Abstract
Purpose
Whole tumor lysates are promising antigen sources for dendritic cell (DC) therapy for they contain many relevant immunogenic epitopes to help prevent tumor escape. Two common methods of tumor lysate preparations are freeze-thaw processing and UVB-irradiation to induce necrosis and apoptosis, respectively. Hypochlorous acid (HOCl)-oxidation is a new method for inducing primary necrosis and enhancing the immunogenicity of tumor cells.
Experimental Design
We compared DCs’ ability to engulf three different tumor lysate preparations, produce Th1-priming cytokines and chemokines, stimulate mixed leukocyte reactions (MLR), and finally elicit T-cell responses capable of controlling tumor growth in vivo.
Results
We demonstrated that DCs engulfed HOCl-oxidized lysate most efficiently, stimulated robust MLRs and elicited strong tumor-specific IFN-γ secretions in autologous T-cells. These DCs produced the highest levels of Th1-priming cytokines and chemokines, including IL-12. Mice vaccinated with HOCl-oxidized ID8-ova lysate pulsed DCs developed T-cell responses that effectively controlled tumor growth. Safety, immunogenicity of autologous DCs pulsed with HOCl-oxidized autologous tumor lysate (OCDC vaccine), clinical efficacy and progression free survival (PFS) were evaluated in a pilot study of five subjects with recurrent ovarian cancer. OCDC vaccination produced few grade 1 toxicities and elicited potent T-cell responses against known ovarian tumor antigens. Circulating T regulatory cells and serum IL-10 were also reduced. Two subjects experienced durable PFS of ≥24 months after OCDC.
Conclusions
This is the first study demonstrating the potential efficacy of a DC vaccine pulsed with HOCl-oxidized tumor lysate, a novel approach in preparing DC vaccine that is potentially applicable to many cancers.
Keywords: Ovarian carcinoma, dendritic cell, HOCl, whole tumor lysate, Th1 responses
Introduction
Accumulating evidence shows that cancer vaccines can induce immune responses and have resulted in clinical benefit in occasional subjects (1–3). However, the true potential of cancer vaccines has yet to be fully reached in the clinic. This is likely due to the difficulty in mounting a significant anti-tumor response in subjects with advanced disease because of pre-existing tolerance mechanisms that actively turn off immune recognition and/or disable effector T-cells in the tumor microenvironment. In addition, most molecularly defined tumor vaccines have used one antigen. One promising approach to address the latter limitation is to use whole tumor lysate for priming the cellular immune response, which could minimize or even prevent tumor escape. Two previous ovarian cancer clinical trials have utilized whole tumor lysate as a source of antigens (4–5). Other cancer trials in melanoma (6), renal cell carcinoma (7) and prostate cancer (8) used UVB-irradiated and/or freeze-thawed allogeneic or autologous tumor cells in combination with keyhole limpet haemocyanin protein for loading DCs, and showed moderate responses. Based on meta-analytical data by Neller et al., enhanced clinical efficacy is consistently observed in subjects vaccinated with DCs pulsed with whole tumor lysate compared to DCs pulsed with defined tumor-associated peptides or proteins (9). In fact, whole tumor lysates offer distinct advantages over defined antigens peptide vaccines, such as all subjects are eligible for the therapy regardless of their human leukocyte antigen (HLA) type. Our increased understanding of private antigens expressed by most tumors favors the use of autologous over allogeneic lysate. Whole tumor lysates also provide a source of all potential antigens, eliminating the need to identify the most optimal antigen to target in a particular type of cancer. Importantly, multiple tumor antigens can be targeted at once, thereby bypassing issues of tumor antigen loss. Whole tumor lysates can be prepared with various methods such as freeze-thaw cycles or ultraviolet B (UVB)-irradiation to induce primary necrosis and apoptosis, respectively. Selection of the optimal and most immunogenic whole tumor lysate preparation is needed to enhance the efficacy of whole tumor lysate vaccines.
Hypochlorous acid (HOCl) is a strong bactericidal oxidant produced by activated neutrophils in acute inflammation. HOCl is also capable of potentiating the immunogenicity of protein antigens to increase their uptake and processing by antigen-presenting cells (10–11) as well as activation of antigen-specific T lymphocytes (12). Treatment of SKOV-3 ovarian tumor cells by HOCl-oxidation induced rapid primary necrosis (13), enhanced the uptake of ovarian tumor cells by DCs and allowed for strong priming of ovarian tumor-specific T-cells (14). HOCl also improves protein antigen immunogenicity by tagging them with aldehydes (15–17) and unfolding them to expose immunogenic peptides to T-cells and for more efficient processing by DCs (18). In this study, we first compared three different types of whole tumor lysate preparations (i.e. HOCl-oxidation, UVB-irradiation or six cycles of freeze-thaw) for loading DCs. Through a series of ex vivo and in vivo mouse experiments, we selected HOCl-oxidation as the method of choice for preparing whole tumor lysate vaccine. We then designed and conducted a pilot clinical study to test the biological effects of HOCl-oxidized autologous whole tumor lysate-pulsed DCs (i.e. OCDC vaccine) in five subjects with recurrent ovarian cancer. We reported here the promising immunological and clinical results.
Materials and Methods
Human DC generation and maturation
DC generation was conducted as previously described (19). Normal donors’ monocytes were cultured in CellGenix DC media (CellGenix, Freiburg, Germany), 2% human AB serum (Valley Biomedical Inc., Winchester, VA), 2 mM L-glutamine, 100units/ml penicillin, 100 µg/ml streptomycin (Cellgro, Manassas, VA), 500 IU/ml human granulocyte-macrophage colony stimulating factor (GM-CSF) and 250 IU/ml interleukin (IL)-4 (PeproTech, Rocky Hill, NJ) after obtaining a written informed consent to a tissue and blood procurement study approved by UPenn’s IRB. Subjects’ elutriated monocytes were cultured with clinical grade GM-CSF (Leukine®, Bayer Healthcare Pharmaceuticals, Wayne, NJ) and animal-free research grade IL-4 (R&D Systems, Inc., Minneapolis, MN). After 4 days, CD11c, CD14 and HLA-DR were determined on DCs and were >70% pure. After lysate-loading, DCs were matured with lipopolysaccharides (LPS) [60 EU/ml; Escherichia coli O:113; gift from Dr. Suffredini at National Institute of Health] and IFN-コ (2000 IU/ml; Intermune, San Francisco, CA).
Human tumor lysate preparations and DC uptake
Methods for preparing autologous and allogeneic tumor lysates by by UVB-irradiation (UVB-L) and freeze-thaw cycles (FTL) have been previously described (20). The detailed methodology of preparing whole tumor lysates by HOCl-oxidation was previously published (13) and is described in supplementary materials and methods. After HOCl-oxidation or UVB-irradiation, tumor cells were subjected to 6 freeze-thaw cycles. For uptake experiment, HOCl-oxidized and UVB-irradiated SKOV-3 cells were not frozen and thawed before coculturing with DCs. Human DCs uptake of tumor cells was performed as previously described (13). SKOV-3 cells labeled with PKH26 (Sigma-Aldrich Corp., CA) were cocultured with normal donor DCs (1:1 ratio) for 4 hours at 37°C for active uptake, or 4°C for passive transfer to DCs. Percentage uptake by DCs was determined by gating on HLA-DR+PKH26+ cells in flow cytometry analysis performed on BD Canto (Becton Dickinson, Franklin Lakes, NJ). Data was analyzed with Pro CellQuest software.
Human DC and T-cell cytokine and chemokine analysis
Normal donor DCs were pulsed with HOCl-L of 3 ovarian tumor lines–SKOV-3, OVCAR5 and A1847 (ratio of 1:1:1 in the lysate mixture), whilst subject DCs were pulsed with HOCl-oxidized autologous tumor lysate in the presence of GM-CSF (500 IU/ml). After 8 hours stimulation with LPS and IFN-γ, supernatants were analysis. For T-cells, subject PBMCs were cocultured with OCDC, unpulsed autologous DCs or media only and the supernatant analyzed after 24 hours.
Mouse bone marrow-derived DC preparation
Bone marrow cells were isolated from hind leg femurs and tibias of mice, and plated at 1×106 cells/ml in complete IMDM media containing 10% FBS, 50 µM 2-mercaptoethanol, 2 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin and 1000 IU/ml GM-CSF. On day 3, floating cells representing granulocytes were removed and replenished with fresh complete IMDM media and 1000 IU/ml GM-CSF. Day 5, IL-4 was added at 100 IU/ml. Day 6, ID8-ova tumor lysate prepared by HOCl-oxidation, UVB-irradiation or 6 cycles of freeze-thawed were cocultured with DCs at 1:1 ratio for 20 to 24 hours. Then DCs were stimulated with LPS (120 EU/ml) and IFN-γ (4000 IU/ml) for 16 hours and used.
