Abstract
Objective: To assess clinically relevant symptom improvement in patients with major depressive disorder (MDD) receiving vilazodone by using the Montgomery-Asberg Depression Rating Scale (MADRS), a clinician-rated scale used to measure MDD symptom severity and improvement.
Method: Pooled data from 2 positive, phase 3, 8-week, double-blind, randomized, placebo-controlled trials in patients with MDD were analyzed. Patients received vilazodone 40 mg/d or placebo; post hoc analyses were conducted on study completers. Depression symptom improvement was evaluated by analyzing the proportions of patients who shifted from the baseline MADRS single-item symptom severity category of ≥ 2 (mild to severe symptoms) to an end-of-study category < 2 (minimal to no symptoms) or from ≥ 4 (moderate to severe symptoms) to ≤ 2 (mild to no symptoms). The proportion of patients who shifted from anxious depression to no anxious depression was also analyzed.
Results: The percentage of patients who completed these studies with severity category shift from baseline ≥ 2 to end of study < 2 was significantly higher for vilazodone versus placebo on all MADRS items (odds ratio [OR] range, 1.4–1.7, P < .05) except reduced appetite (OR = 1.3, P = .232). A significantly greater proportion of vilazodone-treated versus placebo-treated patients shifted from baseline ≥ 4 to end of study ≤ 2 on MADRS items of apparent sadness, reported sadness, inner tension, reduced sleep, and lassitude (OR range, 1.5–2.0, P < .05). Additionally, a significantly greater proportion of vilazodone-treated versus placebo-treated patients shifted from anxious depression at baseline to no anxious depression at end of study (OR = 1.5, P = .031).
Conclusions: These results suggest that vilazodone treatment is associated with clinically relevant changes in depression symptoms in patients with MDD.
Trial Registration: ClinicalTrials.gov identifiers: NCT00285376 and NCT00683592
Clinical Points
⊠ Shifts from greater to lesser severity categories in specific depression and anxiety symptoms associated with major depressive disorder (MDD) may provide an assessment of patient improvement that is more intuitively descriptive than mean changes in total symptom scores.
⊠ Vilazodone is a serotonin (5-HT) reuptake inhibitor and 5-HT1A receptor partial agonist approved by the US Food and Drug Administration for the treatment of MDD in adults.
⊠ In patients treated with vilazodone versus placebo, improvement in depressive symptoms and anxious depression was evident by statistically significant shifts from more severe to less severe symptom categories.
Major depressive disorder (MDD) is one of the most common conditions seen by primary care physicians, accounting for 10%–14% of all patient visits.1–3 Patients with MDD often have poor psychological, physical, and social functioning4; increased prevalence of and complications from chronic medical illness5; and higher rates of health care utilization and costs.6 The core symptoms of MDD include sad mood, diminished interest or pleasure in activities, changes in appetite or weight, sleep disturbances, psychomotor agitation, fatigue, feelings of worthlessness, difficulty concentrating, and suicidal thoughts.7 Additionally, approximately half of all patients with MDD have high levels of comorbid anxiety, which is associated with greater severity of depressive illness and functional impairment, greater chronicity, delayed response to treatment, and increased risk of suicide.8,9
Use of a standardized, measurement-based symptom assessment to evaluate and monitor depressive symptom burden and improvement may enhance outcomes in both primary care and psychiatric practices.10 In clinical trials, mean change from baseline on a depression rating scale is commonly used to show symptom improvement on the basis of statistically significant differences between active treatment and placebo. Since rating scales are not routinely used in clinical practice,10 mean differences on a depression scale may not be the most informative or clinically meaningful metric for a clinician interested in the more broadly formulated question, are my patient’s symptoms improving?
Vilazodone is a selective serotonin (5-HT) reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist approved by the US Food and Drug Administration for the treatment of MDD in adults. Rather than using the mean change from baseline on a standard depression rating scale to evaluate improvement for patients treated with vilazodone or placebo, we explored a novel and intuitive category shift analysis using pooled data from 2 short-term, double-blind, randomized, placebo-controlled phase 3 trials (ClinicalTrials.gov identifiers: NCT00285376 and NCT00683592).11,12 In this post hoc analysis, changes from more severe to less severe symptom categories, rather than simple mean score change, were used to evaluate the treatment effect of vilazodone on depression symptoms as measured by individual items of the Montgomery-Asberg Depression Rating Scale (MADRS).13 Shift to a less severe symptom category or an asymptomatic state demonstrated improvement and the benefits of treatment with vilazodone.
