Abstract
There is a worldwide epidemic of cardiovascular diseases causing not only a public health issue but also accounting for trillions of dollars of healthcare expenditure. Studies pertaining to epidemiology, pathophysiology, molecular biology, gene identification and genetic linkage maps have been able to lay a strong foundation for both the diagnosis and treatment of cardiovascular medicine. Although the concept of ‘epigenetics’ is not recent, the term in current usage is extended from the initial concept of ‘controlling developmental gene expression and signaling pathways in undifferentiated zygotes’ to include heritable changes to gene expression that are not from differences in the genetic code. The impact of epigenetics in cardiovascular disease is now emerging as an important regulatory key player at different levels from pathophysiology to therapeutics. This review focuses on the emerging role of epigenetics in major cardiovascular medicine specialties such as coronary artery disease, heart failure, cardiac hypertrophy and diabetes.
Keywords: cardiac hypertrophy, cardiology, cardiovascular disease, coronary artery disease, diabetes, epigenetics, heart failure
Introduction
Cardiovascular disease (CVD) is considered to be the leading cause of death in all countries [Murray and Lopez, 1997], despite significant disparities related to socioeconomic strata and gender [The Lancet, 2013]. During the past century, there has been dramatic progress in the diagnosis, prevention and treatment in different fields of cardiovascular medicine [Nabel and Braunwald, 2012; Polonsky, 2012], which in turn reduced global and cause-specific mortality [Abi Khalil et al. 2012; Laribi et al. 2012; Nichols et al. 2013; Yeh et al. 2010]. However, current scientific knowledge does not completely explain the complex pathophysiology underlying CVD and other pathways are constantly looked for. Over the past decade, epigenetics has initiated a new era in genetic medicine capable of giving a different approach to human disease [Feinberg, 2010; Portela and Esteller, 2010]. Some international initiatives, including the Human Epigenome Project (HEP) and the International Human Epigenome Consortium (IHEC) have even been launched to catalogue the human epigenome and correlate its relation to pathophysiology [Abbott, 2010; Rakyan et al. 2004]. Epigenetics in CVD is the focus of this review.
Mechanisms of epigenetic imprinting
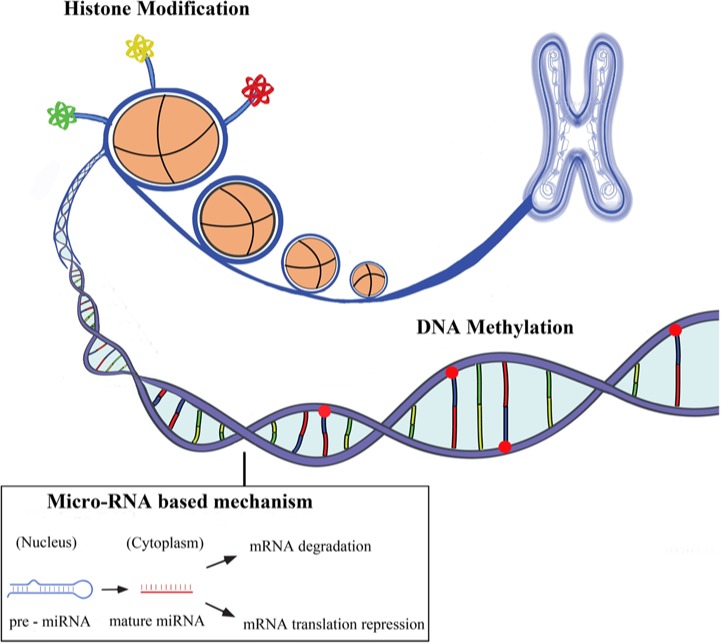
The term epigenetics refers to all the heritable changes in gene expression regulation other than nucleotide sequence and chromatin organization that depend on the DNA sequences itself [Egger et al. 2004; Rodenhiser and Mann, 2006]. Epigenetic inheritance is an essential mechanism that allows the stable propagation of gene activity states from one generation of cells to the next [Kelsey and Feil, 2013]. Epigenetic mechanisms have been involved in the differentiation of many cell types from progenitor or primary cells and with whom they share the same DNA sequence [Ji et al. 2010; Miao et al. 2008]. They also represent a stable cellular memory for the differentiation state of a cell population. The major epigenetic features of human cells include DNA methylation, post-translational histone modifications and RNA-based mechanisms including those controlled by small noncoding micro-RNAs [Wilson, 2008] (see Figure 1).
Figure 1.
The three main areas of epigenetics are DNA methylation, histone modifications (acetylation, methylation, phospholylation, etc.) and micro-RNA based mechanisms. These three processes are distinct but are interrelated and control gene expression.
