Abstract
The autophagic process involves encompassing damaged proteins and organelles within double- or multi-membraned structures and delivering these molecules to the lytic compartments of vacuoles. Sphingolipids (SLs), which are ubiquitous membrane lipids in eukaryotes, participate in the generation of various membrane structures, including rafts, caveolae, and cytosolic vesicles. SLs are a complex family of molecules that have a growing number of members, including ceramide, sphingosine-1-phosphate, and dihydroceramide, which have been associated with the essential cellular process of autophagy. This review highlights recent studies focusing on the regulation and function of SL-associated autophagy and its role in cell fate, diseases, and therapeutic interventions.
Keywords: autophagy, sphingolipids, sphingolipidoses, sphingolipid rheostat, sphingolipid biostat
Facts
Autophagy is generally considered to be a cell survival mechanism and may also contribute to cell death, depending on its various biological contexts.
Sphingolipids were first discovered in brain extracts in 1876, and within a century of intensive research, the chemical structures of thousands of individual sphingolipids had been elucidated.
Sphingolipid-associated autophagy contributes to a range of diseases, including neurodegeneration, tumors, metabolic disorders, and heart diseases.
Open Questions
Sphingolipids are abundant lipid components of eukaryotic plasma membranes that function in a wide range of biological processes. Are sphingolipids involved in the composition of autophagic membranes?
Why do perturbations of sphingolipid metabolism by chemotherapy and nutrition starvation trigger lethal autophagy and protective autophagy, respectively?
Sphingolipid-mediated signals that have long been known to induce apoptosis are now also recognized to cause autophagy. Are sphingolipid-associated apoptosis and autophagy interlinked?
How does sphingolipid-associated autophagy occur and to what extent do alterations in autophagy contribute to disease phenotype?
What drugs or compounds that target sphingolipid metabolism are attractive candidates for therapeutic development?
Autophagy is generally considered to be a cell survival mechanism, and this process also contributes to cell death in several situations.1 Sphingolipids (SLs) are ubiquitous components of membrane structures, and renewed interest in SLs has focused on SL-induced intracellular and extracellular signaling.2 Accumulating evidence indicates that a direct link exists between SL metabolism and autophagy. Moreover, studies have uncovered that a dynamic balance among SL metabolites is significant in cell fate determination.3, 4 Here we review the key aspects of bioactive SLs that have emerged as important effectors in regulating the autophagic pathway, mediating the cross talk between apoptosis and autophagy, and determining the associated cell fate. A deeper understanding of the relationship between SL metabolism and autophagy explains how intact or impaired SL-related autophagic pathways are involved in the malfunctions associated with neurodegeneration, cancer, and other diseases. We also discuss how autophagy-regulating drugs work via cells' SL metabolism and the methods that could be used to monitor SL-related autophagy. Present and future investigations regarding SL-related autophagy will help to develop novel treatment strategies to control autophagy-related diseases.
The SL Family
SLs represent a significant class of lipids that contain a backbone of sphingoid bases and are ubiquitous constituents of membranes in eukaryotes. SLs were first discovered in brain extracts in 1876,3 and within a century of intensive research, the chemical structures of thousands of individual SLs had been elucidated.4 The SL metabolic pathway displays an intricate network of reactions that result in the formation of multiple SLs, including ceramide (Cer), dihydroceramide (dhCer), and sphingosine-1-phosphate (S1P).5
SLs are produced in at least three distinct ways (Figure 1). First, a substantial portion of SLs are derived from de novo biosynthesis in most organisms,2, 6 with the condensation of serine and palmitoyl-CoA catalyzed by serine palmitoyl transferase to generate dehydrosphinganine. Dehydrosphinganine is subsequently reduced to form dihydrosphingosine (sphinganine), which is then N-acylated by dhCer synthase to produce dhCer or Cer. Second, through hydrolysis via a complex lipid-turnover pathway,2, 6 several specific hydrolases are involved in the turnover process, including sphingomyelinases (SMases) and glucocerebrosidase (GCase). In addition to the above two sources, Cer is also recycled.6, 7 In this pathway, sphingosine is recycled into Cer via Cer synthases (CerS); therefore, this pathway is also called the salvage pathway. The cellular metabolic homeostasis of the sphingolipidome is often achieved through the coordination of the biosynthesis and removal of SL species, which requires a delicate balance between de novo biosynthesis, turnover, and recycling.8
Figure 1.
Cer formation through the de novo, turnover, or salvage pathway. Red arrows: de novo pathway; green arrows: turnover pathway; blue arrows: salvage pathway. PM, plasma membrane
SLs are found in cellular membranes, lipoproteins, and other lipid-rich structures; however, they are synthesized in the endoplasmic reticulum (ER) and Golgi apparatus. The scheme in Figure 1 depicts the subcellular localization of SL biosynthesis, turnover, and recycling. In addition, SLs may be incorporated into other intracellular compartments, such as mitochondria or autophagosomes.3 The turnover and recycling of SLs also occur at several intracellular locations, including the endosome, phagosome, and lysosome.3 Thus, SLs travel between organelles, and this transport occurs via either transport vesicles or transfer proteins.9 CERT (Cer transfer protein) and FAPP2 (four-phosphate adaptor protein 2) have proved to be two critical SL-trafficking proteins that regulate the trafficking of SLs to specific compartments within cells.10, 11 Lipids are increasingly implicated in the control of the membrane remodeling and vesicle transport that underlie the biogenesis of autophagosomes.12 However, our knowledge of whether and how SL trafficking is associated with the autophagic process remains incomplete.
SLs are considered to primarily have roles as components of membranes and other biological structures. However, several SL metabolites, including Cer, dhCer, and S1P, have drawn attention as bioactive signaling molecules that mediate cell growth, differentiation, senescence, apoptosis, and autophagy.4, 5
SLs Involved in the Autophagic Pathway
‘Autophagy,' which is derived from Greek and means ‘to eat oneself,' ensures the synthesis, degradation, and recycling of cellular components in eukaryotic cells ranging from yeasts to mammals.13, 14, 15 At present, the following three modes of autophagy have been identified: macroautophagy (which is commonly called ‘autophagy,' including in this review), microautophagy, and chaperone-mediated autophagy. During the autophagic process, nonspecific or targeted cytoplasmic constituents are delivered to and degraded in the lysosome via these autophagic pathways.16, 17 The formation of the autophagosome is a multistep process that includes the biogenesis of the isolation membrane, followed by its elongation and closure.18 Most autophagy-related genes (Atgs) contribute to autophagosome formation, and many are well conserved from yeasts to mammals.19, 20
SLs are abundant lipid components of eukaryotic plasma membranes that function in a wide range of biological processes,21, 22 which make SLs strong candidates as possible autophagosomal lipids. Several studies have implied that SLs, and especially Cer, are autophagosomal membrane components.23, 24 In addition, SLs formed by de novo biosynthesis in the ER might be a driving force for the formation of the autophagosomal vacuole, in what has been referred to as the ‘membrane extension' step,25 which occurs after many of the associated autophagosomal proteins have been recruited. Because the enzymes required for de novo Cer biosynthesis reside in the ER,26 it is possible that they might be recruited into autophagosomes and perhaps continue to produce SLs there.
