Abstract
Recent compelling evidence indicates that Th17 confer host immunity against a variety of microbes, including extracellular and intracellular pathogens. Therefore, understanding mechanisms for the induction and activation of antigen-specific Th17 is important for the rational design of vaccines against pathogens. To study this, we employed an in vitro system in which influenza HA1 was delivered to dendritic cells (DCs) via Dectin-1 using anti-hDectin-1-HA1 recombinant fusion proteins. We found that healthy individuals maintained broad ranges of HA1-specific memory Th17 that were efficiently activated by DCs targeted with anti-hDectin-1-HA1. Nonetheless, these DCs were not able to induce significant level of HA1-specific Th17 response even in the presence of Th17-promoting cytokines, IL-1β and IL-6. We further found that the induction of surface IL-1R1 expression by signals via TCRs and common γ-chain receptors were essential for naïve CD4+ T cell differentiation into HA1-specific Th17. This process was dependent on MyD88, but not IRAK1/4. Thus, interruptions in STAT3 or MyD88 signaling led to substantially diminished HA1-specific Th17 induction. Taken together, the de novo generation of pathogen-specific human Th17 requires complex but complementary actions of multiple signals. Data from this study will help us design new and effective vaccine strategy that can promote Th17-mediated immunity against microbial pathogens.
Keywords: Dendritic cell, T cell, Dectin-1, Th17, IL-17, Vaccine, Immunity
Introduction
IL-17-producing CD4+ T cells (Th17) have been broadly linked to inflammatory diseases (1). However, recent compelling evidence indicates that Th17 also play an important role against both extracellular and intracellular microbial pathogens, including bacteria, fungi, parasites and viruses (2-13). Furthermore, the immunity conferred by Th17 is associated with improved survival of cancer patients (14, 15). Accordingly, Th17-mediated therapeutic immunity has also been demonstrated in murine cancer models (16, 17). Therefore, it is important to understand molecular and cellular mechanisms for the induction and activation of antigen-specific Th17 in the context of T cell receptor (TCR) ligation by peptides and major histocompatibility complexes (MHCs).
The induction of Th17 has been mainly studied in the context of inflammatory cytokine milieu. In mice, TGFβ (18, 19), IL-6 (19), IL-1β (20), IL-21 (21), IL-23 (22), and IL-9 (23) contribute to Th17 induction. In humans, IL-1β with IL-6 was initially reported to induce Th17 differentiation, and this was inhibited by TGFβ and IL-12 (9). TGFβ was also reported to be required for Th17 development (24), but Yang et al. (25) demonstrated that human Th17 could be developed in the presence of TGFβ and IL-21, but not TGFβ and IL-6. In contrast, Volpe et al. (26) showed that pro-inflammatory cytokines were all required and acted synergistically to generate human Th17. These series of findings suggest that each of these cytokines might contribute to Th17 development at certain stages of human T cell differentiation, although a recent finding has shown that IL-1β is essential in Candida albicans-induced human Th17 differentiation (27). Unlike in mice, however, our understanding of the induction and activation of antigen-specific Th17 in humans is still limited. This is mainly due to limitations of reliable experimental systems as well as difficulties in the assessment of antigen-specific T cell responses after in vitro priming of T cells particularly when the frequency of antigen-specific T cells is low. Thus, previous studies (9, 24-27) employed polyclonal T cell activators, such as anti-CD3/CD28 antibodies and phorbol 12-myristate 13-acetate (PMA), to prime and/or reactivate T cells to assess the magnitude and quality of T cell responses. Although these studies led to great progresses in our understanding of human Th17 especially in the context of inflammatory diseases, biology of T cells primed and/or re-activated with such polyclonal activators may not always represent the biology of T cells primed and/or re-activated with MHC II/peptide complexes presented by antigen presenting cells (APCs). Therefore, it is valuable to study the induction and activation of antigen-specific human Th17 in the context of T cell receptor (TCR) ligation by the complexes of MHC II and antigen-derived peptides presented by APCs.
DCs are major APCs that can induce and shape the types of T cell response during microbial infections. DCs express pattern-recognition receptors (PRRs), including toll-like receptors (TLRs) and C-type lectin receptors, which are linked to antimicrobial immunity through the sensing of pathogen-associated molecular patterns (28, 29). Of these PRRs, Dectin-1 is particularly relevant to the Th17-mediated immunity in both mice and humans (3, 7, 30, 31). We and others have shown that DCs can take up protein antigens via Dectin-1 and present antigenic peptides to both CD4+ and CD8+ T cells (32-34). Thus, we established an in vitro system in which HA1 subunit from hemagglutinin (HA) of influenza virus (H1N1, PR8), as a model antigen, could be delivered to DCs via hDectin-1 using recombinant proteins of an agonistic anti-hDectin-1 fused to HA1. This system allowed us for the first time to dissect the complex and dynamic processes of the generation of HA1-specific human Th17 in the context of TCR ligation with MHC II/peptide complexes presented by DCs. In addition, we demonstrated that antigen targeting to DCs via hDectin-1 along with TLR2 ligands could promote antigen-specific Th17 responses in human.
Materials and methods
Cells and culture medium
Blood from healthy volunteers were acquired under a protocol approved by the Institutional Review Board (IRB) of Baylor Research Institute (BRI). Peripheral blood mononuclear cells (PBMCs) of healthy volunteers were isolated by density gradient centrifugation using Ficoll-Paque™ PLUS (GE Healthcare, Sweden). IFNDCs were generated by culturing monocytes from healthy donors in serum free media (Cellgenix, Germany) supplemented with GM-CSF (100 ng/ml) and IFNα (500 units/ml). The medium was replenished with cytokines on day 1. IFNα and GM-CSF were from the Pharmacy at the Baylor University Medical Center (Dallas, TX). Autologous CD4+ T cells were purified using EasySep Human CD4+ T Cell Enrichment Kit (StemCell Technologies, Canada). Naïve (CD45RA+CD45RO−CCR7+), memory (CD45RA−CD45RO+) CD4+ T cells, and mDCs (Lin−HLA-DR+CD11c+CD123−) were sorted by FACS Aria (BD Biosciences, CA) (purity>99.0%). Culture medium consisted of RPMI 1640 (GIBCO, NY) supplemented with HEPES buffer, 2 mM L-glutamine, 1% nonessential amino-acids, sodium pyvurate, 50 units/ml penicillin, 50 μg/ml streptomycin and 10% normal human serum AB (GemCell, TX).
Antibodies and reagents
Anti-CD4-APC Cy7, anti-IFNγ–PE Cy7, anti-CCR5-pacific blue and anti-CCR6-Alexa Fluor 488 were purchased from Biolegend (CA). Anti-IL-1R1-PE, anti-IL-6R-PerCP, anti-CCR9-PE, anti-CXCR3-FITC, anti-IL-23p19 and control IgG were from R&D Systems (MN). Neutralizing anti-IL-1β and anti-IL-6/IL-6R were made in house. Anti-CD45RA-FITC, anti-CD45RO-PE, anti-integrin β7-PE, and anti-CD161-PE were purchased from BD Biosciences. Anti-IL-17-APC (eBioscience, CA) and anti-human IgG-PE (Jackson ImmunoResearch Laboratories, PA) were used. GolgiPlug was purchased from BD Pharmingen (CA). CFSE (Molecular probes, Oregon) was used for measuring CD4+ T cell proliferation. TLR ligands, including Pam(3)CysSK(4) were purchased from Invivogen (Oregon). Jak2 inhibitor (1,2,3,4,5,6-Hexabromocyclohexane), Jak3 inhibitor (4-(4′-Hydroxyphenyl)amino-6,7-dimethoxyquinazoline), pan Jak inhibitor (2-(1,1-Dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one), STAT5 inhibitor (Pimozide) and IRAK-1/4 inhibitor (N-(2-Morpholinylethyl)-2-(3-nitrobenzoylamido)-benzimidazole) were purchased from EMD chemicals (PA). STAT3 inhibitor (Stattic) was from Sigma-Aldrich (MO). MyD88 homodimerization inhibitory peptide (Imgenex, CA) was used. Anti-CD3/CD28 microbeads were purchased from Miltenyi Biotec (Germany).
Cloning and purification of chimeric recombinant mAbs fused to HA1
The Flu HA1 fragment containing nt 52-993 of Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(H1N1)) hemagglutinin (gi|21693168|gb|AF389118.1|) (with A619C and C959T changes) was engineered with a flanking NheI site followed by a flexible sequence (GATACAACAGAACCTGCAACACCTACAACACCTGTAACAACA) at the 5-prime end and 6 X His tag coding sequence followed by stop codon and NotI site at the 3-prime end (35). The NheI and NotI enzyme-digested fragment was fused downstream of and in-frame with the heavy chain coding region at the NheI site by NheI-NotI. Stable CHO-S cells were grown in GlutaMAX and HT media (Life Technologies, NY) and recombinant proteins were purified by protein A colum chromatography. Purified proteins were confirmed by reduced-SDS gel analysis.
Peptides
Overlapping 17-mer peptides (staggered by 11 amino acids) spanning the entire HA1 subunits of hemagglutinin (HA) (A/PR/8/34 H1N1) were purchased from Biosynthesis (TX).
