Abstract
Gamma rhythms are commonly observed in many brain regions during both waking and sleep states, yet their functions and mechanisms remain a matter of debate. Here we review the cellular and synaptic mechanisms underlying gamma oscillations and outline empirical questions and controversial conceptual issues. Our main points are as follows: First, gamma-band rhythmogenesis is inextricably tied to perisomatic inhibition. Second, gamma oscillations are short-lived and typically emerge from the coordinated interaction of excitation and inhibition, which can be detected as local field potentials. Third, gamma rhythm typically concurs with irregular firing of single neurons, and the network frequency of gamma oscillations varies extensively depending on the underlying mechanism. To document gamma oscillations, efforts should be made to distinguish them from mere increases of gamma-band power and/or increased spiking activity. Fourth, the magnitude of gamma oscillation is modulated by slower rhythms. Such cross-frequency coupling may serve to couple active patches of cortical circuits. Because of their ubiquitous nature and strong correlation with the “operational modes” of local circuits, gamma oscillations continue to provide important clues about neuronal population dynamics in health and disease.
Keywords: inhibitory interneurons, interneuronal network, excitatory-inhibitory loop, spike timing, dynamical cell assembly, irregular spiking, cross-frequency coupling, long-distance communication
INTRODUCTION
The precise timing of neuronal-spike discharges is believed to be important for coding of information (O’Keefe & Recce 1993, Buzsáki & Chrobak 1995, Singer & Gray 1995, Singer 1999). The ability of various neuron types to time their action potentials with millisecond precision depends largely on the presence of fast membrane potential fluctuations (Mainen & Sejnowski 1995, Haider & McCormick 2009). In the intact brain, such high-frequency patterns are often brought about by various endogenous oscillations, the most ubiquitous of which are rhythms in the gamma-frequency range (30–90 Hz) (see Origin and Definition of Gamma Oscillation, sidebar below).
Numerous excellent reviews have discussed the biological processes underlying gamma oscillations (Gray 1994, Whittington et al. 2000, Laurent 2002, Traub et al. 2002, Bartos et al. 2007, Tiesinga & Sejnowski 2009, Wang 2010) as well as their role in cognitive operations (Singer & Gray 1995; Engel et al. 2001; Varela et al. 2001; Fries 2005, 2009; Wang 2010) and disease (Llinás et al. 1999, Lewis et al. 2005, Uhlhaas & Singer 2006). The present review focuses on the cellular-synaptic mechanisms of gamma oscillations, their cell-assembly-forming ability in the intact brain, and the subtypes of gamma rhythms. It also examines how gamma-reflected local-circuit operations are temporally coordinated by slower rhythms.
ARE CELL ASSEMBLIES DYNAMICALLY ORGANIZED IN GAMMA CYCLES?
To appreciate the physiological function of the gamma cycle in neural networks, we need to examine the spiking patterns of neurons at this timescale. The exact timing of neuronal spikes can be related to environmental stimuli, overt behavior, local field potential (LFP), or spiking activity of other neurons. Each of these comparisons provides a different “optimum” time window. The best prediction is obtained when information about the spike times of partner neurons are available in the 10- and 30-ms window (Figure 1) (Jensen & Lisman 1996, Borgers & Kopell 2003, Harris et al. 2003, Lisman 2005), i.e., the time window corresponding approximately to a gamma cycle. Neuronal assemblies, i.e., transient neuronal partnerships, can be active repeatedly in successive gamma cycles, or different assemblies can alternate in a rapid sequence.
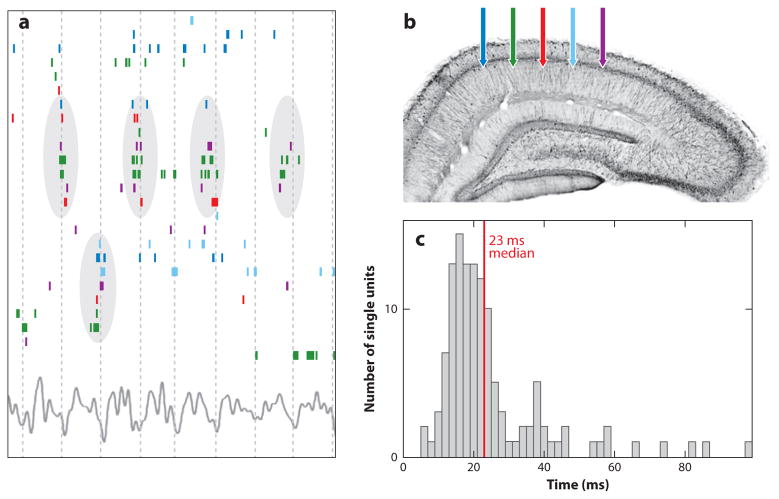
Figure 1.
