Abstract
Background and Purpose
Although the prothrombin G20210A mutation has been implicated as a risk factor for venous thrombosis, its role in arterial ischemic stroke is unclear, particularly among young-adults. To address this issue, we examined the association between prothrombin G20210A and ischemic stroke in a Caucasian case-control population and additionally performed a meta-analysis
Methods
From the population-based Genetics of Early Onset Stroke (GEOS) study we identified 397 individuals of European ancestry aged 15-49 years with first-ever ischemic stroke and 426 matched-controls. Logistic regression was used to calculate odds ratios in the entire population and for subgroups stratified by gender, age, oral contraceptive use, migraine and smoking status. A meta-analysis of 17 case-control studies (n=2305 cases <55 years) was also performed with and without GEOS data.
Results
Within GEOS, the association of the prothrombin G20210A mutation with ischemic stroke did not achieve statistical significance (OR=2.5,95%CI=0.9-6.5,p=0.07). However, among adults aged 15-42 (younger than median age), cases were significantly more likely than controls to have the mutation (OR=5.9,95%CI=1.2-28.1,p=0.03), whereas adults ages 42-49 were not (OR=1.4,95%CI=0.4-5.1,p=0.94). In our meta-analysis, the mutation was associated with significantly increased stroke risk in adults <=55 years (OR=1.4;95%CI=1.1-1.9;p=0.02) with significance increasing with addition of the GEOS results (OR=1.5;95%CI=1.1-2.0;p=0.005).
Conclusions
The prothrombin G20210A mutation is associated with ischemic stroke in young-adults and may have an even stronger association among those with earlier onset strokes. Our finding of a stronger association in the younger-young adult population requires replication.
Keywords: ischemic stroke, young, risk modifiers, risk factors, genetics
Introduction
Prothrombin (coagulation factor II, or FII) G20210A (rs1799963) is a single-nucleotide polymorphism (guanine to adenine; G→A) at position 20210 located at the 3’ untranslated region of the non-coding region of the prothrombin gene on chromosome 11.1 The minor A allele of this polymorphism is found in 2% of individuals of European-ancestry and is slightly less common than the minor A allele associated with Factor V Leiden (3.5%; rs6025).2,3 The prothrombin G20210A mutation (A allele) is exceedingly rare in those of African- or Asian-ancestry.2,3 Prothrombin is a precursor to thrombin, a key regulator of blood coagulation in the clotting cascade, and carriers of the prothrombin G20210A mutation have elevated blood plasma prothrombin levels.2 The prothrombin G20210A mutation plays a role in hypercoagulability and has been associated with a two-to-fourfold higher risk for venous thrombosis.3,4 Although this polymorphism has been well characterized for venous thrombosis, its role in arterial vascular disease still remains uncertain, particularly in young-adults with ischemic stroke. To address this issue, we examined the association between the prothrombin G20210A mutation and first-ever ischemic stroke in young Caucasian adults from the Genetics of Early Onset Stroke (GEOS) study. In addition, we also performed a meta-analysis of 17 previously published association studies of the prothrombin G20210A polymorphism and ischemic stroke in young-adults aged <=55
Methods
GEOS Study
The GEOS Study is a population-based case-control study designed to identify the genetic determinants of early-onset ischemic stroke and to characterize the interactions of identified stroke genetic variants with environmental risk factors such as smoking and oral contraceptive (OC) use. Cases aged 15-49 years with a first ischemic stroke were identified between 1992-2007 by discharge surveillance from one of 59 hospitals in the greater Baltimore/Washington, DC area and by direct referral from regional neurologists. Details of the recruitment of cases and controls have been previously published.5,6,7 In brief, cases and controls were recruited in 3 different time periods: Stroke Prevention in Young Women-1 (SPYW-1) conducted from 1992-1996, Stroke Prevention in Young Women-2 (SPYW-2) conducted from 2001-2003, and Stroke Prevention in Young Men (SPYM) conducted from 2003-2007. SPYW-1 included cases between 15-44 years of age who were recruited within one year of stroke and was designed with a 1:2 case-to-control ratio. SPYW-2 and SPYM included cases 15-49 years of age who were recruited within three years of stroke and was designed with a 1:1 case-to-control ratio. Control participants without a history of stroke were identified by random-digit dialing. Controls were balanced to cases by age and region of residence in each study and were additionally balanced for ethnicity in SPYW-2 and SPYM. Given the rarity of the prothrombin G20210A mutation in non-European ethnicities (only 2 of 392 non-Caucasians in our study had the mutation; both were cases), we limited our analyses to Caucasians, for whom there were 397 cases and 426 matched non-stroke controls.
