Abstract
High-frequency oligonucleotide-directed recombination engineering (recombineering) has enabled rapid modification of several prokaryotic genomes to date. Here, we present a method for oligonucleotide-mediated recombineering in the model eukaryote and industrial production host S. cerevisiae, which we call Yeast Oligo-mediated Genome Engineering (YOGE). Through a combination of overexpression and knockouts of relevant genes and optimization of transformation and oligonucleotide designs, we achieve high gene modification frequencies at levels that only require screening of dozens of cells. We demonstrate the robustness of our approach in three divergent yeast strains, including those involved in industrial production of bio-based chemicals. Furthermore, YOGE can be iteratively executed via cycling to generate genomic libraries up to 105 individuals at each round for diversity generation. YOGE cycling alone, or in combination with phenotypic selections or endonuclease-based negative genotypic selections, can be used to easily generate modified alleles in yeast populations with high frequencies.
Keywords: Yeast, Genome Engineering, Recombineering, MAGE, Saccharomyces cerevisiae, Oligonucleotide transformation
Introduction
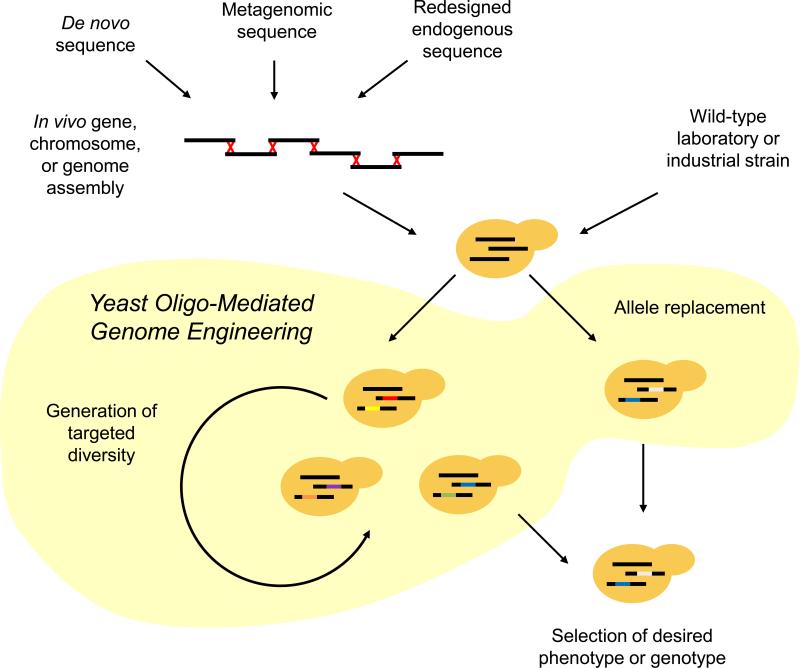
Oligonucleotide-mediated genome engineering is a practical method for performing site-directed mutagenesis to enable the rapid generation of various rationally designed organisms. (1, 2) In Saccharomyces cerevisiae, genome manipulation has had a long and successful history due to its efficient endogenous homologous recombination machinery. In general, high frequency genomic recombination using double stranded DNA (approaching 10−3 transformants per surviving cell) requires large homology arms with a minimum length of ~500 base pairs. (3, 4) The use of shorter homology arms (35 – 70 base pairs) drastically reduces recombination frequency to about 10−6 – 10−5 transformants per surviving cell. (4–6) Directed homologous recombination using single-stranded oligonucleotides has thus far been limited by low recombination frequency in the absence of artificial selection for the modified allele. Nonetheless, short oligonucleotides have significant advantages over large double-stranded DNA constructs since oligonucleotides are inexpensive and easily obtained from commercial sources without the need for PCR amplification and purification. (6–8) An efficient method for oligonucleotide-mediated recombination engineering or allelic replacement in yeast would thus have a variety of applications. Furthermore, high efficiency oligo-mediated recombineering in yeast will further its emerging role as a biological chassis for the de novo assembly and construction of genes, gene clusters, and bacterial genomes.(9, 10) Here, we describe yeast strains with highly efficient oligo-mediated recombination capabilities and identify key factors governing these traits for use in Yeast Oligo-mediated Genome Engineering (YOGE) (Figure 1).
Figure 1.
Diagram of Yeast Oligo-mediated Genome Engineering (YOGE) applications. Synthetically assembled foreign DNA or natural genomes can be modified with YOGE. Iterative rounds can be used to increase the frequency and/or diversity of the directed modifications. Finally, strains can be screened or selected to isolate desired genotypic or phenotypic traits.
Since homologous recombination rates vary in different yeast strains, we sought to develop YOGE in three separate S. cerevisiae haploid strains of different lineages (VL6-48, CEN.PK113-7D, and VTT C-68059). VL6-48 is a 288c strain derivative first developed for transformation-associated recombination (TAR) cloning and has been reported to have high transformation efficiency. (11, 12) S. cerevisiae CEN.PK113-7D is a lab strain widely used in systems biology and metabolic engineering studies that is also physiologically robust in industrial settings. (13) S. cerevisiae (var. diastaticus) VTT C-68059 is an industrial yeast strain that was originally isolated as a brewery contaminant. These three strains represent an array of biological backgrounds that may be encountered for general adoption of YOGE in different experimental settings.