Dynamical cell assemblies are organized in gamma waves. (a) Raster plot of a subset of hippocampal pyramidal cells that were active during a 1-s period of spatial exploration on an open field out of a larger set of simultaneously recorded neurons, ordered by stochastic search over all possible orderings to highlight the temporal relationship between anatomically distributed neurons. Color-coded ticks (spikes) refer to recording locations shown in panel b. Vertical lines indicate troughs of theta waves (bottom trace). Cell-assembly organization is visible, with repeatedly synchronous firing of some subpopulations (circled). (c) Spike timing is predictable from peer activity. Distribution of timescales at which peer activity optimally improved spike-time prediction of a given cell, shown for all cells. The median optimal timescale is 23 ms (red line). Based on Harris et al. (2003).
The gamma-cycle-related lifetime of the cell assembly is closely related to several biophysical properties of neurons, including the time constant of gamma-aminobutyric acid (GABA)A and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Johnston & Wu 1994), the membrane time constant of cortical pyramidal cells (Destexhe & Paré 1999, Leger et al. 2005), and the critical time window of spike-timing-dependent plasticity (Magee & Johnston 1997, Markram et al. 1997). Because these parameters determine the neuron’s ability to integrate inputs from multiple upstream sources, a hypothesized functional role of the cell assembly is to bring together sufficient numbers of peer neurons so that their collective spiking can discharge the postsynaptic neuron (Harris et al. 2003). Consequently, from the point of view of the downstream (“reader” or “integrator”) cell, ensemble activity of upstream neurons whose spikes occur within the gamma-cycle window is classified as a single event (Buzsaki 2010). Upstream neurons whose spikes fall outside this time window become part of another transient assembly.
MODELS OF GAMMA OSCILLATIONS
The similar kinetics of gamma-frequency oscillations in a variety of different brain regions and species have provided clues and constraints about the requirements of their supporting mechanisms. Gamma oscillations have been described in several areas of the neocortex (Gray et al. 1989, Murthy & Fetz 1992, Fries et al. 2001, Sirota et al. 2008), entorhinal cortex (Chrobak & Buzsáki 1998), amygdala (Halgren et al. 1977, Popescu et al. 2009), hippocampus (Buzsáki et al. 1983, Bragin et al. 1995, Whittington et al. 1995, Mann et al. 2005), striatum (Berke et al. 2004, Tort et al. 2008), olfactory bulb (Adrian 1942, Freeman 1975), and thalamus (Pinault & Deschénes 1992) as well as other areas. Common denominators of these brain regions are the presence of inhibitory interneurons and their actions through GABAA synapses. Synchronization of neurons is substantially more effective by perisomatic inhibitory postsynaptic potentials (IPSPs) than dendritic excitatory (E)PSPs (Lytton & Sejnowski 1991). From these considerations, it is reasonable to assume that a key ingredient of gamma oscillations is GABAA receptor–mediated inhibition.