DNA methylation
This primarily happens at specific dinucleotide sites along the genome or at sites where methyl groups attach to cytosine bases followed by guanine (CpG islands). Other chemical modifications to cytosine resulting in hydroxymethylation, formylation and carboxylation also occur but their interrelation with methylation and their impact on epigenetics is not completely understood [Iurlaro et al. 2013]. In fact, 40% of genes contain CpG-rich islands and up to 70% of all CpG dinucleotides in the genome are methylated [Bird, 2002; Wilson, 2008]. Methylated CpGs act as docking sites for methyl binding proteins which have the ability to oligomerize through the DNA in order to recruit chromatin remodeling complexes that, in turn, cause chromatin condensation and gene inactivation and silencing [Ng et al. 1999; Nikitina et al. 2007; Suzuki and Bird, 2008]. Non-CpG island methylation has also been reported to influence protein–DNA interactions, gene expression and chromatin structure and stability [Fouse et al. 2010]. Non-CpG methylation was recently found to be prevalent in human embryonic stem cells [Lister et al. 2009; Ramsahoye et al. 2000] and in neurons [Guo et al. 2013].
Changes in DNA methylation patterns have been observed in association with CVD, inflammation, autoimmune diseases, infections and cancer [Bierne et al. 2012; Dang et al. 2013; Esteller, 2008].
Histone modification
Eukaryotic DNA is wrapped around an octamer of the core histones H2-A, H2-B, H3 and H4, thus building the fundamental unit of chromatin, the nucleosome [Berger, 2007]. These chromatin marks are unstable and they change rapidly in response to any external stimuli, and any permanent changes to DNA can lead to the development of defective organs or the development of a disease. Histones undergo a variety of post-translational modifications, mainly targeting amino acid residues of the N-terminal tails that protrude from the chromatin fiber [Natsume-Kitatani et al. 2011]. Among the different modifications, histone acetylation, methylation and phosphorylation are the most relevant ones to have been associated with transcription and gene expression [Li et al. 2007]. Post-translational modifications of histones also include their binding to specific proteins, called readers, which secondary interfere with chromatin function and mediate processes such as gene expression, apoptosis, and DNA damage repair [Jenuwein and Allis, 2001].
Micro-RNAs
Micro-RNAs (miRNAs) have recently emerged as regulators of gene expression that can simultaneously regulate genes in different biological networks. miRNAs have launched a new area in translational biology at a time when processing of a product was thought to be only by translation and transcription. These small (20–40 nucleotides) noncoding RNAs are highly conserved across species, highlighting the evolutionary importance of these molecules, as modulators of genes expression [Friedman et al. 2009]. More than 2500 miRNAs are currently identified in the human genome (http://www.mirbase.org/) and over 60% of all human genes are predicted to be regulated by these small RNAs (Zhang, 2008).Among miRNAs, many long-coding RNAs had already been identified years ago. Although initially neglected because of a false presumption of a lack of function, recent data show that they also enhance gene expression by modulating chromatin and organization of protein complexes across chromosomes [Geisler and Coller, 2013; Ørom and Shiekhattar, 2013].
Epigenetics in CVD
Epigenetics has been initially studied in patients with CVD for its prominent role in inflammation and vascular involvement [Castro et al. 2003; Stenvinkel et al. 2007]. Furthermore, epigenetic studies in cardiovascular medicine revealed a significant number of modifications affecting the development and progression of CVD. In addition, epigenomics are also involved in cardiovascular risk factors such as smoking [Breitling et al. 2011, 2012; Buro-Auriemma et al. 2013], diabetes (see below), hypertension [Rivière et al. 2011; Smolarek et al. 2010] and age [Fuke et al. 2004].
Epigenetics in heart disease
Cardiac hypertrophy
In experimental models of epigenetics, Cardiac Hypertrophy (CH) has been linked with histone acetylation implicating both histone acetyltransferases (HATs) and histone deacetylases (HDACs). The activity of HATs seems to have a positive role in CH as exemplified by the HAT activity of the transcriptional co-activators CREB binding protein (CBP) and p300. Overexpression of CBP or p300 in cardiomyocytes resulted in hypertrophy, whereas overexpression of mutant CBP and p300 lacking HAT activity did not [Gusterson et al. 2003]. However, the activity of HDACs has been reported in both prohypertrophic and antihypertrophic pathways, resulting in conflicting data. Class IIa HDACs have been shown to suppress CH whereas some animals and in vivo models showed that HDAC inhibitors have a protective role against hypertrophy [Antos et al. 2003; Kee et al. 2006; Kook et al. 2003]. CH has also been linked with histone methylation, in particular with H3K9 [Haddad et al. 2003; Majumdar et al. 2008; Zhang et al. 2011] and H3K4 methylations [Bingham et al. 2007; Stein et al. 2011] in animal models. In a different experimental mice model, hypermethylation associated to hydroxymethylation of epidermal growth factor receptor (EGFR) gene was associated with aortic valve calcification and subsequent ventricular hypertrophy [Barrick et al. 2009]. Parallel to histone modifications, miRNAs are also involved in CH. In neonatal cardiomyocytes, overexpression of miR-23a, miR-23b, miR-24, miR-195 or miR-214 induced CH whereas overexpression of miR-133 inhibited the phenotype [Carè et al. 2007; Latronico and Condorelli, 2009].
