The lungs are remarkably resistant to environmental injury, despite continuous exposure to pathogens, particles, and toxic chemicals in inhaled air. Their resistance depends on a highly effective defense provided by airway mucus,1–7 an extracellular gel in which water and mucins (heavily glycosylated proteins) are the most important components. Airway mucus traps inhaled toxins and transports them out of the lungs by means of ciliary beating and cough (Fig. 1). Paradoxically, although a deficient mucous barrier leaves the lungs vulnerable to injury, excessive mucus or impaired clearance contributes to the pathogenesis of all the common airway diseases.1–4 This review examines the normal formation and clearance of airway mucus, the formation of pathologic mucus, the failure of mucus clearance that results in symptoms and abnormal lung function, and the therapy of mucus dysfunction.
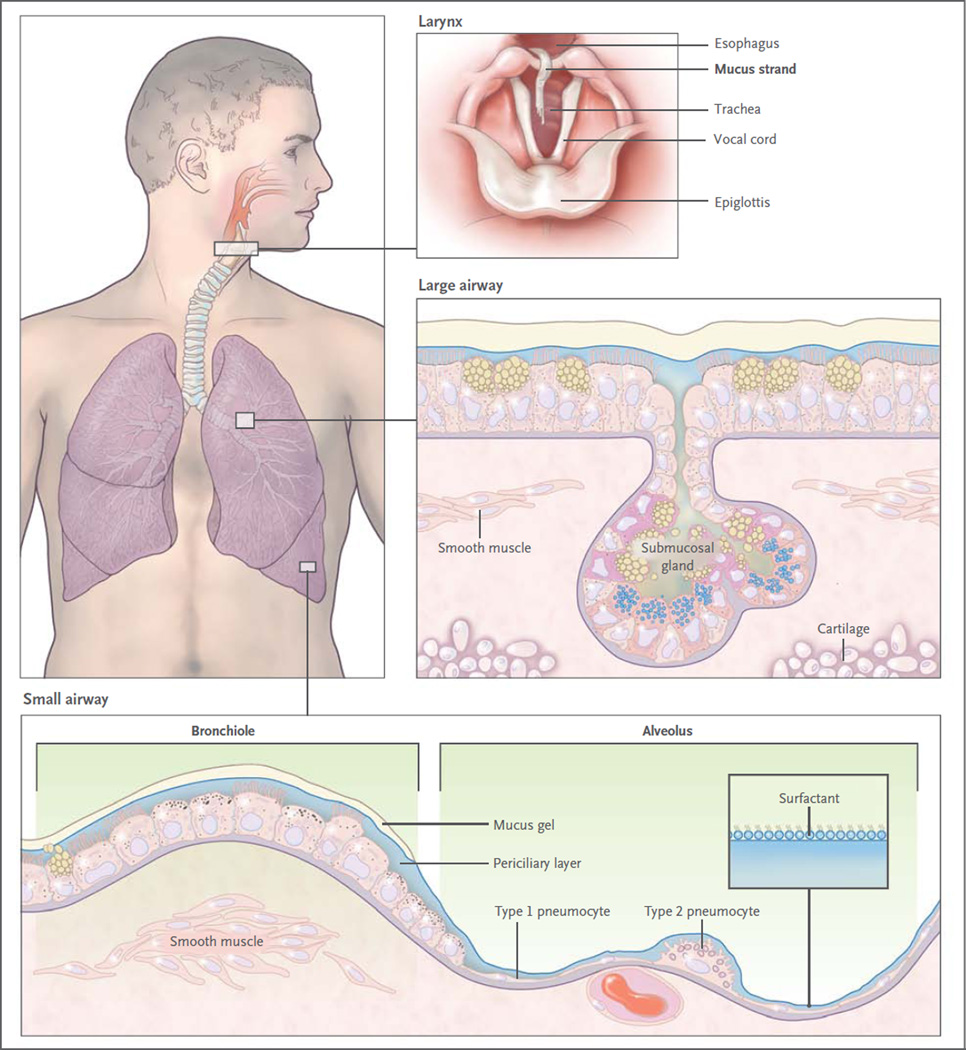
Figure 1. Mucus Clearance in Normal Airways.
Mucus is continuously swept from distal to proximal airways. In the most distal bronchioles, epithelial cells are cuboidal and do not produce mucin (bottom box), and bronchiolar patency is stabilized by surfactant from adjacent alveoli.8 In the adjacent small airways, a thin mucus gel layer is produced by columnar secretory (Clara) cells that do not stain for intracellular mucins because they are produced in low amounts and steadily secreted. In the large airways lined by a pseudostratified epithelium, a thick mucus gel layer (up to 50 µm) accumulates from mucus transported from distal airways and additional mucins are produced by surface secretory cells and glands. After mucus ascends the trachea, it is propelled through the vocal cords by ciliary epithelium in the posterior commissure of the larynx. It then enters the pharynx and is swallowed, with approximately 30 ml of airway mucus eliminated by the gastrointestinal tract daily. The vocal cords are covered by squamous epithelium, so they do not participate in ciliary clearance, although they promote cough clearance by closing while expiratory pressure builds and then opening suddenly so airflow is forceful.
STRUCTURE AND FUNCTION OF THE NORMAL AIRWAY
Epithelial surfaces in contact with the outside environment are protected by mechanical barriers (e.g., keratinized skin) and chemical barriers (e.g., gastric acid). Mucosal surfaces are wet epithelia that have a mucous barrier as part of their protective mechanism.1–7 Mucus layers vary widely in composition and structure; for example, they are thick and adherent to the epithelium in the gut, but thin and mobile in the airway.
SURFACE EPITHELIAL CELLS
The surface epithelium of intrapulmonary airways is composed of two principal cell types — ciliated and secretory (Fig. 2). These cells are present in similar numbers and form a mosaic. Secretory cells have been further divided into subtypes based on their microscopical appearance (e.g., Clara, goblet, and serous cells). However, studies indicate great structural, molecular, and functional plasticity in secretory cells.10–14 Therefore, it is simplest to refer to them generically as “secretory cells.” Besides mucins, secretory cells release a variety of antimicrobial molecules (e.g., defensins, lysozyme, and IgA), immunomodulatory molecules (e.g., secretoglobins and cytokines), and protective molecules (e.g., trefoil proteins and heregulin) constitutively and inducibly; these can become incorporated into mucus.15,16
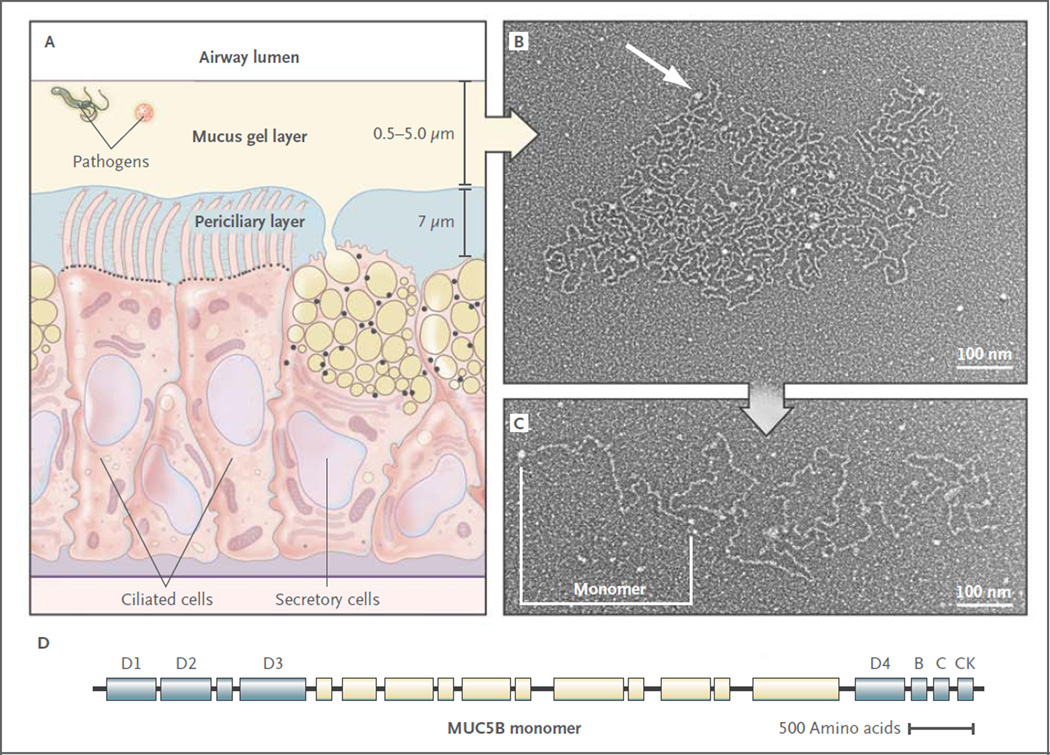
Figure 2. Structure of Airway Mucus.