The abstracted hospital records of cases were reviewed and adjudicated for ischemic stroke subtype by a pair of vascular neurologists according to previously published procedures5,6,7 with disagreements resolved by a third vascular neurologist. Stroke subtypes were classified using the Trial of ORG 10172 in Acute Stroke8 (TOAST) system. Ischemic strokes with the following characteristics were excluded from participation: stroke occurring as an immediate consequence of trauma, stroke within 48 hours after a hospital procedure, stroke within 60 days after the onset of a non-traumatic subarachnoid hemorrhage, and cerebral venous thrombosis. Additional exclusions for these genetic analyses were as follows: known single-gene or mitochondrial disorders recognized by a distinctive phenotype (e.g., cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy [CADASIL], mitochondrial encephalopathy with lactic acidosis and stroke-like episodes [MELAS], homocystinuria, Fabry disease, or sickle cell anemia); mechanical aortic or mitral valve at the time of index stroke; untreated or actively treated bacterial endocarditis at the time of the index stroke; neurosyphilis or other central nervous system infections; neurosarcoidosis; severe sepsis with hypotension at the time of the index stroke; cerebral vasculities by angiogram and clinical criteria; postradiation arteriopathy; left atrial myxoma; major congenital heart disease; and cocaine use in the 48 hours preceding their stroke.
Clinical and medical information, including age, ethnicity, and established stroke risk factors including history of hypertension, diabetes, myocardial infarction (MI), migraine with or without aura, current smoking status, and current OC use (both defined as use within 1 month before the event for cases and at a comparable reference time for controls), were collected during a standardized face-to-face interview and were included as covariates in our analysis. Blood chemistries were not measured among the controls, precluding case-control comparisons of hyperlipidemia.
The prothrombin G20210A polymorphism (rs1799963) was genotyped in all cases and controls at the Centers for Disease Control and Prevention as part of a custom 384-SNP GoldenGate assay according to the manufacturer's protocol (Illumina). Processed Universal-32 Beadchips were imaged on an Illumina BeadArray Reader, and GenomeStudio software (version 2011.1) was used to assess sample and assay quality. The genotyping call rate for rs1799963 was 100%.
Statistical analysis was performed using SAS software (version 9.2; SAS institute, Cary NC). The distributions of prothrombin G20210A and other characteristics among cases and controls were compared using t-tests for continuous variables and Mantel-Haenzel Chi-square tests for categorical variables. The association between prothrombin G20210A and ischemic stroke was then examined within pre-defined subgroups using Chi-square tests (or Fisher exact tests in the event of small sample sizes). Comparisons were repeated using a logistic regression model adjusted for age and gender in a “basic” model and for the basic model plus hypertension, diabetes, history of MI, current OC use, current smoking status, and migraine with aura in a “full” model. Two-tailed P values of <.05 were considered statistically significant.
Meta-Analysis
Using the key words “Prothrombin G20210A mutation”, “ischemic stroke”, and “young-adults” we searched PubMed and Web of Science data bases for case-control studies of ischemic stroke in young-adults published before June 2012. Any identified articles were then hand-searched for references to identify additional relevant studies. Studies were included in the meta-analysis according to standard criteria9 if: (1) neuroimaging was used to confirm clinical diagnoses of ischemic stroke; (2) controls were derived from the same population as cases; (3) prothrombin G20210A genotypes were available for all participants; (4) the numbers of cases and controls with and without prothrombin G20210A were provided in the article; and (5) the study included only cases with first stroke less than or equal to 55 years of age (most identified studies classified young stroke as ≤55 rather than less than or equal to ≤49 as consistent with GEOS), or the number of cases in this age group with and without prothrombin G20210A could be clearly obtained from the study. We also excluded case-series restricted to those with known patent foramen ovale (PFO).