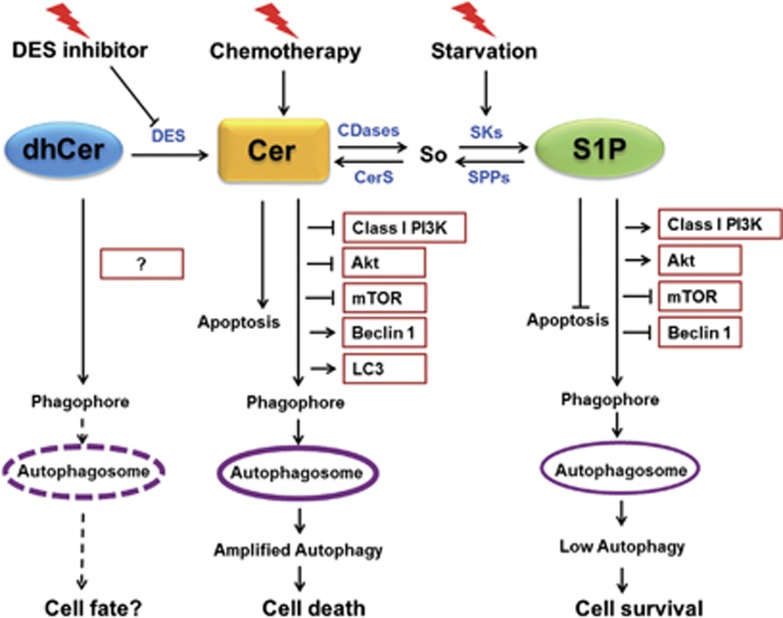
The best-characterized pathway regulating autophagy includes a class I phosphatidyl inositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), which act to inhibit autophagy. Although how autophagy is suppressed by these molecular signals remains to be revealed, mTOR complex 1 (mTORC1) is known to phosphorylate the autophagy regulatory complex, ULK1 (containing unc-51-like kinase 1), the mammalian Atg13 protein, and focal adhesion kinase-interacting protein of 200 kD (FIP200).27, 28 A class III PI3K is needed for the activation of autophagy.4, 16 SLs participate in cell survival and cell death signaling pathways.29, 30 Complex autophagy regulation might be mediated by SLs or SL metabolism. Indeed, different bioactive SL species have been shown to mediate distinct autophagic pathways, described as protective autophagy and autophagy-associated cell death (Figure 2), which have opposing functions in cellular life-or-death decisions.3, 4, 31
Figure 2.
Model of SL-related autophagy and its consequences on cell fate. CerSs, ceramide synthases; DES, dihydroceramide desaturase; So, sphingosine; SPPs, sphingosine-1-phosphate phosphatases
Cer, which is known to induce cell cycle arrest and has been implicated in important physiological roles in cell differentiation, senescence, migration, adhesion, and inflammatory responses, is the key intermediate in SL metabolism.6, 32 Exogenous Cer-related autophagy was shown to trigger autophagy-associated cell death, which occurs in several malignant cell types, including colon cancer, breast cancer, cervical cancer, nasopharyngeal cancer, and glioma cells.3, 33, 34, 35, 36, 37 In addition to exogenous Cer, endogenous Cer species are critical for the induction of autophagy. Tamoxifen, an estrogen receptor antagonist used to treat several types of breast cancer, has been shown to induce autophagy-associated cell death by increasing endogenous Cer levels.33 Exogenous or endogenous Cer induces autophagy through several mechanisms. For example, Cer has been found to stimulate autophagy by regulating classic or atypical autophagic pathways and signals.4 Via these signals, class I PI3K and Akt negatively regulate autophagy, but a class III PI3K is needed for the activation of autophagy. Cer was found to promote the interaction of class III PI3K with other regulators of autophagy38 and to inhibit Akt by activating phosphoprotein phosphatase 2A.33, 39, 40 Moreover, exogenously added Cer and the accumulation of endogenous Cer due to treatment with certain chemotherapeutic drugs, such as tamoxifen, inhibit the mTOR signaling pathway,33 which has a central role in inducing autophagy and increasing the expression of Beclin1,33 an upstream regulator of autophagy.41 Simultaneously, Cer promotes the dissociation of Beclin1 from the Beclin1/Bcl-2 complex.34, 42, 43 A short-chain Cer analog has also been associated with the induction of autophagy-associated cell death by increasing the transcription of the BH3-only protein, a mitochondria-associated protein that induces nonapoptotic cell death.35 A recent study showed that Cer directly interacts with microtubule-associated protein light chain 3 (LC3) on mitochondrial membranes to induce deadly autophagy via an increase in intracellular mitophagy.44
S1P has emerged as a cell-proliferative lipid messenger. S1P has been found to induce survival-mediated or protective autophagy under nutrient starvation, distinct from Cer-associated autophagy-associated cell death.45, 46 In contrast to Cer-related autophagy, S1P-mediated autophagy has not been shown to be related to the accumulation of Beclin1 protein or to the suppression of class I PI3K or Akt.45 However, mTOR was still inhibited by increased S1P levels,45, 46, 47 which suggests that S1P induces autophagy via inhibiting mTOR and is independent of the class I PI3K signal.
dhCer has been used as a negative control for Cer treatments to induce autophagy33, 34, 48 because dhCer has long been thought to be biologically inactive. However, dhCer was recently identified as a novel SL-based mediator of autophagy.3, 49, 50 dhCer induces both protective autophagy and autophagy-associated cell death. Endogenously added dhCer induced a transient, early increase in dhCer levels via inhibition of dhCer desaturase, such as XM462, which successively promoted autophagy and reduced etoposide toxicity in gastric and colon cancer cells.49, 51 However, anticancer agents such as fenretinide (4-hydroxy (phenyl) retinamide or 4-HPR)50 and gamma tocotrienol (a lipophilic antioxidant of vitamin E)52 induced cancer cell death through the elevation of intracellular dhCer levels. Interestingly, none of the reported Cer-mediated autophagic pathways is induced by both Cer and dhCer.33 Intriguingly, the accumulation of dhCer may serve as an additional ‘switch' to regulate cell fate; however, the biological activity of dhCer remains controversial and unclear.
