Highlights
-
•
We generated a hetero parabiosis model of wild type and adiponectin knockout (KO) mice.
-
•
Adiponectin protein was detected in adipose tissues of the KO parabiotic partner.
-
•
High adiponectin levels were found in stromal vascular fraction of the obese KO partner.
-
•
Obese parabiotic mice exhibited marked hypoadiponectinemia.
Abbreviations: APN, adiponectin; KO, adiponectin deficient mice; HF/HS, high fat/high sucrose diet; KO (WT–KO), KO partner of WT–KO; MAF, mature adipocyte fraction; NC, normal chow diet; SVF, stromal vascular fraction; WATmes, mesenteric white adipose tissue; WATsub, subcutaneous white adipose tissue; WT (WT–KO), WT partner of WT–KO; WT (WT–WT), WT partner of WT–WT; WT, wild type mice; WT–KO, parabiosis between WT and KO; WT–WT, parabiosis between WT and WT
Keywords: Adiponectin, Adipose tissue, Obesity, Parabiosis
Abstract
Adiponectin is exclusively synthesized by adipocytes and exhibits anti-diabetic, anti-atherosclerotic and anti-inflammatory properties. Hypoadiponectinemia is associated in obese individuals with insulin resistance and atherosclerosis. However, the mechanisms responsible for hypoadiponectinemia remain unclear. Here, we investigated adiponectin movement using hetero parabiosis model of wild type (WT) and adiponectin-deficient (KO) mice. WT mice were parabiosed with WT mice (WT–WT) or KO mice (WT–KO) and adiponectin levels were measured serially up to 63 days after surgery. In the WT–KO parabiosis model, circulating adiponectin levels of the WT partners decreased rapidly, on the other hand, those of KO partners increased, and then these reached comparable levels each other at day 7. Circulating adiponectin levels decreased further to the detection limit of assay, and remained low up to day 63. However, adiponectin protein was detected in the adipose tissues of not only the WT partner but also WT–KO mice. In the diet-induced obesity model, high adiponectin protein levels were detected in adipose stromal vascular fraction of diet-induced obese KO partner, without changes in its binding proteins. The use of parabiosis experiments shed light on movement of native adiponectin among different tissues such as the state of hypoadiponectinemia in obesity.
1. Introduction
Obesity, especially visceral fat accumulation, is closely associated with chronic inflammation of the adipose tissue, which results in adipocyte dysfunction and dysregulated production of adipocytokines, leading to systemic metabolic disorders and atherosclerosis [1,2]. The adipocytokine, adiponectin [also known as adipocyte complement-related protein of 30 kDa (ACRP30), adipoQ and gelatin binding protein of 28 kDa (GBP28)], was discovered independently by our group and three other groups of investigators [3–7], and is exclusively synthesized by adipocytes. In experimental studies, adiponectin exhibits anti-diabetic, anti-atherosclerotic, and anti-inflammatory properties [8]. Adiponectin is present at high levels (3–30 μg/mL) in the blood of normal subjects, but the circulating levels are low in obese individuals [9]. Studies from our laboratories indicate that reactive oxygen species (ROS), ischemia and adipose hypoxia result in dysregulated production of adiponectin [10,11]. We also reported that hypoadiponectinemia could occur without apparent change in adiponectin mRNA expression [12,13]. Hence, the mechanism of the catabolism and clearance of adiponectin in obesity remains to be elucidated. Although several in vitro and in vivo studies have investigated the short-term effects of adiponectin using adenovirus-induced or recombinant adiponectin [14–16], the relative long-term effects of adiponectin-knockout (KO) mice remains unclear.
Parabiosis is the surgical union of two animals that leads to development of common circulation and allows the investigation of circulating factors in the regulation of physiologic systems [17]. In mice, the reported rate of blood exchange between parabiotic partners is 2.0 ± 0.2% per min (range, 0.71–3.1%) [17]. In the present study, we analyzed the localization of adiponectin using parabiosis model, chronic recruitment of adiponectin, in KO mice.
