Abstract
PLC-isozymes are central elements of cellular signalling downstream of numerous receptors. PLCγ2 is a pivotal component of B cell receptor (BCR) signalling. The regulation of PLCγ2-dependent signalling functions by Tyr-phosphorylation is well characterized, however, the potential role of Ser/Thr phosphorylation events remains undefined. TRPM7 is the fusion of a Ser/Thr kinase with an ion channel, and an essential component of Mg2+-homeostasis regulation. Although the interaction between the C2 domain of several phospholipase C (PLC) isozymes and TRPM7 is well established, previous studies have focused on the effect of PLC-activity on TRPM7. Here, we investigated whether Ser/Thr phosphorylation sites in the C2 domain of PLCγ2 could be identified using TRPM7-kinase. We show that TRPM7-kinase phosphorylates PLCγ2 in its C2-domain at position Ser1164 and in the linker region preceding the C2-domain at position Thr1045. Using a complementation approach in PLCγ2−/− DT40 cells, we found that the PLCγ2-S1164A mutant fully restores BCR mediated Ca2+-responses under standard growth conditions. However, under hypomagnesic conditions, PLCγ2-S1164A fails to reach Ca2+-levels seen in cells expressing PLCγ2 wildtype. These results suggest that Mg2+- sensitivity of the BCR signalling pathway may be regulated by Ser/Thr phosphorylation of PLCγ2.
Keywords: PLCγ2, Ser/Thr phosphorylation TRPM7, Hypomagnesia, B Cell Receptor signalling
1. INTRODUCTION
PLC enzymes are crucial signalling elements that are recruited to the membrane and activated following the ligation of numerous cellular receptors. PLC proteins convert the membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) to the second messengers Diacylglycerol (DAG), a Protein Kinase C activator, and the Ca2+-store mobilizing agent 1,4,5- trisphosphate (InsP3) [1]. The enzymatic activity and membrane recruitment of PLCs are tightly regulated, and the modular architecture of PLC proteins underscores the complex interdomain and protein-protein interactions involved in ensuring their adequate activation in response to receptor stimulation [2]. Beyond their catalytic regions, PLCs additionally contain various combinations of PH-domains (Pleckstrin Homology), Ca2+-binding EF-hands, SH2/SH3 proteinprotein interaction domains, as well as C2 domains that often play the role of Ca2+-sensing phospholipid binding modules [3]. Phosphorylation of specific residues is essential to controlling PLC activity, and the contribution of multiple families of Tyr-kinases in this process is well studied [2, 4]. The influence of Ser/Thr phosphorylation events on PLC-function is however only weakly characterized. In a yeast two-hybrid screen, the C2 domain of phospholipase C β-1 (PLCβ1) was found to interact with the TRPM7 Ser/Thr-kinase [5]. This interaction was confirmed in protein-pulldown assays, which additionally demonstrated that PLCβ2, -β3, and -γ1 also interact with TRPM7-kinase [6].
The vertebrate versions of TRPM7 and its closest homologue TRPM6 are the only known examples of ion channels covalently linked to a kinase domain. Both channels are essential regulators of Mg2+-homeostasis. TRPM6-deficient patients are hypomagnesic and suffer from debilitating seizures, but can live a normal life if supplemented with high levels of Mg2+ [7, 8]. Similarly, TRPM7−/− DT40 B cells and murine embryonic stem cells can be rescued from growth arrest and cell death when cultured in media containing elevated Mg2+ levels [9, 10].
Under physiological conditions, TRPM7 is a divalent cation selective channel inhibited by intracellular Mg2+ or MgATP [11]. This Mg2+-sensitive gating does not require TRPM7- kinase activity [10, 12]. As there are numerous examples of ion channels regulated in trans by phosphorylation events, the presence of a kinase domain in TRPM7 raises the question whether beyond the well-documented autophosphorylation of TRPM7 [10, 13, 14], its kinase domain can also function as a signalling module through phosphorylation of exogenous substrates. In the past years, several substrates of TRPM7-kinase were described, including Annexin I and Myosin IIA [15, 16]. TRPM7-kinase is also involved in adjusting the activity of the eukaryotic elongation factor eEF2 in accordance to the environmental availability of Mg2+ via phosphorylation of its kinase eEF2-k [17].
We propose that the implication of the observed association between TRPM7-kinase and PLCs could thus be two-fold: i) TRPM7 activity might be influenced by PLC and its substrate and/or products, which might not necessarily require the direct interaction between TRPM7 and PLCs. Sensitivity to PIP2 has been ascribed to numerous ion channels, including other TRP family members [18, 19]. Several studies have focused on the effect of PIP2 on TRPM7, some finding TRPM7 to be inhibited by PIP2-hydrolysis, and others reporting the opposite or no effect [6, 20, 21]. ii) PLC enzymes could be substrates of the TRPM7 Ser/Thr kinase, and TRPM7 could be an unsuspected modulator of PLC activity. In B lymphocytes, PLCγ2 is at the heart of BCR signalling. PLCγ2-deficient mouse models exhibit defective B cell development and function, including reduced numbers of mature follicular B cells and weak responses to Tindependent type 2 antigens [22, 23].
