Abstract
Pregnane X receptor (PXR) and constitutive active/androstane receptor (CAR), members of the nuclear receptor superfamily, are two major xeno-sensing transcription factors. They can be activated by a broad range of lipophilic xenobiotics including therapeutics drugs. In addition to xenobiotics, endogenous compounds such as steroid hormones and bile acids can also activate PXR and/or CAR. These nuclear receptors regulate genes that encode enzymes and transporters that metabolize and excrete both xenobiotics and endobiotics. Sulfotransferases (SULTs) are a group of these enzymes and sulfate xenobiotics for detoxification. In general, inactivation by sulfation constitutes the mechanism to maintain homeostasis of endobiotics. Thus, deciphering the molecular mechanism by which PXR and CAR regulate SULT genes is critical for understanding the roles of SULTs in the alterations of physiological and pathophysiological processes caused by drug treatment or environmental exposures.
Keywords: Constitutive active/androstane receptor, gene regulation, pregnane X receptor, sulfotransferase, xeno-sensing nuclear, receptor
Introduction
Constant exposure to numerous xenobiotics (e.g. therapeutics, agricultural and industrial chemicals) often causes significant impacts on human health. To counter toxicity, xenobiotic metabolizing enzymes such as cytochrome P450s (CYPs) and sulfotransferases (SULTs) are coordinately induced to eliminate them from the body (Omiecinski et al., 2011). Pregnane X receptor (PXR, NR1I2) and constitutive active/androstane receptor (CAR, NR1I3) are two major transcription factors that regulate these genes in response to xenobiotics (Willson & Kliewer, 2002). In addition, farnesoid X receptor (FXR, NR1H4), liver X receptor (LXR, NR1H2/3), peroxisome proliferator-activated receptors (PPARs, NR1C1/2/3) and vitamin D receptor (VDR, NR1I1) are also involved in the regulation of genes that encode for xenobiotic metabolizing enzymes (Bouillon et al., 2008; Jakobsson et al., 2012; Modica et al., 2010; Pyper et al., 2010). These nuclear receptors can be co-regulated by xenobiotics and endobiotics and are involved in various physiological and pathophysiological processes such as cell differentiation and development, drug and energy metabolism, immune response and tumorigenesis as well as endocrine homeostasis. Sulfate conjugation is an important reaction in both xenobiotic and endobiotic metabolism and is catalyzed by cytosolic SULTs (Gamage et al., 2006). In most cases, sulfation inactivates both xenobiotics and endobiotics, increasing their water solubility to accelerate excretion (Alnouti, 2009; Gamage et al., 2006). Expression of SULT genes is primarily regulated by the above-mentioned nuclear receptors, in particular by PXR and CAR.
Xeno-sensing nuclear receptors
Liver, kidney and intestines are three major organs that metabolize and excrete xenobiotics and endobiotics. Upon xenobiotic exposures, these organs activate nuclear receptors such as PXR and CAR that regulate SULT expressions.
PXR
PXR (NR1I2) was first cloned from a mouse liver cDNA library (Kliewer et al., 1998). Subsequently PXR orthologs were cloned from various species including human, rat, rabbit and monkey (Blumberg et al., 1998; Moore et al., 2002; Savas et al., 2000; Zhang et al., 1999). The name PXR was given based on its ability to be activated by various natural and synthetic pregnanes. The CYP3A genes were identified as the first PXR target since PXR activators and CYP3A inducers overlapped. It was then determined that ligand specificity was markedly different among various species (Kliewer et al., 2002). PXR adapts a large and flexible structure for ligand binding to accommodate a broad range of hydrophobic man-made and naturally occurring xenobiotics at effective concentrations, which are usually the low μM range. Endobiotics such as steroids and bile acids can also activate PXR. However, it remains elusive as to whether or not these endobiotics actually play any role in regulating PXR in vivo in organs. Un-liganded PXR is predominantly localized in the cytoplasm (Squires et al., 2004). A co-chaperon dubbed cytoplasmic CAR retention protein (CCRP) forms a complex with CAR to enable sequestration of PXR in the cytoplasm (Squires et al., 2004). Liganded PXR translocates into the nucleus, heterodimerizes with retinoid acid X receptor (RXR, NR2B), and binds to response sequences (i.e. DR3, DR4, ER6 and ER8 motifs), thereby activating transcription of targeted genes. Co-activators such as steroid receptor coactivator 1α and PPAR-γ coactivator 1α and co-repressors such as nuclear receptor corepressor 1 and 2 co-regulate PXR-mediated transcription (Bhalla et al., 2004; Sugatani et al., 2005; Synold et al., 2001). A recent report has demonstrated that cell signaling can also activate PXR in the absence of xenobiotic activators (Sivertsson et al., 2013). Cyclin-dependent kinase 2 (CDK2) phosphorylates PXR and sequesters it in the cytoplasm of human hepatocellular carcinoma Huh7 cells. Continuous culture of confluent Huh7 cells decreases CDK2 levels, resulting in the dephosphorylation of PXR. Non-phosphorylated PXR translocates into the nucleus and activates the CYP3A4 gene. Although there are potential phosphorylation sites by protein kinase C, protein kinase A, or p70 S6 kinase in PXR, phosphorylation of these sites in endogenous PXR has not been confirmed (Ding & Staudinger, 2005; Lichti-Kaiser et al., 2009a, b; Pondugula et al., 2009). In addition to phosphorylation, PXR may undergo ubiquitylation, acetylation or SUMOylation (Biswas et al., 2011; Hu et al., 2010; Staudinger et al., 2011). Current understanding of PXR activation is summarized in Figure 1.
Figure 1.
