Abstract
Stimulation of bone regeneration using growth factors is a promising approach for musculoskeletal regenerative engineering. Common limitations with protein growth factors are high manufacturing costs, protein instability, contamination issues, and unwanted immunogenic responses of the host. New strategies for bone regeneration that obviate these problems can have a significant impact on the treatment of skeletal injury and diseases. Over the past decade, a large number of small molecules with the potential of regenerating skeletal tissue have been reported in the literature. Here, we review this literature, paying specific attention to the prospects for small molecule-based bone-regenerative engineering. We also review the preclinical study of small molecules associated with bone regeneration.
Keywords: growth factor, small molecule, orthopedic, osteogenesis, tissue engineering, regenerative engineering
Regenerative engineering is an emerging interdisciplinary field at the convergence of life sciences, engineering technology, and physical sciences. Laurencin et al. defined regenerative engineering as ‘the integration of tissue engineering with advanced material science, stem cell science, and areas of developmental biology’ (Figure 1) [1]. Developmental biology research has uncovered pro-regenerative biological protein-based factors that have the capabilities to modulate stem cell activity towards a final outcome of regenerating injured, damaged, or otherwise impaired tissue [2]. The use of growth factors, such as bone morphogenetic protein-2 (BMP-2), for bone repair and regeneration has been widely researched [3–7]. However, growth factors have significant drawbacks that have so far hindered their practical applications [6,8–12]. Small molecules with osteoinductive potential have been proposed as promising alternatives because they are able to minimize or overcome many of the problems associated with protein-based growth factors [11–18]. For instance, in general, small molecules are often too small in molecular size (<1 000 Da) to induce unwanted immune responses in the host [19]. In addition, unlike protein-based growth factors, structural integrity is usually not required for the bioactivity of small-molecule compounds [10,11,20]. With the advent of high-throughput screening (HTS), a large number of small molecules with osteoinductive potential have been discovered over the past decade [21–26]. A literature survey for osteogenic small molecules based on a search of electronic databases over the past 10 years (Figure 2) clearly indicates the increasing interest in the application of small molecules for bone repair and regeneration: a total of 80 relevant publications appeared in electronic databases from January 2013 to October 2013 versus only one article in 2003.
Figure 1.
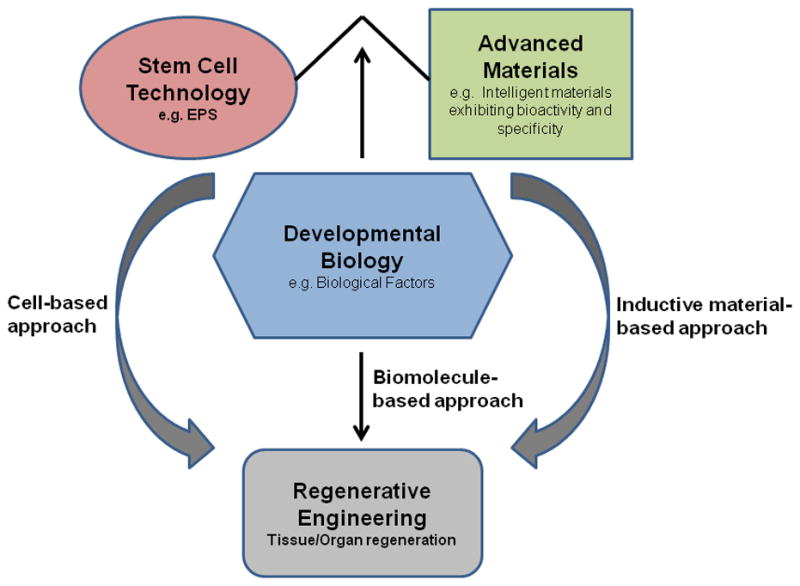
A schematic representation of the emerging field of ‘regenerative engineering’. Advanced materials, stem cells, and biological factors alone or in combination have important roles in regenerating tissue. Abbreviation: EPS, XXXXX. Adapted, with permission, from [18,94].
Figure 2.
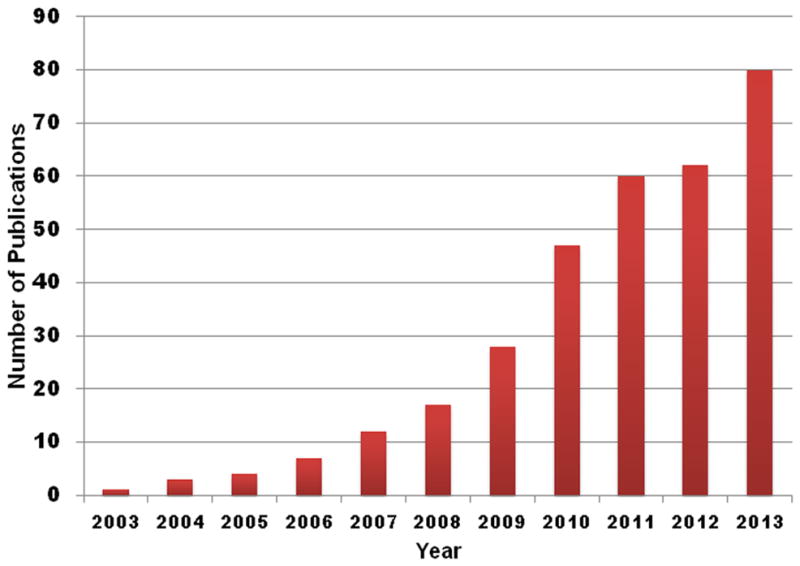
A research survey conducted using different keywords, such as ‘osteogenic small molecules’ or ‘small molecules and bone tissue engineering’ shows an increasing number of publications relating to small-molecule application for bone regenerative engineering over the past 10 years.
