Abstract
Alzheimer’s disease (AD) is characterized by amyloid-beta (Aβ) plaques, hyperphosphorylated tau neurofibrillary tangles (NFTs) and cholinergic dysfunction. Cholinergic degeneration can be mimicked in rats by lesioning cholinergic neurons in medial septum. Hippocampal cholinergic denervation disrupts retrograde transport of nerve growth factor (NGF), leading to its accumulation, which subsequently triggers sprouting of noradrenergic sympathetic fibers from the superior cervical ganglia into hippocampus. Previously we reported that coincident with noradrenergic sprouting is the partial reinnervation of hippocampus with cholinergic fibers, and the maintenance of a M1 mAChR dependent long-term depression at CA3-CA1 synapses that is lost in the absence of sprouting. These findings suggest that sympathetic sprouting and the accompanying cholinergic reinnervation maintains M1 mAChR function. Interestingly, noradrenergic sympathetic and cholinergic sprouting have been demonstrated in AD postmortem human brain. Furthermore, M1 mAChRs have been a recent focus as a therapeutic target for AD given their role in cognition and non-amyloidogenic processing of amyloid beta-protein precursor (AβPP). Here we tested the hypothesis that noradrenergic sympathetic sprouting and the associated increase in cholinergic innervation maintains non-amyloidogenic AβPP processing that is dependent upon M1 mAChRs. Also, we investigated the effect of intrahippocampal Aβ42 infusion on noradrenergic sympathetic and cholinergic sprouting. We found that Aβ42 is not only toxic to central cholinergic fibers innervating hippocampus but prevents and reverses noradrenergic sympathetic sprouting and the accompanying cholinergic reinnervation. These findings reiterate the clinical implications of sprouting as an innate compensatory mechanism and emphasize the importance of M1 mAChRs as an AD therapeutic target.
Keywords: Alzheimer disease, amyloid beta, cholinergic fibers, muscarinic receptor M1, nerve growth factor, amyloid beta-protein precursor
INTRODUCTION
Pathological hallmarks of AD include accumulation of amyloid-beta (Aβ) plaques and hyperphosphorylated tau neurofibrillary tangles (NFTs) along with degeneration of cholinergic innervation in cortex and hippocampus. These pathologies, along with soluble Aβ, blood brain barrier breakdown, oxidative stress, inflammation and gliosis interplay to induce accelerated neuronal degeneration and synaptic dysfunction [1].
Cholinergic dysfunction is the target of the primary treatment, acetylcholinesterase inhibitors, used in AD patients [2]. However, these drugs have limited benefit, likely due to decreased production of acetylcholine with disease progression. Recently, there is direct evidence that M1 muscarinic acetylcholine receptors (M1 mAChRs), which are the most abundant muscarinic receptor in cerebral cortex and hippocampus, play a critical role in cognition and AD pathogenesis [2–4]. Interestingly, activating M1 mAChRs elevates soluble amyloid precursor protein α (sAβPPα), and decreases Aβ plaques and NFTs [3, 5, 6]. On the contrary, pharmacological inhibition or genetic deletion of M1 mAChRs from 3xTgAD mice, which have cholinergic dysfunction, exacerbates cognitive impairments and increases the density of Aβ plaques and NFTs and the magnitude of gliosis [7, 8]. Thus, M1 mAChRs are a viable therapeutic target for AD and a better understanding of the beneficial effects of stimulating M1 mAChRs and preserving their function during cholinergic degeneration is critical [3].
The cholinergic dysfunction found in AD can be mimicked in rats by lesioning the cholinergic cell bodies of the medial septum (MS) [9–12]. Cholinergic denervation of hippocampus leads to nerve growth factor (NGF) accumulation due to loss of retrograde trafficking, and causes sprouting of noradrenergic sympathetic fibers. These fibers normally innervate the cerebral vasculature [13–18] and originate from the superior cervical ganglia (SCG) [16, 19]. We previously showed that coincident with the noradrenergic sympathetic sprouting is the reinnervation of dentate gyrus and area CA1 of hippocampus with cholinergic fibers [20]. Both of these fiber types are completely absent in rats with medial septal lesion combined with bilateral superior cervical ganglionectomy, suggesting that the cholinergic fibers, in addition to the noradrenergic fibers, originate from the SCG, although this remains to be determined [19, 20]. Importantly, we found that concurrent with sympathetic sprouting and cholinergic reinnevation is the maintenance of M1 mAChR dependent long term depression (mLTD) at hippocampal CA3-CA1 synapses [20]. Thus, we hypothesize that this sprouting is compensating for lost cholinergic function and is beneficial.
Importantly, noradrenergic sympathetic sprouting occurs in hippocampus of AD subjects [21]. Additionally, cholinergic sprouting has been demonstrated in close proximity to Aβ plaques in AD post-mortem human brain tissue, although whether this cholinergic sprouting is a consequence of noradrenergic sympathetic sprouting as in rats is not currently known [22, 23]. Given these findings from humans with AD, understanding the role of noradrenergic sympathetic sprouting and the associated cholinergic sprouting and how it interacts with Aβ is imperative and could have clinical implications. Thus, we wanted to investigate how endogenous AβPP processing is affected in rats with noradrenergic sympathetic and cholinergic sprouting compared to rats with complete loss of cholinergic hippocampal innervation and no sprouting. We predicted that sprouting could sustain non-amyloidogenic processing of AβPP because it maintains M1 mAChR function [20]. Additionally, we wanted to determine if intrahippocampal treatment of Aβ42 in vivo could induce or inhibit noradrenergic sympathetic and cholinergic sprouting. On one hand, Aβ leads to degeneration of cholinergic fibers in hippocampus which could increase NGF and stimulate sprouting. However, on the other hand, Aβ purified from AD patient brains has been shown to be toxic to SCG neuronal cultures [24]. Because of the documented benefits of sympathetic sprouting and cholinergic reinnervation on hippocampal synaptic function in rats with cholinergic degeneration, it is critical to determine whether Aβ accumulation will interfere with this potentially clinically relevant compensatory mechanism.