Materials and Methods
Strains and Media
Strain VL6-48 (MATα, his3Δ200, trplΔ1, ura3-52, ade2-101, lys2, psi°, cir°) was chosen for the oligonucleotide recombination experiments, due to its native ade2-101 premature stop codon, VL6-48 was purchased from ATCC (MYA-3666). This strain was chosen due to its reported high transformation ability (7,8). The prototrophic CEN.PK113-7D S. cerevisiae strain (MATa, MAL2-c, SUC2) was chosen since it is a commonly used yeast lab strain. CEN.PK113-7D has recently been shown to have a mosaic genome combining characteristics of laboratory and wild-industrial strains, which likely explains its robust physiological performance and making it a yeast model for industrial applications.(13) CEN.PK113-7D was purchased from EUROSCARF. The VTT C-68059 S. cerevisiae (var. diastaticus) strain (MATa/α) is a diploid industrial strain with good growth characteristics which was originally isolated as a brewery contaminant. VTT C-68059 was obtained from the VTT microbe culture collection.
For transformation, cells were grown in 2X YPAD with additional tryptophan. This media consists of 20 g/L Yeast extract, 40 g/L Peptone, 40 g/L Dextrose, 300 mg/L tryptophan, and 24 mg/L Adenine hemisulfate. To measure recombination frequencies, selections were completed using SCD-arginine + canavanine (60 μg/mL L-canavanine), SCD-lysine + thialysine (100 μg/mL S-aminoethyl-L-cysteine), SCD + FOA (1 mg/mL 5-Fluoroorotic Acid) , SCD-adenine, and SCD-leucine plates. Recombination frequencies were calculated via the ratio of cells that grew on selective media divided by the number that grew on rich non8selective media.
Strain modification
In strain VL6-48, genes MLH1 and MSH2 were initially knocked out using a KanMX cassette amplified from vector pFA6a-KanMX6, using 50 base pair homology arms to the 5’ and 3’ regions of the genes. After finding that the mlh1 knockout and RAD5(K342E) overexpression cassette strain demonstrated the desired phenotype, the KanMX cassette at the MLH1 locus was replaced with a RAD54 overexpression cassette. Overexpression cassettes were generated by cloning yeast codon optimized versions of the λ Red beta protein with and without NLS tag, as well as the yeast gene RAD51 into the XhoI and XmaI cut sites of plasmid p416-TEF. The RAD54 cassette was generated by cloning the yeast gene RAD54 into the XhoI and XmaI cut sites of plasmid p414-TEF. The RAD51(K342E) mutant was generated using an oligonucleotide with the QuikChange Mutagenesis kit (Stratagene). The β, β-NLS, RAD51, and RAD51(K342E) overexpression plasmids were then cloned along with the URA3 marker into a plasmid containing 300 base pair homology arms to the HO locus. These cassettes were them amplified with primers to the 5’ and 3’ regions of the HO flanking sites for transformation into yeast. Transformants were selected on solid synthetic complete media without uracil. Integration was confirmed via PCR of the locus and sequencing. The RAD54 overexpression cassette was amplified from the p414-TEF plasmid along with the TRP1 marker using 50 base pair homology arms to the 5’ and 3’ region of the mlh1 locus, to replace the KanMX marker. The PCR product was DpnI digested before transformation to remove the background of plasmid only transformants, as the p414 vector contains a yeast origin of replication. Transformants were selected on solid synthetic complete media without tryptophan. Integration was confirmed via PCR of the locus and sequencing. A stop codon was inserted into the LEU2 gene of strain VL6-48 ho::RAD51(K342E) mlh1::RAD54, via an oligonucleotide using the described electroporation procedure. Cells were plated on rich media, then replicaplated onto synthetic complete media without leucine to identify leu2 auxtotrophic strains.
To facilitate strain engineering efforts, the VTT C-68059 industrial diploid strain was first sporulated in order to obtain the haploid derivatives VTT C-68059a (MATa) and VTT C- 68059α (MATα). To ensure stable propagation of the VTT C-68059a (MATa) haploid yeast strain, the HO endonuclease gene was deleted from the strain to prevent mating type switching from occurring.
The HO, MLH1 and MSH2 deletion cassettes (ΔHHO-HygR, ΔMLH1-HygR and ΔMSH2-HygR) were generated by PCR amplifying the 5’ and 3’ regions of the HO, MLH1 and MSH2 genes from CEN.PK113-7D genomic DNA and the HygR marker from the SP55-5 plasmid (flanked by the mutant loxP sites, lox66 and lox71). The fragments were gel purified and then Gibson assembled into the PCR-amplified pBlueScript SK(−) plasmid using the NEB Gibson Assembly Kit. The RAD51(K342E) and RAD54 overexpression cassettes [ΔHO-RAD51(K342E)-HygR and ΔHO-RAD54-HygR] were generated by PCR amplifying the RAD51(K342E) and RAD54 genes from the finalized plasmids described above and the PGK1 promoter and CYC1 terminator from the SP55-5 plasmid. The fragments were gel purified and then Gibson assembled into the PCR-amplified ΔHO-HygR plasmid. The combined RAD51(K342E)/RAD54 overexpression cassette [ΔHO-RAD51(K342E)-RAD54-HygR] was generated by PCR amplifying the RAD54 gene from the ΔHO-RAD54-HygR plasmid and the TPI1 promoter and ADH1 terminator from the SP55-5 plasmid. The fragments were gel purified and then Gibson assembled into the PCR-amplified ΔHO-RAD51(K342E)-HygR plasmid. The RAD54 overexpression cassette (ΔPDC6-RAD54-HygR) was generated by PCR amplifying the 5’ and 3’ regions of the PDC6 gene from CEN.PK113-7D genomic DNA, the HygR marker from the SP55-5 plasmid and the RAD54 gene from the ΔHO-RAD54-HygR plasmid. The fragments were gel purified and then Gibson assembled into the PCR-amplified pBlueScript SK(−) plasmid.