Binding of recombinant fusion proteins to hDectin-1 and hDEC205
293F cells transfected with a full-length hDectin-1 or hDEC205 and IFNDCs were incubated with different concentrations of recombinant fusion proteins (anti-hDectin-1-HA1, anti-hDEC205-HA1, and IgG4-HA1) for 25 min on ice. Cells were then washed twice with 2% FCS in phosphate-buffered saline (PBS), and then stained with anti-human IgG-PE for 20 min. Cells were analyzed by flow cytometry.
DC activation
1×105 DCs were incubated with 1 μg/ml anti-hDectin-1-HA1 or 1 μg/ml IgG4-HA1 in the presence or absence of 40 ng/ml Pam(3)CysSK(4) for 24h. Cytokines in the culture supernatants were assessed by the BeadLyte cytokine assay kit (Upstate, MA) as per the manufacturer’s protocol. IL-23 was measured using a human IL-23 ELISA kit (eBiosciences, CA).
DC/CD4+ T cell co-cultures and antigen-specific CD4+ T cell responses
1-2×105 CFSE-labeled purified autologous CD4+ T cells were co-cultured with 5×103 DCs loaded with indicated antibodies, in the absence or presence of different TLR ligands. After 7 days, CD4+ T cell proliferation was measured by CFSE-dilution. In some experiments, anti-IL-23p19, anti-IL-6 and anti-IL-6R, anti-IL-1β antibodies or control IgG (10 μg/ml) were added into the co-cultures of DCs and CD4+ T cells. In some experiments, CD4+ T cells were incubated overnight with 50 units/ml IL-2 (Hoffmann-LaRoche, NJ), 50 units/ml IL-7 (R&D Systems, MN), 50 ng/ml IL-15 (Peprotech, NY) and 50 ng/ml IL-21 (Invitrogen, NY) and then naïve CD4+ T cells were FACS-sorted to obtain IL-1R1− and IL-1R1+ cells. For assessing antigen-specific CD4+ T cell responses, T cells were restimulated with 0.5-1 μM indicated peptides for 6h in the presence of Brefeldin A, and stained with 7-AAD, anti-CD4, anti-IFNγ and anti-IL-17 antibodies labeled with fluorescent dyes. CD4+ T cells expressing intracellular IFNγ and IL-17 were detected by flow cytometry (FACS Canto, BD). In separate experiments, CD4+ T cells were restimulated with indicated peptides for 48h, and cytokines in the supernatants were assessed by the BeadLyte cytokine assay kit (Upstate, MA) as per the manufacturer’s protocol.
Conventional and quantitative Real-time RT-PCRs
Total RNA was isolated from CD4+ T cells using Ambion’s RNAqueous kits (Life Technologies) and cDNA was synthesized with Reverse Transcription System (Promega, CA). Conventional RT-PCR was performed for TBX21, RORC, GATA3, IL-1R1, IL-1B and ACTB (β-actin) using the primers, TBX21 (forward, 5′-GAGGGGCGGGTCCTCGACGG-3′; reverse, 5′-TCGCGGCGGGTAGGCGTAGG-3′), GATA3 (forward, 5′-AACTGTGGGGCAACCTCGAC-3′; reverse, 5′-TTGCAGACAGGGTCCCCATT-3′), RORC (forward, 5′-TCTGGAGCTGGCCTTTCATCATCA-3′; reverse, 5′-TCTGCTCACTTCCAAAGAGCTGGT-3′), IL-1R1 (forward, 5′-CCACAGCCCAAGGGCGGGGCTA-3′; reverse, 5′-CTGCAGCACCTCTCAGGAGAGCCGC-3′), IL1B (forward, 5′-GCAAGGGCTTCAGGCAGGCCG-3′; reverse, 5′-GCTGTGAGTCCCGGAGCGTGC-3′) and ACTB (forward, 5′-CTCGCCTTTGCCGATCCGCCGC-3′; reverse, 5′-GCTCTGGGCCTCGTCGCCCACAT-3′). IL1B, RORC, IL-1R1 and ACTB were also quantified through real-time RT-PCR using the Lightcycler 480 machine (Roche Applied Bioscience, NJ) using SYBR Green master mix (Roche). Expression levels of individual molecules were normalized to the amount of ACTB mRNA.
Statistical Analysis
Statistical significance was determined using the Student’s t-test and significance was set at p<0.05.
Results
Generation and characterization of recombinant protein of HA1 fused to anti-hDectin-1 mAb
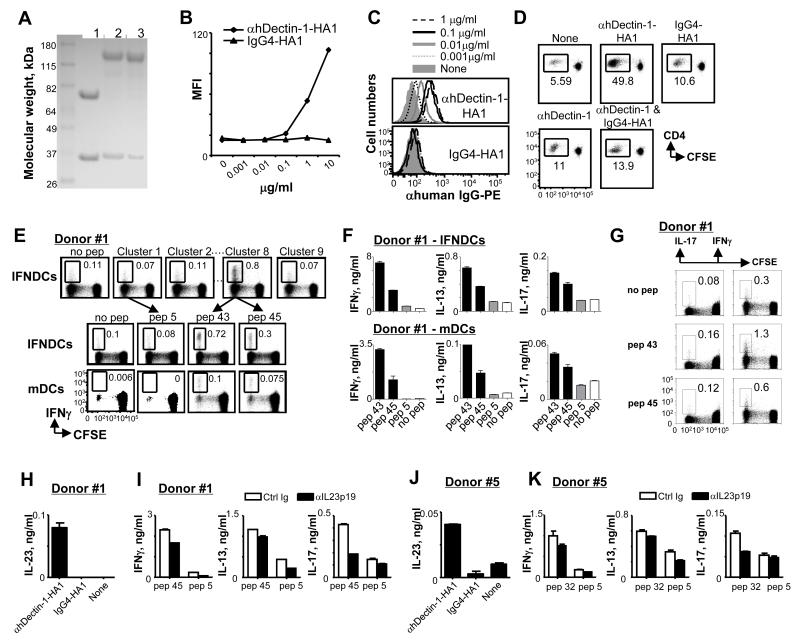
To deliver HA1 to DCs via hDectin-1, a recombinant fusion protein (anti-hDectin-1-HA1) of an agonistic anti-hDectin-1 (34) and HA1 was made on a mouse variable region-human IgG4κ chimera with two site mutations (S228P and L235E) that further diminishes antibody binding to the Fc receptor (36). IgG4-HA1 with the same mutations was made as a control. The putative structure of the recombinant fusion protein is presented in Supplemental Fig. 1. Recombinant proteins were expressed from stable CHO cell lines, purified by Protein A chromatography, and confirmed by the reduced SDS-gel analysis (Fig. 1A). Anti-hDectin-1-HA1, but not IgG4-HA1, specifically bound to 293F cells transfected with hDectin-1 (Fig. 1B). Neither anti-hDectin-1-HA1 nor IgG4-HA1 bound to mock transfectants (data not shown). Anti-hDectin-1-HA1 (upper panel in Fig. 1C) but not IgG4-HA1 (lower panel in Fig. 1C) also bound to the surface of monocyte-derived IFNDCs in a dose-dependent manner. Taken together, HA1 could be efficiently delivered to DCs via hDectin-1 using anti-hDectin-1-HA1 fusion proteins.
FIGURE 1.
DCs loaded with anti-hDectin-1-HA1 can elicit HA1-specific Th1, Th2, and Th17 responses. (A) Reduced SDS-PAGE analysis of anti-hDectin-1 mAb (lane 1), anti-hDectin-1-HA1 (lane 2) and IgG4-HA1 (lane 3). (B and C) Binding of anti-hDectin-1-HA1 and IgG4-HA1 to 293F cells transfected with hDectin-1 (B) and IFNDCs (C). (D) Total CD4+ T cell proliferation induced by IFNDCs alone or IFNDCs loaded with 1 μg/ml anti-hDectin-1-HA1, IgG4-HA1, anti-hDectin-1, or combination of IgG4-HA1 (1 μg/ml) and anti-hDectin-1 (1 μg/ml). (E) CFSE-labeled total CD4+ T cells were co-cultured for 7 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs or blood mDCs. T cells were restimulated with 1 μM of the clusters (upper panels, IFNDCs) or individual peptides from cluster 8 (middle panels, IFNDCs and lower panels, mDCs). (F) CD4+ T cells were restimulated for 48h in the presence or absence of 1 μM peptides indicated. Cytokines in the supernatants were assessed. In (B-F), three independent experiments showed similar results. (G) CFSE-labeled total CD4+ T cells were co-cultured for 7 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs. T cells were restimulated with 1 μM of the indicated peptides and IL-17 and IFNγ expression were analyzed by flow cytometry. Two independent experiments using cells from donors #1 and #2 showed similar results. One representative data generated with donor #1 are presented. (H) 1×105 IFNDCs were incubated overnight with 1 μg/ml HA1 fusion proteins. The amount of IL-23 in the supernatants was assessed. (I) Total CD4+ T cells were co-cultured with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs for 7 days in the presence of 5 μg/ml anti-IL-23p19 or control antibodies. CD4+ T cells were then restimulated with 1 μM peptides for 48h. Cytokines in culture supernatants were assessed. (J and K) Experiments in (H and I) were performed with blood mDCs. In (H-K), Error bars indicate mean±SD of triplicate assay.
