proximal to the body cavity, the femoral artery, vein, and nerve were ligated using 6-0 silk suture, downstream collaterals were severed, and a segment of the ligated vessel was excised. Surgical clips closed the incision site and were removed 14 days after the procedure. Doppler perfusion imaging was used to assess blood flow in both limbs before surgery, immediately after surgery, and at days 3, 7, 14, 21, and 28 post-surgery. Data are presented as a ratio of blood flow in the ischemic limb to the non-ischemic limb to control for variations in room and body temperature.
Score-based Assessment of Limb Function
At the time of laser Doppler measurement (post-surgical day 3), limb coloring and function were assessed by a modified version of a clinical scoring system described previously (39). A score of 0–4 was assigned to each mouse based on characteristics of the feet (0, normal function and coloring; 1, normal function and mild discoloration; 2, normal function and moderate discoloration; 3, impaired function and moderate discoloration; 4, impaired function and necrosis).
Histology
Gastrocnemius muscles were harvested, weighed, and embedded in OCT compound. Sections (5 μm) were generated from the muscle tissue. Hematoxylin and eosin were used to stain the tissue using standard methods after fixing in cold acetone for 10 min. Using ImageJ software, area of viable myofibers was calculated and expressed as a percentage of the total high powered field area. Immediately after sacrifice, liver and adipose tissue were incubated in 10% formalin overnight. Samples were dehydrated in a series of ethanol washes, treated with xylene, and embedded in paraffin. Sections were cut at 7 μm, deparaffinized, and then stained with hematoxylin and eosin. To assess capillary density, frozen gastrocnemius muscle sections were prepared and sectioned as described above. Frozen sections were incubated with CD31 primary antibody (PECAM-1, BD Biosciences) at a 1:50 dilution overnight at 4 °C. Secondary FITC-conjugated antibody was added for 1 h at room temperature. Fluorescent microscope images were randomly taken of three high power fields per genotype. Capillary density was expressed as average number of capillaries per field.
Hydrodynamic Plasmid Delivery
In adiponectin restoration of function experiments, wild-type or AdipoR2-KO mice received a hydrodynamic injection of pLEV113-mADIPO-hFc fusion construct (adiponectin plasmid) or pLEV113-MCS (control plasmid, LakePharma) 1 week before hind limb ischemia surgery. Briefly, a saline solution (0.9% sodium chloride) equal to 10% of the mouse's body weight and containing 24 μg of adiponectin or control plasmid was injected into the tail vein of restrained, unanesthetized mice in 5–8 s as described previously (40, 41). Mice were allowed to recover for 1 week. At the time of surgery, a small amount of blood was harvested by tail vein bleed. Serum was isolated after centrifugation at 7000 rpm. ELISA (B-Bridge) was used to assess serum concentrations of adiponectin according to manufacturer's instructions.
Western Blotting
Blood was collected from AdipoR2-KO mice and wild-type littermates 1 week after hydrodynamic delivery of control or adiponectin plasmid. Serum was resolved by SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with 5% nonfat milk in PBS + 0.1% Tween for 1 h at room temperature. Primary antibody (adiponectin, R&D Systems, 1:1000) was added to the membrane overnight at 4 °C in blocking buffer. The membrane was then incubated with secondary HRP-conjugated antibody (1:5000) for 1 h at room temperature and developed using ECL Prime (GE Healthcare).
Real-time Quantitative PCR
Male C57BL/6J mice (The Jackson Laboratory) were fed a normal chow or a high fat/high sucrose diet for 12 weeks starting at 8 weeks of age. At the time of sacrifice, tissues were harvested and stored in RNA stabilization reagents RNAlater (Ambion) or Allprotect Tissue Reagent (Qiagen). RNA from hind limb and metabolic experiments was isolated using RNeasy Fibrous Tissue Mini kit, RNeasy Mini kit, or RNeasy Lipid Tissue Mini kit (Qiagen). cDNA synthesis was performed using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems) in a final volume of 20 μl according to the manufacturer's instructions. Gene expression was assessed by real-time PCR using an ABI ViiA7 detection system (Applied Biosystems) and TaqMan technology. Relative mRNA expression was determined using TaqMan Master Mix reagent (Applied Biosystems) and the following primer/probe sets: GAPDH (Mm00484668_m1); Adipor1 (Mm01291334_mH), Adipor2 (Mm01184032_m1); T-cadherin (Cdh13) (Mm00490584_m1). Expression was normalized to GAPDH, and relative levels were calculated using the 2−ΔΔCt method.
Metabolic Model and Assessment
Wild-type, AdipoR1-KO, and AdipoR2-KO female mice were fed a high fat/high sucrose diet starting at 9–11 weeks old and continuing for a total of 16 weeks. Mice were fasted overnight before sacrifice. Glucose and insulin tolerance tests were performed at 4, 8, and 12 weeks after the initiation of high fat/high sucrose diet feeding. For the glucose tolerance test, mice were fasted for 16 h, and base-line blood glucose was measured by the Accu-Chek Aviva system. Glucose was administered by intraperitoneal injection (1 g of glucose/kg of body weight). Blood glucose was measured at the following time points: 15, 30, 60, 90, and 120 min after glucose injection. Mice were fasted for 6 h before the start of the insulin tolerance test. An intraperitoneal injection of insulin (0.6 units of insulin/kg body weight) was administered after measurement of baseline blood glucose levels. Glucose measurements were taken at 15, 30, and 60 min post-injection. Serum insulin and leptin levels were measured by ELISA (Crystal Chem, Inc. and R&D Systems, respectively).
Statistical Analysis
Data are presented as the mean ± S.E. Using GraphPad Prism software, data were analyzed using Student's t test or analysis of variance with Bonferroni post-hoc tests as appropriate. In time course experiments a two-way repeated measures analysis of variance was performed. A p value of less than 0.05 was considered statistically significant.