In addition to the major SLs that induce autophagy, gangliosides and several rare SLs are also involved in autophagy-related cell fate decisions. Gangliosides induce autophagy-associated cell death in both isolated astrocytes and astrocytoma cells.53 An increase in the amount of LC3-II and an accumulation of autophagic vacuoles were observed in cells that underwent ganglioside treatment.53, 54 Cer methylaminoethyl phosphonate and sphingadienes are two rare SLs that promote autophagy-associated cell death through the downregulation of PI3K/Akt and the activation of Beclin1, similar to Cer.55, 56
Overall, although Cer, dhCer, and S1P are all able to induce autophagy, the effects of S1P-mediated autophagy are markedly mild compared with the effects elicited by Cer. dhCer-related autophagic effects are moderate, and their intensity level lies between the levels caused by Cer and S1P. Treatment with chemotherapeutic agents often promotes the biosynthesis of intracellular Cer and leads to autophagy-associated cell death; however, elevation in S1P levels is typically a cell response to nutrition starvation and mediates cytoprotective autophagy. Furthermore, cells maintain a dynamic equilibrium between the levels and the effects of Cer, dhCer, and S1P. The conversion of Cer to S1P simultaneously accumulates the survival effects and removes the death signals. This observation has led to the concept of a so-called ‘SL rheostat' or ‘SL biostat,' based on the relative amounts and reciprocal roles of these antagonistic metabolites, which are critical in guiding the destiny of cells.12, 57, 58
Possible SL Involvement in the Cross Talk Between Autophagy and Apoptosis
Apoptosis and autophagy have been recognized as two principal forms of programmed cell death. Apoptosis generally initiates cell death, whereas autophagy is primarily a protective process for the cell that may also contribute to cell death.59 There is a sophisticated and not yet fully understood association between autophagy-related cell death and survival that depends on various biological situations.1 In certain cellular contexts, autophagy functions as a stress response to suppress apoptosis and promote cell survival.16, 60 However, in other cases, autophagy may serve as a mechanism for caspase-dependent or -independent cell death.61, 62
Certain SLs are now thought to elicit autophagy; however, they have also been known to induce apoptosis. Cer is a well-established inducer of apoptosis via activation of the mitochondrial pathway. In a number of different cell types, increased Cer levels arrest cell growth and promote cell apoptosis.63, 64, 65, 66 In contrast to Cer, S1P facilitates cell survival and suppresses cell apoptosis.67, 68, 69 dhCer has also been suggested to exhibit antiapoptotic effects because the lipid inhibits Cer channel assembly in isolated mitochondria.70 In addition, other SL metabolites, such as sphingosine and ganglioside, are involved in the modulation of apoptosis and autophagy.71 SL-associated apoptosis and autophagy are interlinked. The conversion of Cer to S1P switches the cell fate from apoptosis to autophagy-induced survival.57, 58 Several exogenous stimuli, and particularly the activation of the enzymes that interconvert Cer and S1P, such as sphingosine kinases, lead to an increase in S1P levels, a corresponding reduction in Cer levels, and the antagonistic effects of Cer.68 In addition, dhCer has been proposed to exhibit antiapoptotic effects to promote cell survival during hypoxia through the induction of autophagy, while also serving as a lipid reserve for the rapid production of Cer to address cellular damage on reperfusion.72 dhCer serves as a unique regulator of cell fate, controlling the ‘switch' between cytoprotective autophagy and Cer-mediated apoptosis in response to stress. Intriguingly, Cer promotes both apoptosis and autophagy through the inhibition of class I PI3K and Akt signaling or the mTOR pathway.33, 40, 73, 74 Furthermore, suppressors of apoptosis, such as the Bcl-2 family, also directly bind and control Beclin1.75 Disruption of the Beclin1–Bcl-2 complex has also emerged as a common mechanism in Cer-associated autophagy.71 In addition to altering the balance between Beclin1 and Bcl-2 protein levels, endogenous Cer liberates Beclin1 for autophagy induction through the JNK-mediated phosphorylation of Bcl-2.34 Notably, Bcl-2 has emerged as a critical regulator of the Cer-mediated cross talk between autophagy and apoptosis.
SL-Associated Autophagy and Diseases
Although the primary function of autophagy is to aid in adaptation to cellular stress under adverse conditions by enabling cells to degrade cytosolic proteins and organelles to generate a supply of essential nutrients, autophagy has also emerged in the pathological process of many disorders, including neurodegeneration, tumors, immunity responses, and heart diseases.16 SL metabolism-associated autophagy also contributes to a range of diseases.31
Sphingolipidoses are a collection of more than 40 genetically distinct disorders caused by inherited deficiencies of lysosomal hydrolytic activities or lipid transport. These deficiencies result in intracellular accumulations of cholesterol and lipids in the endosomal/lysosomal network, usually leading to signs of neurodegeneration.76, 77 The biochemical abnormalities in sphingolipidoses are complex and lead to the accumulation of SL metabolites by diverse pathways (Table 1).78, 79
Table 1. SL-associated autophagy in SLs.