2. Materials and methods
2.1. Animal models
Male wild-type (WT) mice with a C57BL/6J background were obtained from Clea Japan (Tokyo, Japan). KO mice were generated and backcrossed, as described previously by our group [18]. Mice were acclimated to the new environment for at least one week before the experiments and kept in rooms set at 22 °C with a 12–12 h dark–light cycle (light cycle, 8 am to 8 pm).
At 12–14 weeks of age, WT mice parabiosed with WT mice (WT–WT) or KO mice (WT–KO). Parabiotic surgery was performed using the protocol described previously [17]. Briefly, after anesthesia, the corresponding lateral aspects of each mouse were shaved, and a longitudinal skin incision from the shoulder to the knee joint was made on one side of each animal. The muscles were removed from the scapulae, and the bones were sutured together. The walls of the peritoneal cavities were incised (5 mm long), sutured together to form a peritoneum tunnel. The muscles around the scapulae and femurs were sutured together. Finally, the skin was sutured to create a single paired-animal. After surgery, the partners were housed one pair per cage, and food [Normal chow (NC) or high fat/high sucrose diet (HF/HS) (F2HFD2, Oriental Yeast, Osaka, Japan)] was provided in dish plate on the floor to ensure easy access.
Before surgery and 4–63 days postoperatively, venous blood samples (50 μL each) were collected from the tail vein at the indicated time periods. Serial plasma adiponectin levels were measured by adiponectin enzyme-linked immunosorbent assay (ELISA) kit (Otsuka Pharmaceutical Co. Tokushima, Japan). Serial plasma glucose and insulin levels were measured by the Glucose CII-Test (Wako Pure Chemical, Osaka, Japan) and Ultra Sensitive Insulin ELISA Kit (Morinaga, Kanagawa, Japan), respectively. At 9 weeks after parabiotic surgery, mice under anesthesia were separated immediately from parabiotic-pair and then blood was collected for plasma assay. Next, mice was injected with 20 mL saline into the cardiac apex followed by collection of the entire blood volume. Furthermore, the subcutaneous (WATsub) and mesenteric white adipose tissues (WATmes) as well as various organs were excised for analysis. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Osaka University School of Medicine. Each experiment was repeated at least three times.
2.2. Isolation of mouse mature adipocyte fractions and stromal vascular fractions
WATsub and WATmes from each mice were fractionated, as described previously in detail by our group [19]. The adipose tissues were minced in Krebs-Ringer bicarbonate HEPES (KRBH) buffer [containing 120 mmol/L NaCl, 4 mmol/L KH2PO4, 1 mmol/L MgSO4, 1 mmol/L CaCl2, 10 mmol/L NaHCO3, 30 mmol/L HEPES, 20 μmol/L adenosine, and 4% (wt/vol) bovine serum albumin (Calbiochem, San Diego, CA)]. Tissue suspensions were centrifuged at 500g for 5 min to remove erythrocytes and free leukocytes. Collagenase was added to a final concentration of 2 mg/mL and incubated at 37 °C for 30 min under continuous shaking. The cell suspension was filtered through a 250 μm filter and then spun at 300g for 1 min to separate floating mature adipocytes fraction (MAF) from the stromal vascular cell fraction (SVF) pellet. The fractionation and washing procedures were repeated twice with KRBH buffer. Finally, both fractions were washed with calcium- and magnesium-free phosphate buffered saline (PBS).
2.3. Western blot analysis
Equal amounts of plasma and protein (8–10 μg) samples extracted from various tissues were incubated with a reducing sample buffer (containing 2% SDS, 50 mM Tris–HCl, pH 6.8, and 10% glycerol with 2-mercaptoethanol), boiled for 5 min and separated by 4–20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Immunoblot analysis was performed with first antibody adiponectin at a 1:2000 dilution (Rabbit polyclonal antibody to mouse adiponectin, Otsuka Pharmaceutical Co., Tokyo), followed by incubation with secondary antibody conjugated with horseradish peroxidase at a 1:2000 dilution (Goat anti-rabbit IgG horseradish peroxidase conjugate, GE Healthcare, Uppsala, Sweden). The ECL prime system (GE Healthcare) was used for detection of the protein signal. The intensity of the blots was quantified with the National Institutes of Health NIH image analysis software (v1.62, Bethesda, MD).