Heart failure
Heart failure (HF) results from a complex genetic predisposition and multiple environmental factors. In a series of patients with HF, Movassagh and colleagues reported that three angiogenesis-related genes were differentially methylated, irrespective of the etiology: angiomotin-like 2 gene (AMOTL2) was hypomethylated whereas the 5′ promoter region in the platelet/endothelial cell adhesion molecule gene (PECAM1) and the gene body of Rho GTPase-activating protein 24 (ARHGAP24) were hypermethylated [Movassagh et al. 2010]. The altered epigenomics of the three genes in endstage HF may reflect common epigenetic pathways in heart remodeling and vasculature [Movassagh et al. 2011]. Dilated cardiomyopathy represents about one in three cases of HF. Interestingly, Nguyen and colleagues reported in a mouse model that cardiac specific knockout of Dot1L, the gene encoding for the HKMT Dot1L which catalyzes H3K79 methylation in mammals, caused the appearance of a phenotype similar to dilated cardiomyopathy [Nguyen et al. 2011]. Histone modifications are also involved in HF; in a genome-wide histone methylation of heart tissues, Kaneda and colleagues reported that tri-methylated histone H3H4 and H3K9 were altered in HF [Kaneda et al. 2009]. Micro-RNAs have a particular importance in epigenetics of HF as shown in experimental models highlighting their role in driving gene expression change during the course of the disease [Ikeda et al. 2007; Latronico and Condorelli, 2009].
Arrhythmias
The contribution of epigenetics to heart rhythm disorders has recently been highlighted in common pathologies. Atrial fibrillation is the most prevalent arrhythmia in aging populations. In transgenic mice programmed to develop cardiac hypertrophy, Liu and colleagues showed that an injection of a specific HDAC inhibitor reverses atrial fibrosis and diminishes atrial fibrillation vulnerability following an electrical stimulation [Liu et al. 2008]. In other models, deletion of both HDAC-1 and HDAC-2 from the myocardium resulted in early rodent death by fetal arrhythmia [Montgomery et al. 2007]. Among the complex genetic background of the long QT syndrome, it is thought that a mutation interrupting the CpG island would prevent methylation and silence the paternal allele of the KCNQ1 gene in cardiac tissue [Bokil et al. 2010]. Micro-RNAs are also emerging as regulators of the normal electrophysiology of the heart and could also be implicated in arrhythmias [Kim, 2013]. Among the miRNAs that have been widely explored in experimental models, miR-1 was reported to be essential in normal electrophysiological conduction and its deletion was associated with high rate of sudden death [Zhao et al. 2007]. Overexpression of miR-208a is also associated with arrhythmia and heart death [Oliveira-Carvalho et al. 2013] whereas increase in miR-133a leads to prolonged QT intervals. Several other miRNAs such as the miR-212, miR-17-92, miR-155, miR-181 and miR-181a have been associated with regulation of heart rhythm through regulation of ion channels, transporters and cellular proteins [Kim, 2013].
Epigenetics in vascular diseases
Atherosclerosis and artery disease
Both DNA methylation, miRNAs and epigenetic mechanisms have been described in atherosclerosis. The era of DNA methylation in atherosclerosis was launched initially by two different groups who showed coexisting hypomethylation with atherosclerotic lesions in mice and rabbits [Hiltunen et al. 2002; Laukkanen et al. 1999]. Further studies showed that hypomethylation could even be detected at very early stages of atherosclerosis, even before the appearance of the anatomical manifestation of the disease [Lund et al. 2004]. In human atherosclerosis, hypermethylation of atheroprotective estrogen receptor α (ESR1) and estrogen receptor β (ESR2) in vascular smooth muscle cells were described by two independent groups [Kim et al. 2007; Post et al. 1999]. In cultured tissues of aorta and coronary arteries with varying degrees of atherosclerosis, Zhu and colleagues showed that methylation of the monocarboxylate transporter (MCT3) gene suppresses its transcription and hence allows the passage of smooth muscle cells and the progression of atherosclerosis [Zhu et al. 2005].
The contribution of miRNAs to atherosclerosis has been extensively investigated in the past few years. In fact, miRNAs are key players in programming and modulating gene expression of relevant cell types involved in atherosclerosis. They mediate inflammation, cholesterol influx, cellular differentiation and lipid uptake (Table 1).
Table 1.
