Abstract
Significant advances in maintaining health throughout life can be made through a clear understanding of the fundamental mechanisms that regulate aging. The Oxidative Stress Theory of Aging (OSTA) is likely the most well-studied mechanistic theory of aging and suggests that the rate of aging is controlled by accumulation of oxidative damage. To directly test the OSTA, aging has been measured in several lines of mice with genetic alteration of the expression of enzymatic antioxidants. Under its strictest interpretation, these studies do not support the OSTA, as modulation of antioxidant expression does not generally affect mouse lifespan. However, the incidence of many age-related diseases and pathologies is altered in these models suggesting that oxidative stress does significantly impact some aspects of the aging process. Further, oxidative stress may affect aging in disparate patterns among tissues or under different environmental conditions. In this review, we summarize the current literature regarding aging in antioxidant mutant mice and offer several interpretations on their support of the OSTA.
Keywords: Aging, lifespan, longevity, age-related disease, mouse models
ASSESSING THE QUESTION
Over the last century, modern medicine has made major advances in treatment and eradication of several diseases. This has largely been due to an increased understanding of the mechanistic underpinnings of these diseases and implementation of this knowledge to develop treatment options. Vaccination development, insulin administration, heart medication and others have alleviated major health risks and allowed for healthier lives. However, while the incidence of many potentially fatal diseases has declined over the last few decades, the prevalence of age-related diseases and illness remains high due in part to increasing expected lifespans among the world’s population (1). Frailty and detrimental changes to the organism that occur with age ultimately make that organism more prone to disease and death, but the process leading to aging itself is still not well understood. The lack of a unified definition for the term aging among the general population, biogerontologists and others reflects the lack of understanding of the phenomenon itself (2). For example, does aging affect the systems within the body separately, or the body as a whole? Why do some organisms, and even more interesting, why do some humans, age slower than others? Furthermore, how does one distinguish between aging and age-related diseases? What are the cellular mechanisms leading to aging, and will manipulations to these systems impact lifespan? Delineating these mechanisms has the potential to reduce or even eliminate many of the chronic diseases of aging that lead to mortality (3).
Among the different mechanistic theories of how the aging process is regulated, a general theme has emerged aimed at deciphering the cause(s) of the damage we associate with aging. The most prominent theory to mechanistically explain aging remains the Oxidative Stress Theory of Aging (OSTA). It was first conceptualized by Denham Harman in the 1950’s as the Free Radical Theory of Aging and derived from the idea that oxygen free radicals generated by normal oxygen-utilizing metabolic processes directly regulate the aging process through the accumulation of oxidative damage (4). Subsequent refinements of this theory addressed the roles of other activated oxygen species in aging in the more generalized OSTA.
At its most simple, the OSTA posits that aging is driven by functional decline at a cellular level caused by the accumulation of oxidative damage due to an imbalance between cellular pro-oxidant production and anti-oxidant reduction. An exhaustive literature exists that support the notion that aging is associated with the accumulation of oxidative damage to multiple different cellular macromolecules (reviewed in (5, 6)). This view is also largely supported by studies showing that dietary interventions (i.e., calorie restriction) or genetic mutations that extend longevity in laboratory models are largely associated with reduced accumulation of oxidative damage, reduced production of reactive oxygen species (ROS), increased antioxidant expression or activity, and increased resistance to oxidative stress (reviewed in (5–7)). Perhaps most importantly, human centenarians (those who have reached 100+ years of age) have lower levels of oxidative stress compared to younger (but still aged 70–99 years) controls (8, 9). While, these data generally support the OSTA’s main hypothesis that aging rate is proportional to the accumulation of oxidative damage, it is important to remember that these data are largely correlative and thus generally weak support for the OSTA.
Extrapolation of the OSTA predicts that a shift towards pro-oxidant production and increased damage accumulation will promote aging whereas a shift in the opposite direction will delay aging. In other words, an intervention that modulates the degree of oxidative stress should also directly modulate the aging process. It is through this prediction that direct testing of the OSTA has been approached. Theoretically, direct testing of the OSTA could be mediated equally either by modulation of either pro- or anti-oxidant regulatory factors. However, due to issues of practicality, direct testing of the OSTA has largely focused on models with genetic modulation of major enzymatic cellular antioxidants including the following: Superoxide dismutases (Sod) which reduce superoxide levels in the cell by catalytic conversion of the superoxide radical to molecular oxygen and hydrogen peroxide (10). Glutathione peroxidases (Gpx) which reduce peroxides (including hydrogen peroxide and lipid hydroperoxides) to water and alcohols (11). Catalase (Cat) which catalyzes the decomposition of hydrogen peroxide to oxygen and water (12). Peroxiredoxins (Prdx) which have peroxidase activity that reduces hydrogen peroxide, peroxynitrite and other organic hydroperoxides (13). Thioredoxins (Trx) which catalyze reduction of disulfide bonds in multiple substrate proteins and detoxify peroxides as an essential component of the actions of other oxidoreductases (14). Methionine sulfoxide reductases (Msr) which repair oxidation damage to proteins through catalytic reduce the oxidized (sulfoxide) form of methionine (15).
While this review focuses on studies performed using mammalian models for reasons discussed below, it is important to recognize that direct testing of the oxidative stress theory of aging in invertebrate models has yielded several provocative results. The results from invertebrate models have been discussed in greater detail elsewhere (5), but we briefly discuss here several key findings that have supported the OSTA as well as further muddled the potential role for oxidative stress in aging. For example, deficiency in the cytosolic form of Sod (CuZnSod) shortens lifespan in S. cerevisiae (16) as does the lack of the mitochondrial form, MnSod (17). Conversely, increased expression of either CuZnSod or MnSod, or the protein repair enzyme MsrB, significantly extends the lifespan of this organism (18, 19). Further, administration of agents that modulate mitochondrial oxidant stress production in yeast directly affect lifespan in ways predicted by the OSTA (20, 21). However, several studies in the nematode C. elegans and the fly Drosphila have seriously brought into question whether modulation of antioxidants directly affects aging. For example, it was reported that administration of superoxide dismutase/catalase mimetics to C. elegans is sufficient to extend lifespan (22). However, subsequent reports have failed to confirm this finding and have suggested that these mimitics actually promote higher levels of oxidant stress (23, 24). Administration of other antioxidants has been shown to have narrow therapeutic effects on longevity with some concentrations detrimental and others beneficial (25). In a study by Doonan et al., the modulation of different Sod isoforms in C. elegans had little effect on lifespan with only deletion of the cytosolic form of Sod showing a reduction of lifespan (26). Surprisingly, overexpression of Sod1 in this study extended lifespan but, counterintuitively, increased sensitivity to oxidative stress (26). Similarly, Van Raamsdonk and Hekimi show that lifespan of C. elegans with deletion of MnSod was longer than that of control worms, dramatically opposing the predictions of the OSTA (27). In Drosophila, several groups have reported that overexpression of different antioxidants, including isoforms of Sod, Cat, and Msrs, are sufficient to extend lifespan in flies (28–32). However, others have shown no effect on lifespan of overexpression of several of these antioxidants either alone or in combination and in some cases actually shown that Drosophila lifespan is actually decreased if expression of antioxidants is increased (29, 33–36). Not surprisingly, these dramatic differences among the results of these lifespan studies have been used as support for both challengers and champions of the OSTA.
While invertebrate models have made significant strides towards addressing whether modulation of oxidative stress regulates aging, the main focus of this review is to evaluate the current status of the OSTA in mammals. Ultimately, the goal of biomedical research is to benefit human health. At a basic level, mammalian models such as mice and rodents closer evolutionarily to humans than are invertebrate models suggesting greater chance of similarity in regulation of aging processes. In addition, the regulation of homeostatic mechanisms differs greatly between invertebrates and vertebrate models. Maintaining body temperature in endotherms, for example, requires a significant energetic contribution from mitochondria and thus likely contributes greatly as an intrinsic source of oxidant stress in mammals. Furthermore, the organization of organ systems is much more similar among mammalian models than it is between mammals and invertebrates. The use of invertebrate models also precludes investigators from studying many of the diseases of aging, such as cardiovascular disease, cancer, neurodegeneration and metabolic dysfunction, at least without significant alterations to the model. As will be discussed below, addressing the incidence or rate of many of these age-related diseases can help better understand the aging process as a whole. For these reasons, the remainder of this review will focus on direct testing of the OSTA in mice.