2.4. Quantification of mRNA level
Total RNA was isolated from mouse tissues by using RNA STAT-60 (Tel-Test, Inc., Friendswood, TX) according to the protocol supplied by the manufacturer. The quality and quantity of total RNA were determined by spectrophotometry (model ND-1000, Nano Drop Technologies, Wilmington, DE). First-strand cDNA was synthesized from total RNA using Thermoscript RT (Invitrogen, Carlsbad, CA) and oligodT primer. Real-time quantitative PCR amplification was conducted with the 7900HT system (Applied Biosystems, Forester City, CA) using Thunderbird qPCR mix (Toyobo, Osaka, Japan) according to the protocol recommended by the manufacturer. Table 1 lists the sequences of the primers used in the present study.
Table 1.
Sequences of the primers used in the present study.
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Adiponectin | GATGGCAGAGATGGCACTCC | CTTGCCAGTGCTGCCGTCAT |
| AdipoR1 | ATGGAGAAGATGGAGGAGTTCGT | TCTTGAAGCAAGCCCGAAA |
| AdipoR2 | TCCCAGGAAGATGAAGGGTTTAT | TTCCATTCGTTCGATAGCATGA |
| Calreticulin | TACGCACTGTCCGCCAAAT | GTCCAAACCACTCGGAAACAG |
| T-cadherin | GCCCTCGTGAGCCTTCTTC | CACCCTGAGGTCCGTGATGT |
| 36B4 | GGCCAATAAGGTGCCAGCT | ATCAGCCCGAAGGAGAAG |
2.5. Histological examination
Paraffin samples were dehydrated through an ethanol series, washed three times in xylene, embedded in paraffin and stored at 4 °C. Paraffin samples were sectioned at 8 μm. The tissue sections were processed for routine hematoxylin–eosin staining. Data were collected from five individual views per WAT. The area of adipocytes was traced manually and measured in 200 cells per mouse by using Win ROOF 5.5 software (Mitani Co, Fukui, Japan).
2.6. Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were examined for statistical significance using the Student t-test or ANOVA with the Fisher protected least significant difference test. In all cases, p values <0.05 were considered statistically significant. All statistical analyses were performed with The Statistical Package for Social Sciences (version 11.0, SPSS Inc, Chicago, IL).
3. Results
3.1. Parabiosis between WT and KO mice
To investigate the effects of long-term adiponectin recruitment in adipose tissues, WT mice were parabiosed with WT mice (WT–WT) or KO mice (WT–KO) (Fig. 1A). There was no significant difference in body weight of WT–WT and WT–KO pairs (Fig. 1B). Serial measurements of plasma glucose and insulin levels showed similar levels in WT partners of WT–WT [WT (WT–WT)], WT partners of WT–KO [WT (WT–KO)] and KO partners of WT–KO [KO (WT–KO)] (Fig. 1C). Serial measurements of circulating adiponectin levels by ELISA showed increased levels at day 14 in WT (WT–WT), followed by plateau at 15–18 μg/mL (Fig. 1C). This was in contrast to WT (WT–KO) parabiotic mice, which showed rapid fall in circulating adiponectin levels. On the other hand, the levels in KO (WT–KO) increased initially and then at day 7 reached levels comparable to those of WT (WT–KO) (Fig. 1C). Previous reports showed that cross-circulation was established by day 3 after parabiosis surgery [20,21]. Our results showed that adiponectin migrated from WT mice to KO mice through the bloodstream. Interestingly, since day 7, circulating adiponectin levels decreased markedly below the detection limit of ELISA assay, and remained at those low levels up to day 63 (Fig. 1C).
Fig. 1.