Panel A shows a distal bronchus with a single layer of columnar epithelial cells. The ciliated cells express approximately 200 cilia that are about 7 µm in length. The secretory cells show mixed features of Clara cells (small, black, apical granules containing proteins) and goblet cells (large granules up to 1 µm in diameter containing yellow mucins and black proteins). The mucus gel layer increases in thickness from distal to proximal airways, whereas the periciliary layer is approximately 7 µm deep throughout the conducting airways. Panel B shows an electron micrograph of a partially expanded MUC5B polymer after secretion, intermediate between its condensed form within a secretory granule and its expanded linear structure.9 Nodes where monomers are bonded appear as white globules (arrow). Panel C shows an electron micrograph of an extended MUC5B polymer. A MUC5B monomer is approximately 450 nm in length, and polymers contain 2 to 20 subunits. Panel D shows the structure of MUC5B. It is organized into an N-terminal region containing von Willebrand factor D1–3 domains involved in N–N polymerization (blue), a central region containing glycosylated mucin domains (pale yellow), and a C-terminal region containing von Willebrand factor D4, B, C, and CK domains involved in C-C polymerization.1,3 The structure of MUC5A is similar (not shown). Electron micrographs provided courtesy of Mehmet Kesimer and John K. Sheehan, University of North Carolina.
SUBMUCOSAL GLANDS
In large airways (luminal diameter, >2 mm), submucosal glands contribute to the secretion of mucins and liquid (Fig. 1). Each gland is connected to the airway lumen by a superficial ciliated duct that propels secretions outward and a deeper nonciliated collecting duct.17,18 The body of the gland is located between the spiral bands of smooth muscle and the cartilage plates. Mucous cells constitute approximately 60% of the gland volume, and based on studies in primates, it has been estimated that half as much intracellular mucin is stored in submucosal glands as is stored in surface epithelial cells.19 Serous cells, located distally, make up the remaining approximately 40% of the gland and secrete proteoglycans and numerous antimicrobial proteins. In pathologic states, the volume of submucosal glands can increase to several times the normal volume.20,21
MUCUS GEL LAYER
A gel is a dilute network that holds shape; thus, although it is composed mostly of liquid, it has many physical characteristics of a solid. Mucus is a gel with properties of both a soft (deformable), elastic solid and a viscous fluid.1,4,5,22,23 Normal mucus is 97% water and 3% solids (mucins, non-mucin proteins, salts, lipids, and cellular debris). Mucins, exceedingly large glycoproteins (up to 3×106 D per monomer) with regions rich in serine and threonine residues linked by their hydroxyl side groups to sugar chains (O-glycosylation), account for less than 30% of the solids.3,4,6,15,24 Mucins are 50 to 90% carbohydrate, and they are highly anionic because most of their terminal sugars contain carboxyl or sulfate groups. There are 17 genes encoding mucins in the human genome, of which the gene products of seven are secreted and the remainder is membrane-bound.3,4,6 Five of the secreted mucins have terminal cysteinerich domains that can form disulfide bonds resulting in polymers that impart the properties of a gel (Fig. 2). Two of these polymers, MUC5AC and MUC5B, are strongly expressed in the airways and are detected in similar quantities in human mucus.3,4
MUC5AC and MUC5B form homotypic polymers (i.e., MUC5AC monomers bond only with MUC5AC, and MUC5B monomers bond only with MUC5B), structured as long single chains rather than branches (Fig. 2). They form the mucus gel both by entanglement in a mesh and by noncovalent calcium-dependent cross-linking of adjacent polymers.1,3 The glycan side chains bind large amounts of liquid (hundreds of times their weight), which allows mucus to act as a lubricant and the gel layer to serve as a liquid reservoir for the periciliary layer.2 The hydration of mucus dramatically affects its viscous and elastic properties, which in turn determine how effectively it is cleared by ciliary action and cough.1–5,22 Healthy mucus contains 3% solids, with the consistency of egg white. However, mucin hypersecretion or dysregulation of surface liquid volume may increase the concentration of solids up to 15%, resulting in viscous and elastic mucus that is not easily cleared.1,5,22 In addition, dehydrated mucus adheres more readily to the airway wall.23,25
Since infection is often initiated by the recognition of host epithelial surfaces by microbial sugar-binding proteins, mucin glycans help sequester pathogens by providing a diverse “glycoprotein landscape” for interaction with these microbial proteins, and patterns of glycosylation can change during inflammation.3,5,26 In addition, the mucus gel layer acts as a solid physical barrier to most pathogens.1,3,5,7 However, the pore size of the gel mesh is sufficiently large (approximately 500 nm) that it is readily penetrated by small viruses with hydrophilic capsids; this has implications for microbial infection and gene therapy.5
MUCIN PRODUCTION
In healthy persons, MUC5AC is produced predominantly in proximal airways by surface goblet cells, whereas MUC5B is produced in surface secretory cells throughout the airways and by submucosal glands.3,4,14,27–29 In the airways of normal mice, which resemble human distal airways, almost no Muc5ac is produced,10–12,30–32 and mice with Muc5ac deletion are healthy, whereas Muc5b is produced constitutively in airway surface secretory cells,11–13,29 and mice with Muc5b deletion die from lung inflammation (Evans CM: personal communication). This finding suggests that Muc5b mediates baseline barrier and clearance functions in mice, and MUC5B probably does the same in human distal airways.28,33 Since MUC5AC is produced constitutively in human proximal airways, it may augment proximal barrier and clearance functions.