All cassettes were sequenced to ensure their correctness. All integration cassettes were digested from their pBlueScript vector backbone with SbfI and NarI and subsequently gel-purified prior to transformation into their respective strains. Transformants were selected on solid YPD(+200 μg/mL Hygromycin B) media and proper integration was confirmed via PCR of the locus. To remove the HygR selectable marker from the strains to allow for its reuse between successive integrations, the plasmid pSH47-KanMX, containing the Cre recombinase under control of a GAL1 promoter, was transformed into the strains which were selected on YPD(+200 ug/mL G418) solid media. Positive transformants were grown in YP(+200 ug/mL G418, +2 % galactose) liquid media for five hours to induce expression of the Cre recombinase and then grown overnight in YPD medium in order to lose the pSH47-KanMX plasmid. Correct transformants were established based on their inability to grow on YPD(+200 μg/mL Hygromycin B) and YPD(+200 Mg/mL G418) solid media and the absence of a HygR gene in their genome, as determined by PCR. All presented strain modifications were also performed in the α mating type equivalents of CEN.PK113-7D and VTT C-68059.
Gene Cassette Transformation
Gene replacement and gene cassette integration was performed using standard lithium acetate-PEG transformation procedures by Gietz et al. (14)
Oligonucleotide Electroporation Optimization Protocol
Cultures were grown to saturation overnight in 2X YPAD (Yeast extract-Peptone-Adenine-Dextrose), see Supplemental Information for recipe. The next morning, a 7 mL culture was inoculated in 2X YPAD to an OD600 = 0.3. Inoculated cells were grown in a roller drum at 30 °C until the OD600 reached 1.6 – 1.8 after ~5 hours. Cells were pelleted at 2250 × g for 3 minutes and the media was removed. The cell pellet was washed once by 10 mL of ice-cold water and once by 10 mL of ice-cold electroporation buffer (1 M Sorbitol / 1 mM CaCl2). The cells were conditioned by re-suspending the cell pellet in 2 mL of 500 mM lithium acetate/10 mM dithiotheitol solution and placed in a roller drum for 30 minutes at 30 °C. Conditioned cells were pelleted by centrifugation, and washed once by 10 mL of ice-cold electroporation buffer. The cell pellet was re-suspended to a final volume of 1.6 mL in electroporation buffer. 400 μL of cells were used per electroporation with 2.5 μM of oligonucleotide per cuvette (or varying amounts depending on the experiment). Cells were electroporated at 2.5 kV, 25 uF, 200Ω. Electroporated cells were transferred from each cuvette into 7 mL of 1:1 mix of 1 M sorbitol / 2X YPAD media. The cells were incubated in a roller drum at 30 °C for 12 – 16 hours. Cells were diluted appropriately to obtain ~50 – 500 colonies on selective media and on rich media. The ratio of colony count on selective plates over rich plates was used as a measure of oligonucleotide incorporation frequency.
Oligonucleotide Electroporation Cycling Protocol
The cycling procedure was the same as above except for the following changes. Inoculated cells were grown in a roller drum at 30 °C until the OD600 reached 0.6 – 0.8 after ~3.5 hours. The conditioned cell pellet was re-suspended to a final volume of 400 uL of electroporation buffer with 2.5 μM of oligonucleotide (or varying amounts depending on the experiment). Electroporated cells were transferred from each cuvette into 5 mL of 1:1 mix of 1 M sorbitol / 2X YPAD media. The cells were incubated in a roller drum at 30 °C for 12 – 16 hours until the OD reached 0.6 – 0.8, and the cycle was repeated.
Oligonucleotide Design
Oligonucleotides used for oligonucleotide recombination and PCR were purchased from Integrated DNA Technologies (Coralville, IA, USA). Mutations were always placed in the center of the oligonucleotide, allowing as much flanking region as possible. See Supplementary Data for the list of oligonucleotides. Double-stranded oligonucleotides were made by annealing equimolar amounts of complementary single-stranded oligonucleotides. To anneal, single-strand oligonucleotides were mixed, denatured at 100°C for 5 minutes, and then cooled to 25°C with a ramp of 0.1°C per second.
Results and Discussion
Mismatch Repair Knockout
In yeast, mismatch repair (MMR) is a multistep process that identifies and fixes incorrectly matched Watson-Crick base pairs in the genome. A mismatched DNA heteroduplex is first recognized by MutSα and MutSβ heterodimeric complexes, which is composed of Msh2p/Msh3p and Msh2p/Msh6p, respectively. MutS heterodimers then interact with MutL homolog proteins Mlh1p and Pms1p to continue the recognition cascade and to recruit the correction machinery.(15, 16) Since the incorporation of mutagenic oligonucleotide DNA into the target genomic site produces a heteroduplex that is recognized by the MMR machinery, avoidance of this system is important to facilitate efficient oligo incorporation (17, 18).