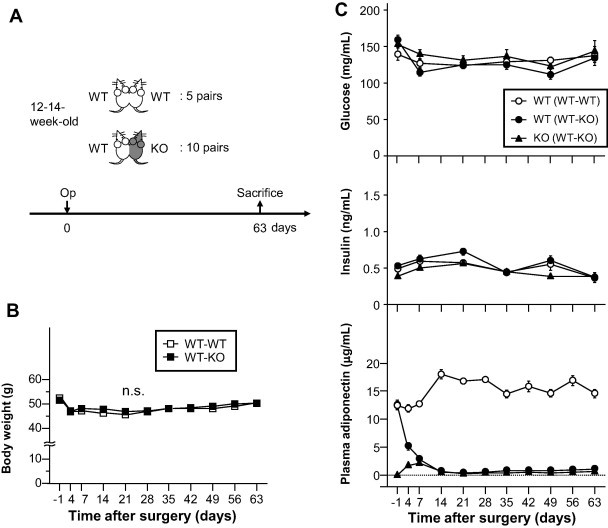
Effect of parabiosis. (A) Experimental protocol. WT mice were parabiosed with WT mice (WT–WT) or adiponectin knockout mice (WT–KO). At 63 days after surgery, the mice were sacrificed and their tissues analyzed. WT; wild type mice, KO; adiponectin knockout mice. (B) Body weight of parabiosed mice. n.s.; not significant. (C) Plasma glucose, insulin and adiponectin levels were measured in partners of parabiosed mice. WT (WT–WT); WT mice of WT–WT pair, WT (WT–KO); WT mice of WT–KO pair, KO (WT–KO); KO mice of WT–KO pair. Data are mean ± SEM. Each experiment was repeated at least three times.
3.2. Adiponectin protein in parabiosis mice
At day 63 after surgical parabiosis, each pair mice was sacrificed and analyzed. There was no significant difference in tissue weight of WATsub and WATmes between WT (WT–WT), WT (WT–KO) and KO (WT–KO) (Fig. 2A). Next, we examined the tissue distribution of adiponectin protein in these mice. Both western blotting and tissue ELISA assay showed abundant adiponectin protein levels in WATsub, WATmes and aorta of WT (WT–KO), similar to WT (WT–WT) (Fig. 2B). However, lower adiponectin protein levels were detected in WATsub, WATmes and aorta of KO (WT–KO) (Fig. 2B), which lacks adiponectin mRNA expression (Fig. 2C).
Fig. 2.
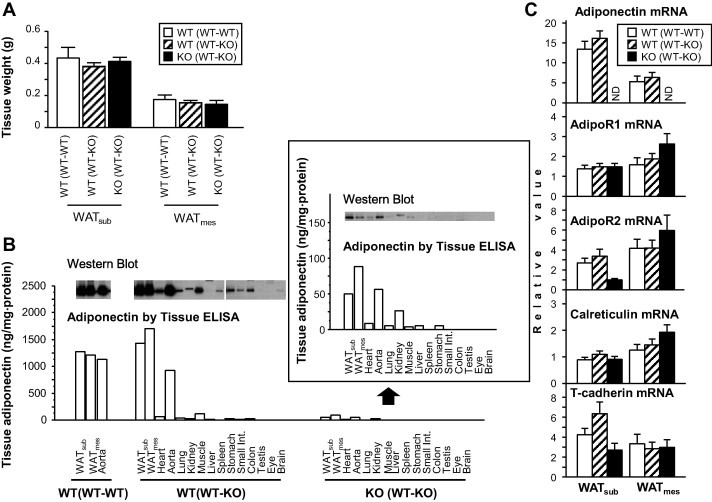
Accumulation of adiponectin in WAT of parabiosis mice. (A) WAT tissue weight in parabiosis mice. WATmes; mesenteric white adipose tissue, WATsub; subcutaneous white adipose tissue. n.s.; not significant. (B) Adiponectin protein levels in various tissues. Western blot analysis (top). Quantitative data measured by ELISA (bottom). Data of KO (WT–KO) are also shown in enlarged view. (C) Gene expression levels of adiponectin and its binding molecules or receptors in WAT. (n = 4–6, each group). ND; not detected. Data are mean ± SEM. Each experiment was repeated at least three times.
To further investigate adiponectin accumulation in WAT, we determined mRNA gene expression of adiponectin, adiponectin receptors and binding proteins genes in WATsub and WATmes (Fig. 2C). Adiponectin mRNA levels were exclusively expressed in WT (WT–WT) and WT (WT–KO), but not in KO (WT–KO). There was no difference in mRNA levels between WT (WT–WT) and WT (WT–KO) (Fig. 2C). Furthermore, there were no significant difference in mRNA gene expression levels of putative adiponectin receptors and binding molecules, AdipoR1, AdipoR2, calreticulin and T-cadherin [22–25], between WATsub and WATmes of the three mice groups (Fig. 2C).