Micro-RNAs involved in atherosclerosis.
| miRNA | Gene target | Cell type | Biological process | Reference |
|---|---|---|---|---|
| miR – 33a/b | ABCA1, ABCG1, CPTA, CROT, HADHB | Monocytes/ macrophages | Cholesterol efflux, fatty acid beta-oxidation | Rayner et al. [2010] |
| Marquart et al. [2010] | ||||
| Najafi-Shoushtari et al. [2010] | ||||
| miR – 758, 26, 106 and 10b | ABCA1 | Monocytes/ macrophages | Cholesterol efflux | Rayner et al. [2010] |
| Horie et al. [2012] | ||||
| Ramirez et al. [2011] | ||||
| Sun et al. [2012a] | ||||
| miR 155 | LOX-1, CD36, CD68 MyD88, BCL-6 | Monocytes/ macrophages | Lipid uptake and inflammation | Kim et al. [2012] |
| Huang et al. [2010] | ||||
| miR 125a-5p | ORP9 | Monocytes/ macrophages | Lipid uptake and inflammation | Wang et al. [2012] |
| miR-26a | SMAD1, SMAD 4 | Vascular smooth muscle cells | Vascular differentiation and apaptosis | Chen et al. [2009] |
| miR-29b | DNMT3b | Vascular smooth muscle cells | Migration | Nazari-Jahantigh et al. [2012] |
| miR-145 | KLF4 | Vascular smooth muscle cells | Differentiation | Leeper et al. [2011] |
| miR-125b | SP7 | Vascular smooth muscle cells | Calcification | Chen et al. [2011] |
| miR-21 | PDC4 | Vascular smooth muscle cells | Apoptosis | Li et al. [2009] |
| miR-221/222 | P27(kip1), p57(kip2), c-kit | Vascular smooth muscle cells | Proliferation, migration and apoptosis | Qin et al. [2011] |
| miR-126 | RGS16 | Endothelial cells | Progenitor cells, apoptosis | Zernecke et al. [2009] |
| miR143/145 | ELK1, KLF4, CAMK2d,SSH2, PHACTR4, CFL1 | Endothelial cells | Differentiation | Hergenreider et al. [2012] |
| miR-10a/miR-181b | HOXA1,M βTRC, AP3K7, KPNA1 | Endothelial cells | Inflammation and dysfunction | Fang et al. [2010] |
| Sun et al. [2012b] | ||||
| miR 155/miR22 | eNOS | Endothelial cells | Neovascularization | Sun et al. [2012a] |
| STAT5A | Kim et al. [2012] |
miRNA, micro-RNA.
Clinical studies are aligned to experimental ones. In a follow up of elderly subjects, Baccarelli and colleagues showed that hypomethylation of long interspersed nucleotide elements was associated with higher incidence of ischemic heart disease events [Baccarelli et al. 2010]. Friso and colleagues showed that hypomethylation of the promoter of coagulation factor VII was associated with coronary artery disease in patients with angiographically proven coronary lesions [Friso et al. 2012].
Diabetic vascular disease
Chronic exposure to hyperglycemia induces specific chromatin changes and transcriptional responses, indicating epigenetic events that mediate signaling pathways relevant to diabetic CVD [El-Osta, 2012]. Genome-wide analysis of human vascular cells showed that histone modifications and DNA methylation occur simultaneously during hyperglycemic conditions and predict gene expression [Pirola et al. 2011].
Cell culture studies allow a direct comprehension of epigenetics and gene expression. The transcriptional co-activator PPARGC1A coordinates gene expression, which in its turn stimulates secretion of mitochondrial reactive oxygen species in different tissues in humans [Puigserver and Spiegelman, 2003]. In pancreatic β-cells of patients with type 2 diabetes (T2D), the PPARGC1A promoter is more methylated compared with nondiabetic individuals [Ling et al. 2008]. As a result, its expression is reduced, which confirms the theory that epigenomics have a critical part in regulating gene expression. In addition, Mutsktov and colleagues showed that insulin gene in human islet-derived precursor cells displayed high levels of histone modifications (H4 hyperacetylation and dimethylation of H3 lysine 4), which are typical of active genes [Mutskov et al. 2007].