RESULTS
Differential Effects of AdipoR1 and AdipoR2 Deficiency on Limb Reperfusion
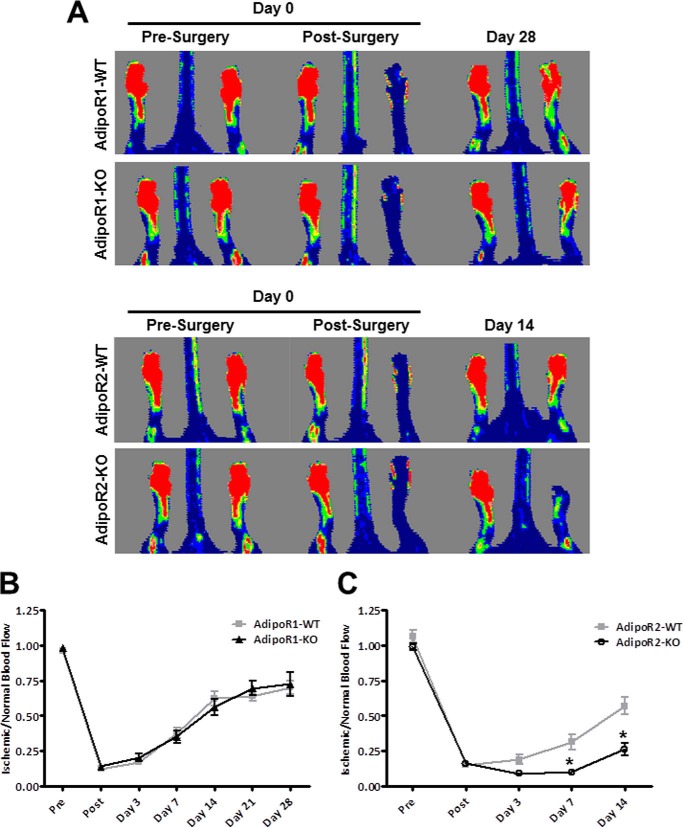
It has been previously reported that adiponectin-deficient mice display impaired revascularization in a model of chronic hind limb ischemia (17, 29–31). To identify a functional receptor for adiponectin in the vasculature, unilateral hind limb ischemia surgery was performed on mice deficient in AdipoR1 or AdipoR2 and their wild-type littermates. AdipoR1-KO mice recovered limb perfusion at a similar rate as wild-type mice (Fig. 1A). After 28 days both wild-type and AdipoR1-KO mice had recovered 70–75% of blood flow in their ischemic limbs (Fig. 1B). In contrast, AdipoR2-KO mice had notably delayed recovery, and flow improvement was not detectable until 14 days after induction of ischemia. Limb retraction and atrophy in AdipoR2-KO mice are visible in the laser Doppler image (Day 14, Fig. 1A). Because of the severe limb necrosis observed, ∼30% of the AdipoR2-KO mice were euthanized by day 14 post-surgery per veterinarian recommendations. The surviving AdipoR2-deficient mice displayed significantly impaired blood flow recovery compared with their littermate controls (Fig. 1C).
FIGURE 1.
Differential effects of AdipoR1 and AdipoR2 on limb reperfusion. A, representative Doppler images of foot blood flow before surgery, immediately after surgery and at day 14 (AdipoR2-KO) or day 28 (AdipoR1-KO) post-surgery. B, blood flow recovery quantified from laser Doppler perfusion images of wild-type (gray line, squares) and AdipoR1-KO mice (black line, triangles) after hind limb ischemia surgery. Data are the mean ± S.E., n = 10–12, p > 0.05. C, blood flow recovery quantified from laser Doppler perfusion images of wild-type (gray line, squares) and AdipoR2-KO mice (black line, open circles) after hind limb ischemia surgery. Data are the mean ± S.E. n = 6. *, p < 0.05.
AdipoR2-deficient Mice Exhibit Limb Necrosis and Impaired Function
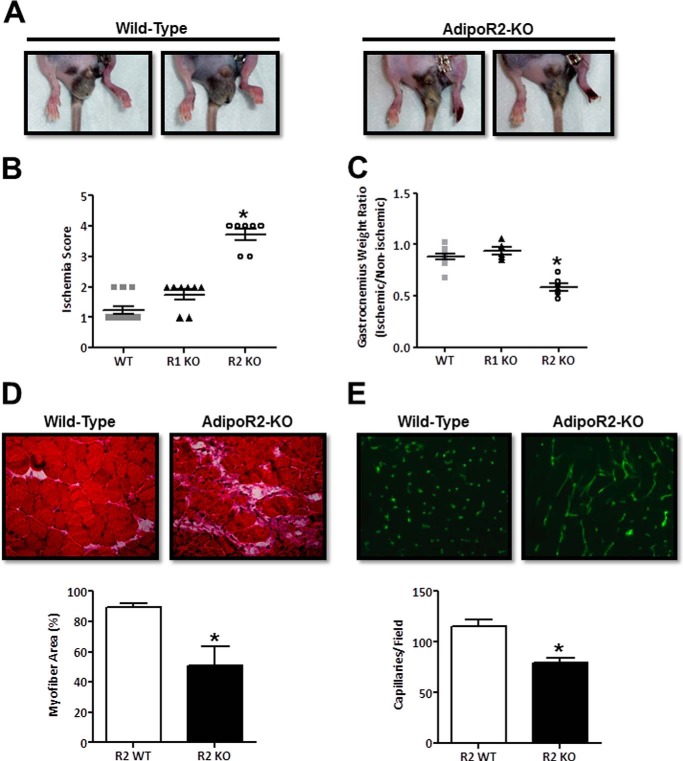
In wild-type and AdipoR1-deficient mice, toe necrosis is rare, and limb function appears to normalize 3–7 days after limb ischemia surgery. In contrast, the necrosis observed in AdipoR2-KO mice can extend past the toe nail and throughout the toes at 1 week post-surgery (Fig. 2A). In some AdipoR2-KO mice the foot completely necroses leading to auto-amputation. Marked necrosis and impaired limb function were found in AdipoR2-deficient but not in wild-type or AdipoR1-deficient mice. Evidence of toe necrosis was observed in >70% of AdipoR2-KO mice as early as 3 days after surgery (Fig. 2B).
FIGURE 2.
AdipoR2-deficient mice exhibit limb necrosis and impaired function after hind limb ischemia surgery. A, representative images of wild-type and AdipoR2-KO limbs 7 days post-hind limb ischemia surgery. B, clinical scoring assessment of limb appearance and function at day 3 post-surgery (0, normal function and coloring; 1, normal function and mild discoloration; 2, normal function and moderate discoloration; 3, impaired function and moderate discoloration; 4, impaired function and necrosis). Data are the mean ± S.E. n = 7–10. *, p < 0.05. C, gastrocnemius muscle weight 28 days post-surgery. Data are expressed as a ratio of ischemic/non-ischemic skeletal muscle weights. Data are the mean ± S.E. n = 6–10. *, p < 0.05. D, histological H&E stain of ischemic gastrocnemius muscle harvested 28 days post-surgery and quantitation of percent viable myofiber area per field. Data are the mean ± S.E. n = 5. *, p < 0.05. E, representative CD31 immunofluorescence images and capillary density analysis of gastrocnemius muscle harvested from the ischemic limb at day 28. Data are the mean ± S.E. n = 3. *, p < 0.05.
A supplemental video provides a visual comparison of the ambulatory activity of wild-type and AdipoR2-KO mice 3 days after hind limb ischemia surgery. At this time point wild-type mice can utilize both their ischemic and non-ischemic limbs equally. It can be observed that wild-type mice are capable of lifting, applying weight, and pushing off of both their non-ischemic and ischemic hind limbs. In marked contrast, AdipoR2-KO mice were consistently observed dragging their ischemic limb while walking. Unlike the AdipoR2-KO mice, no functional deficit was observed in the AdipoR1-KO mice relative to wild-type mice. A semi-quantitative scoring system was employed to evaluate limb condition. In this system, a higher score indicates more severe discoloration (necrosis) and loss of ischemic limb function, and the score is predictive of long term recovery after chronic hind limb ischemic injury (39). Consistent with the laser Doppler imaging analysis, wild-type and AdipoR1-KO mice did not differ in their ischemia score 3 days post-surgery. In contrast, AdipoR2-deficient mice had a significantly higher score compared with wild-type controls (Fig. 2B).