3.3. Accumulation of adiponectin in stromal vascular fraction of HF/HS diet parabiosis mice
Does adiponectin protein accumulate in WAT of obese mice more than that of lean mice after long-term adiponectin recruitment? To answer this question, we compared high fat/high sucrose (HF/HS) diet-induced obese parabiosis WT and APN-KO mice and parabiosis mice fed NC diet (Fig. 3A, B). HF/HS diet load was provided at day 8 after operation, based on the finding of comparable levels of plasma adiponectin levels at day 7 (Fig. 3C bottom). The body weight of parabiotic partners slightly, but significantly, increased during the 8-week period of HF/HS feeding (Fig. 3B). Plasma glucose levels were significantly higher in WT (WT–KO) fed HF/HS than WT (WT–KO) fed NC at days 14 and 21 (p = 0.0006, p < 0.0001 each), however, no such difference was observed at day 63. Plasma insulin levels were significantly higher in HF/HS-fed mice than in NC-fed mice [WT (WT–KO) fed NC vs. WT (WT–KO) fed HF/HS; p = 0.0094 at day 14, p = 0.0222 at day 21, p = 0.0256 at day 63, KO (WT–KO) fed NC vs. KO (WT–KO) fed HF/HS; p = 0.0070 at day 14, p = 0.0079 at day 21, p = 0.0120 at day 63]. In the HF/HS fed mice, plasma insulin levels were higher in WT (WT-KO) than in KO (WT-KO). These data suggest that adiponectin derived from WT (WT-KO) seems to improve insulin resistance in KO (WT-KO), which lack native adiponectin.
Fig. 3.
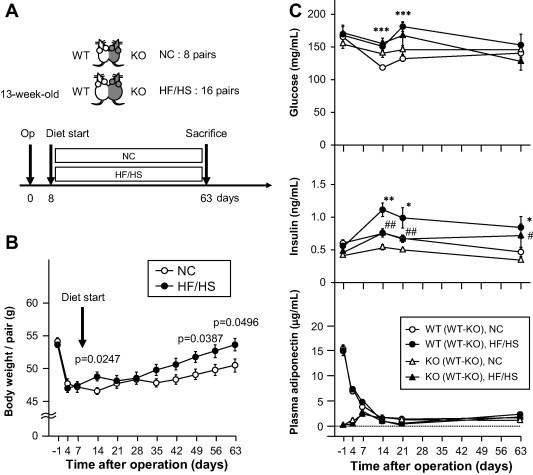
Diet-induced obese parabiotic mice. (A) Experimental protocol. WT mice were parabiosed with KO mice (WT–KO). Starting day 8, mice were fed either normal chow (NC, n = 8) or high fat/high sucrose diet (HF/HS, n = 16) for 8 weeks. At 63 days, the mice were sacrificed and their tissues analyzed. (B) Body weight of a pair of parabiosed mice. Plasma adiponectin levels were measured by ELISA in partners of parabiosed mice (bottom). (C) Effect of obesity on blood glucose, insulin and serum adiponectin. Data are mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; NC- vs. HF/HS-fed WT (WT–KO) mice (C), #p < 0.05, ##p < 0.01, NC vs. HF/HS fed KO (WT–KO) mice (C). Each experiment was repeated at least three times.
Movement of circulating adiponectin levels in HF/HS-fed mice was similar to that in NC-fed mice. At day 63, tissue weights of WATsub and WATmes of mice fed HF/HS diet was significantly higher than of mice fed NC diet (Fig. 4A). There was no significant difference of tissue weight of WAT between WT and KO partners fed both diets (Fig. 4A). Histological examination showed that adipocytes in WATsub and WATmes of HF/HS-fed mice were larger than those of NC-fed mice (Fig. 4B).
Fig. 4.