It is already known that inflammation is part of the complex biochemical process of initiating and further developing cardiovascular complications of diabetes. Exposure to hyperglycemia could induce epigenomic changes in inflammatory pathways. The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls DNA transcription and regulation of the activity of genes implicated in inflammation and atherothrombosis [Miao et al. 2004]. In individuals with T2D, chronic uncontrolled hyperglycemia enhances NF-κB activity in white blood cells, therefore affecting the activity of cytokines [Shanmugam et al. 2003]. HATs that acetylate lysine amino acids by transferring an acetyl group from acetyl CoA to form e-N-acetyl lysine on histone proteins interact with NF-κB, which causes hyperacetylation of inflammation-related genes promoters such as tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 [Bird, 2007; Hofmann et al. 1998]. Other histone transferases that work in a similar way to HATs could also interfere in the regulation of pro-inflammatory genes by recruiting NF-kB to gene promoters. In animal models, such as the ones done on diabetic db/db mice, Reddy and colleagues reported that a hyperglycemic milieu enhances the expression of inflammation-related genes and precipitates pro-atherogenic responses in smooth muscle cells of the vascular system [Reddy et al. 2008]. This was also confirmed by Villeneuve and colleagues in a similar model [Villeneuve et al. 2008]. They reported that the expression of inflammatory genes in vascular cells cultured in high glucose was increased whereas that of H3K9me3, a histone H3 known to protect against the biochemical state of diabetic inflammation, were decreased at their promoters. Most importantly, El-Osta and colleagues reported that even a transient exposure to high levels of glucose induced sustained epigenomic changes in the p65 subunit promoter of the NF-κB, modifying thereafter the epigenomics of cultured aortic endothelial cells [El-Osta et al. 2008]. That might explain, at least partially, the irreversibility of cardiovascular complications of long-standing uncontrolled diabetes often encountered in clinical care. Finally, MicroRNAs are also implicated in the pathophysiology of diabetes and its complications and could even be potential pharmacological targets [Mao et al. 2013].
Conclusion
Epigenetics is likely to be involved in the biology of major disciplines of cardiovascular medicine. To date, there are no significant reports of epigenetics contribution in clinical practice or therapeutics in CVD whereas in cancer, DNA methylation inhibitors, histone methylation inhibitors and histone deacetylase inhibitors have been successful so far [Gal-Yam et al. 2008]. Understanding the epigenetic mechanisms, their interactions and alterations using translational research approaches or large human cohorts promises to make a significant contribution to our understanding of CVDs.
Acknowledgments
The author would like to thank Marie-Joe Dib, BS for editorial and technical support.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares no conflict of interest in preparing this article.
References
- Abbott A. (2010) Project set to map marks on genome. Nature 463: 596–597 [PubMed] [Google Scholar]
- Abi Khalil C., Roussel R., Mohammedi K., Danchin N., Marre M. (2012) Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol 19: 374–381 [DOI] [PubMed] [Google Scholar]
- Antos C., McKinsey T., Dreitz M., Hollingsworth L., Zhang C., Schreiber K., et al. (2003) Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J Biol Chem 278: 28930–28937 [DOI] [PubMed] [Google Scholar]
- Baccarelli A., Wright R., Bollati V., Litonjua A., Zanobetti A., Tarantini L., et al. (2010) Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology 21: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick C., Roberts R., Rojas M., Rajamannan N., Suitt C., O’Brien K., et al. (2009) Reduced EGFR causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in C57BL/6J but not 129S1/SvImJ mice. Am J Physiol Heart Circ Physiol 297: H65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. (2007) The complex language of chromatin regulation during transcription. Nature 447: 407–412 [DOI] [PubMed] [Google Scholar]
- Bierne H., Hamon M., Cossart P. (2012) Epigenetics and bacterial infections. Cold Spring Harbor Perspect Med 2: a010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham A., Ooi L., Kozera L., White E., Wood I. (2007) The repressor element 1-silencing transcription factor regulates heart-specific gene expression using multiple chromatin-modifying complexes. Mol Cell Biol 27: 4082–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16: 6–21 [DOI] [PubMed] [Google Scholar]
- Bird A. (2007) Perceptions of epigenetics. Nature 447: 396–398 [DOI] [PubMed] [Google Scholar]
- Bokil N., Baisden J., Radford D., Summers K. (2010) Molecular genetics of long QT syndrome. Mol Genet Metab 101: 1–8 [DOI] [PubMed] [Google Scholar]
- Breitling L., Salzmann K., Rothenbacher D., Burwinkel B., Brenner H. (2012) Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur Heart J 33: 2841–2848 [DOI] [PubMed] [Google Scholar]
- Breitling L., Yang R., Korn B., Burwinkel B., Brenner H. (2011) Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 88: 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buro-Auriemma L., Salit J., Hackett N., Walters M., Strulovici-Barel Y., Staudt M., et al. (2013) Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Hum Mol Genet 22: 4726–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carè A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., et al. (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618 [DOI] [PubMed] [Google Scholar]