Vaccination of mice and tumor challenge
Mouse ID8-ova tumor line was generated to express surface SIINFEKL-H-2Kb complex by lentiviral transduction. Ovalbumin (OVA) plasmid vector pAC-Neo-OVA (21) [plasmid 22533; deposited by Dr. Michael Bevan] was obtained from Addgene (Cambridge, MA), and the full length OVA amplified with polymerase chain reaction and inserted into the pELNS lentiviral vector (22) [kind gift of Dr. Carl June from University of Pennsylvania]. MOI 5 was used, and cell surface SIINFEKL-H-2Kb complexes on transduced ID8 cells were detected by anti-mouse H-2Kb bound to SIINFEKL (Biolegend) after 24 hours of IFN-γ treatment. Positive cells were sorted by flow cytometery, and SIINFEKL-H-2Kb expressions were again confirmed after 1 week of culture and found to be 100% pure. Tumor lysates of ID8-ova were prepared (i.e. by HOCl-L UVB-L or FTL), cocultured with mouse DCs and matured as described above. Each mouse was inoculated with 5×106 live ID8-ova cells intraperitoneally 3 weeks prior to the first tumor-lysate-pulsed DC vaccination. Vaccine (1×106 DCs/dose in 50 µl PBS) was given intradermally once weekly for 3 weeks. For IFN-γ ELISPOT, SIINFEKL pentamer analysis, sera IL-10 and Treg cell phenotyping (see supplementary data), mice were sacrificed 2 weeks after the third vaccine. For tumor challenge, mice were sacrificed when they became distressed and moribund per University of Pennsylvania (UPenn) guidelines.
Clinical trial design and subjects
We performed a pilot study (UPCC-19809) in recurrent ovarian cancer subjects to determine the safety, immunogenicity and clinical efficacy of OCDC (i.e. HOCl-oxidized autologous whole tumor lysate-pulsed dendritic cell) vaccine. Eligible subjects were ≥18 years old; had undergone cytoreductive surgery with sufficient tumor harvested for lysate; had undergone physician’s choice post-operative chemotherapy; and had a baseline Eastern Cooperative Oncology Group (ECOG) performance status ≤1. Enrollment was allowed ≥4 weeks after surgery. The study (NCT01132014) was approved by the Food and Drug Administration under (BB-IND-14269) and UPenn’s Institutional Review Board (IRB). All patients gave written informed consent prior to initiation of any study procedures. Leukapheresis was performed 15 to 30 days prior to harvesting peripheral blood mononuclear cells (PBMCs) for OCDC vaccine manufacturing at Clinical Cell and Vaccine Production Facility (CVPF) at UPenn as described in supplemental material. Vaccines were cryopreserved at −140°C, thawed and washed before administration. Subjects received 5 doses of vaccines (~5–10 × 106 DCs/dose) intranodally every two weeks, and continued on a monthly maintenance regimen until disease progression or exhaustion of vaccine supply. Subjects were evaluated every 2 weeks. Safety was determined using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Subjects underwent computed tomography (CT) scan at enrollment (i.e. pre-vaccine) and ~4 weeks post-vaccination Day 86 (i.e. end of study [EOS]). Clinical response was determined using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. PBMCs were assessed at enrollment and at EOS with IFN-γ ELISPOT, HER-2/neu tetramer staining, tumor-specific T-cell cytokine assay, peripheral blood T regulatory cells (Treg cells) phenotyping, and sera IL-10 [described in supplemental data].
Statistical analysis
Comparisons among groups in Figures 1 and 2, and Supplementary Figure S1 were performed on natural log transformed data using ANOVA. For those comparisons found to be statistically significant by ANOVA, Tukey post hoc testing was used to conduct pairwise comparisons. The Kaplan–Meier method was used to estimate survival curves in Figure 1D and was compared using the log-rank test. In Figure 3, mean value was indicated by a line. In Figures 5 and 6, statistical significance was assessed using Student’s paired t test. A significance level of 0.05 or less was considered statistically significant. Analyses were performed in SPSS v19 (SPSS Inc, Chicago, IL).
Figure 1.
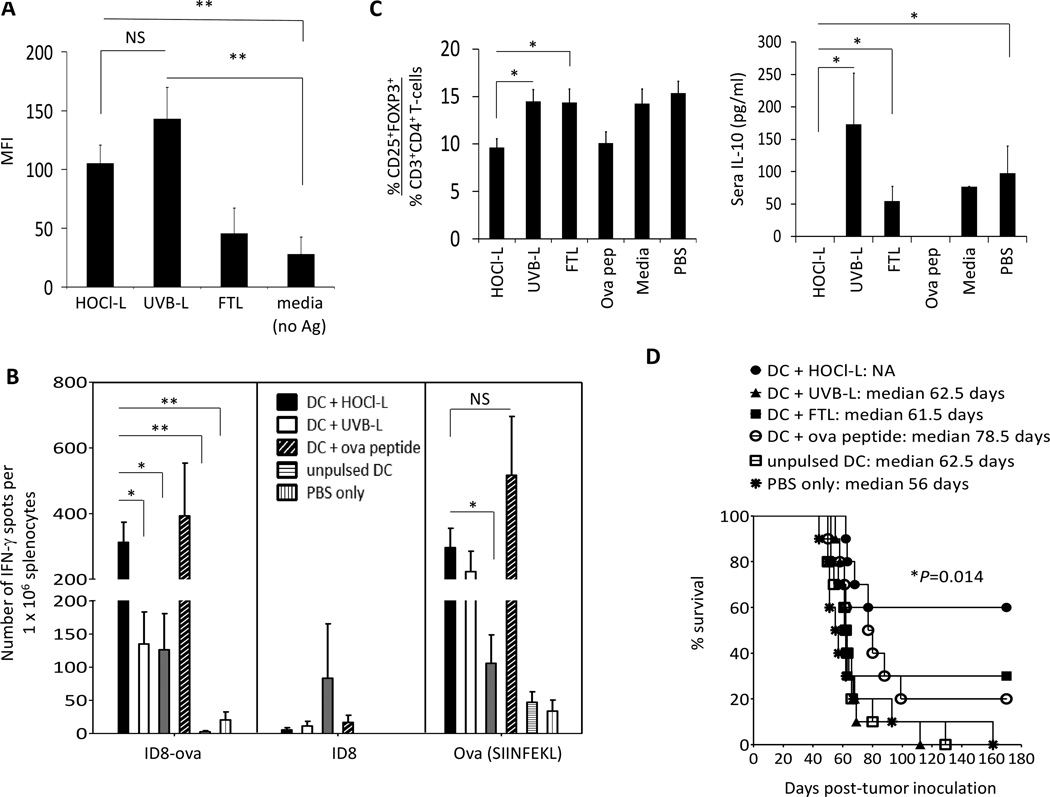
DCs pulsed with HOCl-L efficiently present tumor-associated SIINFEKL, stimulated SIINFEKL-specific CD8+ T-cell and prolonged survival in mice. A, mouse bone marrow-derived DCs were pulsed with HOCl-L, UVB-L or FTL lysate for 20 to 24 hours, matured with LPS and FIN-γ and evaluated for the expression of ovalbumin immunodominant SIINFEKL on cell surface (n per group=6). B, evaluation of the number of IFN-γ secreting tumor-reactive cells in the spleens of mice following DC-lysate vaccinations. Each vaccination group was represented by different color spot and the mean result was indicated as a line in each group. Data was from 2 independent experiments with 5 mice in each group. C, left panel, the % of CD3+CD4+CD25+ FOXP3+ Treg cells was quantified in spleens 2 weeks after the 3rd vaccine. Data was the mean of 3 independent experiments with 5 mice in each experiment. C, right panel, evaluation of IL-10 in sera 2 weeks following 3rd vaccine (n per group=15). D, 3 weeks after live ID8-ova tumor cell inoculation in the peritoneal, mice were vaccinated with different DC-lysate vaccines and their % survival was evaluated. PBS only (star) and unpulsed DC (open square) groups served as controls. Each curve represented data from 2 independent experiments of 5 mice per group per experiment (n per group=10). Data was presented as mean ± standard error of the mean (SEM). Double asterisks indicate results that were highly significant at P<0.001 and single asterisks indicate results that were significant at P<0.05.