In this review, we discuss the results from mouse aging studies that largely have met criteria that generate the strongest support for the OSTA. First, the genetic mutation in antioxidant expression should have a significant effect on oxidative stress or accumulation of oxidative damage in vivo. Second, these mutations should not cause dramatic developmental effects or extremely early death. While some disagree on the temporal pattern of aging (2), it is difficult to argue that either of these conditions would be representative of “accelerated” aging. Third, the sample sizes of mice tested should be sufficient to eliminate the effects of uncontrolled variables, such as maternal- or paternal-specific effects on lifespan. While the number necessary will differ depending on the strength of the effect, the final sample size should have sufficient power to detect significant differences in lifespan. Last, aging studies should be performed under conditions that maximize longevity by reducing non-aging related deaths. This last criteria may be the most critical for interpretation of lifespan studies; for example, while lifespan may be shortened by infection with pneumonia, smallpox or parasites, this likely does not occur due to fundamental changes in the aging process. The most significant question addressed by these studies asks whether altering oxidative stress (through modulation of antioxidant expression) significantly impacts the aging process. Currently, several studies have tested the functional effects of these genetic modulations on aging and age-related diseases in mice. A review of this literature suggests several possible conclusions that could be interpreted from these data regarding the OSTA in mice.
POSSIBLE CONCLUSION 1: Altering expression of antioxidant genes in mice largely does not affect aging
The “gold-standard” parameter utilized to measure changes in aging is lifespan or longevity. That is, the rate of aging of a group of study should be represented by the median/mean/maximum lifespan of this group. Thus, a change in lifespan of a mouse strain with reduced or increased expression of a particular antioxidant enzyme would represent the strongest support for the OSTA. However, as summarized in TABLE 1, studies with complete (or nearly complete) lifespan analysis of antioxidant mutant mice have largely shown no significant alterations in the aging process.
TABLE 1.
Published lifespan observations of mice with genetic alteration in antioxidant expression.
| REDUCEDexpression | Antioxidant | INCREASEDexpression | ||
|---|---|---|---|---|
| (ref) | Lifespan | Lifespan | (ref) | |
| Superoxide dismutase | ||||
| (38, 47) | ↓(30%) | Sod1 | NC | (39, 51, 52) |
| (37) | NC | Sod2 | 1.NC; 2.↑(4%) | (53, 54) |
| (38) | NC | Sod3 | ||
| Glutathione peroxidase | ||||
| (39, 40) | NC | Gpx1 | ||
| (43) | ↑(7%) | Gpx4 | NC | (39) |
| Thioredoxin | ||||
| Trx1 | 1.↑(35%); 2.NC | (55, 56) | ||
| (41) | ↓(7%) | Trx2 | ||
| Catalase | ||||
| Cat | NC | (39, 52) | ||
| mitochondria-targeted (mCat) | ↑(20%) | (57) | ||
| peroxisome-targeted (pCat) | NC | (57) | ||
| nucleus-targeted (nCat) | NC | (57) | ||
| Peroxiredoxin | ||||
| (46) | ↓(20%) | Prdx1 | ||
| Methionine sulfoxide reductase | ||||
| (44, 45) | 1.↓(40%); 2.NC | MsrA | ||
| (42) | NC | MsrB1 | ||
↑ = increased in lifespan reported, ↓ = reduction in lifespan reported, NC = no change in lifespan reported. Percentages given in parenthesis represent difference in median or maximum lifespan reported. Shaded boxes represent no data for indicated mutant mouse model.
Reduced antioxidant expression does not accelerate aging
In support of this possible conclusion, several studies have shown that lifespan is unchanged in mice with genetic reduction of the mitochondrial form (MnSod, Sod2) or extra-cellular form (ECSod, Sod3) of superoxide dismutase (37, 38) or glutathione peroxide 1 (Gpx1) (39, 40). While mice with reduced thioredoxin 2 (Trx2) showed a tendency towards a shorter lifespan, these data did not reach statistical significance (39, 41). Mice lacking the protein oxidation repair enzyme methionine sulfoxide reductase B (MsrB1) have show no change in lifespan through at least 20 months of age (42). Paradoxically, mice with a reduction in glutathione peroxidase 4 (Gpx4) showed a slight (~7%) increase in median lifespan, though no change in maximum lifespan (43).
There have been reports that reduction of the expression of some antioxidants causes a significant shortening of lifespan. While these results would support the OSTA, closer examination of these reports dampens some of the enthusiasm for these studies. The first example is that of mice lacking methionine sulfoxide reductase A (MsrA). An initial report by Moskovitz et al. suggested a 40% reduction in lifespan in mice lacking this protein oxidation repair enzyme (44). However, our group later reported that MsrA−/− mice maintained in our animal facility show no change in lifespan (45). The reason for the discrepancy between the two studies is unclear, but one suggestion might be differences in animal husbandry that exist between the two facilities. Control mice in Moskovitz’s 2001 study were ~1/3 shorter-lived than the control mice of the same strain in our 2009 study suggesting a form of chronic stress or disease that may shorten lifespan. This example exemplifies the challenges of differentiating effects of disease from those of aging without studying lifespan under optimal husbandry conditions.
A second example of this is reports of significant reduction of lifespan in mice lacking peroxiredoxin 1 (Prdx1) or the cytosolic form of superoxide dismutase (CuZnSod, Sod1). Lack of Prdx1 was reported to significantly shorten (20%) lifespan in mice due largely to an increased incidence in osteosarcoma, fibrosarcoma and hemolytic anemia (46). Similarly, mice lacking Sod1 (Sod1−/−) have been reported to live 30% shorter than control mice due in part to increased hepatocarcinoma (38, 39, 47). However, these are uncommon end of life pathologies in the particular strain of mouse for these studies (B6) and may suggest that lack of these antioxidants promotes the acceleration of these diseases rather than alters the fundamentals of aging per se. On the other hand, Sod1−/− mice have been reported to show acceleration in the incidence of age-related diseases including hearing loss, cataracts and sarcopenia (48–50). As this shows, disentangling the effects of antioxidants on aging from those on age-related disease are often not simple and may require assessments of aging beyond lifespan.
Increased antioxidant expression does not slow aging
Data from transgenic mice with increased antioxidant expression also do not support a role for these enzymes in the regulation of longevity. Lifespan was reportedly unchanged in mice that overexpress Sod1 (39, 51, 52) or Gpx4 (39). Overexpression of Sod2 has been reported to have no effect on lifespan in one study using B6 mice (53) and a small (4%) increase in lifespan in a second study using B6C3 mice (54), though no formal lifespan analyses were reported in this study. In a situation similar to that presented for MsrA−/− mice above, a report from Mitsui et al. suggested that overexpression of thioredoxin 1 (Trx1) extended lifespan ~35% (55). However, control mice in this study were relatively short-lived and a second lifespan study by Ikeno and colleagues performed under conditions that optimized control mouse longevity showed no effect of Trx1 overexpression on lifespan (56). As above, a cautious interpretation of these findings would be little effect of Trx1 on the aging process.
Increasing expression of the antioxidant catalase has been perhaps the most-comprehensively studied set of models in regards to effects on lifespan. Perez et al. reported that overexpression of catalase in its native cellular locations (the peroxisome) had no effect on lifespan (39, 52). However, Schriner et al. found that lifespan of mice could be significantly extended if increased catalase expression was targeted specifically to the mitochondria (57). These mCAT mice lived ~20% longer than control mice and significantly reduced age-related increases in oxidative damage. Schriner confirmed that overexpression of catalase specifically in the peroxisome had no effect on lifespan and also showed that increasing catalase expression in the nucleus had no effect. Thus, this finding makes a major contribution to OSTA in showing that lifespan is extended by increasing the expression of an antioxidant. At the same time though, this study also raises many questions including why this only occurs with increased catalase and why only when expressed in the mitochondria, a cellular organelle not typically associated with catalase.