The proportion of MUC5AC and MUC5B varies with the state of health. For example, during allergic mucous metaplasia of the surface epithelium in humans, the production of MUC5AC increases greatly (40 to 200 times as high as normal levels) within the same cells that produce MUC5B at baseline, with similar findings in mice.11,12,14,24,29,30,33–36 Muc5b production increases moderately (3 to 10 times as high as normal levels) during allergic inflammation in mice,14,30 and MUC5B transcripts and protein increase in the distal airways of patients with asthma and smokers,28,33 though MUC5B transcripts actually decrease in proximal airways.35,37,38 Hyperplasia plays only a minor role in augmented surface epithelial mucin production, since epithelial-cell numbers increase by 30% or less during inflammation.11,12,27,39 However, hyperplasia may play a major role in augmented submucosal gland MUC5B production in chronic obstructive pulmonary disease (COPD) and cystic fibrosis, since gland volume increases by up to four times the normal volume,20,21 though the relative contributions of hyperplasia and hypertrophy have not been defined. The resolution of surface epithelial mucous metaplasia occurs when secretory cells down-regulate mucin production after the withdrawal of inflammatory stimuli, and the resolution of hyperplasia occurs through apoptosis of excess secretory cells.4,11,27
Since MUC5AC and Muc5ac production is highly regulated at the transcriptional level, its control is of great clinical interest. ErbB-receptor signaling appears to play a ubiquitous role, since inhibitors block MUC5AC and Muc5ac up-regulation by diverse stimuli.10,36,40–42 Interleukin-13 greatly increases MUC5AC and Muc5ac expression,34,36,43,44 and key downstream transcription factors have been identified,30,39,44,45 although the pathways that connect them are not yet fully established (Fig. 3). Many other stimuli that increase MUC5AC and Muc5ac expression, such as viruses,31 the smoke component acrolein,40 and the cytokines interleukin-4, 9, 17, 23, and 25,52–54 do so, at least in part, through interleukin-13. Overexpression of proinflammatory cytokine interleukin-1β or interleukin-17 increases Muc5ac expression,32,54 whereas interleukin-6 and tumor necrosis factor α do not themselves increase Muc5ac expression but do so indirectly by augmenting the intensity of allergic inflammation.55,56 The control of MUC5B and Muc5b expression is less well understood.57
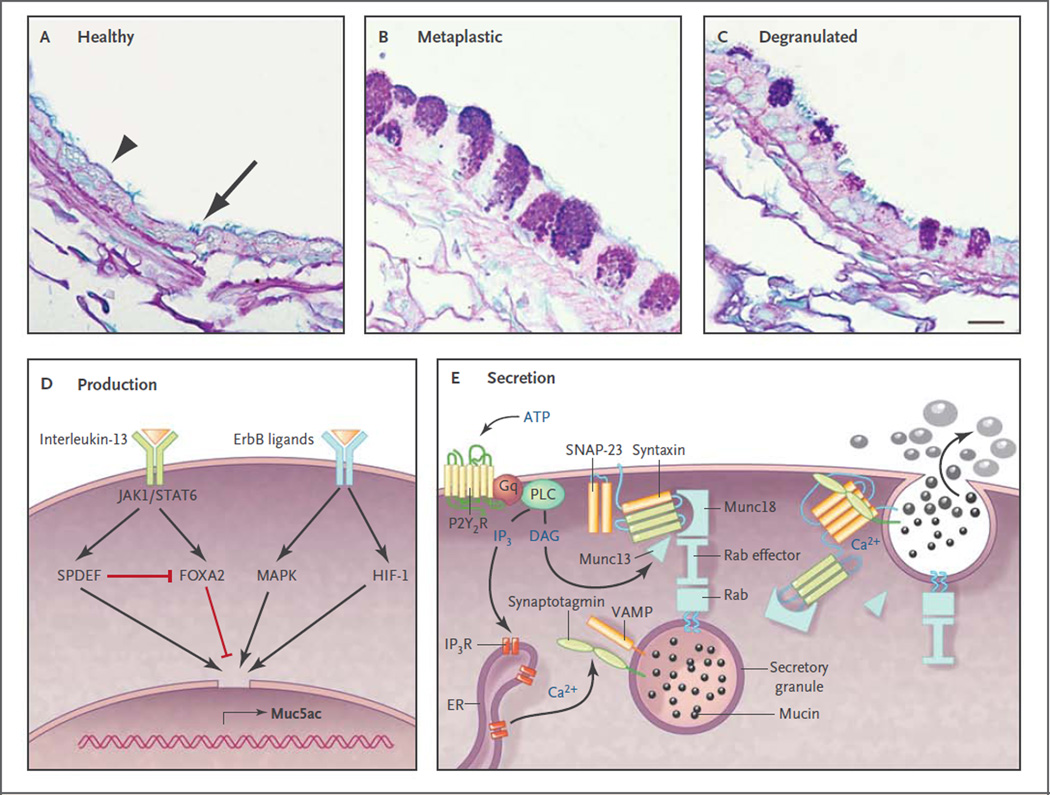
Figure 3. Production and Secretion of Polymeric Mucins.
Panels A through C show axial sections through bronchi of mice, which are similar in size and structure to human bronchioles. Panel A shows an airway under healthy conditions, in which polymeric mucin production is low so that the secretory cells (arrowhead) do not show mucin granules when stained with Alcian blue and periodic acid–Schiff reagent. Nonetheless, antibodies indicate that the cells do produce small amounts of Muc5b (not shown), although Muc5ac is undetectable.14,29 Ciliated cells are interspersed among the secretory cells (arrow). Panel B shows an airway 2 days after the induction of mucous metaplasia by asthmalike allergic inflammation due to sensitization and challenge with ovalbumin.11 Mucin-containing granules are visible in the secretory cells as a result of greatly increased Muc5ac and moderately increased Muc5b production.14,29,30 Panel C shows a metaplastic airway 10 minutes after stimulation of mucin secretion by an ATP aerosol (scale bar, 10 µm). Panel D shows ligands and transcription factors that are important in Muc5ac expression. Interleukin-13 binds to a receptor that includes the interleukin-4Rα subunit, activating Janus kinase 1 (Jak1), leading to the phosphorylation of Stat6. There is no consensus Stat6 binding site in the MUC5AC and Muc5ac promoter, but Stat6 activation leads to increased expression of SPDEF (SAM pointed domain-containing Ets transcription factor), which up-regulates multiple genes involved in mucous metaplasia,39 and inhibits expression of Foxa2, which negatively regulates Muc5ac.45 Several ligands bind ErbB receptors, including epidermal growth factor, transforming growth factor α, amphiregulin, and neuregulin, activating mitogen-activated protein kinases (MAPK).42,46 Hypoxia-inducible factor 1 (HIF-1) also can be activated downstream of ErbB receptors, and there is a conserved HIF-1 binding site in the proximal MUC5AC and Muc5ac promoter,30 but whether this is the dominant mechanism of up-regulation by ErbB ligands is not known. Not shown are complement C3 and β2-adrenergic–receptor signaling, which amplify Muc5ac production,29,47–49 or transcription factors such as Sox2, Notch, E2f4, and Math, which primarily regulate development. Panel E shows the molecular mechanism of mucin exocytosis. A mucin-containing secretory granule is docked to the plasma membrane by the interaction of a granule-bound Rab protein with an effector protein that acts as a tether to Munc18, which binds the closed conformation of Syntaxin anchored to the plasma membrane. Secretion is triggered when ATP binds to P2Y2 purinergic receptors (P2Y2R) coupled to Gq, activating phospholipase C (PLC), which generates the second messengers diacylglycerol (DAG) and inositol triphosphate (IP3). DAG activates Munc1314 to open Syntaxin so it can form a four-helix SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) complex with SNAP-23 (synaptosomal-associated protein 23) and VAMP (vesicle-associated membrane protein), drawing together the granule and plasma membranes.50 IP3 induces the release of calcium from IP3 receptors (IP3R) in the endoplasmic reticulum (ER), activating Synaptotagmin51 to induce final coiling of the SNARE complex, which results in fusion of the membranes and release of the mucins. The photomicrographs are courtesy of Dr. Michael J. Tuvim.