MATERIAL AND METHODS
All experiments were performed with Institutional Animal Care and Use Committee approval at the University of Alabama at Birmingham in accordance with NIH guidelines.
Animals
Adult male Sprague-Dawley rats, 2–5 months old (Charles River Laboratories) were used in all experiments. Animals were housed two per cage and were kept on a 12 hour light/dark cycle with ad libitum food and water. These studies include 4 animal groups: sham lesion with intact ganglia (control), MS lesion with intact ganglia (MS), MS lesion with ganglionectomy (MSGx) and sham lesion with ganglionectomy (Gx).
MS lesion and superior cervical ganglionectomy
Medial septal lesions and ganglionectomies were performed at 8–9 weeks of age. Rats were anesthetized using a ketamine (100 mg/kg) and xylazine (13 mg/kg) mixture administered intraperitoneally. Superior cervical ganglionectomies were performed as previously reported [10, 11, 20, 25–27]. After a neck incision, the SCG were visualized using a surgical microscope and bilaterally excised. Sham ganglionectomies involved the SCG being exposed but not removed. After SCG removal, medial septal lesions were performed. Cholinergic neurons in the medial septum were lesioned using either electrolytic stimulus (2 mA, 10 seconds) for immunohistochemistry experiments or using the immunotoxin 192-IgG-Saporin (Advanced Targeting Systems, San Diego, CA) which binds the low affinity pan neurotrophin receptor (p75NTR) for western blot experiments. 192-IgG-Saporin (0.5 μg toxin/ul PBS, total volume 2 uL) was injected stereotaxically over 5 minutes using a Hamilton syringe (AP +0.2, DV −5.8, L=0). Sham lesioned rats underwent the same procedure but received PBS only or electrode insertion with no current. Although rats with MS lesions induced either via electrolytic or saporin are used here, both lesion methods have been shown to have the same effect on M1 mAChR coupling [10, 25, 26].
Dicyclomine administration
Control and MS rats received intraperitoneal injections of M1 mAChR antagonist, dicyclomine (Sigma-Aldrich, St. Louis, MO, 8 mg/kg), each day for 4 weeks. Previously, this method was used in 3xTgAD mice and shown to increase Aβ pathology [28].
Cannulation and Aβ administration
Rats with MS lesion and intact ganglia were anesthetized via ketamine/xylazine (100 mg/13mg/kg, ip). Bilateral cannulas (Plastics One, Roanoke, VA) were implanted into region CA1 of hippocampus (AP-3.6 mm, DV-3.2mm, L ±2.1mm). Synthetic Aβ peptide (300 pM/day) was administered using an infusion pump (Alzet, Cupertino, CA) to control rats for 28 days. Two groups of MS rats received Aβ. One group received Aβ for 14 days beginning 16 days post-lesion after sprouting has been established. The second MS group received Aβ for 28 days starting 7 days post lesion prior to the onset of sprouting.
Western blot
Rats were anesthetized using 2.5% isoflurane and were decapitated and brain removed. The brain was partitioned such that the medial septum block was immediately stored in 4% PFA and acute hippocampal slices were prepared from the remaining block in artificial cerebral spinal fluid (aCSF) (in mM): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26 NaHCO3, and 11 glucose. Following this incubation, the CA1 and dentate gyrus regions were immediately dissected from the slices. The tissue was then homogenized in lysis buffer (T-PER Tissue Protein Extraction Reagent, ThermoScientific, Rockford, IL), including protease inhibitor. Samples containing 15 μg of protein were resolved on 4–20% precast gels (Invitrogen) and transferred to polyvinylidene difluoride membrane, blocked and incubated with anti-Aβ (1:750, Zymed) antibody overnight at 4°C. Blots were then incubated in HRP-conjugated secondary antibody (1:3000; Bio-Rad, Hercules, CA) for 2 hours at RT. Bands were detected using Western Lightning Plus-ECL chemiluminescence (Perkin Elmer, Waltham, MA). Densitometric quantification of immunopositive bands was performed with ImageJ and ImageQuant software. The ratio of α C-terminal fragment (αCTF, 8kDa) to β C-terminal fragment (βCTF, 10 kDa) to actin was determined and graphed.
Transcardial perfusion
Rats were anesthetized using 2.5% isoflurane and were transcardially perfused to remove blood from the brain with ice cold phosphate buffered saline (PBS) for 10 minutes followed by 4% paraformaldehyde (PFA) for 10 minutes. After perfusion, brain was removed and fixed in 4% PFA at 4°C for 48 hours.
Immunohistochemistry
Medial Septum
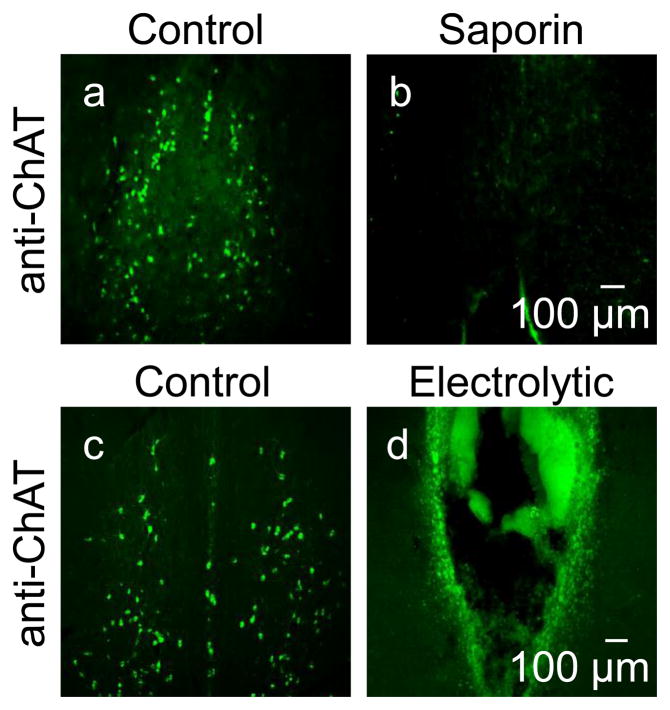
To confirm lesion completeness, serial sections (50μm in 6 series) of medial septum from all rats were prepared by vibratome and stored in antifreeze solution until staining. One series of sections (8 sections per rat) were washed and incubated in blocking buffer (0.3% Triton X-100, 10% normal donkey serum in PBS) for 90 minutes and then incubated in anti-choline acetyltranserase (ChAT) (1:300, EMD Millipore, Billerica, MA, AB144P) at 4°C overnight. The next day, sections were washed and then incubated in donkey anti-goat Alexa 488 (1:500, Invitrogen) for 90 minutes at RT followed by washing and a 10 minute application of DAPI. Sections were then mounted and coverslipped with Aquamount reagent and imaged on a fluorescent light microscope. A complete lesion was defined as 90% cholinergic cell loss from medial septum (Figure 1).