DCs loaded with anti-hDectin-1-HA1 can elicit HA1-specific Th17 responses
Consistent with their bindings to DCs (Fig. 1C), anti-hDectin-1-HA1-loaded IFNDCs induced greater proliferation of CD4+ T cells than did IgG4-HA1-loaded IFNDCs (Fig. 1D, upper panel). Anti-hDectin-1 mAb alone or combination of anti-hDectin-1 and IgG4-HA1 induced slightly enhanced CD4+ T cell proliferation compared to DCs alone (Fig. 1D, lower panel). To test antigen specificity of the proliferating CD4+ T cells, T cells were restimulated with clusters of HA1 peptides (6 peptides in each cluster; 17-mers overlapping by 11 amino acids) (Fig. 1E, upper panels). Cluster 8 showed the greatest percentage of IFNγ+CD4+ T cells. Individual peptides in cluster 8 were further tested in separate experiments (Fig. 1E, middle panels), and pep 43 and pep 45 resulted in substantially increased percentage of IFNγ+CD4+ T cells. Pep 5 from cluster 1 and no peptide were negative controls. Experiments performed with blood myeloid DCs (mDCs) showed similar results (Fig. 1E, lower panels), although IFNDCs were more efficient than mDCs at eliciting CD4+ T cell responses. IFNDCs also resulted in a greater by-stander proliferation of CD4+ T cells than did mDCs. We (35) and others (37) previously showed that DCs induced by-stander T cell proliferation, as shown in Fig. 1D, and this by-stander proliferation is further enhanced when the antigen-specific T cells are activated (35, 37).
Total CD4+ T cells co-cultured with anti-hDectin-1-HA1-loaded IFNDCs (Fig. 1F, upper panels) or mDCs (Fig. 1F, lower panels) were restimulated with pep 43, pep 45, control pep 5 or no peptide for 48h and the amounts of IFNγ, IL-13, and IL-17 in the supernatants were assessed. They secreted increased amount of IFNγ and IL-13 in response to pep 43 and pep 45. In addition, they also secreted increased amount of IL-17 in response to both pep 43 and pep 45 compared to control pep 5 or no peptide. Although the level of IL-17 was lower than those of IFNγ and IL-13, our data suggested the presence of HA1-specific Th17 in the cultures. In separate experiments using cells from the same donor (donor #1), we were also able to detect HA1-specific IL-17-producing CD4+ T cells (Fig. 1G, left panel), although the frequency of IL-17+CD4+ T cells were far less than that of IFNγ+CD4+ T cells (Fig. 1G, right panel) that are specific for HA1.
IL-23 secreted from IFNDCs (Fig. 1H) and mDCs (Fig. 1J) contributed to HA1-specific Th17 and Th1 responses (Fig. 1I, 1K), as blocking IL-23p19 during the co-cultures of DCs and CD4+ T cells resulted in decreased IL-17- and slightly decreased IFNγ-producing CD4+ T cell responses. DCs loaded with anti-hDectin-1 alone did not result in HA1-specific CD4+ T cell responses (data not shown).
Taken together, we concluded that anti-hDectin-1-HA1-loaded DCs can efficiently activate HA1-specific IFNγ-, IL-13-, and IL-17-producing CD4+ T cells. HA1-derived peptides used throughout this study were characterized by performing the experiments in Fig. 1E and their corresponding HLA class II types are summarized in Table 1.
Table 1.
MHC class II types of healthy subjects and peptide binding scores of individual peptides to corresponding MHC class II
| Donors | HLA types | Peptides | Amino acid residues |
Peptide sequence | ARB score (IC50 values) |
|---|---|---|---|---|---|
| #1 (Fig. 1E, 1F, 1G, 1H; Fig. 2A, 2B; Fig. 3A, 3B; Fig. 7B, 7C, 7D) |
HLA-DRB1*01 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 10.7 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 9.6 | ||
| HLA-DQB1*05:01 | NA | NA | |||
| #2 (Fig. 2B, 2D; Fig.4A 4C, 4D; Fig. 6A, 6B, 6C; Fig. 7A, 7B, 7C) |
HLA-DRB1*13:02 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 0 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 10004 | ||
| HLA-DRB1*15:01 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 41.6 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 54.6 | ||
| HLA-DRB3*03:01 | NA | NA | |||
| HLA-DQB1*06:04 | NA | NA | |||
| #3 (Fig. 2B, 2C, 2D; Fig, 4D, 4E; Fig. 7C) |
HLA-DRB1*01 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 826 |
| pep54 | 316-332 | IDECPKYVRSAKLRMVT | 6.7 | ||
| HLA-DRB1*13 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 25784.9 | |
| pep54 | 316-332 | IDECPKYVRSAKLRMVT | 1354 | ||
| HLA-DQB1*03:01 | NA | NA | |||
| HLA-DQB1*05 | NA | NA | |||
| #4 (Fig. 2B; Fig. 4D Fig. 7B) |
HLA-DRB1*04:01 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 346.8 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 10.4 | ||
| HLA-DRB1*09 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 378.9 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 6.1 | ||
| HLA-DQB1*03:01 | NA | NA | |||
| HLA-DQB1*03:03 | NA | NA | |||
| #5 (Fig. 1I, 1J; Fig. 2D; Fig. 4E) |
HLA-DRB1*04:02 | NA | NA | ||
| HLA-DRB1*13 | pep32 | 186-202 | EKEVLVLWGVHHPPNIG | 351.1 | |
| HLA-DQB1*06:04 | NA | NA | |||
| HLA-DQB1*03:02 | NA | NA | |||
| #6 (Fig. 7B) |
HLA-DRB1*07 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 103.4 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 1.6 | ||
| HLA-DRB1*08 | pep 43 | 250-266 | LEPGDTIIFEANGNLIA | 79.5 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 596.9 | ||
| HLA-DQB1*02 | NA | NA | |||
| HLA-DQB1*04:02 | NA | NA | |||
| #7 (Fig. 7B) |
HLA-DRB1*01 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 826 |
| pep 54 | 316-332 | IDECPKYVRSAKLRMVT | 6.7 | ||
| HLA-DRB1*11 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 50.6 | |
| pep 54 | 316-332 | IDECPKYVRSAKLRMVT | 6.3 | ||
| HLA-DQB1*03 | NA | NA | |||
| HLA-DQB1*05 | NA | NA | |||
| #8 (Fig. 7C) |
HLA-DRB1*03 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 16976.1 |
| pep 54 | 316-332 | IDECPKYVRSAKLRMVT | 726.5 | ||
| HLA-DRB1*11 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 50.6 | |
| pep 54 | 316-332 | IDECPKYVRSAKLRMVT | 6.3 | ||
| HLA-DQB1*02 | NA | NA | |||
| HLA-DQB1*06 | NA | NA | |||
| # 9 (Fig. 7C) |
HLA-DRB1*03 | pep 7 | 37-53 | LEKNVTVTHSVNLLEDS | 478.4 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 53285.8 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 61616.3 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 7485.1 | ||
| HLA-DRB1*07 | pep 7 | 37-53 | LEKNVTVTHSVNLLEDS | 13.6 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 1.6 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 1.6 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 13.6 | ||
| HLA-DQB1*02 | NA | NA | |||
| # 10 (Fig. 7C) |
HLA-DRB1*07 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 2641.5 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 1.6 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 1.6 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 13.6 | ||
| HLA-DRB1*11 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 50.6 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 104.5 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 104.5 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 2981.4 | ||
| HLA-DQB1*02 | NA | NA | |||
| HLA-DQB1*03 | NA | NA | |||
| # 11 (Fig. 7D) |
HLA-DRB1*03 | pep 7 | 37-53 | LEKNVTVTHSVNLLEDS | 478.4 |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 53285.8 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 61616.3 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 7485.1 | ||
| HLA-DRB1*11 | pep 22 | 126-142 | SSFERFEIFPKESSWPN | 50.6 | |
| pep 45 | 262-278 | GNLIAPWYAFALSRGFG | 104.5 | ||
| pep 46 | 268-284 | WYAFALSRGFGSGIITS | 104.5 | ||
| pep 52 | 304-320 | SSLPFQNVHPVTIGECP | 2981.4 | ||
| HLA-DQB1*02 | NA | NA | |||
| HLA-DQB1*03 | NA | NA | |||
Peptides were predicted by the algorithm in the web: (http://tools.immuneepitope.org/analyze/cgi-bin/mhc_II_binding.py).
NA: alleles are not available in the algorithm.