MUCIN SECRETION
The secretion of polymeric mucins is regulated separately from mucin production.50,58 The most important secretagogue for surface epithelium appears to be ATP, which acts on apical membrane P2Y2 receptors.59–61 It is not yet clear whether other agonists such as acetylcholine or histamine directly activate receptors on airway epithelial cells or induce airway smooth-muscle contraction leading to ATP release.59,62–64 The continuous presence of low levels of ATP in airway-surface liquid (see below) causes continuous low activity of the secretory machinery, resulting in the steady release of mucins that provide a normal barrier. When mucin production is increased so that mucins accumulate intracellularly (Fig. 3B), and secretion of a large number of granules is then triggered (mucus hypersecretion) (Fig. 3C), airway luminal occlusion can occur.13,65–67 It might seem that the secretion of a mucin granule would result in no net change in the volume of luminal air space if epithelial-cell volume decreased by the same amount as the volume of secreted mucin. However, mucins are stored in dehydrated form within secretory granules, and they swell to several hundred times their dehydrated volume after secretion as a result of hydration and the exchange of each calcium counterion within the granule for two sodium ions in the extracellular space.9,68 Rapid secretion can deplete airway-surface liquid, resulting in the formation of concentrated, rubbery mucus that is resistant to dilution once the mucin network is formed.1,5,17 Submucosal glands continuously secrete polymeric mucins at a low level and can be further stimulated by adrenergic, cholinergic, and nonadrenergic, noncholinergic nerves.17
PERICILIARY LAYER
The airway mucus gel lies atop a periciliary layer approximately 7 µm deep (Fig. 2A). The depth of this layer is critically important for mucociliary clearance (see below). Since the airway epithelium is highly permeable to water, liquid volume is determined by the amount of sodium chloride in the airway lumen.63 In turn, the amount of sodium chloride is regulated primarily by sodium absorption through the epithelial sodium channel and chloride extrusion through the cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channels.63,69 As mucus is propelled proximally, there is net salt and water absorption (>90%) commensurate with the decreasing total cross-sectional area of the airways.2 Locally, the depth of the periciliary layer is fine-tuned by the concentrations of adenine and uridine nucleotides and the metabolite adenosine. Adenine nucleotides are released through channels from ciliated cells that sense mechanical stress during ventilation63,70 and by exocytosis along with uridine nucleotides from secretory cells.60,61 These nucleotides activate P2Y2 receptors and adenosine activates A2b receptors on the apical membrane of ciliated cells, causing changes in intracellular second messengers that promote chloride release and inhibit sodium absorption; as a result, water moves into the airway lumen.60,61
Membrane-bound mucins contribute to the physical properties of liquid near the cell surface, conferring features of a “grafted gel” rather than a fluid on the periciliary layer (Boucher RC, University of North Carolina: personal communication). MUC4 is densely expressed on cilia, configured like parallel bottle brushes, where it prevents penetration by the mucus gel layer and provides lubrication through bound water.4,6 MUC1 is much smaller than MUC4 and is present on the cell surface and microvilli of both ciliated and secretory cells. It has a cytoplasmic tail capable of intracellular signaling, and it modulates pathogen defense and inflammation.6,71 MUC16, the largest mucin, is expressed by both ciliated and secretory cells, and it can be cleaved and incorporated into the mobile gel layer.6,72
CLEARANCE MECHANISMS
The mucus gel is propelled in a proximal direction by ciliary beating, clearing inhaled particles, pathogens, and dissolved chemicals that might damage the lungs.2 Polymeric mucins are continuously synthesized and secreted to replenish the gel layer. Normal cilia beat 12 to 15 times per second, resulting in a velocity of the gel layer of approximately 1 mm per minute.73 The rate of mucociliary clearance increases with greater hydration,2,73 and the rate of ciliary beating can be increased by purinergic, adrenergic, cholinergic, and adenosine-receptor agonists,60,73 as well as irritant chemicals.74
A second mechanism for the expulsion of mucus from the airways is cough clearance. This may help explain why lung diseases caused by impaired ciliary function are less severe than those caused by dehydration, which impedes both clearance mechanisms.2 Although cough contributes beneficially to the clearance of mucus in diseases of excessive production or impaired ciliary function, it can also be a troublesome symptom.75,76
MUCUS DYSFUNCTION IN DISEASE
Effective mucus clearance is essential for lung health, and airway disease is a consistent consequence of poor clearance. Healthy mucus is a gel with low viscosity and elasticity that is easily transported by ciliary action, whereas pathologic mucus has higher viscosity and elasticity and is less easily cleared.5,38 The conversion from healthy to pathologic mucus occurs by multiple mechanisms that change its hydration and biochemical constituents; these include abnormal secretion of salt and water, increased production of mucins, infiltration of mucus with inflammatory cells, and heightened bronchovascular permeability (Fig. 4). The accumulation of mucus results from some combination of overproduction and decreased clearance, and persistent accumulation can lead to infection and inflammation by providing an environment for microbial growth.
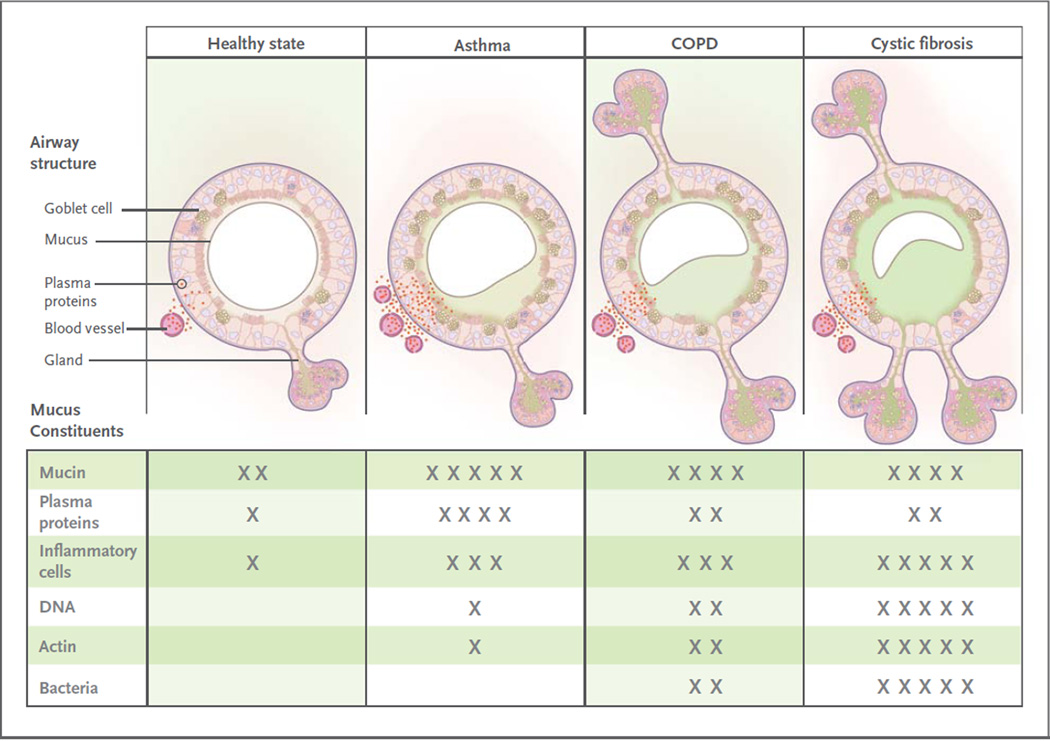
Figure 4. Airway Mucosal Disease and Mucus Characteristics.
The top panels show the contribution of mucosal disease to abnormal mucus. In asthma, airway remodeling is characterized by increases in epithelial mucin stores because of surface epithelial mucous metaplasia with modest hyperplasia and increased numbers of subepithelial bronchial microvessels that become leaky during inflammation. Changes in submucosal glands are not prominent except in severe disease. In chronic obstructive pulmonary disease (COPD), increased mucin stores occur because of surface epithelial mucous metaplasia and some hyperplasia, together with increases in the volume and number of the submucosal glands. Bronchial microvessel remodeling is not as prominent as in asthma. In cystic fibrosis, epithelial mucin stores are similar to normal levels (possibly because of increased secretion), but submucosal glands are very prominent. Bronchial microvessel remodeling is not as prominent as in asthma. Not shown in the top panels are the increased numbers of inflammatory cells in the airway wall and lumen, which occur in all airway diseases. Products of inflammatory cell death include DNA and actin polymers, which are important constituents of pathologic mucus. The bottom panels list some of the constituents of mucus in health and in airway disease. The degree of cellular inflammation and biochemical constituents of mucus differ among airway diseases. The data for asthma reflect changes that occur in acute severe asthma. The number of Xs indicates the relative abundance of the constituents in each disease state.