Gastrocnemius muscle was harvested from wild-type, AdipoR1-KO, and AdipoR2-KO mice 28 days after hind limb ischemia surgery to evaluate muscle weight and histological features. In the ischemic hind limbs of wild-type mice, it is typical to observe ischemic muscle swelling (day 3) followed by atrophy (day 7), and ultimately, recovery to 85–90% of the muscle mass in the non-ischemic limb by day 28 (data not shown). Although this trend was observed in AdipoR1-KO mice, muscle atrophy remained unresolved in AdipoR2-KO mice at 28 days post-surgery (Fig. 2C). In wild-type mice, hematoxylin and eosin-stained gastrocnemius muscle sections display a centralized region of necrotic tissue surrounded by infiltrating inflammatory cells 3 days post-hind limb ischemia surgery. At days 7 and 14, angiogenesis and myocyte proliferation occur in the ischemic limb. Nascent myocytes, identified by their centralized nuclei, are abundant during this regenerative phase (data not shown). By day 28, necrosis is resolved, nascent myocytes are rare, and few inflammatory cells remain in wild-type skeletal muscle tissue. Immunohistochemical analysis revealed widespread, unresolved necrosis in AdipoR2-deficient skeletal muscle at day 28 (Fig. 2D). Within the ischemic lesion, the area of skeletal muscle tissue containing viable myofibers was reduced by 50% compared with wild type. In contrast to wild-type tissue, the majority of myocytes present in AdipoR2-KO tissue at 28 days are nascent, identified by their centralized nuclei, indicating an ongoing regenerative response. Strikingly, AdipoR2-KO skeletal muscle has a significant amount of acellular area containing cell debris and residual inflammatory infiltrate.
Consistent with measures of impaired perfusion by laser Doppler imaging analysis, ischemic gastrocnemius muscle from AdipoR2-KO mice has a reduced capillary density (Fig. 2E). The vessels present in the AdipoR2-KO tissue appear incompletely formed as evidenced by the irregular and elongated CD31 endothelial immunofluorescence. In contrast, vessels in the wild-type ischemic limb have a normal punctate CD31 staining pattern (Fig. 2E). Collectively, these data describe an attenuated blood vessel and skeletal muscle-regenerative response in AdipoR2-deficient mice in response to chronic ischemia.
AdipoR2 Is Necessary for the Revascularization Actions of Adiponectin
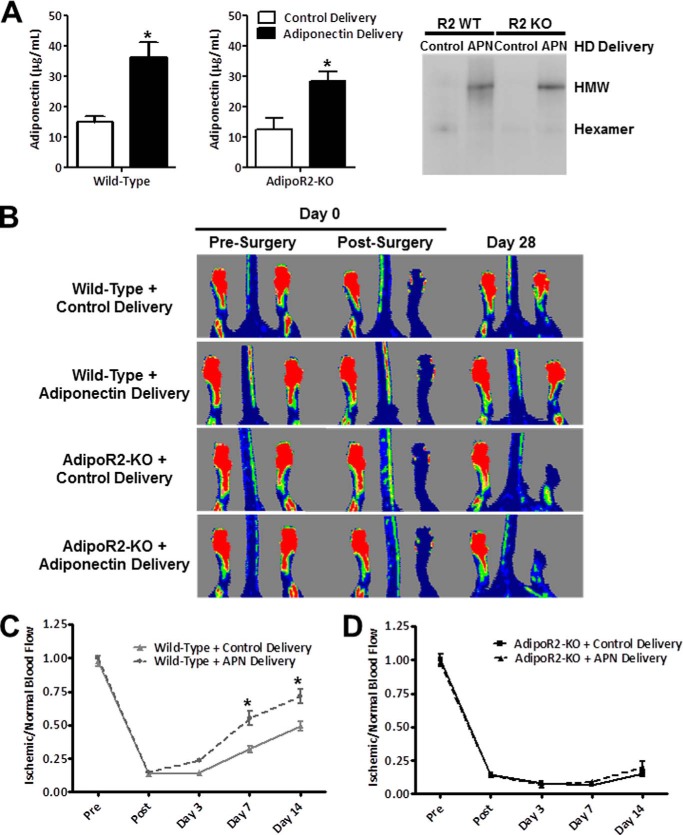
We previously reported that hydrodynamic delivery of adiponectin plasmid rescued the impairment in blood flow recovery observed in adiponectin-deficient mice subjected to hind limb ischemia (17). Thus, to test for a functional ligand-receptor interaction between adiponectin and AdipoR2, we determined if treatment with exogenous adiponectin would rescue the impairment in blood flow recovery in AdipoR2-KO mice. In the current study we employed the hydrodynamic method to deliver a plasmid-encoding adiponectin or a control plasmid to wild-type and AdipoR2-KO mice 1 week before hind limb ischemia surgery. No significant difference was detected in baseline serum adiponectin between wild-type and AdipoR2-KO mice. Treatment with adiponectin plasmid increased circulating levels of adiponectin by 2-fold in both wild-type and AdipoR2-KO mice. Serum levels of the hexameric isoform were unchanged, whereas the high molecular weight isoform increased in both wild-type and AdipoR2-KO mice (Fig. 3A). As anticipated, blood flow recovery was improved in adiponectin-treated wild-type mice compared with control-treated wild-type mice (Fig. 3B). At 14 days post-surgery, the ischemic limb of control-treated wild-type mice showed 50% blood flow recovery compared with the non-ischemic limb. Adiponectin treatment accelerated this recovery to 70% blood flow relative to the control limb (Fig. 3C). However, under conditions of AdipoR2 deficiency, adiponectin treatment did not promote limb reperfusion, and no difference in Doppler blood flow was observed between the ischemic limbs of control- and adiponectin-treated AdipoR2-deficient mice (Fig. 3D). Compared with the non-ischemic limb, Doppler blood flow in the ischemic limbs of AdipoR2-KO mice did not exceed 25% in either treatment group (Fig. 3D). Because adiponectin has an inhibitory effect on TNF-α, levels of this proinflammatory cytokine were measured in serum after hind limb ischemia surgery. Serum TNF-α levels were below the minimum detectable level of the kit in all wild-type samples tested. AdipoR2-KO mouse serum contained low levels of TNF-α that were not significantly different between control and adiponectin plasmid-treated AdipoR2-KO mice (data not shown). Taken together, these data show that expression of AdipoR2 is essential for adiponectin-mediated revascularization.
FIGURE 3.
AdipoR2 is necessary for the revascularization actions of adiponectin. A, wild-type and AdipoR2-KO mice were administered control (pLEV113-MCS) or adiponectin plasmid (pLEV113-mADIPO-hFc fusion construct, LakePharma) by hydrodynamic delivery (HD) 1 week before hind limb ischemia surgery. Adiponectin (APN) levels were measured by ELISA in serum samples harvested at the time of surgery. Data are the mean ± S.E. n = 5–9. *, p < 0.05 versus control treatment. Representative native Western blot of adiponectin isomers in serum harvested from mice at the time of surgery. HMW, high molecular weight adiponectin. B, representative Doppler images pre-surgery, immediately post-surgery, and 14 days post-surgery. C, laser Doppler perfusion analysis of control or adiponectin plasmid-treated wild-type mice. Data are the mean ± S.E. n = 6–9, *, p < 0.05. D, laser Doppler perfusion analysis of control or adiponectin plasmid-treated AdipoR2-KO mice. Data are the mean ± S.E., n = 5–7, p > 0.05.