Activation mechanism of PXR and CAR. (A) Current understanding of PXR activation. Being part of the Hsp90–CCRP complex, PXR is predominantly localized in the cytoplasm. Upon ligand binding, PXR translocates into the nucleus, heterodimerizes with RXR, and transactivates response sequences such as XREM in the CYP3A4 gene. Cell signaling pathways have been implicated to modulate PXR activity, either positively or negatively, where PXR undergoes post-translational modifications including phosphorylation, acetylation and SUMOylation. (B) Current understanding of CAR activation. Under un-stimulated condition, threonine 38 of CAR is phosphorylated. Phosphorylation status of threonine 38 determines the intercellular localization of CAR, and this phosphorylated form is inactive and mostly localized in the cytoplasm as a part of Hsp90– CCRP complex. Dephosphorylation of threonine 38 allows CAR to translocate into the nucleus, which in turn heterodimerizes with RXR and transactivates response sequences such as PBREM in the Cyp2b10 gene. Upon stimulation, PP2A catalyzes dephosphorylation of threonine 38, whereas RACK1 serves as the specific regulatory subunit. Phenobarbital, a typical indirect CAR activator, binds to EGF receptor antagonizes EGFR signaling to promote the RACK1-regulated dephosphorylation. On the other hand, how ligands activate CAR remains unknown. XREM: xenobiotic responsive enhancer module; PBREM: phenobarbital responsive enhancer module; PP2A: protein phosphatase 2A; RACK1: receptor for activated protein C kinase 1; CCRP: cytoplasmic CAR retention protein; EGFR: epidermal growth factor receptor.
PXR is also known to be expressed in lung, ovary, normal and cancerous breast tissues and peripheral blood mononuclear cells (PMBCs) (Masuyama et al., 2001; Schote et al., 2007; Siest et al., 2008). PXR appears to induce CYPs in the ovary and PMBCs. PXR may regulate growth and death of breast cancer cells, in addition to increasing drug resistance by inducing metabolism. Activation of PXR may affect various metabolic diseases such as acetaminophen and bilirubin toxicities, steatosis, cholestasis, diabetes and osteomalacia (Kakizaki et al., 2008; Konno et al., 2008; Staudinger et al., 2001a; Zhou et al., 2009). In the other cases, activation can be beneficial acting as an anti-inflammatory factor to bowel disease and biliary primary cirrhosis (Shah et al., 2007; Wallace et al., 2010).
CAR
CAR (NR1I3), nominally called constitutive activate receptor, constitutive androstane receptor or CAR, was cloned from human liver cDNA library and initially named as MB67 (Baes et al., 1994). Because of its ability to transactivate retinoic acid responsive elements constitutively, the term constitutive activator of retinoid response was first given to CAR, later shortened to constitutive active receptor (Choi et al., 1997). Subsequently, after two androstane metabolites, 5α-androst-16-en-3α-ol and 5α-androstan-3α-ol were identified as inverse agonists that repress this constitutive activity in cell-based reporter assays, constitutive androstane receptor was conferred to CAR (Forman et al., 1998). However, in actuality it has not been confirmed that androstanes repress CAR in in vivo environments, such as liver and primary hepatocytes, thereby regulating CAR activation. The only known mechanism to repress CAR in vivo is phosphorylation of threonine 38 (Mutoh et al., 2009). Phosphorylated CAR at threonine 38 is an inactive form of CAR that is sequestered in the cytoplasm in a complex with heat shock protein 90 and CCRP (Kawamoto et al., 1999; Kobayashi et al., 2003; Mutoh et al., 2009; Yoshinari et al., 2003). In response to activators, threonine 38 is dephosphorylated to activate CAR and translocate it into the nucleus. This dephosphorylation is catalyzed by protein phosphatase 2A which utilizes receptor for activated C kinase 1 (RACK1) as the specific regulatory subunit (Mutoh et al., 2013). The first CAR response sequence characterized was the phenobarbital (PB)-responsive enhancer module (PBREM) characterized within the Cyp2b10 gene (Honkakoski et al., 1998a,b). There are two different types of CAR activators, direct and indirect activators. PB and phenytoin indirectly activate CAR without direct binding, represent one group. Another group is represented by TCPOBOP for mouse CAR and CITCO for human CAR; these are ligands of CAR (Maglich et al., 2003; Sueyoshi et al., 1999; Tzameli et al., 2000). As to the indirect activation mechanism, PB binds to and represses epidermal growth factor receptor (EGFR) signaling in mouse hepatocytes. Repression of EGFR transduces the PB induction signal to promote the RACK1-regulated dephosphorylation of threonine 38, thereby activating CAR and translocating it into the nucleus (Mutoh et al., 2013). How ligands activate phosphorylated CAR remains unknown. However, the fact is that, as long as threonine 38 is phosphorylated, ligands are not able to activate CAR (Mutoh et al., 2009). Current understanding of CAR activation is summarized in Figure 1.
SULTs in biological homeostasis
SULTs catalyze transfer of a sulfonate ( ) group from the universal sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to hydroxyl or amino-groups of acceptor substrates (Gamage et al., 2006; Strott, 2002). SULTs sulfate both xenobiotics (e.g. p-nitrophenol, diethylstilbestrol, α-hydroxytamoxifen, 6-hydroxymethylbenzo[a]pyrene) and endobiotics such as steroid hormones (Gamage et al., 2006; Glatt, 1997). Their expression is robustly influenced by xenobiotics and regulated by PXR and CAR, thereby altering biological homeostasis (Maglich et al., 2002; Rosenfeld et al., 2003).