Acute activation events following BCR ligation typically involve phosphorylation of selected tyrosine residues, which are well-characterized in PLCγ2 [24]. From our review of the literature, little is known about the potential modulation of PLC-activity via Ser/Thr phosphorylation. Here, we hypothesized that TRPM7-kinase could be used to identify new Ser/Thr phosphorylation sites in the C2-domain of PLCγ2. We present data indicating that TRPM7 phosphorylates PLCγ2 at position Ser1164 in the C2-domain, and at position Thr1045 in the linker between the catalytic region and the C2 domain. Mutation of the Ser1164 position to Alanine leads to a similar Ca2+-response under physiological Mg2+-conditions, but a lower level of cytoplasmic Ca2+-elevation under hypomagnesic conditions. These data thus provide first clues that Ser/Thr phosphorylation of PLCγ2 might contribute to adjusting the signalling intensity of PLCγ2-dependent pathways according to the availability nutrients, such as the biologically essential ion Mg2+.
2. MATERIAL AND METHODS
2.1 Molecular biology and cell line generation
Human (h)PLCγ2 was subcloned into pcDNA4/TO (Invitrogen), and the C2-domain of PLCγ2 into pcDNA4/TO-NFlag. The PLCγ2 S1164A mutation in full-length and C2-domain constructs, and the nine mutants individually replacing the Thr-residues in the C2-domain construct by Ala, were generated by PCR and verified by sequencing. TRPM7 constructs were previously described [10, 17]. PLCγ2−/− DT40 cells were stably transfected by electroporation with hPLCγ2 wildtype (WT) or PLCγ2 S1164A constructs.
Cell culture
DT40 cells [25] were maintained in RPMI, 10% FBS, 1% chicken serum. For Mg2+-deprivation experiments cells were grown in complete chemically defined HyQ CCM1 media (Hyclone) containing 1% chicken serum, with 0 or 1 mM MgCl2. HEK293 cells (Invitrogen T-REx system, and [11]) were maintained in DMEM 10% FBS, with Blasticidin S (5 µg/ml, Invivogen). Zeocin (Invitrogen) was added for HEK293 cell lines (400 µg/ml) or DT40 cells (1 mg/ml) stably transfected with pcDNA4/TO constructs. Protein overexpression was induced with 100–1000 ng/ml doxycycline for 24–48 h.
2.2. Immunoprecipitations, immunoblotting, and in vitro phosphorylation assays
Lysis, in vitro phosphorylation, SDS gel electrophoresis and immunoblotting study conditions have been described previously [17]. Immunoprecipitations were performed from HEK293 or DT40 WT cell lysates using antibodies against Flag (Sigma), HA (Sigma), PLCγ2 (Santa Cruz) or IgG conjugated to protein G beads. Membranes were probed with these antibodies or a motif specific anti phospho-Ser antibody (RxY/FxpS) (Cell Signaling). Western Blot analysis was performed using the Li-Cor ODYSSEY Infrared Imaging System or Immobilon Western Chemiluminescent HRP Substrate (Millipore) and Super-RX Medical X-ray film (Fuji).
2.3. Ca2+ measurements
Cytosolic [Ca2+] was evaluated using stable PLCγ2−/− DT40 cell lines expressing PLCγ2 WT or PLCγ2 S1164A grown in chemically defined media with 0 or 1 mM MgCl2. At 15–24 h 4 × 106 cells were loaded with 1 µg/ml Fura-2 (Invitrogen) for 30 min at 25°C in Ringer buffer and analyzed using a bulk assay as previously described [14]. Anti-chicken IgM mAb M4 (1.2 µg/ml) was added at 40 sec.
2.4, Calculations and statistical analysis
All experiments were repeated at least 3 times with consistent results. The area under the curve was calculated by subtracting the baseline average (the average fluorescence ratio of the first 39 sec) from each time point in the response (40 sec – 420 sec) and these values were summed. The SEM was calculated for each group and paired two-tailed t-test performed.
3. RESULTS
3.1. PLCγ2 is phosphorylated in its C2-domain on Ser1164 by TRPM7-kinase
Biochemical studies tested the interaction of TRPM7-kinase with several PLC isozymes, and found that whereas PLCβ1, -β2, and -β3, and -γ1 associate with TRPM7-kinase, PLCβ4 and -δ1 do not [6]. Because we are interested in TRPM7 function in B lymphocytes, we first asked the question whether PLCγ2 associates with TRPM7. The DT40 chicken DT40 B cell line shows high rate of homologous recombination allowing for the efficient targeting of genomic regions to disrupt genes of interest. It has been extensively used to study the effect of deleting specific molecules on the B cell receptor signalling pathway [26]. We found that available TRPM7-specific antibodies do not allow for detection of native TRPM7 levels in DT40s, prohibiting the verification of native PLCγ2/TRPM7 association. We thus looked at this association in TRPM7−/− DT40 B cells complemented with HA-tagged human TRPM7 we generated previously [10], as it comes closest to the physiological situation. Native chicken PLCγ2 can be analyzed in DT40s, and we found TRPM7-currents to be only 2–3× WT levels in these cells (Supporting Fig. S1A), which is substantially less than typical current amplitudes observed in HEK293 cells overexpressing TRPM7 (10–50×) [11]. We precipitated PLCγ2 and subsequently tested for co-association with HA-TRPM7 by Western blot (Fig. S1B). Using this experimental system, we documented that native PLCγ2 and full-length HA-tagged TRPM7 interact in DT40 cells. Encouraged by this finding, we decided to next analyze whether PLCγ2 is a substrate of TRPM7-kinase.