Data analysis was performed using Comprehensive Meta-Analysis version 2.0 by Biostat (http://www.meta-analysis.com). The numbers of cases and controls stratified by prothrombin G20210A carrier status were extracted from each study, and odds ratios calculated. A pooled odds ratio was calculated using a variance-weighted approach under both fixed-effects and random-effects models. The fixed-effects model assumes the effects are the same across studies while the random-effect model allows for heterogeneity of effects. The calculation for the genetic effect of prothrombin variant assuming fixed-effects model was repeated with each of the studies individual removed from the analysis to confirm that no single study was principally responsible for the findings. Between-study heterogeneity was assessed using the Q-test, which is based on comparing the estimated study-specific treatment effects to the estimated overall treatment effect. We additionally computed the I2 statistic, which describes the proportion of variation across studies attributable to heterogeneity rather than chance.10
Results
GEOS
Clinical characteristics of cases (n=397) and controls (n=426) are summarized in Table 1. Cases were older than controls and were more likely to report a history of hypertension, diabetes, myocardial infarction, to be current smokers, to have history of migraine with aura and, among women, to be oral contraceptive users. A total of 20 subjects were carriers of a prothrombin G20210A minor A allele, 14 cases (3.5%) and 6 controls (1.4%). This difference was statistically significant (p = 0.05). Controls were in Hardy-Weinberg equilibrium for the prothrombin G20210A mutation. There was 1 homozygote in the study population, a case, which was also a Factor V Leiden heterozygote. As an established hypercoaulable state, by TOAST8 subtype criteria, this homozygote case was classified as a stroke of other determined etiology. Evaluating the distribution of 13 heterozygote cases with the prothrombin 20210A allele by TOAST8 stroke subtype demonstrated 1 cardioembolic, 1 large-artery atherosclerotic, 4 small vessel, and 7 undetermined etiology (cryptogenic).
Table 1.
Demographic and clinical characteristics of cases and controls of European ancestry in the Genetics of Early Onset Stroke (GEOS) Study.
| Cases (n=397) | Controls (n=426) | P value | |
|---|---|---|---|
| Gender, male | 62.7% | 55.4% | 0.03 |
| Mean age, years | 41.1 | 39.4 | 0.0004 |
| Hypertension | 31.7% | 16.0% | <.0001 |
| Diabetes Mellitus | 11.4% | 2.1% | <.0001 |
| Previous MI | 5.1% | 0.7% | 0.0002 |
| Current Oral contraceptive use* | 23.0% | 10.5% | 0.002 |
| Current smoking | 42.6% | 24.2% | <.0001 |
| History of migraines | 33.2% | 29.1% | 0.21 |
| Migraine with aura | 26.8% | 20.2% | 0.03 |
| Migraine without aura | 6.4% | 8.9% | 0.17 |
| Prothrombin G20210A mutation | 3.5% | 1.4% | 0.05 |
women only
Analysis of prothrombin G20210A and ischemic stroke risk are presented in Table 2 stratified by demographic and established risk factors for stroke, including gender, current smoking status, migraine, and OC use. In the overall Caucasian population, cases had more than a 2-fold greater odds of having the prothrombin G20210A mutation than controls, although this difference did not achieve statistical significance (14/397 cases vs. 6/426 controls; OR=2.5;95%=CI 0.9-6.5;p=0.07). Stratification by gender, current OC use (among women), current smoking status, and migraine did not alter these results. However, in a post-hoc analysis, the association was more pronounced in younger-onset cases (i.e., < the median age of 42 years; OR=5.9;95%CI=1.2-28.1;p=0.03) than in older onset cases (i.e., ≥ age 42; OR=1.4;95%CI=0.4-5.1;p=0.94).
Table 2.