Scores on the individual items of the MADRS were organized into 3 descriptive severity categories: “no or minimal symptoms,” “mild to no symptoms,” and “moderate to severe symptoms.” Improvement in depressive symptoms was evaluated by the percentage of patients who shifted from a category that denoted greater depressive symptom severity to a category that denoted less severe symptoms. In other words, did patients with symptomatic depression improve to no or minimal symptoms at the end of the study? Did patients with more severe symptoms improve to mild to no symptoms?
Vilazodone was approved for the treatment of depression on the basis of the positive results of the 2 studies that were pooled for these post hoc analyses. In both studies, vilazodone showed significantly greater improvement relative to placebo on the primary efficacy outcomes mean change from baseline to week 8 on the MADRS total score. Additionally, the long-term safety and tolerability of vilazodone was supported in a 1-year, open-label trial (ClinicalTrials.gov identifier: NCT00644358).14 Vilazodone was generally well tolerated in clinical trials; adverse events were transient and generally mild or moderate in severity. On the basis of the pooled data from the individual 8-week phase 3 studies,15 common adverse events that occurred in > 5% of vilazodone-treated patients and at twice the rate of placebo were diarrhea, nausea, and insomnia. Vilazodone was associated with a relatively low impact on sexual function.16
In this post hoc pooled analysis, MADRS single-item scores for each item were grouped into categories on the basis of severity; symptomatic improvement was evaluated by the percentage of patients who shifted from a more severe to a less severe category of depressive symptoms at the end of 8 weeks of treatment. The percentage of patients who shifted from baseline anxious depression to no anxious depression at the end of the study was also evaluated.
METHOD
Study Design and Dosing
This was a post hoc analysis of pooled data from the 2 randomized, placebo-controlled, double-blind, multicenter, parallel-group trials to assess the treatment effects of vilazodone in moderate to severe MDD. All patients who participated in the studies provided written informed consent; protocols were approved by the institutional review board of each center. Detailed methods, efficacy, and safety analyses have been previously published.11,12 Briefly, the studies consisted of a washout period of at least 2 weeks, 1-week screening, and an 8-week double-blind treatment period. Patients were randomly assigned (1:1) to receive placebo or vilazodone 40 mg once daily taken with food; vilazodone was titrated to the target dose according to a fixed titration schedule (10 mg once daily for 7 days, 20 mg once daily for the next 7 days, and 40 mg once daily thereafter). Efficacy assessments were conducted at weeks 0 (baseline), 1, 2, 4, 6, and 8 or at early discontinuation.
Inclusion and Exclusion Criteria
Eligible patients were men and women 18 to 70 years of age who met the diagnostic criteria for MDD according to the DSM-IV-TR.7 Patients had a current major depressive episode of 4 weeks’ to 2 years’ duration, a 17-item Hamilton Depression Rating Scale (HDRS-17)17 score ≥ 22, and a HDRS-17 item 1 (depressed mood) score ≥ 2.
Exclusion criteria included an Axis I disorder other then MDD (eg, schizophrenia, schizoaffective disorder, bipolar disorder, substance abuse/dependence); generalized anxiety disorder, social phobia, or simple phobia were allowed. Patients with serious suicidal risk or suicide attempt within 6 months before screening were excluded. Additionally, patients with clinically significant comorbid medical disorders, including myocardial infarction in the past year, insulin-dependent diabetes mellitus, and renal or hepatic impairment, were excluded.
Post Hoc Efficacy Outcomes: Analyses of Categorical Improvement
Analysis of categorical improvement in depression symptom severity was conducted by comparing the percentage of patients treated with vilazodone versus placebo who shifted from a more severe baseline single-item MADRS symptom category to a less severe category at week 8. The MADRS single-item symptom severity categories were numerically based, and descriptors representing a range of scores were assigned: no/minimal = 0–1, mild = 2–3, moderate = 4–5, and severe = 6 (Table 1). Depression symptom improvement was evaluated by analyzing the percentage of vilazodone-treated and placebo-treated patients who shifted from the baseline MADRS single-item symptom severity category of ≥ 2 (mild to severe symptoms) to an end-of-study symptom severity category of < 2 (minimal to no symptoms) or the percentage of patients who shifted from the baseline category of ≥ 4 (moderate to severe symptoms) to an end-of-study category score ≤ 2 (mild to no symptoms).