Figure 1.
MS lesions confirmed using immunohistochemistry with anti-ChAT (green) antibodies. In control rats (a, c) cell bodies of MS can be identified. However, with saporin (b) or electrolytic lesions (d), most of these cells are no longer present. A lesion was considered complete if less than 10% of cells were remaining.
Hippocampus
One series of hippocampal sections (8 sections per rat) were washed and blocked in universal blocking buffer (UBB: 1% BSA, 0.2% non-fat powdered skim milk, 0.35% Triton X-100 in PBS) and then stored in anti-p75 neurotrophin receptor (p75-NTR) receptor [MC-192] (1:250, abcam, ab6172) and either anti-tyrosine hydroxylase (TH) (1:500, EMD Millipore, Billerica, MA, ab152) or anti-dopamine β hydroxylase (DβH) (1:500, abcam, Cambridge, MA, ab43868) at 4°C overnight. After washing, the sections were incubated in ImmPRESS anti-mouse Ig (1:10, Vector Labs, Burlingame, CA, MP-7402) and donkey-anti-rabbit Alexa 594 (1:500, Invitrogen, Grand Island, NY) in UBB for 90 minutes at RT. Next, the sections were washed and then stored in FITC Tyramide Plus in amplification buffer (1:1,500, Perkin Elmer) for 30 minutes at RT followed by washing and a 10 minute application of DAPI. Sections were then mounted and coverslipped with ProLong Gold antifade reagent (Invitrogen, Grand Island, NY) and imaged on an Olympus Fluoview 1000 laser scanning confocal microscope acquiring 30 stacks at 0.5 μm increments. For each rat, all 8 sections in the series were inspected for the presence of positive immunostaining in both regions CA1 and DG. At least 2 series were stained per rat and at least an N=4 rats were included in each data set. To verify that our anti-p75NTR staining was indeed working, we investigated the nucleus basalis region in the same sections and found the expected immunopositive cell bodies and processes (Supplemental 1d). Furthermore, p75NTR positive fibers could clearly be seen in sections stained together with those above from a control rat with no Aβ42. (Supplemental 1a–c). To confirm the absence of nonspecific binding from secondary antibodies, we stained one series of sections in unison with the above experiment eliminating the addition of primary antibody (data not shown).
Statistics
All data are expressed as mean ± SEM. Statistical significance of optical density measured between groups by western blot was determined using one-way ANOVA, followed by Tukey post-hoc test using Origin software (OriginLab). The accepted level of significance was p < 0.05.
RESULTS
Sympathetic sprouting can be identified by the colocalization of antibodies to p75 neurotrophin receptor (p75NTR) and tyrosine hydroxylase (TH) in rats with a medial septal lesion
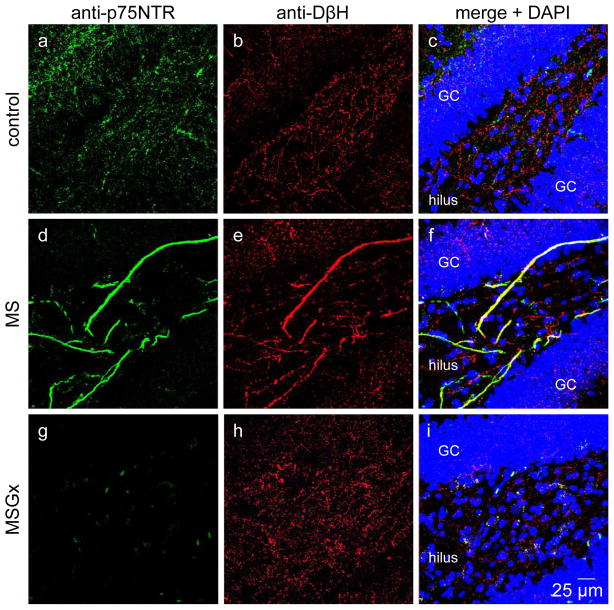
Previously, our lab and others have identified noradrenergic sympathetic sprouting induced by cholinergic denervation by morphological characterization using an antibody to tyrosine hydroxylase (TH), the rate limiting enzyme in the synthetic pathway for dopamine and norepinephrine. (TH) [14, 20, 27]. However, hippocampus is normally innervated by central noradrenergic fibers arising from the locus coeruleus, which are also positively labeled with the anti-TH antibody, preventing an unambiguous identification of peripheral versus central noradrenergic fibers. Therefore, to conclusively distinguish noradrenergic sympathetic sprouting from central noradrenergic fibers, we used a double staining strategy taking advantage of the selective expression of the pan neurotrophin receptor, p75NTR (green) by peripheral but not central noradrenergic fibers [29]. Thus, we performed double immunofluoresence histochemistry using anti-p75NTR (green) and anti-TH (red) antibodies to identify peripheral versus central noradrenergic fibers. Using this approach, we find thick ribbon-like double labeled fibers in the hilar region of the dentate gyrus, separately identifying noradrenergic sympathetic axons (both green and red; yellow in merged image) from central noradrenergic axons (red only) (Figure 2d–f). Central cholinergic fibers and the cholinergic reinnervation that occurs as a consequence of noradrenergic sympathetic sprouting are also identified (green only) because central cholinergic neurons also express p75NTRs [29]. Double labeled fibers were absent in control (Figure 2a–c) and MSGx (Figure 2g–i) rats.