Odds ratios for Prothrombin G20210A and ischemic stroke in young-adults of European Ancestry as stratified by risk factors.
| No. FII/No. of Cases | No. FII/No. of Controls | OR (basic model) | 95%CI | P value (*basic model) | P value (ψfull model) | ||
|---|---|---|---|---|---|---|---|
| Entire Population | 14/397 | 6/426 | 2.5 | 0.9 - 6.5 | 0.07 | 0.07 | |
| Gender | Male | 11/249 | 4/236 | 2.8 | 0.9 - 8.9 | 0.09 | 0.09 |
| Female | 3/148 | 2/190 | 1.9 | 0.3 - 11.8 | 0.47 | 0.47 | |
| OC use | Yes | 1/34 | 0/20 | NE | NE | NE | NE |
| No | 2/114 | 2/170 | 1.7 | 0.2 - 12.0 | 0.62 | 0.45 | |
| Current Smoking | Yes | 4/169 | 1/103 | 2.2 | 0.2 - 20.3 | 0.49 | 0.38 |
| No | 10/228 | 5/323 | 3.0 | 1.0 - 8.8 | 0.05 | 0.12 | |
| Migraine with Auraξ | Yes | 3/105 | 0/86 | NE | NE | NE | NE |
| No | 10/287 | 6/340 | 2.0 | 0.7 - 5.5 | 0.20 | 0.24 | |
| Age | <42 | 8/167 | 2/235 | 5.9 | 1.2 - 28.1 | 0.03 | 0.03 |
| ≥42 | 6/230 | 4/191 | 1.4 | 0.4 - 5.1 | 0.63 | 0.94 |
Includes: age and gender.
Includes: age, gender, hypertension, diabetes, previous myocardial infraction, current oral contraceptive use, current smoking, migraine with aura. NE = not estimable
Five case subjects did not have migraine information available.
Meta-analyses without and with GEOS data
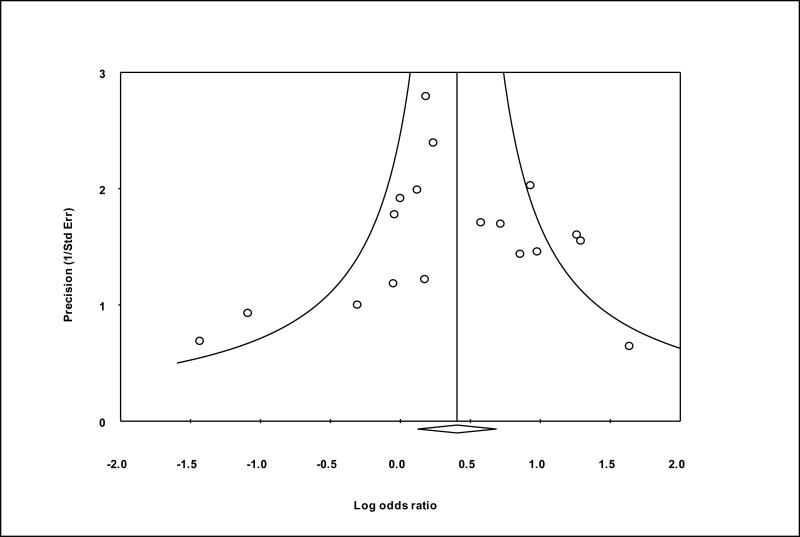
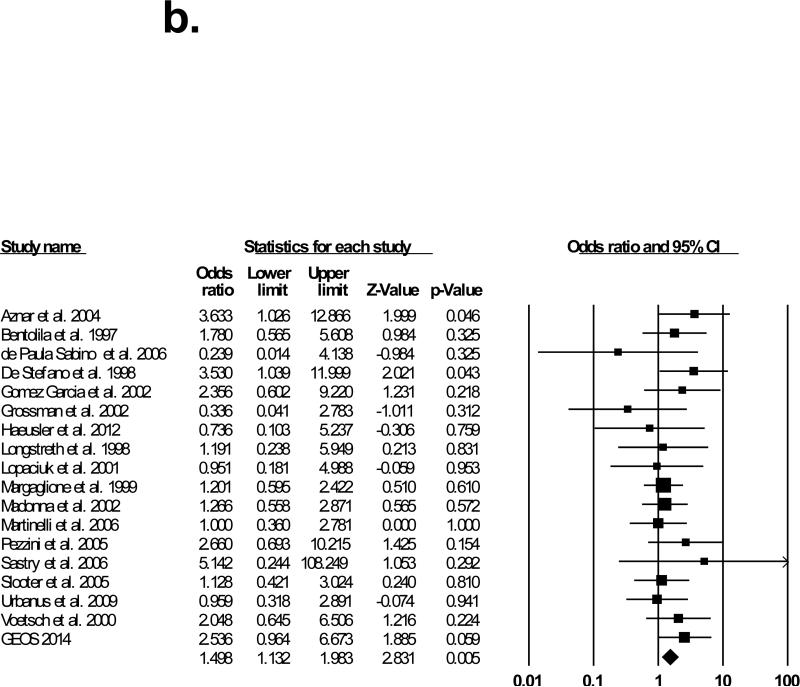
Seventeen studies matched our selection criteria and were included in the meta-analysis.11-27 Table 3 shows the demographic characteristics of studies included in the meta-analysis. The results of the meta-analysis performed with and without GEOS data are shown in Figure 1. The studies were conducted in several European countries (prothrombin G20210A prevalence is highest in Southern European countries2), the United States, and Brazil, and the majority of participants were of Caucasian ancestry. In 13 of these studies, the reported odds ratio for prothrombin G20210A and ischemic stroke was greater or equal to 1, and in two of these studies the odds ratio was significantly greater than 1 (p<.05). Across prior studies, prothrombin G20210A was detected in 80 out of 1908 cases (4.2%) and in 175 out of 5551 controls (3.2%) yielding an OR of 1.4 (95%CI=1.1-1.9;p-value=0.02). After including GEOS data, prothrombin G20210A was present in 94 of 2305 cases (4.0%) and in 181 of 5977 controls (3.0%), yielding a