Anti-hDectin-1-HA1-loaded DCs expand memory Th17 but cannot efficiently induce HA1-specific Th17
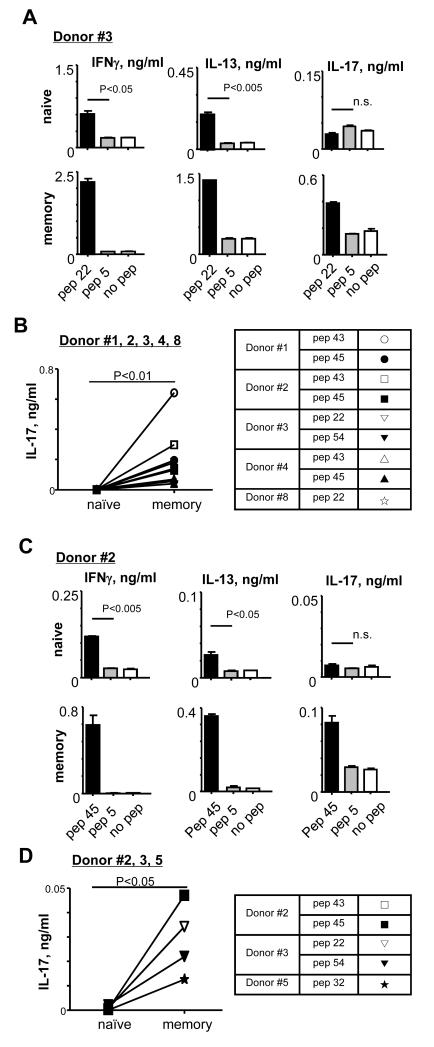
To test whether anti-hDectin-1-HA1-loaded DCs could induce HA1-specific Th17, FACS-sorted naïve and memory CD4+ T cells were co-cultured with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs for 7 days. CD4+ T cells were then restimulated with indicated peptides for 48h and the amounts of IFNγ, IL-13, and IL-17 in the supernatants were assessed. Compared to unstimulated or control pep 5-stimulated CD4+ T cells, both naïve (Fig. 2A, upper left and middle) and memory CD4+ T cells (lower left and middle) stimulated with pep 22 secreted significantly increased amounts of both IFNγ and IL-13. Interestingly, however, increased amount of IL-17 by pep 22 was observed only in the culture supernatant of memory, but not naïve CD4+ T cells. Data from experiments using cells from five healthy donors further demonstrated that IFNDCs loaded with anti-hDectin-1-HA1 could efficiently activate HA1-specific memory Th17 cells, but they could not efficiently prime HA1-specific Th17 responses (Fig. 2B). Similar observations were made from experiments using blood mDCs (Fig. 2C, 2D). Naïve and memory CD4+ T cells were co-cultured with 1 μg/ml anti-hDectin-1-HA1-loaded mDCs for 7 days. CD4+ T cells were then restimulated for 48h with indicated peptides. Pep 45-stimulated naïve T cells secreted increased amounts of both IFNγ (Fig. 2C, upper left panel) and IL-13 (Fig. 2C, upper middle panel), but not IL-17 (Fig. 2C, upper right panel), compared to naïve T cells stimulated with control pep 5 or no peptide. Pep 45-stimulated memory T cells secreted increased amounts of the all three cytokines, including IL-17, compared to memory T cells stimulated with control pep 5 or no peptide (Fig. 2C, lower panel). Data from experiments using cells from 3 healthy donors (Fig. 2D) further showed that anti-hDectin-1-HA1-loaded mDCs efficiently activate HA1-specific memory Th17, but they are not efficient to induce naïve CD4+ T cell differentiation into HA1-specific Th17. Thus, we concluded that DCs loaded with anti-hDectin-1-HA1 can efficiently activate HA1-specific memory Th17, but cannot efficiently prime HA1-specific Th17 responses in vitro.
FIGURE 2.
DCs loaded with anti-hDectin-1-HA1 activate memory Th17, but cannot efficiently induce HA1-specific Th17. (A and C) FACS-sorted naïve and memory CD4+ T cells were co-cultured for 7 days with anti-hDectin-1-HA1-loaded IFNDCs (A) or blood mDCs (C). T cells were restimulated for 48h in the presence or absence of indicated peptides (1 μM). Pep 5 was used as a control peptide. Cytokines in the supernatants were assessed. Error bars indicate mean±SD of triplicate assay. (B and D) Summarized data generated with IFNDCs (B) and blood mDCs (D) from healthy donors. Background values acquired with pep 5 were subtracted. P values were acquired by the Student’s t-test. Indications of donors and peptides tested in (B and D) are summarized in the table.
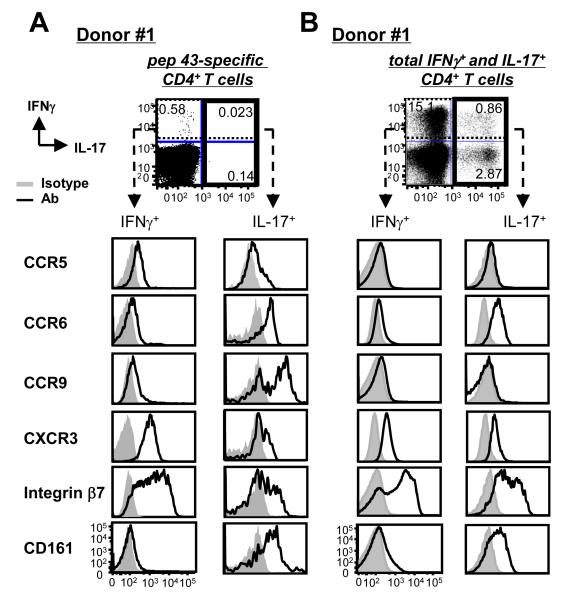
Phenotypes of HA1-specific Th17 are different from those of other Th17
HA1 peptide-specific IFNγ+CD4+ and IL-17+CD4+ T cells were further characterized by assessing the expression levels of chemokine receptors, β7 integrin, and CD161 (Fig. 3A). Compared to IFNγ+CD4+ T cells (Fig. 3A, left panel) expressing CXCR3, IL-17+CD4+ T cells (Fig. 3A, right panel) expressed increased levels of CCR6 and CD161 (38, 39). Interestingly, fractions of the HA1-specific IL-17+CD4+ T cells also expressed a high level of CCR9, which could support the presence of Th17 in the gut mucosa (40). Fractions of IFNγ+CD4+ and IL-17+CD4+ T cells expressed β7 integrin. CCR5 was similarly expressed on both IFNγ+CD4+ and IL-17+CD4+ T cells.
FIGURE 3.
Phenotypes of HA1-specific and PMA/ionomycin-activated Th17. Total CD4+ T cells were co-cultured for 7 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs. T cells were restimulated with 1 μM pep 43 (A) or PMA/ionomycin (B) for 6h. Cells were stained for intracellular IFNγ and IL-17 as well as surface receptors indicated. Four independent experiments using cells from two different healthy donors showed similar results.
We also assessed the expression levels of these receptors on IFNγ+CD4+ and IL-17+CD4+ T cells restimulated with PMA and ionomycin (Fig. 3B). Similar to the HA1-specific IFNγ+CD4+ T cells (Fig. 3A, left panel), PMA/ionomycin-induced IFNγ+CD4+T cells (Fig. 3B, left panel) expressed CCR6, CXCR3, β7-integrin, and CD161. Both pep 43- (Fig. 3A, right panel) and PMA/ionomycin-induced IL-17+CD4+ T cells (Fig. 3B, right panel) also expressed similar levels of these receptors. However, PMA/ionomycin-induced IL-17+CD4+ T cells did not express CCR9 that was expressed on pep 43-induced IL-17+CD4+ T cells. Pep 43-induced IFNγ+CD4+ and IL-17+CD4+ T cells also expressed increased levels of CCR5 compared to those induced with PMA/ionomycin.
In conclusion, these data demonstrated that phenotypes of HA1-specific human Th17 cells are not the same to those of other Th17 cells activated with polyclonal activators.
IL-1β and IL-6 are insufficient to induce naïve CD4+ T cell differentiation into HA1-specific Th17
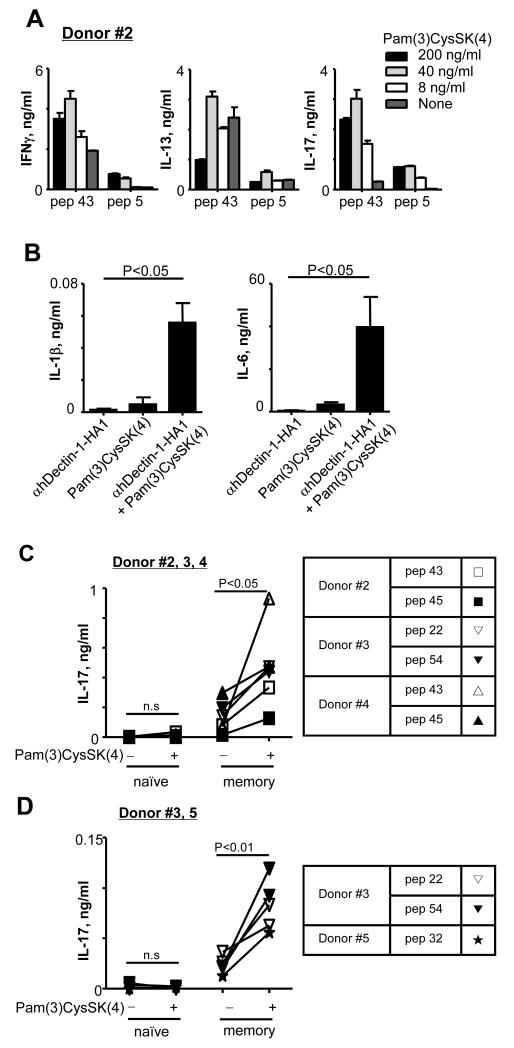
Both IL-1β with IL-6 have been known to contribute to the induction of human Th17 differentiation (9, 27). We have shown that anti-hDectin-1 mAb and TLR2 ligand synergistically act on DCs to secrete IL-6 and IL-1β (34). Synergistic actions of signals via Dectin-1 and TLR2 can also increase IL-23, but decrease IL-12 secretion from DCs (41), thereby favoring Th17 responses (9). Thus, we first tested whether DCs loaded with anti-hDectin-1-HA1 plus TLR2 ligand could enhance HA1-specific Th17 responses (Fig. 4A). Total CD4+ T cells were co-cultured for 7 days with IFNDCs loaded with anti-hDectin-1-HA1 in the presence or absence of different concentrations of Pam(3)CysSK(4). CD4+ T cells were then restimulated for 48h with the indicated peptides and cytokines in the supernatants were assessed. HA1-specific Th17 (Fig. 4A, right panel) and Th1 responses (Fig. 4A, left panel) were enhanced by Pam(3)CysSK(4). Of the concentrations tested, 40 ng/ml Pam(3)CysSK(4) resulted in the highest levels of IL-17 production. Accordingly, DCs loaded with 1 μg/ml anti-hDectin-1-HA1 plus 40 ng/ml Pam(3)CysSK(4) secreted increased amounts of both IL-1β (Fig.4B, left panel) and IL-6 (Fig. 4B, right panel).