Considering the growing number of osteoinductive small molecules that have been reported in the literature, some of them might represent the next generation of therapies for clinical bone repair and regeneration. In this review, we focus on the prospective future of small-molecule delivery to bone tissue as well as on current preclinical studies associated with small molecules for bone repair and regeneration.
Delivery of small molecules
Despite the fact that emerging small molecules show promise in various orthopedic applications, their use is limited by their nonspecific adverse effects on nontarget tissues and organs [11,27]. The key to success with utilizing small molecules for bone regeneration is designing suitable delivery systems to localize and sustain the controlled release of small molecules to target sites. Although many types of biomaterial, from biologically derived constructs to those of synthetic origin, have been developed to address this need, constructs that are biocompatible and biodegradable are of the utmost interest. Biodegradability is of particular importance because small molecules can be entrapped during construct fabrication and released during scaffold degradation [28–33].
Scaffolds have been used as vehicles for the controlled delivery of small-molecule drugs, proteins, and nucleic acid for engineering various musculoskeletal tissues, such as bone, skin, nerve, cartilage, ligament, and muscle [34]. For bone tissue-engineering applications, scaffolds are usually biocompatible, 3D, and highly porous, as well as being able to mimic the extracellular matrix of bone in both physical architecture and chemical composition [34]. Such scaffolds provide an elegant system for osteoblasts or stem cells to adhere to the implant surface and respond to small molecules loaded within 3D matrices that initiate the cascade of osteogenic molecular signaling [35]. Many natural and synthetic materials have been used for scaffolds, but within the realm of small-molecule delivery, calcium phosphate (CaP) ceramics have garnered much attention because of their inherent properties that make them attractive as osteoinductive materials [36,37]. There are several types of CaP compound, namely hydroxyapatite (HAp), tricalcium phosphate (TCP), amorphous calcium phosphates (ACPs), and biphasic calcium phosphates (BCPs), which differ slightly in physical and chemical properties. Properties such as surface roughness, crystallinity, solubility, and surface charge stimulate varying effects on cells in vitro and the treatment of osteoporosis and healing of long bone fractures, non-unions, and spinal injuries in vivo in preclinical animal models. However, the overall chemical nature of these materials is such that they all mainly comprise calcium and phosphate ions, the same ions that make up the bulk of natural bone mineral. Thus, when implanted in bone, these materials are capable of participating in calcium phosphate solid–solution equilibrium at their surface, wherein the requisite calcium and phosphate ions needed to establish this equilibrium can be derived from the implant or surrounding bone, or both. This process has been shown to enhance bone regeneration at defect and injury sites in several preclinical orthopedic applications.
In addition to CaP compounds, polymeric scaffolds have emerged as prime candidates for the delivery of small molecules because of the ease of their processibility and tailorability towards specific chemical and physical properties that, among other design considerations, lead to adjustable pharmacokinetic release profiles [38]. For instance, synthetic polymers, such as poly(lactic-co-glycolic) acid (PLAGA), have been among the most attractive candidates used for drug delivery device fabrication [39]. Natural polymers, such as chitosan, have also been used as drug delivery platforms because of the simple preparation methods for their encapsulation of drugs [40]. Furthermore, there are several 3D scaffold fabrication and encapsulation techniques, including but not limited to microspheres, nanospheres, and nanofibers [36,41]. Such scaffolds are favorable because they have more design flexibility because of their inherent ‘bottom-up’ fabrication method.
There are several types of incorporation technique, including covalent bonding, physical adsorption, and entrapment (Figure 3) [42]. Each method has inherent advantageous depending on the biomolecule chemical structure, intended scaffold fabrication method, and desired drug release profile. Given that small molecules are usually highly thermally stable and soluble in various organic solvents, small molecules can be conjugated or loaded into polymeric scaffold by a variety of methods that are not feasible with more fragile full-length protein growth factors [11,13]. Nuttelman et al. covalently grafted small-molecule dexamethasone, a supplement used in osteoinductive culture medium, to poly(ethylene glycol) (PEG) through a degradable lactic acid linker using the cross-linker di-isopropyl carbodiimide. Dexamethasone was released from the polymer upon degradation of the lactide bone, which was shown to be biologically active because it enhanced the osteogenic differentiation of human mesenchymal stem cells in vitro [43].
Figure 3.
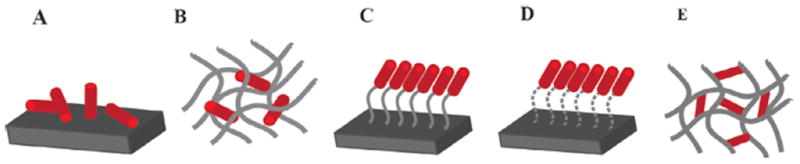
Schematic depictions of physical adsorption (A) and entrapment (B), or chemical covalent binding (C,D), and crosslinking (E) as strategies to incorporate small molecules onto biomaterials. Adapted, with permission, from [42].