Bile acid homeostasis
Bile acids, the end product of hepatic cholesterol catabolism, emulsify dietary fats and vitamins to promote their absorption in the small intestine. Bile acids also play important roles in the energy homeostasis of endobiotics such as glucose and lipids (Li & Chiang, 2012). Bile acids, the secondary bile acid lithocholic acid (LCA) in particular, are toxic and can cause cholestasis in rodent models (Fisher et al., 1971; Javitt, 1966). LCA levels are elevated in patients with chronic cholestatic liver disease and are suggested to be pathogenic in colorectal cancer (Fischer et al., 1996; Kishida et al., 1997). PXR activating drugs have been used to treat cholestatic liver disease in humans (Kliewer et al., 2002). Bile acid homeostasis is tightly maintained through synthesis and degradation. Nuclear receptors PXR, CAR and FXR play various roles in regulating bile acid homeostasis. In synthesis, CYP7A1 is the key enzyme that synthesizes bile acids from cholesterol. Activation of PXR represses hepatic CYP7A1 and protects mouse livers from developing LCA-induced hepatotoxicity and mortality (Kliewer & Willson, 2002; Staudinger et al., 2001b; Xie et al., 2001).
For degradation processes, SULTs are the enzymes that play a major role in the detoxification and elimination of LCA and other bile acids (Alnouti, 2009). Sulfated bile acids are less toxic and more easily excreted than un-sulfated ones. In fact, 40–75% of LCA is sulfated in human bile acids (Alnouti, 2009). SULT2A, also known as dehydroepiandrosterone SULT, is the major enzyme to sulfate LCA (Radominska et al., 1990). PAPS synthase 2 (PAPSS2) is the enzyme that synthesizes sulfate donor PAPS and can also play a role in this sulfation-mediated degradation of LCA. PXR and CAR regulate expression of these enzymes to regulate the hepatic ability of sulfating LCA. Treatment with PXR activator PCN induces hepatic expression of SULT2A in rodents (Liu & Klaassen, 1996; Sonoda et al., 2002). Treatment also co-induces PAPSS2, thereby increasing hepatic LCA sulfation capability. Transgenic mice expressing VP-CAR increase resistance to developing LCA-induced hepatotoxicity. This CAR-mediated protection appears to be largely associated with LCA sulfation, although it remains unknown which SULT sulfates LCA in mouse liver.
Over decades, PB has been used for treatment of pruritus associated with intrahepatic cholestasis (Jenkins & Boothby, 2002). Therefore, what the role of CAR observed in mice may be conserved in humans. However, whether or not PXR regulates SULT2A1 in humans as observed in mice remains controversial (Duanmu et al., 2002; Echchgadda et al., 2007; Fang et al., 2007). Rifampicin treatment induced SULT2A1 at least 1.5-fold in only 12 out of 23 primary hepatocytes (Fang et al., 2007). Eleven other primary hepatocytes either repressed SULT2A1 or did not alter levels. In fact, PXR repressed the SULT2A1 promoter in HepG2-based assays in vitro. PXR, on the other hand, was shown to activate the SULT2A1 expression in human colon adenocarcinoma cells (Echchgadda et al., 2007).
FXR, can be activated by cholic acid, chenodeoxycholic acid, or their conjugated derivatives at their physiological concentrations, is a physiological bile acid sensor to maintain bile acid homeostasis by controlling bile acid synthesis, metabolism and excretion (Modica et al., 2010). Activated FXR induces small heterodimer partner (SHP, NR0B2) and SHP then inactivates transcription factors that activate the CYP7A1 gene such as liver receptor homolog 1 (LRH1, NR5A2) and hepatocyte nuclear factor 4α (HNF4α, NR2A1) (Chiang, 2002; Goodwin et al., 2000). FXR also prompts bile acid elimination by up-regulating SULT2A1 and transporters (Song et al., 2001). FXR, PXR and CAR appear to exert coordinated efforts to regulate bile acid homeostasis. In this, FXR acts as physiological bile acid sensor, while PXR works as pathophysiological bile acid sensor. CAR, over-expressed in FXR-KO mice and FXR/PXR-double KO mice, compensates the functions of FXR and PXR (Guo et al., 2003). In addition to these three nuclear receptors, LXRs, VDR and PPARs were also reported to up-regulate SULTs (Echchgadda et al., 2004b; Fang et al., 2005b; Uppal et al., 2007).
Thyroid hormone homeostasis
3,5,3′-Triiodothyronine (T3) is the active form of thyroid hormone (TH) (Brent, 2012; Gereben et al., 2008). By binding TH receptors TRα (NR1A1) and TRβ (NR1A2), T3 plays important roles in liver regeneration and overall energy expenditure (Brent, 2012; Lopez-Fontal et al., 2010). The pro-hormone 3,5,3′,5′-tetraiodothyronine (T4) is synthesized in and secreted from the thyroid gland. Thyroid-stimulating hormone (TSH) stimulates T4 synthesis and secretion. In peripheral target organs such as liver, T4 is converted to T3 through outer ring deiodination by type 1 deiodinase (D1). The levels of free T3 are in vivo a diagnostic indicator for TH activity in the body. T3 can be inactivated by conversion to diiodothyroxines (T2s), 3,3′,5-triidothyronine (rT3; reverse T3) or sulfated or glucuronide-conjugates (Gereben et al., 2008; Kaptein et al., 1997; Visser, 1994; Visser et al., 1993, 1998). In particular, T4 sulfation by SULTs is the quite important step that could determine the fate of T4 metabolism. T4 sulfation inhibits the outer ring deiodination of T4 while it stimulates the inner ring deiodination of T4, resulting in a rapid and irreversible inactivation of THs (Visser, 1994).