Tissue mRNA Expression Analysis of Candidate Adiponectin Receptors
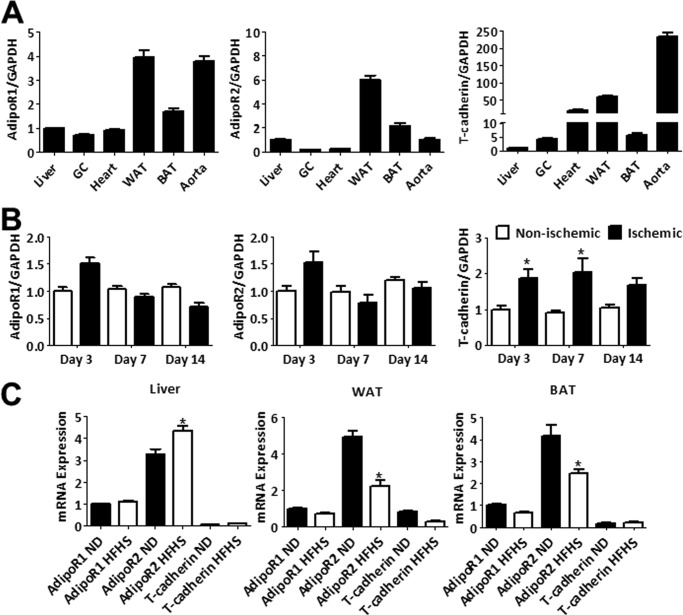
A distribution analysis was performed for AdipoR1 and AdipoR2 mRNA expression in vascular and metabolically important mouse tissues. AdipoR1 is abundant in white adipose tissue and aorta (Fig. 4A). AdipoR2 is most abundant in white and brown adipose tissue with relatively low transcript levels in cardiovascular tissues. This analysis was also performed on the adiponectin-binding protein, T-cadherin, that has recently been identified as essential for mediating the cardiac-protective and revascularization actions of adiponectin by localizing adiponectin to cardiovascular tissues (17, 42). T-cadherin mRNA is abundant in the heart and aorta and comparatively low in metabolically active skeletal muscle and liver tissues (Fig. 4A).
FIGURE 4.
Tissue mRNA expression analysis of candidate adiponectin receptors. A, tissue distribution of AdipoR1, AdipoR2, and T-cadherin was assessed in liver, skeletal muscle (gastrocnemius (GC)), heart, epididymal white adipose tissue (WAT), brown adipose tissue (BAT), and aorta by measuring relative mRNA expression. Data are the mean ± S.E. n = 6. B, ischemic and non-ischemic gastrocnemius muscles were harvested at the following time points after hind limb ischemia: day 3 (n = 6), day 7 (n = 5), and day 14 (n = 3). Expression of AdipoR1, AdipoR2, and T-cadherin mRNA was measured at each time point. Data are the mean ± S.E. *, p < 0.05. C, relative mRNA levels of the candidate adiponectin receptors in tissue isolated from wild-type mice fed a normal diet (ND) or high fat/high sucrose (HFHS) diet for 12 weeks. Data are the mean ± S.E. n = 6. *, p < 0.05.
Expression of each candidate receptor was also analyzed after hind limb ischemia surgery (Fig. 4B) and high fat/high sucrose diet (Fig. 4C) by real-time PCR. T-cadherin mRNA levels increased in ischemic muscle 3 days after hind limb ischemia surgery compared with expression in the non-ischemic muscle. A trend was observed for increased ischemic limb expression of AdipoR1 and AdipoR2 at day 3. T-cadherin mRNA levels remain elevated in the ischemic limb at 7 and 14 days post-surgery, whereas AdipoR1 and AdipoR2 levels return to base line at these time points. After 12 weeks of high fat/high sucrose diet, AdipoR2 levels were significantly increased in liver and decreased in white and brown adipose tissue. In contrast, no diet-induced changes in AdipoR1 or T-cadherin gene expression were observed.
High Fat/High Sucrose Diet Induces Metabolic Dysfunction in AdipoR1-deficient Mice
The importance of AdipoR1 and AdipoR2 in mediating the metabolic actions of adiponectin remains controversial (18, 22, 23). Because we observed a divergent role for AdipoR1 and AdipoR2 in the vascular actions of adiponectin, the metabolic consequences of eliminating AdipoR1 or AdipoR2 were examined.
When fed a normal chow diet, AdipoR1-deficient mice had an increased body weight, whereas AdipoR2-deficient mice had a reduced body weight compared with wild-type littermates at 9–11 weeks old. Importantly, no differences in glucose tolerance or insulin sensitivity were detected among the different experimental groups at base line (data not shown).
AdipoR1-KO and wild-type littermate controls were fed a high fat/high sucrose diet for 16 weeks starting at 9–11 weeks of age. After only 4 weeks of high fat/high sucrose diet, AdipoR1-KO mice exhibited significantly elevated body weight compared with wild-type littermate controls. After acute administration of glucose, wild-type mice cleared glucose more efficiently than AdipoR1-KO mice (data not shown). The increased body weight and impaired glucose tolerance observed in AdipoR1-KO mice persisted and became greater after 8 weeks (data not shown) and 12 weeks on the diet (Fig. 5, A and B). After injection of insulin, the effect on blood glucose levels was initially similar between groups. However, after 12 weeks of high fat/high sucrose diet, AdipoR1-KO mice displayed impaired sensitivity to insulin compared with wild-type mice (Fig. 5C).
FIGURE 5.
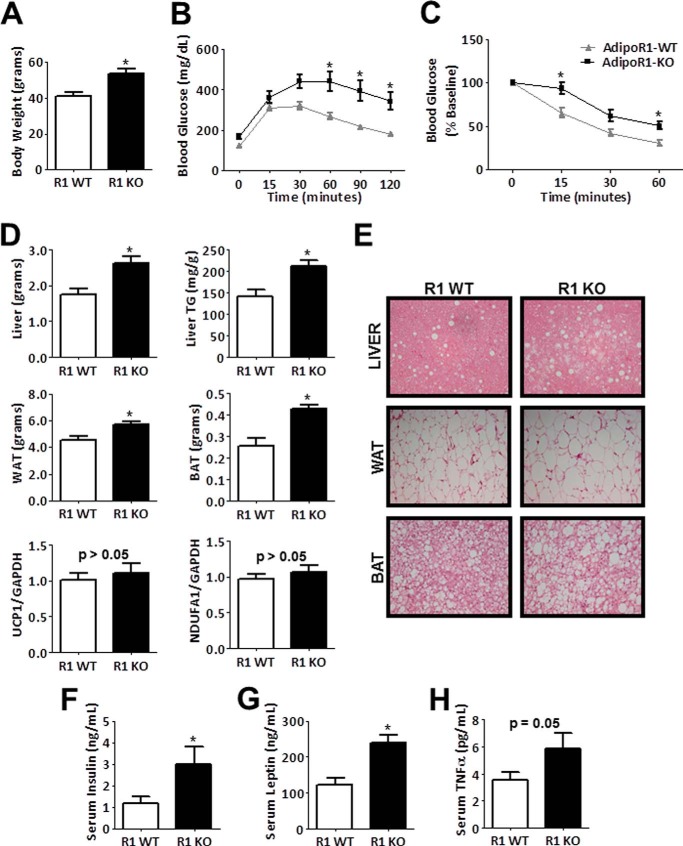
High fat/high sucrose diet induces metabolic dysfunction in AdipoR1-deficient mice. A–C, body weight, glucose tolerance test, and insulin tolerance test results of wild-type and AdipoR1-KO mice fed a high fat/high sucrose diet for 12 weeks. Data are the mean ± S.E. n = 8–12. *, p < 0.05. D, metabolic tissue weights after 16 weeks of diet, triglyceride (TG) content in liver, and relative mRNA expression of UCP1 and NDUFA1 in brown adipose tissue (BAT). Data are the mean ± S.E. n = 6–8. *, p < 0.05. TG, triglyceride. WAT, white reproductive adipose tissue; BAT, brown adipose tissue. E, representative images of hematoxylin and eosin-stained liver, white reproductive adipose tissue, and interscapular brown adipose tissue. F–H, serum levels of insulin, leptin, and TNF-α. Data are the mean ± S.E. n = 6–8. *, p < 0.05.