Figure 2.
Noradrenergic sympathetic sprouting can be identified using double immunohistochemistry by the colocalization (yellow) of anti-p75NTR (green) and anti-DβH (red) antibodies. Double labeling is absent in dentate gyrus from control (a–c) and MSGx (g–i) rats. Double labeled fibers are only present in MS rats (d–f). Confocal images obtained at 40x. GC, granule cell
Non-amyloidogenic processing is maintained in MS rats with noradrenergic sympathetic sprouting and cholinergic reinnervation but not in MSGx rats where sprouting is absent
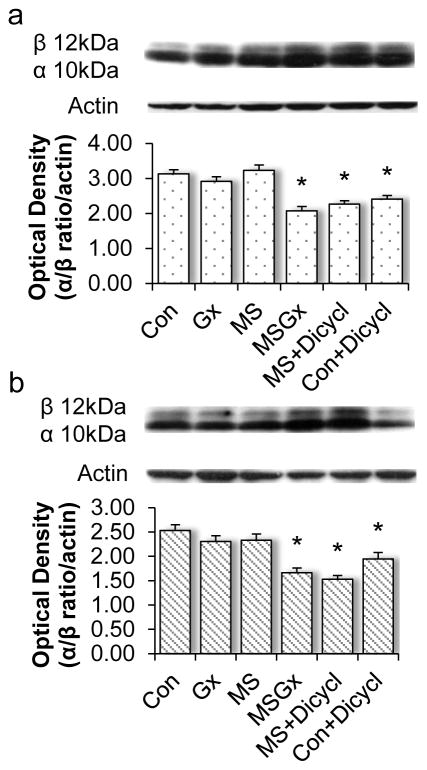
We have shown that sympathetic sprouting and the consequent increase in cholinergic innervation maintains M1 mAChR LTD [20], therefore we tested the hypothesis that sprouting should also maintain non-amyloidogenic AβPP processing, which requires M1 mAChRs. To determine this, we measured αCTF and βCTF protein levels in synaptosomal fractions from dissected CA1 and dentate gyrus subfields from all four animal groups: control (sham lesion + intact ganglia, N=7), Gx (sham lesion + ganglionectomy, N=7), MS (medial septal lesion + intact ganglia, N=7), and MSGx (medial septal lesion + ganglionectomy, N=7), and calculated the αCTF:βCTF ratio. The αCTF is generated by α-secretase cleavage of AβPP in the non-amyloidogenic pathway and the βCTF fragment is generated by β-secretase cleavage in the amylodiogenic pathway. Therefore, a higher αCTF:βCTF ratio indicates predominant non-amyloidogenic processing [30]. The αCTF:βCTF ratio is not different between control, Gx, and MS rats in area CA1 (Figure 3a, p=1) or DG (Figure 3b, p=1), indicating that non-amyloidogenic processing is maintained in MS rats that have sprouting. However, MSGx rats that do not have sprouting have a significant decrease in the αCTF:βCTF ratio in area CA1 (p<0.001) and DG (p<0.005) as compared to control, Gx and MS (Figure 3). These results emphasize the benefit of noradrenergic sympathetic and cholinergic sprouting in compensating for the lost cholinergic input to maintain non-amyloidogenic processing of AβPP.
Figure 3.
M1 mAChR dependent non-amyloidogenic processing of AβPP is maintained in rats with noradrenergic sympathetic sprouting and cholinergic reinnervation. The αCTF/βCTF/actin ratio in area CA1 (a) and DG (b) was not different between control, GX or MS rats, demonstrating a compensatory role of sprouting on non-amyloidogenic AβPP metabolism. Control and MS rats treated systemically with the M1 mAChR antagonist dicyclomine had a significant decrease in the αCTF/βCTF/actin ratio, indicating that the maintenance of non-amyloidogenic AβPP processing is dependent upon M1 mAChRs. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell
M1 mAChRs maintain nonamyloidogenic processing in control and MS rats
M1 mAChRs are required for non-amyloidogenic AβPP processing [3, 5, 6]. To determine whether M1 mAChRs are involved in the maintained nonamyloidogenic processing, control (N=7) and MS (N=6) rats were treated in vivo with dicyclomine, the M1 mAChR antagonist previously shown to exacerbate Aβ pathology in 3xTgAD mice [28], and αCTF and βCTF protein levels were measured in synaptosomal fractions by Western blot. We found that dicyclomine decreases the αCTF:βCTF ratio in both the control (p<0.001 in CA1 and DG) and MS group (p<0.001 in CA1 and DG) (Figure 3a, b). This reconfirms our previous findings that M1 mAChRs are maintained in MS rats [20] that have noradrenergic sympathetic sprouting and the accompanying cholinergic sprouting and emphasizes the importance of M1 mAChRs on processing of endogenous AβPP.