I-I Model
Only three requirements are needed for gamma oscillations to emerge, as illustrated by a “stripped-down” network model consisting of only inhibitory interneurons (Figure 2a) (Wang & Rinzel 1992, Whittington et al. 1995, Wang & Buzsáki 1996, Traub et al. 1996b): mutually connected inhibitory interneurons, a time constant provided by GABAA receptors, and sufficient drive to induce spiking in the interneurons. Gamma oscillations in inhibitory-inhibitory (I-I) neuron models can emerge in two different ways (see Irregular Activity of Single Neurons and Gamma Oscillations of Neuron Groups, sidebar below). When the input drive is relatively tonic, neurons can fire spikes with a well-defined periodicity (Figure 2a) (Kopell & Ermentrout 2002). By contrast, when neurons receive stochastic inputs and fire spikes irregularly, sufficiently strong recurrent synaptic interactions will make the asynchronous state unstable against random fluctuations, and oscillations emerge (Figure 2b) (Brunel & Hakim 1999, Brunel 2000, Brunel & Wang 2003, Geisler et al. 2005, Ardid et al. 2010, Economo & White 2012). In both cases, the emerging synchrony is caused when a subset of the interneurons begins to discharge together and generates synchronous IPSPs in the partner neurons. In turn, the inhibited neurons will spike again with increased probability when GABAA receptor–mediated hyperpolarization has decayed, and the cycle repeats (Figure 2a,b). Because the duration of IPSCs (inhibitory postsynaptic current) is determined by the subunit composition of the GABAA receptor (cf. Farrant & Nusser 2005), the frequency of gamma oscillations in the I-I model is determined mainly by the kinetics of the IPSPs and the net excitation of interneurons (Whittington et al. 1995, Wang & Buzsáki 1996).
Figure 2.
I-I and E-I models of gamma oscillations. (a) Clock-like rhythm of coupled oscillators in an interneuronal (I-I) population. (Upper panel) Single interneurons fire spikes periodically at ~40 Hz. Mutual inhibition via GABAA receptors quickly brings them to zero-phase synchrony; (lower panel) two example neurons. Adapted from Wang & Buzsáki (1996). (b,c) Sparsely synchronous oscillations in a neural circuit where single neuronal spiking is stochastic. Adapted from Geisler et al. (2005). (b) Interneuronal population in noise-dominated regime typically exhibits gamma power in the higher frequency range, in contrast to (a) the clock-like rhythmic case. (c) Reciprocally connected E-I network where pyramidal cells send fast excitation via AMPA receptors to interneurons, which in turn provide inhibition via GABAA receptors, leading to coherent oscillations in the gamma-frequency range.
ORIGIN AND DEFINITION OF GAMMA OSCILLATION.
Berger (1929) introduced the Greek letters alpha and beta to refer to the larger amplitude rhythmic patterns below 12 Hz and the lower amplitude faster than 12-Hz patterns, respectively. Jasper & Andrews (1938) first used the term gamma waves to designate low-amplitude beta-like waves at 35–45 Hz. Other synonyms referring to this band are the 40-Hz oscillation or cognitive rhythm, both introduced by Das & Gastaut (1955). The phrase gamma oscillation became popular in the 1980s, mostly through papers by Walter Freeman (Bressler & Freeman 1980). Proper taxonomy of brain rhythms should eventually be based on mechanisms. Because mechanisms are not fully understood in most cases, the names of the brain rhythms respect historical traditions. We refer to periodic events in the 30–90-Hz band as gamma oscillations and the band above this frequency as epsilon (ε) (Freeman 2007) (also see the Supplemental Text: follow the Supplemental Material link in the online version of this article at http://www.annualreviews.org.).
In vitro experiments provided support for the sufficient role of mutual inhibition among interneurons for the generation of gamma rhythm, for instance sustained by activation of metabotropic glutamate receptors (Whittington et al. 1995). Gamma oscillations can be induced by other means as well, such as activation of muscarinic-cholinergic receptors (Fisahn et al. 1998) or kainate receptors (Fisahn et al. 2004, Hájos & Paulsen 2009). Common to all these conditions is the increased firing of synaptically coupled interneurons. When pyramidal cells and other interneuron types are added to the I-I model network, the entire network can become phase-locked to the gamma oscillations.
E-I Model
The earliest model of gamma oscillations is based on the reciprocal connections between pools of excitatory pyramidal (E) and inhibitory (I) neurons (Wilson & Cowan 1972, Freeman 1975, Leung 1982, Ermentrout & Kopell 1998, Borgers & Kopell 2003, Brunel & Wang 2003, Geisler et al. 2005). In such two-neuron pool models (Figure 2c), fast excitation and the delayed feedback inhibition alternate, and with appropriate strength of excitation and inhibition, cyclic behavior may persist for a while. E-I models can also exhibit two distinct regimes, depending on whether single neurons behave periodically or highly stochastically. In the model, axon conduction and synaptic delays lead to a phase shift (~5 ms or up to 90°) between the pyramidal and interneuron spikes, and these delays determine the frequency of the gamma rhythm (Freeman 1975, Leung 1982). An appeal of the E-I model is that the delay between the timing of pyramidal cell and interneuron spikes is a prominent feature of gamma oscillations both in vivo and in vitro (Figure 3) (Bragin et al. 1995, Csicsvari et al. 2003, Hasenstaub et al. 2005, Mann et al. 2005, Hájos & Paulsen 2009, Tiesinga & Sejnowski 2009). In further support of the model, weakening the E-I connection by genetic knock down of AMPA receptors on fast spiking interneurons reduces the amplitude of gamma oscillations (Fuchs et al. 2007). The mainstream I-I and E-I models have been developed to explain gamma oscillations in the cortex but other gamma frequency oscillations may possibly arise from other mechanisms as well (Wang 1993, Gray & McCormick 1996, Wang 1999, Minlebaev et al. 2011).