- Castro R., Rivera I., Struys E., Jansen E., Ravasco P., Camilo M., et al. (2003) Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem 49: 1292–1296 [DOI] [PubMed] [Google Scholar]
- Chen K., Wang Y., Hu C., Chang W., Liao Y., Dai C., et al. (2011) OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J 25: 1718–1728 [DOI] [PubMed] [Google Scholar]
- Chen T., Huang Z., Wang L., Wang Y., Wu F., Meng S., et al. (2009) MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83: 131–139 [DOI] [PubMed] [Google Scholar]
- Dang M., Buzzetti R., Pozzilli P. (2013) Epigenetics in autoimmune diseases with focus on type 1 diabetes. Diabetes Metab Res Rev 29: 8–18 [DOI] [PubMed] [Google Scholar]
- Egger G., Liang G., Aparicio A., Jones P. (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 [DOI] [PubMed] [Google Scholar]
- El-Osta A. (2012) Glycemic memory. Curr Opin Lipidol 23: 24–29 [DOI] [PubMed] [Google Scholar]
- El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P., Roeder R., et al. (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. (2008) Epigenetics in cancer. N Engl J Med 358: 1148–1159 [DOI] [PubMed] [Google Scholar]
- Fang Y., Shi C., Manduchi E., Civelek M., Davies P. (2010) MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A 107: 13450–13455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. (2010) Epigenomics reveals a functional genome anatomy and a new approach to common disease. Nat Biotechnol 28: 1049–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouse S., Nagarajan R., Costello J. (2010) Genome-scale DNA methylation analysis. Epigenomics 2: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R., Farh K., Burge C., Bartel D. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso S., Lotto V., Choi S., Girelli D., Pinotti M., Guarini P., et al. (2012) Promoter methylation in coagulation F7 gene influences plasma FVII concentrations and relates to coronary artery disease. J Med Genet 49: 192–199 [DOI] [PubMed] [Google Scholar]
- Fuke C., Shimabukuro M., Petronis A., Sugimoto J., Oda T., Miura K., et al. (2004) Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet 68: 196–204 [DOI] [PubMed] [Google Scholar]
- Gal-Yam E., Saito Y., Egger G., Jones P. (2008) Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med 59: 267–280 [DOI] [PubMed] [Google Scholar]
- Geisler S., Coller J. (2013) RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14: 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Su Y., Shin J., Shin J., Li H., Xie B., et al. (2013) Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci 17: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson R., Jazrawi E., Adcock I., Latchman D. (2003) The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J Biol Chem 278: 6838–6847 [DOI] [PubMed] [Google Scholar]
- Haddad F., Bodell P., Qin A., Giger J., Baldwin K. (2003) Role of antisense RNA in coordinating cardiac myosin heavy chain gene switching. J Biol Chem 278: 37132–37138 [DOI] [PubMed] [Google Scholar]
- Hergenreider E., Heydt S., Tréguer K., Boettger T., Horrevoets A., Zeiher A., et al. (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256 [DOI] [PubMed] [Google Scholar]
- Hiltunen M., Turunen M., Häkkinen T., Rutanen J., Hedman M., Mäkinen K., et al. (2002) DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med 7: 5–11 [DOI] [PubMed] [Google Scholar]
- Hofmann M., Schiekofer S., Kanitz M., Klevesath M., Joswig M., Lee V., et al. (1998) Insufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care 21: 1310–1316 [DOI] [PubMed] [Google Scholar]
- Horie T., Baba O., Kuwabara Y., Chujo Y., Watanabe S., Kinoshita M., et al. (2012) MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE-/- mice. J Am Heart Assoc 1: e003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Hu G., Lin B., Lin Z., Sun C. (2010) MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J Invest Med 58: 961–967 [DOI] [PubMed] [Google Scholar]
- Ikeda S., Kong S., Lu J., Bisping E., Zhang H., Allen P., et al. (2007) Altered microRNA expression in human heart disease. Physiol Genomics 31: 367–373 [DOI] [PubMed] [Google Scholar]
- Iurlaro M., Ficz G., Oxley D., Raiber E., Bachman M., Booth M., et al. (2013) A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biol 14: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Allis C. (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Ji H., Ehrlich L., Seita J., Murakami P., Doi A., Lindau P., et al. (2010) Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 467: 338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda R., Takada S., Yamashita Y., Choi Y., Nonaka-Sarukawa M., Soda M., et al. (2009) Genome-wide histone methylation profile for heart failure. Genes Cells 14: 69–77 [DOI] [PubMed] [Google Scholar]
- Kee H., Sohn I., Nam K., Park J., Qian Y., Yin Z., et al. (2006) Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–59 [DOI] [PubMed] [Google Scholar]
- Kelsey G., Feil R. (2013) New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos Trans R Soc Lond B 368: 20110336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. (2013) MicroRNA regulation of cardiac conduction and arrhythmias. Transl Res J Lab Clin Med 161: 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim J., Song K., Lee Y., Seo J., Jelinek J., et al. (2007) Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim Biophys Acta 1772: 72–80 [DOI] [PubMed] [Google Scholar]