Chronic Aβ is deleterious to central cholinergic fibers in hippocampus and does not induce sympathetic sprouting in control rats
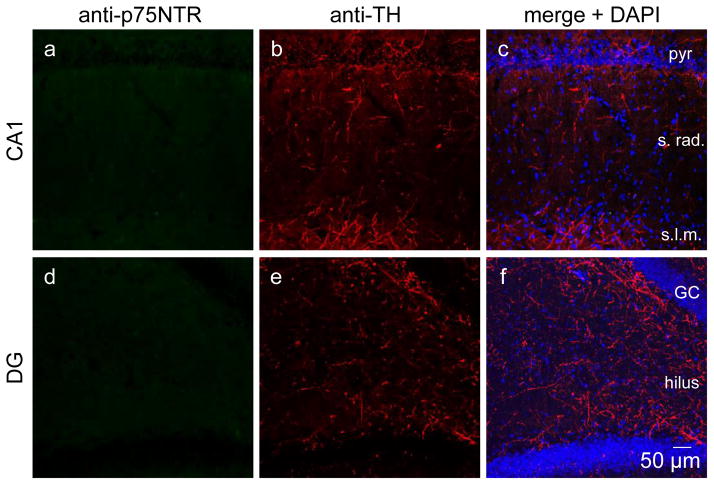
Aβ is toxic to central cholinergic neurons which should cause accumulation of NGF and stimulate noradrenergic sympathetic and cholinergic sprouting [15–17]. Therefore, we next wanted to determine if cholinergic degeneration caused by chronic intrahippocampal Aβ administration stimulates sprouting. Using double anti-p75NTR and anti-TH immunohistochemistry, we found a complete loss of fibers positive only for p75NTR, indicating a complete loss of cholinergic axons in area CA1 (Figure 4a–c) and DG (Figure 4d–f) in rats following 28 days of Aβ treatment. Furthermore, we found no fibers double labeled with anti-p75NTR and anti-TH, indicating the absence of noradrenergic sympathetic sprouting (Figure 4, N=5).
Figure 4.
Aβ42 is toxic to central cholinergic fibers in control rats. Intrahippocampal infusion of synthetic Aβ42 for 28 days into hippocampus is detrimental to all central cholinergic axons in hippocampus. Using p75NTR immunohistochemistry, no cholinergic fibers can be identified in area CA1 (a–c) or DG (d–f) (N=5). Confocal images obtained at 20x. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell
Chronic intrahippocampal infusion of synthetic Aβ limits noradrenergic sympathetic and cholinergic sprouting
The above data demonstrates that Aβ42 does not induce sympathetic sprouting as a consequence of the cholinergic degeneration. Next to determine whether Aβ42 interferes with the establishment of sprouting induced by electrolytic MS lesion synthetic Aβ42 was delivered via cannula to area CA1 post-medial septum lesion at two different time points in separate groups of rats. In the first group, intrahippocampal Aβ42 infusion began 7 days post-lesion and continued for 28 days (Figure 5, N=4). The goal of this experiment was to determine whether Aβ42 administration at the time that noradrenergic sympathetic sprouting and cholinergic reinnervation are just beginning would prevent additional sprouting. In the second group, intrahippocampal Aβ42 infusion began 16 days post-lesion and continued for 14 days (Figure 6, N=4). The goal of this was to determine whether the Aβ42 administration after the establishment of sprouting would cause degeneration of the sprouted axons. Using double anti-p75NTR and anti-TH immunohistochemistry, we rarely observed noradrenergic sympathetic or cholinergic sprouting in rats that received intrahippocampal Aβ42 infusion just as sprouting was beginning (Figure 5, 4/32 sections from 4 rats) or after the establishment of sprouting (Figure 6, 2/32 sections from 4 rats). Furthermore, in the few sections where sprouting was present, only 1 or 2 double labeled fibers were observed and the morphology was thin and delicate compared to the ribbon-like appearance that is more typical of sympathetic sprouting (Figure 2). These findings reveal that Aβ42 is not only toxic to central cholinergic neurons, but also to peripheral sympathetic axons in vivo.
Figure 5.
Aβ42 prevents noradrenergic sympathetic and cholinergic sprouting in MS rats. Intrahippocampal infusion of synthetic Aβ42 began 7 days post-lesion and continued for 28 days. Noradrenergic sympathetic or cholinergic sprouting could not be identified in area CA1 (a–c). Rarely, noradrenergic sympathetic sprouting could be identified in DG (d–f, arrow) however, the abundance is drastically reduced compared to sprouting seen without Aβ42 (Figure 2d–f) (N=4). Confocal images obtained at 20x. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell
Figure 6.
Aβ42 interferes with noradrenergic sympathetic and cholinergic sprouting in MS rats. Intrahippocampal infusion of synthetic Aβ42 began 16 days post-lesion and continued for 14 days. Noradrenergic sympathetic or cholinergic sprouting could not be identified in area CA1 (a–c) or DG (d–f) (N=4). Confocal images obtained at 20x. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell
DISCUSSION
Here, we report the novel finding that noradrenergic sympathetic sprouting and the concomitant cholinergic sprouting maintain nonamyloidogenic processing of AβPP that is dependent upon M1 mAChRs. Also, we found that, even though reports show increased noradrenergic sympathetic sprouting in AD patients compared to age matched control subjects [21], chronic Aβ42 infusion to hippocampus causes cholinergic axon degeneration but does not trigger noradrenergic sympathetic or cholinergic sprouting in control rats, suggesting that Aβ42 accumulation is likely not the trigger for sprouting in humans. Furthermore, we found that Aβ42 prevents and is deleterious to sprouting with only rare incidences of sprouting being identified. Aβ has been shown to be toxic to SCG neurons in vitro [24], but to the best of our knowledge, this is the first report to demonstrate that Aβ42 is toxic to peripheral sympathetic axons in vivo. Previously, our lab has shown that noradrenergic sympathetic and cholinergic sprouting are able to maintain M1 mAChR dependent mLTD [20]. Altogether, these findings suggest that noradrenergic sympathetic and cholinergic sprouting is compensatory; maintaining otherwise lost M1 mAChR function.