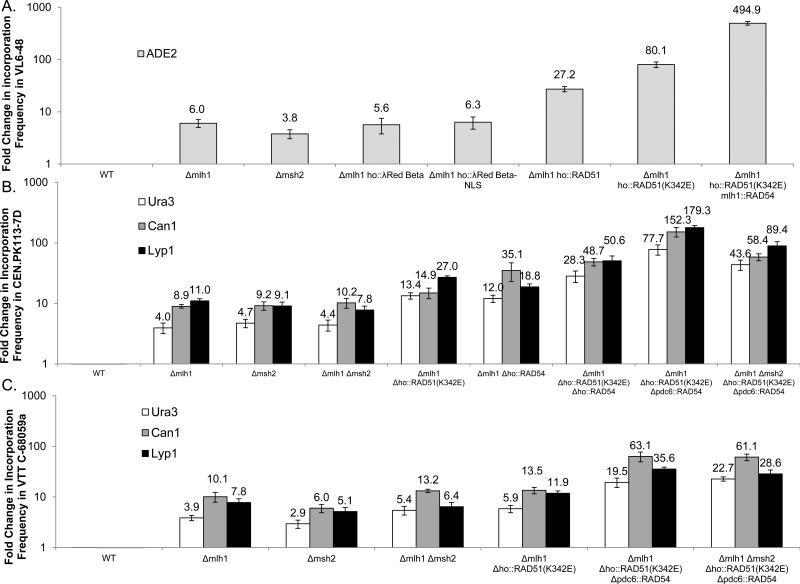
Previous work has shown that mlh1 and msh2 knockouts can increase oligonucleotide incorporation in yeast. However, these knockouts result in varying recombination frequencies depending on the characteristics of the locus and oligonucleotide transformed. (15) Figure 2 A, B, and C show the efficiency of oligonucleotide allelic replacement using oligonucleotides with a single point mutation in the center of the target sequence when using strains with mlh1 and msh2 knockouts. Across all strains and loci, mlh1 deletions increased median oligonucleotide incorporation from 3.9 to 11.0 fold, while msh2 deletions increased median oligonucleotide incorporation from 2.9 to 9.2 fold. The double knockouts of mlh1 and msh2 in the CEN.PK113-7D and VTT C-68059a strains showed no significant effect in oligonucleotide recombination. We proceeded to use the strains with a single knockout in mlh1 for further optimization, as this modification generally gave the highest increase of oligonucleotide incorporation across the different strains in 5 out of 7 tested loci.
Figure 2.
Oligonucleotide recombination frequency across all strains and loci tested. Recombinations were completed using 90mer oligonucelotides with the optimal sense strand targeted. All recombinations were completed in triplicate, with values plotted to represent the mean values and error bars corresponding to the standard deviation. A. Oligonucleotide incorporation frequency in strain VL6-48 B. Oligonucleotide incorporation frequency in strain CEN.PK113-7D C. Oligonucleotide incorporation frequency in strain VTT C-68059a.
DNA Recombinase Overexpression
Single-stranded DNA recombinases are crucial for oligonucleotide recombineering in prokaryotic organisms, able to increase the rate of oligo incorporation by ~10,000-fold in some cases. (1, 2) Endogenous proteins crucial to the homologous recombination pathway in yeast also stimulate oligonucleotide incorporation. Overexpression of Rad51 and Rad54 proteins, as well as particular point mutants of Rad51, has previously been shown to affect oligonucleotide integration in yeast. (7, 19, 20)
Starting with the Δmlh1 knockout strains, we constitutively expressed heterologous and endogenous DNA recombinases to examine their effect on oligonucleotide incorporation. In strain VL6-48, we expressed a yeast codon optimized version of the λ Red beta-protein, both with and without a nuclear localization signal tagged to the C-terminus of the protein. We were unable to observe a positive effect on oligonucleotide incorporation by overexpression either verisons of the λ Red beta8protein. (Figure 2A) -In a Δmlh1 mutant background strain we overexpressed these proteins starting with RAD51, a DNA strand exchange protein crucial in homologous recombination. Lu et al. showed that a K342E mutant of RAD51 enhanced oligonucleotide recombination when compared to wild-type RAD51. This particular mutation was shown to affect the DNA-binding profile of RAD51 by removing its intrinsic inhibition to form filaments around single-stranded DNA in the presence of double8stranded DNA. (7, 19, 20) To examine if this effect held true in a mismatch repair deficient strain, we constitutively overexpressed both proteins genomically in the VL6-48 Δmlh1 strain and found nearly a 3 fold increase in oligonucleotide incorporation in the RAD51(K342E) mutant as compared to the wild type RAD51. (Figure 2 A) Furthermore, RAD51(K342E) overexpression resulted in increased oligonucleotide incorporation compared to wild type by up to 80.1 fold in VL6-48, 13.4 to 27 fold in CEN.PK113-7D, and 5.9 to 13.5 fold in VTT C-68059a. We additionally overexpressed RAD54, a helicase and chromatin remodeler, in strain CEN.PK113-7D Hmlh1 in an attempt to further improve oligo incorporation efficiency. Overexpression of RAD54 in the mlh1 background showed a 12 to 35 fold increase in oligonucleotide incorporation compared to wild-type. (Figure 2 B) Overexpression of both RAD51(K342E) and RAD54 in an Δmlh1 background across all three strains resulted in the highest increase in oligonucleotide recombination of 19.5 to 494.9 fold across all strains and loci (Figure 2) compared to wild-type. The variability of these fold increases are likely due to: i) the different promoters used for gene overexpression (i.e., TEF1 promoters in VL6-48 versus PGK1 and TPI1 promoters in CEN.PK113-7D and VTT C-68059a), ii) the site of genomic incorporation of the RAD54 overexpression cassette (i.e., the mlh1 locus for VL6-48 versus the pdc6 locus for CEN.PK113-7D and VTT C-68059a), and iii) the inherent variability of oligonucleotide incorporation across the loci tested. In the CEN.PK113-7D Δmlh1 strain, we overexpressed both RAD51(K342E) and RAD54 from the same HO locus and from separate loci (i.e., HO and PDC6) to see if overexpression of both proteins in close proximity would affect oligonucleotide incorporation. Compared to wild-type, expression of the genes from separate loci resulted in a 77.7 to 179.3 fold increase in oligonucleotide incorporation, which was higher than the 28.3 to 50.6 fold increase in oligonucleotide incorporation when they were expressed at the same locus. (Figure 2B) However, the differences in expression levels could come from many sources other than mere distance between expression cassettes, such as transcriptional activity of each locus.