Preclinical studies and potential clinical applications
Typically, drug development can be divided into three major steps: drug discovery, preclinical animal studies, and clinical trials in humans [44]. Recently, several preclinical studies detailing the use of small molecules in bone regeneration have been reported in the literature. Here, we summarize the recent literature in the area of preclinical evaluations of osteoinductive small molecules over the past 5 years. These preclinical studies focused on animal models of osteoporosis, long bone fractures and non-unions, and spinal fusion (Table 1).
Table 1.
Small molecules that have been investigated in preclincal bone regenerative engineering studies
| Small molecule | MW (Da) | Pathway | Delivery system | Loading | Animal model | Refs |
|---|---|---|---|---|---|---|
| Osteoporosis | ||||||
| Simvastatin | 418.6 | BMP/Smad | CaP nanocapsules, including deoxycholate micelles containing simvastatin | 1:3 (v/v) | Ovariectomized mouse | [52] |
| Bisphosphonate | 270 | Mevalonate | Hydroxyapatite plasma-coated titanium alloy cylinders | Soaked in 2.25 × 10−5 mol/l bisphosphonate solution for 48 h | Ovariectomized sheep | [55] |
| Long bone fractures and non-unions | ||||||
| FTY720 | 343.9 | S1P | 50:50 PLAGA-coated demineralized allograft | 1:200 (wt/wt) | Critical-sized rat tibial defect | [60] |
| Purmorphamine | 520.6 | Hedgehog | HAp beads | Soaked in 200 mM purmorphamine solution for 24 h | Fetal chick femur | [64] |
| SVAK-12 | 137.14 | BMP | Not applicable | 200 to 250 mg SVAK-12 percutaneously injected | Rat femoral fracture model | [67] |
| Simvastatin | 418.6 | BMP/Smad | Calcium sulfate/simvastatin/MSC sheet | CS mixed with 2.5 ml of 200 mg/ml simvastatin solution, then 0.5 mg simvastatin was applied in each scaffold | Rat tibia osteotomy | [95] |
| Simvastatin | 418.6 | BMP/Smad | Simvastatin/PLGA/HAp microspheres | 77.7% ± 10.3% (encapsulation efficiency) | Mouse model of gap fracture bridging with a graft of necrotic bone | [73] |
| Lovastatin | 404.5 | BMP/Smad | Lovastatin microparticle-loaded polyurethane scaffolds | 25 or 100 mg LV-MP in PBS injected into PUR scaffolds | Femoral plug defects and critical-sized segmental defects | [96] |
| Rosuvastatin | 481.5 | BMP/Smad | Adsorbable collagen sponge | Soaked in 0.1 mg/ml | Critical-sized rabbit cortical bone defect | [97] |
| Helioxanthin derivative (TH) | 348 | BMP/Smad | Tetrapod-shaped alpha tricalcium phosphate granules (Tetrabone®) | Soaked in 1 mM TH solution in DMSO overnight | Rat femur bone defect model | [98] |
| N-acetyl cysteine | 163.19 | Unknown | Commercial collagen sponge (Teruplug®) | Soaked in 5.0 mM NAC solution | Critical sized rat femur cortical bone defect | [76] |
| Spinal fusions | ||||||
| Simvastatin | 418.6 | BMP/Smad | Not applicable | Orally administered at a dose of 120 mg/kg/day | Rat spinal fusion | [80] |
| Oxysterols | 420 | Hedgehog | Adsorbable collagen sponge | Soaked in 50 ml Oxy34 or Oxy49 | Rat spinal fusion | [82] |
| Oxysterols | 420 | Hedgehog | Adsorbable collagen sponge | Soaked in 40 ml Oxy4, 18, or 21 for 1–2 h | Rat spinal fusion | [83] |
| Rolipram | 275.4 | PKA | Alginate-microfibrous patch | 25 or 50 g/ml rolipram-loaded hydrogels | Rat hemisection spinal cord injury | [99] |
Osteoporosis
Osteoporosis is the most common form of metabolic bone disorders. In the USA, nearly half of American Caucasian women over the age of 50 have osteoporosis [45]. Although it is often thought of as a disease that affects women, osteoporosis also significantly affects men [46]. Recent US Food and Drug Administration (FDA)-approved medications for osteoporosis, such as recombinant human parathyroid hormone peptide (rhPTH 1-34) (trade name: Forteo®), have been shown to be effective in treating osteoporosis [47]. However, they can be problematic because, for example, rhPTH 1-34 can degrade during its shelf life [48]. These observations suggest that small molecules could be utilized as alternative novel bone anabolic agents for the treatment of osteoporosis. In recent years, statins, a family of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors originally developed to treat hypercholesterolemia, have received significant attention in the area of osteoporosis treatment because of their osteoinductivity shown in osteoblasts and bone marrow stromal cells via the bone morphogenetic protein (BMP) signaling pathway [49]. Although statins are the most prescribed medication in western countries, recent evidence from an epidemiological study demonstrated an association between long-term statin use and a reduced risk of low-energy hip fractures in middle-aged and older women [50]. Sustained-release drug delivery systems for statins have been proposed as an effective strategy for osteoporosis. For instance, Ito et al. fabricated an injectable delivery device containing the small-molecule simvastatin encapsulated in CaP nanoparticles [51,52]. This novel drug delivery construct was shown to reduce simvastatin cytotoxicity significantly in osteoclast-like MLC-6 cells.