Considerable data including the present work supports that M1 mAChRs are a viable therapeutic target for AD [3, 20, 27, 31–35]. Due to this, selective M1 mAChR pharmaceuticals are being developed [36]. Interestingly, TBPB, a M1 mAChR allosteric potentiator, was reported to increase non-amyloidogenic processing of AβPP [36]. Also, the mechanism by which M1 mAChRs regulate AβPP processing is beginning to be understood. Specifically, M1 mAChRs and interacts with BACE1 to regulate its proteasomal degradation and regulate AβPP processing through a mitogen-activated protein kinase-dependent and protein kinase C (PKC)-dependent pathway [6, 37]. Additionally, disrupted coupling between M1 mAChRs and Gq proteins correlates with reductions of PKC activity and NMDA receptor density in post-mortem human brain from AD subjects [38]. While the relationship between M1 mAChRs and AβPP processing is now appreciated, the strongest recent evidence showing therapeutic potential of M1 mAChRs is that the removal of M1 mAChRs exacerbates all AD related phenotypes found in the 3xTgAD mice [8].
The finding that Aβ42 is toxic to noradrenergic sympathetic and cholinergic sprouting is not surprising. Even though Aβ42 is toxic to cholinergic fibers, which should increase NGF and trigger sprouting, Aβ can interact with p75NTRs to induce neuritic dystrophy [15, 39, 40]. In fact, application of Aβ isolated from AD patient brains has been shown to be toxic to superior cervical ganglia neurons, further supporting our findings [24]. Interestingly, noradrenergic sympathetic sprouting has been found predominately in Aβ plaque poor areas of post-mortem brain sections from AD subjects [41]. This raises the question of the timing of noradrenergic sympathetic sprouting in the AD disease process. Our data would suggest that chronic Aβ42 both prevents and is deleterious to sprouting. Knowing that noradrenergic sympathetic sprouting is found in AD post-mortem human brain in regions that are Aβ plaque poor would suggest that this sprouting likely occurs early in the disease process [21, 41]. Taken together with our finding that noradrenergic sympathetic sprouting and the associated cholinergic sprouting maintain physiological function suggests that sprouting could be a novel therapeutic target improving M1 mAChR function for AD patients [20]. Importantly, NGF treatment, the trigger for noradrenergic sympathetic and cholinergic sprouting, is currently in clinical trials for AD patients and has shown benefit [42–44].
Here, we employed a cholinergic lesion rat model that recapitulates many of the hallmarks of AD [9, 10, 12, 45–49]. The intriguing aspect of this animal model is the ingrowth of noradrenergic sympathetic and cholinergic fibers into hippocampus, both of which have been identified in post-mortem AD hippocampus [11, 21, 22, 50]. We show that this sprouting maintains M1 mAChR function sustaining non-amyloidogenic processing of AβPP. Future studies should explore the benefit of noradrenergic sympathetic and cholinergic sprouting on other AD related deficits including hippocampal dependent learning. Altogether, our findings reiterate the potential for M1 mAChRs as a therapeutic target for AD and highlight the compensatory mechanisms of noradrenergic sympathetic and cholinergic sprouting.
Supplementary Material
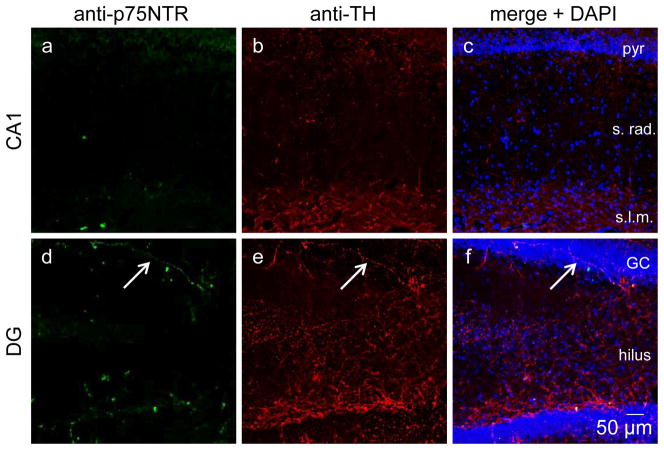
Positive controls demonstrating that p75NTR and TH is abundant in a control rat that received no Aβ that was stained in unison with tissue from Figures 4–6(a–c) and also in nucleus basalis of the same section from the control rat with 28 days Aβ42 in Figure 4. Confocal images obtained at 20x. pyr, GC, granule cell
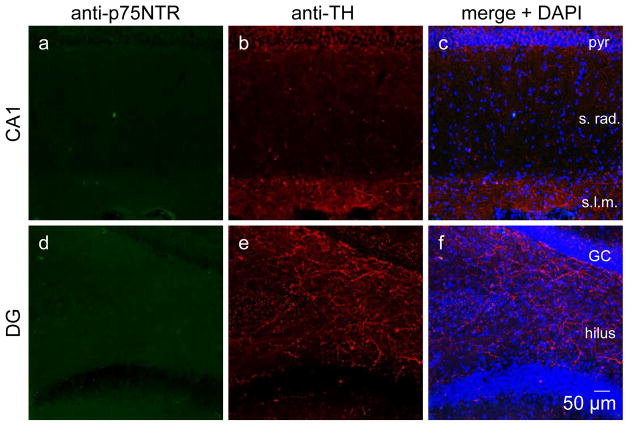
Negative controls demonstrating the absence of non-specific binding of secondary antibodies in area CA1 (a–c) and DG (d–f). One series of sections was stained in parallel to Figure 4–6. Confocal images obtained at 20x. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell
Acknowledgments
We would like to thank Dr. Lindy Harrell for scientific discussion related to this manuscript. Also, we would like to thank the Center for Glial Biology in Medicine Imaging Core and UAB-High Resolution Imaging Facility for use of their confocal microscope. This work was funded by NIH R01 AG021612 awarded to L.L. McMahon.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Selkoe DJ. Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res. 2013 doi: 10.1007/s12272-013-0036-3. [DOI] [PubMed] [Google Scholar]
- 3.Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):22–33. doi: 10.1111/j.1471-4159.2011.07507.x. [DOI] [PubMed] [Google Scholar]