The principal symptoms of impaired mucus clearance are cough and dyspnea. Cough is caused by the stimulation of vagal afferents in the intrapulmonary airways or the larynx and pharynx.75,76 Patients often infer that laryngopharyngeal stimulation, described as “a tickle in the throat,” results from “postnasal drip,” since they recognize that gravity causes mucus to descend from the nasopharynx but are generally unaware that it also ascends from the lungs by ciliary action. Dyspnea is caused when mucus obstructs airflow by occupying the lumen of numerous airways.21,65–67 Physical signs of impaired mucus clearance include cough, bronchial breath sounds, rhonchi, and wheezes. Retained mucus and inflammatory exudates may appear as localized atelectasis or linear or branched opacities on plain radiographs of the chest, and as luminal filling in proximal airways or tree-in-bud opacities in peripheral airways on computed tomographic examination.77 It is important to recognize the role of mucus in clinical presentation. It is necessary to clear mucus from the airway lumen in order to resolve symptoms and allow effective delivery of aerosol therapies. In addition, the presence of mucus may be a sign of underlying inflammation or infection that may warrant additional treatment.
CYSTIC FIBROSIS
Cystic fibrosis is caused by mutations in the gene encoding CFTR, which result in reduced chloride secretion and increased sodium absorption. Together, these result in insufficient airway luminal liquid.78,79 In addition, reduced bicarbonate secretion may result in excessive mucin cross-linking by calcium.80 In transgenic mice with overexpression of a subunit of the epithelial sodium channel in airway epithelial cells, airway luminal liquid is insufficient and a cystic fibrosis–like phenotype develops.81 This finding, coupled with a large amount of supportive data from in vitro studies of human airway epithelial-cell function, has led to a broad consensus that the major consequences of CFTR dysfunction in the airway are dehydration of mucus and reduction in the height of the periciliary layer, particularly in response to infectious or toxic insults.63,69,78,79 These changes result in poor mucus clearance, which sets up a vicious cycle of infection, inflammation, and injury. In patients with cystic fibrosis, mucus has the following characteristics: infiltration with neutrophils and high concentrations of neutrophil-derived DNA and filamentous actin22,82,83; infection with organisms such as Pseudomonas aeruginosa, Staphylococcus aureus, and aspergillus species, often in biofilms at the epithelial-cell surface; and dehydrated, highly entangled polymeric macromolecules that form a gel matrix with a pore size reduced from the normal size of approximately 500 nm to approximately 150 nm.5 Decreased pore size is postulated to immobilize microbes within the mucus gel, thereby promoting biofilm formation, and to inhibit the movement of neutrophils that might otherwise clear the infection.5,69 The net effects of these processes are manifested radiographically as bronchiectasis; pathologically as neutrophilic inflammation, airway fibrosis, and increased numbers of mucin-secreting cells, especially in the submucosal glands; and clinically as cough, purulent sputum, hemoptysis, dyspnea, recurrent lung infections, and rapid loss of lung function.78,79,84
ASTHMA
The central role of diffuse mucus plugging of the airways in the pathophysiology of asthma has been recognized by pathologists for more than 100 years.65,66,85 However, mucus dysfunction in asthma is often underappreciated by clinicians, possibly because cough in asthma infrequently results in expectoration or because the unavailability of therapies to clear mucus plugs has diverted attention exclusively toward reversing bronchoconstriction and inflammation.86 Mucous metaplasia (i.e., increased surface epithelial mucin production) and an increased number of bronchial microvessels are important components of the airway remodeling in asthma that confers a predisposition to mucus dysfunction.86 These changes occur in patients with airway inflammation characterized by infiltration of the airway wall and luminal mucus with CD4+ T cells, eosinophils, and innate immune cells that secrete Th2 cytokines,85,87 although neutrophil infiltration may also be prominent in acute exacerbations.84 Airway occlusion by mucus plugs can cause localized atelectasis that is evident radiographically in patients with acute asthma exacerbations, and widespread mucus plugging is consistently detected at autopsy in patients with fatal asthma.65,66 Diffuse airway narrowing from a combination of concentric smooth-muscle contraction and luminal obstruction with mucus makes asthma uniquely dangerous among airway diseases in its propensity for sudden exacerbations.
Airway mucus in severe asthma has a rubbery quality that contributes to impaired clearance and plug formation. Biochemical analysis of these plugs shows high concentrations of mucins and plasma proteins,84,88 and biophysical analysis shows high entanglement density and elastic modulus.89 Another important pathologic role of plasma proteins in forming these highly elastic mucus plugs is shielding mucins from protease digestion.89 Since neutrophil elastase activity is increased in the airways of patients with asthma in the recovery phase of near-fatal exacerbations, it may help to digest mucus plugs in these patients.90 A history of persistent symptoms related to sputum is associated with more severe disease phenotypes in chronic asthma,91 and mucus hypersecretion is especially problematic in allergic bronchopulmonary aspergillosis.85
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Small-airway mucus obstruction is characteristic of COPD, even in patients who do not expectorate sputum or who have an emphysematous phenotype.92,93 Conversely, patients with COPD who have copious expectoration may have little airflow obstruction, probably because the mucus comes from large airways and causes minimal occlusion. Despite this weak correlation with sputum production, airflow obstruction does correlate with changes in mucin gene expression,38 increases in goblet-cell number and size,38 the occlusion of small airways with mucus,88 and expansion of the submucosal glands.21,92 Mucus dysfunction induced by cigarette smoke is complex and incompletely understood, but it involves adverse effects on the structure and function of cilia,94–96 activation of ErbB receptors,41 decreased function of CFTR,97 and proinflammatory effects that increase mucin production while decreasing mucus hydration and clearance. Cigarette smoke contains multiple toxins, including particulate matter, oxidative chemicals, and organic compounds, among which acrolein is important because it potently induces mucin production.40
Increased mucin production and decreased luminal liquid in COPD have deleterious consequences for airway health, as they do in asthma and cystic fibrosis, including mucus stasis and airway infection. Haemophilus influenzae, P. aeruginosa, Streptococcus pneumoniae, and Moraxella catarrhalis are detected in sputum in 25 to 50% of adults with COPD. The infection rate increases with increasing disease severity, and the acquisition of new bacterial strains is associated with COPD exacerbations.98 On the basis of studies in a mouse model in which H. influenzae lysate elicited airway inflammation and fibrosis but not mucous metaplasia,56 one may speculate that in COPD, a reduction in mucus clearance that is related to cigarette smoke leads to airway infection, which, in turn, leads to inflammation and fibrosis.
OTHER AIRWAY DISEASES ASSOCIATED WITH MUCUS DYSFUNCTION
Mucus dysfunction occurs in virtually all inflammatory airway diseases. Acute viral and bacterial infections and chronic diseases such as primary ciliary dyskinesia, non–cystic fibrosis bronchiectasis (which is often caused by atypical mycobacterial infection), panbronchiolitis, and immunodeficiency states (e.g., hypogammaglobulinemia, human immunodeficiency virus infection, organ transplantation, and hematologic malignant conditions) all have a component of mucus dysfunction. In addition, retained mucus is a problem in intubated patients and those in whom lung mechanics are disrupted as a result of paralysis, immobilization, or surgery; atelectasis and pneumonia are common complications in such patients. Genomic markers in chromosomal region 11p15.5 (which encompasses MUC5AC and MUC5B) have been reported to be associated with asthma severity,99 and panbronchiolitis,100 although mechanisms leading to disease susceptibility have not yet been defined.