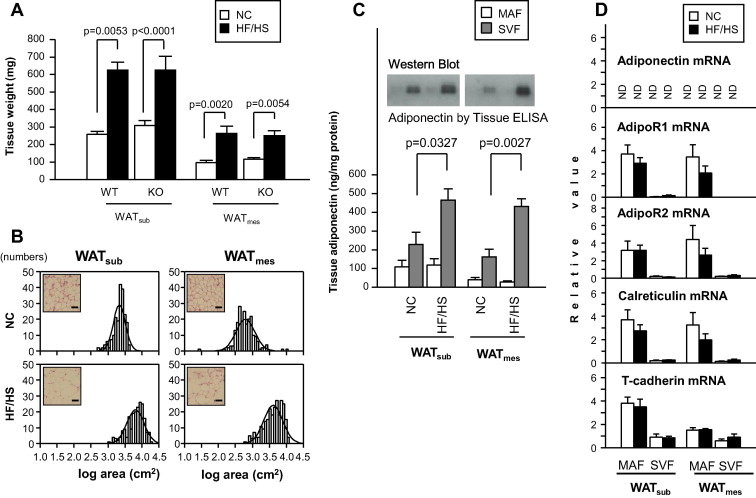
Accumulation of adiponectin in SVF of obese KO mice. (A) WAT tissue weight in parabiosis mice. (B) Hematoxylin and eosin-stained sections and adipocyte cell size distribution. Scale bar: 100 μm. (C) Adiponectin levels in MAF and SVF of adipose tissues. Western blot analysis (top). Quantitative data measured using adiponectin ELISA kit (n = 4–6, each group, bottom). (D) Gene expression of adiponectin and its binding molecules and receptors in MAF and SVF (n = 4–6). Data are mean ± SEM. Each experiment was repeated at least three times.
Finally, we identified the cell type that preferentially contributed to the observed adiponectin levels in both MAF and SVF isolated from WAT. Adiponectin protein level was significantly higher in the SVF of HF/HS-fed mice than of NC-fed mice (WATsub: p = 0.0327, WATmes: p = 0.0027, Fig. 4C). Furthermore, the mRNA gene expression levels of AdipoR1, AdipoR2 and calreticulin in both WATsub and WATmes and T-cadherin in WATsub were dominant in MAF, and T-cadherin in WATmes was also higher in MAF than SVF (Fig. 4D). However, there were no differences in the mRNA levels of these putative adiponectin receptors and binding genes in SVF as well as MAF between NC- and HF/HS-fed mice (Fig. 4D). These results suggest that adiponectin accumulation in obese adipose tissues may be accounted for by unknown adiponectin binding machinery.
4. Discussion
In the present study, we investigated the recruitment of adiponectin in various tissues over a relatively long period of time. The experiments were conducted in hetero parabiosis animals of WT and KO mice. The results showed the presence of adiponectin protein in the plasma of KO (WT–KO) mice at day 4 after parabiosis, suggesting adiponectin recruitment via the circulation from WT mouse to the KO partner. Survey of adiponectin tissue distribution demonstrated its accumulation in various tissues, especially the adipose tissue. In KO (WT–KO), adiponectin protein was detected in both MAF and SVF of WAT, and SVF adiponectin levels increased in HF/HS diet-induced obesity. To clarify the mechanism of such increase, we examined the gene expression levels of four putative adiponectin receptors and binding proteins; AdipoR1, AdipoR2, calreticulin and T-cadherin. Our recent experimental study demonstrated that adiponectin accumulation in adipose SVF of obese mice was mediated at least in part through ROS-upregulated T-cadherin [26]. In present study, plasma thiobarbituric acid reactive substance (TBARS) levels were slightly, but significantly, higher in KO (WT–KO) mice fed HF/HS than in those fed NC (2.1 ± 0.2 vs. 1.7 ± 0.3 nmolMDA/mL, mean ± SD, p = 0.0058). However, we did not find any change in adiponectin binding proteins, including T-cadherin by diet-induced obesity in parabiosis mice. The exact mechanism of adiponectin accumulation remains to be elucidated.