Table 1.
Montgomery-Asberg Depression Rating Scale (MADRS) Score Definitionsa
| MADRS Single Items | MADRS Symptom Severity Categories | ||||
| MADRS Score of 0–1 “No/Minimal Symptoms” | MADRS Score of 2–3 “Mild Symptoms” | MADRS Score of 4–5 “Moderate Symptoms” | MADRS Score of 6 “Severe Symptoms” | ||
| 1. | Apparent sadness: despondency, gloom, and despair reflected in speech, facial expression, and posture | No sadness | Looks dispirited | Appears sad and unhappy most of the time | Looks miserable all of the time |
| 2. | Reported sadness: depressed mood, regardless of whether it is reflected in appearance or not; includes low spirits, despondency, or feeling of being beyond help without hope | Occasional sadness | Sad or low | Pervasive feelings of sadness | Continuous or unvarying sadness |
| 3. | Inner tension: feelings of ill-defined discomfort, edginess, inner turmoil mounting to either panic, dread, or anguish | Placid | Occasional feelings of edginess and ill-defined discomfort | Continuous feelings of inner tension or intermittent panic | Unrelenting dread or anguish |
| 4. | Reduced sleep: experience of reduced duration or depth of sleep compared to the subject’s own normal pattern when well | Sleeps as usual | Slight difficulty dropping off to sleep | Sleep reduced/broken by ≥ 2 h | < 2–3 h of sleep |
| 5. | Reduced appetite: feeling of loss of appetite compared with when well | Normal or increased appetite | Slightly reduced appetite | No appetite, food is tasteless | Needs persuasion to eat |
| 6. | Concentration difficulties: difficulties in collecting one’s thoughts mounting to incapacitating lack of concentration | No difficulties in concentrating | Occasional difficulties in collecting one’s thoughts | Difficulties in concentrating and sustaining thought | Unable to read or converse without great initiative |
| 7. | Lassitude: difficulty getting started or slowness initiating and performing everyday activities | Hardly any difficulty in getting started | Difficulties in starting activities | Difficulties in starting simple routine activities | Unable to do anything without help |
| 8. | Inability to feel: subjective experience of reduced interest in the surroundings or activities that normally give pleasure; ability to react with adequate emotion to circumstances or people is reduced | Normal interest in surroundings and other people | Reduced ability to enjoy usual interest | Loss of interest in surroundings | Emotionally paralyzed, inability to feel anger, grief, or pleasure |
| 9. | Pessimistic thoughts: thoughts of guilt, inferiority, self-reproach, sinfulness, remorse, and ruin | No pessimistic thoughts | Fluctuating ideas of failure, self-reproach, or self-deprecation | Persistent self-accusations or definite but still rational ideas of guilt or sin | Delusions of ruin, remorse, or unredeemable sin |
| 10. | Suicidal thoughts: feeling that life is not worth living, feeling that a natural death would be welcome, suicidal thoughts, and the preparations for suicide; suicide attempts should not in themselves influence the rating | Enjoys life or takes it as it comes | Only fleeting suicidal thoughts | Suicidal thoughts are common but without specific plans or intention | Explicit plans for suicide when there is an opportunity |
Based on Khan et al.11
Analysis of categorical improvement in anxious depression was evaluated by the HDRS-17 anxiety/somatization subscale, which comprises the following items: psychic anxiety, somatic anxiety, gastrointestinal somatic symptoms, general somatic symptoms, hypochondriasis, and insight. A HDRS-17 anxiety/somatization score > 7 was used to denote the presence of anxious depression. Categorical improvement in anxious depression was demonstrated by the percentage of patients in each treatment group with a HDRS-17 anxiety/somatization score > 7 at baseline and ≤ 7 (no anxious depression) at the end of the study.
Statistical Analyses
All analyses and summaries were produced using SAS version 9.1.3 (SAS Institute, Cary, North Carolina). The intent-to-treat population included all patients who received study drug (safety population) and had ≥ 1 postbaseline MADRS total score assessment in either of the primary studies that were pooled. The completer population included patients in the intent-to-treat population who completed 8 weeks of double-blind treatment. Descriptive statistics were used to analyze baseline demographics and disease characteristics, baseline depressive symptom severity category for each MADRS single item, and presence of anxious depression in the completer population. Categorical improvement analyses were performed on the completer population. For MADRS single-item analyses, odds ratios (ORs) were estimated, and Fisher exact test was used to obtain 2-sided nominal P values for comparisons between vilazodone and placebo; adjustments for multiplicity were not performed. HDRS-17 anxious depression analyses were based on a logistic regression model, with study and treatment group as factors and corresponding baseline HDRS-17 anxiety/somatization score as explanatory variable.