Previous studies have focused on the role of PLC enzymes as regulators of phosphoinositide metabolism on the channel activity of TRPM7. However, in contrast to other ion channels, the relationship between TRPM7 and PLC enzymes is unique as it involves the kinase domain of TRPM7. We thus hypothesized that PLC proteins interacting with TRPM7 might be substrates of TRPM7-kinase. To test this idea, we performed in vitro phosphorylation reactions with immunoprecipitated epitope-tagged TRPM7-kinase and the C2 domain of PLCγ2 (schematic representation of the constructs in Fig. 1A). To detect potential Ser-phosphorylation of PLCγ2, we used an anti-phospho-Ser motif antibody (RxY/FxpS). We found that PLCγ2 was only recognized by this antibody in the presence of TRPM7-kinase WT, but not of its kinasedead counterpart (K1648R) (Fig. 1B, lanes 2 and 4). This observation shows for the first time TRPM7-kinase mediated phosphorylation of a PLC isozyme, in this case of PLCγ2.
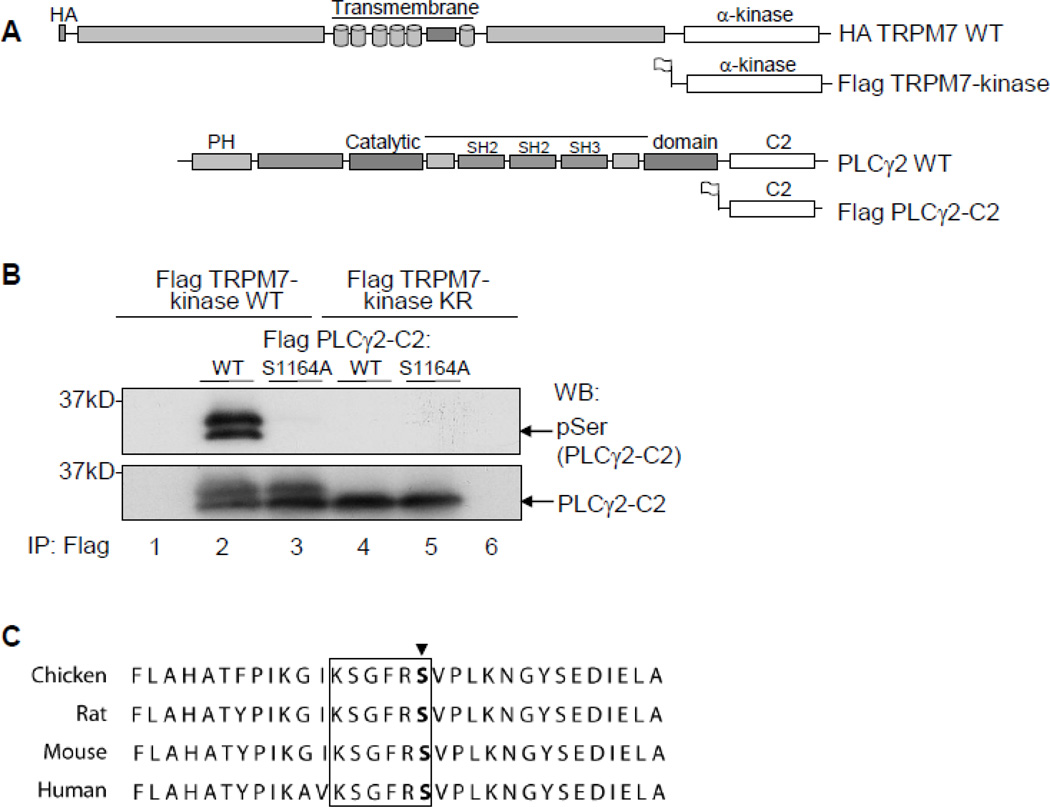
FIGURE 1. The PLCγ2-C2 domain residue, Ser1164, is phosphorylated by TRPM7-kinase.
A. Schematic of the recombinant TRPM7 and PLCγ2 constructs used. B. Phosphorylation of PLCγ2 determined using HEK293 cells transiently transfected with N-terminally Flag-tagged constructs of PLCγ2-C2 WT, PLCγ2-C2 S1164A, TRPM7-kinase WT or TRPM7-kinase dead (KR). Flag dual-immunoprecipitations performed followed by in-vitro phosphorylation reactions. Detection of Ser1164 phosphorylation in PLCγ2 was determined after SDS-PAGE, and immunoblotting with anti-Phospho-Ser motif antibody. C. Alignment of a C-terminal portion of PLCγ2 for chicken, mouse, rat, and human is shown, site Ser1164 marked by arrow, motif specific region is boxed in black.
We next aimed at identifying which serine position(s) in the C2-domain of PLCγ2 is phosphorylated by TRPM7. After screening the PLCγ2-C2 protein sequence, we concluded that from the 15 serine residues present, only two, Ser1164 and Ser1265, were in a context that is similar to the recognition motif of the phospho-Ser antibody (RxY/FxpS). Moreover, Ser1164 and the surrounding region are conserved in chicken, murine, rat, and human PLCγ2 sequences (Fig. 1C). We therefore introduced a mutation resulting in a serine to alanine exchange at position 1164 (S1164A) to test whether this mutation would abrogate TRPM7-dependent serinephosphorylation of PLCγ2-C2. Our results show that indeed, following an in vitro phosphorylation reaction with WT TRPM7-kinase, the S1164A mutant fails to be detected by the phospho-Ser specific antibody (Fig. 1B, lane 3). Additionally, to investigate the specificity of TRPM7-mediated Ser-phosphorylation of PLCγ2, we asked whether eEF2-kinase, a protein closely related to TRPM7-kinase, can also phophorylate PLCγ2. Although we found that eEF2-k was recognizing and phosphorylating its cognate substrate eEF2, it had no effect on PLCγ2 Serphosphorylation level (Fig. S2). Our data thus indicate that TRPM7-kinase specifically phosphorylates PLCγ2 in its C2-domain on Ser1164.