- 4.Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janickova H, Rudajev V, Zimcik P, Jakubik J, Tanila H, El-Fakahany EE, Dolezal V. Uncoupling of M1 muscarinic receptor/G-protein interaction by amyloid beta(1–42) Neuropharmacology. 2013;67:272–283. doi: 10.1016/j.neuropharm.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Jiang S, Wang Y, Ma Q, Zhou A, Zhang X, Zhang YW. M1 muscarinic acetylcholine receptor interacts with BACE1 and regulates its proteosomal degradation. Neurosci Lett. 2012;515:125–130. doi: 10.1016/j.neulet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Perez SE, He B, Muhammad N, Oh KJ, Fahnestock M, Ikonomovic MD, Mufson EJ. Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol Dis. 2011;41:338–352. doi: 10.1016/j.nbd.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros R, Kitazawa M, Caccamo A, Baglietto-Vargas D, Estrada-Hernandez T, Cribbs DH, Fisher A, LaFerla FM. Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am J Pathol. 2011;179:980–991. doi: 10.1016/j.ajpath.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolasa K, Harrell LE, Parsons DS. Effect of hippocampal sympathetic ingrowth and cholinergic denervation on hippocampal phospholipase C activity and G-protein function. Neuroscience. 1997;77:111–120. doi: 10.1016/s0306-4522(96)00438-1. [DOI] [PubMed] [Google Scholar]
- 10.Harrell LE, Kolasa K, Parsons DS, Ayyagari V. Hippocampal sympathetic ingrowth and cholinergic denervation uniquely alter muscarinic receptor subtypes in the hippocampus. Brain Res. 1995;676:386–393. doi: 10.1016/0006-8993(95)00070-7. [DOI] [PubMed] [Google Scholar]
- 11.Harrell LE, Parsons D, Kolasa K. Hippocampal sympathetic ingrowth occurs following 192-IgG-Saporin administration. Brain Res. 2001;911:158–162. doi: 10.1016/s0006-8993(01)02626-9. [DOI] [PubMed] [Google Scholar]
- 12.Kolasa K, Parsons DS, Harrell LE. Effect of phospholipase C and protein kinase C following cholinergic denervation and hippocampal sympathetic ingrowth in rat hippocampus. Neuroscience. 2000;99:25–31. doi: 10.1016/s0306-4522(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 13.Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- 14.Crutcher KA, Brothers L, Davis JN. Sympathetic noradrenergic sprouting in response to central cholinergic denervation; a histochemical study of neuronal sprouting in the rat hippocampal formation. Brain Res. 1981;210:115–128. doi: 10.1016/0006-8993(81)90889-1. [DOI] [PubMed] [Google Scholar]
- 15.Kawaja MD, Crutcher KA. Sympathetic axons invade the brains of mice overexpressing nerve growth factor. J Comp Neurol. 1997;383:60–72. [PubMed] [Google Scholar]
- 16.Springer JE, Loy R. Intrahippocampal injections of antiserum to nerve growth factor inhibit sympathohippocampal sprouting. Brain Res Bull. 1985;15:629–634. doi: 10.1016/0361-9230(85)90212-6. [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Crutcher KA. Nerve growth factor immunoreactivity and sympathetic sprouting in the rat hippocampal formation. Brain Res. 1995;672:55–67. doi: 10.1016/0006-8993(94)01344-h. [DOI] [PubMed] [Google Scholar]
- 18.Stenevi UaBA. Growth of vascular sympathetic axons into the hippocampus after lesions of the septo-hippocampal pathway: a pitfall in brain lesion studies. Neurosci Lett. 1978;7:219–224. doi: 10.1016/0304-3940(78)90171-4. [DOI] [PubMed] [Google Scholar]
- 19.Crutcher KA, Madison R, Davis JN. A study of the rat septohippocampal pathway using anterograde transport of horseradish peroxidase. Neuroscience. 1981;6:1961–1973. doi: 10.1016/0306-4522(81)90036-1. [DOI] [PubMed] [Google Scholar]
- 20.Scheiderer CL, McCutchen E, Thacker EE, Kolasa K, Ward MK, Parsons D, Harrell LE, Dobrunz LE, McMahon LL. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3-CA1 synapses. J Neurosci. 2006;26:3745–3756. doi: 10.1523/JNEUROSCI.5507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booze RM, Mactutus CF, Gutman CR, Davis JN. Frequency analysis of catecholamine axonal morphology in human brain. II. Alzheimer’s disease and hippocampal sympathetic ingrowth. J Neurol Sci. 1993;119:110–118. doi: 10.1016/0022-510x(93)90198-8. [DOI] [PubMed] [Google Scholar]
- 22.Masliah E, Alford M, Adame A, Rockenstein E, Galasko D, Salmon D, Hansen LA, Thal LJ. Abeta1–42 promotes cholinergic sprouting in patients with AD and Lewy body variant of AD. Neurology. 2003;61:206–211. doi: 10.1212/01.wnl.0000073987.79060.4b. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 24.Roher AE, Ball MJ, Bhave SV, Wakade AR. Beta-amyloid from Alzheimer disease brains inhibits sprouting and survival of sympathetic neurons. Biochem Biophys Res Commun. 1991;174:572–579. doi: 10.1016/0006-291x(91)91455-l. [DOI] [PubMed] [Google Scholar]
- 25.Roberson MR, Kolasa K, Parsons DS, Harrell LE. Cholinergic denervation and sympathetic ingrowth result in persistent changes in hippocampal muscarinic receptors. Neuroscience. 1997;80:413–418. doi: 10.1016/s0306-4522(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 26.Ayyagari V, Harrell LE, Parsons DS, Kolasa K. The effect of cholinergic denervation and hippocampal sympathetic ingrowth on the internalization of muscarinic receptors in rat hippocampus. Brain Res. 1995;676:394–397. doi: 10.1016/0006-8993(95)00146-h. [DOI] [PubMed] [Google Scholar]
- 27.McCoy PA, McMahon LL. Sympathetic sprouting in visual cortex stimulated by cholinergic denervation rescues expression of two forms of long-term depression at layer 2/3 synapses. Neuroscience. 2010;168:591–604. doi: 10.1016/j.neuroscience.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Barrett GL, Greferath U, Barker PA, Trieu J, Bennie A. Co-expression of the P75 neurotrophin receptor and neurotrophin receptor-interacting melanoma antigen homolog in the mature rat brain. Neuroscience. 2005;133:381–392. doi: 10.1016/j.neuroscience.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 30.Liu RM, van Groen T, Katre A, Cao D, Kadisha I, Ballinger C, Wang L, Carroll SL, Li L. Knockout of plasminogen activator inhibitor 1 gene reduces amyloid beta peptide burden in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32:1079–1089. doi: 10.1016/j.neurobiolaging.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beach TG, Walker DG, Potter PE, Sue LI, Fisher A. Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res. 2001;905:220–223. doi: 10.1016/s0006-8993(01)02484-2. [DOI] [PubMed] [Google Scholar]