RESULTS
Patient Disposition and Demographics
The intent-to-treat population comprised 864 patients who received ≥ 1 dose of double-blind study medication and had ≥ 1 postbaseline MADRS assessment (placebo: n = 432, vilazodone: n = 432). The completer population consisted of 349 placebo patients (80.8%) and 345 vilazodone patients (79.9%) in the intent-to-treat population who completed the study. Similar proportions of patients in the placebo versus vilazodone groups discontinued prematurely (19.4% vs 20.9%, respectively); the most common reasons for discontinuation for placebo versus vilazodone were lost to follow-up (6.7% vs 7.3%, respectively), adverse events (3.2% vs 7.1%, respectively), and withdrawal of consent (3.7% vs 2.8%, respectively).
In the intent-to-treat population, the mean MADRS score at baseline was 31.4 in both treatment groups. Of patients in the completer population, over 80% had anxious depression (HDRS-17 anxiety/somatization score > 7) at baseline. Baseline demographic characteristics were similar between groups (Table 2).
Table 2.
Baseline Demographics and Disorder Characteristics (completer population)
| Variable | Placebo (n = 349) | Vilazodone 40 mg/d (n = 345) |
| Women, % | 56 | 60 |
| White, % | 80 | 83 |
| Age, mean (SD), y | 41.9 (12.7) | 41.3 (11.8) |
| Weight, mean (SD), kga | 87.4 (20.8) | 86.3 (25.2) |
| Duration of current depressive episode ≤ 12 mo, % | 83 | 79 |
| First lifetime MDD episode, % | 34 | 32 |
| Patients with anxious depression (HDRS-17 anxiety/somatization score > 7), % | 83 | 81 |
Value in pounds is 193 (46) lb for placebo and 190 (56) lb for vilazodone.
Abbreviations: HDRS-17 = 17-item Hamilton Depression Rating Scale, MDD = major depressive disorder.
Analysis of Depressive Symptoms at Baseline
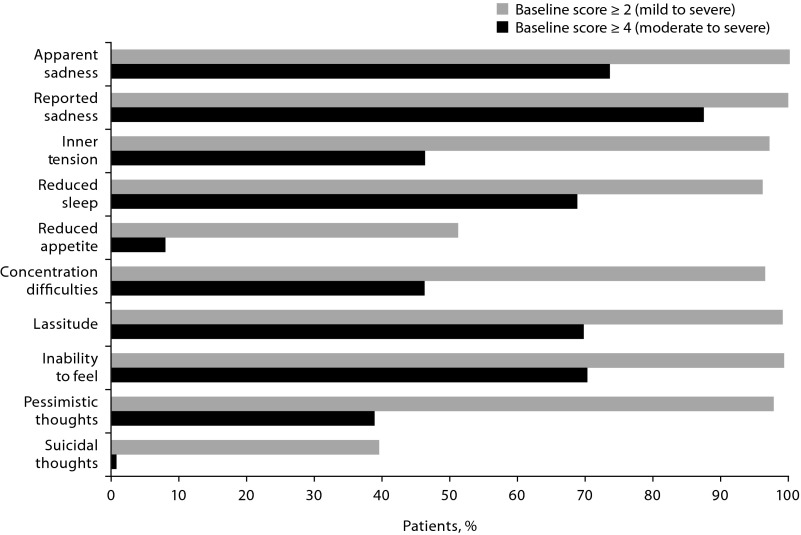
Almost all placebo and vilazodone patients in the completer population had a baseline score ≥ 2 indicating mild to severe symptoms on the MADRS items of apparent sadness (n = 694), reported sadness (n = 694), inner tension (n = 674), reduced sleep (n = 669), concentration difficulties (n = 666), lassitude (n = 686), inability to feel (n = 688), and pessimistic thoughts (n = 677) (Figure 1). Baseline scores ≥ 4 indicating moderate to severe symptoms were reported by most placebo and vilazodone patients (∼ 70%) for apparent sadness (n = 511), reported sadness (n = 606), reduced sleep (n = 477), lassitude (n = 484), and inability to feel (n = 488) (Figure 1). The number of patients with baseline score ≥ 4 on the suicidal thoughts item (item 10, placebo: n = 3, vilazodone: n = 4) was too small for evaluation since patients with serious suicidal risk or suicide attempt within 6 months before screening were excluded. For all MADRS items, the proportion of patients with baseline scores ≥ 2 was similar between the vilazodone and placebo groups; this was also true for patients with baseline scores ≥ 4.