Altering expression of multiple antioxidants does not affect aging
A key caveat of the studies described above is the assumption that modulating a single antioxidant gene will have significant impact on a complex process such as aging. The antioxidant defense is composed of both enzymatic and non-enzymatic components that may compensate for the loss (or gain) of a single constituent. One might hypothesize that altering multiple components might have more of an effect on oxidative stress-sensitive traits than would altering a single antioxidant. However, reducing several different combinations of antioxidants also had no overall effect on lifespan. These combinations include Sod2 × Gpx1, Sod2 × Gpx4, and Gpx1 × Gpx4 (39, 40). Interestingly, reducing the expression of different antioxidants in combination with Sod1 (i.e., Sod1 × Sod2, Sod1 × Sod3, Sod1 × Gpx1, Sod1 × Gpx4) resulted in lifespans no different from those of the relatively short-lived Sod1−/− mice (38, 39). Similarly, increasing expression of multiple antioxidants also has little effect on mouse lifespan; Perez et al. found that lifespan was unaffected by increased Sod1 and Sod2 expression or by increased Sod1 and catalase expression (39, 52). However, Schriner et al. found that mice with increased Sod1 and catalase in the peroxisome did show increased median, but not maximum lifespan (57).
In general, the results from lifespan studies largely support the conclusion that genetic modulation of antioxidant genes largely does not affect aging. Since these genetic modulations have generally been reported to alter oxidative stress in vivo (reviewed in (7)), these data would tend to refute the most basic interpretation of the OSTA.
POSSIBLE CONCLUSION 2: Altering expression of antioxidant genes in mice does affect the aging process, but has limited effects on longevity
As described above and in TABLE 1, lifespan of mice is largely unaffected by genetic modification of several different antioxidant genes with a few possible exceptions. Under the interpretation that changes in lifespan are equivalent to changes in aging, these data would support the conclusion that aging is likely not regulated by oxidative stress. While lifespan is the most easily discernable marker effects on aging, it must be reminded that this is not the only marker. Aging is a gradual process that turns young, healthy individuals into old, frail individuals prone to sickness and disease. Throughout this process, nearly every organ, tissue and cell type undergoes a multitude of biochemical and physical changes that we attribute to aging (58). It is the entirety of these changes that promote the declines in physiological function, increased incidence of disease and increased risk of mortality that occur with age. Therefore, monitoring general markers of healthy aging, or how long individuals live relatively free of disease and pathology, can be important determinants of altered aging rate. For example, the incidence of various forms of neoplasia, cardiovascular disease, nephropathology, metabolic disease, cataracts, osteoarthritis, etc. have all been clearly defined to increase with age in rodent models (59–63). Manipulations known to extend lifespan, such as dietary restriction or inhibition of GH/IGF1 signaling, have also been shown to delay the incidence of many of these age-related pathologies suggesting a general slowing of the aging process (59, 61, 63).
Reduced antioxidant expression accelerates age-related disease
While the evidence above shows mouse lifespan is largely unchanged by altering antioxidant expression, a more thorough examination of physiological changes with age in these mice does support a role for antioxidants in the modulation of age-related diseases. For example, even though mice with reduced MnSod (Sod2+/−) are not short-lived compared to control mice, they do have a significant increase in the incidence of neoplasia in old age (37). Sod2+/− mice also have increased incidence of cardiac hypertrophy (64), and age-related deficits in both glucose metabolism (65) and hearing (66). Reduction of Sod2 has also been shown to accelerate neurological disorders when introduced into mouse models of Alzheimer’s disease (67) or amyotrophic lateral sclerosis (68). Similarly, the lack of Gpx1 (Gpx1−/−) has been shown to exacerbate the pathological effects of hypertension (69), atherosclerosis (70), and brain ischemia/reperfusion (71) in different mouse models. Somewhat paradoxically, Gpx1−/− mice are also protected from defects in glucose metabolism associated with obesity (72) as well as from the pathologies associated with Parkinson’s disease in a mouse model of this disease (73). In both cases, this protective effect of Gpx1−/− has been attributed to reactive oxygen species-mediated signaling through cell survival pathways. Mice lacking MsrA (MsrA−/−) have been shown to have increased susceptibility to mouse models of cardiac dysfunction (74–76), obesity and diabetes (77), and neurodegeneration (44, 78).
Increased antioxidant expression delays age-related disease
Conversely, overexpression of antioxidant enzymes in mice has been shown to reduce or delay the development of several age-related diseases without significantly altering lifespan. Increased expression of Sod1 (Sod1 TG) has been shown to be beneficial in mouse models of atherosclerosis (79), glucose metabolic dysfunction (80), Alzheimer’s disease (81) and Parkinson’s disease (82). Similarly, increased Sod2 expression (Sod2 TG) prevents cardiac myopathy (83), declines in glucose metabolism (80), and Alzheimer’s disease (84, 85). Overexpression of Gpx4 (Gpx4 TG) prevents cardiac dysfunction in an ischemia/reperfusion model (86) and preserves pancreatic β-cell function to maintain glucose metabolism under high lipid conditions (87). Similarly, overexpression of Trx1 (Trx1 TG) preserves glucose metabolism under high fat feeding conditions in part through reduction of adipose inflammation (88). Increased Trx1 expression also prevents cardiac dysfunction caused either by cardiotoxin or ischemia/reperfusion (89, 90) and neurological pathologies associated with ischemia or excitotoxicity (91, 92). Somewhat surprisingly, increased expression of Sod2 had no effect on incidence of neoplasia as a cause of death and increasing Trx1 expression actually increased the numbers of neoplastic tumors in old mice (53, 56, 93).
The above data generally support the notion that genetic modulation of antioxidants can affect aspects of the aging process without affecting lifespan overall. However, these types of study can also support whether or not those mutations that do alter lifespan do so by altering the rate of aging. For example, Sod1−/− mice are relatively (−30%) short-lived but have high incidence of a form of neoplasia not often found in this mouse’s background strain. Thus, the lack of Sod1 may shorten lifespan simply by inducing a rare fatal disease and not by accelerating the aging process. If this were the case, we would expect that physiological changes that occur with age would proceed at the same rate in both Sod1−/− and WT mice and Sod1−/− mice would simply start dying sooner than WT mice as hepatocarcinoma develops. On the contrary though, the incidence of several age-related pathological changes in Sod1−/− mice have been reported to be increased and/or accelerated including sarcopenia and neuromuscular junction degeneration (48, 94), hearing loss (49), macular degeneration and cataracts (50, 95), pancreatic β-cell dysfunction (96) and neuronal degeneration (97). While not direct proof, these data could be cautiously interpreted as evidence for an acceleration of aging rate in Sod1−/− mice because the lack of this antioxidant promotes pathologies among many different tissue systems. Similarly, evidence from the incidence of age-related disease and pathologies in mCAT mice suggest that the increase in their lifespan (+20%) is due to a general slowing of aging and not by preventing a single disease or pathological cause of death. For example, aging mCAT mice showed reduced non-hematopoietic tumor burden, cardiac lesions, and systemic inflammation as compared to WT mice (98). In addition, mitochondrial catalase overexpression prevents age-related decline in cardiac function (99), age- and obesity-related declines in glucose metabolism (100, 101), and prevents neuronal decline both natively and in an Alzheimer’s disease model (102, 103). Again, these data are not definitive proof but do suggest that the mCAT mutation does delay the incidence of pathologies among several tissue and organ systems and thus may slow the aging process.
The data in this section are summarized in TABLE 2 and tend to support the hypothesis that antioxidant gene expression in mice does significantly impact the incidence of physiological declines or pathological insults associated with aging. While mutations in these genes may not (or may) affect mouse lifespan, these data suggest that modulation of antioxidant enzymes may significantly alter the aging process in measureable ways. Further, these changes tend to be consistent among each of the different systems; i.e., reduction of antioxidants generally increases susceptibility to cancer, cardiovascular diseases, metabolic defects and neurological disorders whereas increasing antioxidant expression prevents these (TABLE 2). Systematic studies of how hallmarks of aging are altered in these models would give significant support to this possible conclusion (58).
TABLE 2.
Published observations on incidences of age-related pathologies in antioxidant mutant mice.
| REDUCED expression | |||||
|---|---|---|---|---|---|
| Enzyme | Cancer | Cardiovascular | Metabolic | Neuronal | Refs |
| Sod1−/− | ↑ | ↑ | ↑ | (47, 94, 96, 97) | |
| Sod2+/− | ↑ | ↑ | ↑ | ↑ | (37, 64, 65, 67) |
| Gpx1−/− | ↑ | ↑ | ↓ | ↓↑ | (40, 69–73) |
| MsrA−/− | ↑ | ↑ | ↑ | (44, 74–78) | |
| INCREASED expression | |||||
| Sod1 TG | ↓ | ↓ | ↓ | (79–82) | |
| Sod2 TG | - | ↓ | ↓ | ↓ | (53, 54, 80, 83–85) |
| Gpx4 TG | ↓ | ↓ | (86, 87) | ||
| Trx1 TG | ↑ | ↓ | ↓ | ↓ | (56, 88–93) |
| mCAT TG | ↓ | ↓ | ↓ | ↓ | (98–103) |
↑ = increased incidence of pathology reported, ↓ = reduced incidence of pathology reported, ↓↑ = both increased and reduced incidence of pathology reported, - = no difference reported. Shaded boxes = no data published on pathological state for given model.