| Disease | Gene mutations | Accumulated SL substrates | Autophagic status | Ref |
|---|---|---|---|---|
| Niemann-Pick disease type C | NPC1 NPC2 | Sphingomyelin Glucosylceramide Lactosylceramide Sphingosine | Accumulated autophagosomes and ubiquitinated proteins; impaired autophagic flux | 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 |
| Gaucher disease | GBA Sap C | Glucosylceramide Glucosylsphingosine | Accumulated autophagosomes and autophagic substrates; deficient degradation of autolysosome cargo | 76, 88, 89, 90, 91 |
| Fabry disease | GLA | Globotriaosylceramide | Induction of autophagy; impaired autophagic flux | 76, 91 |
Abbreviations: ref, references; SL, sphingolipids
Niemann–Pick disease type C (NPC) is a complex neurodegenerative sphingolipidosis characterized by the accumulation of unesterified cholesterol, SLs, and complex gangliosides in late endosomes and lysosomes.80 The disease is caused by mutations in either the NPC1 or the NPC2 gene,81, 82 which disturbs not only the regular transport of endocytosed lipids but also the autophagic flux, leading to an accumulation of autophagic vacuoles in cells.83, 84 NPC1 and NPC281, 82 are genes whose protein products mediate proper intracellular lipid transport through pathways that are incompletely understood. The brains of afflicted humans and NPC-deficient animal models are marked by a loss of neurons.85, 86 Histological studies and cell culture experiments have shown that defective lysosomal degradation of autophagosomes may contribute to the abnormal autophagic flux.84, 87 The deficiency present in NPC promotes both the generation of autophagosomes and the impairment of autophagic flux. An imbalance between induction and flux through the autophagic pathway contributes to cell stress and neuronal loss in NPC.77
Gaucher disease (GD), a kind of sphingolipidosis, is characterized by the accumulation of glucosylceramide or glucosylsphingosine in the lysosomes of the cells of the monocyte/macrophage system.88, 89 In GD, the point mutations within the GBA (glucosidase, beta, acid) gene lead to the production of acid β-glucosidase with functional, kinetic, trafficking, and/or stability defects and a resultant decrease in lysosomal function and increase in the accumulation of glucosylceramide and glucosylsphingosine.76 GD can be classed into three subsets, based on the age of onset and the presence of central nervous system abnormalities. Type 1 is known as the non-neuronopathic form, and types 2 and 3 are differentiated from type 1 by neurodegeneration of the central nervous system with either rapid or chronic progression. Vaccaro et al.89 reported that mutation of saposin (Sap) C, and not a direct GBA gene mutation, was associated with the type 3 or type 1 phenotype in cases of GD. The decrease in/absence of Sap C affected GCase intracellular localization, resulting in lysosomal lipid accumulation and enhanced autophagy. This study suggested that Sap C might have a role in intralysosomal SL transport, as indicated by the accumulation of glucosylceramide and Cer in the lysosomes of the Sap C-mutant cells. In addition, deficient degradation of autophagic substrates in cells in GD can lead to an increased risk of Parkinson disease.90 For NPC, GD, and other sphingolipidoses,76, 91 the relevance of the impairment of autophagic flux to disease pathogenesis remains poorly defined. In addition, the extent to which the accumulation of autophagic substrates contributes to neuron dysfunction needs to be determined in future studies.
Dysregulated SL metabolism occurs in numerous cancers and has been shown to contribute to cancer progression and chemoresistance.92, 93 Several species of SLs have been shown to have aberrant expression or metabolism in cancer cells.94 The tumor suppressor Cer and the tumor promoter S1P are generally recognized to trigger autophagy; however, Cer and S1P have different outcomes regarding cell death and survival.29 Nevertheless, recent studies have suggested that de novo-generated Cer-associated autophagy can be lethal as well as protective for cells.92 For example, Beljansky et al.95 determined that enhancement of Cer levels through treatment with a SphK 2-selective inhibitor suppresses tumor growth by leading to autophagy-associated cell death. Conversely, Park et al.96 suggested that Cer-CD95-PERK signaling promotes cell death via the cascade activation of caspase proteins; however, gene silencing of Atg5 further strengthened the cell death process, indicating that the autophagy was protective. The contradictory activities may correspond to variations among Cers in their carbon chain lengths, double bond numbers, subcellular distributions, and versatile targets. In addition, Cer is metabolized into S1P by SphK 1 or SphK 2, which are associated with protective autophagy and other biological behaviors, including survival, infiltration, angiogenesis, metastasis, and resistance to anticancer drugs in cancer cells.92
Intact and impaired SL-related autophagy has significant implications for many other diseases and pathological processes. For example, CerS 5-mediated autophagy has recently been implicated in lipotoxic cardiomyopathy and hypertrophy.97 In addition, Kdo 2-lipid A induced substantial alterations in SL metabolism and composition in RAW264.7 cells, a mouse macrophage-like cell line. These changes apparently promote the de novo SL biosynthesis that is required for autophagosome generation, which is suggested to have an essential role in the processes of the innate immune response.24 Cer also mediates the augmentation of interleukin-1(IL-1) levels and the release of tumor necrosis factor (TNF) alpha that are induced by toll-like receptor 4, and this process may contribute to the enhanced inflammatory response in metabolic diseases, including obesity and diabetes, which are characterized by dyslipidemia.98
Overall, the aberrations in the bioactive SLs that mediate autophagy have been associated with diverse pathological conditions, including neurodegeneration, carcinogenesis, metabolic diseases, and inflammatory responses. Understanding the specific mechanism connecting SL-related autophagy and these diseases has substantial implications for revealing these diseases' biochemical characteristics and for designing and developing new therapeutic strategies.
Compounds and Drugs that Might Trigger Autophagy-Associated Cell Death Through Regulation of SL Metabolism
Many human disease states may be caused by the aberrant regulation of SL metabolism-associated autophagy; therefore, drugs and compounds that target SL metabolism, including chemotherapeutic drugs, sphingomimetics, and enzyme inhibitors, are attractive candidates for therapeutic development (Table 2).
Table 2. Summary of antitumor compounds that might regulate autophagy through SL metabolism.