Figure 1.
Proportion of Patients With Montgomery-Asberg Depression Rating Scale (MADRS) Single-Item Scores ≥ 2 (mild to severe) or ≥ 4 (moderate to severe) at Baseline (completer population, n = 694)
MADRS Single-Item Categorical Improvement Analyses
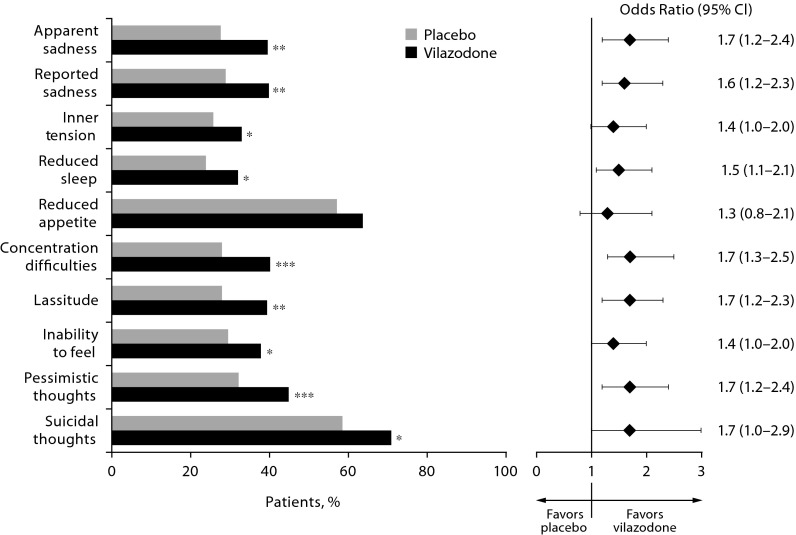
In patients who completed the study, a significantly greater percentage of vilazodone-treated relative to placebo-treated patients improved from the baseline ≥ 2 category (mild to severe symptoms) to end of study < 2 category (minimal to no symptoms) on 9 of 10 MADRS single items (Figure 2); the between-group difference was not significant for the reduced appetite item (OR = 1.3, P = .232). Odds ratios (vilazodone vs placebo) for category shift improvements from ≥ 2 to < 2 were 1.5 or higher on most MADRS single items.
Figure 2.
Categorical Improvement: Baseline Montgomery-Asberg Depression Rating Scale (MADRS) Single-Item Score ≥ 2 to < 2 at End of Study (completer population, n = 694)
*P < .05 vs placebo.
**P < .01 vs placebo.
***P < .001 vs placebo.
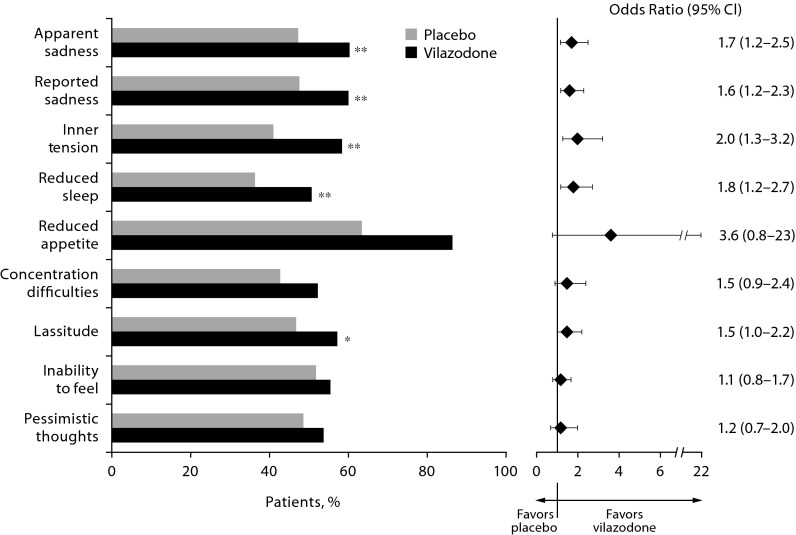
On 5 of 9 evaluable MADRS single items (suicidal thoughts [item 10] was not evaluable due to low number of observations), a significantly greater proportion of vilazodone-treated versus placebo-treated patients improved from baseline ≥ 4 (moderate to severe symptoms) to end of study ≤ 2 (mild to no symptoms) (Figure 3). Between-group differences were not significant for reduced appetite (OR = 3.6, P = .074), concentration difficulties (OR = 1.5, P = .095), inability to feel (OR = 1.2, P = .468), and pessimistic thoughts (OR = 1.2, P = .465). For the suicidal thoughts item (item 10), all 3 placebo-treated patients and 3 of 4 vilazodone-treated patients with baseline score ≥ 4 improved to end of study score ≤ 2. For the majority of MADRS single items, the ORs (vilazodone vs placebo) in achieving a categorical improvement from baseline ≥ 4 to end of study ≤ 2 were 1.5 or greater (Figure 3).