In the CEN.PK113-7D Δmlh1 ho::RAD51(K342E) pdc6::RAD54 and VTT C-68059a Δmlh1 ho::RAD51(K342E) pdc6::RAD54 strain backgrounds, msh2 was also knocked out, which provided no significant increase in oligonucleotide incorporation in the strains CEN.PK113-7D and VTT C-68059a. (Figure 2 B and C) We designate the most recombinogenic strains with the suffix, “.RR” to refer to their overexpression of RAD51 and RAD54. Hence, VL6-48 ho::RAD51(K342E) mlh1::RAD54, CEN.PK113-7D Δmlh1 Δho::RAD51(K342E) Δpdc6::RAD54, and VTT C-68059a Δmlh1 Δho::RAD51(K342E) Δpdc6::RAD54, are referred to as VL6-48.RR, CEN.PK.RR and VTT.RR, respectively.
External Factors Affecting Oligonucleotide Recombination
We further examined other factors that affect oligonucleotide recombination in yeast, including oligonucleotide length, amount of oligonucleotide transformed, and amount of homology to the site of integration. The intrinsic differences in transformation frequency between strains were also measured with the transformation of a plasmid having a centromeric origin of replication. Several studies have reported a variety of base values for oligonucleotide incorporation, depending on the locus. (5, 6, 16, 21) To obtain a range of oligonucleotide incorporation frequencies in each strain examined, we measured the frequency at three loci on separate chromosomes in each strain; ADE2, LEU2 and CAN1 for strain VL6-48, and URA3, LYP1, and CAN1 for strains CEN.PK113-7D and VTT C-68059a.
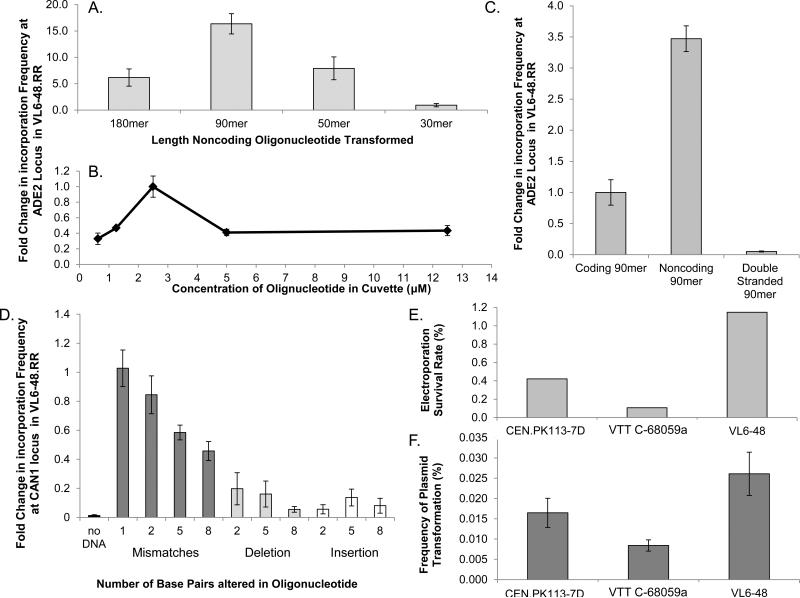
Optimal oligonucleotide length for oligonucleotide-mediated recombination in yeast has varied across different studies. Sherman et al. initially reported an optimum oligonucleotide length of 50 base pairs, while several other reports have used 70 base pair oligonucleotides for generating changes.(5, 6, 21) In our most efficient strain, VL6-48.RR, we found that 90 base pair oligonucleotides gave the greatest increase in incorporation frequency when tested at the ADE2 locus. (Figure 3A) Other oligo-mediated recombination studies in E. coli have also found the 90mer to be optimal.(1) Hence 90mer oligonucleotides were used in all further recombination experiments. For VL6-48.RR, incorporation of 90mer oligonucleotides containing the noncoding sequence of the ADE2 gene was ~3.5-fold higher in frequency than oligos with the coding sequence. Annealed doubled stranded 90mer oligonucleotides gave the lowest incorporation frequency in this strain, resulting in recombination frequencies that were ~10-fold worse than the coding sequence oligonucleotides. (Figure 4 B) Furthermore, we titrated the amount of 90mer noncoding oligonucleotide used for each transformation and found that an oligonucleotide concentration of 2.5 μM in the 400 μl of cell electroporation mixture resulted in the highest transformation frequency. (Figure 3 B) The amount of homology to the locus of integration also affects the frequency of recombination. A premature stop codon was encoded in the 90mer oligonucleotides along with various other modifications of varying lengths such as mismatches, deletions and insertions. In the strain VL6-48.RR, mismatches were more tolerated than either insertions or deletions. (Figure 3 C) This is likely due to the preservation of the oligonucleotide length in the mismatched DNA. We observed that the oligo incorporation frequency decreases as the number of mismatches increases, consistent with previous observations in E. coli.(1) (Figure 3 C)
Figure 3.
External factors affecting oligonucleotide recombination. All values represent mean values of three replicates with error bars representing the standard deviation. A. Effect of oligonucleotide length on recombination at the ADE2 locus in strain VL6-48.RR B. Effect of oligonucleotide concentration on recombination at the ADE2 locus in strain VL6-48.RR C. Effect of mismatches, deletions and insertions on the oligonucleotide recombination frequency at the CAN1 locus in strain VL6-48.RR (the 1 mismatch, all deletion, and all insertion experiments were completed with 5 replicates) D. The survival rate of cells following electroporation across the three strains tested. E. Frequency of plasmid transformation in all three strains using a centromeric plasmid containing hygromycin resistance.