FIGURE 4.
IL-1β and IL-6 can promote HA1-specific memory Th17 responses but fail to induce naïve CD4+ T cell differentiation into HA1-specific Th17. (A) Total CD4+ T cells were cultured for 7 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs in the presence of different concentrations of Pam(3)CysSK(4). T cells were then restimulated for 48h with 1 μM pep 43 or pep 5 (control peptide). Cytokines in the supernatants were assessed. Error bars indicate mean±SD of triplicate assay. Two independent experiments showed similar results. (B) IL-1β and IL-6 levels in the culture supernatants of 1×105 IFNDCs incubated with 1 μg/ml anti-hDectin-1-HA1, 40 ng/ml Pam3CysSK4, or 1 μg/ml anti-hDectin-1-HA1 plus 40 ng/ml Pam(3)CysSK(4). Data are pooled from three independent experiments. (C and D) FACS-sorted naïve and memory CD4+ T cells were co-cultured for 7 days with IFNDCs (C) and blood mDCs (D) loaded with 1 μg/ml anti-hDectin-1-HA1 in the presence or absence of 40 ng/ml Pam(3)CysSK(4). T cells were restimulated for 48h with 1 μM peptides. The amount of IL-17 in the supernatants was assessed. In (C) and (D), background values generated with pep 5 were subtracted. P values were acquired by the Student’s t-test. Indications of donors and peptides tested in (C and D) are summarized in the table.
We next tested whether DCs loaded with anti-hDectin-1-HA1 plus 40 ng/ml Pam(3)CysSK(4) could induce HA1-specific Th17 (Fig. 4C, 4D). FACS-sorted naïve (CD45RA+CD45RO−CCR7+) and memory CD4+ T cells (CD45RA−CD45RO+) were co-cultured with either IFNDCs or blood mDCs loaded with anti-Dectin-1-HA1 in the absence or presence of 40 ng/ml Pam(3)CysSK(4). Both IFNDCs (Fig. 4C) and mDCs (Fig. 4D) were able to activate HA1-specific memory Th17, and these Th17 responses were further enhanced by Pam(3)CysSK(4). However, neither IFNDCs nor blood mDCs induced HA1-specific Th17 even in the presence of Pam(3)CysSK(4). Thus, the HA1-specific Th17 responses observed in Fig. 4A were due to the activation of HA1-specific memory CD4+ T cells. TLR2 is known to be expressed in human T cells (42), but Pam(3)CysSK(4) alone did not induce CD4+ T cells to secrete significant amount of cytokines tested, although it slightly increased the amount of TNFα secreted by anti-CD3/CD28-activated CD4+ T cells (data not shown). DCs washed after Pam(3)CysSK(4) treatment were still able to enhance anti-hDectin-1-HA1-loaded DC-mediated HA1-specific Th17 responses (data not shown). Therefore, the enhanced HA1-specific memory Th17 responses by the combination of anti-hDectin-1-HA1 and Pam(3)CysSK(4) were due to the activation of DCs via Dectin-1 and TLR2.
Taken together, we concluded that DCs loaded with anti-hDectin-1-HA1 and TLR2 ligand could greatly promote HA1-specific memory Th17 responses. However, they were not efficient to induce naïve CD4+ T cell differentiation into HA1-specific Th17 even in the presence of IL-1β and IL-6. These results suggested that naïve CD4+ T cells require additional signals to differentiate into HA1-specific Th17.
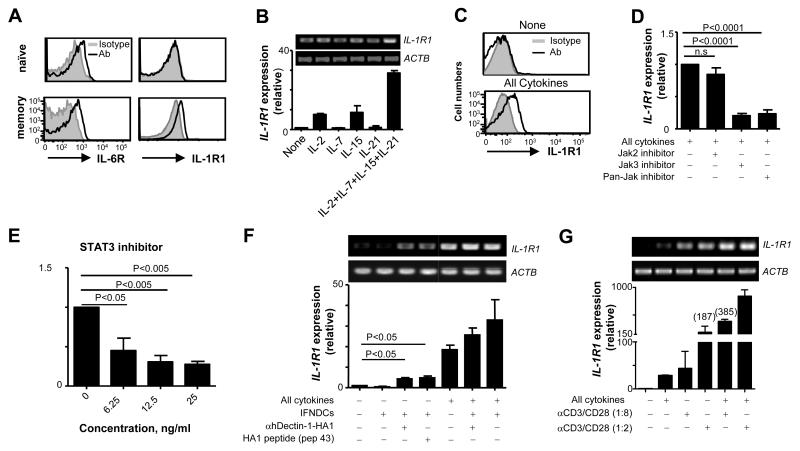
Naïve CD4+ T cells express IL-6R, but not IL-1R1 that is inducible by synergistic actions of signals via common γ-chain receptors and TCRs
Despite IL-1β and IL-6 secretion by DCs after stimulation with anti-hDectin-1-HA1 and TLR2 ligand, there was no significant enhancement of the induction of HA1-specific Th17 (Fig. 4C, 4D). We therefore investigated the expression levels of cytokine receptors on the surface of naïve and memory CD4+ T cells in peripheral blood of healthy donors. Both naïve and memory CD4+ T cells expressed similar levels of surface IL-6R (Fig. 5A, left panel). However, surface IL-1R1 was detected only on memory CD4+ T cells (Fig. 5A, right panel). The presence of IL-6 in the culture medium (Fig. 4B) and the constitutive expression of IL-6R on naïve CD4+ T cell (Fig. 5A, top left panel), but no significant level of HA1-specific Th17 response (Fig. 4C, 4D) suggested that IL-6 is not the key factor that turns on the program for the differentiation of naïve CD4+ T cells into HA1-derived peptide-specific Th17. Thus, we hypothesized that the induction of surface IL-1R1 expression on naïve CD4+ T cells could be a prerequisite for the enhanced de novo induction of antigen-specific Th17.
FIGURE 5.
Naïve CD4+ T cells do not express IL-1R1 that is inducible by synergistic actions of signals from TCRs and common-γ chain receptors. (A) IL-1R1 and IL-6R expression on naïve and memory CD4+ T cells in the peripheral blood. CD4+ T cells from 6 healthy donors showed similar results. (B) Real-time (lower panel) and conventional (upper panel) RT-PCR analysis of IL-1R1 expression in naïve CD4+ T cells treated for 18h with indicated cytokines. (C) Surface IL-1R1 expression on naïve CD4+ T cells treated for 36h with the combination of the cytokines in (B). (D and E) Real-time RT-PCR analysis of IL-1R1 expression in naïve CD4+ T cells treated with the combination of the cytokines in (B) in the presence or absence of indicated inhibitors. Data are pooled from three independent experiments. P values were acquired by the Student’s t-test. (F and G) Real-time (lower panel) and conventional (upper panel) RT-PCR analysis of IL-1R1 expression in naïve CD4+ T cells co-cultured overnight with IFNDCs alone, IFNDCs loaded with HA1 peptide (Pep 43, 1 μM), or IFNDCs loaded with 1 μg/ml anti-hDectin-1-HA1 in the presence or absence of the combination of common γ-chain cytokines (F) or different amounts of anti-CD3/CD28-coated microbeads (G). (F) Error bars indicate mean±SD of duplicate assay of two independent experiments. In (B), (C), and (G), Error bars indicate mean±SD of triplicate assay and three independent experiments showed similar results. P values were acquired by the Student’s t-test.
Induction of IL-1R1 expression on the surface of naïve CD4+ T cells is known to be very modest and takes 5-6 days after TCR stimulation in the presence of IL-2 (43). Naïve CD4+ T cells from cord blood started to express IL-1R1 on 6 days of culture in the presence of TGFβ, IL-7 and IL-15 (44). Therefore, we first sought cytokine signaling that could rapidly induce IL-1R1 expression on the surface of naïve CD4+ T cells. Fig. 5B showed that naïve CD4+ T cells from the peripheral blood of healthy donors expressed IL-1R1, although it was not detected on the cell surface (Fig. 5A). IL-1R1 expression was upregulated within 18h by treating naïve CD4+ T cells with the combination of IL-2, IL-7, IL-15, and IL-21 (Fig. 5B). In contrast to the previously published data (44), naïve CD4+ T cells treated with the combination of common γ-chain cytokines also expressed surface IL-1R1 within 36h (Fig. 5C) and this was consistent with the mRNA expression levels (Fig. 5B). The individual cytokines showed variable effects on IL-1R1 expression (Fig. 5B), but they were not able to induce surface IL-1R1 expression on naïve CD4+ T cells (data not shown). This was further confirmed by the data showing that Janus kinase 3 (Jak 3) and pan-Jak inhibitors but not Jak 2 inhibitor suppressed the common-γ chain cytokine-induced IL-1R1 upregulation (Fig. 5D). Accordingly, STAT3 inhibitor resulted in decreased expression of IL-1R1 (Fig. 5E). These data indicated that IL-2, IL-7, IL-15 and IL-21 act synergistically to induce surface IL-1R1 expression on naïve CD4+ T cells.