Figure 2.
HOCl-oxidized tumor lysates enhanced DC uptake, improved cytokine and chemokine productions of DCs, stimulated potent MLRs and elicited tumor-specific FIN-γ secretions from autologous T-cells. A, normal donor DCs were cocultured with HOCl-oxidized SKOV-3 cells [HOCl-L], UVB-irradiated SKOV-3 cells [UVB-L] or freeze-thawed SKOV-3 lysate [FTL] for 4 hours at 37°C or 4°C to determine the % tumor cells/ lysate uptake by DCs. Bar chart was the quantitative representation of the average uptake from 6 different normal individuals. B, cytokine and chemokine profiles of lysate-pulsed DCs from 6 different normal individuals were assessed following 8 hours of LPS and FIN-γ activation. C, stimulation of MLRs by DCs (the averaged of six different normal individuals) pulsed with different tumor lysates preparations or unpulsed mature or immature. D, FIN-γ responses from autologous T-cells after 10 days of coculture with lysate-pulsed DCs or unpulsed DCs (i.e. media, no antigen) to live breast (MDA-231) and ovarian (OVCAR2) tumor lines, and T2 cells pulsed with HER-2/neu369–377 peptide or unpulsed. Data was presented as mean ± SEM. Asterisks indicate results that were significant at P<0.05.
Figure 3.
Subject DCs that were pulsed with HOCl-L matured normally with LPS and FIN-γ and exhibited a Th1 cytokine and chemokine profile. DCs prepared for subjects and pulsed with HOCl-oxidized autologous tumor lysate (i.e. OCDC vaccine) in the clinics were thawed and cultured in CellGenix DC media without GM-CSF and IL-4 for 24 hours and evaluated for cytokine and chemokine secretions. Each subject was represented by different color data point.
Figure 5.
Tumor-specific T-cells were elicited in subjects following OCDC vaccination. A, subjects’ PBMCs from pre-vaccine and EOS were evaluated for FIN-γ secreting cells in response to autologous DCs pulsed with autologous HOCl-oxidized lysate or to unpulsed autologous DCs. PBMCs alone were used as specificity control. The results were expressed as number of FIN-γ spots per 1×105 human PBLs. B, subjects’ PBMCs taken from pre-vaccine and EOS were assessed for Th1 (FIN-γ), Th2 cytokines (IL-10, IL4 and IL-5) and IL-17. C, ratio of tumor-reactive IFN-g secreting CD3+ cells and Treg cells in subjects’ PBMCs pre- and post-vaccinations. D, measurement of serum IL-10 in subjects at pre-vaccination and EOS.
Figure 6.
OCDC vaccines were Th1 priming and primed HER-2/neu specific T-cells that could be expanded ex vivo. A, PBMCs from HLA-A2+ subjects S3 and S4 were further tested for recognition to various HLA-A2 restricted ovarian TAAs. B, five rounds of OCDC vaccinations were able to prime HER-2/neu 369-specific CD8+ T-cells that could further be expanded in vitro following 10 days of stimulation with HER-2/neu369–377 peptide, IL-7 and IL-15 (10ng/ml each). C, expression of various ovarian TAAs was confirmed in the primary tumor of subject S4 with immunohistochemistry. Asterisks indicate results that were significant at P<0.05.
Results
Mouse DCs efficiently process HOCl-oxidized lysate
ID8 murine ovarian tumor cell line transduced with ovalbumin (ID8-ova) was used to compare the efficacy of mouse DCs pulsed with whole tumor lysate prepared in three different ways: 1) HOCl-oxidation followed by freeze-thaw (HOCl-L), 2) UVB-irradiation followed by freeze-thaw (UVB-L), and 3) 6 cycles of freeze-thaw (FTL). Ovalbumin is chosen as the model antigen because it is a well-characterized research tool, which is readily available to assess immune responses. Mouse bone marrow-derived DCs were cocultured with these different lysates, and matured with LPS and IFN-γ. DCs (gated on CD11c and MHC class II) were analyzed for crosspresentation of the MHC-I restricted immunodominant epitope OVA257–264 (SIINFEKL) using a specific antibody which recognized the SIINFEKL peptide bound to H-2Kb. Unpulsed DCs and DCs pulsed with the SIINFEKL peptides were used as controls. DCs pulsed with HOCl-L (**P=<0.001) and UVB-L (**P=<0.001) efficiently processed and presented the peptide SIINFEKL compared to unpulsed DCs (Figure 1A). DCs pulsed with HOCl-L or UVB-L were equally efficient in presenting the SIINFEKL peptide (NS; not significant) [Figure 1A]. All lysate-pulsed DCs produced IL-12p70 (data not shown).
HOCl-oxidized lysates yield improved DC vaccines
To test the efficacy of DCs pulsed with the different lysates, C56BL/6 mice were inoculated intraperitoneally with live ID8-ova tumor cells and allowed 3 weeks for tumor establishment. Mice were then vaccinated with DCs pulsed with HOCl-L, UVB-L, FTL or SIINFEKL peptide, while control mice received unpulsed mDCs or PBS only. Some mice were sacrificed 2 weeks post-vaccination (i.e. 56 days post-tumor inoculation) for immune analysis, while groups of mice were followed for survival.
To determine whether DCs pulsed with whole tumor lysate were able to cross-present and elicit T-cell responses against tumor-associated antigens, we cocultured splenocytes from vaccinated mice with live target ID8-ova cells at a 10:1 effector to target ratio. Mice vaccinated with HOCl-L DCs exhibited the highest mean number of FIN-γ secreting T-cells specific for ID8-ova cells (~300 spots/106 splenocytes) compared to mice vaccinated with DCs pulsed with UVB-L (~125 spots/106 splenocytes; *P=<0.05) or FTL (~120 spots/106 splenocytes; *P=<0.05), unpulsed DCs (~7 spots/106 splenocytes; **P=<0.001), or PBS only (~18 spots/106 splenocytes; **P=<0.001) [Figure 1B]. To test whether these tumor-reactive T-cells were directed towards OVA epitopes and also to additional tumor epitopes, we cocultured splenocytes from these mice with parent ID8 cells at the same effector to target ratio. We found that most of the IFN-γ tumor-reactive responses from mice in the HOCl-L-pulsed DC group seemed to be directed against OVA with very little reactivity against ID8 epitopes (Figure 1B).
To verify the presence of T-cells specific to OVA epitopes, we pulsed splenocytes with the SIINFEKL peptide. We found that HOCl-L-pulsed DCs elicited a relatively high frequency of SIINFEKL-reactive IFN-γ secreting CD8+ T-cells, which was comparable to those elicited by SIINFEKL peptide-pulsed DCs (NS; not significant) [Figure 1B]. UVB-L-pulsed DCs elicited a similar number of SIINFEKL-reactive IFN-γ secreting CD8+ T-cells as HOCl-L-pulsed DCs, but FTL-pulsed DCs yielded a smaller frequency of such cells (*P=<0.05 when compared to HOCl-L-pulsed DCs), in agreement with the relative ability of DCs to crosspresent the SIINFEKL peptide. The superior ability of HOCl-L-pulsed DCs to elicit SIINFEKL-specific CD8+ T-cells was confirmed by pentamer staining (Supplementary Figure S1A).
Given that whole tumor lysate potentially contains large number of potential epitopes for priming CD4+ and CD8+ T-cell responses, we investigated whether CD4+ tumor-specific IFN-γ secreting T-cells were also being elicited by the DC-whole tumor lysate vaccines. Using intracellular staining, we found that mice that were vaccinated with DCs pulsed with HOCl-L developed a higher percent of CD4+ T-cells that recognized and secreted IFN-γ in response to live ID8-ova cells (0.31%) compared to mice that were vaccinated with DCs pulsed with UVB-L (0.14%) or FTL (0.13%) [Supplementary Figure S1B]. Vaccination of mice with DCs pulsed with HOCl-L stimulated significantly higher percent of CD4+ T-cells compared to vaccination with unpulsed DCs or PBS only (*P=<0.05). Interestingly, a higher frequencies of SIINKEFL-specific CD8+ and overall tumor-specific IFN-γ T-cell responses were also elicited in the mice that received DCs pulsed with HOCl-L (Figure 1B and Supplementray Figure S1A), suggesting that the development of stronger anti-tumor CD4+ T-cell responses by the DCs pulsed with HOCl-L might help in stimulating stronger overall anti-tumor Th1 responses in this tumor model. Mice vaccinated with HOCl-L-pulsed DCs showed the lowest mean level of splenic Treg cells (9.61%), while mice that received UVB-L- or FTL-pulsed DCs had 14.45% (*P=<0.05) and 14.34% (*P=<0.05) splenic Treg cells, respectively [Figure 1C; left panel]. In addition, mice vaccinated with HOCl-L-pulsed DCs had no detectable IL-10 in sera, while mice received UVB-L-pulsed DCs (mean of 173.22 pg/ml; *P=<0.05) or FTL-pulsed DCs (mean of 54.67 pg/ml; *P=<0.05) had detectable serum levels of IL-10 [Figure 1C, right panel]. Mice receiving only PBS exhibited a mean 15.35% of splenic Treg cells and had detectable serum IL-10 (mean of 97.3 pg/ml; *P=<0.05).