The present study documented movement of circulating adiponectin in hetero parabiosis of WT and KO mice. Plasma adiponectin in WT (WT–KO model) decreased rapidly after parabiosis with KO mice (Fig. 1). One possible reason for the fall in adiponectin in WT mice could be dilution related to the increase in blood volume. It is conceivable that adiponectin blood levels in WT–KO model diminish by 50% half at early postoperative period due to the doubling of circulating blood volume after surgery. However, our analysis showed that adiponectin blood concentrations reduced to around the detection limit of the assay. In our study, adiponectin mRNA levels in WATsub and WATmes were not different between WT (WT–WT) and WT (WT–KO) (Fig. 2C), suggesting that the reduction of adiponectin in blood was not due to failure in production. Another group conducted infusion study of labeled adiponectin in mice, and reported that the main site for clearance of adiponectin was the liver, and that the kidney might excrete the final degradation products [27]. Excess degradation of adiponectin might have occurred in parabiosis between WT and KO mice, but we could not clarify that point.
Adiponectin levels did not “equilibrate” between the WT and KO mouse over a 63 day period (Fig. 1). It appeared that adiponectin derived from the WT mice fell into “bottomless pit” in KO mice in this WT–KO parabiosis model. A potential explanation could be that adiponetin KO mice have a vast number of binding sites in the body, and that after adiponectin is restored it might take an exceedingly long time, beyond the experimental period of 63 days, to occupy these binding sites and to raise the plasma levels. However, this explanation seems implausible.
We reported recently that adiponectin formed a protein complex with structurally-related complement C1q [28]. Exogenous adiponection from WT may form such complexes with other proteins in the blood of KO mice, which are then rapidly removed from circulation.
We and others have reported that adiponectin accumulates in injured organs, such as arterial walls [29,30], kidney [16], and liver [31]. Parabiosis experiments might cause severe injury in specific organs and accumulation of adiponectin, although there was no difference in the gross appearance or size of organs between WT and KO mice in the WT–KO model in the present study (data not shown). The pattern of tissue distribution of adiponectin protein was similar in these mice (Fig. 2) and we did not find adiponectin accumulated in the organs specific for parabiosis.
The present study demonstrated that adiponectin protein from WT mice was distributed in various tissues of the body of KO mice, especially in its source tissue (i.e. adipose tissue), similar to WT. Exogenous adiponectin may be utilized and catabolized quite rapidly in KO mouse by unknown mechanism because of the lack of endogenous adiponectin. The molecular mechanism of such “adiponectin-inducible clearance system” may be clarified in the future. To our knowledge, this report is the first to investigate blood and tissue adiponectin levels in parabiosis mice.
Interestingly, the present study found that plasma insulin levels were significantly higher in WT (WT–KO model) HF/HS fed mice than in KO (WT–KO model) HF/HS fed mice (Fig. 3). These data suggest that reduced adiponectin levels in WT (WT–KO model) HF/HS fed (native adiponectin is presented) rather than replaced adiponectin in KO (WT–KO model) HF/HS fed mice (native adiponectin is not presented) may partly lead to hyper-insulinemia.
In conclusion, the present study demonstrated that hetero parabiosis of WT and KO mice resulted in hypoadiponectinemia in WT mice, as well as adiponectin accumulation in various tissues, including WAT, in the KO partner, which lack adiponectin gene expression, probably through recruitment of adiponectin from the WT partner. Furthermore, diet-induced obesity increased adiponectin protein level in adipose SVF of KO partner. These findings shed light on the movement of native adiponectin among different tissues such as the state of hypoadiponectinemia in obesity.
Conflict of interest
T.F. is a member of the “Department of Metabolism and Atherosclerosis”, a sponsored course endowed by Kowa Co., Ltd. All other authors declare no competing interests.
Contributions
H.N. and K.K. researched and analyzed the data. K.K. participated in the concept and design of the study, interpretation of data and reviewed/edited the manuscript. R.S. collected the data. T.F. and I.S. contributed to the discussion. All authors read and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Yoshihiro Tochino and Mrs. Miyuki Nakamura for the excellent technical assistance. This research was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas (Research in a proposed research area) “Molecular Basis and Disorders of Control of Appetite and Fat Accumulation” (#22126008, to T.F. and K.K.).