TCR ligation by peptide/MHC complexes is an inevitable process for the induction of antigenic peptide-specific T cell responses. Thus, we tested whether TCR signaling contributed to the IL-1R1 expression in naïve CD4+ T cells (Fig. 5F, 5G). FACS-sorted naïve CD4+ T cells were co-cultured for 18h with IFNDCs alone, anti-hDectin-1-HA1-loaded IFNDCs, and HA1 peptide-loaded IFNDCs in the presence or absence of the combination of common γ-chain cytokines (Fig. 5F). DCs loaded with either anti-hDectin-1-HA1 or HA1 peptide slightly, but significantly (p= 0.0107 and p= 0.0239 for anti-Dectin-HA1 and pep43 loaded IFNDCs, respectively) increased the expression of IL-1R1 in naïve CD4+ T cells. However, the combination of common γ-chain cytokines was more efficient than the antigen-loaded DCs at inducing IL-1R1 expression in naïve CD4+ T cells. This was not surprising because the frequency of HA1 antigen-specific CD4+ T cells in the naïve pool is low. In contrast, the combination of common γ-chain cytokines acts on majority of the naïve T cells in the cultures.
To further explore the roles of signaling via TCR in the induction of IL-1R1 expression, naïve CD4+ T cells were cultured with different ratios of anti-CD3/CD28-coated beads. Fig. 5G shows that anti-CD3/CD28 microbeads increased IL-1R1 expression and this increase was anti-CD3/CD28 dose-dependent. Thus, naïve CD4+ T cells treated with the beads at 1:2 ratio expressed higher levels of IL-1R1 than those treated with the beads at 1:8 ratio, demonstrating that IL-1R1 expression is positively correlated to the strength of TCR signaling. Furthermore, anti-CD3/CD28-induced upregulation of IL-1R1 expression was enhanced by the combination of common γ-chain cytokines, showing a synergistic effect of signals from common γ-chain receptors and TCRs on naïve CD4+ T cells to upregulate IL-1R1 expression.
Taken together, naïve CD4 T cells express IL-6R, but not IL-1R1 on their surface. Nevertheless, IL-1R1 expression can be rapidly induced by the synergistic actions of signals via common γ-chain receptors and TCRs.
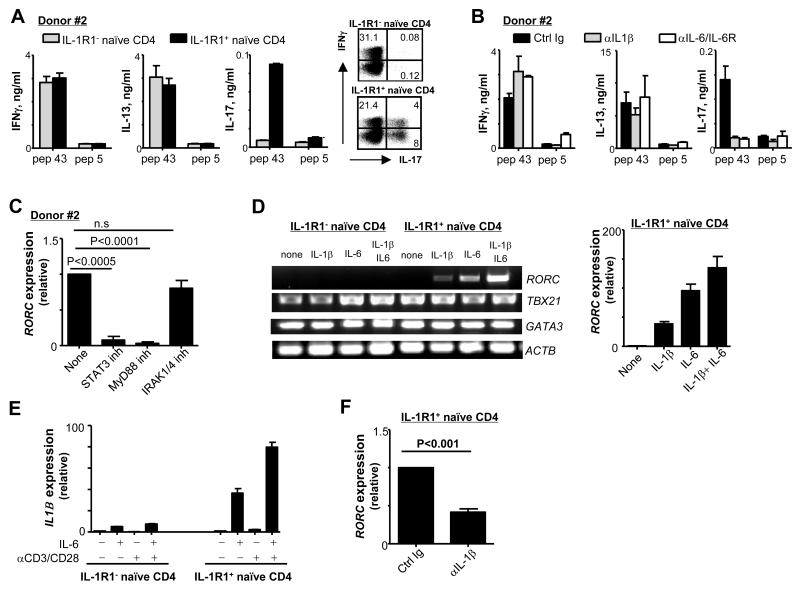
IL-1R1+, but not IL-1R1−, naïve CD4+ T cells differentiate into HA1-specific Th17
To test whether anti-hDectin-1-HA1-loaded DCs were able to induce IL-1R1+ naïve CD4+ T cell differentiation into HA1-specific Th17, IL-1R1− and IL-1R1+ naïve CD4+ T cells were co-cultured with anti-hDectin-1-HA1-loaded IFNDCs. After 7 days, both IL-1R1− and IL-1R1+ naïve CD4+ T cells secreted IFNγ and IL-13 in response to pep 43 during 48h restimulation (Fig. 6A, left and middle panels). HA1-specific Th17 responses were observed only in the cultures of IL-1R1+ naïve CD4+ T cells (Fig. 6A, right panel). We were not able to detect HA1-specific IL-17+CD4+ T cells by FACS. However, we observed that fraction of IL-1R1+ naïve CD4+ T cells co-cultured with DCs expressed intracellular IL-17 in response to PMA/ionomycin (Fig. 6A). Blocking IL-1β during the co-cultures of IL-1R1+ naïve CD4+ T cells and DCs substantially impaired HA1-specific Th17 responses (Fig. 6B, right panel). Thus, the surface IL-1R1 induction on naïve CD4+ T cells by signals via both TCR and common γ-chain receptors is a crucial for the enhanced induction of HA1-specific human Th17.
FIGURE 6.
Anti-hDectin-1-HA1-loaded DCs induce IL-1R1+ naïve CD4+ T cell differentiation into HA1-specific Th17. (A) IL-1R1− and IL-1R1+ naïve CD4+ T cells were co-cultured for 7 days with anti-hDectin-1-HA1-loaded IFNDCs. (left panels) T cells were then restimulated for 48h with 1 μM pep 43 or pep 5. Cytokines in the supernatants were assessed. (right panels) T cells were then restimulated for 6h with PMA and ionomycin. Cells were stained for intracellular IFNγ and IL-17 expression. (B) Experiments in (A) were performed with IL-1R1+ naïve CD4+ T cells in the presence or absence of indicated antibodies (10 μg/ml). T cells were restimulated for 48h with 1 μM pep 43 or pep 5. In (A) and (B), three independent experiments showed similar results. Error bars indicate mean±SD of triplicate assay. (C) Real time RT-PCR analysis of RORC expression in IL-1R1+ naïve CD4+ T cells co-cultured for 5 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs in the absence or presence of indicated inhibitors. (D) IL-1R1− and IL-1R1+ naïve CD4+ T cells were incubated with anti-CD3/CD28-coated microbeads (beads: cells=1:40) in the absence or presence of indicated cytokines for 5 days. mRNA was analyzed by conventional RT-PCR (left panel) and real time RT-PCR (right panel). Two independent experiments showed similar results. Error bars indicate mean±SD of triplicate assay. (E) IL-1R1− and IL-1R1+ naïve CD4+ T cells were incubated for 4h with 50 ng/ml IL-6, anti-CD3/CD28-coated microbeads or combination of IL-6 and anti-CD3/CD28-coated microbeads. IL1B expression was assessed by real time RT-PCR. Error bars indicate mean±SD of triplicate assay. One representative of three experiment is shown. (F) IL-1R1+ naïve CD4+ T cells were incubated with anti-CD3/CD28-coated microbeads in the presence of indicated antibodies for 5 days. mRNA was analyzed by real time RT-PCR. In (C) and (F), Data are pooled from three independent experiments. P values were acquired by the Student’s t-test.
Blocking IL-6 also greatly decreased the induction of HA1-specific Th17 from IL-1R1+ naïve CD4+ T cells (Fig. 6B, right panel). Thus, STAT3 inhibitors, which block IL-6-mediated signals, also decreased RORC expression in the IL-1R1+ naïve CD4+ T cells on day 5 of the co-cultures with anti-hDectin-1-HA1-loaded DCs (Fig. 6C). Fig. 6C also demonstrated that IL-1β-induced RORC expression is dependent on myeloid differentiation primary response gene 88 (MyD88), but not IL-1R-associated kinase 1 (IRAK1) or IRAK4.
Taken together, we concluded that both IL-1β and IL-6 contribute to the induction of HA1-specific Th17. However, surface IL-1R1 expression on naïve CD4+ T cells is essential for the enhanced induction of HA1-specific Th17. Surface IL-1R1− naïve CD4+ T cells did not efficiently differentiate into HA1-specific Th17 (Fig. 2 and Fig. 6A).