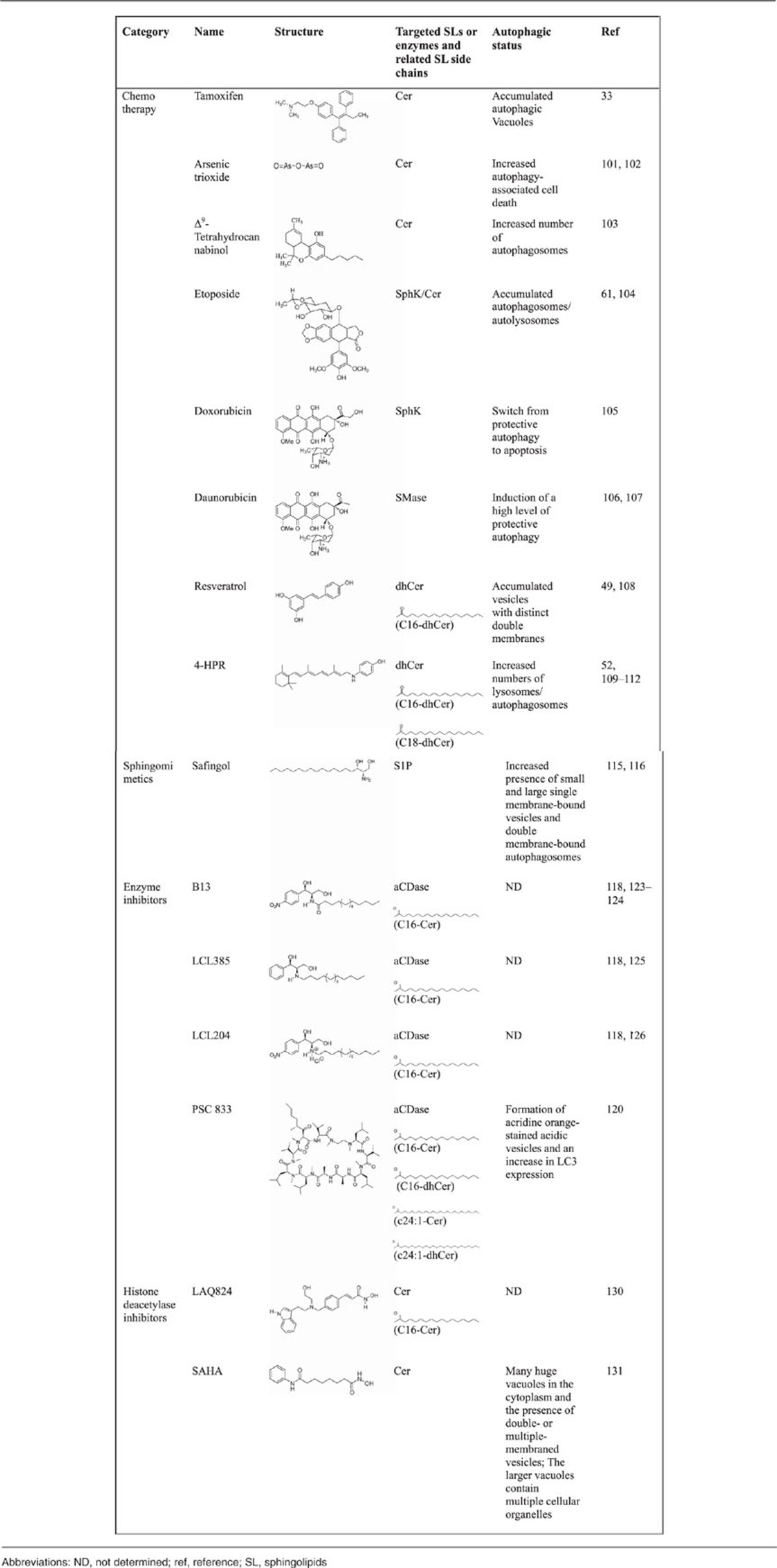
Many chemotherapeutic drugs elicit autophagy and exert their anticancer properties by increasing the intracellular levels of SLs. A large number of malignant cells often acquire genetic deletions or mutations that render them resistant to the classic apoptotic cell death that is induced by many anticancer therapies, which ultimately results in poor prognosis.99, 100 In these cases, the use of autophagy-inducing drugs offers an alternative way to induce cell death in apoptosis-resistant tumors.99, 100 As reviewed in the present paper, one of the major results of the chemotherapy-mediated increase in the levels of intracellular SLs, such as Cer and dhCer, is cell death induction by autophagy in various human cancer cells.
Autophagy is initiated by tamoxifen, an antagonist of the estrogen receptor, leading to an increase in endogenous Cer levels that acts against breast cancer cells.33 Arsenic trioxide, a potent antineoplastic agent, has been used clinically for the treatment of certain types of leukemia. Arsenic trioxide was shown to induce the biosynthesis of Cer in both acute promyelocytic leukemia and adult T-cell leukemia cells, and the cytotoxic effects were attributed to the induction of both apoptosis and autophagy.101, 102 Δ9-Tetrahydrocannabinol, the major active component of marijuana, was reported to induce the activation of autophagy-mediated apoptotic cell death by increasing de novo Cer biosynthesis.103 Moreover, SL metabolism may be involved in the regulation of autophagy by other chemotherapeutic drugs, including, but not limited to, etoposide,61, 104 doxorubicin,105 and daunorubicin.106, 107
Resveratrol is a natural polyphenolic phytoalexin produced by grapes and other berries that exerts its cancer-preventive properties in several animal models and exhibits its potent anticancer activities in leukemia and many solid tumors. The capacity to trigger dhCer biosynthesis contributes to resveratrol-induced autophagy-associated cell death. Puissant et al.108 found that resveratrol initiates autophagy in chronic myelogenous leukemia cells through the AMP-activated protein kinase (AMPK)-dependent induction of autophagy and that p62 is also required for resveratrol-mediated autophagy. Whether other polyphenolic compounds, such as curcumin, which has two phenolic hydroxyl groups: genistein, which has three phenolic hydroxyl groups; and quercetin, which has four phenolic hydroxyl groups, are implicated in SL metabolism and SL-mediated autophagy remains to be determined.