Figure 3.
Categorical Improvement: Baseline Montgomery-Asberg Depression Rating Scale (MADRS) Single-Item Score ≥ 4 to ≤ 2 at End of Study (completer population, n = 694)a
aThe suicidal thoughts item (item 10) was not evaluable due to low number of observations (placebo = 3, vilazodone = 4).
*P < . 05 vs placebo.
**P < .01 vs placebo.
Anxious Depression Categorical Improvement Analysis
In patients with baseline anxious depression (HDRS-17 anxiety/somatization score > 7), a significantly greater percentage of vilazodone-treated versus placebo-treated patients met the no anxious depression criterion (score ≤ 7) at end of study (73.8% vs 66.3%, respectively, P = .031); the OR (95% CI) (vilazodone vs placebo) in achieving a shift from baseline anxious depression to no anxious depression at end of study was 1.5 (1.0–2.2).
DISCUSSION
In patients who began the study with mild to severe symptoms, a significantly greater proportion of vilazodone-treated compared with placebo-treated patients improved to minimal or no symptoms on 9 of 10 MADRS single items; a greater proportion of vilazodone-treated patients who began the study with moderate to severe symptoms improved to mild to no symptoms on 5 items. Additionally, in patients with anxious depression, significantly more vilazodone-than placebo-treated patients met the criterion for no anxious depression at end of study; the clinical relevance of this finding was demonstrated by an OR of 1.5, indicating 50% greater odds of improving with vilazodone relative to placebo.
Since the introduction of SSRIs, primary care physicians have played a major role in depression treatment. The majority of patients with MDD are diagnosed by and receive treatment from a primary care physician18; of note, primary care physicians prescribe 62% of all antidepressant medications.19 Additionally, there is a higher incidence and prevalence of major depression in patients with chronic medical conditions that are commonly seen in primary care settings, including diabetes, pulmonary disease, heart disease, or arthritis.6 Patients with depression and chronic medical illness have significantly more medical symptoms, as well as higher morbidity and mortality than patients with chronic illness alone.5
The perception that patients presenting to primary care settings have less severe and less chronic depressive illness than patients presenting to specialty care has been largely debunked; newly diagnosed patients presenting to primary or specialty care have been shown to have generally similar depression characteristics including illness severity and psychiatric comorbidity.18,20 Although specialty care patients are twice as likely to have made a prior suicide attempt, suicidal ideation is reported by about half of patients in both settings.18,20 Approximately half of all patients who present with MDD in either primary or specialty care settings also have high levels of comorbid symptoms of anxiety.8,9 Of particular interest to primary care physicians, in an analysis of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) database, treatment with open-label citalopram in the first treatment step was shown to produce equivalent outcomes in primary (n = 1,091) and specialty care settings (n = 1,785).18,20 In this highly representative sample of outpatients with nonpsychotic MDD, the likelihood of response or remission, and the time to reach the outcome, were similar regardless of treatment in a primary or specialty care setting.
The presence of even mild residual depressive symptoms after acute treatment of MDD increases the likelihood of relapse, recurrence, and poorer outcomes.21 Additional factors that are associated with posttreatment residual symptoms include more severe depression, comorbid anxiety, chronicity, and medical comorbidities,9,22 which are characteristics of MDD that are likely to be seen in a primary care setting.18 Deconstructing depression into symptom components may be a valuable way to monitor persistent, minor, or residual depressive symptoms in both clinical research and practice.