IL-6 contributes to the induction of HA1-specific Th17 through IL-1β induction in TCR-activated T cells
IL-6 has been known to contribute to Th17 induction in both mice (19) and humans (9). However, IL-6 can contribute to the induction of HA1-specific Th17 only when naïve CD4+ T cells express IL-1R1 (Fig. 6B), although IL-6R is expressed on the surface of naïve CD4+ T cells (Fig. 5A, upper left panel). To further investigate the roles of IL-6 in the induction of Th17, both IL-1R1− and IL-1R1+ naïve CD4+ T cells were activated with anti-CD3/CD28-coated microbeads at a 1:40 ratio in the presence or absence of IL-1β, IL-6, or both IL-1β and IL-6 (Fig. 6D). Anti-CD3/CD28-coated microbeads at 1:40 did not upregulate IL-1R1 expression in naïve CD4+ T cells (data not shown). After 5 days, the expression levels of RORC, TBX21, and GATA3 in the two groups of naïve CD4+ T cells were assessed. Consistent with the data in Fig. 6A, IL-1R1− naïve CD4+ T cells expressed both TBX21 and GATA3, but not RORC, in any tested condition. The expression levels of TBX21 and GATA3 in IL-1R1− CD4+ T cells treated with either IL-6 alone or combination with IL-1β were variable in different experiments (data not shown). However, IL-1R1+ naïve CD4+ T cells expressed RORC in the presence of either IL-1β or IL-6 alone. IL-1β plus IL-6 induced the greatest level of RORC expression. In line with the data in Fig. 6B (right panel) and 6C, IL-6 alone could also induce RORC expression in IL-1R1+ naïve CD4+ T cells (Fig. 6D, right panel). Indeed, IL-6 was more efficient than IL-1β at inducing RORC expression in IL-1R1+ naïve CD4+ T cells. Therefore, we hypothesized that IL-6 could induce these naïve CD4+ T cells to express IL-1β followed by the induction of RORC expression, and thus could amplify IL-1β-induced RORC expression. As shown in Fig. 6E, exogenous IL-6 was able to induce IL-1R1− and particularly IL-1R1+ naïve CD4+ T cells to upregulate IL1B expression. Furthermore, blocking IL-1β led to decreased IL-6-mediated RORC expression in IL-1R1+ naïve CD4+ T cells (Fig. 6F).
Taken together, IL-1R1+ naïve CD4+ T cells were able to differentiate into HA1-derived peptide-specific Th17 when they were activated via both TCRs and IL-1R1. IL-6 could argument Th17 responses by inducing IL-1β and thus enhanced RORC expression in the TCR-activated IL-1R1+ naïve CD4+ T cells.
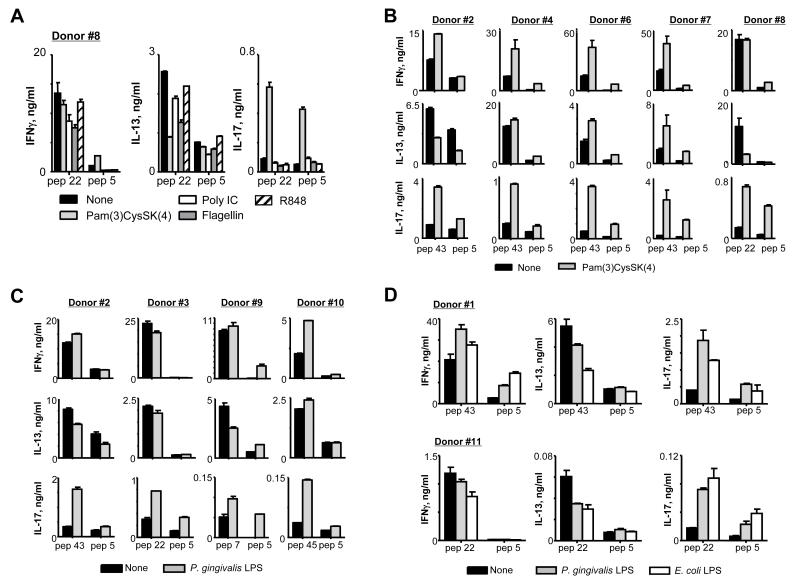
Targeting antigens to DCs via Dectin-1 can elicit antigen-specific human Th17 responses that can be further promoted by TLR2 and TLR4 ligands
Antigen targeting to DCs via Dectin-1 using recombinant fusion protein of an agonistic anti-hDectin-1 mAb and antigen could elicit antigen-specific Th17 responses. IL-23 secreted from anti-hDectin-1-HA1-loaded DCs contributed to HA1-specific Th17 responses (Fig. 1H, 1J). In addition, anti-hDectin-1-HA1 and TLR2 ligands synergized to activate DCs to secrete IL-1β and IL-6, resulting in the enhanced HA1-specific Th17 responses (Fig. 4A, 4B).
We further tested the effects of other TLR ligands on the magnitude of HA1-specific Th17 responses elicited by DCs loaded with anti-hDectin-1-HA1 (Fig. 7A). Compared to other TLR ligands tested, Pam(3)CysSK(4) was the most efficient at enhancing the HA1-specific Th17 responses, as it synergizes with anti-hDectin-1-HA1 to induce increased amounts of both IL-1β and IL-6 secretion from DCs (Fig. 4B). The enhanced HA1-specific Th17 responses by the combination of anti-hDectin-1-HA1 and TLR2 ligands were further confirmed (Fig. 7B, 7C). Total CD4+ T cells from eight healthy donors were co-cultured for 7 days with DCs loaded with 1 μg/ml anti-hDectin-1-HA1 in the absence or presence of TLR2 ligands, Pam(3)CysSK(4) (Fig. 7B) and P. gingivalis lipopolysaccharide (LPS) (Fig. 7C). Consistent with the data in Fig. 4B and Fig. 7A, both Pam(3)CysSK(4) and P. gingivalis LPS promoted HA1-specific Th17 responses elicited by anti-hDectin-1-HA1-loaded DCs. E. coli LPS was also able to enhance HA1-specific Th17 responses elicited by anti-hDectin-1-HA1-loaded IFNDCs (Fig 7D, upper panels) and mDCs (Fig. 7D, lower panels). We previously reported that anti-hDectin-1 mAb and E. coli LPS synergize to activate DCs to secrete increased amount of both IL-1β and IL-6 (34). Taken together, healthy individuals tested in this study maintained HA1-specific memory Th17, although the magnitudes of Th17 responses in the healthy individuals were variable. In addition, a vaccine model made of an agonistic anti-hDectin-1 and antigens along with TLR2 or TLR4 ligands could offer great potential in promoting Th17-mediated host immunity against infections.
FIGURE 7.
Healthy individuals maintain influenza HA1-specific Th17 that can be promoted by the combination of anti-hDectin-1-HA1 and TLR2 ligands. (A) Total CD4+ T cells were co-cultured for 7 days with IFNDCs loaded with 1 μg/ml anti-hDectin-1-HA1 in the presence or absence of indicated TLR ligands (40 ng/ml Pam(3)CysSK(4), 1μg/ml poly IC, 50 ng/ml flagellin, 500 ng/ml R848). T cells were then restimulated for 48h with 1 μM of indicated peptides. Cytokines in the culture supernatants were assessed. Two independent experiments showed similar results. (B and C) Total CD4+ T cells from healthy donors were co-cultured for 7 days with 1 μg/ml anti-hDectin-1-HA1-loaded IFNDCs in the presence or absence of 40 ng/ml Pam(3)CysSK(4) (B) or P. gingivalis LPS (C). T cells were then restimulated with 1 μM indicated peptides. Cytokines in the culture supernatants were assessed. (D) Total CD4+ T cells were co-cultured for 7 days with IFNDCs (top panels) or mDCs (lower panels) loaded with 1 μg/ml anti-hDectin-1-HA1 in the presence or absence of P. gingivalis LPS (40 ng/ml) or E. Coli LPS (100 ng/ml). T cells were then restimulated for 48h with 1 μM of indicated peptides. Cytokines in the culture supernatants were assessed. Two independent experiments showed similar results. In (A-D), Error bars indicate mean±SD of triplicate assay.
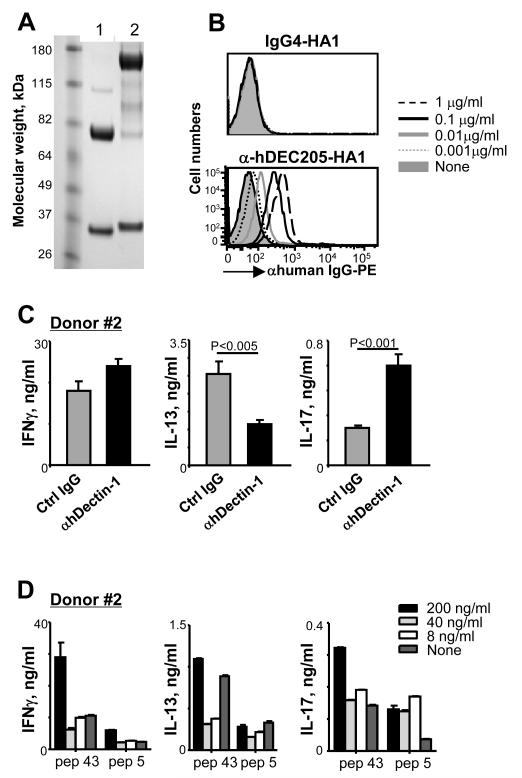
Data in Fig. 8 further support our notion that targeting antigens to DCs via Dectin-1 using recombinant fusion protein of an agonistic anti-hDectin-1 mAb and antigen is an efficient strategy to elicit antigen-specific Th17 responses. Anti-hDEC205-HA1 fusion protein was made (Fig. 8A). Anti-hDEC205-HA1 (Fig. 8B, lower panel) but not IgG4-HA1 (Fig. 8B, upper panel) bound to the surface of monocyte-derived IFNDCs in a dose-dependent manner. IFNDCs loaded with anti-hDEC205-HA1 resulted in HA1-specific Th1, Th2, and Th17 responses (Fig. 8C). More importantly, HA1-specific Th17 responses elicited with anti-DEC205-HA1 were further enhanced by anti-hDectin-1 mAb (Fig. 8C, right panel). Anti-hDectin-1 mAb did not increase HA1-specific IFNγ+CD4+ T cell responses (Fig. 8C, left panel), but significantly decreased IL-13+CD4+ T cell responses (Fig. 8C, middle panel). We next tested whether anti-hDEC205-HA1 and Pam(3)CysSK(4) synergized to promote HA1-specific CD4+ T cell responses (Fig. 8D). Only the higher concentration (200 ng/ml) of Pam(3)CysSK(4) could enhance HA1-specific Th1, Th2, and Th17 responses. These data showed that TLR2 signaling does not synergize with DEC205 to promote HA1-specific Th17 responses.