Serum rT3 levels were significantly increased after partial hepatectomy (PH) in both wild-type and CAR-KO mice (Tien et al., 2007). PB treatment decreased these levels in wild-type but not CAR-KO mice. No significant changes were observed in the levels of total T3, free T3, total T4 or TSH in either wild-type or CAR-KO mice. While D1 was repressed in PH liver of both wild-type and CAR-KO mice, PB treatment induced D1 in wild type but not CAR-KO mice. Therefore, CAR-regulated induction of D1 appears to be a major pathway to increase rT3 levels, thereby modulating expression of TR-targeted genes in PH livers (Tien et al., 2007).
Treatment with CAR activator TCPOBOP is reported to stimulate thyroid follicular cell proliferation in rodents (Diwan et al., 1996; Qatanani et al., 2005). CAR-mediated increase of SULTs and serum TSH levels are suggested to cause a TH-related like disorder (Qatanani et al., 2005). Caloric restriction is shown to decrease serum total T3 and T4 levels only in wild-type but not in CAR-KO mice (Maglich et al., 2004). This induction of SULTs is speculated to decrease T4 and T3. However, neither were the levels of sulfated T3 and T4 determined in that report nor was the particular SULT enzyme responsible for T3 and T4 sulfation described (Maglich et al., 2004; Qatanani et al., 2005).
Steroid hormone homeostasis
Steroid hormones are essential in various normal physiological processes. Steroid hormones are inactivated by sulfation and reactivated by desulfation. Hence, the expression of SULTs could have significant impact on the levels of active steroid hormones.
Androgen homeostasis
Androgens, testosterone, dihydrotestosterone and androstenedione, activate androgen receptor (AR, NR3C4) to regulate the development and maintenance of male characteristics (Wang et al., 2009). Dysregulation of the androgen/AR signal is associated with the development and progression of prostate cancer (Balk & Knudsen, 2008). SULT2A1 is responsible for sulfation of androgens. SULT2A1 is predominantly expressed in the liver, intestine, adrenal glands prostate and ovary in humans (Chen et al., 2003; Falany, 1997a,b; Falany et al., 1995; Javitt et al., 2001). SULT2A1 is expressed in the mouse liver (Alnouti & Klaassen, 2006). Overexpression of rat SULT2A1 attenuated AR-mediated trans-activation in human prostate cancer-derived PC-3 cells (Chan et al., 1998).
Activation of PXR has been implicated in androgen deprivation in mice in vivo (Zhang et al., 2010). The study utilized genetically engineered mice either expressing constitutively activated PXR (VP-PXR) in the liver and small intestine or lacking the expression of PXR. Both VP-PXR and activation of PXR by PCN partially inhibited prostate regeneration by the exogenous testosterone administration in castrated mice, in which the serum testosterone levels were lowered in a PXR-dependent manner. Activation of PXR significantly inhibited the proliferation of human androgen-responsive prostate cancer cells. Activation of PXR was reported to inactivate androgens by up-regulating the expression of SULT2A1 for sulfation. Thus, PXR may antagonize the dysregulation caused by the androgen/AR signal, particularly in the development and progression of prostate cancer.
LXRα and LXRβ are sterol sensors and are activated by endogenous oxysterols, oxidized derivatives of cholesterol (Zhao & Dahlman-Wright, 2010). LXRα is highly expressed in organs such as liver, adipose tissue, kidney and macrophages, whereas LXRβ is ubiquitously expressed. In experiments with VP-LXRα transgenic and LXRα/LXRβ double-KO mice demonstrated that LXRs directly activate the hepatic expression of SULT2A1 and attenuate hepatotoxicity and cholestasis caused by LCA (Uppal et al., 2007). The LXRSULT2A1 pathway is linked to androgen deprivation and also to decreasing serum testosterone levels and decrease prostate epithelial proliferation in castrated mice and in human androgen-responsive prostate cancer cells (Lee et al., 2008). Moreover, LXRs also promote androgen inactivation by down-regulating the expression of steroid sulfatase in mouse prostate and cancer cells.
Estrogen homeostasis
By activating estrogen receptors ERα (NR3A1) and ERβ (NR3A2), estrogens play key roles in a wide range of physiological aspects throughout life; the development and maintenance of female characteristics, male characteristics such as spermatogenesis and libido and energy homeostasis (Barros & Gustafsson, 2011; Carreau & Hess, 2010; Nilsson & Gustafsson, 2011; Robertson et al., 1999). Aromatase synthesizes active estrogens, while SULTs sulfates estrogens to inactivate them. Sulfated estrogens can be re-activated by sulfatases (Suzuki et al., 2003a). Estrogen SULT (SULT1E1) is the major enzyme that sulfates estrogens in reproductive tissues (Alnouti & Klaassen, 2006). SULT1E1 is considered to be a potent prognostic factor in human breast cancer (Suzuki et al., 2003b). SULT1E1-KO mice develop placental thrombosis and spontaneous fetal loss in female mice (Tong et al., 2005) and an age-dependent structural and functional lesions in the male reproductive systems (Qian et al., 2001). SULT1E1 is also expressed in human livers (Miki, 2002). Both PXR and CAR repress SULT1E1 expression in human primary hepatocytes (Kodama et al., 2011; Lambert et al., 2009). SULT1E1, expressed at low levels in normal mouse livers, increases its expression levels in diabetogenic db/db mice (Song et al., 1995). CAR activates the Sult1e1 gene in mouse livers (Aleksunes & Klaassen, 2012; Sueyoshi et al., 2011). Although CAR-mediated increase of SULT1E1 enzyme facilitates clearance of exogenously administrated estrogen, levels of circulating estrogens remain constant (Sueyoshi et al., 2011). Whether or not PXR also regulates the Sult1e1 gene in mouse livers has not been confirmed. Utilizing VP-LXRα transgenic and LXRα/LXRβ double-KO mice, LXRα is shown to activate directly the Sult1e1 gene and induce estrogen SULT activity in livers (Gong et al., 2007). VP-LXRα transgenic females exhibit resistance against uterine epithelial cell proliferation induced by estrogen at pharmacological doses as well as growth of xenocrafted estrogen-responsive breast cancer cells. These functions of LXR and SULT1E1 have not been confirmed in humans.