Novel post hoc categorical shift analyses using pooled data from 2 phase 3 studies11,12 were used to compare improvement in symptoms of depression and anxious depression in patients treated with vilazodone 40 mg or placebo. Instead of the traditional mean change from baseline assessment, depressive symptom improvement and anxious depression were evaluated by a shift from more to less severe symptom categories to provide a more intuitively descriptive assessment of patient improvement.
In patients treated with vilazodone, improvement across symptom domains was evident by statistically significant shifts to less severe symptom categories. This finding is noteworthy since almost all patients were in at least mild to severe individual symptom categories at baseline, with most patients (70%–87%) having more severe symptom ratings. Depressed mood, sleep disturbances, and fatigue are among the most common residual symptoms following depression treatment.23,24 Significantly more vilazodone-treated than placebo-treated patients improved to a less severe symptom category on the MADRS items that correspond to these symptoms (apparent and reported sadness, reduced sleep, and lassitude). For most symptoms, patients receiving vilazodone were at least 1.5 times as likely as patients receiving placebo to shift to minimal or no symptoms, suggesting that patients receiving vilazodone had clinically relevant improvements in depressive symptoms.
Even though more than 80% of the patients in this analysis had anxious depression at baseline, significantly more vilazodone-treated than placebo-treated patients achieved the threshold for no anxious depression (HDRS-17 anxiety/somatization score ≤ 7) at end of study; patients receiving vilazodone were 1.5 times as likely as patients receiving placebo to shift from anxious depression to no anxious depression. Significant shifts to lesser symptom severity were also demonstrated on the inner tension item, the MADRS single item that most closely corresponds to the symptom of anxiety, in patients with mild to severe or moderate to severe symptoms at baseline. Improvements in anxiety-related measures may be clinically important since patients with MDD and comorbid anxiety have greater functional impairments, more severe course of illness, and increased risk for suicide than patients with MDD alone.25
Limitations include the post hoc nature and pooled design of these analyses; of note, the primary trials were not specifically designed to analyze single symptoms. Additionally, multiple comparisons were not controlled for, and the conventional P < .05 to indicate statistical significance was used. Analysis of the completer population may underestimate or overestimate treatment benefits since those who dropped out of the study may have done so due to lack of efficacy or adverse events. Duration of full-dose treatment was limited by the dose titration schedule, which meant that patients only received the full vilazodone 40-mg daily dose during the final 6 weeks of the 8-week trial. Additionally, somatic symptoms that frequently complicate diagnosis and treatment of MDD were not evaluated in this analysis since the MADRS assesses only core symptoms and cognitive features of depression. Inclusion/exclusion criteria may also limit the ability to generalize these findings to a more diverse population. Additionally, because significant suicidality was a criterion for exclusion from the study, the sample size for the group of patients with a baseline score ≥ 4 (moderate to severe symptom category) on the MADRS suicidal thoughts item was too small to evaluate (placebo: n = 3, vilazodone: n = 4); however, significant improvement was demonstrated in favor of vilazodone versus placebo on the suicidal thoughts item for patients with a baseline score ≥ 2 (mild to severe symptom category).
Strengths of these analyses include the availability of a robust data set from 2 similarly designed large clinical trials, which allowed pooling of data. Despite the inherent limitations of pooling data, doing so for these analyses created a large sample size that allowed for increased statistical power to evaluate MADRS single items and subgroups. The category shifts analysis presents a novel way to evaluate symptoms of depression; tracking symptom improvement from a categorical perspective of worse to better may be a more intuitive way to assess patient well-being than mean change from baseline on a depression rating scale.
The progression from clinically symptomatic MDD to wellness is marked by the resolution of many symptoms of depression and comorbid anxiety that impair mood and functioning. In these analyses, individual symptom improvement, evaluated by a shift from symptomatic depression or anxious depression to minimal or no symptoms, was observed in a significantly greater proportion of vilazodone-treated patients compared with placebo. These results suggest that vilazodone treatment is associated with clinically relevant changes across depression symptoms in adult patients with MDD.
Drug names: citalopram (Celexa and others), vilazodone (Viibryd).
Funding/support: Support for this publication was funded by Forest Laboratories, Inc, New York, New York.
Role of the sponsor: Forest Laboratories, Inc, contributed to the design and interpretation of this study and preparation, review, and approval of the manuscript.