To test the efficacy of vaccination in vivo, mice were followed for survival. Mice vaccinated with HOCl-L-pulsed DCs showed the longest overall survival, with median survival not reached at 180 days (60% alive at 180 days), whereas the median survival of all other groups was 78.5 days or less (P=0.014; log-rank test) [Figure 1F]. Interestingly, although strong responses against SIINFEKL were detected in mice vaccinated with DCs pulsed with SIINFEKL peptide or with UVB-L [Fig, 1B and C], this was insufficient for tumor control, and 80% and 100% of the mice from these groups, respectively, succumbed to ascites and tumor progression (Figure 1D). Thus, HOCl-L-pulsed DCs were able to crosspresent tumor-associated immunodominant epitopes, elicited the highest frequency of IFN-γ secreting tumor-reactive CD8+ and CD4+ T-cells, and achieved the best tumor response in vivo. This was also associated with a lower frequency of detectable splenic Treg cells, and absence of serum IL-10. These results were encouraging and worthy of further clinical translation.
HOCl oxidation enhances the uptake of tumor lysate by human DCs
To define the optimal whole tumor lysate for clinical application, we compared the above three methods of whole tumor lysate preparation for pulsing human DCs – i.e. HOCl-L, UVB-L and FTL. Due to the limited availability of PBMCs from ovarian cancer subjects, we evaluated PBMC-derived immature (i)DCs from normal donors to take up the different lysate preparations. We cocultured iDCs with HOCl-L, UVB-L or FTL of SKOV-3 cells. DCs took up more HOCl-L compared to UVB-L or FTL (*P=<0.05) by 4 hours at 37°C (Figure 2A). Passive transfer of tumor fragments to DC surfaces at 4°C was negligible, confirming that lysate uptake was an active process.
Following lysate uptake, DCs matured within 16 hours of stimulation with LPS and IFN-γ, as assessed by upregulation of HLA-DR, CD40, CD80, CD86, ICAM-1 and CCR7 to levels similar to those of control unpulsed mature (m)DCs stimulated with LPS and IFN-γ (Supplementary Figure S2), which were significantly higher than baseline expression in control unpulsed iDCs. There were no significant differences amongst DCs pulsed with the three different lysate types.
Human DCs pulsed with HOCl-L produce high levels of Th1 priming cytokines and chemokines, and primed strong responses to ovarian tumor-associated antigen
We further characterized the cytokine and chemokine profiles of DCs from normal donors pulsed with tumor lysates of three ovarian tumor lines (i.e. SKOV-3, A1847 and OVCAR5; used at 1:1:1 ratio for DC loading). Culture supernatants were evaluated 8 hours following treatment with LPS and IFN-ΰ. HOCl-L-pulsed DCs produced higher levels of IL-12, MIP-1a and MIG (100-fold, 20-fold and 15-fold higher, respectively) when compared to unpulsed mDCs [Figure 2B]. It was noted that HOCl-L-pulsed DCs produced significantly higher level of IL-12 compared to UVB-L- or FTL- (*P=<0.05) pulsed DCs [Figure 2B]. More than 2-fold increases were also detected in IL-1Ra, IL-1β, IL-15, IFN-0α, IP-10 and RANTES in the HOCl-L-pulsed DC supernantants when compared to unpulsed mDCs (Figure 2B). IL-10 secretion from HOCl-L-pulsed DCs was increased by about 2-fold but was very low compared to the 100-fold increase observed with IL-12, which is a Th1-polarizing cytokine (Figure 2B). A 2-fold increase in IL-8 level was also observed from HOCl-L-pulsed DCs, however it was very low compared to the 100-fold increase in IL-12. No increase was seen with IL-6 and Th2-priming cytokines such as IL-4, IL-5 and IL-13 from HOCl-L-pulsed DCs (data not shown). We did not assess TGF-β in our Luminex analysis. Comparable robust mixed leukocyte reactions (MLRs) were observed from DCs pulsed with HOCl-L, UVB-L or unpulsed mDCs [Figure 2C]. Poor MLRs were observed from DCs pulsed with FTL or unpulsed iDC. We further observed that DCs pulsed with HOCl-L made from SKOV-3 cells were more potent than DCs pulsed with UVB-L or FTL of SKOV-3 (P=0.030) in priming IFN-γ production in naïve autologous normal donor T-cells following 10 days of coculture. These primed T-cells efficiently recognized live MDA-231 breast and OVCAR2 ovarian tumor lines that overexpressed HER-2/neu, as well as HER-2/neu369–379 peptide pulsed onto HLA-A2-expressing T2 lymphoblasts (compared to T-cells primed with UVB-L- or FTL-pulsed DCs: *P=<0.05) [Figure 2D]. Low IFN-γ production was observed in the presence of unpulsed T2. Unpulsed mDCs (i.e. media [no antigen]) were used as control. In summary, human DCs pulsed with HOCl-L exhibited favorable lysate uptake and cytokine/chemokine profiles, stimulated robust MLRs and ovarian tumor reactivity, and were therefore suitable for clinical translation.
Autologous HOCl-L-pulsed matured DCs are suitable for therapy
We tested HOCl-L-pulsed DCs for vaccine production in a pilot study for recurrent ovarian cancer. Elutriated monocytes were obtained from subjects’ PBMCs after leukapheresis, and cultured in cell factories with GM-CSF and IL-4 as described in Materials and Methods and in Supplementary Figure S3. On day 4 of culture, DCs were pulsed with HOCl-oxidized autologous tumor lysate for 16 to 20 hours, followed by treatment with LPS and IFN-γ for 6 to 8 hours. On day 5, matured oxidized ovarian cancer lysate-pulsed DCs (OCDC) were harvested and cryopreserved until clinical use.
To test the functionality of OCDC, vaccine aliquots from 5 subjects (S1-S5; see Table 1 for subjects’ demographics) were rapidly thawed, washed and cultured in fresh complete CellGenix DC media for 24 hours without GM-CSF and IL-4. Culture supernatants were then analyzed by Luminex cytokine and chemokine array. Similar to fresh HOCl-L-pulsed DCs of normal donors, thawed OCDC produced high levels of Th1-priming cytokines and chemokines such as IL-1Ra, IL-12, MIP-α, MIP-β, MCP-1, MIG, IP-10, RANTES and TNF-α, and low levels of Th2 cytokines such as IL-4, IL-5, IL-10 and IL-13 (Figure 3). These results also indicated that cryopreservation did not affect the ability of OCDC vaccine to produce strong Th1 proinflammatory cytokines and chemokines. A complete cytokine and chemokine analysis of each subject was presented in Supplementary Table S1.
Table 1.
Patient demography and outcome of OCDC vaccine treatment.
| Subject ID |
Age | Stage of disease |
Number
of Prior ChemoX |
Number
of vaccines received |
Purity of vaccine |
Response to vaccine | |
|---|---|---|---|---|---|---|---|
| %CD86 | %HLA-DR | ||||||
| S1 | 48 | IIIC | 3 | 3 | 92.45% | 72.42% | PD |
| S2 | 46 | IV | 4 | 5 | 88.20% | 71.10% | SD |
| S3 | 49 | IIC | 2 | 5 | 94.97% | 88.75% | SD |
| S4 | 63 | IIIC | 7 | 5 | 82.20% | 74.51% | mixed response |
| S5 | 59 | IIIC | 2 | 5 | 81.11% | 82.53% | PD |
Note: PD- progressing disease; SD- stable disease.