pooled OR of 1.5 (95%CI=1.1-2.0;p-value=0.005), based on fixed effect. There was no significant heterogeneity between the studies (Q-value=13.2;I2=0.0). Repeating the meta-analysis with each of the studies removed individually did not significantly alter the calculated odds ratio (data not shown). To assess publication bias, we created a funnel plot of all the studies used in the meta-analysis (Figure 2), which conformed to the expected shape of the curve and demonstrated overall left-right symmetry.28
Table 3.
Demographic Characteristics of Studies Included in Meta-Analysis
| Author(Year) | Country | N | Males/Females | Ethnicity | Mean Age (case/control) |
|---|---|---|---|---|---|
| Aznar(2004) | Spain | 343 | 105/238 | White | <50 |
| Bentolila(1997) | France | 259 | 139/120 | White | 40.6/34 |
| de Paula Sabino(2006) | Brazil | 328 | 72/256 | White/black/mestizo | 31/33.5 |
| De Stefano(1998) | Italy | 270 | 113/157 | White | 33.9/49 |
| Gomez Garcia 2002) | Netherlands | 136 | 61/75 | White | 36/37 |
| Grossman(2002) | Germany | 279 | 138/141 | White | 36/33 |
| Haeusler(2012) | Germany | 326 | 18/26 | White | 36/38.5 (median) |
| Longstreth(1998) | USA | 496 | 0/496 | White/black | 36.6/37.7 |
| Lopaciuk(2001) | Poland | 338 | 207/131 | White | 38.1/33.2 |
| Madonna(2002) | Italy | 394 | 183/211 | White | 38.4/36 |
| Margaglione(1999) | Italy | 1238 | 545/693 | White | 39/35 (median) |
| Martinelli(2006) | Italy | 398 | 0/398 | White | 34.7/34.9 |
| Pezzini(2005) | Italy | 321 | 169/152 | White | 35.0/34.8 |
| Sastry(2006) | UK | 202 | * | White | 33.2/33.2 |
| Slooter(2005) | Netherlands | 960 | 0/960 | White | 38.6/39.7 |
| Urbanus(2009) | Netherlands | 779 | 0/779 | White | 39/39 |
| Voetsch(2000) | Brazil | 378 | 153/217 | White/black | 33.3/34.1 |
Data Not Available
Figure 2.
Funnel plot of precision by log odds ratio
Discussion
Our meta-analysis of 2305 young-onset stroke cases reveals a moderately strong association between the prothrombin 20210A minor allele and young-onset ischemic stroke. While the GEOS results were not significant, they were of a similar magnitude and direction, and adding them to the meta-analysis increased the significance of the association. Moreover, stratifying the GEOS data by age of stroke-onset revealed the effect of the prothrombin allele to be most pronounced in the youngest subjects.