Metabolic tissues were harvested and weighed at the time of sacrifice. AdipoR1-deficient mice had increased reproductive white adipose tissue, interscapular brown adipose tissue, and liver weights compared with wild-type littermates (Fig. 5D). Gastrocnemius muscle weight was comparable between groups (data not shown). Lipid droplets were more abundant in hematoxylin and eosin-stained liver sections of AdipoR1-deficient mice (Fig. 5E). After tissue digestion, liver extracts from AdipoR1-KO mice have a higher concentration of triglycerides (Fig. 5D). The observed increases in liver weight and lipid accumulation in AdipoR1-KO mice indicate development of hepatic steatosis. In hematoxylin and eosin-stained sections from AdipoR1-KO mice, adipocytes in white adipose tissue have a larger diameter, and lipid droplets are more abundant in brown adipose tissue (Fig. 5E). However, mRNA expression of genes important for mitochondrial function, uncoupling protein 1 (UCP1) and NADH dehydrogenase [ubiquinone] 1α subcomplex subunit 1 (NDUFA1), was not different in brown adipose tissue between genotypes (Fig. 5D). In the serum, AdipoR1-deficient mice have elevated insulin, leptin, and TNF-α levels (Fig. 5, F–H), which are also indicative of metabolic dysfunction. In summary, AdipoR1-KO mice are more susceptible to diet-induced obesity complications including expansion of adipose depots, glucose intolerance, insulin resistance, and fatty liver.
AdipoR2-deficient Mice Are Protected from Diet-induced Weight Gain and Metabolic Dysfunction
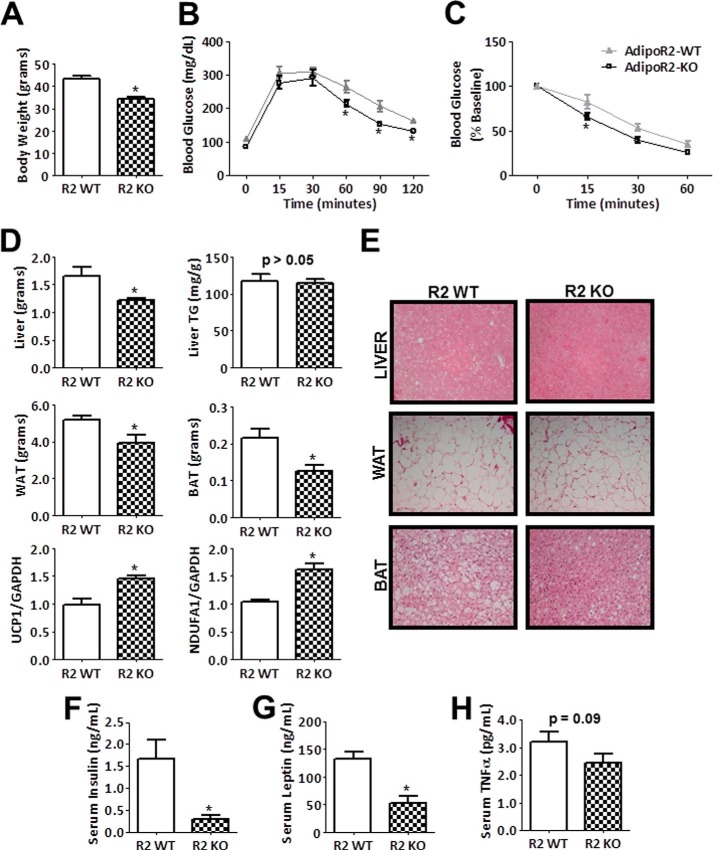
In contrast to AdipoR1-KO mice, AdipoR2-KO mice were resistant to high fat/high sucrose diet-induced weight gain after 4 and 8 weeks on diet. Glucose tolerance and insulin tolerance test performance did not differ between AdipoR2-KO mice and wild-type littermate controls at these early time points (data not shown). AdipoR2-KO mice maintained a significantly lower body weight than wild-type mice after 12 weeks of diet (Fig. 6A). At this point, peripheral tissues in AdipoR2-deficient mice had a significantly improved ability to clear of blood glucose compared with wild-type mice (Fig. 6B). In addition, tissue responsiveness to insulin in AdipoR2-KO mice was superior to that of wild-type mice (Fig. 6C). Consistent with these data, weights of reproductive white adipose tissue, interscapular brown adipose tissue, and liver tissue were significantly less than littermate controls (Fig. 6D). Lipid accumulation, as measured by liver triglyceride levels, was not significantly different between AdipoR2-KO and wild-type mice (Fig. 6D). However, adipocyte size in reproductive adipose tissue is slightly smaller, and the frequency of lipid droplets is reduced in brown adipose tissue (Fig. 6E). Transcript levels of UCP1 and NDUFA1 were elevated in AdipoR2-KO brown adipose tissue compared with wild-type littermate controls (Fig. 6D). In the serum AdipoR2-deficient mice have lower levels of insulin, leptin, and TNF-α compared with wild-type mice (Fig. 6, F–H), highlighting their preserved metabolic function. Collectively, these data reveal highly divergent metabolic roles for AdipoR1 and AdipoR2, with AdipoR2-deficient mice displaying improved parameters after exposure to high fat/high sucrose diet including enhanced glucose tolerance and insulin sensitivity and resistance to weight gain compared with wild-type littermate controls.
FIGURE 6.
AdipoR2-deficient mice are protected from diet-induced weight gain and metabolic dysfunction. A–C, body weight, glucose tolerance test, and insulin tolerance test results of wild-type and AdipoR2-KO mice fed a high fat/high sucrose diet for 12 weeks. Data are the mean ± S.E. n = 9–11. *, p < 0.05. D, metabolic tissue weights after 16 weeks of diet, triglyceride (TG) content in liver, and relative mRNA expression of UCP1 and NDUFA1 in brown adipose tissue (BAT). Data are the mean ± S.E. n = 4–9. *, p < 0.05. WAT, white reproductive adipose tissue. E, representative images of hematoxylin and eosin-stained liver, white reproductive adipose tissue, interscapular brown adipose tissue. F–H, serum levels of insulin, leptin, and TNF-α. Data are the mean ± S.E. n = 7–9. *, p < 0.05.