Moreover, implants with local delivery of small molecules have been studied in animal osteoporotic models. The therapeutic efficacy of the simvastatin-loaded CaP coated delivery vehicle observed in an osteoporotic mice model resulted in increased body weight, bone mineral content, and bone mechanical strength compared with controls [52]. Additionally, the therapeutic effect of small-molecule simvastatin on osteogenesis around titanium implants was demonstrated in an ovariectomized model [53,54]. CaP-coated implants releasing bisphosphonate osteoporotic drug increased periprosthetic bone density and implant pull force, compared with control implants in an osteoporotic sheep model [55]. Taken together, these observations indicate that local delivery of small-molecule drugs has great potential to heal osteoporotic bones.
Long bone fractures and non-unions
In the event of a bone fracture or break, the osseous tissue has the unique repair ability to remodel mineralized tissue to heal the site internally. However, in instances of trauma, congenital defects, or bone tumors, resulting large bone defects often cannot heal and require bone replacement to restore mobility and function [56]. Although bone replacements have traditionally been from autologous or allogenic sources, both types are usually associated with myriad issues, such as donor site morbidity, risks of infection, immune response, and lack of adequate supply [57–59]. Therefore, alternative synthetic options, which are more readily accessible, easily modified, and cost effective, have been explored [1,59]. Tissue-engineered scaffolds loaded with small molecules have been proposed for long bone fracture and non-union applications [11]. For instance, FTY720 is an osteoinductive small-molecule analog of sphingosine-1-phosphate (S1P) that targets the receptors S1P1 and S1P3-5, and has been shown to increase new bone and microvascular formation within a rat cranial defect model [60–62]. To demonstrate the potential of FTY720 to enhance current clinical repair practices for non-unions, investigators from the Botchwey research group coated devitalized bone allografts with a continuous phase of small-molecule FTY720-loaded PLAGA polymer. Results showed that, within a massive rat tibial defect model, the osseointegration, mechanical stability, and smooth muscle cell recruitment of the coated allograft was enhanced by the local delivery of FTY720. At 6 weeks, FTY720-treated groups demonstrated higher bone density at the allograft–implant interface, superior mechanical properties in both elastic modulus and compressive strength, and increased smooth vessel incorporation compared wit unloaded controls [60]. The results of this study warrant further investigation into the localized delivery of S1P agonists for improved incorporation of porous, mechanically relevant bone allografts.
Small-molecule purmorphamine has been shown to induce in vitro osteogenesis by activation of the Hedgehog signaling pathway [63]. Recently, CaP-bound purmorphamine was fabricated to determine whether the local administration of this small molecule could accelerate bone growth and repair in an in vivo chick embryo chorioallantoic membrane (CAM) assay. Purmorphamine was adsorbed to CaP and then injected into a defect site made within the donor femurs. After 7 days, the proportion of trabecular bone area to overall bone area was significantly higher than in the control [64].
Small-molecule BMP-signaling activators have been recently discovered and their in vitro and/or in vivo effects on osteogenesis in the presence of low dosages of exogenous rhBMPs have been reported [26,65–67]. It is believed that application of these small molecules can significantly lower the dosages of exogenous rhBMPs required or eliminate the need for exogenous rhBMPs, thus significantly reducing overall treatment cost [67]. In one study, Wong et al. demonstrated that a single dose of the small-molecule BMP activator, SVAK-12, could accelerate fracture healing in a rat femoral fracture model without the need for exogenous rhBMPs [67].
Several small molecules extracted from traditional Chinese herbal products have shown potential clinical effects. Interestingly, Wong et al. evaluated several of these herbally derived small molecules in a rabbit critical-sized bone defect model. In each instance, the authors first created 5 mm × 10 mm critical defect sites in parietal bone in rabbits and the defects were then grafted with collagen matrix carriers preloaded with various small-molecule candidates. Their results revealed that, in each case, delivering small molecules extracted from herbs such as buguzhi [68], psoralen [69], daidzein [70], genistin [71], and quercetin [72], increased bone formation in the critical defect site compared with the control groups. These interesting results indicate the promising effects of herbally derived small molecules to heal bone defects.
As described in the previous section, simvastatin has been investigated extensively for its ability to induce osteogenesis in osteoporotic models. It is believed that simvastatin also has an important role in healing bone fracture. In a recent study, Tai et al. incorporated simvastatin in PLAGA/HAp microspheres to induce bone formation in a mouse fracture gap model bridging with a necrotic bone graft (i.e., dead bone). More specifically, at 2 and 4 weeks, it was shown that simvastatin-loaded PLAGA/HAp significantly increased callus formation around the implanted area and increased blood vessel formation and cell ingrowths in the necrotic bone graft, thereby substituting the dead bone [73].