OCDC vaccine is safe, and may offer clinical benefit in subjects with advanced recurrent ovarian cancer
Five subjects (age 48–63) were enrolled in UPCC-19809. The OCDC vaccine was tested and found to be negative for mycoplasma, bacterial and fungi, contained <5 EU/ml endotoxin and >70% CD86+ HLA-DR+ (see supplementary materials and methods). The OCDC vaccine was also found to be free from residual HOCl solution (data not shown). The HOCl-oxidized whole tumor lysate was non-toxic to subjects’ DCs as the DCs remained highly viable after lysate-pulsing, cryopreservation and thawed (please see Supplementary Fig. S2, last column). Thus the vaccine product met release criteria in all subjects (Table 1) and the yield of DCs was correlated with monocyte count derived from the leukapheresis (Supplementary Table S2). OCDC were administered through direct injection into the one to two groin lymph nodes bilaterally under ultrasound guidance. All subjects completed 5 vaccinations (Figure 4A), except S1 who withdrew after three vaccinations due to disease progression. A total of 45 intranodal vaccinations were performed. All vaccines were well tolerated and most toxicities were <grade 2 (Supplementary Table S3). A common side effect was flu-like symptomatology (i.e. fatigue, fever and chills), which was attributed to systemic cytokine activation induced by the vaccine (see below) and possibly by any residual LPS that remained in the OCDC vaccine.
Figure 4.
A, OCDC vaccine schedule of subjects. B, line graph showed regression or stabilization of 6 of 13 tumor metastatic deposits at 128 days (i.e. at EOS II) in subject S4, and CT imaging showed 3 additional nodules with central necrosis/cavitation (enlarged picture) at 128 days. C, taking into account the entire salvage treatment sequence of surgery, adjuvant therapy and ODCD, subject S2 experienced a second progression-free survival (PFS) of 36 versus an initial PFS of 26 months
Three subjects (S1, S4 and S5) entered the study with radiographically measurable disease, and two subjects (S1 and S5) progressed while one subject (S4) showed a mixed response by RECIST criteria at EOS. Subject S4 showed radiographic progression in 13 lesions at EOS (Day 86) via CT [Figure 4B]. However 6 weeks later in the absence of additional therapy, she underwent repeat CT imaging (day 128; i.e. EOS II) and showed regression or stabilization of 6 of 13 tumor metastatic deposits. Interestingly, 3 additional nodules, which were measured as increased, at this time showed central necrosis (cavitation) [Figure 4B; see enlarged CT].
Two subjects (S2 and S3) entered the study with no evidence of disease (NED) after tumor recurrence, debulking surgery and adjuvant chemotherapy. These subjects experienced durable progression-free intervals of 36 and 44 months. Both subjects achieved remission inversion [i.e. subjects experienced a longer second progression-free survival (PFS2) after vaccine therapy compared to the PFS after previous chemotherapy (PFS1)] in response to OCDC vaccination. PFS is important in assessing studies of novel chemotherapy or immunologic treatment strategies for patients with relapsed disease, and was first used was first used by Lee et al to predict PFS2 in patients with relapsed acute myeloid leukemia (23) (Figure 4C). As the historic remission inversion rate (PFS2 > PFS1) in ovarian cancer is 3% (24), these result are encouraging given the propensity of recurrent ovarian cancer to commonly recur in shorter intervals than the previous remission interval.
OCDC vaccine elicits a tangible Th1 antitumor immune response
To analyze the effects of vaccination, we quantified tumor-specific T-cells in pre- and post-vaccine PBLs by 40 hours IFN-γ ELISpot using aliquots of OCDC vaccine (DCs pulsed with autologous tumor lysate) as the antigen-presenting platform presenting whole tumor antigen. Unpulsed autologous DCs developed exactly as OCDC (with GM-CSF/IL-4 followed by LPS/IFN-γ, except for lysate pulsing) were used as controls. We detected a significant increase in the frequency of tumor-reactive T-cells post-vaccination at EOS in subjects S1 (P=0.044), S2 (P=0.025 at EOS and P=0.018 at 6 months post-EOS), S3 (P=0.005) and S4 (P=0.031), while all except subject S1 exhibited clinical benefit (Figure 5A). We did not detect a significant increase in tumor-reactive T-cells in subject S5 post-vaccination (P=0.312; NS).
In similar experiments as above, we characterized the cytokine profile of T-cells at EOS by ex vivo stimulation with OCDC vaccine for 24 hours. OCDC vaccination induced strong Th1 responses as assessed by the high levels of IFN-γ secreted by EOS T-cells over 24 hours (Figure 5B, first panel). Importantly, these EOS T-cells did not exhibit features of Th2, Th3 or Th17 cells, as judged by the almost undetectable IL-4, IL-5 and IL-10, and very low levels of IL-17, respectively. Very low or undetectable levels of IFN-γ, IL-4, IL-5, IL-10 and IL-17 were observed from OCDC vaccines alone that served as controls (Figure 5B; diagonal bar). The above results also indicated that the OCDC vaccine generated ex vivo were robust antigen-presenting cells as demonstrated by their ability to stimulate strong tumor-specific IFN-³ secretions from T-cells of subjects at EOS.
Treg cells have been associated with poor outcome in ovarian cancer (25) and experimentally depleting them has shown to be critical for tumor suppression (26). We measured the effects of vaccination on peripheral Treg cells. We observed a reduction in peripheral blood Treg cells at EOS in subjects S3, S4 and S5 (Supplementary Figure S4A). We also determined the tumor-reactive IFN-γ secreting CD3+ cells to CD3+CD4+CD25+FOXP3+ Treg cells ratio in the peripheral blood and observed a 1.5 to 3.6-fold increase in the ratio in subjects S2, S3, S4 and S5 at EOS. Subject S1 showed no change in the ratio (Figure 5C, left panel). For subject S2, we followed T-cells beyond EOS during maintenance vaccination and observed that peripheral Treg cells were decreased to approximately 2% at 6 months post-vaccination (Supplementary Figure S4B). At the same time, the number of IFN-γ secreting tumor-reactive T-cells in subject S2 was increased by 1.7-fold (Figure 5A), resulting in a 7.5-fold increase in the tumor-reactive IFN-γ secreting CD3+ cells to total peripheral Treg cell ratio (Figure 5C, right panel). In summary, peripheral Treg cells were reduced in subjects S2, S3, S4 and S5 following OCDC vaccinations and during maintenance.
Lastly, we characterized the serum cytokine changes following intranodal administration of OCDC vaccine. We detected a potent systemic inflammatory activation with ≥0.5-fold increase in serum IL-1β, IL-2, IL-15, TNF-α, MCP-1, MIP-1α, MIG and eotaxin in at least 2 out of 5 subjects (Supplementary Figure S4C). There was no increase in Th2-associated cytokines, including IL-4, IL-5 and IL-13 in the sera of all the subjects (data not shown). In particular, we observed decreases in serum IL-10 levels ranging from 0.24 to 2.28 pg/ml in subjects S1 to S4 at EOS [Figure 5D] who also demonstrated tumor-reactive IFN-³ responses after OCDC vaccinations.
OCDC vaccine efficiently crosspresents and elicits polyclonal CD8+ responses to common ovarian cancer antigens
Ovarian cancers express a variety of well-characterized tumor-associated antigens [TAAs] (27). To test whether such response was directed towards any known TAAs, we took advantage of the finding that subjects S3 and S4 were HLA-A*02-positive. We detected a post-vaccine increase in T-cells directed against several HLA-A2-restricted epitopes of known ovarian-specific antigens, including a MHC class II-restricted HER-2/neu epitope (S4: P=0.045), as well as MHC class I-restricted epitopes of MUC-1 (S4: P=0.047), NY-ESO-1 (S3: P=0.033, and S4: P=0.02), WT1 (S4: P=0.001), mesothelin (S4: P=0.008) survivin (S4: P=0.041) and hTERT (S4: P=0.016) [Figure 6A]. Detection of these polyclonal tumor-specific CD8+ T-cell responses thus indicated that the OCDC vaccine was highly efficient in crosspresentation of the ovarian TAAs. We confirmed the increase in HER-2/neu 369-specific CD8+ T-cells in subjects S3 and S4 post-vaccination by tetramer analysis, and we expanded these cells when PBMCs taken at EOS were pulsed with HER-2/neu369–377 peptide (Figure 6B). Expressions of HER-2/neu, mesothelin and WT1 were further confirmed by immunohistochemistry on pre-vaccine tumor sample for subject S4 (Figure 6C).