In clinical trials, 4-HPR has been encouraging as a chemopreventive and therapeutic agent for breast cancer.109, 110 The promise of 4-HPR as a therapeutic agent is enhanced by its unique ability to activate the autophagy-associated cell death pathway when the apoptotic pathway is deregulated, which is a characteristic that is not shared by other chemotherapeutic agents, including cisplatin and etoposide.109 Furthermore, the accumulation of dhCer following treatment with 4-HPR was found to induce cell cycle arrest in neuroblastoma cells.111, 112
SL metabolism is an exploitable target for the discovery of novel chemotherapeutics, and this target has been validated through the development of several sphingomimetics.8, 113, 114 Safingol, a synthetic sphinganine, triggers autophagy in several tumor cell types by the suppression of class I PI3K and Akt signaling.115 Safingol has been assessed in phase I clinical trials alone or in combination with cisplatin for adults with advanced solid tumors and for children with neuroblastoma. Safingol treatment was shown to trigger a dose-dependent decrease in S1P levels, and safingol is a promising representative drug whose primary mechanism for promoting tumor cell death is autophagy.116
Targeting enzymes in SL metabolism is another attractive avenue to provide therapeutic benefits in SL-related human diseases.117, 118 Acid SMases (aSMases) and acid ceramidases (aCDases) are crucial enzymes that modulate the synthesis and degradation of Cer in SL metabolism.2, 6 aCDase overexpression has been observed in cancer cell lines and primary tumors and contributes to resistance to chemotherapy and radiation.119, 120, 121 The consequence of aCDase overexpression is the ability to convert Cer, which is often produced as a proapoptotic response to stress, to sphingosine, which can then be converted to S1P.122 In addition to the ability to metabolize the Cer produced in response to stress, Turner et al.119 found that prostate cancer cell lines overexpressing aCDase also have an increased lysosomal density and increased levels of autophagy. Morad et al.120 identified an analog of cyclosporin A, PSC 833 (Valspodar, Novartis Pharma AG, Basel, Switzerland), a second-generation, non-immunosuppressive P-glycoprotein antagonist that inhibits aCDase and generates autophagy-associated cytotoxicity in pancreatic cancer cells. B13 was found to be a potent CDase inhibitor that induced colon cancer cell death123 and the inhibition of colon tumor growth in a xenograft model.124 Furthermore, LCL385, a B13 analog, enhanced the sensitivity of prostate cancer cells to radiation and inhibited tumor growth in a nude mouse model.125 Another novel B13 analog, LCL204, has been shown to overcome the resistance of head and neck squamous carcinoma cells to Fas-induced cell death in both in vitro and in vivo experiments.126 Most aCDase inhibitors are lysosomotropic agents and cause lysosomal destabilization and a change in SL metabolism enzymes in the lysosomal compartment.118
aSMase hydrolyzes sphingomyelin into Cer and phosphorylcholine. For the most part, aSMase seems to reside in classic lysosomes, where it mediates the catabolism of sphingomyelin.117 aSMase activity is essential for lysosomal stability and the survival of cancer cells, as well as for the multidrug-resistant phenotype. Because of their ability to inhibit autophagic flux, certain aSMase inhibitors, such as chloroquine, siramesine, and clomipramine, are currently being tested as anticancer agents in several clinical trials and laboratory studies.127 aSMase was also found to be a target for the treatment of Niemann–Pick disease type A (NPA), which is caused by loss-of-function mutations in the aSMase gene and is a lysosomal storage disorder leading to neurodegeneration. Fibroblasts from NPA patients and aSMase-knockout mouse brains show similar autophagolysosome accumulation and impaired autophagy.128 A recent study has suggested that the control of lysosome trafficking and fusion by aSMase is essential to normal autophagic flux in coronary arterial smooth muscle cells and has a protective role in atherosclerosis.129
Other chemotherapeutic treatments, such as several histone deacetylase inhibitors, were also demonstrated to lead to leukemic cell death by enhancing Cer production via the degradation of sphingomyelin.130 One of these histone deacetylase inhibitors is suberoylanilide hydroxamic acid, which was demonstrated to trigger autophagy-associated cell death in apoptosis-deficient tumor cells, such as chondrosarcoma cells.131
Conclusion
Numerous well-developed and convenient experimental methods and techniques that can be used to detect autophagy in different species systems exist.132, 133 To establish that an autophagic response is occurring in SL-related autophagy, multiple methods have been used, including cellular ultrastructure studies by transmission electron microscopy,132, 133 an LC3-puncta formation assay combined with immunoblots for autophagic cargoes (for example, p62 and NBR1), and the detection of autophagy-related molecules.132, 134 In addition, performing a large-scale and comprehensive sphingolipidomic analysis has been challenging in the study of SL-associated autophagy.135, 136 Both nets and hooks are indispensable tools to allow efficient ‘fishing' for multiple intermediates and products directly from the sophisticated process of SL metabolism.
Because there is an obvious overlap between the subcellular localization of enzymes needed for SL biosynthesis and autophagosome formation, SLs have been increasingly implicated in the composition of the phagophore and the autophagosomal membrane.
Autophagy-associated cell death and cell survival represent the so-called ‘Yin-Yang' regulatory mechanism. The balance has been exemplified by interconvertible SLs that produce opposite autophagic effects on cells that were termed as ‘SL rheostat' or ‘SL biostat'. In addition, the increase in intracellular Cer levels usually results from chemotherapy and often stimulates autophagy-associated cell death, although this increase has also been found to be cytoprotective. In contrast, starvation treatment triggers protective autophagy via increasing S1P levels. However, the underlying mechanisms by which chemotherapy and nutrient deprivation mediate different regulation are still largely unknown.
In addition to autophagy, SLs have long been identified to induce apoptosis. SLs mediate the cross talk between apoptosis and autophagy because certain SLs share several common kinase signaling pathways that regulate cell fate that affect both apoptosis and autophagy or execute a seesaw type of regulation of key molecules.
Increasing new knowledge on the biochemistry and cell biology of SL-mediated autophagy is beneficial for deepening our understanding of SL-associated diseases, including neurodegeneration, cancer pathogenesis, and inflammatory responses. An imbalance between induction of and flux through the autophagic pathway contributes to cell stress and neuronal loss in NPC. Intact and impaired SL-related autophagy has significant implications for the development of malignant tumors.
In addition, the current understanding of the effects of multiple SLs on the modulation of various autophagy-related cell fates has primarily arisen from exogenous SLs or pharmacological perturbations of SL metabolism. The identification of SL-mediated cytoprotective autophagy that can be shifted to cell death may provide a novel strategy for cancer therapy, including chemotherapy, sphingomimetics, SL metabolic enzyme inhibitors, and histone deacetylase inhibitors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, Grants 81100764, 81172287, 81202139, and 81230043.
Glossary
- 4-HPR
4-hydroxy (phenyl) retinamide
- aCDase
acid ceramidase
- Atg
autophagy-related gene
- CDase
ceramidase
- Cer
ceramide
- CerS
Cer synthase
- dhCer
dihydroceramide
- ER
endoplasmic reticulum
- GCase
glucocerebrosidase
- LC3
microtubule-associated protein light chain 3
- mTOR
mammalian target of rapamycin
- NPC
Niemann–Pick disease type C
- PI3K
phosphatidyl inositol 3-kinase
- S1P
sphingosine-1-phosphate
- SL
sphingolipid
- SMase
sphingomyelinase
- SphK
sphingosine kinase
The authors declare no conflict of interest.