3.2. TRPM7-kinase phosphorylates PLCγ2 on Thr1045 in the linker region connecting its catalytic domain to the C2-domain
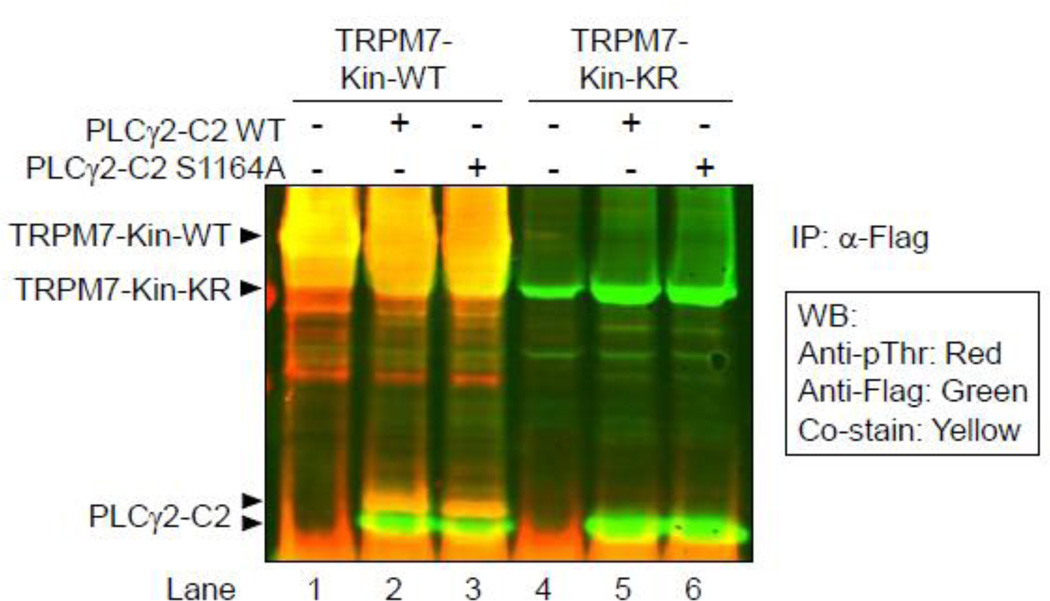
When performing the phosphorylation experiments described in the previous section, we noticed the formation of a doublet band in the samples where PLCγ2-C2 was phosphorylated by TRPM7-kinase. Both bands are equally detected by the anti phospho-Ser antibody, and the doublet can also be seen in the S1164A mutant (Fig. 1B), indicating that this migratory shift does not originate from the S1164 phosphorylation and may thus be caused by one or multiple additional phosphorylation sites. Because the higher PLCγ2 band was not recognized by the antiphospho- Ser motif antibody we used, we concluded that the potential site(s) might be a Thr residue(s). To test this, we performed a phosphorylation experiment as previously, but used a phospho-Thr specific antibody to detect phosphorylation. We developed the Western blot membrane using an infrared scanner allowing for simultaneous visualization of Flag-specific stain (in green), and phospho-Thr specific signals (in red). This approach clearly demonstrated that only the upper band of the phospho-PLCγ2 doublet is recognized both by the anti-flag and the anti phospho-Thr antibodies (co-stained in yellow, Fig. 2). Analyses of the PLCγ2-C2 S1164A mutant additionally showed that the same migration shift and phospho-Thr specific detection can be seen, indicating that the observed Thr-phosphorylation is independent of S1164 phosphorylation. Control reactions using the kinase-dead TRPM7-KR mutant showed no Thr-phosphorylation of PLCγ2-C2 (Fig. 2 lanes 5, 6). We next therefore aimed at further defining the Thr residues(s) phosphorylayed by TRPM7-kinase.
FIGURE 2. TRPM7-kinase not only phosphorylates Ser1164 in PLCγ2-C2, but also a Thr residue(s).
Phosphorylation of PLCγ2 determined using HEK293 cells transiently transfected with N-terminally Flag-tagged constructs of PLCγ2-C2 WT, PLCγ2-C2 S1164A, TRPM7-kinase WT or TRPM7-kinase dead (KR). Flag dual-immunoprecipitations performed followed by in vitro phosphorylation reactions. Detection of Ser1164 phosphorylation in PLCγ2 was determined after SDS-PAGE, and immunoblotting with an anti-phospho-Thr antibody. Positive signals on the Western blot membrane were visualized using the Li-Cor Odyssey Infrared Imaging System.