Discussion
Whole tumor lysates are a promising source of antigen for DC-based vaccine therapy as they contain many relevant immunogenic epitopes for priming the immune system against tumors (28). Autologous whole tumor lysates that carry private tumor-associated antigens might be especially important in stimulating long-lasting anti-tumor responses in cancer subjects. However, whole tumor lysates can be prepared in several ways, and the methods of inducing cell death or the chemical modification of proteins could impact the immunogenicity and efficacy of the therapy (28). We sought to address which type of whole tumor lysate preparation, if any, is optimal for use with DCs.
Freeze-thaw cycles and UVB-irradiation are the two most commonly used methods of whole tumor lysate preparations in the clinic. HOCl-oxidation induces primary necrotic tumor cell death (13). HOCl is also unique in its ability to enhance protein immunogenicity allowing for improved tumor protein antigen processing and presentation by DCs (13–14). In our mouse study, we found that DCs pulsed with HOCl-oxidized ID8-ova lysate were able to cross-present SIINFEKL efficiently. In comparison, DCs pulsed with FTL were inefficient in presenting SIINFEKL. Accordingly, DCs pulsed with HOCl-oxidized ID8-ova lysate produced the best results with respect to tumor suppression in vivo, and led to 60% survival in mice. We found that most of the tumor-reactive T-cells elicited by the vaccine were directed against OVA epitopes (directly confirmed by CD8+ T-cells recognizing SIINFEKL), since vaccine-primed T-cells directly recognized ID8-ova cells but not the parental ID8 cells. This finding was interpreted as encouraging, since it proved that DCs could cross-present an immunodominant epitope admixed with the whole tumor lysate as efficiently as DCs pulsed only with the purified peptide. The fact that DCs did not elicit a potent response against other ID8 antigens was not considered disappointing since immunodominant epitopes of xenoantigens can easily skew immune responses at the expense of less immunogenic tumor antigens. We detected a higher frequency of tumor-specific IFN-γ CD4+ T-cell responses in mice that received DCs pulsed with HOCl-L compared to mice receiving DCs pulsed with UVB-L or FTL. As CD4+ T-cells help is required in the generation of antigen-specific CD8+ T-cell responses, it is reasonable to postulate that the development of a stronger anti-tumor CD4+ T-cell response by the HOCl-L pulsed vaccine helped in stimulating a stronger overall anti-tumor Th1 response.
Although human monocyte-derived DCs can behave differently than mouse bone marrow-derived DCs, we confirmed the superior immunogenicity of human DCs pulsed with HOCl-L over human DCs pulsed with UVB-L or FTL due to a) their ability to maintain a phenotype that had been correlated with the induction of a type 1 antitumor response, i.e. expressing high levels of MHC class I and II molecules, high levels of costimulatory surface molecules of CD80, CD86 and CD40, and high levels of CCR7, and secreting high amounts of IL-12 and other Th1-polarizing cytokines and chemokines, and low amounts of Th2-priming cytokines (such as IL-4, IL-5, IL-10 and IL-13); and b) the ability to elicit a significant increase in tumor-specific IFN-γ secreting T-cells in peripheral blood assays, including ELISPOT. Our DC vaccine met all these criteria in the subjects S2, S3 and S4 who experienced clinical benefit or mixed response.
We tested the above DC vaccine in five subjects with stage II/IV recurrent ovarian in a pilot clinical study, using a short DC manufacturing protocol optimized for generating HOCl-oxidized autologous tumor lysate vaccine (OCDC) as previously reported (19). Subjects’ DCs behaved similar to normal donor DCs, producing high levels of IL-12 and other important Th1 cytokines and chemokines such as IL-1Ra, IL-12, MIP-1α, MIP-1β, MCP-1, MIG, IP-10 and RANTES. Following vaccination, we confirmed a potent systemic inflammatory activation in these subjects, as judged by increased serum IL-1β, IL-2, IL-15, TNF-α, MCP-1, MIP-1α, MIG and eotaxin in at least 2 out of 5 subjects. Serum IL-10 was decreased in 4 out of 5 patients at EOS. These were associated with induction of a significant anti-tumor T-cell response in 4 out of 5 subjects. Tumor-reactive T-cells exhibited a strong Th1 polarization and made little or no IL-17, IL-10, IL-4 or IL-5. As documented in subjects S3 and S4, the vaccine-elicited FIN-γ secreting tumor-specific T-cells were polyclonal and were directed against several known HLA-A2-restricted epitopes of ovarian cancer-specific antigens including HER-2/neu, mesothelin, NY-ESO-1, MUC1 and WT1. Thus the results demonstrated that OCDC vaccine was highly efficient in crosspresentation to CD8+ T-cells. OCDC vaccine also efficiently primed tumor-specific CD4+ T-cell response as we detected a significant IFN-γ response in the post-vaccine PBMCs of Subject S4 to a HER-2/neu class II-derived peptide. These observations were further supported by two previous studies that demonstrated that HOCl-oxidation promoted crosspresentation and elicited tumor-specific CD4+ and CD8+ T-cells (13–14). These studies demonstrated that DCs efficiently crosspresented HOCl-oxidized SKOV-3 tumor cells (which are HLA-A2 negative and allogeneic to the HLA-A2+ DC and T-cells that were used in these studies), and elicited IFN-γ responses from CD8+ T-cells to HLA-A2 restricted epitopes of HER-2/neu and MUC-1, as well as CD4+ T-cells to MHC Class II-derived HER-2/neu peptides. Thus we demonstrated that the OCDC vaccine generated ex vivo were robust antigen-presenting cells by producing strong Th1-polarizing cytokines and chemokines, efficiently crosspresenting relevant tumor-associated antigens and eliciting a sharply Th1-polarized T-cell response.
The ability of HOCl to increase the immunogenicity of whole tumor lysate, and subsequently enhancing DC ability to produce strong IL-12 following LPS and IFN-γ could be explained by different potential mechanisms – a) as HOCl induces necrotic tumor cell death (13), numerous danger signals such as DNA, RNA, ATP, uric acid, high mobility group box 1 and heat shock proteins could be released to activate and mature DCs. One study showed that SKOV-3 tumor cells that were treated with 40 to 60 µM HOCl induced upregulation of human DC maturation markers including CD40, CD83, CD86 and HLA-DR (13). Thus, HOCl-oxidized tumor cells partially activate DCs and could promote stronger IL-12 production following stimulation with LPS and IFN-γ; b) The danger signals released from the HOCl-L might help increase the uptake of HOCl-L by the DCs. We performed 6 cycles of freeze and thaw following HOCl-oxidation of tumor cells, which could release more danger signals to activate DCs. We showed in this study that DC uptake of HOCl-L was significantly better compared to the uptake of UVB-L or FTL; c) As tumor cells contain a whole array of biomolecules including proteins, lipids and glyoproteins that are targets of HOCl-oxidation, these oxidized products could simultaneously engage and activate numerous types of scavengers receptors such as LOX-1 (12), CD36 and MARCO, and possibly Toll-like receptors on DCs. The simultaneous activation of these receptors might help enhance the IL-12 production from DCs loaded with HOCl-L followed by LPS and IFN-γ stimulation; d) ovarian tumor cells frequently produce suppressive molecules that could inhibit DC activation, including prostaglandin E2, TGF-β and IL-10 (29–30). Treatment of whole tumor cell lysate with HOCl-oxidation and not UVB-irradiation or freeze-thaw, might inactivate these suppressive molecules, leading to improved DC maturation by LPS and IFN-γ stimulation and hence IL-12 production.