Last but not least, other recent preclinical studies that focus on small-molecule delivery, such as octahydrochloride hydrate (AMD3100) [74], osteogenic inducible compound-active 006 (OIC-A006) [75], and N-acetyl cysteine [76], highlight the exciting preclinical developments of small molecules. Such work has the potential to revolutionize strategies for long bone and non-union treatment and repair.
Spinal fusions
Interverterbral discs (IVDs) give flexibility to the spine and enable the body to twist and bend into a wide range of postures. Approximately 80% of the human population has back problems at some point during their lives [77]. IVD degeneration leads to chronic back pain, is a major health problem in the USA, and causes substantial disability [78]. Spinal fusion is an effective surgical approach following the removal of degenerated IVDs to treat spinal disorders [77]. It is estimated that approximately more than 300 000 lumbar spinal fusions occur each year in the USA [79]. Osteoinductive small molecules are expected to impact on spinal fusion surgeries. Several studies have investigated various small molecules to determine their capacity to aid in spinal fusion. In a recent study, a single dose of simvastatin (120 mg/kg/day) was administered orally to rats and the results showed significantly improved spinal fusion grades in rats through histological examination. More specifically, the rats administered with simvastatin had a mean of 9.30 +/− 0.949 Newtons fusion mass compared with a mean of 6.82 +/ 2.044 Newtons fusion mass of untreated rats. The histological data were further confirmed by radiographic examination [80]. Interestingly, an earlier study reported by Yee et al. revealed that rabbits that were orally administrated simvastatin did not exhibit a statistically significant increase in spinal fusion mass compared with the control group [81]. These contradicting studies suggest that there are interspecies differences in the effect of simvastatin on rats and rabbits. Future studies are needed to determine the effectiveness of small-molecule simvastatin on a larger animal model to provide further evidence of its potential as a spinal fusion agent.
Two novel analogs of oxysterols, Oxy 34 and Oxy 49, have recently been shown to induce bone formation in a rat spinal fusion model. This study revealed that rats receiving collagen implants loaded with Oxy 34 or Oxy 49 showed comparable osteogenic efficacy to BMP2/collagen implants as evaluated by manual inspection, micro-computerized tomography, radiography, and histological analysis [82]. More recently, a similar study conducted by Stappenbeck et al. demonstrated that three novel analogs of oxysterols, Oxy 4, Oxy 18, and Oxy 21, were able to stimulate bone formation in a rat spinal fusion model [83]. These observations demonstrate the promise of using small-molecule oxysterols in spinal fusion.
To our knowledge, literature reporting the delivery of small molecules in animal spinal fusion models is limited. Therefore, further research is needed to investigate which specific spinal fusion model, delivery method, and dosage, as well as choice of small molecule, could enhance the result of spinal fusions and, thus, provide the foundation for future clinical usage.
Concluding remarks and future directions
The use of small molecules for bone regenerative medicines has been largely overlooked. There are several rate-limiting factors for progress in the field of small molecule-based bone regeneration. In general, small molecules are small enough to penetrate nontarget cells and trigger unwanted signaling cascades [84]. Thus, the major concern associated with small-molecule therapeutics is their nonspecific adverse effects [10,11,85–87]. In addition, a lack of an effective delivery strategy for small molecules is another issue, given that one of the objectives of small molecule-based bone regenerative engineering is to develop an engineered scaffold system that provides adequate doses of the small molecule and/or acts as a structural support for infiltrating cells [11,17,60]. Sustained release from ceramic and polymeric carriers has been proposed as a viable strategy for numerous biomolecules, including protein and small-molecule drugs [88–91]. However, the key problem with using ceramic and polymeric carriers for drug delivery is the difficulty in controlling sustained delivery rates [92]. This is particularly true for low-molecular-weight drugs because the diffusion rate is faster and most of the drug diffusion occurs during the first 24 h [93]. More sophisticated drug delivery systems will be valuable for imparting spatiotemporal control over small-molecule release kinetics. Although small molecule-based bone-regenerative engineering holds tremendous promise in various orthopedic applications, several questions for small-molecule researches need to be addressed before clinical translation, including: what is the signaling mechanism of these small molecules in cells? How can one minimize the nonspecific adverse effects of small-molecule drugs? How can one combine stem cell sciences and/or advanced materials for small molecule-based bone regenerative engineering?
Highlights.