DISCUSSION
We previously reported that mice lacking adiponectin display impaired revascularization in a model of peripheral artery disease (17, 29–31). In the present study we provide the first evidence for the roles of the candidate adiponectin receptors AdipoR1 and AdipoR2 in mediating the revascularization-stimulatory actions of adiponectin in a murine model of peripheral artery disease. In this surgical model of chronic hind limb ischemia, AdipoR1 deficiency had no detectable effect on the blood flow recovery response compared with wild-type, littermate control mice. In striking contrast, AdipoR2-KO mice displayed a marked impairment in revascularization after hind limb ischemia surgery as measured by laser Doppler perfusion imaging and skeletal muscle capillary density. The hemodynamic deficit was so severe that the AdipoR2-KO mice developed necrosis and a loss of limb function. Administration of exogenous adiponectin significantly improved blood flow recovery in wild-type mice but failed to rescue the impaired phenotype of AdipoR2-KO mice. Taken together, these mouse genetic data indicate that expression of AdipoR2, but not AdipoR1, is essential for the revascularization actions of adiponectin.
A functional divergence between AdipoR1 and AdipoR2 was also observed in a model of diet-induced obesity. Mice fed a high fat/high sucrose diet for 12 weeks develop metabolic dysfunction, and in the context of this model, AdipoR1 deficiency led to more severe metabolic dysfunction. AdipoR1-KO mice had greater body weight and fat mass, hepatic steatosis, impaired glucose disposal, and elevations in serum insulin and leptin. Because AdipoR1 deficiency had no detectable impact in the hind limb ischemia model of revascularization as assessed by multiple anatomical and functional measures, these data document that murine AdipoR1 and AdipoR2 have exclusive roles in controlling metabolic and vascular function, respectively. Furthermore, in contrast to AdipoR1 deficiency, AdipoR2 deficiency was protective in the model of diet-induced obesity. Although AdipoR2-deficient mice displayed a severe regenerative deficiency in the revascularization model, they were protected against metabolic dysfunction in the obesity model. Collectively, these markedly divergent phenotypes document that AdipoR1 and AdipoR2 have functionally distinct roles in cardiovascular and metabolic disease models.
Despite numerous reports on the actions of adiponectin in model systems, relatively few studies have employed mouse genetic methods to study the roles of the AdipoR1/AdipoR2 receptors. To date, no mouse genetic studies have examined the roles of AdipoR1/AdipoR2 in ischemic cardiovascular models. However, a limited number of studies have examined metabolic function in AdipoR1- and AdipoR2-deficient mice. Bjursell et al. (22) observed that AdipoR1-KO mice exhibited increased body weight and glucose intolerance, whereas AdipoR2-KO mice were resistant to diet-induced weight gain and displayed improved glucose tolerance compared with wild-type controls. These findings mirror the metabolic data that are reported here. Also, consistent with the results presented here, Liu et al. (23) have reported that AdipoR2-KO mice are resistant to weight gain and glucose and insulin intolerance after 8 weeks of high fat diet feeding (AdipoR1-KO mice were not examined). In contrast to the metabolic phenotypes reported here and by others (22, 23), Yamauchi et al. (18) reported that either AdipoR1-deficient or AdipoR2-deficient mice developed glucose intolerance in the absence of changes in body weight. Similarly, a separate study found that adenoviral overexpression of either AdipoR1 or AdipoR2 improves insulin sensitivity in mice fed a high fat diet (43). The reasons for these discrepant results on AdipoR1/AdipoR2 deficiency in metabolic dysfunction are unknown. Theoretically, these differences could be due to variations in mouse models, mouse gender, and testing conditions among the laboratories (discussed in Ye and Scherer (44)).
Although no studies have examined the revascularization properties of AdipoR1 and AdipoR2 in vivo, numerous in vitro experiments have examined the roles of these receptors on angiogenic-like activities in cultured endothelial cells. For example, a dual siRNA knockdown of both AdipoR1 and AdipoR2 attenuates adiponectin-induced endothelial nitric-oxide synthase phosphorylation in human umbilical vein endothelial cells (13). In a similar study siRNA knockdown of AdipoR1 but not AdipoR2 was found to attenuate the activating phosphorylation of AMP-activated protein kinase after adiponectin treatment (30). In addition, we and others have found that reduced expression of either AdipoR1 or AdipoR2 inhibits adiponectin-mediated endothelial cell migration and proliferation (17), surrogate measures of angiogenesis. Of course, other cell types participate in limb regenerative response to ischemia. In this regard, adiponectin actions on progenitor cells (36), vascular smooth muscle cells (45), macrophages (46), and skeletal muscle (47) have all been described, but the relative roles of AdipoR1 versus AdipoR2 have generally not been defined in these systems. Thus, further studies involving the targeted ablation of AdipoR1 and AdipoR2 in various cardiovascular (and metabolic) cell types will ultimately be required to better define the divergent actions of AdipoR1 and AdipoR2 within the model organism.
Various mechanistic possibilities exist that could explain the divergent actions of AdipoR1 and AdipoR2. We examined differences in tissue mRNA expression of these widely expressed and structurally similar membrane proteins. Although AdipoR1 has important metabolic actions, its expression tends to be highest in cardiovascular tissues such as aorta and heart. AdipoR2 expression is high in liver and adipose depots despite having important vascular roles. Thus, the expression data is opposite of what may be expected based on the relative functional importance of each receptor. A second possibility for the divergent actions of AdipoR1 and AdipoR2 could be differences in intracellular signaling. Yamauchi et al. (18) reported that in terms of metabolic function AdipoR1 signals through AMP-activated protein kinase, whereas peroxisome proliferator-activated receptor-α is important for AdipoR2 signaling. However, both AMP-activated protein kinase and peroxisome proliferator-activated receptor-α also have well described roles in angiogenesis (29, 48–50). A third scenario is that currently unidentified adaptor proteins confer specific presentation of adiponectin to a receptor, thereby providing different functionalities. One likely candidate for this role is T-cadherin, a glycosylphosphatidylinositol-anchored adiponectin-binding protein. T-cadherin localizes hexameric and high molecular weight adiponectin to cardiovascular tissues, and its expression is essential for the protective actions of adiponectin in these depots (17, 42). Similar to the results presented here with AdipoR2, we have previously reported that mice lacking T-cadherin display impaired revascularization after hind limb ischemia that cannot be rescued by administration of adiponectin protein (17). Here, we report that T-cadherin displays relatively high expression in aorta, consistent with a vascular role. Thus, we speculate that T-cadherin presents high molecular weight adiponectin to AdipoR2 on endothelial cells to promote revascularization under conditions of ischemic stress. Interacting partners for AdipoR1 have been recently identified and include caveolins 1 and 3 (51, 52). Future studies are required to clarify these complex adiponectin receptor-mediated signaling mechanisms and how they may influence physiological outcomes.