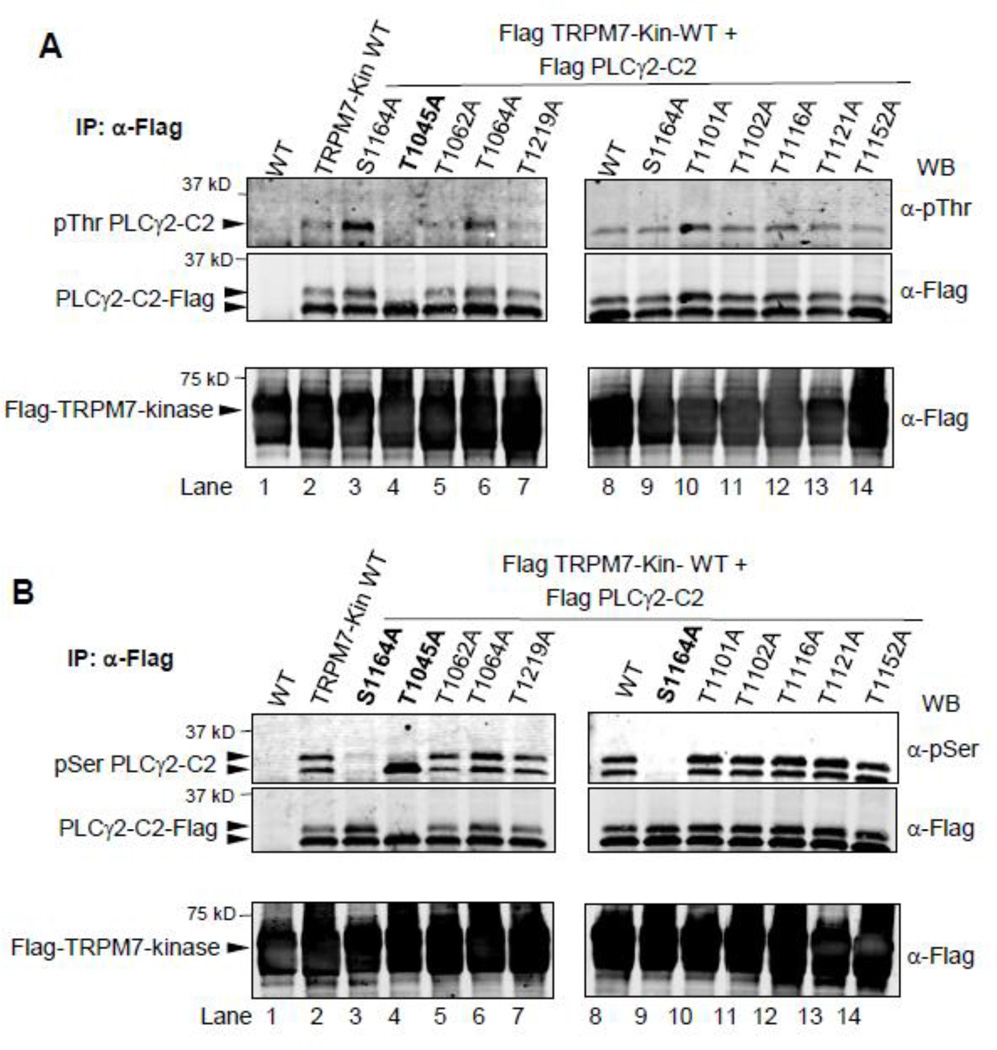
Nine threonine residues are present in the PLCγ2-C2 domain construct used in this study (Thr1045, Thr1062, Thr1101, Thr1102, Thr 1116, Thr1121, Thr1152, and Thr1219, see also Fig. S3). Because we could not discern a particular Thr residue that would be a good candidate phosphorylation site, we generated nine mutants of PLCγ2-C2 by site-directed PCR mutagenesis, in which each of the Threonine residues was singly mutated to Alanine. Phosphorylation experiments with TRPM7-kinase revealed that only one of the mutations, T1045A, resulted in loss of the electrophoretic migration shift, as well as of the phospho-Thr signal (Fig. 3A). In contrast, when the same set of Thr-mutants was analyzed using the anti phospho-Ser antibody and the S1164A construct as a negative control, the phosphorylation signal was not affected (Fig. 3B). Thus, the TRPM7-mediated S1164 and T1045 phosphorylation events do not appear to be interdependent.
FIGURE 3. TRPM7-kinase phosphorylates Thr1045 in the linker region between the catalytic and C2-domain of PLCγ2.
A. Phosphorylation of PLCγ2-C2 determined using HEK293 cells transiently transfected with N-terminally Flag-tagged constructs of PLCγ2-C2 WT or indicated mutants, TRPM7-kinase WT or TRPM7-kinase dead (KR). Flag dual-immunoprecipitations performed followed by in-vitro phosphorylation reactions. Detection of threonine phosphorylation of PLCγ2-C2 WT and mutants was determined after SDSelectrophoresis, and immunoblotting with antibody against phospho-Thr or Flag. B. Same experimental design as in A, except that immunoblotting was performed using antibody against anti-Phospho-Ser motif or Flag.
In sum, our results show that T1045 and S1164 represent two previously unknown phosphorylation sites in PLCγ2 that are potential substrates of the TRPM7 channel-kinase. Whereas S1164 is located in the C2-domain itself, Thr1045 is at the very N-terminal edge of the PLCγ2-C2 construct we designed and used here, and is described as being located in the linker region between the catalytic region and the C2 domain [27]. Because the original goal of the present study was to characterize the effect of Ser/Thr phosphorylation in the C2-domain of PLCγ2, we focused in the following on characterizing the potential importance of Ser1164 for PLCγ2-mediated Ca2+-signalling.