TREATMENT
The development of rationally designed treatments for pathologic mucus has been hindered by a lack of understanding of the mechanisms of mucus dysfunction. Over-the-counter medications for airway mucus dysfunction, including guaifenesin, have not been rigorously evaluated in clinical trials, and they are not recommended in treatment guidelines for cystic fibrosis, asthma, or COPD.65,85,93,101–103 Multiple additional agents with uncertain mechanisms are used worldwide without clear evidence of a benefit.104,105 Asthma, COPD, and cystic fibrosis have important differences in pathologic mucus, and mucus treatment should be tailored accordingly. Current therapies do this to an extent, but they may be facilitated by therapies that are currently under development. Therapies can be subdivided into those that decrease mucin production, those that decrease mucin secretion, those that promote mucus clearance, and those that treat airway infection (Table 1).
Table 1.
Treatment of Mucus Hypersecretion.
| Purpose and treatment | Comments |
|---|---|
| Decrease mucin production | |
| Available treatments | |
| Glucocorticoids | Allergen-induced increases in numbers of airway goblet cells are inhibited by glucocorticoids. In acute severe asthma, glucocorticoids may also reduce bronchovascular permeability and promote neutrophildriven mucus turnover.89 Glucocorticoids are much less effective in treating mucus in other airway diseases.93,107 |
| Agents in development | |
| ErbB-receptor inhibitors | A recent trial of inhaled BIBW 2948 BS, an epidermal growth factor receptor inhibitor (ClinicalTrials.gov number, NCT00423137), did not show a benefit in reducing airway mucin gene expression or epithelial mucin stores in patients with COPD, and the treatment was associated with adverse effects on lung and liver function.108 |
| Decrease mucin secretion | |
| Available treatments | |
| None | |
| Agents in development | |
| MARCKS inhibitors | MARCKS regulates the reconfiguration of actin filaments at the apical pole of goblet cells during mucin secretion.50,109 A peptide derived from the MARCKS N-terminal that is myristoylated so that it enters cells inhibits stimulated mucin secretion in mice.109 In a phase 2 trial (NCT00648245), this peptide is being administered by aerosol in patients with COPD who have mucus hypersecretion. |
| Botulinum neurotoxins | Botulinum neurotoxins are zinc proteases that cleave SNARE proteins to inhibit release of synaptic vesicles. The C and E neurotoxins have been engineered so that they are active against non-neuronal SNARE isoforms, inhibiting epithelial-cell mucin secretion, and the modified C toxin has been conjugated to epidermal growth factor to promote delivery to goblet cells, but no related clinical trial has been registered.110,111 |
| Promote mucus clearance | |
| Available treatments | |
| Physical measures | Chest percussion and postural drainage improve clearance of purulent airway mucus in cystic fibrosis.103 The value of alternative methods, including positive expiratory pressure, flutter valves, or high-frequency chest-compression vests, is difficult to assess objectively, although trials are in progress (e.g., NCT01057524). Mucus clearance is probably aided by any maneuver that promotes coughing and increased minute ventilation, including exercise. Since airflow generates shear stress on airway cell surfaces that stimulate release of nucleotides that interact with P2Y2 receptors to regulate mucus hydration, there are both mechanical and biochemical mechanisms of benefit from nonpharmacologic approaches to mucus clearance.63,103 |
| Bronchodilators | Bronchodilation with beta-adrenergic agonists or anticholinergic drugs may improve mucus clearance in the short term because of an enlarged luminal diameter. In addition, beta-adrenergic agonists increase the frequency of ciliary beats, and anticholinergic drugs may decrease surface mucin secretion and mucin secretion from the submucosal gland.17,62,64,73 However, beta-adrenergic agonists up-regulate mucin production in animal models,29,49 so they are not recommended for long-term treatment of mucus hypersecretion. The long-term effects of both classes of bronchodilators on mucus clearance warrant further study. |
| Inhaled dornase alfa | Inhaled dornase alfa hydrolyzes DNA, improves lung function, and decreases the frequency of exacerbation in patients with cystic fibrosis, in whom airway mucus concentrations of DNA are very high (5–10 mg/ml).84,101 The concentration of DNA in other airway diseases, including non−cystic fibrosis bronchiectasis, COPD, and asthma, is 1/5 to 1/10 as great84,88,112; dornase alfa does not have beneficial effects in these diseases and may even be harmful.113 |
| Inhaled hypertonic saline | Treatment twice daily with aerosolized 7% hypertonic saline solution is associated with significant improvements in mucus clearance, modest improvements in airflow, and clinically meaningful reductions in rates of exacerbation among patients with cystic fibrosis.114,115 The mechanism of benefit is thought to be rehydration of the periciliary layer through the drawing of water from epithelial cells, but other mechanisms such as promotion of cough and direct effects on mucus elasticity and entanglement may also contribute.69,116 Trials are under way for the treatment of other airway diseases with 3 to 7% hypertonic saline; these diseases include infantile bronchiolitis (NCT01016249, NCT00677729, NCT00729274, NCT00619918, NCT00151905, and NCT00696540), COPD (NCT00639236), atelectasis (NCT00671723), non−cystic fibrosis bronchiectasis (NCT00484263), and asthma (NCT01073527). |
| N-acetylcysteine | N-acetylcysteine breaks the disulfide bonds that link mucin monomers to polymers, and it is very effective in vitro in solubilizing sputum. Case reports attest to its usefulness when applied through the bronchoscope to break up mucus plugs.117 Clinical studies of N-acetylcysteine and carbocysteine in COPD have shown some promise.118 Aerosolized N-acetylcysteine also can be irritating to the airway,119 so its routine use is not recommended. Oral N-acetylcysteine is under study as an antiinflammatory (glutathione-replenishing and antioxidant) treatment in cystic fibrosis and COPD (NCT00969904 and NCT00809094). |
| Agents in development | |
| Mannitol | Nonabsorbable osmotic agents have a theoretical advantage of drawing liquid into the periciliary layer for longer periods than sodium chloride. Inhaled mannitol is undergoing safety and efficacy testing in cystic fibrosis, COPD, and bronchiectasis (NCT00446680, NCT00117208, and NCT00669331). Its use in children with cystic fibrosis is associated with bronchoconstriction and cough, but a 3-month treatment protocol showed similar efficacy to that of dornase alfa.120 |
| P2Y2 agonists | P2Y2 agonists promote the activity of calcium-activated chloride channels and inhibit the activity of epithelial sodium channels, so they may normalize the height of the periciliary layer and improve mucus clearance, especially in cystic fibrosis. Phase 2 and 3 trials of denufusol in cystic fibrosis are ongoing (NCT00625612 and NCT00357279). |
| CFTR modulation | Several therapeutic agents are in development to augment the function of mutant CFTR, including ataluren to promote read-through of premature termination codons (NCT00803205), VX-809 to promote transport of misfolded CFTR protein to the cell surface (NCT00865904 and NCT00966602), and VX-770 to promote the opening of CFTR proteins expressed on the cell surface (NCT00909532 and NCT00966602). |
| Epithelial sodium-channel modulation | An aerosolized inhibitor of epithelial sodium-channel function, GS-9411, is being evaluated as a potential therapy to improve airway hydration and mucociliary clearance in cystic fibrosis (NCT00800579, NCT009999531, NCT01025713, and NCT00951522). |
| Actin filament depolymerizing agents, proteases, and antiproteases | Gelsolin and thymosin β4 depolymerize actin filaments,23,83 which could be helpful in promoting mucus clearance in cystic fibrosis; however, the results of clinical studies of recombinant human plasma gelsolin in cystic fibrosis in the 1990s were not promising, and no trials are currently registered. Proteases that digest gel-forming mucins could be helpful in treating mucus plugs in severe asthma,89 although no trials are yet under way. Inhaled alpha1-antitrypsin is being studied to prevent lung parenchymal damage from leukocyte proteases in cystic fibrosis (NCT00499837). |
| Treat airway infection | |
| Available treatments | |
| Inhaled antibiotics | Treatment with inhaled tobramycin is associated with improved lung function and reduced exacerbation frequency in patients with cystic fibrosis.101 |
| Oral antibiotics | Prolonged use of azithromycin improves lung function in cystic fibrosis, although it is associated with increased nausea and diarrhea.101 Long-term treatment with oral erythromycin reduces the frequency of exacerbation in COPD,121 and several groups, including the COPD Clinical Research Network (NCT00119860), are studying azithromycin in patients with this condition. The value of oral antibiotics in the short-term treatment of COPD exacerbations is questionable.122 In patients with bronchiectasis who do not have cystic fibrosis, long-term treatment with low-dose azithromycin reduces the frequency of exacerbation and improves lung function.123 |
| Intravenous antibiotics | Intravenous antibiotics directed against pseudomonas species infections are effective in the treatment of pulmonary exacerbations of cystic fibrosis.102,124 |
| Agents in development | |
| Inhaled antibiotics | Inhaled aztreonam reduced the time to exacerbation in cystic fibrosis in two phase 3 studies (NCT00112359, NCT00104520),125,126 and a study comparing inhaled aztreonam with inhaled tobramycin in cystic fibrosis is ongoing (NCT00757237). Also under study are inhaled ciprofloxacin (NCT00645788), liposomal amikacin (NCT00558844), and tobramycin combined with fosfomycin (NCT00794586). |
CFTR denotes cystic fibrosis transmembrane conductance regulator, COPD chronic obstructive pulmonary disease, MARCKS myristoylated alanine-rich C-kinase substrate, and SNARE soluble N-ethylmaleimide–sensitive factor attachment protein receptor.