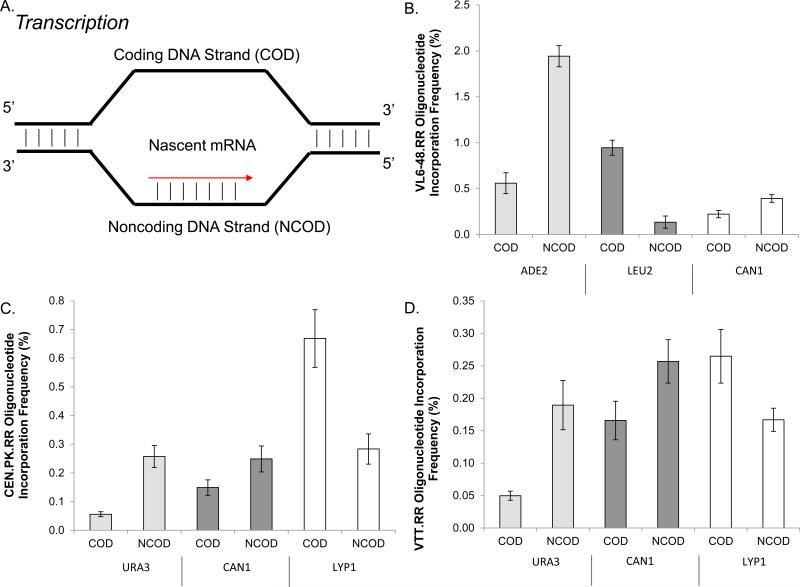
Figure 4.
Variability in oligonucleotide recombination across DNA strands, loci and strains. All values represent the mean of three replicates with error bars representing the standard deviation. A. Diagram of the transcription fork with the coding and noncoding strands indicated. Oligonucleotides are named based on the sequence they correspond to in the transcription fork. B. Strand bias variability in oligonucleotide recombination across the ADE2, LEU2, and CAN1 loci of the VL6-48.RR strain. C. Strand bias variability in oligonucleotide recombination across the URA3, CAN1, and LYP1 loci of the CEN.PK.RR strain D. Strand bias variability in oligonucleotide recombination across the URA3, CAN1, and LYP1 loci of the VTT.RR strain.
Additionally, we assayed transformation and survival frequencies via the transformation of a replicating plasmid to determine if oligonucleotide incorporation frequencies may be biased by intrinsic strain variability in DNA transformation. Indeed, we found that the wild-type VL6-48 strain had the highest survival and transformation frequency, which was consistent with its higher oligo-mediated recombination frequency. (Figures 3 D and E)
Locus Variability
We examined the variability of oligonucleotide recombination frequency across the genome in each strain studied. In strains CEN.PK.RR and VTT.RR three negatively selectable alleles were generated (using oligonucleotides containing premature stop codons), and in strain VL6-48.RR one negatively selectable allele and two positively selectable alleles were generated (using oligonucleotides correcting genomic premature stop codons). To designate which oligonucleotide was transformed, we denoted the sequences containing the coding or transcribed strand of the gene as COD, and the sequences containing the noncoding or non-transcribed strand of the gene as NCOD. (Figure 4 A) Taking the ratio of the most recombinogenic oligonucleotide and loci to the least, we found a ~40 fold difference in recombination frequency across all alleles and strains tested, indicating that certain loci are more recombinogenic than others. (Figure 4) The highest recombination frequency, ~2%, was observed in strain VL6-48.RR at the ADE2 locus using a NCOD oligonucleotide (Figure 4 B). CEN.PK.RR exhibited the next highest recombination frequencies at the loci tested (Figure 4 C) and the lowest recombination frequencies were observed in the industrial strain VTT.RR. (Figure 4 D) Furthermore, we found that across all loci and strains there seems to be a lack of transcriptional bias towards rates of oligonucleotide-mediated recombination, potentially supporting a mechanism that oligonucleotides become incorporated during DNA replication. Unfortunately, because of an absence of empirical data regarding replication fork directionality in the loci examined, it is difficult to definitively conclude that either the leading or lagging strand is favored for oligonucleotide incorporation, however our data does not contradict a replication based model. We found that variability in strand bias in the examined loci was consistent between strains and could not be solely explained by transcriptional effects (Figure 4), which lend further mechanistic support for lagging strand incorporation of oligos at the DNA replication fork.(15, 21, 22)
Multiplex Modifications and Cycling
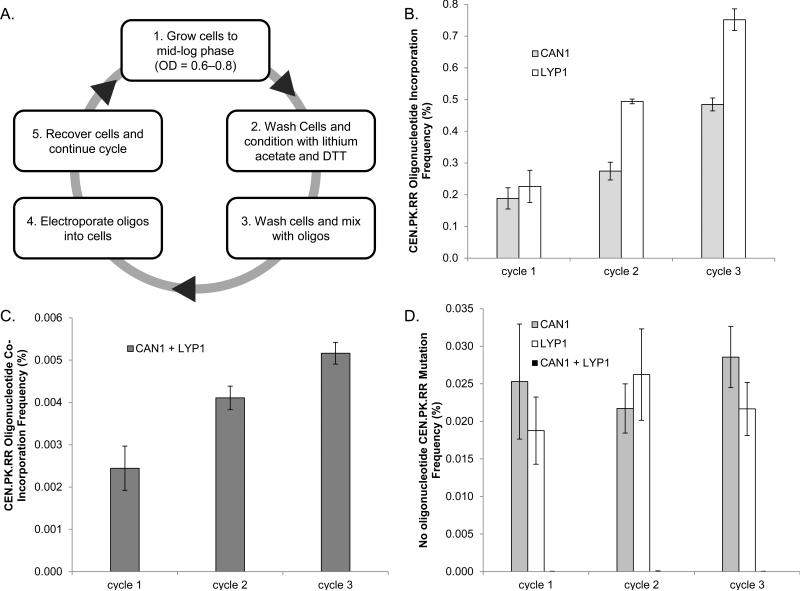
For the practical oligo-mediated directed evolution of yeast genomes, the ability to target multiple sites simultaneously is important. Furthermore, given that most of the single recombination frequencies observed in this study were below 1%, additional cycles of transformation may be required to enrich the population with modifications. To demonstrate multiplexing and cycling, we used strain CEN.PK.RR to target two negatively selectable genes CAN1 and LYP1 with the most efficient oligonucleotides to introduce premature stop codons (a NCOD oligonucleotide for CAN1 and a COD oligonucleotide for LYP1, Figure 4 C). We developed a cycling protocol allowing iterative transformation and recovery of the population with a turnaround of 12 to 16 hours. (Figure 5 A) We completed three cycles of oligonucleotide transformation and assayed the recombination frequency after each cycle. The transformed oligo pool contained a mixture of 1.25 μM of each oligonucleotide yielding a total of 2.5 μM of oligonucleotides. We observed, as expected, that the frequency of incorporation increased as the number of cycles increased. (Figure 5 B) In cycle one, the individual oligonucleotide recombination frequencies were at most half of the individual recombination frequencies previously observed, which is likely due to the decreased concentration of the individual oligonucleotides used in the multiplexing experiment. (Figure 5 B) We observed that the co-incorporation frequency per cycle is less than the individual frequencies. (Figure 5 C) Additionally, the number of nonsense CAN1 or LYP1 mutants remained constant when no oligonucleotides were added to the transformation mix. (Figure 5 D)
Figure 5.