POSSIBLE CONCLUSION 3: There are still too many poorly answered questions to accurately determine whether altering expression of antioxidant genes in mice affects the aging process
QUESTION 1. Does degree of oxidative damage matter?
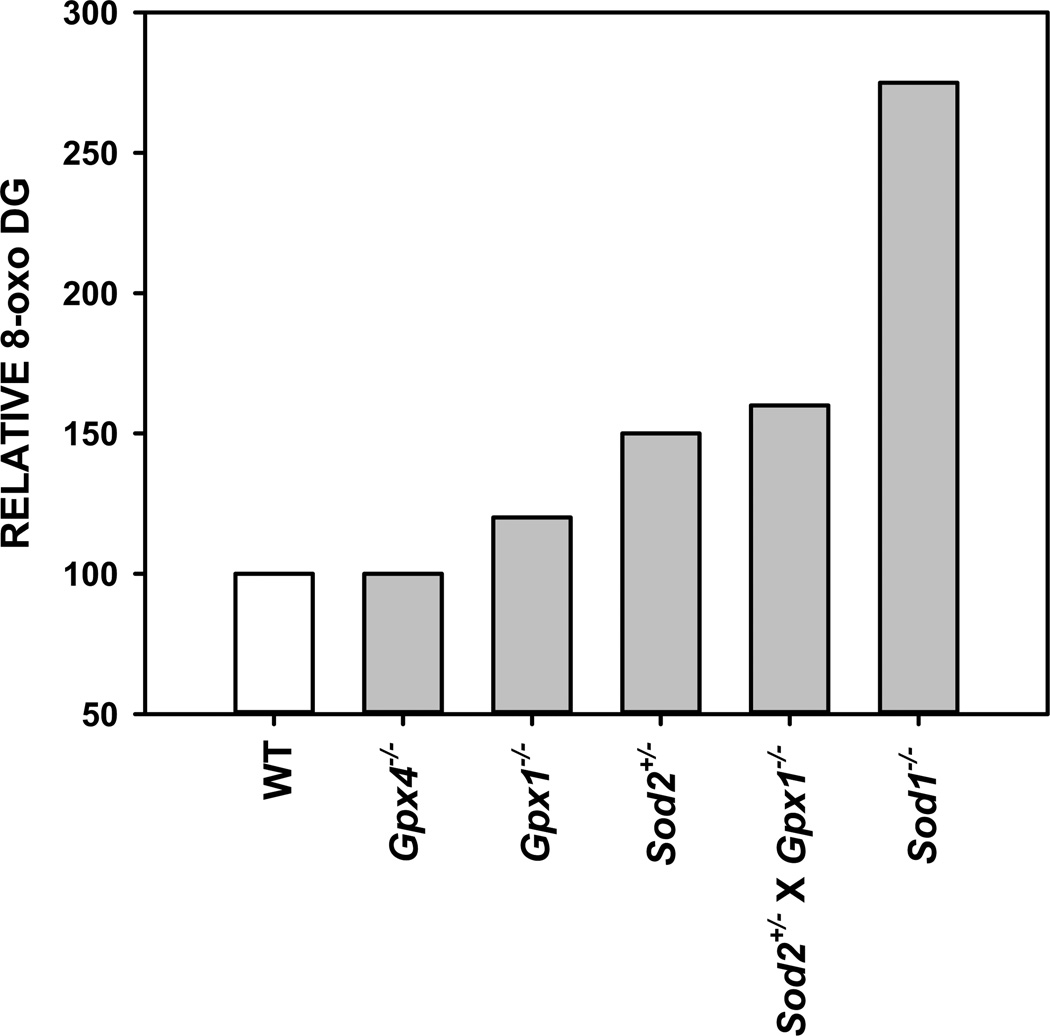
As has been well-established, aging does promote a measurable accumulation of oxidative damage (reviewed in (6)). Further, this accumulation of damage is mitigated or exacerbated by genetic modulation of antioxidants (40, 47, 48, 53, 57). This raises the question of why modulating antioxidant expression largely has no effect on lifespan in mice (TABLE 1). At its most basic, the OSTA posits that aging is caused directly by the accumulation of cellular oxidative damage. One interpretation of this suggests a relationship between aging and oxidative stress that extrapolates to a defined, negative correlation; i.e., a relationship where lifespan/aging is directly predicted based on accumulation of oxidative damage. However, another possibility may be that this relationship is not linear and that some threshold level of oxidative damage must be reached before detrimental effects on aging are readily apparent. That is, levels of oxidative damage throughout the lifespan of mammals exist across a “normal” range and aging rates will only be altered when levels fall outside of this range. Of the mutant mice with reduced antioxidant enzyme expression, only one model, mice lacking Sod1 (Sod1−/−), has consistently shown a reduction in lifespan as predicted by the OSTA (TABLE 1). Interestingly, the increase in oxidative damage in Sod1−/− mice compared to WT mice seems to be far greater than that of other antioxidant knockout mice (FIGURE 1). An examination of the literature regarding levels of the DNA oxidation marker 8-Oxo-2'-deoxyguanosine (8-oxo DG) in the liver of young mice shows a >250% increase in 8-oxo DG in Sod1−/− mice (47, 104). In contrast, mutant mice with no reduction in lifespan (Gpx4+/−, Gpx1−/−, or Sod2+/−) are reported to show increases in liver 8-oxo DG of <50% (37, 40, 43, 104). Reduction of both Sod2 and Gpx1 in mice (Sod2+/− × Gpx1−/−) does not further potentiate accumulation of liver 8-oxo DG (with an increase of only ~60% over WT) and has no detrimental effect on lifespan (40). Other markers of oxidative damage, including damage to proteins and lipids, also suggest considerably greater increases in Sod1−/− mice than in other antioxidant mutant mice (40, 47, 104, 105). And while markers of several different forms of oxidative damage do increase with age in WT mice, these levels are further exacerbated with aging in Sod1−/− mice (40, 47, 48, 53).
Figure 1.
8-Oxo-2'-deoxyguanosine (8-oxo DG) levels in liver of young antioxidant mutant mice. Data are presented as relative to levels measured for wild-type (WT) mice as reported in (37, 40, 43, 47, 104). Gpx4 = glutathione peroxide isoform 4, Gpx1 = glutathione peroxide isoform 1, Sod2 = superoxide dismutase 2, Sod1 = superoxide dismutase 1.
It might be argued then that Sod1−/− mice are short-lived because levels of oxidative stress/damage in this model are sufficiently beyond this hypothetical “pro-aging threshold”. By extension, reducing oxidative damage in this model would be predicted to prevent the shortening of lifespan. Directly supporting this hypothesis is the finding of Zhang et al. which shows that dietary restriction of Sod1−/− mice is sufficient to both reduce oxidative stress and rescue the lifespan attenuation in this mouse model (106). Zhang et al. also found that dietary restriction was sufficient to reduce the high incidence of liver pathology and neoplasia in Sod1−/− mice. Further, Jang et al. found in a similar study that dietary restriction inhibited the neuromuscular decline with age in Sod1−/− mice (107). These studies suggest that dietary restriction can mitigate the high levels of oxidative stress that cause the accelerated aging phenotype of the Sod1−/− mouse. Further, these studies suggest that some forms of age-related disease are directly regulated by the accumulation of oxidative damage with age. On the other hand, these studies do not directly address whether it is the reduction of oxidative stress that is key for the dietary restriction-mediated extension of lifespan in WT mice, or whether other functional pathways affected by dietary restriction are more critical. Moreover, it is still unclear why oxidative stress/damage is much higher in Sod1−/− mice compared to other mouse mutants with reduced antioxidants. The lack of Sod1 in mice does not cause significant changes in the expression of other antioxidant enzymes (47, 48, 96). However, whether Sod1−/− mice have significant reduction in levels of cellular non-enzymatic antioxidants and reducing agents has not yet be thoroughly investigated.
QUESTION 2. Does the tissue site of oxidative stress matter?