FIGURE 8.
HA1-specific Th17 responses elicited by anti-hDEC205-HA1 can be enhanced by the activation of DCs via Dectin-1 but not TLR2. (A) Reduced SDS-PAGE analysis of anti-hDEC205 mAb (lane 1) and anti-hDEC205-HA1 (lane 2). (B) Binding of anti-hDEC205-1-HA1 and IgG4-HA1 to IFNDCs. (C) Total CD4+ T cells were co-cultured for 7 days with IFNDCs loaded with 1 μg/ml anti-hDEC205-HA1 in the presence 5 ug/ml control IgG or anti-hDectin-1 mAb. T cells were then restimulated for 48h with 1 μM of pep 43 or control pep 5. Cytokines in the culture supernatants were assessed. Values acquired with control pep5 were subtracted. Two independent experiments showed similar results. (D) Effects of different concentrations of Pam(3)CysSK(4) on HA1-specific CD4+ T cell responses were assessed.
Discussion
This study dissected the pathways for the de novo generation of protein antigen-derived peptide-specific human Th17 in the context of TCR ligation by MHC II/peptide complexes presented by DCs. It appears that the induction of protein antigen-specific Th17 requires more than the actions of previously known Th17-promoting cytokines, but rather occurs through the complementary actions of signals delivered from both innate and adaptive immune cells. Notably, synergistic actions of signals via both TCR and common γ-chain receptors play pivotal roles in programming naïve CD4+ T cells to respond to IL-1β followed by the induction of RORC expression. It was also important to note that phenotypes of Th17 activated with HA1 peptides and those activated with polyclonal activators are not the same. Finally, this study demonstrates that targeting antigens to DCs via Dectin-1 using an agonistic anti-hDectin-1 mAb, particularly with TLR2 ligands, can efficiently promote antigen-specific Th17 where antigen-specific memory T cells have already been established.
It has been known that the immune system can favor Th17 responses to certain microbial pathogens, including fungi and bacteria, and this relies mainly on recognition of such pathogens via PRRs, particularly Dectin-1, followed by the induction of Th17-promoting cytokines from APCs (3, 45). However, Th17 are now considered as important immune arms against both extracellular and intracellular pathogens (2-11, 13, 30) as well as cancers (14-17). The assumption that human Th17 are mainly effector T cells with a short life span, as they are often found in peripheral tissues and organs (46), had raised a question as to the value of such vaccine-induced Th17-mediated immunity. However, recent studies have shown that human Th17 consist of long lived-effector memory cells (47, 48), and thus can contribute to long-lasting immunity. We also found that healthy individuals maintained influenza HA1-specific memory Th17, although the levels of Th17 among donors varied. Such memory Th17 could contribute to the protective immunity against influenza infections, presumably by enhancing CD8+ T cell and antibody responses (6, 13, 16, 49). More importantly, those Th17 responses can be greatly enhanced by the vaccine model, recombinant fusion protein of anti-hDectin-1 and antigens along with TLR2 and TLR4 ligands.
The mechanisms of human Th17 differentiation remained obscure, but previous studies have revealed several key cytokines that promote Th17 differentiation mainly in the context of inflammatory diseases. Data from this study are in agreement with the previous data, showing that IL-1β/IL-1R and IL-6 play key roles in human (9) and mouse Th17 differentiation (19, 20). The major role of IL-23, expanding memory Th17 (1, 50), is recapitulated by our data. We could detect active forms of TGFβ in the co-cultures of DCs and T cells (data not shown). This suggests that TGFβ may not be a key cytokine that determines the generation of antigen-specific human Th17, while the induction of IL-1R1 expression and signals by IL-1β play key roles. It is important to note that experimental systems used in many of the previous studies were designed to test Th17 differentiation mainly in the context of inflammatory diseases. Thus, T cells were activated with polyclonal stimuli in polarized conditions made with exogenous cytokines or neutralizing antibodies specific for targeted cytokines.
We have shown that the induction of antigen-specific Th17 requires more than the actions of currently known Th17-promoting cytokines. Foremost, the induction of surface IL-1R1 expression was the key step for naïve CD4+ T cell differentiation into pathogen-specific Th17. A rapid induction of surface IL-1R1 expression on naïve CD4+ T cells is directed by the synergistic actions of signals via TCRs and common γ-chain receptors.
In support of the important roles of common γ-chain cytokines, inhibitors of STAT3 abolished the upregulation of IL-1R1 expression. Although IL-2 and STAT5 showed suppressive functions in Th17 development (51), our data indicated that these could also contribute to the generation of antigen-specific Th17 by enhancing the expression of IL-1R1. Furthermore, IL-2 supports T cell proliferation. The contribution of STAT5 in the upregulation of IL-1R1 expression could not be measured because STAT5 inhibitor (52) suppressed the phosphorylation of STAT1, 3, and 5, as measured by phospho-flow cytometry (data not shown). IL-21 also uses the common γ-chain receptors and thus can contribute to the generation of pathogen-specific Th17 by promoting IL-1R1 expression. The roles of other common-γ chain cytokines, including IL-9 (23) as well as IL-7 and IL-15 (53) in Th17 responses have also been reported.
Although IL-1β/IL-1R1 was the key axis for the induction of RORC, IL-6-mediated STAT3 activation was required for efficient generation of pathogen-specific human Th17. This was further supported by the facts that mutations in STAT1 or STAT3 resulted in the deficiency of Th17-mediated immunity in patients with fungal infections (12, 54). Interestingly, however, IL-6 promotes the induction Th17 only when naïve CD4+ T cells express IL-1R1. Further experiments show that IL-6 contributes to the IL-1β-induced RORC expression by enhancing IL-1β expression in TCR-activated IL-1R1+ naïve CD4+ T cells. The key role of IL-1β in the induction of RORC expression through the action of MyD88 is supported by the previous studies, showing that MyD88 deficiency results in the lack of Th17-mediated immunity against chlamydial infection (55, 56). Moreover, defective IL-1R/MyD88 signaling is associated with impaired Th17 responses (57).
In conclusion, this study revealed the complex but complementary mechanisms for the induction of protein antigen-specific human Th17 in the context of TCR ligation by MHC/peptide complexes. Although there could be alternative pathways for the generation of antigen-specific human Th17, the pathway characterized with DCs, major immune inducers and modulators, could represent the major one. In addition, this study provides a rational strategy that can potentially enhance Th17-mediated host immunity against infections and certain types of cancers in humans.
Supplementary Material
Acknowledgments
We thank the FACS Core, Sample Core, Cell Processing Core, and Luminex Core at BIIR. We thank Xiao-Hua Li and Amy O’Bar for providing anti-hDectin-1 reagents. We thank Dr. Carson Harrod and Mr. Jerome Ellis for proofreading and editing of this manuscript.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 4.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 27.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 29.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011;14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, Brossart P. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 33.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 34.Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O’Bar A, Clayton S, Palucka AK, Zurawski G, Banchereau J, Oh S. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, O’Garra A, Zurawski G, Banchereau J, Oh S. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy MP, Kinney CA, Chaikin MA, Payne A, Fishman-Lobell J, Tsui P, Dal Monte PR, Doyle ML, Brigham-Burke MR, Anderson D, Reff M, Newman R, Hanna N, Sweet RW, Truneh A. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J Immunol. 2000;164:1925–1933. doi: 10.4049/jimmunol.164.4.1925. [DOI] [PubMed] [Google Scholar]
- 37.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W, Jr., Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercer F, Kozhaya L, Unutmaz D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PLoS ONE. 2010;5:e8639. doi: 10.1371/journal.pone.0008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R, van der Meer JW, Joosten LA, Netea MG. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 46.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O’Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, Terrell S, Klitgaard JL, Santo L, Addorio MR, Ebert BL, Griffin JD, Frank DA. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan Q, Kozhaya L, ElHed A, Ramesh R, Carlson TJ, Djuretic IM, Sundrud MS, Unutmaz D. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J Exp Med. 2011;208:1875–1887. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, Kullberg BJ, van der Meer JW, Lilic D, Veltman JA, Netea MG. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. Mice deficient in MyD88 Develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol. 2010;184:2602–2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- 56.Nagarajan UM, Sikes J, Prantner D, Andrews CW, Jr., Frazer L, Goodwin A, Snowden JN, Darville T. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect Immun. 2011;79:486–498. doi: 10.1128/IAI.00843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenten D, Nish SA, Yu S, Yan X, Lee HK, Brodsky I, Pasman L, Yordy B, Wunderlich FT, Bruning JC, Zhao H, Medzhitov R. Signaling through the adaptor molecule MyD88 in CD4+ T cells is required to overcome suppression by regulatory T cells. Immunity. 2014;40:78–90. doi: 10.1016/j.immuni.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.