Figure 3.
A critical role of parvalbumin (PV) basket cells in gamma oscillations. (a) Local field potential (LFP) recording from the CA1 pyramidal layer (top) and dentate hilus (bottom) and unit recording from a fast-spiking putative interneuron in the hilus (middle trace). Note the trains of spikes at gamma frequency, repeating periodically at theta frequency. (b) Power spectrum of the unit shown in panel a. Note the peak at theta and a broader peak at 50–80 Hz (gamma). (c) Spike-triggered average of the LFP in the hilus. Note the prominent phase locking of the interneuron to gamma wave phase and the cross-frequency coupling between gamma and theta waves.
(a–c) Recordings from a behaving rat. (d) Camera-lucida reconstruction of the axon arbor of an immunocytochemically identified CA1 basket cell in vivo. The axon arbor outlines the CA1 pyramidal layer, showing (circles) putative contacts with other PV-positive neurons, (inset) averages of the intracellularly recorded Vm (membrane potential) and the LFP, (triangle) peak of the mean preferred discharge of the surrounding pyramidal cells, and (arrow) peak of the mean preferred discharge of the basket cell. Note the short delay between the spikes of pyramidal cells and the basket neuron. Current source density (CSD) map is superimposed on the pyramidal layer. Arrow points to current source of gamma wave (red). (e) Continuous display (110) of integrated and rectified gamma activity of the LFP and the fast intracellularly recorded Vm fluctuation (20–80 Hz; after digital removal of spikes) in a CA1 pyramidal neuron. Vm was biased by the intracellular current injection: (dashed line) resting membrane potential. Note the increase of the intracellular Vm gamma during both depolarization (inset) and hyperpolarization as well as the smallest Vm gamma power at resting membrane potential (asterisks) against the steady background of LFP gamma power. (d, e) In vivo recordings under urethane anesthesia. (f) Excitatory (E) and inhibitory (I) postsynaptic currents (PSCs) in a pyramidal cell, triggered by LFP gamma (top) and the spike timing of a pyramidal cell (P) and a basket interneuron (B) during carbachol-induced gamma oscillation in a hippocampal slice in vitro. Note that maximum discharge of the basket cell precedes the hyperpolarization of the pyramidal cell. (g) Intracellular recordings in a ferret prefrontal pyramidal cell in vivo illustrating the large amplitude, inhibition-dominated barrages recorded at 0 mV (brown) and smaller amplitude, excitation-dominated, synaptic barrages recorded at −80 mV (tan) for two representative UP states. Membrane potentials are expanded further (inset). EPSP, excitatory postsynaptic potential; IPSP, inhibitory postsynaptic potential. Reproduced with permission from (a–c) Buzsáki et al. (1983), (d–e) after Penttonen et al. (1998), (f) after from Mann et al. (2005), and (g) after Hasenstaub et al. (2005).