All lifespan studies discussed above have been performed mutant mice in which the given antioxidant has been altered ubiquitously among all tissues and throughout life. While such models simplify the question addressed, they also make interpretation of the models more complex. For example, if a particular antioxidant has a beneficial effect for one stage of life (perhaps early in life or development) but has detrimental effect at another (late in life), the net result may be no change in lifespan. An example of this may be found in the study by Ikeno and colleagues that showed increased levels of Trx1 expression were beneficial to lifespan early in life (increased median lifespan) but detrimental late in life (increased age-related neoplasia) (56). Altering antioxidants or oxidative stress may also have different affects determined by tissue-specific context. For example, increased expression of an antioxidant may benefit one tissue, but be detrimental to another again potentially confounding the issue of oxidative stress in aging. While challenging to address in ubiquitous transgenic models, the development of Cre-lox mediated genetic recombination mouse models has made tissue- and temporal-specific antioxidant modulation possible. While the use of these models in the aging field is still relatively new, some insight has been made into the role of oxidative stress and aging over the last few years.
The complete ablation of some antioxidant enzymes (i.e., Sod2 and Gpx4) is lethal to mice either at the embryonic or peri-natal stage of development (108, 109). Because of this absolute requirement for these antioxidants during development, lifespan studies have been performed only on heterozygous mutants; i.e., 50% reduction in the expression of either gene (37, 43). While oxidative stress was increased in these mice, lifespan was essentially unchanged, raising the possibility that a 50% reduction in either gene was not sufficient to decrease lifespan (see QUESTION 1 above). However, tissue-specific knockout mice (or perhaps more appropriately, knockdown), especially those that can be induced after development, can be utilized to make great progress in understanding the roles of these antioxidants in aging. For example, Cre-lox mediated knockout of Sod2 in some tissues may directly impact that particular tissue, but have little impact on aging at least through ~1 year of age. Published examples of this include Sod2 knockdown in the liver (using the AFP promoter), skeletal muscle (HAS promoter), Type IIB skeletal fibers (TniFast promoter), kidney (Ksp-Cadherin promoter), post-natal motor neurons (VAChT promoter) and mammary gland (MMTV promoter) (110–115). However, complete longevity studies have not been performed in these models and their effect on longevity is not yet known. In contrast, some Cre-lox mediated tissue-specific mice have been shown to have dramatic effects on survival. Sod2 knockout in the brain (Nestin promoter) or peripheral nervous system (Nestin promoter) cause peri-natal death before approximately 4 weeks of age (116, 117). Heart- and skeletal musclespecific knockout of Sod2 (MCK promoter) causes severe cardiac abnormalities and survive only until ~150 days (118). Because these mice die extremely early, these models likely do not provide evidence for Sod2’s role in aging. However, a recent report from Trieber et al. suggests that the lack of Sod2 specifically in connective tissue (α2(I) collagen promoter) may accelerate the rate of aging (119). Lifespan of Sod2 knockout mice in this study was ~15% shorter than their controls and these mice showed accelerated rates of sarcopenia, osteoporosis, skin atrophy and accumulation of senescent cells. These interesting findings therefore suggest that knockdown of Sod2 in connective tissue does promote aging and certainly supports a role for oxidative stress in aging.
Tissue-specific knockout mice might be also be used to isolate the role of antioxidants in the aging of particular tissues or cells. For example, as discussed above Sod1−/− mice develop muscle atrophy with aging, but it has been unclear whether this is due to oxidative stress-mediated failure of the muscle or the motor neuron (48, 94). Zhang et al. recently reported that mice that lack Sod1 only in the skeletal muscle (but retain Sod1 expression elsewhere) do not phenocopy the sarcopenia of Sod1−/− mice (120). These data suggest that sarcopenia in Sod1−/− mice is driven largely due to oxidative damage in other tissues, most likely the peripheral nerve system. Further supporting this is a recent report by Sakellariou et al. that showed restoring Sod1 activity in the brain, spinal cord and peripheral nervous system of Sod1−/− mice is sufficient to prevent the neuromuscular junction degeneration and sarcopenia that occur in mice completely lacking Sod1 (121). Tissuespecific antioxidant mutant mice could also be utilized to test cell non-autonomous roles of antioxidants in the aging process. For example, Parkes et al. showed that overexpression of Sod1 only in the motorneuron was sufficient to extend Drosophila lifespan suggesting that reduced oxidative stress in this one tissue had body-wide effects on aging (30). Similar studies have not yet been reported with antioxidants in mice; however, lifespan extension has been reported in mice with brain-specific knockout of insulin receptor substrate–2 (Irs2) and fat-specific knockout of insulin receptor (122, 123)
QUESTION 3. Do environmental conditions matter?
As discussed above, it is generally thought that studies on mouse aging should strive to utilize optimal animal husbandry to minimize any potential effects of animal stress on these studies. Under such conditions, lifespan should be solely directed by the rate of the aging process and not driven by chronic disease or pathology. However, it is also likely that the mechanisms that regulate aging and disease cannot be completely disentangled; as described above, the incidence of several diseases are clearly associated with advancing age (59–63). Under this possibility, the environmental conditions may have a profound impact on lifespan through both modulation of oxidative stress and the aging process.
While it is clearly a much more complex question, there are some previously published hints on the combined role of oxidative stress and environment in regulating aging and lifespan. From a review of Drosophila studies, Orr and Sohal meticulously showed that the lifespan extending effects of increased Sod1 expression were largely dependent on the longevity of the control fly strain (29). That is, Sod1 overexpression in a short-lived Drosophila background promoted a significant extension of lifespan whereas the same level of Sod1 increase had little to no effect in a long-lived Drosophila background. It isn’t clear, but one could surmise that short-lived flies were an “at-risk” group in terms of health or longevity and were protected by Sod1 expression. In contrast, the “robust” long-lived group may have been at their maximum of health and longevity and thus increasing Sod1 had no further effect. Revisiting the data in mice, the studies reporting discrepancies in the lifespan of MsrA−/− and Trx1 TG mice might be similarly interpreted. Moskovitz et al. reported that the lack of MsrA shortens lifespan when control mice have a mean survival of ~680 days (44). However, we reported if the mean survival of control mice of the same genetic background is increased to ~925 days, the lack of MsrA has no effect on lifespan (45). In other words, simply improving the husbandry conditions to extend the control group’s lifespan ~1/3 longer completely ablated the lifespan effect of MsrA. While no pathology data were reported in either study, it seems reasonable that mice studied by Moskovitz et al. may have been chronically stressed or ill and that the lack of MsrA exacerbated this condition. Similarly, Mitsui et al. reported that overexpression of Trx1 extended lifespan ~35% if the mean survival of control mice was ~23 months of age (55). However, Ikeno and colleagues later reported that the same mouse showed no effect on longevity if the mean survival was ~30 months (56). These studies again show that the effects of modulating antioxidant expression on lifespan may be largely dependent on environmental conditions.