Potential conflicts of interest: Dr Culpepper has served as an advisor to or consultant for Boehringer Ingelheim, Forest, Janssen, Jazz, H. Lundbeck A/S, Merck, Pfizer, Reckitt Benckiser, Sunovion, and Takeda; has made presentations regarding a federally funded study of methods to reduce hospital readmissions (with no mention of pharmaceutical agents) supported through Merck’s speaker’s bureau; and is a stock shareholder in M3 (My Mood Monitor), a mental health IT screening and management tool. Drs Mathews and Edwards are full-time employees of Forest Research Institute. Dr Ghori is a contractor for Forest Research Institute.
Previous presentations: American Association of Nurse Practitioners National Conference; June 19–23, 2013; Las Vegas, Nevada · Society of General Internal Medicine Annual Meeting; April 24–27, 2013; Denver, Colorado New Clinical Drug Evaluation Unit Annual Meeting; May 28–31, 2013; Hollywood, Florida · College of Psychiatric and Neurologic Pharmacists Annual Meeting; April 21–24, 2013; Colorado Springs, Colorado.
Acknowledgments
The authors thank Dalei Chen, PhD, of Forest Research Institute, Jersey City, New Jersey, for assistance with statistical analyses. Writing and editorial support for the preparation of the manuscript was provided by Roderick Gedey, MS, and Carol Dyer, MS, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute. Dr Chen, Mr Gedey, and Ms Dyer report no other conflicts of interest related to the subject of this article.
Footnotes
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
References
- 1.Goodwin RD, Kroenke K, Hoven CW, et al. Major depression, physical illness, and suicidal ideation in primary care. Psychosom Med. 2003;65(4):501–505. doi: 10.1097/01.psy.0000041544.14277.ec. [DOI] [PubMed] [Google Scholar]
- 2.Leon AC, Olfson M, Broadhead WE, et al. Prevalence of mental disorders in primary care: implications for screening. Arch Fam Med. 1995;4(10):857–861. doi: 10.1001/archfami.4.10.857. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer RL, Kroenke K, Linzer M, et al. Health-related quality of life in primary care patients with mental disorders: results from the PRIME-MD 1000 Study. JAMA. 1995;274(19):1511–1517. [PubMed] [Google Scholar]
- 4.Daly EJ, Trivedi MH, Wisniewski SR, et al. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. 2010;22(1):43–55. [PubMed] [Google Scholar]
- 5.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29(2):147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54(3):216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. Fourth Edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 8.Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 9.Fava M, Rush AJ, Alpert JE, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51(13):823–835. doi: 10.1177/070674370605101304. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi MH, Rush AJ, Wisniewski SR, et al. STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441–447. doi: 10.4088/JCP.10m06596. [DOI] [PubMed] [Google Scholar]
- 12.Rickels K, Athanasiou M, Robinson DS, et al. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(3):326–333. doi: 10.4088/jcp.08m04637. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643–646. doi: 10.1097/JCP.0b013e31822c6741. [DOI] [PubMed] [Google Scholar]
- 15.Liebowitz M, Croft HA, Kajdasz DK, et al. The safety and tolerability profile of vilazodone, a novel antidepressant for the treatment of major depressive disorder. Psychopharmacol Bull. 2011;44(3):1–19. [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton AH, Kennedy SH, Edwards JB, et al. The effect of vilazodone on sexual function during the treatment of major depressive disorder. J Sex Med. 2013;10(10):2465–2476. doi: 10.1111/jsm.12004. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaynes BN, Rush AJ, Trivedi MH, et al. Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med. 2007;5(2):126–134. doi: 10.1370/afm.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60(9):1167. doi: 10.1176/ps.2009.60.9.1167. [DOI] [PubMed] [Google Scholar]
- 20.Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551–560. doi: 10.1007/s11606-008-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2–3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 22.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 23.Fava M. Pharmacological approaches to the treatment of residual symptoms. J Psychopharmacol. 2006;20(suppl):29–34. doi: 10.1177/1359786806064325. [DOI] [PubMed] [Google Scholar]
- 24.Menza M, Marin H, Opper RS. Residual symptoms in depression: can treatment be symptom-specific? J Clin Psychiatry. 2003;64(5):516–523. doi: 10.4088/jcp.v64n0504. [DOI] [PubMed] [Google Scholar]
- 25.Rao S, Zisook S. Anxious depression: clinical features and treatment. Curr Psychiatry Rep. 2009;11(6):429–436. doi: 10.1007/s11920-009-0065-2. [DOI] [PubMed] [Google Scholar]