Regulation of the SULT genes
Activation mechanism
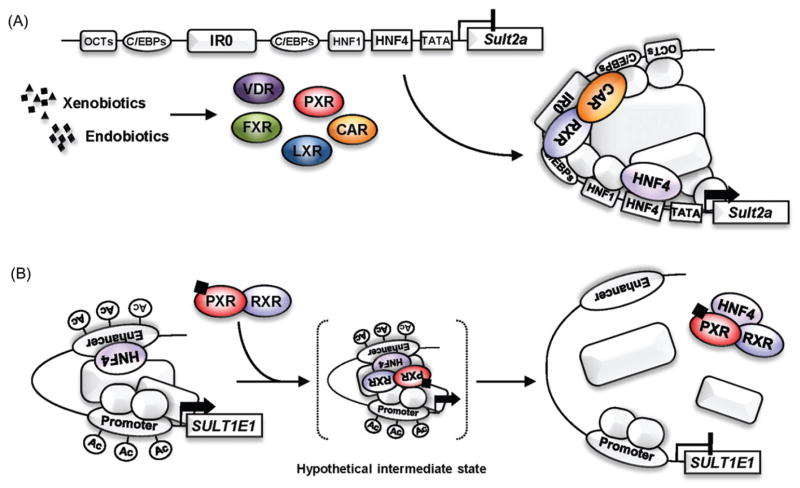
The regulatory mechanism by which nuclear receptors regulate the SULT2A genes has been investigated in both rodents and humans (Runge-Morris & Kocarek, 2005; Runge-Morris et al., 2013). The proximal promoters of Sult2a genes are well conserved in rodents and contain binding sites for liver-enriched transcription factors HNF1 and CCAAT/enhancer-binding proteins (C/EBPs) (Song et al., 1998). Androgen receptor indirectly represses the rat Sult2a1 gene by preventing the OCT-1 transcription factor and C/EBPs from activating its promoter (Song et al., 1998). Glucocorticoid induces expression of C/EBPα and C/EBPβ, which in turn activate the rat Sult2a1 promoter in primary hepatocytes (Fang et al., 2005a). The Sult2a promoter conserves an IR0 (inverted repeat without a spacing nucleotide) motif, through which PXR, CAR, VDR, FXR or LXR activate the Sult2a genes (Echchgadda et al., 2004a,b; Saini et al., 2004; Sonoda et al., 2002; Uppal et al., 2007). Figure 2(A) summarizes current views of how Sult2a genes are regulated.
Figure 2.
Regulation mechanism of the SULT genes. (A) Activation of the rodent Sult2a promoters by nuclear receptors. In rodents, the proximal promoters of Sult2a genes are well conserved. Nuclear receptors (e.g. PXR, CAR, VDR, FXR and LXR) bind to an IR0 and activate the Sult2a genes in concert with liver-enriched transcription factors. (B) Repression of the human SULT1E1 promoter by PXR. HNF4α is a transcription factor that determines the basal expression level of SULT1E1 gene in human primary hepatocytes. The binding of HNF4α to the enhancer prompts the SULT1E1 promoter to form a transcriptionally active chromatin structure by looping it position close to the proximal promoter. Upon activation by ligand, PXR interacts with HNF4α and dissociate it from the enhancer, resulting in disruption of the active chromatin structure, concomitant with deacetylation of histone H3 on both the enhancer and proximal promoter regions.
The proximal promoter of human SULT2A1 gene contains an IR2 (inverted repeat with two spacing nucleotides), DR4 (direct repeat with four spacing nucleotides), C/EBP and DR1 (direct repeat with a spacing nucleotide) motifs (Echchgadda et al., 2007; Song et al., 2006). PXR, CAR or VDR activates the SULT2A1 gene through these motifs in human colon adenocarcinoma-derived cells (Echchgadda et al., 2007; Song et al., 2006). For instance, vitamin D3 treatment recruits VDR and C/EBPα to the human SULT2A1 promoter, thereby activating the SULT2A1 gene (Song et al., 2006). PXR binds to both IR2 and DR4 motifs to activate this gene (Echchgadda et al., 2007). On the other hand, CAR can activate the promoter by binding to either IR2 or DR4 (Echchgadda et al., 2007). HNF4α binds to DR1 motif and determines the basal promoter activity of the SULT2A1 gene as well as synergizes the PXR- and CAR-mediated activation (Echchgadda et al., 2007). PPARα constitutively binds to a distal DR1 motif (at-5949 bp upstream) and activates SULT2A genes in human but not rat primary hepatocytes (Fang et al., 2005b).