When we analyzed our GEOS population as a whole, we failed to find an overall statistically significant association between the prothrombin G0210A polymorphism and ischemic stroke in those of European ancestry. We further hypothesized that prothrombin G20210A might be associated with ischemic stroke risk in specific subpopulations, such as those with cryptogenic stroke or those having one or more vascular risk factors such as hypertension, diabetes, history myocardial infarction, oral contraceptive use, migraine headache, and current smoking status. We did not find an association among cryptogenic stroke patients, or those with cardiovascular risk factors as stratified individually or when analyzed in aggregate (results not shown). This could be due to the fact that stratification of the GEOS study population yielded small sample sizes with limited power to detect such associations.
However, in the GEOS population, we demonstrated that the prothrombin G20210A mutation is a significant risk factor in the youngest population of young-adults with ischemic stroke (age less than the median age of 42 in our study). This suggests that the prothrombin minor A allele has a stronger association among patients with earlier onset strokes. In the youngest group of young-adults with a ‘light’ disease burden, the prothrombin minor A allele could be a significant risk factor for ischemic stroke whereas in older individuals, the progression of disease and other risk factors makes the presence of the prothrombin minor A allele less of a contributing stroke risk factor. In other words, in the absence of traditional risk factors (e.g. smoking, oral contraceptive use, heart disease), the heritability of stroke risk may be enhanced. We also emphasize that from a clinical standpoint it is generally not advocated to perform screening “hypercoagulability workups” in all stroke patients.29, 30 Typically, patients to be screened for coagulation defects will have a prior history of one or more unexplained thromboembolic events. The yield for diagnosing a hypercoagulable state is typically greatest for young stroke patients or those with a family history of thrombosis and who have no other explanations for their stroke (i.e. cryptogenic stroke). 29, 30
While the GEOS study is among the largest study to date to have examined the association between prothrombin G20210A and ischemic stroke in young-adults, our study has several limitations. First, the low frequency of prothrombin 20210A minor allele in our study population limits our power to detect an association with ischemic stroke, further limiting subsequent stratified analysis by stroke subtype and vascular risk factors. Furthermore, because of the low frequency of the mutation in non-Caucasians, our study does not provide useful information about other ethnic subgroups. Second, because all but one of those with the prothrombin 20210A minor allele were heterozygotes, we do have statistical power to evaluate the effect of homozygosity. Third, the population-based design of the GEOS study with recruitment at more than 50 regional hospitals precluded consistent assessment of the presence of patent foramen ovale (PFO) and potential paradoxical embolism among cases. This is important because the prothrombin minor A allele can cause ischemic stroke through venous thrombosis and paradoxical venous-to-arterial embolus through a PFO, or potentially via the PFO in and of itself. In our meta-analysis, we specifically excluded studies that considered only stroke patients with the prothrombin 20210A minor allele and PFO, as risk has been shown to be consistently higher in this setting.31 However it is important to note that among the studies included in our meta-analyses, the absence or presence of a PFO was not consistently reported. As such, paradoxical embolism may have played a significant role in the pathogenesis of cryptogenic stroke occurring in those studies. Lastly, because blood chemistries were not obtained for the GEOS controls, we were unable to evaluate potential relationships between dislipidemia and the prothrombin 20210A mutation.
All studies included in this meta-analysis were case-control association studies. The case-control approach is an efficient design for studying genetic risk factors for early-onset stroke because the outcome, stroke in young-adults, is rare with a potentially long latency period. Although case-control studies in general can be prone to selection bias, this is unlikely in our study because the exposure is genotype. However, unlike cohort studies, case-control studies may be subject to survival bias because cases characterized by high fatality rates are less likely to be included in the study sample. Lastly, to reduce genotyping error, GEOS cases and controls were plated together for genotyping.
In conclusion, we report that the prothrombin G20210A mutation is associated with ischemic stroke in young-adults and may have an even higher association among the youngest group of young-adults. Specific to the GEOS data, in adults with first ever ischemic stroke before the age of 42, the prothrombin G20210A mutation may be a contributing factor. In PFO cases where a venous source is not identified, positive prothrombin G20210A screening might increase the likelihood that the PFO was involved. Our results suggest the need for the studies included in the meta-analysis to stratify their data by age groups to determine if the association is truly stronger in the younger age ranges, as suggested by the GEOS data.