Footnotes
Edited by C Munoz-Pinedo
References
- Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- Hla T, Dannenberg AJ. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012;16:420–434. doi: 10.1016/j.cmet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bedia C, Levade T, Codogno P. Regulation of autophagy by sphingolipids. Anticancer Agents Med Chem. 2011;11:844–853. doi: 10.2174/187152011797655131. [DOI] [PubMed] [Google Scholar]
- Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50 (Suppl:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Becker KP, Kitatani K, Idkowiak-Baldys J, Bielawski J, Hannun YA. Selective inhibition of juxtanuclear translocation of protein kinase C betaII by a negative feedback mechanism involving ceramide formed from the salvage pathway. J Biol Chem. 2005;280:2606–2612. doi: 10.1074/jbc.M409066200. [DOI] [PubMed] [Google Scholar]
- Merrill AH., Jr Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Lisman Q. Sphingolipid transport: rafts and translocators. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- Futerman AH. Intracellular trafficking of sphingolipids: relationship to biosynthesis. Biochim Biophys Acta. 2006;1758:1885–1892. doi: 10.1016/j.bbamem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Armi C, Devereaux KA, Di Paolo G. The role of lipids in the control of autophagy. Curr Biol. 2013;23:R33–R45. doi: 10.1016/j.cub.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 (Suppl 2:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Tanida I. Autophagy basics. Microbiol Immunol. 2011;55:1–11. doi: 10.1111/j.1348-0421.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Codogno P, Cuervo AM, Deretic V, Elazar Z, Fueyo-Margareto J, et al. A comprehensive glossary of autophagy-related molecules and processes. Autophagy. 2010;6:438–448. doi: 10.4161/auto.6.4.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Baehrecke EH, Brumell JH, Chu CT, Codogno P, Cuervo AM, et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition) Autophagy. 2011;7:1273–1294. doi: 10.4161/auto.7.11.17661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassme H, Riethmuller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46:161–170. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Schenck M, Carpinteiro A, Grassme H, Lang F, Gulbins E. Ceramide: physiological and pathophysiological aspects. Arch Biochem Biophys. 2007;462:171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Obara K, Kihara A. Sphingolipid synthesis is involved in autophagy in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2011;410:786–791. doi: 10.1016/j.bbrc.2011.06.061. [DOI] [PubMed] [Google Scholar]
- Sims K, Haynes CA, Kelly S, Allegood JC, Wang E, Momin A, et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem. 2010;285:38568–38579. doi: 10.1074/jbc.M110.170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Oku M, Wasada Y, Ano Y, Sakai Y. PI4P-signaling pathway for the synthesis of a nascent membrane structure in selective autophagy. J Cell Biol. 2006;173:709–717. doi: 10.1083/jcb.200512142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal. 2009;2:pe51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci USA. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- Aoki H, Kondo Y, Aldape K, Yamamoto A, Iwado E, Yokoyama T, et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–475. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- Sun T, Li D, Wang L, Xia L, Ma J, Guan Z, et al. c-Jun NH2-terminal kinase activation is essential for up-regulation of LC3 during ceramide-induced autophagy in human nasopharyngeal carcinoma cells. J Transl Med. 2011;9:161. doi: 10.1186/1479-5876-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119 (Pt 2:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Schubert KM, Scheid MP, Duronio V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J Biol Chem. 2000;275:13330–13335. doi: 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–991. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, et al. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol Pharmacol. 2009;76:342–355. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Saba JD. Cancer treatment strategies targeting sphingolipid metabolism. Adv Exp Med Biol. 2010;688:185–205. doi: 10.1007/978-1-4419-6741-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Ho MC, Lee PH, Hsu CY, Huang WP, Lee H. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am J Physiol Cell Physiol. 2009;297:C451–C458. doi: 10.1152/ajpcell.00586.2008. [DOI] [PubMed] [Google Scholar]
- Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, et al. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer. 2012;130:685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliostro V, Casas J, Caretti A, Abad JL, Tagliavacca L, Ghidoni R, et al. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int J Biochem Cell Biol. 2012;44:2135–2143. doi: 10.1016/j.biocel.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- Hwang J, Lee S, Lee JT, Kwon TK, Kim DR, Kim H, et al. Gangliosides induce autophagic cell death in astrocytes. Br J Pharmacol. 2010;159:586–603. doi: 10.1111/j.1476-5381.2009.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Lee HJ, Lee WH, Suk K. NF-kappaB as a common signaling pathway in ganglioside-induced autophagic cell death and activation of astrocytes. J Neuroimmunol. 2010;226:66–72. doi: 10.1016/j.jneuroim.2010.05.037. [DOI] [PubMed] [Google Scholar]
- Chintalapati M, Truax R, Stout R, Portier R, Losso JN. In vitro and in vivo anti-angiogenic activities and inhibition of hormone-dependent and -independent breast cancer cells by ceramide methylaminoethylphosphonate. J Agric Food Chem. 2009;57:5201–5210. doi: 10.1021/jf803818y. [DOI] [PubMed] [Google Scholar]
- Fyrst H, Oskouian B, Bandhuvula P, Gong Y, Byun HS, Bittman R, et al. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res. 2009;69:9457–9464. doi: 10.1158/0008-5472.CAN-09-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol B Biochem Mol Biol. 2012;163:26–36. doi: 10.1016/j.cbpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Hannun YA.Ceramide and apoptosis Trends Biochem Sci 199924224–225.author reply 227. [DOI] [PubMed] [Google Scholar]
- Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why. Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335 (Pt 3:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349 (Pt 2:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476:55–57. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis. 2006;11:773–780. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54:5–19. doi: 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin CM, Lahm T, Hubbard WC, Van Demark M, Wang KC, Wu X, et al. Dihydroceramide-based response to hypoxia. J Biol Chem. 2011;286:38069–38078. doi: 10.1074/jbc.M111.297994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Li J, Qiu Y, Sun H. 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) facilitates curcumin-induced melanoma cell apoptosis by enhancing ceramide accumulation, JNK activation, and inhibiting PI3K/AKT activation. Mol Cell Biochem. 2012;361:47–54. doi: 10.1007/s11010-011-1086-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, et al. Regulation of autophagy and its associated cell death by ‘sphingolipid rheostat': reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem. 2012;287:39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8:719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco CD, Lieberman AP. The pathogenesis of Niemann-Pick type C disease: a role for autophagy. Expert Rev Mol Med. 2008;10:e26. doi: 10.1017/S146239940800080X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M. Pathology and current treatment of neurodegenerative sphingolipidoses. Neuromolecular Med. 2010;12:362–382. doi: 10.1007/s12017-010-8133-7. [DOI] [PubMed] [Google Scholar]