Cycling and multiplex modifications using YOGE in strain CEN.PK.RR at the CAN1 and LYP1 loci. All values represent the mean of three replicates with error bars representing the standard deviation. A. Diagram of YOGE electroporation cycling and recovery. B. Singleplex recombination frequencies for the CAN1 and LYP1 loci. C. Multiplex recombination frequency for both loci simultaneously. D. Rate of gene conversion without oligonucleotide added during electroporation, demonstrating that enrichment in loci modification is caused from transformation and not enrichment due to fitness advantage of any genotype.
Future Considerations
Generating targeted diversity or allelic replacement in yeast is crucial for rational strain engineering. Here we have identified the combinatorial strain modifications and optimized the key parameters important for oligonucleotide recombination. Furthermore, YOGE has achieved a single optimum oligonucleotide genomic incorporation frequency of 0.2 to 2.0% across all strains and loci tested without selection for the modification or stimulation for recombination by cleavage, which is at least 20 to 1000 fold higher than previously reported (4, 5, 7, 20, 21). In many cases, these levels of recombination may be suitable for moderate screening efforts to isolate correct recombinants. Furthermore, this method is multiplexable and the population can be enriched for the desired mutations with additional cycles. Given the most and least recombinogenic strains and loci recombination frequencies, (VL6-48.RR with 2% at the ADE2 locus and VTT.RR with 0.15% at the URA3 locus), along with their respective survival rate post8electroporation (VL6-48 with ~1.0% and VTT C-68059a with 0.1%) and a constant number of 108 cells used per electroporation, we can estimate a range of potential library size of 102 to 105 genomic recombinants per locus per cycle. As several yeast library generation protocols use 10-fold more cells for electroporation, it is likely that these values could increase if more diversity is required. While all transformations in this study were accomplished using electroporation, chemical methods (such as those relying solely on PEG and Lithium Acetate) don't require electroporation apparatus may be more easily adapted to future automated regimes. (14)
The generation of genetic diversity in a rational, site-specific way would allow for higher fitness mutant libraries that could be screened for desirable phenotypes. For industrial strain evolution, using mild selections to relevant phenotypes, such as crude feedstock tolerance or cellular waste product resistance, the relevant modifications in the populations could be further enriched. Furthermore, easy generation of multiple alleles would allow for investigation of epistastic interactions between DNA regulatory regions and proteins. For example, pathways for desirable compounds could be optimized via promoter tuning using oligonucleotides.(23) Additionally, changes could be made to protein coding regions to generate mutant protein variants. (24)
Enrichment of modified cells, without a phenotypic selection, would greatly ease recovery of generated mutants using YOGE. Employment of site directed DNA double-strand breaks at unmodified genomic loci could be used to enrich for correctly modified cells. Unrepaired double strand breaks cause cell arrest and reduce cellular fitness, meaning that cells containing the cut sites will be at a disadvantage, allowing for a fitness advantage to cells without these cut sites. By using designable site-specific endonucleases, such as the RNA-guided endonuclease Cas9 or transcription activator like endonucleases (TALENs), targeted to cleave wild-type unmodified alleles, a negative selection can be applied against these alleles to enrich the population for desired genotypes. (25)
The variability associated with YOGE across strains may stem from many factors. Individual differences between oligonucleotide recombination frequencies at different loci could be affected by protein occupancy at the locus, secondary structure of the donor oligonucleotide DNA, as well as replication dynamics proximal to the integration site. Furthermore, differences in strain oligonucleotide recombination profiles may relate to endogenous recombination proteome differences, along with reported transformation variability between strains. In addition we observed that the location of the integration of RAD51(K342E) and RAD54 expression cassettes can affect oligonucleotide recombination profiles. In the CEN.PK113-7D strain, integration of RAD51(K342E) and RAD54 expression cassettes in the same and at different safe harbor loci, result in different oligonucleotide recombination profiles, with the separate safe harbor loci integration method, resulting in a higher oligonucleotide recombination profile.
While the variability of oligonucleotide recombination between loci and strains may range by a factor of 10, cycling YOGE allows for step-by-step enrichment of desired genotypes allowing modifications to attain higher frequency with each cycle. In summary, YOGE is a useful method for integrating oligonucleotides with high frequency in yeast, with the ability to rapidly cycle the procedure. In combination with mild selections for desirable phenotypes or targeted endonuclease based DNA negative selections, the targeted modified sequences or desired phenotypes could be enriched to easily screenable levels.