Figure 1a.
Meta-analysis results without GEOS data
Figure 1b.
Meta-analysis with GEOS data
Acknowledgements
None.
Sources of Funding
This work was supported in part by the Department of Veterans Affairs, Baltimore, Office of Research and Development, Medical Research Service; the Department of Veterans Affairs Stroke Research Enhancement Award Program; the Department of Veterans Affairs, Baltimore, Geriatrics Research, Education, and Clinical Center of Excellence; the National Institute of Neurological Disorders and Stroke (NINDS) (Grants U01 NS069208-01 and R01 NS39987); the NIH Office of Research on Women's Health (ORWH) (Grant R01 NS45012); the National Human Genome Research Institute (NHGRI) (Grant U01 HG004436). The Centers for Disease Control and Prevention partially supported data collection and genotyping. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
Footnotes
Disclosures/Conflicts of Interest: None.
References
- 1.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- 2.Rosendaal FR, Doggen CJ, Zivelin A, Arruda VR, Aiach M, Siscovick DS, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79:706–8. [PubMed] [Google Scholar]
- 3.Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am. Soc. Hematol. Educ. Program. 2005;1:1–12. doi: 10.1182/asheducation-2005.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Bank I, Libourel EJ, Middeldorp S, Van Pampus EC, Koopman MM, Hamulyák K, et al. Prothrombin 20210A mutation: a mild risk factor for venous thromboembolism but not for arterial thrombotic disease and pregnancy-related complications in a family study. Arch Intern Med. 2004;164:1932–1937. doi: 10.1001/archinte.164.17.1932. [DOI] [PubMed] [Google Scholar]
- 5.Hamedani AG, Cole JW, Cheng Y, Sparks MJ, O'Connell JR, Stine OC, et al. Factor V Leiden and Ischemic Stroke Risk: The Genetics of Early Onset Stroke (GEOS) Study. Stroke. 2013;22:419–23. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CJ, Kittner SJ, McCarter RJ, Sloan MA, Stern BJ, Buchholz D, et al. Interrater reliability of an etiologic classification of ischemic stroke. Stroke. 1995;26:46–51. doi: 10.1161/01.str.26.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–74. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kapelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Dichgans M, Markus HS. Genetic association studies in stroke: Methodological issues and proposed standard criteria. Stroke. 2005;35:2027–31. doi: 10.1161/01.STR.0000177498.21594.9e. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aznar J, Mira Y, Vayá A, Corella D, Ferrando F, Villa P, Estellés A. Factor V Leiden and prothrombin G20210A mutations in young-adults with cryptogenic ischemic stroke. Thromb Haemost. 2004;91:1031–4. doi: 10.1160/TH03-11-0690. [DOI] [PubMed] [Google Scholar]
- 12.de Paula Sabino A, Ribeiro DD, Carvalho MG, Cardoso J, Dusse LM, Fernandes AP. Factor V Leiden and increased risk for arterial thrombotic disease in young Brazilian patients. Blood Coagul Fibrinolysis. 2006;17:271–5. doi: 10.1097/01.mbc.0000224846.35001.64. [DOI] [PubMed] [Google Scholar]
- 13.De Stefano V, Chiusolo P, Paciaroni K, Casorelli I, Rossi E, Molinari M, et al. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood. 1998;91:3562–5. [PubMed] [Google Scholar]
- 14.Gómez Garcia EB, van Goor MP, Leebeek FW, Brouwers GJ, Koudstaal PJ, Dippel DW. Elevated prothrombin is a risk factor for cerebral arterial ischemia in young-adults. Clin Neurol Neurosurg. 2002;104:285–8. doi: 10.1016/s0303-8467(01)00202-5. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann R, Geisen U, Merati G, Müllges W, Schambeck CM, Walter U, et al. Genetic risk factors in young-adults with 'cryptogenic' ischemic cerebrovascular disease. Blood Coagul Fibrinolysis. 2002;13:583–90. doi: 10.1097/00001721-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Haeusler KG, Herm J, Hoppe B, Kasabov R, Malzahn U, Endres M, et al. Thrombophilia screening in young patients with cryptogenic stroke: Prevalence of gene polymorphisms compared to healthy blood donors and impact on secondary stroke prevention. Hämostaseologie. 2012;32:147–52. doi: 10.5482/ha-1175. [DOI] [PubMed] [Google Scholar]