3.3. The PLCγ2 mutant S1164A supports normal BCR mediated Ca2+-responses under physiological Mg2+-levels, but reduced responses under hypomagnesic conditions
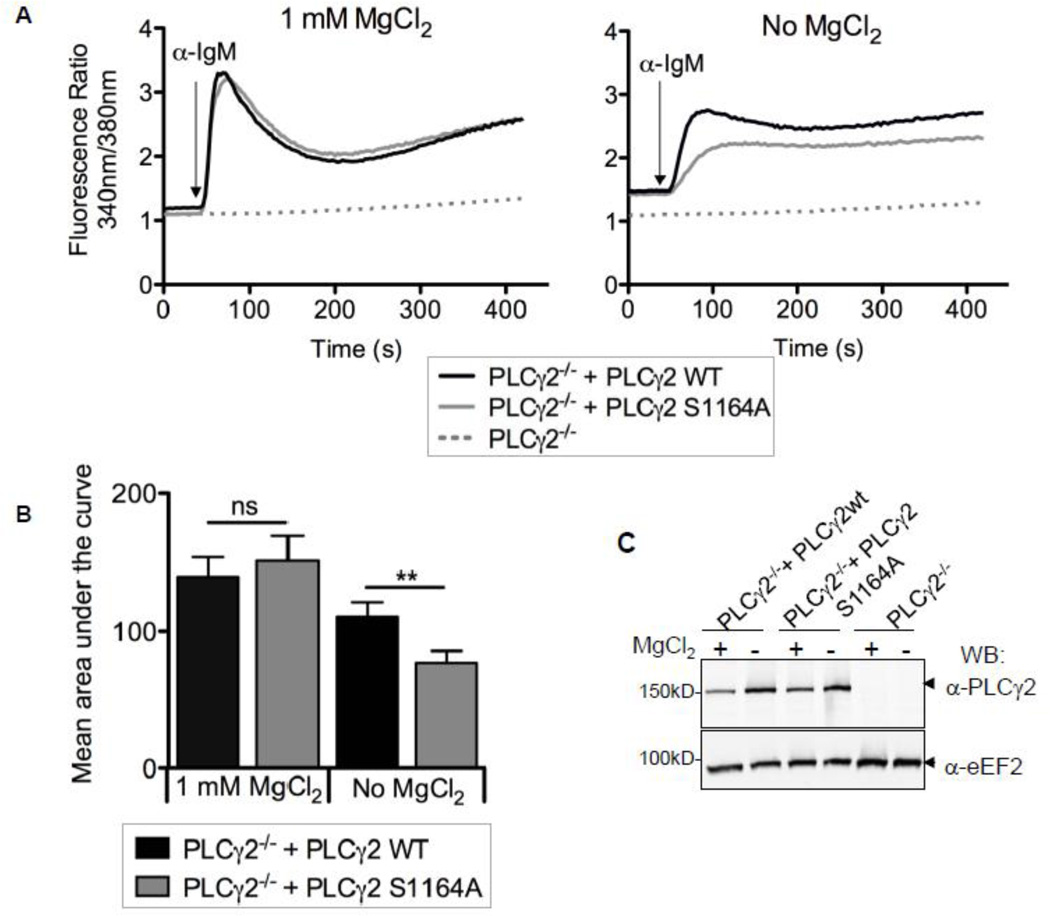
To investigate the effect of mutating Ser1164 on PLCγ2 function in the context of BCR signalling, we chose a complementation approach in a PLCγ2-deficient DT40 B cell line [25]. To this end, we generated DT40 B cells with stable expression of WT or S1164A full-length hPLCγ2 in the PLCγ2−/− background. We first tested the ability of PLCγ2 S1164A to restore Ca2+-responses following BCR stimulation with anti-IgM using the Ca2+-sensitive fluorescent dye Fura-2. Under standard growth conditions, we found no difference in the amplitude or shape of the Ca2+- response between PLCγ2−/− DT40 cells complemented with PLCγ2 WT or S1164A (Fig. 3A left panel). Since TRPM7 is proposed to play the role of an environmental sensor of Mg2+- availability, we hypothesized that the S1164A mutation might exhibit altered signalling properties in comparison to PLCγ2 WT under suboptimal Mg2+-conditions. We therefore cultured the PLCγ2−/− cells expressing either PLCγ2 WT or S1164A in Mg2+-free medium for 15–25 h, and repeated the Ca2+-measurements following BCR activation. We found the BCR mediated Ca2+-response to be reduced when DT40 cells were grown under hypomagnesic conditions (Supporting Fig. S2B, Fig. 4A and B). The amplitude of the Ca2+-signal in Mg2+- starved PLCγ2−/− cells that express the S1164A mutant was consistently even further diminished (Fig. 4A right panel, quantification in Fig. 4B). This effect was not due to a difference in protein expression levels between PLCγ2 WT and PLCγ2-S1164A, as the protein amounts appear well matched (Fig. 4C). Additionally, it is important to mention that the Mg2+-deprivation protocol the cells were exposed to did not result in altered BCR surface expression (Supporting Fig. S4B). Combined with the lack of effect of the S1164A mutation at physiological 1 mM MgCl2 levels, these results indicate that the S1164A PLCγ2 mutant exhibits a changed sensitivity to suboptimal Mg2+-conditions. These findings provide a novel molecular mechanism that may explain the relationship between insufficient availability of Mg2+ and the efficiency of immune responses.