Supplementary Material
Acknowledgements
The authors would like to thank Brad Zamft, Alejandro Chavez, Nikolai Eroshenko, and Xavier Rios for valuable discussions about results and future directions. We would also like to thank Fred Winston and Daniel Spatt for their generous donation of plasmids and knowledge.
Funding Sources
This work was funded by the Department of Energy [DE-FG02-02ER63445]; the Synthetic Biology Engineering Research Center from the National Science Foundation [SA5283-11210]. HHW is funded through the National Institutes of Health Director's Early Independence Award [Grant 1DP5OD009172-01].
Footnotes
Conflict of Interest
The authors declare no competing financial interest.
References
- 1.Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, Forest CR, Church GM. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijkeren J.-P. van, Britton RA. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wach A, Brachat A, Pöhlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 4.Storici F, Durham CL, Gordenin DA, Resnick MA. Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast. Proc. Natl. Acad. Sci. 2003;100:14994–14999. doi: 10.1073/pnas.2036296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moerschell RP, Tsunasawa S, Sherman F. Transformation of yeast with synthetic oligonucleotides. Proc. Natl. Acad. Sci. U. S. A. 1988;85:524–528. doi: 10.1073/pnas.85.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brachman EE, Kmiec EB. Targeted Nucleotide Repair of cyc1 Mutations in Saccharomyces cerevisiae Directed by Modified Single-Stranded DNA Oligonucleotides. Genetics. 2003;163:527–538. doi: 10.1093/genetics/163.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Cheng S, van Brabant AJ, Kmiec EB. Rad51p and Rad54p, but not Rad52p, elevate gene repair in Saccharomyces cerevisiae directed by modified single-stranded oligonucleotide vectors. Nucleic Acids Res. 2002;30:2742–2750. doi: 10.1093/nar/gkf397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storici F, Lewis LK, Resnick MA. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 2001;19:773–776. doi: 10.1038/90837. [DOI] [PubMed] [Google Scholar]
- 9.Gibson DG. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 2009;37:6984–6990. doi: 10.1093/nar/gkp687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson DG, Benders GA, Axelrod KC, Zaveri J, Algire MA, Moodie M, Montague MG, Venter JC, Smith HO, Hutchison CA. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome. Proc. Natl. Acad. Sci. 2008;105:20404–20409. doi: 10.1073/pnas.0811011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larionov V, Kouprina N, Solomon G, Barrett JC, Resnick MA. Direct isolation of human BRCA2 gene by transformation-associated recombination in yeast. Proc. Natl. Acad. Sci. 1997;94:7384–7387. doi: 10.1073/pnas.94.14.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larionov V, Kouprina N, Nikolaishvili N, Resnick MA. Recombination during transformation as a source of chimeric mammalian artificial chromosomes in yeast (YACs). Nucleic Acids Res. 1994;22:4154–4162. doi: 10.1093/nar/22.20.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijkamp JF, Broek M. van den, Datema E, Kok S. de, Bosman L, Luttik MA, Daran-Lapujade P, Vongsangnak W, Nielsen J, Heijne WH, Klaassen P, Paddon CJ, Platt D, Kötter P, Ham R. C. van, Reinders MJ, Pronk JT, Ridder D. de, Daran J-M. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb. Cell Factories. 2012;11:36. doi: 10.1186/1475-2859-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 15.Kow YW, Bao G, Reeves JW, Jinks-Robertson S, Crouse GF. Oligonucleotide transformation of yeast reveals mismatch repair complexes to be differentially active on DNA replication strands. Proc. Natl. Acad. Sci. 2007;104:11352–11357. doi: 10.1073/pnas.0704695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. Visualization of Eukaryotic DNA Mismatch Repair Reveals Distinct Recognition and Repair Intermediates. Cell. 2011;147:1040–1053. doi: 10.1016/j.cell.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HH, Xu G, Vonner AJ, Church G. Modified bases enable high-efficiency oligonucleotide-mediated allelic replacement via mismatch repair evasion. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costantino N, Court DL. Enhanced levels of λ Red-mediated recombinants in mismatch repair mutants. Proc. Natl. Acad. Sci. 2003;100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Maguire KK, Kmiec EB. Genetic re-engineering of Saccharomyces cerevisiae RAD51 leads to a significant increase in the frequency of gene repair in vivo. Nucleic Acids Res. 2004;32:2093–2101. doi: 10.1093/nar/gkh506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X-P, Galkin VE, Yu X, Egelman EH, Heyer W-D. Loop 2 in Saccharomyces cerevisiae Rad51 protein regulates filament formation and ATPase activity. Nucleic Acids Res. 2009;37:158–171. doi: 10.1093/nar/gkn914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez GP, Song JB, Crouse GF. Transformation with Oligonucleotides Creating Clustered Changes in the Yeast Genome. Plos One. 2012;7:e42905. doi: 10.1371/journal.pone.0042905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Rice MC, Drury M, Cheng S, Gamper H, Kmiec EB. Strand Bias in Targeted Gene Repair Is Influenced by Transcriptional Activity. Mol. Cell. Biol. 2002;22:3852–3863. doi: 10.1128/MCB.22.11.3852-3863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HH, Kim H, Cong L, Jeong J, Bang D, Church GM. Genome-scale promoter engineering by coselection MAGE. Nat. Methods. 2012;9:591–593. doi: 10.1038/nmeth.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HH, Huang P-Y, Xu G, Haas W, Marblestone A, Li J, Gygi SP, Forster AC, Jewett MC, Church GM. Multiplexed in Vivo His-Tagging of Enzyme Pathways for in Vitro Single-Pot Multienzyme Catalysis. Acs Synth. Biol. 2012;1:43–52. doi: 10.1021/sb3000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.