Recent insights into the formation of pathologic mucus in disease have led to the introduction of tailored therapies such as hydration by means of aerosolized hypertonic saline solutions or the reduction of mucus viscosity and elasticity by aerosolized dornase alfa. Targeted treatment of pathologic airway mucus not only improves symptoms of cough and dyspnea but also decreases the frequency of disease-related exacerbations and slows disease progression. Elucidation of how mucin production is controlled is still needed, since that might allow the development of additional therapies to prevent overproduction.
Acknowledgments
Dr. Dickey reports receiving consulting fees from BioMarcks Pharmaceuticals; Dr. Fahy, serving on a scientific advisory board for Cytokinetics and receiving consulting fees from Five Prime Therapeutics, Amira, Oxagen, Gilead, GlaxoSmithKline, and Amgen, grant support to the University of California, San Francisco, from Genentech, Boehringer Ingelheim, and Aerovance, and travel fees from GlaxoSmithKline, Merck, Amira, and Amgen. Dr. Fahy reports being named on a provisional patent application submitted for a gene signature for type 2 helper T cell–high asthma (with Genentech).
We thank Kenneth B. Adler, Richard C. Boucher, Stephen D. Carrington, C. William Davis, Kyubo C. Kim, Christopher M. Evans, Susan J. Muller, Jay A. Nadel, Mary C. Rose, David J. Thornton, and Jeffrey J. Wine for their careful reading of an earlier version of the manuscript; and members of the Fahy and Dickey laboratories for helpful insights.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 5.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 7.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macklem PT, Proctor DF, Hogg JC. The stability of peripheral airways. Respir Physiol. 1970;8:191–203. doi: 10.1016/0034-5687(70)90015-0. [DOI] [PubMed] [Google Scholar]
- 9.Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gelforming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol. 2010;298:L15–L22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Shim JJ, Burgel PR, et al. IL-13-induced Clara cell secretory protein expression in airway epithelium: role of EGFR signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2002;283:L67–L75. doi: 10.1152/ajplung.00404.2001. [DOI] [PubMed] [Google Scholar]
- 11.Evans CM, Williams OW, Tuvim MJ, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reader JR, Tepper JS, Schelegle ES, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T, Ishii A, Nakai S, Hasegawa K. Ultrastructure of goblet-cell metaplasia from Clara cell in the allergic asthmatic airway inflammation in a mouse model of asthma in vivo. Virchows Arch. 2004;444:66–73. doi: 10.1007/s00428-003-0926-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Ehre C, Abdullah LH, et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesimer M, Kirkham S, Pickles RJ, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 18.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plopper CG, Heidsiek JG, Weir AJ, George JA, Hyde DM. Tracheobronchial epithelium in the adult rhesus monkey: a quantitative histochemical and ultrastructural study. Am J Anat. 1989;184:31–40. doi: 10.1002/aja.1001840104. [DOI] [PubMed] [Google Scholar]
- 20.Hays SR, Fahy JV. Characterizing mucous cell remodeling in cystic fibrosis: relationship to neutrophils. Am J Respir Crit Care Med. 2006;174:1018–1024. doi: 10.1164/rccm.200603-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 22.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin BK. Mucus and mucins. Otolaryngol Clin North Am. 2010;43:27–34. doi: 10.1016/j.otc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Imberty A, Varrot A. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol. 2008;18:567–576. doi: 10.1016/j.sbi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Tesfaigzi Y. Regulation of mucous cell metaplasia in bronchial asthma. Curr Mol Med. 2008;8:408–415. doi: 10.2174/156652408785160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Zhao YH, Di YP, Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am J Respir Cell Mol Biol. 2001;25:542–553. doi: 10.1165/ajrcmb.25.5.4298. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LP, Omoluabi O, Parra S, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtzman MJ, Byers DE, Benoit LA, et al. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol. 2009;102:245–276. doi: 10.1016/S0065-2776(09)01205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 33.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am J Respir Cell Mol Biol. 2009;40:277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [Erratum, Am J Respir Crit Care Med 2009; 180:796.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhen G, Park SW, Nguyenvu LT, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Innes AL, Woodruff PG, Ferrando RE, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Korfhagen TR, Xu Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deshmukh HS, Shaver C, Case LM, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008;38:446–454. doi: 10.1165/rcmb.2006-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeyama K, Jung B, Shim JJ, et al. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2001;280:L165–L172. doi: 10.1152/ajplung.2001.280.1.L165. [DOI] [PubMed] [Google Scholar]
- 42.Kettle R, Simmons J, Schindler F, et al. Regulation of neuregulin 1beta1-induced MUC5AC and MUC5B expression in human airway epithelium. Am J Respir Cell Mol Biol. 2010;42:472–481. doi: 10.1165/rcmb.2009-0018OC. [DOI] [PubMed] [Google Scholar]
- 43.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 44.Kuperman DA, Huang X, Nguyenvu L, Hölscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol. 2005;175:3746–3752. doi: 10.4049/jimmunol.175.6.3746. [DOI] [PubMed] [Google Scholar]
- 45.Wan H, Kaestner KH, Ang SL, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 46.Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J. 2008;32:1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 47.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 48.Humbles AA, Lu B, Nilsson CA, et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen LP, Lin R, Parra S, et al. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci U S A. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 51.Tuvim MJ, Mospan AR, Burns KA, et al. Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum. J Biol Chem. 2009;284:9781–9787. doi: 10.1074/jbc.M807849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42:268–275. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng J, Yang XO, Chang SH, Yang J, Dong C. IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res. 2010;20:62–71. doi: 10.1038/cr.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hung LY, Velichko S, Huang F, Thai P, Wu R. Regulation of airway innate and adaptive immune responses: the IL-17 paradigm. Crit Rev Immunol. 2008;28:269–279. doi: 10.1615/critrevimmunol.v28.i4.10. [DOI] [PubMed] [Google Scholar]
- 55.Moghaddam SJ, Clement CG, De la Garza MM, et al. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neveu WA, Allard JB, Dienz O, et al. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol. 2009;183:1732–1738. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol. 2001;25:397–400. doi: 10.1165/ajrcmb.25.4.f214. [DOI] [PubMed] [Google Scholar]
- 59.Kim KC, Hisatsune A, Kim DJ, Miyata T. Pharmacology of airway goblet cell mucin release. J Pharmacol Sci. 2003;92:301–307. doi: 10.1254/jphs.92.301. [DOI] [PubMed] [Google Scholar]
- 60.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163:208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bateman ED, Rennard S, Barnes PJ, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009;22:533–542. doi: 10.1016/j.pupt.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 64.Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130:219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hogg JC. The pathology of asthma. APMIS. 1997;105:735–745. doi: 10.1111/j.1699-0463.1997.tb05079.x. [DOI] [PubMed] [Google Scholar]