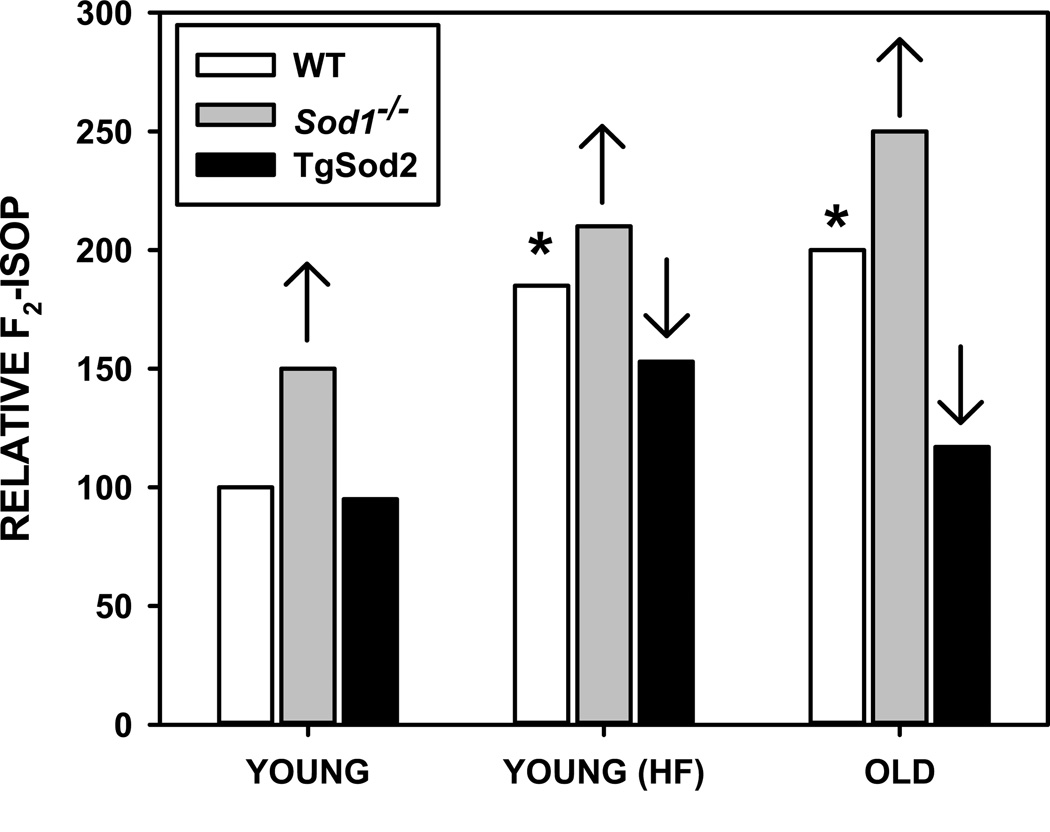
A case could be made for assessing lifespan of antioxidant mice not only under optimal husbandry conditions, but also under conditions that expose these mice to some form of chronic, low-grade stress to better understand the potential environmental impact. Such studies would have challenges with the most significant being maintaining control of these conditions over long periods of time. One possibility would be to determine lifespan in obese, high fat fed mice. Obesity is associated with increased levels of oxidative stress in both rodents and humans (23, 124–126). Data from our lab show that levels of F2-isoprostanes, a marker of lipid oxidation, in the muscle of mice fed high fat diets for 3–4 months are not different from those found in muscle of old mice (48, 53, 80, 96). That is, feeding mice a high fat diet for the equivalent of ~1/8 of their lifespan is sufficient to promote levels of oxidative stress normally found in mice older than 30 months. Furthermore, we have shown that these levels can be further modulated by altering the expression of antioxidants (80, 96). The lack of Sod1 further exacerbates the accumulation of oxidative damage both in high fat feeding and aging whereas overexpression of Sod2 prevents oxidative damage in both cases (FIGURE 2). While lifespan of high fat-fed antioxidant mutant mice has not been determined under these conditions, other reports suggest that a high fat diet moderately diminishes mouse lifespan without significantly altering the incidence of uncommon fatal rodent pathologies (127, 128). Thus, this feeding protocol might be a solution to deliver a robust, chronic oxidative stress in a controlled manner.
Figure 2.
Modulation of F2-isoprostane (F2-isop) levels in muscle by age, obesity, and genetic mutation of antioxidant enzymes. Data are presented as relative to levels measured in WT mice as reported in (48, 53, 80, 96). Asterisks represent F2-isop in WT increased significantly with either high fat diet or age. Up arrow represents significant increase in F2-isop in Sod1−/− mice compared to WT for indicated condition. Down arrow represents significant decrease in F2-isop in TgSod2 mice compared to WT for indicated condition.
CONCLUDING REMARKS
The current status of the OSTA in mammals likely depends on the viewpoint of the reader interpreting the literature regarding antioxidant mutant mice aging studies published over the last two decades. Under the strictest interpretation, i.e., modulating oxidative stress or damage should directly impact lifespan, these data generally fail to support the OSTA in that most of these mutant mice show little to no alteration in lifespan. However, this interpretation fails to account for alterations to the aging process that do not directly impact longevity such as accumulation of damage (both oxidative and cellular), development of frailty, incidence of age-related diseases and other measurements. An interpretation of the OSTA that includes these parameters of aging has much more support from the literature on these markers in aging antioxidant mutant mice. Furthermore, oxidative stress may affect certain tissue/organs to a much greater degree than others which could significantly impact the maintenance of health with advancing age. Lastly, the current state of aging studies has necessarily taken a very narrow view on the impact of environmental challenges on the aging process. While human innovation has protected society from many of these challenges, our population is still not comparable to mice in a stable animal facility and we face constantly changing environmental impacts. It is reasonable to assume these impact our rate of healthy aging, if not aging directly. It would seems that the time is ripe for further modification of the OSTA to address a precise definition of what aging is, as well as what tools are best for its measurements. While somewhat battered, the OSTA is likely not dead yet and may still provide us with key findings regarding the fundamental mechanisms of aging.
Highlights.
-
-
Direct testing in mouse models has challenged the oxidative stress theory of aging.
-
-
Lifespan in largely unaffected by genetic modulation of antioxidants.
-
-
Antioxidants do alter the development of age related disease and pathology.
-
-
Can we differentiate “longevity” from “pathological” effects in aging studies?
ACKNOWLEDGEMENTS
The authors wish to thank Arlan Richardson, Holly Van Remmen and Yuji Ikeno for discussions that led to the compilation of this review and their yeoman’s work over the last 15+ years in regards to testing the OSTA under the criteria above. This work was supported by the Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care Systems, a pilot grant from the San Antonio Nathan Shock Center (NIA 5 P30 AG013319-19) and a Biomedical Research Grant from the San Antonio Area Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Yashin AI. Time trends of incidence of age-associated diseases in the US elderly population: medicare-based analysis. Age and Ageing. 2013;42:494–500. doi: 10.1093/ageing/aft032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood TBL. Understanding the Odd Science of Aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the Longevity Dividend. Annals of the New York Academy of Sciences. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Aging: A Theory Based on Free Radical and Radiation Chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 5.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radical Biology and Medicine. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mechanisms of ageing and development. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radical Biology and Medicine. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paolisso G, Tagliamonte MR, Rizzo MR, Manzella D, Gambardella A, Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. Journal of the American Geriatrics Society. 1998;46:833–838. doi: 10.1111/j.1532-5415.1998.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri M, Rizzo MR, Manzella D, Grella R, Ragno E, Carbonella M, Abbatecola AM, Paolisso G. Glucose regulation and oxidative stress in healthy centenarians. Experimental Gerontology. 2003;38:137–143. doi: 10.1016/s0531-5565(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. Fundamental Aspects of Reactive Oxygen Species, or What's the Matter with Oxygen? Annals of the New York Academy of Sciences. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 11.Tappel AL. Glutathione peroxidase and hydroperoxides. Methods in enzymology. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 12.Deisseroth A, Dounce AL. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiological reviews. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 13.Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 14.Powis G, Montfort WR. PROPERTIES AND BIOLOGICAL ACTIVITIES OF THIOREDOXINS. Annual Review of Pharmacology and Toxicology. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- 15.Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Wawryn J, Krzepilko A, Myszka A, Bilinski T. Deficiency in superoxide dismutases shortens life span of yeast cells. Acta biochimica Polonica. 1999;46:249–253. [PubMed] [Google Scholar]
- 17.Longo VD, Gralla EB, Valentine JS. Superoxide Dismutase Activity Is Essential for Stationary Phase Survival in Saccharomyces cerevisiae: MITOCHONDRIAL PRODUCTION OF TOXIC OXYGEN SPECIES IN VIVO. Journal of Biological Chemistry. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 18.Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS letters. 2004;557:136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- 19.Harris N, Costa V, MacLean M, Mollapour M, Moradas-Ferreira P, Piper PW. Mnsod overexpression extends the yeast chronological (G(0)) life span but acts independently of Sir2p histone deacetylase to shorten the replicative life span of dividing cells. Free radical biology & medicine. 2003;34:1599–1606. doi: 10.1016/s0891-5849(03)00210-7. [DOI] [PubMed] [Google Scholar]
- 20.Longo VD, Liou LL, Valentine JS, Gralla EB. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999;365:131–142. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 21.Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. The Journal of biological chemistry. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- 22.Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of Life-Span with Superoxide Dismutase/Catalase Mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 23.Keaney JF, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in The Framingham Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 24.Keaney M, Gems D. No increase in lifespan in Caenorhabditis elegans upon treatment with the superoxide dismutase mimetic EUK-8. Free Radical Biology and Medicine. 2003;34:277–282. doi: 10.1016/s0891-5849(02)01290-x. [DOI] [PubMed] [Google Scholar]
- 25.Pun PB, Gruber J, Tang SY, Schaffer S, Ong RL, Fong S, Ng LF, Cheah I, Halliwell B. Ageing in nematodes: do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology. 2010;11:17–30. doi: 10.1007/s10522-009-9223-5. [DOI] [PubMed] [Google Scholar]