Regenerative engineering has been recently proposed to regenerate skeletal tissue
Regenerative small molecules play a vital role in bone regenerative engineering
Numerous small molecules have been recently discovered for bone regeneration
Polymeric scaffolds play an important role to delivery small molecules in vivo
Acknowledgments
This work was supported by funding from the NIH (R21-AR060480) and the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences to C.T.L., a grant from the State of Connecticut Stem Cell Research Foundation to K.W-HL. C.T.L. was the recipient of the Presidential Faculty Fellowship Award from President William Clinton and the Presidential Award for Excellence in Science, Mathematics, and Engineering Mentorship from President Barack Obama.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med. 2012;4:160ed169. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 2.Evans CH. Advances in regenerative orthopedics. Mayo Clin Proc. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JR, et al. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Bessa PC, et al. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 6.Lo KWH, et al. Studies of bone morphogenetic protein based surgical repair. Adv Drug Deliv Rev. 2012;64:1277–1291. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Q, et al. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr Pharm Des. 2013;19:3374–3383. doi: 10.2174/1381612811319190004. [DOI] [PubMed] [Google Scholar]
- 8.Cho MJ, Juliano R. Macromolecular versus small-molecule therapeutics: drug discovery, development and clinical considerations. Trends Biotechnol. 1996;14:153–158. doi: 10.1016/0167-7799(96)10024-X. [DOI] [PubMed] [Google Scholar]
- 9.Baker M, Carr F. Pre-clinical considerations in the assessment of immunogenicity for protein therapeutics. Curr Drug Saf. 2010;5:308–313. doi: 10.2174/157488610792246000. [DOI] [PubMed] [Google Scholar]
- 10.Lo KW, et al. Current patents on osteoinductive molecules for bone tissue engineering. Rec Pat Biomed Eng. 2011;4:153–167. [Google Scholar]
- 11.Lo KWH, et al. The role of small molecules in the musculoskeletal regeneration. Regen Med. 2012;7:535–549. doi: 10.2217/rme.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han QQ, et al. The role of small molecules in bone regeneration. Future Med Chem. 2013;5:1671–1684. doi: 10.4155/fmc.13.133. [DOI] [PubMed] [Google Scholar]
- 13.Fu K, et al. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18:24–25. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 14.Kessler M, et al. Immunogenicity of biopharmaceuticals. Nephrol Dial Transplant. 2006;21 (Suppl 5):v9–v12. doi: 10.1093/ndt/gfl476. [DOI] [PubMed] [Google Scholar]
- 15.Hwang CJ, et al. Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine. 2009;10:443–451. doi: 10.3171/2009.1.SPINE08473. [DOI] [PubMed] [Google Scholar]
- 16.Egusa H, et al. A small-molecule approach to bone regenerative medicine in dentistry. J Oral Biosci. 2010;52:107–118. [Google Scholar]
- 17.Segar CE, et al. Regulation of angiogenesis and bone regeneration with natural and synthetic small molecules. Curr Pharm Des. 2013;19:3403–3419. doi: 10.2174/1381612811319190007. [DOI] [PubMed] [Google Scholar]
- 18.Lo KW-H, et al. Small molecule based musculoskeletal regenerative engineering. Trends Biotechnol. doi: 10.1016/j.tibtech.2013.12.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaich G, et al. Overview: differentiating issues in the development of macromolecules compared with small molecules. In: XXXX Y, editor. Handbook of Pharmaceutical Biotechnology. John Wiley & Sons; 2006. http://dx.doi.org/10.1002/9780470117118.ch1c. [Google Scholar]
- 20.Wieghaus KA, et al. Small molecule inducers of angiogenesis for tissue engineering. Tissue Eng. 2006;12:1903–1913. doi: 10.1089/ten.2006.12.1903. [DOI] [PubMed] [Google Scholar]
- 21.Hojo H, et al. Development of high-throughput screening system for osteogenic drugs using a cell-based sensor. Biochem Biophys Res Commun. 2008;376:375–379. doi: 10.1016/j.bbrc.2008.08.167. [DOI] [PubMed] [Google Scholar]
- 22.Han CY, et al. Small molecules with potent osteogenic-inducing activity in osteoblast cells. Bioorg Med Chem Lett. 2009;19:1442–1445. doi: 10.1016/j.bmcl.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Brey DM, et al. High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol Bioeng. 2011;108:163–174. doi: 10.1002/bit.22925. [DOI] [PubMed] [Google Scholar]
- 24.Alves H, et al. High-throughput assay for the identification of compounds regulating osteogenic differentiation of human mesenchymal stromal cells. PLoS ONE. 2011;6:e26678. doi: 10.1371/journal.pone.0026678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darcy A, et al. A novel library screen identifies immunosuppressors that promote osteoblast differentiation. Bone. 2012;50:1294–1303. doi: 10.1016/j.bone.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vrijens K, et al. Identification of small molecule activators of BMP signaling. PLoS ONE. 2013;8:e59045. doi: 10.1371/journal.pone.0059045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11 (Suppl 2):S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 28.Lin SY, et al. Microencapsulation and controlled release of insulin from polylactic acid microcapsules. Biomater Med Devices Artif Organs. 1985;13:187–201. doi: 10.3109/10731198509118850. [DOI] [PubMed] [Google Scholar]
- 29.Ustariz-Peyret C, et al. Cephradin-plaga microspheres for sustained delivery to cattle. J Microencapsul. 1999;16:181–194. doi: 10.1080/026520499289167. [DOI] [PubMed] [Google Scholar]
- 30.Alonso MJ, et al. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 31.Birnbaum DT, et al. Controlled release of beta-estradiol from PLAGA microparticles: the effect of organic phase solvent on encapsulation and release. J Control Release. 2000;65:375–387. doi: 10.1016/s0168-3659(99)00219-9. [DOI] [PubMed] [Google Scholar]
- 32.Boisdron-Celle M, et al. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47:108–114. doi: 10.1111/j.2042-7158.1995.tb05760.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen ZG. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol Med. 2010;16:594–602. doi: 10.1016/j.molmed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhandayuthapani B, et al. Polymeric scaffolds in tissue engineering application: a review. Int J Polymer Sci. 2011 http://dx.doi.org/10.1155/2011/290602.