- Kim J., Yoon H., Ramírez C., Lee S., Hoe H., Fernández-Hernando C., et al. (2012) MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp Neurol 235: 476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook H., Lepore J., Gitler A., Lu M., Wing-Man Yung W., Mackay J., et al. (2003) Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest 112: 863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laribi S., Aouba A., Nikolaou M., Lassus J., Cohen-Solal A., Plaisance P., et al. (2012) Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail 14: 234–239 [DOI] [PubMed] [Google Scholar]
- Latronico M., Condorelli G. (2009) MicroRNAs and cardiac pathology. Nat Rev Cardiol 6: 419–429 [DOI] [PubMed] [Google Scholar]
- Laukkanen M., Mannermaa S., Hiltunen M., Aittomäki S., Airenne K., Jänne J., et al. (1999) Local hypomethylation in atherosclerosis found in rabbit ec-sod gene. Arterioscler Thromb Vasc Biol 19: 2171–2178 [DOI] [PubMed] [Google Scholar]
- Leeper N., Raiesdana A., Kojima Y., Chun H., Azuma J., Maegdefessel L., et al. (2011) MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol 226: 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Carey M., Workman J. (2007) The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Li Z., Hassan M., Jafferji M., Aqeilan R., Garzon R., Croce C., et al. (2009) Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284: 15676–15684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C., Del Guerra S., Lupi R., Rönn T., Granhall C., Luthman H., et al. (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R., Pelizzola M., Dowen R., Hawkins R., Hon G., Tonti-Filippini J., et al. (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Levin M., Petrenko N., Lu M., Wang T., Yuan L., et al. (2008) Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 45: 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund G., Andersson L., Lauria M., Lindholm M., Fraga M., Villar-Garea A., et al. (2004) DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem 279: 29147–29154 [DOI] [PubMed] [Google Scholar]
- Majumdar G., Johnson I., Kale S., Raghow R. (2008) Epigenetic regulation of cardiac muscle-specific genes in H9c2 cells by Interleukin-18 and histone deacetylase inhibitor m-carboxycinnamic acid bis-hydroxamide. Mol Cell Biochem 312: 47–60 [DOI] [PubMed] [Google Scholar]
- Mao Y., Mohan R., Zhang S., Tang X. (2013) MicroRNAs as pharmacological targets in diabetes. Pharmacol Res 75: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart T., Allen R., Ory D., Baldán A. (2010) miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A 107: 12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao F., Gonzalo I., Lanting L., Natarajan R. (2004) In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem 279: 18091–18097 [DOI] [PubMed] [Google Scholar]
- Miao F., Wu X., Zhang L., Riggs A., Natarajan R. (2008) Histone methylation patterns are cell-type specific in human monocytes and lymphocytes and well maintained at core genes. J Immunol 180: 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R., Davis C., Potthoff M., Haberland M., Fielitz J., Qi X., et al. (2007) Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21: 1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassagh M., Choy M., Goddard M., Bennett M., Down T., Foo R. (2010) Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PloS One 5: e8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassagh M., Choy M., Knowles D., Cordeddu L., Haider S., Down T., et al. (2011) Distinct epigenomic features in end-stage failing human hearts. Circulation 124: 2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C., Lopez A. (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349: 1498–1504 [DOI] [PubMed] [Google Scholar]
- Mutskov V., Raaka B., Felsenfeld G., Gershengorn M. (2007) The human insulin gene displays transcriptionally active epigenetic marks in islet-derived mesenchymal precursor cells in the absence of insulin expression. Stem Cells 25: 3223–3233 [DOI] [PubMed] [Google Scholar]
- Nabel E., Braunwald E. (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366: 54–63 [DOI] [PubMed] [Google Scholar]
- Najafi-Shoushtari S., Kristo F., Li Y., Shioda T., Cohen D., Gerszten R., et al. (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328: 1566–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume-Kitatani Y., Shiga M., Mamitsuka H. (2011) Genome-wide integration on transcription factors, histone acetylation and gene expression reveals genes co-regulated by histone modification patterns. PloS One 6: e22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R., et al. (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 122: 4190–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H., Zhang Y., Hendrich B., Johnson C., Turner B., Erdjument-Bromage H., et al. (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet 23: 58–61 [DOI] [PubMed] [Google Scholar]
- Nguyen A., Xiao B., Neppl R., Kallin E., Li J., Chen T., et al. (2011) DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev 25: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M., Townsend N., Scarborough P., Rayner M. (2013) Cardiovascular disease in Europe: epidemiological update. Eur Heart J 34: 3028–3034 [DOI] [PubMed] [Google Scholar]
- Nikitina T., Shi X., Ghosh R., Horowitz-Scherer R., Hansen J., Woodcock C. (2007) Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin. Mol Cell Biol 27: 864–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Carvalho V., Carvalho V., Bocchi E. (2013) The emerging role of miR-208a in the heart. DNA Cell Biol 32: 8–12 [DOI] [PubMed] [Google Scholar]