Repression mechanism
Rifampicin treatment represses expression of the SULT1E1 gene in human primary hepatocytes (Kodama et al., 2011). Activity of PXR-mediated repression was delineated to the distal enhancer (−1000/−901) that contains overlapping DR1 and DR2 (direct repeat with two spacing nucleotides) motifs and the proximal promoter within the SULT1E1 promoter (Kodama et al., 2011). When HNF4α binds to distal DR1 and DR2 motifs, the promoter loops to locate distal enhancer to juxtapose the proximal promoter, thereby forming a transcriptionally active chromatin structure. This looping associates with an increase histone H3 acetylation in both distal and proximal promoter regions. Upon rifampicin activation, PXR disrupts this active chromatin structure and represses the SULT1E1 gene (Figure 2B). Chromatin immunoprecipitation assays did not detect PXR binding to either regions of the promoter. PXR may transiently interact with HNF4α and dissociate from the promoter together with HNF4α. In human primary hepatocytes, rifampicin treatment results in either induction or repression of SULT2A1 (Fang et al., 2007). Although reasons for these different responses are not known, studies with in vitro cell-based analyses suggest that PXR prevents HNF4α from activating the SULT2A1 promoter via both distal (at −6160 bp) and proximal DR1 motifs, thereby repressing the SULT2A1 gene. Therefore, HNF4α can be a common target when PXR represses SULT genes. CITCO treatment also decreases SULT1E1 mRNA levels in human primary hepatocytes. Whether or not CAR utilizes the same mechanism as described for the PXR-mediated repression remains as a possibility at the present time.
Conclusion
By cross talking with various nuclear receptors (e.g. HNF4α, FXR, LXRs, PPARs and VDR), PXR and CAR regulate SULT genes in response to both xeno- and endobiotics. Through the regulation of SULT expression, these nuclear receptors are involved not only in metabolic detoxification and excretion of xenobiotics but also in maintaining biological homeostasis (Figure 3). The molecular mechanism by which SULT genes can be regulated by these nuclear receptors is beginning to be understood but further investigations for complete understanding is required.
Figure 3.
Biological role of nuclear receptors in regulation of the SULT genes. Through the regulation of SULT expression, nuclear receptors are involved in xenobiotic metabolism and endobiotic homeostasis, and thereby play important roles in physiological and pathophysiological processes. NRRM: nuclear receptor responsive module.
Footnotes
Declaration of interest
This work was supported by Grant-in-Aid for Young Scientists from Japan Society for the Promotion of Science: 24790142 (S.K.) and by the Intramural Research Program of the National Institutes of Health, and National Institute of Environmental Health Sciences: Z01ES1005-01 (M.N.). The authors report no declarations of interest.
References
- Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab Dispos. 2012;40:1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y. Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, et al. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Ozalp C, Fang S, et al. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- Biswas A, Pasquel D, Tyagi RK, Mani S. Acetylation of pregnane X receptor protein determines selective function independent of ligand activation. Biochem Biophys Res Commun. 2011;406:371–376. doi: 10.1016/j.bbrc.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Song CS, Matusik RJ, et al. Inhibition of androgen action by dehydroepiandrosterone sulfotransferase transfected in PC-3 prostate cancer cells. Chem Biol Interact. 1998;109:267–278. doi: 10.1016/s0009-2797(97)00138-5. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang D, Jing N, et al. Human gastrointestinal sulfotransferases: Identification and distribution. Toxicol Appl Pharmacol. 2003;187:186–197. doi: 10.1016/s0041-008x(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acid regulation of gene expression: Roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- Choi HS, Chung M, Tzameli I, et al. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J Biol Chem. 1997;272:23565–23571. doi: 10.1074/jbc.272.38.23565. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Henneman JR, Rice JM, Nims RW. Enhancement of thyroid and hepatocarcinogenesis by 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene in rats at doses that cause maximal induction of CYP2B. Carcinogenesis. 1996;17:37–43. doi: 10.1093/carcin/17.1.37. [DOI] [PubMed] [Google Scholar]
- Duanmu Z, Locke D, Smigelski J, et al. Effects of dexamethasone on aryl (SULT1A1)- and hydroxysteroid (SULT2A1)-sulfotransferase gene expression in primary cultured human hepatocytes. Drug Metab Dispos. 2002;30:997–1004. doi: 10.1124/dmd.30.9.997. [DOI] [PubMed] [Google Scholar]
- Echchgadda I, Song CS, Oh T, et al. The xenobiotic-sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4alpha in the regulation of human steroid-/bile acid-sulfotransferase. Mol Endocrinol. 2007;21:2099–2111. doi: 10.1210/me.2007-0002. [DOI] [PubMed] [Google Scholar]
- Echchgadda I, Song CS, Oh TS, et al. Gene regulation for the senescence marker protein DHEA-sulfotransferase by the xenobiotic-activated nuclear pregnane X receptor (PXR) Mech Ageing Dev. 2004a;125:733–745. doi: 10.1016/j.mad.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004b;65:720–729. doi: 10.1124/mol.65.3.720. [DOI] [PubMed] [Google Scholar]
- Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997a;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- Falany CN. Sulfation and sulfotransferases. Introduction: Changing view of sulfation and the cytosolic sulfotransferases. FASEB J. 1997b;11:1–2. doi: 10.1096/fasebj.11.1.9034159. [DOI] [PubMed] [Google Scholar]
- Falany CN, Comer KA, Dooley TP, Glatt H. Human dehydroepiandrosterone sulfotransferase. Purification, molecular cloning, and characterization. Ann N Y Acad Sci. 1995;774:59–72. doi: 10.1111/j.1749-6632.1995.tb17372.x. [DOI] [PubMed] [Google Scholar]
- Fang HL, Abdolalipour M, Duanmu Z, et al. Regulation of glucocorticoid-inducible hydroxysteroid sulfotransferase (SULT2A-40/41) gene transcription in primary cultured rat hepatocytes: Role of CCAAT/enhancer-binding protein liver-enriched transcription factors. Drug Metab Dispos. 2005a;33:147–156. doi: 10.1124/dmd.104.000281. [DOI] [PubMed] [Google Scholar]