- 35.Salgado AJ, et al. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 36.Khan Y, et al. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90 (Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 37.Samavedi S, et al. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater. 2013;9:8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Makadia HK, Siege SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu L, et al. Advances in chitosan-based drug delivery vehicles. Nanoscale. 2013;5:3103–3111. doi: 10.1039/c3nr00338h. [DOI] [PubMed] [Google Scholar]
- 41.Chung YI, et al. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007;121:91–99. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Maia FR, et al. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomater. 2013;9:8773–8789. doi: 10.1016/j.actbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Nuttelman CR, et al. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J Biomed Mater Res A. 2006;76:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 44.Steinmetz KL, Spack EG. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009;9 (Suppl 1):S2. doi: 10.1186/1471-2377-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honig S. Osteoporosis: new treatments and updates. Bull NYU Hosp Jt Dis. 2010;69:253–256. [PubMed] [Google Scholar]
- 46.Mosekilde L, et al. The pathogenesis, treatment and prevention of osteoporosis in men. Drugs. 2013;73:15–29. doi: 10.1007/s40265-012-0003-1. [DOI] [PubMed] [Google Scholar]
- 47.Deal C, Gideon J. Recombinant human PTH 1-34 (Forteo): an anabolic drug for osteoporosis. Cleve Clin J Med. 2003;70:585–601. doi: 10.3949/ccjm.70.7.585. [DOI] [PubMed] [Google Scholar]
- 48.Kothari R, et al. Modes of degradation and impurity characterization in rhPTH (1-34) during stability studies. PDA J Pharm Sci Technol. 2011;65:348–362. doi: 10.5731/pdajpst.2011.00745. [DOI] [PubMed] [Google Scholar]
- 49.Mundy G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 50.Helin-Salmivaara A, et al. Statins and hip fracture prevention: a population based cohort study in women. PLoS ONE. 2012;7:e48095. doi: 10.1371/journal.pone.0048095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito T, et al. Preparation of injectable auto-forming alginate gel containing simvastatin with amorphous calcium phosphate as a controlled release medium and their therapeutic effect in osteoporosis model rat. J Mater Sci Mater Med. 2012;23:1291–1297. doi: 10.1007/s10856-012-4597-3. [DOI] [PubMed] [Google Scholar]
- 52.Ito T, et al. Preparation of calcium phosphate nanocapsules including simvastatin/deoxycholic acid assembly, and their therapeutic effect in osteoporosis model mice. J Pharm Pharmacol. 2013;65:494–502. doi: 10.1111/jphp.12008. [DOI] [PubMed] [Google Scholar]
- 53.Du Z, et al. Effects of simvastatin on bone healing around titanium implants in osteoporotic rats. Clin Oral Implants Res. 2009;20:145–150. doi: 10.1111/j.1600-0501.2008.01630.x. [DOI] [PubMed] [Google Scholar]
- 54.Yang G, et al. Bone responses to simvastatin-loaded porous implant surfaces in an ovariectomized model. Int J Oral Maxillofac Implants. 2012;27:369–374. [PubMed] [Google Scholar]
- 55.Stadelmann VA, et al. Implants delivering bisphosphonate locally increase periprosthetic bone density in an osteoporotic sheep model. A pilot study Eur Cell Mater. 2008;16:10–16. doi: 10.22203/ecm.v016a02. [DOI] [PubMed] [Google Scholar]
- 56.Buckwalter JA, et al. Bone biology. I: structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371–386. [PubMed] [Google Scholar]
- 57.Khan SN, et al. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77–86. [PubMed] [Google Scholar]
- 58.Laurencin CT, et al. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 59.Laurencin CT, et al. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 60.Petrie Aronin CE, et al. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31:6417–6424. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sefcik LS, et al. Selective activation of sphingosine 1-phosphate receptors 1 and 3 promotes local microvascular network growth. Tissue Eng A. 2011;17:617–629. doi: 10.1089/ten.tea.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C, et al. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell Tissue Res. 2012;347:553–566. doi: 10.1007/s00441-011-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu X, et al. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 2004;11:1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Gellynck K, et al. Small molecule stimulation enhances bone regeneration but not titanium implant osseointegration. Bone. 2013;57:405–412. doi: 10.1016/j.bone.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Park KW, et al. The small molecule phenamil induces osteoblast differentiation and mineralization. Mol Cell Biol. 2009;29:3905–3914. doi: 10.1128/MCB.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato S, et al. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Mol Cell Biochem. 2011;349:97–106. doi: 10.1007/s11010-010-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong E, et al. A novel low-molecular-weight compound enhances ectopic bone formation and fracture repair. J Bone Joint Surg Am. 2013;95:454–461. doi: 10.2106/JBJS.L.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong RW, Rabie AB. Effect of Buguzhi (Psoralea corylifolia fruit) extract on bone formation. Phytother Res. 2010;24 (Suppl 2):S155–S160. doi: 10.1002/ptr.3049. [DOI] [PubMed] [Google Scholar]