- Ørom U., Shiekhattar R. (2013) Long noncoding RNAs usher in a new era in the biology of enhancers. Cell 154: 1190–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola L., Balcerczyk A., Tothill R., Haviv I., Kaspi A., Lunke S., et al. (2011) Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res 21: 1601–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky K. (2012) The past 200 years in diabetes. N Engl J Med 367: 1332–1340 [DOI] [PubMed] [Google Scholar]
- Portela A., Esteller M. (2010) Epigenetic modifications and human disease. Nat Biotechnol 28: 1057–1068 [DOI] [PubMed] [Google Scholar]
- Post W., Goldschmidt-Clermont P., Wilhide C., Heldman A., Sussman M., Ouyang P., et al. (1999) Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43: 985–991 [DOI] [PubMed] [Google Scholar]
- Puigserver P., Spiegelman B. (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90 [DOI] [PubMed] [Google Scholar]
- Qin B., Xiao B., Liang D., Xia J., Li Y., Yang H. (2011) MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem Biophys Res Commun 410: 127–133 [DOI] [PubMed] [Google Scholar]
- Rakyan V., Hildmann T., Novik K., Lewin J., Tost J., Cox A., et al. (2004) DNA methylation profiling of the human major histocompatibility complex: a pilot study for the human epigenome project. PLoS Biol 2: e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez C., Dávalos A., Goedeke L., Salerno A., Warrier N., Cirera-Salinas D., et al. (2011) MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol 31: 2707–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsahoye B., Biniszkiewicz D., Lyko F., Clark V., Bird A., Jaenisch R. (2000) Non-CpG methylation is prevalent in embryonic stem cells and May be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci U S A 97: 5237–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K., Suárez Y., Dávalos A., Parathath S., Fitzgerald M., Tamehiro N., et al. (2010) MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328: 1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M., Villeneuve L., Wang M., Lanting L., Natarajan R. (2008) Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res 103: 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rivière G., Lienhard D., Andrieu T., Vieau D., Frey B., Frey F. (2011) Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 6: 478–489 [DOI] [PubMed] [Google Scholar]
- Rodenhiser D., Mann M. (2006) Epigenetics and human disease: translating basic biology into clinical applications. CMAJ 174: 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam N., Reddy M., Guha M., Natarajan R. (2003) High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52: 1256–1264 [DOI] [PubMed] [Google Scholar]
- Smolarek I., Wyszko E., Barciszewska A., Nowak S., Gawronska I., Jablecka A., et al. (2010) Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Monit 16: CR149–155 [PubMed] [Google Scholar]
- Stein A., Jones T., Herron T., Patel S., Day S., Noujaim S., et al. (2011) Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J Clin Invest 121: 2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvinkel P., Karimi M., Johansson S., Axelsson J., Suliman M., Lindholm B., et al. (2007) Impact of inflammation on epigenetic DNA methylation - a novel risk factor for cardiovascular disease? J Intern Med 261: 488–499 [DOI] [PubMed] [Google Scholar]
- Sun D., Zhang J., Xie J., Wei W., Chen M., Zhao X. (2012a) MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett 586: 1472–1479 [DOI] [PubMed] [Google Scholar]
- Sun X., Icli B., Wara A., Belkin N., He S., Kobzik L, et al. (2012b) MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 122: 1973–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Bird A. (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9: 465–476 [DOI] [PubMed] [Google Scholar]
- The Lancet (2013) Cardiovascular health for all. Lancet 382: 572. [DOI] [PubMed] [Google Scholar]
- Villeneuve L., Reddy M., Lanting L., Wang M., Meng L., Natarajan R. (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A 105: 9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Xia M., Yan X., Li D., Wang L., Xu Y., et al. (2012) Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res 111: 967–981 [DOI] [PubMed] [Google Scholar]
- Wilson A. (2008) Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 79: 1514–1519 [DOI] [PubMed] [Google Scholar]
- Yeh R., Sidney S., Chandra M., Sorel M., Selby J., Go A. (2010) Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362: 2155–2165 [DOI] [PubMed] [Google Scholar]
- Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., et al. (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2: ra81. [DOI] [PubMed] [Google Scholar]
- Zhang C. (2008) MicroRNomics: a newly emerging approach for disease biology. Physiol Genomics 33: 139–147 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Chen H., Wang L., Liu D., Hill J., Liu Z. (2011) The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J Clin Invest 121: 2447–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Ransom J., Li A., Vedantham V., von Drehle M., Muth A., et al. (2007) Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129: 303–317 [DOI] [PubMed] [Google Scholar]
- Zhu S., Goldschmidt-Clermont P., Dong C. (2005) Inactivation of monocarboxylate transporter MCT3 by DNA methylation in atherosclerosis. Circulation 112: 1353–1361 [DOI] [PubMed] [Google Scholar]