References
- 1.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 2.Després J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., Matsubara K. CDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem. Biophys. Res. Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Maeda K., Okubo K., Shimomura I., Mizuno K., Matsuzawa Y., Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997;190:227–235. doi: 10.1016/s0378-1119(96)00730-5. [DOI] [PubMed] [Google Scholar]
- 5.Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 6.Hu E., Liang P., Spiegelman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y., Tobe T., Choi-Miura N.H., Mazda T., Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K., Funahashi T., Bodkin N.L., Ortmeyer H.K., Arita Y., Hansen B.C., Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa Y., Kishida K., Kihara S., Yoshida R., Funahashi T., Shimomura I. Nocturnal falls of adiponectin levels in sleep apnea with abdominal obesity and impact of hypoxia-induced dysregulated adiponectin production in obese murine mesenteric adipose tissue. J. Atheroscler. Thromb. 2011;18:240–247. doi: 10.5551/jat.6593. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto Y., Kihara S., Ouchi N., Nishida M., Arita Y., Kumada M., Ohashi K., Sakai N., Shimomura I., Kobayashi H., Terasaka N., Inaba T., Funahashi T., Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 15.Fujita K., Maeda N., Sonoda M., Ohashi K., Hibuse T., Nishizawa H., Nishida M., Hiuge A., Kurata A., Kihara S., Shimomura I., Funahashi T. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi K., Iwatani H., Kihara S., Nakagawa Y., Komura N., Fujita K., Maeda N., Nishida M., Katsube F., Shimomura I., Ito T., Funahashi T. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 17.Harris R.B. Loss of body fat in lean parabiotic partners of ob/ob mice. Am. J. Physiol. 1997;272:R1809–R1815. doi: 10.1152/ajpregu.1997.272.6.R1809. [DOI] [PubMed] [Google Scholar]
- 18.Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 19.Sekimoto R., Kishida K., Nakatsuji H., Nakagawa T., Funahashi T., Shimomura I. High circulating levels of S100A8/A9 complex (calprotectin) in male Japanese with abdominal adiposity and dysregulated expression of S100A8 and S100A9 in adipose tissues of obese mice. Biochem. Biophys. Res. Commun. 2012;419:782–789. doi: 10.1016/j.bbrc.2012.02.102. [DOI] [PubMed] [Google Scholar]
- 20.Wright D.E., Wagers A.J., Gulati A.P., Johnson F.L., Weissman I.L. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 21.Koh Y.J., Kang S., Lee H.J., Choi T.S., Lee H.S., Cho C.H., Koh G.Y. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J. Clin. Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N.H., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 23.Takemura Y., Ouchi N., Shibata R., Aprahamian T., Kirber M.T., Summer R.S., Kihara S., Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hug C., Wang J., Ahmad N.S., Bogan J.S., Tsao T.S., Lodish H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi T., Adachi Y., Ohtsuki Y., Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med. Mol. Morphol. 2007;40:115–120. doi: 10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- 26.Nakatsuji, H., Kishida, K., Sekimoto, R., Komura, N., Kihara, S., Funahashi, T., Shimomura, I. (2014) Accumulation of adiponectin in inflamed adipose tissues of obese mice, Metabolism (in press). [DOI] [PubMed]
- 27.Halberg N., Schraw T.D., Wang Z.V., Kim J.Y., Yi J., Hamilton M.P., Luby-Phelps K., Scherer P.E. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatsuji H., Kobayashi H., Kishida K., Nakagawa T., Takahashi S., Tanaka H., Akamatsu S., Funahashi T., Shimomura I. Binding of adiponectin and C1q in human serum, and clinical significance of the measurement of C1q-adiponectin / total adiponectin ratio. Metabolism. 2013;62:109–120. doi: 10.1016/j.metabol.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto Y., Arita Y., Nishida M., Muraguchi M., Ouchi N., Takahashi M., Igura T., Inui Y., Kihara S., Nakamura T., Yamashita S., Miyagawa J., Funahashi T., Matsuzawa Y. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm. Metab. Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda M., Shimomura I., Sata M., Arita Y., Nishida M., Maeda N., Kumada M., Okamoto Y., Nagaretani H., Nishizawa H., Kishida K., Komuro R., Ouchi N., Kihara S., Nagai R., Funahashi T., Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J. Biol. Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 31.Kamada Y., Tamura S., Kiso S., Matsumoto H., Saji Y., Yoshida Y., Fukui K., Maeda N., Nishizawa H., Nagaretani H., Okamoto Y., Kihara S., Miyagawa J., Shinomura Y., Funahashi T., Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]