- 32.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 33.Sadot E, Gurwitz D, Barg J, Behar L, Ginzburg I, Fisher A. Activation of m1 muscarinic acetylcholine receptor regulates tau phosphorylation in transfected PC12 cells. J Neurochem. 1996;66:877–880. doi: 10.1046/j.1471-4159.1996.66020877.x. [DOI] [PubMed] [Google Scholar]
- 34.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 35.Caccamo A, Fisher A, LaFerla FM. M1 agonists as a potential disease-modifying therapy for Alzheimer’s disease. Curr Alzheimer Res. 2009;6:112–117. doi: 10.2174/156720509787602915. [DOI] [PubMed] [Google Scholar]
- 36.Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haring R, Fisher A, Marciano D, Pittel Z, Kloog Y, Zuckerman A, Eshhar N, Heldman E. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to beta-amyloid precursor protein secretion. J Neurochem. 1998;71:2094–2103. doi: 10.1046/j.1471-4159.1998.71052094.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsang SW, Pomakian J, Marshall GA, Vinters HV, Cummings JL, Chen CP, Wong PT, Lai MK. Disrupted muscarinic M1 receptor signaling correlates with loss of protein kinase C activity and glutamatergic deficit in Alzheimer’s disease. Neurobiol Aging. 2007;28:1381–1387. doi: 10.1016/j.neurobiolaging.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Hochstrasser T, Hohsfield LA, Sperner-Unterweger B, Humpel C. beta-Amyloid induced effects on cholinergic, serotonergic, and dopaminergic neurons is differentially counteracted by anti-inflammatory drugs. J Neurosci Res. 2013;91:83–94. doi: 10.1002/jnr.23126. [DOI] [PubMed] [Google Scholar]
- 40.Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM. The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo. J Neurosci. 2009;29:10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolar M, Scott SA, Crutcher KA. Sympathetic neurite outgrowth is greater on plaque-poor vs. plaque-rich regions of Alzheimer’s disease cryostat sections. Brain Res. 1998;787:49–58. doi: 10.1016/s0006-8993(97)01455-8. [DOI] [PubMed] [Google Scholar]
- 42.Tuszynski MH. Nerve growth factor gene therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:179–189. doi: 10.1097/WAD.0b013e318068d6d2. [DOI] [PubMed] [Google Scholar]
- 43.Tuszynski MH. Nerve growth factor gene delivery: animal models to clinical trials. Dev Neurobiol. 2007;67:1204–1215. doi: 10.1002/dneu.20510. [DOI] [PubMed] [Google Scholar]
- 44.Tuszynski MH, Thal L, Pay M, Salmon DP, UHS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 45.Ayyagari PV, Harrell LE, Parsons DS, Kolasa K. Sympathetic sprouting reverses decreases in membrane-associated activity of protein kinase C following septohippocampal denervation of the rat hippocampus. Brain Res. 1996;708:205–208. doi: 10.1016/0006-8993(95)01364-4. [DOI] [PubMed] [Google Scholar]
- 46.Harrell LE, Parsons DS, Kolasa K. Pro- and anti-apoptotic evidence for cholinergic denervation and hippocampal sympathetic ingrowth in rat dorsal hippocampus. Exp Neurol. 2005;194:182–190. doi: 10.1016/j.expneurol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Harrell LE, Parsons DS, Kolasa K. The effect of central cholinergic and noradrenergic denervation on hippocampal sympathetic ingrowth and apoptosis-like reactivity in the rat. Brain Res. 2005;1033:68–77. doi: 10.1016/j.brainres.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Kolasa K, Harrell LE. Apoptotic protein expression and activation of caspases is changed following cholinergic denervation and hippocampal sympathetic ingrowth in rat hippocampus. Neuroscience. 2000;101:541–546. doi: 10.1016/s0306-4522(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 49.Scheiderer CL, Kolasa K, Ward M, Parsons D, Harrell LE, Dobrunz LE, McMahon LL. Sympathetic sprouting rescues plasticity at a central synapse. 2003 doi: 10.1523/JNEUROSCI.5507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crutcher KA. Cholinergic denervation of rat neocortex results in sympathetic innervation. Exp Neurol. 1981;74:324–329. doi: 10.1016/0014-4886(81)90172-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Positive controls demonstrating that p75NTR and TH is abundant in a control rat that received no Aβ that was stained in unison with tissue from Figures 4–6(a–c) and also in nucleus basalis of the same section from the control rat with 28 days Aβ42 in Figure 4. Confocal images obtained at 20x. pyr, GC, granule cell
Negative controls demonstrating the absence of non-specific binding of secondary antibodies in area CA1 (a–c) and DG (d–f). One series of sections was stained in parallel to Figure 4–6. Confocal images obtained at 20x. pyr, pyramidal cell; s. rad., stratum radiatum; s.l.m., stratum lacunosom moleculare; GC, granule cell