- 66.Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med. 2003;115:68–69. doi: 10.1016/s0002-9343(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 67.Bossé Y, Riesenfeld EP, Paré PD, Irvin CG. It’s not all smooth muscle: non-smooth-muscle elements in control of resistance to airflow. Annu Rev Physiol. 2010;72:437–462. doi: 10.1146/annurev-physiol-021909-135851. [DOI] [PubMed] [Google Scholar]
- 68.Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 69.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim KC, Lillehoj EP. MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol. 2008;39:644–647. doi: 10.1165/rcmb.2008-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies JR, Kirkham S, Svitacheva N, Thornton DJ, Carlstedt I. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol. 2007;39:1943–1954. doi: 10.1016/j.biocel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 74.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canning BJ. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl):33S–47S. doi: 10.1378/chest.129.1_suppl.33S. [DOI] [PubMed] [Google Scholar]
- 76.Rubin BK. The role of mucus in cough research. Lung. 2010;188(Suppl):S69–S72. doi: 10.1007/s00408-009-9198-7. [DOI] [PubMed] [Google Scholar]
- 77.Martinez S, Heyneman LE, McAdams HP, Rossi SE, Restrepo CS, Eraso A. Mucoid impactions: finger-in-glove sign and other CT and radiographic features. Radiographics. 2008;28:1369–1382. doi: 10.1148/rg.285075212. [DOI] [PubMed] [Google Scholar]
- 78.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 79.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 80.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 82.Potter JL, Spector S, Matthews LW, Lemm J. Studies on pulmonary secretions. 3. The nucleic acids in whole pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1969;99:909–916. doi: 10.1164/arrd.1969.99.6.909. [DOI] [PubMed] [Google Scholar]
- 83.Vasconcellos CA, Allen PG, Wohl ME, Drazen JM, Janmey PA, Stossel TP. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994;263:969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- 84.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 85.Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. ( http://www.nhlbi.nih.gov/guidelines/asthma.) [Google Scholar]
- 86.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–305. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 87.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 88.Sheehan JK, Richardson PS, Fung DC, Howard M, Thornton DJ. Analysis of respiratory mucus glycoproteins in asthma: a detailed study from a patient who died in status asthmaticus. Am J Respir Cell Mol Biol. 1995;13:748–756. doi: 10.1165/ajrcmb.13.6.7576713. [DOI] [PubMed] [Google Scholar]
- 89.Innes AL, Carrington SD, Thornton DJ, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–210. doi: 10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 91.Siroux V, Boudier A, Bousquet J, et al. Phenotypic determinants of uncontrolled asthma. J Allergy Clin Immunol. 2009;124:681–687. doi: 10.1016/j.jaci.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 92.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 93.The Global Initiative for Chronic Obstructive Lung Disease. 2009 updates. ( http://www.goldcopd.com.) [Google Scholar]
- 94.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS ONE. 2009;4(12):e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23:117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 96.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Crémoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 97.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 98.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 99.The Collaborative Study on the Genetics of Asthma (CSGA) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 100.Kamio K, Matsushita I, Hijikata M, et al. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med. 2005;171:949–957. doi: 10.1164/rccm.200409-1168OC. [DOI] [PubMed] [Google Scholar]
- 101.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 102.Flume PA, Mogayzel PJ, Jr, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 103.Flume PA, Robinson KA, O’Sullivan BP, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522–537. [PubMed] [Google Scholar]
- 104.Boogaard R, de Jongste JC, Merkus PJ. Pharmacotherapy of impaired mucociliary clearance in non-CF pediatric lung disease: a review of the literature. Pediatr Pulmonol. 2007;42:989–1001. doi: 10.1002/ppul.20693. [DOI] [PubMed] [Google Scholar]
- 105.Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care. 2007;52:1176–1193. [PubMed] [Google Scholar]
- 106.Southam DS, Ellis R, Wattie J, Glass W, Inman MD. Goblet cell rebound and airway dysfunction with corticosteroid withdrawal in a mouse model of asthma. Am J Respir Crit Care Med. 2008;178:1115–1122. doi: 10.1164/rccm.200801-084OC. [DOI] [PubMed] [Google Scholar]
- 107.Kapur N, Bell S, Kolbe J, Chang AB. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2009;1:CD000996. doi: 10.1002/14651858.CD000996.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Woodruff PG, Wolff M, Hohlfeld JM, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/rccm.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 109.Singer M, Martin LD, Vargaftig BB, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 110.Chen S, Barbieri JT. Engineering botulinum neurotoxin to extend therapeutic intervention. Proc Natl Acad Sci U S A. 2009;106:9180–9184. doi: 10.1073/pnas.0903111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foster KA, Adams EJ, Durose L, et al. Re-engineering the target specificity of Clostridial neurotoxins — a route to novel therapeutics. Neurotox Res. 2006;9:101–107. doi: 10.1007/BF03354881. [DOI] [PubMed] [Google Scholar]
- 112.Fahy JV, Steiger DJ, Liu J, Basbaum CB, Finkbeiner WE, Boushey HA. Markers of mucus secretion and DNA levels in induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1132–1137. doi: 10.1164/ajrccm/147.5.1132. [DOI] [PubMed] [Google Scholar]
- 113.O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest. 1998;113:1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 114.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 115.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 116.Levin MH, Sullivan S, Nielson D, Yang B, Finkbeiner WE, Verkman AS. Hypertonic saline therapy in cystic fibrosis: evidence against the proposed mechanism involving aquaporins. J Biol Chem. 2006;281:25803–25812. doi: 10.1074/jbc.M604332200. [DOI] [PubMed] [Google Scholar]
- 117.Shridharani M, Maxson TR. Pulmonary lavage in a patient in status asthmaticus receiving mechanical ventilation: a case report. Ann Allergy. 1982;49:156–158. [PubMed] [Google Scholar]
- 118.Decramer M, Janssens W. Mucoactive therapy in COPD. Eur Respir Rev. 2010;19:134–140. doi: 10.1183/09059180.00003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rao S, Wilson DB, Brooks RC, Sproule BJ. Acute effects of nebulization of N-acetylcysteine on pulmonary mechanics and gas exchange. Am Rev Respir Dis. 1970;102:17–22. doi: 10.1164/arrd.1970.102.1.17. [DOI] [PubMed] [Google Scholar]
- 120.Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax. 2010;65:51–56. doi: 10.1136/thx.2009.116970. [DOI] [PubMed] [Google Scholar]
- 121.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 122.Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:150–157. doi: 10.1164/rccm.200906-0837OC. [DOI] [PubMed] [Google Scholar]
- 123.Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med. 2008;102:1494–1496. doi: 10.1016/j.rmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Smyth AR, Bhatt J. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2010;1:CD002009. doi: 10.1002/14651858.CD002009.pub3. [DOI] [PubMed] [Google Scholar]
- 125.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135:1223–1232. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]