- Fang HL, Strom SC, Cai H, et al. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol. 2005b;67:1257–1267. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- Fang HL, Strom SC, Ellis E, et al. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: Roles of hepatocyte nuclear factor 4alpha and pregnane X receptor. J Pharmacol Exp Ther. 2007;323:586–598. doi: 10.1124/jpet.107.124610. [DOI] [PubMed] [Google Scholar]
- Fischer S, Beuers U, Spengler U, et al. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–186. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- Fisher MM, Magnusson R, Miyai K. Bile acid metabolism in mammals. I. Bile acid-induced intrahepatic cholestasis. Lab Invest. 1971;25:88–91. [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, et al. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, et al. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci. 2006;90:5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt H. Sulfation and sulfotransferases 4: Bioactivation of mutagens via sulfation. FASEB J. 1997;11:314–321. doi: 10.1096/fasebj.11.5.9141497. [DOI] [PubMed] [Google Scholar]
- Gong H, Guo P, Zhai Y, et al. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Moore R, Washburn KA, Negishi M. Activation by diverse xenochemicals of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol Pharmacol. 1998a;53:597–601. doi: 10.1124/mol.53.4.597. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998b;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Xu C, Staudinger JL. Pregnane X receptor is SUMOylated to repress the inflammatory response. J Pharmacol Exp Ther. 2010;335:342–350. doi: 10.1124/jpet.110.171744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson T, Treuter E, Gustafsson JA, Steffensen KR. Liver X receptor biology and pharmacology: New pathways, challenges and opportunities. Trends Pharmacol Sci. 2012;33:394–404. doi: 10.1016/j.tips.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Javitt NB. Cholestasis in rats induced by taurolithocholate. Nature. 1966;210:1262–1263. doi: 10.1038/2101262a0. [DOI] [PubMed] [Google Scholar]
- Javitt NB, Lee YC, Shimizu C, et al. Cholesterol and hydroxycholesterol sulfotransferases: Identification, distinction from dehydroepiandrosterone sulfotransferase, and differential tissue expression. Endocrinology. 2001;142:2978–2984. doi: 10.1210/endo.142.7.8244. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Boothby LA. Treatment of itching associated with intrahepatic cholestasis of pregnancy. Ann Pharmacother. 2002;36:1462–1465. doi: 10.1345/aph.1A479. [DOI] [PubMed] [Google Scholar]
- Kakizaki S, Yamazaki Y, Takizawa D, Negishi M. New insights on the xenobiotic-sensing nuclear receptors in liver diseases – CAR and PXR. Curr Drug Metab. 2008;9:614–621. doi: 10.2174/138920008785821666. [DOI] [PubMed] [Google Scholar]
- Kaptein E, van Haasteren GA, Linkels E, et al. Characterization of iodothyronine sulfotransferase activity in rat liver. Endocrinology. 1997;138:5136–5143. doi: 10.1210/endo.138.12.5555. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, et al. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida T, Taguchi F, Feng L, et al. Analysis of bile acids in colon residual liquid or fecal material in patients with colorectal neoplasia and control subjects. J Gastroenterol. 1997;32:306–311. doi: 10.1007/BF02934485. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43:359–364. [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, et al. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol. 2003;64:1069–1075. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- Kodama S, Hosseinpour F, Goldstein JA, Negishi M. Liganded pregnane X receptor represses the human sulfotransferase SULT1E1 promoter through disrupting its chromatin structure. Nucleic Acids Res. 2011;39:8392–8403. doi: 10.1093/nar/gkr458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet. 2008;23:8–13. doi: 10.2133/dmpk.23.8. [DOI] [PubMed] [Google Scholar]
- Lambert CB, Spire C, Claude N, Guillouzo A. Dose- and time-dependent effects of phenobarbital on gene expression profiling in human hepatoma HepaRG cells. Toxicol Appl Pharmacol. 2009;234:345–360. doi: 10.1016/j.taap.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gong H, Khadem S, et al. Androgen deprivation by activating the liver X receptor. Endocrinology. 2008;149:3778–3788. doi: 10.1210/en.2007-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY. Bile acid signaling in liver metabolism and diseases. J Lipids. 2012;2012:754067. doi: 10.1155/2012/754067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Brobst D, Xu C, Staudinger JL. A systematic analysis of predicted phosphorylation sites within the human pregnane X receptor protein. J Pharmacol Exp Ther. 2009a;331:65–76. doi: 10.1124/jpet.109.157180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Xu C, Staudinger JL. Cyclic AMP-dependent protein kinase signaling modulates pregnane x receptor activity in a species-specific manner. J Biol Chem. 2009b;284:6639–6649. doi: 10.1074/jbc.M807426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Klaassen CD. Regulation of hepatic sulfotransferases by steroidal chemicals in rats. Drug Metab Dispos. 1996;24:854–858. [PubMed] [Google Scholar]
- Lopez-Fontal R, Zeini M, Traves PG, et al. Mice lacking thyroid hormone receptor Beta show enhanced apoptosis and delayed liver commitment for proliferation after partial hepatectomy. PLoS One. 2010;5:e8710. doi: 10.1371/journal.pone.0008710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Watson J, McMillen PJ, et al. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–19838. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, Mizutani Y, et al. The expression of pregnane X receptor and its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell Endocrinol. 2001;172:47–56. doi: 10.1016/s0303-7207(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Miki Y. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87:5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Osabe M, Inoue K, et al. Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3) J Biol Chem. 2009;284:34785–34792. doi: 10.1074/jbc.M109.048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, et al. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 2013;6:ra31. doi: 10.1126/scisignal.2003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Gustafsson JA. Estrogen receptors: Therapies targeted to receptor subtypes. Clin Pharmacol Ther. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 2011;120:S49–S75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula SR, Brimer-Cline C, Wu J, et al. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab Dispos. 2009;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: Energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- Qian YM, Sun XJ, Tong MH, et al. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology. 2001;142:5342–5350. doi: 10.1210/endo.142.12.8540. [DOI] [PubMed] [Google Scholar]