- 26.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, Matscheski A, Vanfleteren JR, Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes & Development. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Raamsdonk JM, Hekimi S. Deletion of the Mitochondrial Superoxide Dismutase<italic>sod-2</italic>Extends Lifespan in<italic>Caenorhabditis elegans</italic>. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim D-H, Han JY, Kim J-R, Lee YS, Kim H-Y. Methionine sulfoxide reductase B in the endoplasmic reticulum is critical for stress resistance and aging in Drosophila. Biochemical and biophysical research communications. 2012;419:20–26. doi: 10.1016/j.bbrc.2012.01.099. [DOI] [PubMed] [Google Scholar]
- 29.Orr WC, Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Experimental Gerontology. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 30.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature genetics. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 31.Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Molecular and cellular biology. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 34.Bayne AC, Mockett RJ, Orr WC, Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. The Biochemical journal. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free radical biology & medicine. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- 36.Shchedrina VA, Vorbrüggen G, Lee BC, Kim H-Y, Kabil H, Harshman LG, Gladyshev VN. Overexpression of methionine-R-sulfoxide reductases has no influence on fruit fly aging. Mechanisms of ageing and development. 2009;130:429–443. doi: 10.1016/j.mad.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiological genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 38.Sentman M-L, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of Mice Lacking Extracellular Superoxide Dismutase and Copper- and Zinc-containing Superoxide Dismutase. Journal of Biological Chemistry. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 39.Pérez VI, Bokov A, Remmen HV, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez VI, Lew CM, Cortez LA, Webb CR, Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, Richardson A, Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free radical biology & medicine. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Fomenko DE, Novoselov SV, Natarajan SK, Lee BC, Koc A, Carlson BA, Lee T-H, Kim H-Y, Hatfield DL, Gladyshev VN. MsrB1 (Methionine-R-sulfoxide Reductase 1) Knockout Mice: ROLES OF MsrB1 IN REDOX REGULATION AND IDENTIFICATION OF A NOVEL SELENOPROTEIN FORM. Journal of Biological Chemistry. 2009;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ, 2nd, Wolf N, Van Remmen H, Richardson A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 44.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proceedings of the National Academy of Sciences. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmon AB, Pérez VI, Bokov A, Jernigan A, Kim G, Zhao H, Levine RL, Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. The FASEB Journal. 2009;23:3601–3608. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 47.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 48.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang T-T, Epstein CJ, Roberts Ii LJ, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radical Biology and Medicine. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 49.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiology of Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 50.Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Experimental Eye Research. 2004;79:859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 52.Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiology of learning and memory. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxidants & redox signaling. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 56.Perez VI, Cortez LA, Lew CM, Rodriguez M, Webb CR, Van Remmen H, Chaudhuri A, Qi W, Lee S, Bokov A, Fok W, Jones D, Richardson A, Yodoi J, Zhang Y, Tominaga K, Hubbard GB, Ikeno Y. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:1286–1299. doi: 10.1093/gerona/glr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of Murine Life Span by Overexpression of Catalase Targeted to Mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 58.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Lopez EF, Jin Y, Van Remmen H, Bauch T, Han H-C, Lindsey ML. Age-related cardiac muscle sarcopenia: Combining experimental and mathematical modeling to identify mechanisms. Experimental Gerontology. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S-i, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. The Journal of Clinical Investigation. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Ikeno Y, Lew C, Cortez L, Webb C, Lee S, Hubbard G. Do long-lived mutant and calorie-restricted mice share common anti-aging mechanisms?—a pathological point of view. AGE. 2006;28:163–171. doi: 10.1007/s11357-006-9007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richters L, Lange N, Renner R, Treiber N, Ghanem A, Tiemann K, Scharffetter-Kochanek K, Bloch W, Brixius K. Exercise-induced adaptations of cardiac redox homeostasis and remodeling in heterozygous SOD2-knockout mice. Journal of Applied Physiology. 2011;111:1431–1440. doi: 10.1152/japplphysiol.01392.2010. [DOI] [PubMed] [Google Scholar]
- 65.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proceedings of the National Academy of Sciences. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinoshita M, Sakamoto T, Kashio A, Shimizu T, Yamasoba T. Age-Related Hearing Loss in Mn-SOD Heterozygous Knockout Mice. Oxidative Medicine and Cellular Longevity. 2013;2013:12. doi: 10.1155/2013/325702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esposito L, Raber J, Kekonius L, Yan F, Yu G-Q, Bien-Ly N, Puoliväli J, Scearce-Levie K, Masliah E, Mucke L. Reduction in Mitochondrial Superoxide Dismutase Modulates Alzheimer's Disease-Like Pathology and Accelerates the Onset of Behavioral Changes in Human Amyloid Precursor Protein Transgenic Mice. The Journal of Neuroscience. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller FL, Liu Y, Jernigan A, Borchelt D, Richardson A, Van Remmen H. MnSOD deficiency has a differential effect on disease progression in two different ALS mutant mouse models. Muscle & Nerve. 2008;38:1173–1183. doi: 10.1002/mus.21049. [DOI] [PubMed] [Google Scholar]
- 69.Ardanaz N, Yang X-P, Cifuentes ME, Haurani MJ, Jackson KW, Liao T-D, Carretero OA, Pagano PJ. Lack of Glutathione Peroxidase 1 Accelerates Cardiac-Specific Hypertrophy and Dysfunction in Angiotensin II Hypertension. Hypertension. 2010;55:116–123. doi: 10.1161/HYPERTENSIONAHA.109.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr H-A, Blankenberg S, Förstermann U, Münzel T, Lackner KJ. Deficiency of Glutathione Peroxidase-1 Accelerates the Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 71.Crack PJ, Taylor JM, Flentjar NJ, De Haan J, Hertzog P, Iannello RC, Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. Journal of Neurochemistry. 2001;78:1389–1399. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 72.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell metabolism. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hennis MR, Marvin MA, Taylor Ii CM, Goldberg MS. Surprising behavioral and neurochemical enhancements in mice with combined mutations linked to Parkinson's disease. Neurobiology of Disease. 2014;62:113–123. doi: 10.1016/j.nbd.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erickson JR, Joiner M-lA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochemical and biophysical research communications. 2008;366:775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 76.Nan C, Li Y, Jean-Charles P-Y, Chen G, Kreymerman A, Prentice H, Weissbach H, Huang X. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochemical and biophysical research communications. 2010;402:608–613. doi: 10.1016/j.bbrc.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 77.Styskal J, Nwagwu FA, Watkins YN, Liang H, Richardson A, Musi N, Salmon AB. Methionine sulfoxide reductase A affects insulin resistance by protecting insulin receptorfunction. Free Radical Biology and Medicine. 2013;56:123–132. doi: 10.1016/j.freeradbiomed.2012.10.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal R, Oien D, Ersen F, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- 79.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of Atherosclerosis by Overexpression of Catalase or Both Cu/Zn-Superoxide Dismutase and Catalase in Mice Lacking Apolipoprotein E. Circulation Research. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Qi W, Richardson A, Van Remmen H, Ikeno Y, Salmon AB. Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochemical and biophysical research communications. 2013;438:78–83. doi: 10.1016/j.bbrc.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borg J, Chereul E. Differential MRI patterns of brain atrophy in double or single transgenic mice for APP and/or SOD. Journal of Neuroscience Research. 2008;86:3275–3284. doi: 10.1002/jnr.21778. [DOI] [PubMed] [Google Scholar]
- 82.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. Overexpression of Superoxide Dismutase or Glutathione Peroxidase Protects against the Paraquat + Maneb-induced Parkinson Disease Phenotype. Journal of Biological Chemistry. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 83.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of Cardiac Mitochondria by Overexpression of MnSOD Reduces Diabetic Cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 84.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. The FASEB Journal. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radical Biology and Medicine. 2008;45:855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 87.Koulajian K, Ivovic A, Ye K, Desai T, Shah A, Fantus IG, Ran Q, Giacca A. Overexpression of glutathione peroxidase 4 prevents beta-cell dysfunction induced by prolonged elevation of lipids in vivo. American journal of physiology. Endocrinology and metabolism. 2013;305:E254–E262. doi: 10.1152/ajpendo.00481.2012. [DOI] [PubMed] [Google Scholar]