CELLULAR-NETWORK MECHANISMS OF GAMMA OSCILLATIONS
Perisomatic Inhibition Is Critical for Gamma Oscillations
The first support for the involvement of fast-spiking interneurons in gamma oscillations came from the correlation (spike-field coherence) between their spikes and locally recorded LFP gamma oscillations in the hippocampus of behaving rats (Figure 3) (Buzsáki et al. 1983). Putative fast-spiking interneurons and histologically verified parvalbumin (PV)-immunoreactive basket cells often show a broad peak in their autocorrelograms and spectrograms at gamma frequency (Figure 3b), and the occurrence of their spikes follows those of the surrounding pyramidal neurons by a few milliseconds (Figure 3d, f) (Bragin et al. 1995, Csicsvari et al. 2003, Mann et al. 2005, Hájos & Paulsen 2009), as in E-I models. As expected from the spike-LFP relationship (Figure 3a,c) postsynaptic potentials phase-locked to the LFP gamma rhythm are present in pyramidal neurons. These gamma-correlated postsynaptic potentials in pyramidal cells reverse their polarity close to the equilibrium potential of Cl− (Figure 3e,g), indicating that the gamma-rhythm-related inhibition is mediated by GABAA receptors (Soltesz & Deschênes 1993, Whittington et al. 1995, Penttonen et al. 1998, Hasenstaub et al. 2005, Mann et al. 2005). The IPSPs paced by the PV basket cells produce coherent transmembrane fluctuations in the target pyramidal cell population (Penttonen et al. 1998, Gloveli et al. 2005, Hasenstaub et al. 2005, Mann et al. 2005, Quilichini et al. 2010) and can be detected as a strong current source in the cell-body layer (Figure 3d) (Csicsvari et al. 2003, Mann et al. 2005). The interconnected PV-basket interneuron network with its divergent output to pyramidal cells provides an anatomical substrate for coherent timing of the pyramidal cells (Figure 3d) (Kisvárday et al. 1993, Buhl et al. 1994, Sik et al. 1995). Altogether, these findings support the hypothesis that extracellularly recorded gamma waves largely correspond to synchronous IPSPs in pyramidal cells, brought about by fast-spiking interneurons (Buzsáki et al. 1983, Bragin et al. 1995, Hasenstaub et al. 2005, Freund & Katona 2007, Hájos & Paulsen 2009).
IRREGULAR ACTIVITY OF SINGLE NEURONS AND GAMMA OSCILLATIONS OF NEURON GROUPS.
A fruitful debate persists between researchers who study population gamma oscillations and ponder their functions, and researchers who study single-neuron data and observe that neuronal-spike trains are often irregular and by some measures approximate a Poisson process (Softky & Koch 1993). Recent work has offered a novel theoretical framework in which population rhythms can arise from irregularly firing neurons, thereby bridging these contrasting dynamical aspects of cortical dynamics (c.f., Wang 2010).
Several other findings support the critical role of fast-spiking basket neurons in gamma oscillations. Basket cells have several distinctive features among the interneuron family, including (a) low spike threshold (Gulyás et al. 1993), (b) ability to fire rapidly without fatigue (Buzsáki et al. 1983, McCormick et al. 1985, Kawaguchi & Kubota 1997), (c) narrow spikes conferred by a large density of KV3.1/3.2 channels (Lien & Jonas 2003), (d) a unique spike-conductance trajectory (Tateno & Robinson 2009), and (e) resonance at gamma frequency in response to stochastic excitatory conductance inputs (Figure 4) (Pike et al. 2000, Cardin et al. 2009, Sohal et al. 2009). Overall, these findings support the hypothesis that gamma oscillations can be induced by activation of interconnected PV interneurons by multiple means.
Figure 4.
“Synthetic” gamma rhythm in vivo. (a) Local field potential (LFP) recordings in anesthetized mouse, expressing ChR2 selectively in either parvalbumin (PV) neurons (ChR2-PV-Cre) or pyramidal cells (ChR2-αCamKII-Cre).
Stimulation at 8 Hz evoked rhythmic activity in the αCamKII-Cre but not the PV-Cre mouse. Conversely, stimulation at 40 Hz induced gamma oscillation in the PV-Cre but not in αCamKII-Cre mouse. (b) Mean LFP power ratio measured in multiple frequency bands in response to rhythmic light activation of ChR2-PV-Cre expressing neurons (blue) or ChR2-αCamKII-Cre expressing neurons (purple) at various frequencies. Reprinted from Cardin et al. (2009).