We presented here the clinical and immunological data of the first cohort of an ambitious phase I study which will treat 35 subjects with ovarian cancer. The results were highly encouraging as we observed a close correlation in this cohort between clinical benefit and immune response to vaccine. The two subjects (S2 and S3), who were in clinical remission when enrolled in the study, remained in remission for a period of time much longer than expected based on historic observations of recurrent ovarian cancer treated with available second or third line chemotherapeutic drugs. Both subjects demonstrated a PFS2 > PFS1 in response to OCDC vaccination as second-line therapy following relapse from first-line therapy. The comparison of second versus first PFS is important in assessing studies of novel chemotherapy or immunologic treatment strategies for patients with relapsed disease including ovarian cancers, and is used to model second response based on first-response duration (31) and interpret the clinical efficacy of second-line therapies (32). Especially interesting is the durable remission in S3, whom after two recurrences remains to date in remission for a total of 44 months, of which 24 months on no additional therapy since the last vaccine. Furthermore, we were encouraged by the response observed in subject S4, who in spite of progression of disease by RECIST, showed stabilization or initial regression in half of the tumor deposits and cavitation in three additional tumor nodules. It is interesting to note that in the three subjects (S2, S3 and S4) with clinical benefit or mixed response we noticed favorable immune parameters, including high levels of IL-12 (>40 ng/ml in 24 hours) produced by OCDCs ex vivo, induction of FIN-γ secreting tumor-reactive cells, induction of polyclonal tumor-reactive T-cells directed against known tumor antigens, and similar to the mouse, reduction in peripheral blood Treg cells and serum IL-10. High levels of serum IL-10 have been shown to correlate with poor prognosis in subjects with hepatocellular carcinoma (33–34) and hindered the proper maturation of circulating DCs in these subjects (35). Given that Treg cells are known to produce IL-10, it is reasonable to postulate that the significant reduction in periphery Treg cells observed in our subjects following repeat OCDC vaccination might have contributed to the reduction of serum IL-10 level in the same subject. Furthermore, the overall strong polarization to Th1 responses after OCDC vaccinations could impact myeloid cells to produce less IL-10. A surprising finding of our analyses is that DCs from S2, S3 and S4 secreted low IFN-α (<0.02 ng/ml), which contrasted with the high IFN-α produced by OCDC from S1 and S5 who progressed. This finding will be confirmed in a larger phase II study, which is ongoing.
In this trial, we chose the intranodal route of administration because it allows us to administer a defined quantity of DCs directly to the site of T-cell sensitization. This approach also allows the peak IL-12 secretion to be synchronized with their proximity to T-cells, where IL-12 can exert its full effects during antigen presentation (36). In a murine tumor model, DC pulsed with tumor lysate and injected intranodally resulted in greater sensitization of T-cells and improved anti-tumor responses (37). In a randomized, Phase I, dose-escalation trial Lambert et al (37) compared different administration routes (intravenous, intranodal and intradermal) in metastatic melanoma receiving four autologous peptide-pulsed DC vaccinations. The results showed that intranodal administration led to superior T-cell sensitization as measured by de novo target-cell recognition and delayed type hypersensitivity priming, indicating that intranodal injection may be the preferred route of administration for matured DC vaccines (38). We used LPS to mature the DCs of cancer subjects because it was available in clinical grade from the National Institute of Health, and together with IFN-γ had consistently induced strong IL-12 production from the HOCl-L-pulsed DCs. We found that MPLA, a detoxified version of LPS widely used in human therapy, was less efficient than LPS when used at the same concentration alone or in combination with IFN-γ in stimulating IL-12p70 production from DCs (Chiang L-L.C, unpublished data). DCs stimulated with MPLA produced ~2-fold less IL-12p70 than DCs stimulated with LPS. We could, however, obtain similar IL-12p70 levels as achieved with LPS stimulation by doubling the concentration of MPLA (Chiang L-L.C, unpublished data). One study compared the differential stimulatory effects of various lipid A derivatives (including LPS from E. Coli and MPLA from S. Minnesota) on mouse DCs and macrophages and found that LPS had stronger proinflammatory effects than MPLA (39–41). The main reason could lie with the structural differences of the lipids and the signal transduction pathways, and the type of proinflammatory cytokines and chemokines that they activate.
Two recent clinical trial studies described the used of a similar DC vaccine platform. Although there were differences in the DC preparation; antigen used for loading DCs; and maturation stimuli for activating DCs, these studies described the positive correlation of IL-12 production to anti-tumor responses and length of progression-free survival of the subjects. In a clinical trial conducted by Okada et al., of 22 subjects with recurrent malignant glioma, it was showed that increased IL-12 production by DCs was positively correlated to time to progression. In a clinical trial conducted by Alfado et al. on 28 subjects with metastatic cancers, it was demonstrated that DC vaccination induced increased IL-12p70 levels in sera. The type of maturation stimuli for activating DCs may affect the production of different T-cell polarizing cytokines and chemokines. Hardwick et al. observed that the use of poly I:C alone to mature DCs loaded with HOCl-oxidized tumor cells led to the induction of significant TGF-β production from DCs (15). Our study demonstrated that LPS and IFN-γ induce strong production of IL-12 by HOCl-oxidized whole tumor lysate-pulsed DCs. Thus, robust IL-12 production by the DC vaccine might be a key criterion for successful anti-tumor vaccination.
In conclusion, through methodical preclinical experimentation we developed an immunogenic whole tumor lysate preparation for DC-based immunotherapy. This vaccine platform proved encouraging in mice and in human preclinical experiments, and was translated to the clinic where it showed early encouraging results in subjects with recurrent ovarian cancer. The data showed are part of cohort 1 of a planned Phase I clinical study, where OCDC vaccine is subsequently tested in combinations (NCT01132014). We reported the results from this first cohort to show the preclinical development of OCDC as monotherapy and its promising biological effects in the treated cancer subjects. Further improvements can be developed in the clinic to increase the vaccine efficacy. For example, we showed that angiogenesis blockade can enhance the homing of T-cells in tumors and increase the potency of vaccine (42–43). The addition of antiangiogenesis therapy, such as bevacizumab, could prove useful. Furthermore, although our vaccine was associated with a reduction of Treg cells in blood, further therapy aiming at attenuating Treg cells, for example with low dose cyclophosphamide (44–46), could prove beneficial. These hypotheses are tested in the ongoing phase I study. Finally, checkpoint blockade could significantly enhance the efficacy of vaccine, and this can be tested as these agents become available.
Supplementary Material
Translational Relevance.
Whole tumor lysates can potentially elicit polyclonal anti-tumor responses via presentation of numerous immunogenic epitopes by DCs to CD4+ and CD8+ T-cells, but their potency may vary. We developed a novel method of whole tumor lysate preparation with hypochlorous acid (HOCl) oxidation to induce rapid necrosis and increase the immunogenicity of tumor cells. Dendritic cells (DC) pulsed with HOCl-oxidized autologous whole tumor lysate (OCDC) elicited robust anti-tumor effects in mouse and human preclinical studies. In a pilot clinical study of five subjects with recurrent ovarian cancers, OCDC vaccine produced high IL-12 ex vivo, and OCDC vaccination induced polyclonal anti-tumor IFN-γ secreting cells, and reduced peripheral blood Treg cells and serum IL-10 in vivo. Two subjects experienced durable progression-free survival intervals of 36 and 43 months after OCDC vaccinations. This is the first study in human clinical practice demonstrating the immune effects of DC-HOCL-oxidized tumor lysate therapy, and it is potentially applicable to many cancer types.
Acknowledgements
We thank our subjects for participating in this trial. We also thank the University Laboratory Animal Resources (ULAR) at UPenn for animal maintenance, Mr. Thomas Garrabrant for flow cytometer technical support, the CVPF staff and Mr. Andrew Best for OCDC productions, our Ovarian Cancer Research Center (OCRC) clinical nursing group, UPenn’s IRB and Clinical Trials Scientific Review and Monitoring Committee (CTSRMC), and Ms. Li-Ping Wang and Ms. Amy Ziober for performing immunohistochemistry staining. We also thank Drs. Stefano Ugel and Francesco De Sanctis at OCRC for advices on mice experiments, and Dr. Jaikumar Duraiswamy (OCRC) for providing HLA-A2-restricted peptides.