FIGURE 4. Under hypomagnesic conditions BCR mediated Ca2+-responses are reduced in PLCγ2−/− DT40 cells complemented with PLCγ2-S1164A.
A, Changes in free cytosolic Ca2+ in PLCγ2−/− cells alone or with stable expression of PLCγ2 WT or PLCγ2 S1164A. Cell lines were cultured in 0 or 1 mM MgCl2 for 15–25 h and loaded with Fura-2 for 30 min. Cells were stimulated with 1.2 µg anti-chicken IgM and analyzed by a fluorometer. Shown traces are representative of 4 separate experiments. B, Quantification of the results presented in A and B where bars represent the mean area under the curve and a paired two-tailed t-test was performed, mean ± SEM; **p < 0.01. C, Immunoblot of 2.5 × 105 equivalent cells from whole cell lysates made from A and probed with anti-PLCγ2 and anti-eEF2 (loading control).
4. DISCUSSION
Although the interaction between TRPM7-kinase and several PLC isozymes has been known for several years [5, 6], subsequent studies have focused on the modulation of TRPM7 channel activity by the substrate of PLC isozymes, PIP2 [6, 20, 21]. The reverse scenario, the phosphorylation and regulation of PLC proteins by TRPM7’s Ser/Thr-kinase, has to our knowledge not been previously considered. The data presented here show that PLCγ2 can be phosphorylated by the Ser/Thr kinase domain of TRPM7, allowing us to identify two novel phosphorylation sites in PLCγ2: The conserved Serine residue 1164, located in the C2 domain of PLCγ2 (Fig. 1), and a Thr residue (Fig. 2), Thr 1045, located in the linker region between the catalytic region and the C2-domain of PLCγ2 (Fig. 3). Mutating Ser1164 in hPLCγ2 and expressing this mutant in PLCγ2−/− DT40 B cells results in a full restoration of the BCR mediated Ca2+-signal under standard growth conditions. However, when compared to WT PLCγ2, the S1164A mutant can only sustain a reduced Ca2+-response following Mg2+-deprivation (Fig. 4).
PLC enzymes are crucial elements of cellular signal transduction. The modular structural features and complex regulation of these enzymes reflect the diversity of their function, from cell proliferation and survival, to immunity [2]. PLCs are mainly cytosolic, and translocate to the membrane upon receptor stimulation. Membrane recruitment is thus critically regulated. The C2 domain of PLCγ2 contains several Ca2+-binding motifs, and using a complementation approach in PLCγ2−/− DT40 cells, it was shown to mediate the Ca2+-dependent increase in PLCγ2 translocation to the membrane following the initial release of the Ca2+-stores [28]. Therefore, Ser/Thr phosphorylation in this domain might influence the intensity and sustainability of the Ca2+-signal downstream of BCR ligation, as we observe in the S1164A PLCγ2 mutant. The potential modulation of PLCγ enzymes via phosphorylation on Ser/Thr residues is very poorly characterized. Although members of the PLCβ subgroup have been found to be phosphorylated by various Ser/Thr kinases, including ERK [29], and CaMK II [30], from our review of the literature, no functional characterization of Ser/Thr phosphorylation events have been described in PLCγ2.
Given the essential and ubiquitous nature of Mg2+ in biology, it is perhaps not surprising that B cells adjust their level of BCR responsiveness to Mg2+ availability. The decrease in BCR dependent Ca2+-influx we observe under hypomagnesic conditions is accentuated in the S1164A mutant indicating that phosphorylation of Ser1164 may have an enhancing effect on PLCγ2- activation. However, Ser1164 phosphorylation might not be reflective of the total effect of TRPM7-mediated phosphorylation of PLCγ2, as the remaining electrophoresis migratory shift we observe in PLCγ2-S1164A suggested at least one additional site, which we identified as Thr1045. Also, beyond TRPM7, multiple kinases might be involved in modulating the Ser/Thr phosphorylation levels of PLC enzymes in intact cells.
It is interesting to note that beyond its role as a “maintenance ion”, Mg2+ is also discussed as having more specific signalling functions in lymphocytes. In B cells the elevation of free [Mg2+]i follows the [Ca2+]i mobilization in response to receptor activation or ionophore treatment [31]. In B cell blasts, a Mg2+ dependent PLC activity was discovered [32]. In a recent study, immunodeficient patients were found to exhibit a genetic deficiency in MagT1 [33], a Mg2+- transporter that we have shown to partially complement for TRPM7-deficiency in DT40s [34]. MagT1 was shown to mediate a transient Mg2+-influx that is crucial for T cell activation [33]. Collectively, these findings and the data presented here suggest that responsiveness to alterations in intra- and extracellular Mg2+ levels are an integral part of immune receptor signalling.
Our current working model is that TRPM7 via its kinase domain is ideally poised to act as an environmental sensor of Mg2+-availability to guide the cell in mounting a global response for example in situations of hypomagnesia. This could include measures to conserve Mg2+ by reducing the rates of translational elongation [17], but also as implied by the phenotype of the S1164A PLCγ2 mutant, by ensuring that crucial signalling pathways maintain a sufficient level of activation. In analogy to the “two-signal” model and the role of the mTOR Ser/Thr kinase in T cells [35], Ser/Thr phophorylation of PLC isozymes via TRPM7 and other kinases might therefore contribute to the integration of environmental cues into the activation of immune responses.