- 69.Wong RW, Rabie AB. Effect of psoralen on bone formation. J Orthop Res. 2011;29:158–164. doi: 10.1002/jor.21124. [DOI] [PubMed] [Google Scholar]
- 70.Wong RW, Rabie AB. Effect of daidzein on bone formation. Front Biosci. 2009;14:3673–3679. doi: 10.2741/3479. [DOI] [PubMed] [Google Scholar]
- 71.Wong RW, Rabie AB. Effect of genistin on bone formation. Front Biosci. 2010;2:764–770. doi: 10.2741/e136. [DOI] [PubMed] [Google Scholar]
- 72.Wong RW, Rabie AB. Effect of quercetin on preosteoblasts and bone defects. Open Orthop J. 2008;2:27–32. doi: 10.2174/1874325000802010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tai IC, et al. Local delivery of controlled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int J Nanomed. 2013;8:3895–3905. doi: 10.2147/IJN.S48694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012–1018. doi: 10.1016/j.bone.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao H, et al. OIC-A006-loaded true bone ceramic heals rabbit critical-sized segmental radial defect. Pharmazie. 2012;67:247–252. [PubMed] [Google Scholar]
- 76.Yamada M, et al. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials. 2013;34:6147–6156. doi: 10.1016/j.biomaterials.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 77.Peng B, et al. Diagnosis and surgical treatment of back pain originating from endplate. Eur Spine J. 2009;18:1035–1040. doi: 10.1007/s00586-009-0938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 79.Becker C. Spine-tingling prospects: artificial disc implants are among the new technologies expected to revolutionize the outcomes of back surgery. Mod Healthc. 2003;33:30–32. [PubMed] [Google Scholar]
- 80.Bostan B, et al. Simvastatin improves spinal fusion in rats. Acta Orthop Traumatol Turc. 2011;45:270–275. doi: 10.3944/AOTT.2011.2526. [DOI] [PubMed] [Google Scholar]
- 81.Yee AJ, et al. The use of simvastatin in rabbit posterolateral lumbar intertransverse process spine fusion. Spine J. 2006;6:391–396. doi: 10.1016/j.spinee.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 82.Johnson JS, et al. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J Cell Biochem. 2011;112:1673–1684. doi: 10.1002/jcb.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stappenbeck F, et al. Novel oxysterols activate the Hedgehog pathway and induce osteogenesis. Bioorg Med Chem Lett. 2012;22:5893–5897. doi: 10.1016/j.bmcl.2012.07.073. [DOI] [PubMed] [Google Scholar]
- 84.Brouwers L, et al. Network neighbors of drug targets contribute to drug side-effect similarity. PLoS ONE. 2011;6:e22187. doi: 10.1371/journal.pone.0022187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weiss WA, et al. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat Chem Biol. 2007;3:739–744. doi: 10.1038/nchembio1207-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lo K-WH, et al. one-day treatment of small molecule 8-bromo-cyclic amp analogue induces cell-based VEGF production for in vitro angiogenesis and osteoblastic differentiation. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1839. http://dx.doi.org/10.1002/term.1839. [DOI] [PMC free article] [PubMed]
- 87.Lo K-WH, et al. Short-term treatment of small molecule phenamil induced a protracted osteogenic effect on osteoblast-like MC3T3-E1 cells. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1786. http://dx.doi.org/10.1002/term.1786. [DOI] [PubMed]
- 88.Ravivarapu HB, et al. Sustained activity and release of leuprolide acetate from an in situ forming polymeric implant. AAPS PharmSciTech. 2000;1:E1. doi: 10.1208/pt010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruhe PQ, et al. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 90.Agarwal P, Rupenthal ID. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Ulery BD, et al. Facile fabrication of polyanhydride/anesthetic nanoparticles with tunable release kinetics. Adv Healthc Mater. 2013 doi: 10.1002/adhm.201300521. http://dx.doi.org/10.1002/adhm.201300521. [DOI] [PMC free article] [PubMed]
- 92.Liechty WB, et al. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bibby DC, et al. Poly(acrylic acid) microspheres containing beta-cyclodextrin: loading and in vitro release of two dyes. Int J Pharm. 1999;187:243–250. doi: 10.1016/s0378-5173(99)00190-8. [DOI] [PubMed] [Google Scholar]
- 94.Deng M, et al. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans Nanobiosci. 2012;11:3–14. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- 95.Qi Y, et al. Mesenchymal stem cell sheet transplantation combined with locally released simvastatin enhances bone formation in a rat tibia osteotomy model. Cytotherapy. 2013;15:44–56. doi: 10.1016/j.jcyt.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Yoshii T, et al. Local injection of lovastatin in biodegradable polyurethane scaffolds enhances bone regeneration in a critical-sized segmental defect in rat femora. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1547. http://dx.doi.org/10.1002/term.1547. [DOI] [PubMed]
- 97.Monjo M, et al. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010;6:1405–1412. doi: 10.1016/j.actbio.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 98.Maeda Y, et al. Bone healing by sterilizable calcium phosphate tetrapods eluting osteogenic molecules. Biomaterials. 2013;34:5530–5537. doi: 10.1016/j.biomaterials.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 99.Downing TL, et al. Drug-eluting microfibrous patches for the local delivery of rolipram in spinal cord repair. J Control Release. 2012;161:910–917. doi: 10.1016/j.jconrel.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