- Staretz-Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123:1191–1207. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Peake KB, Vance JE. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta. 2009;1791:659–670. doi: 10.1016/j.bbalip.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Elrick MJ, Yu T, Chung C, Lieberman AP. Impaired proteolysis underlies autophagic dysfunction in Niemann-Pick type C disease. Human Mol Genet. 2012;21:4876–4887. doi: 10.1093/hmg/dds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Yamazaki T, Okamoto K. Association of autophagy with cholesterol-accumulated compartments in Niemann-Pick disease type C cells. J Clin Neurosci. 2009;16:954–959. doi: 10.1016/j.jocn.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Fahey M, Desmond P, Wood A, Seal ML, Steward C, et al. White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology. 2010;75:49–56. doi: 10.1212/WNL.0b013e3181e6210e. [DOI] [PubMed] [Google Scholar]
- Yamada A, Saji M, Ukita Y, Shinoda Y, Taniguchi M, Higaki K, et al. Progressive neuronal loss in the ventral posterior lateral and medial nuclei of thalamus in Niemann-Pick disease type C mouse brain. Brain Dev. 2001;23:288–297. doi: 10.1016/s0387-7604(01)00209-1. [DOI] [PubMed] [Google Scholar]
- Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A, et al. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 -/- mouse brain. Am J Pathol. 2007;171:962–975. doi: 10.2353/ajpath.2007.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:13–18. doi: 10.1182/asheducation-2012.1.13. [DOI] [PubMed] [Google Scholar]
- Vaccaro AM, Motta M, Tatti M, Scarpa S, Masuelli L, Bhat M, et al. Saposin C mutations in Gaucher disease patients resulting in lysosomal lipid accumulation, saposin C deficiency, but normal prosaposin processing and sorting. Human Mol Genet. 2010;19:2987–2997. doi: 10.1093/hmg/ddq204. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier M, Brakch N, Celine L, Genty D, Ramdani Y, Moll S, et al. Autophagosome maturation is impaired in Fabry disease. Autophagy. 2010;6:589–599. doi: 10.4161/auto.6.5.11943. [DOI] [PubMed] [Google Scholar]
- Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, et al. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Norris J, Hylemon PB, Fisher PB, Grant S, et al. Regulation of autophagy by ceramide-CD95-PERK signaling. Autophagy. 2008;4:929–931. doi: 10.4161/auto.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, et al. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem. 2013;288:2923–2932. doi: 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14:376–391. doi: 10.1007/s10495-008-0307-5. [DOI] [PubMed] [Google Scholar]
- Schleicher SM, Moretti L, Varki V, Lu B. Progress in the unraveling of the endoplasmic reticulum stress/autophagy pathway and cancer: implications for future therapeutic approaches. Drug Resist Updat. 2010;13:79–86. doi: 10.1016/j.drup.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Kfoury Y, Darwiche N, Panjarian S, Kozhaya L, Nasr R, et al. Arsenic trioxide induces accumulation of cytotoxic levels of ceramide in acute promyelocytic leukemia and adult T-cell leukemia/lymphoma cells through de novo ceramide synthesis and inhibition of glucosylceramide synthase activity. Haematologica. 2007;92:753–762. doi: 10.3324/haematol.10968. [DOI] [PubMed] [Google Scholar]
- Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res. 2007;31:329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- Lepine S, Allegood JC, Edmonds Y, Milstien S, Spiegel S. Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated autophagy-related gene 5 (Atg5) cleavage. J Biol Chem. 2011;286:44380–44390. doi: 10.1074/jbc.M111.257519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Sun J, Feng L, Wang K, Li D, Pan Q, et al. Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS One. 2011;6:e28491. doi: 10.1371/journal.pone.0028491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrezou JP, Levade T, Bettaieb A, Andrieu N, Bezombes C, Maestre N, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–2424. [PMC free article] [PubMed] [Google Scholar]
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- Fazi B, Bursch W, Fimia GM, Nardacci R, Piacentini M, Di Sano F, et al. Fenretinide induces autophagic cell death in caspase-defective breast cancer cells. Autophagy. 2008;4:435–441. doi: 10.4161/auto.5669. [DOI] [PubMed] [Google Scholar]
- Sogno I, Vene R, Ferrari N, De Censi A, Imperatori A, Noonan DM, et al. Angioprevention with fenretinide: targeting angiogenesis in prevention and therapeutic strategies. Crit Rev Oncol Hematol. 2010;75:2–14. doi: 10.1016/j.critrevonc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011;11:138–149. doi: 10.4161/cbt.11.2.14624. [DOI] [PubMed] [Google Scholar]
- Barth BM, Cabot MC, Kester M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem. 2011;11:911–919. doi: 10.2174/187152011797655177. [DOI] [PubMed] [Google Scholar]
- Kester M, Kolesnick R. Sphingolipids as therapeutics. Pharmacol Res. 2003;47:365–371. doi: 10.1016/s1043-6618(03)00048-3. [DOI] [PubMed] [Google Scholar]
- Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, et al. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–193. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Carvajal RD, Merrill AH, Jr, Gonen M, Cane LM, Schwartz GK. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res. 2011;17:2484–2492. doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, et al. Novel analogs of D-e-MAPP and B13. Part 2: signature effects on bioactive sphingolipids. Bioorg Med Chem. 2008;16:1032–1045. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LS, Cheng JC, Beckham TH, Keane TE, Norris JS, Liu X. Autophagy is increased in prostate cancer cells overexpressing acid ceramidase and enhances resistance to C6 ceramide. Prostate Cancer Prostatic Dis. 2011;14:30–37. doi: 10.1038/pcan.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad SA, Messner MC, Levin JC, Abdelmageed N, Park H, Merrill AH, Jr, et al. Potential role of acid ceramidase in conversion of cytostatic to cytotoxic end-point in pancreatic cancer cells. Cancer Chemother Pharmacol. 2013;71:635–645. doi: 10.1007/s00280-012-2050-4. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des. 2006;12:4625–4635. doi: 10.2174/138161206779010422. [DOI] [PubMed] [Google Scholar]
- Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- Liu G, Wang W, Sun G, Ma X, Liu Z, Yang J. Nystatin interferes with the effects of N-methyl-N'-nitro-N-nitrosoguanidine on sphingolipid metabolism in human FL cells. Lipids. 2008;43:867–875. doi: 10.1007/s11745-008-3209-y. [DOI] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, et al. Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol Ther. 2009;17:430–438. doi: 10.1038/mt.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15:1259–1263. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 2013;24:379–393. doi: 10.1016/j.ccr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Gabande-Rodriguez E, Boya P, Labrador V, Dotti CG, Ledesma MD. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014;21:864–875. doi: 10.1038/cdd.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu M, Pitzer AL, Xia M, Boini KM, Li PL, et al. Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med. 2014;92:473–485. doi: 10.1007/s00109-014-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato RR, Maggio SC, Almenara JA, Payne SG, Atadja P, Spiegel S, et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006;69:216–225. doi: 10.1124/mol.105.017145. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tanaka K, Sakimura R, Okada T, Nakamura T, Li Y, et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 2008;28:1585–1591. [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Haynes CA, Allegood JC, Park H, Sullards MC. Sphingolipidomics: methods for the comprehensive analysis of sphingolipids. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2696–2708. doi: 10.1016/j.jchromb.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]