In both mouse models of peripheral artery disease and metabolic dysfunction, the phenotypes of AdipoR2-KO mice and AdipoR1-KO mice, respectively, are much more severe compared with that of adiponectin deficiency. In other words, the receptor deficiency has a greater phenotypic effect than the ligand deficiency. In the model of hind limb ischemia, adiponectin-deficient mice have impaired blood flow recovery but rarely develop necrosis, whereas foot necrosis occurred in almost three-quarters of AdipoR2-KO mice tested. It is possible that the in vivo consequences of adiponectin deficiency are diminished by a compensatory mechanism involving the up-regulation of other anti-inflammatory adipokines. Although termed adiponectin receptors 1 and 2, these transmembrane proteins may have adiponectin-independent actions. For example, AdipoR1 or AdipoR2 may have constitutive functions or bind other ligands in addition to adiponectin. The C1q/TNF-related proteins (CTRPs) share structural and functional similarity with adiponectin. A recent in vitro study found that expression of AdipoR1 is important for the actions of C1q/TNF-related proteins in endothelial cells (53).
Although metabolic dysfunction is a strong risk factor for peripheral artery disease and other cardiovascular diseases, drugs that improve metabolic function have either failed to improve or worsen cardiovascular outcomes. For instance, clinical trials with TZDs have shown that their ability to lower glucose is generally uncoupled from a reduction in the risk of cardiovascular disease, as would be expected from improved glycemic control (54–56). Because TZDs work in part through their ability to modulate adiponectin expression (6, 33, 37, 38), our findings of distinct roles for adiponectin receptors 1 and 2 in models of ischemia and obesity may provide a molecular framework to understand aspects of the complex interplay between these co-morbidities.
Using identical mouse strains in side-by-side experiments of ischemic repair and diet-induced obesity, our results show for the first time that AdipoR1 and AdipoR2 have divergent roles in mediating revascularization and metabolic function in vivo. These experiments set the stage for future studies aimed at dissecting the mechanistic details that can account for the differential regulation conferred by AdipoR1 and AdipoR2.
Supplementary Material
Acknowledgments
We are grateful to Taina Rokotuiveikau Woodward and Matthew Phillippo for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AG34972, HL81587, and HL68758 (to K. W.), K01 AR055965-02 (to T. A.), and by Pre-doctoral Training Grants in Cardiovascular Biology HL007969 and Biomolecular Pharmacology GM008541 (to J. L. P.-D.).

This article contains a supplemental video.
- TZD
- thiazolidinedione
- AdipoR1 and AdipoR2
- adiponectin receptor 1 and 2, respectively
- UCP1
- uncoupling protein 1
- NDUFA1
- NADH dehydrogenase [ubiquinone] 1α subcomplex subunit 1.
REFERENCES
- 1. Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ouchi N., Kihara S., Funahashi T., Matsuzawa Y., Walsh K. (2003) Obesity, adiponectin, and vascular inflammatory disease. Curr. Opin. Lipidol. 14, 561–566 [DOI] [PubMed] [Google Scholar]
- 3. Hu E., Liang P., Spiegelman B. M. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 [DOI] [PubMed] [Google Scholar]
- 4. Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., Matsuzawa Y. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 5. Kubota N., Terauchi Y., Yamauchi T., Kubota T., Moroi M., Matsui J., Eto K., Yamashita T., Kamon J., Satoh H., Yano W., Froguel P., Nagai R., Kimura S., Kadowaki T., Noda T. (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 277, 25863–25866 [DOI] [PubMed] [Google Scholar]
- 6. Nawrocki A. R., Rajala M. W., Tomas E., Pajvani U. B., Saha A. K., Trumbauer M. E., Pang Z., Chen A. S., Ruderman N. B., Chen H., Rossetti L., Scherer P. E. (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 281, 2654–2660 [DOI] [PubMed] [Google Scholar]
- 7. Ma K., Cabrero A., Saha P. K., Kojima H., Li L., Chang B. H., Paul A., Chan L. (2002) Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J. Biol. Chem. 277, 34658–34661 [DOI] [PubMed] [Google Scholar]
- 8. Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., Jelicks L. A., Mehler M. F., Hui D. Y., Deshaies Y., Shulman G. I., Schwartz G. J., Scherer P. E. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aprahamian T. R. (2013) Elevated adiponectin expression promotes adipose tissue vascularity under conditions of diet-induced obesity. Metabolism 62, 1730–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang W. S., Jeng C. Y., Wu T. J., Tanaka S., Funahashi T., Matsuzawa Y., Wang J. P., Chen C. L., Tai T. Y., Chuang L. M. (2002) Synthetic peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 25, 376–380 [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N. H., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 [DOI] [PubMed] [Google Scholar]
- 12. Lee M. H., Klein R. L., El-Shewy H. M., Luttrell D. K., Luttrell L. M. (2008) The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry 47, 11682–11692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng K. K., Lam K. S., Wang Y., Huang Y., Carling D., Wu D., Wong C., Xu A. (2007) Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56, 1387–1394 [DOI] [PubMed] [Google Scholar]
- 14. Adya R., Tan B. K., Chen J., Randeva H. S. (2012) Protective actions of globular and full-length adiponectin on human endothelial cells: novel insights into adiponectin-induced angiogenesis. J. Vasc. Res. 49, 534–543 [DOI] [PubMed] [Google Scholar]
- 15. Zhang P., Wang Y., Fan Y., Tang Z., Wang N. (2009) Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29, 67–74 [DOI] [PubMed] [Google Scholar]
- 16. Fasshauer M., Klein J., Kralisch S., Klier M., Lössner U., Blüher M., Paschke R. (2004) Growth hormone is a positive regulator of adiponectin receptor 2 in 3T3-L1 adipocytes. FEBS Lett. 558, 27–32 [DOI] [PubMed] [Google Scholar]
- 17. Parker-Duffen J. L., Nakamura K., Silver M., Kikuchi R., Tigges U., Yoshida S., Denzel M. S., Ranscht B., Walsh K. (2013) T-cadherin is essential for adiponectin-mediated revascularization. J. Biol. Chem. 288, 24886–24897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., Ito Y., Kamon J., Tsuchida A., Kumagai K., Kozono H., Hada Y., Ogata H., Tokuyama K., Tsunoda M., Ide T., Murakami K., Awazawa M., Takamoto I., Froguel P., Hara K., Tobe K., Nagai R., Ueki K., Kadowaki T. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 [DOI] [PubMed] [Google Scholar]
- 19. Iwabu M., Yamauchi T., Okada-Iwabu M., Sato K., Nakagawa T., Funata M., Yamaguchi M., Namiki S., Nakayama R., Tabata M., Ogata H., Kubota N., Takamoto I., Hayashi Y. K., Yamauchi N., Waki H., Fukayama M., Nishino I., Tokuyama K., Ueki K., Oike Y., Ishii S., Hirose K., Shimizu T., Touhara K., Kadowaki T. (2010) Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 20. Patel S. A., Hoehn K. L., Lawrence R. T., Sawbridge L., Talbot N. A., Tomsig J. L., Turner N., Cooney G. J., Whitehead J. P., Kraegen E. W., Cleasby M. E. (2012) Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 153, 5231–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo N., Chung B. H., Wang X., Klein R. L., Tang C. K., Garvey W. T., Fu Y. (2013) Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis 228, 124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bjursell M., Ahnmark A., Bohlooly-Y M., William-Olsson L., Rhedin M., Peng X. R., Ploj K., Gerdin A. K., Arnerup G., Elmgren A., Berg A. L., Oscarsson J., Lindén D. (2007) Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56, 583–593 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y., Michael M. D., Kash S., Bensch W. R., Monia B. P., Murray S. F., Otto K. A., Syed S. K., Bhanot S., Sloop K. W., Sullivan J. M., Reifel-Miller A. (2007) Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology 148, 683–692 [DOI] [PubMed] [Google Scholar]