5. CONCLUSIONS
hPLCγ2 interacts with hTRPM7 in DT40 B cells.
The Ser/Thr kinase domain of TRPM7 phosphorylates PLCγ2 on several sites.
Thr1045 and Ser1164 are two novel phosphorylation sites in PLCγ2 that were identified using TRPM7-kinase.
PLCγ2−/− DT40 B cells complemented with the PLCγ2-S1164A mutant show normal BCR mediated Ca2+-responses under physiological Mg2+-levels, but reduced responses under hypomagnesic conditions.
Supplementary Material
Highlights.
PLCγ2 interacts with the Mg2+-sensing channel-kinase TRPM7.
The C2-domain of PLCγ2 is phosphorylated by TRPM7’s Ser/Thr kinase.
TRPM7-mediated PLCγ2 phosphorylation reveals two novel phospho-sites, T1045 and S1164.
PLCγ2-S1164A mutation results in diminished BCR-mediated Ca2+-signal under low Mg2+.
AKNOWLEDGEMENTS
We would like to thank Dr. Andrew Scharenberg for his support and the characterization of TRPM7 currents in DT40s, Dr. Tomohiro Kurosaki for providing the DT40 PLCg2−/− cells and the anti-IgM M4 antibody, and Dr. John Cambier for making these reagents available, as well as Dr. Fujio Sekiya (Dr. Sue Goo Rhee’s group, NHLBI) for the hPLCγ2 pRc/CMV construct.
This work was supported by the following awards from the National Institutes of Health: Predoctoral Traineeship NIH T32 AI07405 to FDT, R01GM068801 to ALP, K08AI060926, R21AI0088421 (both from NIAID), and R01GM90123 (includes funding from NIGMS and the Office of Dietary Supplements, ODS) to CS.
Abbreviations
- TRPM7
Transient Receptor Potential Melastatin 7
- PLCγ2
Phospholipase C γ2
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Vines CM. Adv Exp Med Biol. 2012;740:235–254. doi: 10.1007/978-94-007-2888-2_10. [DOI] [PubMed] [Google Scholar]
- 2.Bunney TD, Katan M. Trends Biochem Sci. 2011;36(2):88–96. doi: 10.1016/j.tibs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. BMB Rep. 2008;41(6):415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mol Cell Biol. 2004;24(22):9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runnels LW, Yue L, Clapham DE. Science. 2001;291(5506):1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 6.Runnels LW, Yue L, Clapham DE. Nat Cell Biol. 2002;4(5):329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 7.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Nat Genet. 2002;31(2):171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 8.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Nat Genet. 2002;31(2):166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 9.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Cell. 2003;114(2):191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 11.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. Nature. 2001;411(6837):590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC. J Biol Chem. 2005;280(21):20793–20803. doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- 13.Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN. PLoS One. 2008;3(3):e1876. doi: 10.1371/journal.pone.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. J Biol Chem. 2005;280(45):37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 15.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. EMBO J. 2006;25(2):290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorovkov MV, Ryazanov AG. J Biol Chem. 2004;279(49):50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 17.Perraud AL, Zhao X, Ryazanov AG, Schmitz C. Cell Signal. 2011;23(3):586–593. doi: 10.1016/j.cellsig.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilius B, Owsianik G, Voets T. EMBO J. 2008;27(21):2809–2816. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B. J Physiol. 2010;588(Pt 17):3179–3185. doi: 10.1113/jphysiol.2010.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Proc Natl Acad Sci U S A. 2004;101(16):6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. J Biol Chem. 2007;282(1):232–239. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, Ihle JN. Immunity. 2000;13(1):25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto A, Takeda K, Inaba M, Sekimata M, Kaisho T, Ikehara S, Homma Y, Akira S, Kurosaki T. J Immunol. 2000;165(4):1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 24.Scharenberg AM, Humphries LA, Rawlings DJ. Nat Rev Immunol. 2007;7(10):778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takata M, Homma Y, Kurosaki T. J Exp Med. 1995;182(4):907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosaki T. Mol Immunol. 2011;48(11):1287–1291. doi: 10.1016/j.molimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Nalefski EA, Falke JJ. Protein Sci. 1996;5(12):2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishida M, Sugimoto K, Hara Y, Mori E, Morii T, Kurosaki T, Mori Y. EMBO J. 2003;22(18):4677–4688. doi: 10.1093/emboj/cdg457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu A, Suh PG, Marmy-Conus N, Pearson RB, Seok OY, Cocco L, Gilmour RS. Mol Cell Biol. 2001;21(9):2981–2990. doi: 10.1128/MCB.21.9.2981-2990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue C, Sanborn BM. Mol Cell Endocrinol. 2001;175(1–2):149–156. doi: 10.1016/s0303-7207(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 31.Rijkers GT, Griffioen AW. Biochem J. 1993;289(Pt 2):373–377. doi: 10.1042/bj2890373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien MM, Cambier JC. J Biol Chem. 1990;265(16):9201–9207. [PubMed] [Google Scholar]
- 33.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Nature. 2011;475(7357):471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deason-Towne F, Perraud AL, Schmitz C. FEBS Lett. 2011;585(14):2275–2278. doi: 10.1016/j.febslet.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell JD, Delgoffe GM. Immunity. 2010;33(3):301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.