- 17.Margaglione M, D'Andrea G, Giuliani N, Brancaccio V, De Lucia D, Grandone E, et al. Inherited prothrombotic conditions and premature ischemic stroke: Sex difference in the association with factor V Leiden. Arterioscler Thromb Vasc Biol. 1999;19:1751–6. doi: 10.1161/01.atv.19.7.1751. [DOI] [PubMed] [Google Scholar]
- 18.Madonna P, de Stefano V, Coppola A, Cirillo F, Cerbone AM, Orefice G, et al. Hyperhomocysteinemia and other inherited prothrombotic conditions in young-adults with a history of ischemic stroke. Stroke. 2002;33:51–6. doi: 10.1161/hs0102.100483. [DOI] [PubMed] [Google Scholar]
- 19.Martinelli I, Battaglioli T, Burgo I, Di Domenico S, Mannucci PM. Oral contraceptive use, thrombophilia and their interaction in young women with ischemic stroke. Haematologica. 2006;91:844–7. [PubMed] [Google Scholar]
- 20.Bentolila S, Ripoll L, Drouet L, Mazoyer E, Woimant F. Thrombophilia due to 20210 G-->A prothrombin polymorphism and cerebral ischemia in the young. Stroke. 1997;28:1846–7. [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Rosendaal FR, Siscovick DS, Vos HL, Schwartz SM, Psaty BM, et al. Risk of stroke in young women and two prothrombotic mutations: Factor V leiden and prothrombin gene variant (G20210A). Stroke. 1998;29:577–580. doi: 10.1161/01.str.29.3.577. [DOI] [PubMed] [Google Scholar]
- 22.Lopaciuk S, Bykowska K, Kwiecinski H, Mickielewicz A, Czlonkowska A, Mendel T, et al. Factor V leiden, prothrombin gene G20210A variant, and methylenetetrahydrofolate reductase C677T genotype in young-adults with ischemic stroke. Clin Appl Thromb Hemost. 2001;7:346–350. doi: 10.1177/107602960100700418. [DOI] [PubMed] [Google Scholar]
- 23.Pezzini A, Grassi M, Del Zotto E, Archetti S, Spezi R, Vergani V, et al. A. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young-adults. Stroke. 2005;36:533–539. doi: 10.1161/01.STR.0000155741.31499.c2. [DOI] [PubMed] [Google Scholar]
- 24.Sastry S, Riding G, Morris J, Taberner D, Cherry N, Heagerty A, et al. Young Adult Myocardial Infarction and Ischemic Stroke: The Role of Paradoxical Embolism and Thrombophilia (The YAMIS Study). J Am Coll Cardiol. 2006;48:686–691. doi: 10.1016/j.jacc.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 25.Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213–7. doi: 10.1111/j.1538-7836.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 26.Urbanas R, Siegerink B, Roest M, Rosendaal F, de Groot P, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurology. 2009;8:998–1005. doi: 10.1016/S1474-4422(09)70239-X. [DOI] [PubMed] [Google Scholar]
- 27.Voetsch B, Damasceno BP, Camargo EC, Massaro A, Bacheschi LA, Scaff M, et al. Inherited thrombophilia as a risk factor for the development of ischemic stroke in young-adults. Thromb Haemost. 2000;83:229–33. [PubMed] [Google Scholar]
- 28.Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More than numbers: The power of graphs in meta analysis. Am J Epidemiol. 2009;169:249–55. doi: 10.1093/aje/kwn340. [DOI] [PubMed] [Google Scholar]
- 29.Tatlisumak T, Fisher M. Hematologic disorders associated with ischemic stroke. J Neurol Sci. 1996;140:1–11. doi: 10.1016/0022-510x(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 30.Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr. 2005;10:567–78. doi: 10.1017/s109285290001021x. [DOI] [PubMed] [Google Scholar]
- 31.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–5. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]