- 24. Pischon T., Girman C. J., Hotamisligil G. S., Rifai N., Hu F. B., Rimm E. B. (2004) Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291, 1730–1737 [DOI] [PubMed] [Google Scholar]
- 25. Ho D. Y., Cook N. R., Britton K. A., Kim E., Creager M. A., Ridker P. M., Pradhan A. D. (2011) High-molecular-weight and total adiponectin levels and incident symptomatic peripheral artery disease in women: a prospective investigation. Circulation 124, 2303–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouchi N., Ohishi M., Kihara S., Funahashi T., Nakamura T., Nagaretani H., Kumada M., Ohashi K., Okamoto Y., Nishizawa H., Kishida K., Maeda N., Nagasawa A., Kobayashi H., Hiraoka H., Komai N., Kaibe M., Rakugi H., Ogihara T., Matsuzawa Y. (2003) Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42, 231–234 [DOI] [PubMed] [Google Scholar]
- 27. Shibata R., Izumiya Y., Sato K., Papanicolaou K., Kihara S., Colucci W. S., Sam F., Ouchi N., Walsh K. (2007) Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J. Mol. Cell Cardiol. 42, 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D. R., Kumada M., Sato K., Schiekofer S., Ohashi K., Funahashi T., Colucci W. S., Walsh K. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10, 1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 30. Ohashi K., Ouchi N., Sato K., Higuchi A., Ishikawa T. O., Herschman H. R., Kihara S., Walsh K. (2009) Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol. Cell. Biol. 29, 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kondo M., Shibata R., Miura R., Shimano M., Kondo K., Li P., Ohashi T., Kihara S., Maeda N., Walsh K., Ouchi N., Murohara T. (2009) Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J. Biol. Chem. 284, 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higuchi A., Ohashi K., Kihara S., Walsh K., Ouchi N. (2009) Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-α expression. Circ. Res. 104, 1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higuchi A., Ohashi K., Shibata R., Sono-Romanelli S., Walsh K., Ouchi N. (2010) Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 30, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kobayashi H., Ouchi N., Kihara S., Walsh K., Kumada M., Abe Y., Funahashi T., Matsuzawa Y. (2004) Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 94, e27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shibata R., Skurk C., Ouchi N., Galasso G., Kondo K., Ohashi T., Shimano M., Kihara S., Murohara T., Walsh K. (2008) Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett. 582, 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong W. T., Tian X. Y., Xu A., Yu J., Lau C. W., Hoo R. L., Wang Y., Lee V. W., Lam K. S., Vanhoutte P. M., Huang Y. (2011) Adiponectin is required for PPARγ-mediated improvement of endothelial function in diabetic mice. Cell Metab. 14, 104–115 [DOI] [PubMed] [Google Scholar]
- 38. Aprahamian T., Bonegio R. G., Richez C., Yasuda K., Chiang L. K., Sato K., Walsh K., Rifkin I. R. (2009) The peroxisome proliferator-activated receptor γ agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J. Immunol. 182, 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zbinden S., Clavijo L. C., Kantor B., Morsli H., Cortes G. A., Andrews J. A., Jang G. J., Burnett M. S., Epstein S. E. (2007) Interanimal variability in preexisting collaterals is a major factor determining outcome in experimental angiogenesis trials. Am. J. Physiol. Heart Circ. Physiol. 292, H1891–H1897 [DOI] [PubMed] [Google Scholar]
- 40. Zhang G., Gao X., Song Y. K., Vollmer R., Stolz D. B., Gasiorowski J. Z., Dean D. A., Liu D. (2004) Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 11, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu F., Song Y., Liu D. (1999) Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 [DOI] [PubMed] [Google Scholar]
- 42. Denzel M. S., Scimia M. C., Zumstein P. M., Walsh K., Ruiz-Lozano P., Ranscht B. (2010) T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Invest. 120, 4342–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holland W. L., Miller R. A., Wang Z. V., Sun K., Barth B. M., Bui H. H., Davis K. E., Bikman B. T., Halberg N., Rutkowski J. M., Wade M. R., Tenorio V. M., Kuo M. S., Brozinick J. T., Zhang B. B., Birnbaum M. J., Summers S. A., Scherer P. E. (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye R., Scherer P. E. (2013) Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2, 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding M., Carrão A. C., Wagner R. J., Xie Y., Jin Y., Rzucidlo E. M., Yu J., Li W., Tellides G., Hwa J., Aprahamian T. R., Martin K. A. (2012) Vascular smooth muscle cell-derived adiponectin: a paracrine regulator of contractile phenotype. J. Mol. Cell Cardiol. 52, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohashi K., Parker J. L., Ouchi N., Higuchi A., Vita J. A., Gokce N., Pedersen A. A., Kalthoff C., Tullin S., Sams A., Summer R., Walsh K. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Satoh H., Nguyen M. T., Trujillo M., Imamura T., Usui I., Scherer P. E., Olefsky J. M. (2005) Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes 54, 1304–1313 [DOI] [PubMed] [Google Scholar]
- 48. Rizvi Y. Q., Mehta C. S., Oyekan A. (2013) Interactions of PPAR-α and adenosine receptors in hypoxia-induced angiogenesis. Vascul. Pharmacol. 59, 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagata D., Mogi M., Walsh K. (2003) AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 50. Ouchi N., Shibata R., Walsh K. (2005) AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ. Res. 96, 838–846 [DOI] [PubMed] [Google Scholar]
- 51. Wang Y., Wang X., Lau W. B., Yuan Y., Booth D., Li J. J., Scalia R., Preston K., Gao E., Koch W., Ma X. L. (2014) Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res. 114, 792–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y., Wang X., Jasmin J. F., Lau W. B., Li R., Yuan Y., Yi W., Chuprun K., Lisanti M. P., Koch W. J., Gao E., Ma X. L. (2012) Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler. Thromb. Vasc. Biol. 32, 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng Q., Yuan Y., Yi W., Lau W. B., Wang Y., Wang X., Sun Y., Lopez B. L., Christopher T. A., Peterson J. M., Wong G. W., Yu S., Yi D., Ma X. L. (2011) C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 31, 2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dormandy J. A., Charbonnel B., Eckland D. J., Erdmann E., Massi-Benedetti M., Moules I. K., Skene A. M., Tan M. H., Lefèbvre P. J., Murray G. D., Standl E., Wilcox R. G., Wilhelmsen L., Betteridge J., Birkeland K., Golay A., Heine R. J., Korányi L., Laakso M., Mokán M., Norkus A., Pirags V., Podar T., Scheen A., Scherbaum W., Schernthaner G., Schmitz O., Skrha J., Smith U., Taton J., and PROactive investigators (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 [DOI] [PubMed] [Google Scholar]
- 55. Lincoff A. M., Wolski K., Nicholls S. J., Nissen S. E. (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 56. Singh S., Loke Y. K., Furberg C. D. (2007) Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298, 1189–1195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.