Grant Support
This work was supported by National Cancer Institute (NCI) P01-CA83638 Ovarian Specialized Program of Research Excellence (SPORE), the Immunotherapy Initiative for Ovarian Cancer (ITI-OC) and the Markus Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: G. Coukos, CL-L. Chiang, L.E. Kandalaft
Development of methodology: CL-L. Chiang, G. Coukos
Acquisition of data (provided animals, acquired and managed subjects, provided facilities, etc.): CL-L. Chiang, L.E. Kandalaft, J. Tanyi, A.R. Hagemann, G.T. Motz, K. Montone, G.M. Mantia-Smaldone, H.L. Nisenbaum, D.A. Torigian, B.L. Levine, M. Kalos, , D. Powell. Jr., B.J. Czerniekie, G. Coukos
Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): CL-L. Chiang, L.E. Kandalaft, R. Mick, G.T. Motz, M. Kalos, K. Montone
Writing, review, and/or revision of the manuscript: CL-L. Chiang, L.E. Kandalaft, R. Mick, G. Coukos
Administrative, technical and material support (i.e. reporting or organizing data, constructing databases): A. Best, T. Gabarrant, N. Svoronos
Generated data: CL-L. Chiang, L.E. Kandalaft
Study supervision: G. Coukos, L.E. Kandalaft
References
- 1.Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T-cells. J Clin Oncol. 2009;27:945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 2.Barth RJ, Jr., Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, et al. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16:5548–5556. doi: 10.1158/1078-0432.CCR-10-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadul CE, Fisher JL, Hampton TH, Lallana EC, Li Z, Gui J, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother. 2011;34:382–389. doi: 10.1097/CJI.0b013e318215e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernando JJPT, Kübler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother. 2002;51:45–52. doi: 10.1007/s00262-001-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandalaft LE, Powell DJ, Jr., Chiang CL-L, Tanyi J, Kim S, Bosch M, et al. Autologous Lysate-pulsed Dendritic Cell Vaccination Followed by Adoptive Transfer of Vaccine-primed Ex Vivo Costimulated T-cells in Recurrent Ovarian Cancer. Oncoimmunology. 2013;1(2):e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, et al. A Phase I Trial of Tumor Lysate-pulsed Dendritic Cells in the Treatment of Advanced Cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 7.Oosterwijk-Wakka JC, Tiemessen DM, Bleumer I, de Vries IJM, Jongmans W, Adema GJ, et al. Vaccination of Patients With Metastatic Renal Cell Carcinoma With Autologous Dendritic Cells Pulsed With Autologous Tumor Antigens in Combination With Interleukin-2: A Phase 1 Study. J Immunother. 2002;25:500–508. doi: 10.1097/00002371-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, et al. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94:412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 9.Neller MA, López JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–295. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Allison MED, Fearon DT. Enhanced immunogenicity of aldehyde-bearing antigens: a possible link between innate and adaptive immunity. Eur J Immunol. 2000;30:2881–2887. doi: 10.1002/1521-4141(200010)30:10<2881::AID-IMMU2881>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Marcinkiewicz J, Chain BM, Olszowska E, Olszowski S, Zgliczynski JM. Enhancement of immunogenic properties of ovalbumin as a result of its chlorination. Int J Biochem. 1991;23:1393–1395. doi: 10.1016/0020-711x(91)90280-z. [DOI] [PubMed] [Google Scholar]
- 12.Prokopowicz ZM, Arce F, Biedron R, Chiang CL-L, Ciszek M, Katz DR, et al. Hypochlorous Acid: A Natural Adjuvant That Facilitates Antigen Processing, Cross-Priming, and the Induction of Adaptive Immunity. J Immunol. 2010;184:824–835. doi: 10.4049/jimmunol.0902606. [DOI] [PubMed] [Google Scholar]
- 13.Chiang C, Ledermann J, Rad AN, Katz D, Chain B. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T-cells. Cancer Immunol Immunother. 2006;55:1384–1395. doi: 10.1007/s00262-006-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang CL, Ledermann JA, Aitkens E, Benjamin E, Katz DR, Chain BM. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T-cells that recognize autologous primary tumor. Clin Cancer Res. 2008;14:4898–4907. doi: 10.1158/1078-0432.CCR-07-4899. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Humanneutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids intoglycolaldehyde, 2-hydroxypropanalacrolein A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nε-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–113. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco-Marín sE, Paz-Miguel J, López-Mato P, Alvarez-Domínguez C, Leyva-Cobián F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T-cells. Immunology. 1998;95:314–321. doi: 10.1046/j.1365-2567.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang C, Maier D, Kandalaft L, Brennan A, Lanitis E, Ye Q, et al. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J Transl Med. 2011;9:198. doi: 10.1186/1479-5876-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang CL-L, Hagemann AR, Leskowitz R, Mick R, Garrabrant T, Czerniecki BJ, et al. Day-4 Myeloid Dendritic Cells Pulsed with Whole Tumor Lysate Are Highly Immunogenic and Elicit Potent Anti-Tumor Responses. PLoS ONE. 2011;6:e28732. doi: 10.1371/journal.pone.0028732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 22.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Tallman MS, Oken MM, Cassileth PA, Bennett JM, Wiernik PH, et al. Duration of second complete remission compared with first complete remission in patients with acute myeloid leukemia. Leukemia. 2000;14:1345–1348. doi: 10.1038/sj.leu.2401853. [DOI] [PubMed] [Google Scholar]
- 24.Markman M, Markman J, Webster K, Zanotti K, Kulp B, Peterson G, et al. Duration of Response to Second-Line, Platinum-Based Chemotherapy for Ovarian Cancer: Implications for Patient Management and Clinical Trial Design. J Clin Oncol. 2004;22:3120–3125. doi: 10.1200/JCO.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T-cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 27.Chu CS, Kim SH, June CH, Coukos G. Immunotherapy opportunities in ovarian cancer. Expert Rev Anticancer Ther. 2008;8:243–257. doi: 10.1586/14737140.8.2.243. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CL-L, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol. 2010;22:132–143. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagemann AR, Cadungog M, Hagemann IS, Hammond R, Adams SF, Chu CS, et al. Tissue-based immune monitoring I: Tumor core needle biopsies allow in-depth interrogation of the tumor microenvironment. Cancer Biol Ther. 2011;12:357–366. doi: 10.4161/cbt.12.4.16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol. 1997;28:321–331. doi: 10.1016/s0046-8177(97)90131-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee CK, Simes RJ, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. Br J Cancer. 2011;105:1144–1150. doi: 10.1038/bjc.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong DK, White AJ, Weil SC, Phillips M, Coleman RL. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol Oncol. 2013;129:452–458. doi: 10.1016/j.ygyno.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Hattori E, Okumoto K, Adachi T, Takeda T, Ito J-i, Sugahara K, et al. Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res. 2003;27:309–314. doi: 10.1016/j.hepres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Chau G-Y, Wu C-W, Lui W-Y, Chang T-J, Kao H-L, Wu L-H, et al. Serum Interleukin-10 But Not Interleukin-6 Is Related to Clinical Outcome in Patients With Resectable Hepatocellular Carcinoma. Ann Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, et al. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clin Cancer Res. 2004;10:7260–7269. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 36.Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 37.Lambert LA, Gibson GR, Maloney M, Durell B, Noelle RJ, Barth RJ., Jr. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–646. [PubMed] [Google Scholar]
- 38.Bedrosian I, Mick R, Xu S, Nisenbaum H, Faries M, Zhang P, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Gaekwad J, Zhang Y, Zhang W, Reeves J, Wolfert MA, Boons G-J. Differential Induction of Innate Immune Responses by Synthetic Lipid A Derivatives. J Biol Chem. 2010;285:29375–29386. doi: 10.1074/jbc.M110.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okemoto K, Kawasaki K, Hanada K, Miura M, Nishijima M. A Potent Adjuvant Monophosphoryl Lipid A Triggers Various Immune Responses, but Not Secretion of IL-1β or Activation of Caspase-1. J Immunol. 2006;176:1203–1208. doi: 10.4049/jimmunol.176.2.1203. [DOI] [PubMed] [Google Scholar]
- 41.ten Brinke A, Schijndel G, Visser R, Gruijl T, Zwaginga J, Ham SM. Monophosphoryl lipid A plus IFNγ maturation of dendritic cells induces antigen-specific CD8+ cytotoxic T-cells with high cytolytic potential. Cancer Immunol Immunother. 2010;59:1185–1195. doi: 10.1007/s00262-010-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T-cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 43.Kandalaft LE, Motz GT, Busch J, Coukos G. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol. 2011;344:129–148. doi: 10.1007/82_2010_95. [DOI] [PubMed] [Google Scholar]
- 44.Ghiringhelli F, Menard C, Puig P, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T-cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T-cells allows the recruitment of mesothelin-specific CD8 T-cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–2518. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A. 1995;92:432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, et al. Identification of HLA-A2-restricted T-Cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 50.Chen J-L, Dunbar PR, Gileadi U, Jäger E, Gnjatic S, Nagata Y, et al. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 51.Gao L, Bellantuono I, Elsässer A, Marley SB, Gordon MY, Goldman JM, et al. Selective elimination of leukemic CD34+ progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 52.Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernatchez C, Zhu K, Li Y, Andersson H, Ionnides C, Fernandez-Vina M, et al. Altered decamer and nonamer from an HLA-A0201-restricted epitope of Survivin differentially stimulate T-cell responses in different individuals. Vaccine. 2011;29:3021–3030. doi: 10.1016/j.vaccine.2011.01.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 55.Röpke M, Hald J, Guldberg P, Zeuthen J, Nørgaard L, Fugger L, et al. Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. Proc Natl Acad Sci U S A. 1996;93:14704–14707. doi: 10.1073/pnas.93.25.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.