- Radominska A, Comer KA, Zimniak P, et al. Human liver steroid sulphotransferase sulphates bile acids. Biochem J. 1990;272:597–604. doi: 10.1042/bj2720597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA. Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab. 2005;6:299–307. doi: 10.2174/1389200054633871. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA, Falany CN. Regulation of the cytosolic sulfotransferases by nuclear receptors. Drug Metab Rev. 2013;45:15–33. doi: 10.3109/03602532.2012.748794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini SP, Sonoda J, Xu L, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- Savas U, Wester MR, Griffin KJ, Johnson EF. Rabbit pregnane X receptor is activated by rifampicin. Drug Metab Dispos. 2000;28:529–537. [PubMed] [Google Scholar]
- Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: Expression and correlations. Mol Immunol. 2007;44:1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, et al. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–G1122. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- Siest G, Jeannesson E, Marteau JB, et al. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab Dispos. 2008;36:182–189. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- Sivertsson L, Edebert I, Palmertz MP, et al. Induced CYP3A4 expression in confluent Huh7 hepatoma cells as a result of decreased cell proliferation and subsequent pregnane X receptor activation. Mol Pharmacol. 2013;83:659–670. doi: 10.1124/mol.112.082305. [DOI] [PubMed] [Google Scholar]
- Song CS, Echchgadda I, Baek BS, et al. Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid X receptor. J Biol Chem. 2001;276:42549–42556. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- Song CS, Echchgadda I, Seo YK, et al. An essential role of the CAAT/enhancer binding protein-alpha in the vitamin D-induced expression of the human steroid/bile acid-sulfotransferase (SULT2A1) Mol Endocrinol. 2006;20:795–808. doi: 10.1210/me.2005-0428. [DOI] [PubMed] [Google Scholar]
- Song CS, Jung MH, Kim SC, et al. Tissue-specific and androgen-repressible regulation of the rat dehydroepiandrosterone sulfotransferase gene promoter. J Biol Chem. 1998;273:21856–21866. doi: 10.1074/jbc.273.34.21856. [DOI] [PubMed] [Google Scholar]
- Song WC, Moore R, McLachlan JA, Negishi M. Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology. 1995;136:2477–2484. doi: 10.1210/endo.136.6.7750469. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Xie W, Rosenfeld JM, et al. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci USA. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279:49307–49314. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, et al. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001a;29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001b;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Xu C, Biswas A, Mani S. Post-translational modification of pregnane x receptor. Pharmacol Res. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Green WD, Vinal K, et al. Garlic extract diallyl sulfide (DAS) activates nuclear receptor CAR to induce the Sult1e1 gene in mouse liver. PLoS One. 2011;6:e21229. doi: 10.1371/journal.pone.0021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, et al. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Nishitani S, Yamakawa K, et al. Transcriptional regulation of human UGT1A1 gene expression: Activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol Pharmacol. 2005;67:845–855. doi: 10.1124/mol.104.007161. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miki Y, Nakata T, et al. Steroid sulfatase and estrogen sulfotransferase in normal human tissue and breast carcinoma. J Steroid Biochem Mol Biol. 2003a;86:449–454. doi: 10.1016/s0960-0760(03)00356-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakata T, Miki Y, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res. 2003b;63:2762–2770. [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Tien ES, Matsui K, Moore R, Negishi M. The nuclear receptor constitutively active/androstane receptor regulates type 1 deiodinase and thyroid hormone activity in the regenerating mouse liver. J Pharmacol Exp Ther. 2007;320:307–313. doi: 10.1124/jpet.106.112706. [DOI] [PubMed] [Google Scholar]
- Tong MH, Jiang H, Liu P, et al. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med. 2005;11:153–159. doi: 10.1038/nm1184. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal H, Saini SP, Moschetta A, et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology. 2007;45:422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- Visser TJ. Role of sulfation in thyroid hormone metabolism. Chem Biol Interact. 1994;92:293–303. doi: 10.1016/0009-2797(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, Glatt H, et al. Characterization of thyroid hormone sulfotransferases. Chem Biol Interact. 1998;109:279–291. doi: 10.1016/s0009-2797(97)00139-7. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, van Raaij JA, et al. Multiple UDP-glucuronyltransferases for the glucuronidation of thyroid hormone with preference for 3,3′,5′-triiodothyronine (reverse T3) FEBS Lett. 1993;315:65–68. doi: 10.1016/0014-5793(93)81134-l. [DOI] [PubMed] [Google Scholar]
- Wallace K, Cowie DE, Konstantinou DK, et al. The PXR is a drug target for chronic inflammatory liver disease. J Steroid Biochem Mol Biol. 2010;120:137–148. doi: 10.1016/j.jsbmb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, et al. Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/s0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]
- Zhang B, Cheng Q, Ou Z, et al. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology. 2010;151:5721–5729. doi: 10.1210/en.2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, LeCulyse E, Liu L, et al. Rat pregnane X receptor: Molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7:e001. doi: 10.1621/nrs.07001. [DOI] [PMC free article] [PubMed] [Google Scholar]