- 88.Salmon AB, Flores LC, Li Y, Van Remmen H, Richardson A, Ikeno Y. Reduction of glucose intolerance with high fat feeding is associated with anti-inflammatory effects of thioredoxin 1 overexpression in mice. Pathobiology of aging & age related diseases. 2012;2 doi: 10.3402/pba.v2i0.17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S-i, Yodoi J. Overexpression of Thioredoxin-1 in Transgenic Mice Attenuates Adriamycin-Induced Cardiotoxicity. Circulation. 2002;106:1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 90.Turoczi T, Chang VW-H, Engelman RM, Maulik N, Ho Y-S, Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. Journal of Molecular and Cellular Cardiology. 2003;35:695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 91.Takagi Y, Hattori I, Nozaki K, Mitsui A, Ishikawa M, Hashimoto N, Yodoi J. Excitotoxic hippocampal injury is attenuated in thioredoxin transgenic mice. J Cerebr Blood F Met. 2000;20:829–833. doi: 10.1097/00004647-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 92.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proceedings of the National Academy of Sciences. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flores L, Ortiz M, Dube S, Hubbard G, Lee S, Salmon A, Zhang Y, Ikeno Y. Thioredoxin, oxidative stress, cancer and aging. Longevity & Healthspan. 2012;1:4. doi: 10.1186/2046-2395-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. The FASEB Journal. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proceedings of the National Academy of Sciences. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muscogiuri G, Salmon AB, Aguayo-Mazzucato C, Li M, Balas B, Guardado-Mendoza R, Giaccari A, Reddick RL, Reyna SM, Weir G, DeFronzo RA, Van Remmen H, Musi N. Genetic Disruption of SOD1 Gene Causes Glucose Intolerance and Impairs β-cell Function. Diabetes. 2013 doi: 10.2337/db13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischer LR, Igoudjil A, Magrané J, Li Y, Hansen JM, Manfredi G, Glass JD. SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse. Brain. 2011;134:196–209. doi: 10.1093/brain/awq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of Age-Associated Pathology in Old Mice by Overexpression of Catalase in Mitochondria. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 99.Dai D-F, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H-Y, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI. Targeted Expression of Catalase to Mitochondria Prevents Age-Associated Reductions in Mitochondrial Function and Insulin Resistance. Cell Metabolism. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of Clinical Investigation. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP, Shirendeb U, Lo H-H, Rabinovitch PS, Reddy PH. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Human Molecular Genetics. 2012;21:2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reid HJO, Lance AJ, Damian GZ, Charles LL, Jacob R. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. Journal of Neurochemistry. 2013;125:303–313. doi: 10.1111/jnc.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han E-S, Muller FL, Pérez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts LJ, Van Remmen H, Richardson A. The in vivo gene expression signature of oxidative stress. Physiological genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Busuttil RA, Garcia AM, Cabrera C, Rodriguez A, Suh Y, Kim WH, Huang T-T, Vijg J. Organ-Specific Increase in Mutation Accumulation and Apoptosis Rate in CuZn-Superoxide Dismutase–Deficient Mice. Cancer Research. 2005;65:11271–11275. doi: 10.1158/0008-5472.CAN-05-2980. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Ikeno Y, Bokov A, Gelfond J, Jaramillo C, Zhang H-M, Liu Y, Qi W, Hubbard G, Richardson A, Van Remmen H. Dietary restriction attenuates the accelerated aging phenotype of Sod1−/− mice. Free Radical Biology and Medicine. 2013;60:300–306. doi: 10.1016/j.freeradbiomed.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, Chaudhuri A, Qi W, Li Y, Huang J-Y, Verdin E, Richardson A, Van Remmen H. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11:770–782. doi: 10.1111/j.1474-9726.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radical Biology and Medicine. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature genetics. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 110.Lenart J, Dombrowski F, Görlach A, Kietzmann T. Deficiency of manganese superoxide dismutase in hepatocytes disrupts zonated gene expression in mouse liver. Archives of Biochemistry and Biophysics. 2007;462:238–244. doi: 10.1016/j.abb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 111.Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radical Biology and Medicine. 2010;48:1252–1262. doi: 10.1016/j.freeradbiomed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 112.Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parajuli N, Marine A, Simmons S, Saba H, Mitchell T, Shimizu T, Shirasawa T, MacMillan-Crow LA. Generation and characterization of a novel kidney-specific manganese superoxide dismutase knockout mouse. Free Radical Biology and Medicine. 2011;51:406–416. doi: 10.1016/j.freeradbiomed.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Misawa H, Nakata K, Matsuura J, Moriwaki Y, Kawashima K, Shimizu T, Shirasawa T, Takahashi R. Conditional knockout of Mn superoxide dismutase in postnatal motor neurons reveals resistance to mitochondrial generated superoxide radicals. Neurobiology of Disease. 2006;23:169–177. doi: 10.1016/j.nbd.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Case AJ, Domann FE. Manganese superoxide dismutase is dispensable for post-natal development and lactation in the murine mammary gland. Free Radic Res. 2012;46:1361–1368. doi: 10.3109/10715762.2012.715370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sasaki T, Shimizu T, Koyama T, Sakai M, Uchiyama S, Kawakami S, Noda Y, Shirasawa T, Kojima S. Superoxide dismutase deficiency enhances superoxide levels in brain tissues during oxygenation and hypoxia-reoxygenation. Journal of Neuroscience Research. 2011;89:601–610. doi: 10.1002/jnr.22581. [DOI] [PubMed] [Google Scholar]
- 117.Oh SS, Sullivan KA, Wilkinson JE, Backus C, Hayes JM, Sakowski SA, Feldman EL. Neurodegeneration and early lethality in superoxide dismutase 2-deficient mice: A comprehensive analysis of the central and peripheral nervous systems. Neuroscience. 2012;212:201–213. doi: 10.1016/j.neuroscience.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative Stress Causes Heart Failure with Impaired Mitochondrial Respiration. Journal of Biological Chemistry. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 119.Treiber N, Maity P, Singh K, Kohn M, Keist AF, Ferchiu F, Sante L, Frese S, Bloch W, Kreppel F, Kochanek S, Sindrilaru A, Iben S, Högel J, Ohnmacht M, Claes LE, Ignatius A, Chung JH, Lee MJ, Kamenisch Y, Berneburg M, Nikolaus T, Braunstein K, Sperfeld A-D, Ludolph AC, Briviba K, Wlaschek M, Scharffetter-Kochanek K. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell. 2011;10:239–254. doi: 10.1111/j.1474-9726.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Davis C, Sakellariou GK, Shi Y, Kayani AC, Pulliam D, Bhattacharya A, Richardson A, Jackson MJ, McArdle A, Brooks SV, Van Remmen H. CuZnSOD gene deletion targeted to skeletal muscle leads to loss of contractile force but does not cause muscle atrophy in adult mice. The FASEB Journal. 2013;27:3536–3548. doi: 10.1096/fj.13-228130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sakellariou GK, Davis CS, Shi Y, Ivannikov MV, Zhang Y, Vasilaki A, Macleod GT, Richardson A, Van Remmen H, Jackson MJ, McArdle A, Brooks SV. Neuron-specific expression of CuZnSOD prevents the loss of muscle mass and function that occurs in homozygous CuZnSOD-knockout mice. The FASEB Journal. 2013 doi: 10.1096/fj.13-240390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taguchi A, Wartschow LM, White MF. Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 123.Blüher M, Kahn BB, Kahn CR. Extended Longevity in Mice Lacking the Insulin Receptor in Adipose Tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 124.Frohnert BI, Long EK, Hahn WS, Bernlohr DA. Glutathionylated Lipid Aldehydes are Products of Adipocyte Oxidative Stress and Activators of Macrophage Inflammation. Diabetes. 2013 doi: 10.2337/db13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grimsrud PA, Picklo MJ, Griffin TJ, Bernlohr DA. Carbonylation of Adipose Proteins in Obesity and Insulin Resistance: Identification of Adipocyte Fatty Acid-binding Protein as a Cellular Target of 4-Hydroxynonenal. Molecular & Cellular Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 126.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muller A, de Oliveira Dietrich M, Martimbianco de Assis A, Souza D, Portela L. High saturated fat and low carbohydrate diet decreases lifespan independent of body weight in mice. Longevity & Healthspan. 2013;2:10. doi: 10.1186/2046-2395-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]