The involvement of other interneuron types (Freund & Buzsáki 1996, Klausberger & Somogyi 2008) in gamma generation is understood less well. Chandelier cells are likely not critical in I-I models, because they innervate only principal cells. The somatostatin-containing O-LM interneurons and Martinotti cells mainly target distal dendrites, establish few connections among themselves (Gibson et al. 1999), and have resonance at theta, rather than gamma, frequencies (Pike et al. 2000, Gloveli et al. 2005). The postsynaptic receptor targets of CCK basket cells contain slower α2 subunits (Glickfield & Scanziani 2006, Freund & Katona 2007), and CCK interneurons are not effective in maintaining gamma oscillations (Hájos et al. 2004, Tukker et al. 2007). Hippocampal CA1 bistratified neurons showed stronger phase locking of spikes to gamma waves than did PV basket cells (Tukker et al. 2007). Their phase locking may be “inherited” from the CA3 output (Csicsvari et al. 2003), but the IPSPs they produce in the dendrites of pyramidal cells may not be faithfully transferred to the soma (Lytton & Sejnowski 1991). These other types of interneurons appear better suited to contribute to slower oscillations and, by controlling basket cells, are likely critical in establishing cross-frequency coupling (see below) between gamma and slower rhythms.
Do I-I and E-I Mechanisms Compete or Cooperate in the Brain?
Both I-I and E-I models have merits and disadvantages (Whittington et al. 2000, Tiesinga & Sejnowski 2009, Wang 2010). Because the oscillation frequency of individual neurons in the I-I model is at least partially determined by the amount of excitation, a heterogeneous input can result in a wide range of oscillation frequencies. In the face of such frequency dispersion, the population synchrony inevitably decreases. This shortcoming can be effectively compensated for by gap-junction-enhanced synchrony (Gibson et al. 1999, Hormuzdi et al. 2001, Buhl et al. 2003, Traub et al. 2004), resonant properties of basket cells, and fast and strong shunting inhibition between interneurons (Bartos et al. 2007). However, heterogeneity of neuronal firing rates may be beneficial. In networks consisting of neurons with different firing patterns and rates, gamma oscillation may function as a selection mechanism, because transient synchrony would emerge only among those neurons that are activated to approximately the same level.
In most E-I models, there is no need for I-I connections (Wilson & Cowan 1972, Whittington et al. 2000, Borgers & Kopell 2003, Brunel & Wang 2003, Geisler et al. 2005). In support of this prediction, experimentally disconnecting many I-I links in knockout mice did not strongly affect gamma power in the hippocampal CA1 region (Wulff et al. 2009). In the E-I models, the driving force of the oscillation is the activity of pyramidal cells. Note that gamma rhythms are also prominent in structures, which lack dense local E-I connections, such as the basal ganglia or ventral tegmental area (Brown et al. 2002, Berke et al. 2004, Tort et al. 2008, Fujisawa & Buzsáki 2011). E-I models require a time delay between E spikes and I spikes, since timing of the interneurons is “inherited” from the pyramidal cells. In contrast, in I-I models the spike phase of pyramidal cells largely reflects the intensity of their tonic drive. In the hippocampal CA1 region, interneurons show both phase delay or advance relative to the spikes of pyramidal cells (Bragin et al. 1995, Csicsvari et al. 2003, Tukker et al. 2007, Senior et al. 2008, Mizuseki et al. 2011). These results suggest that E-I and I-I hybrid gamma networks may work together to generate gamma frequency oscillations (Brunel & Wang 2003, Geisler et al. 2005, Tiesinga & Sejnowski 2009, Belluscio et al. 2012).
The role of recurrent excitatory (E-E) connections between principal cells in gamma models are not well-understood (Kopell et al. 2000, Whittington et al. 2000, Brunel & Wang 2003, Geisler et al. 2005). In the cortex, gamma oscillations are more prominent in the superficial, rather than the deep, layers where local recurrent connections are abundant (Chrobak & Buzsáki 1998, Quilichini et al. 2010, Buffalo et al. 2011). By contrast, the largest-amplitude gamma rhythm in the hippocampus is observed in the dentate gyrus (Buzsáki et al. 1983), even though granule cells lack recurrent excitation onto themselves. Decreasing recurrent excitatory synaptic currents in dynamic clamp studies had little effect on gamma power (Morita et al. 2008). The less critical role of E-E recurrent excitation may liberate the pyramidal cells from the timing constraints of the rhythm; therefore, they could fire spikes stochastically at various cycle phases in an input drive–dependent manner without interrupting rhythm.
LONG-RANGE SYNCHRONIZATION OF GAMMA OSCILLATIONS
Although gamma oscillations typically arise locally, patches of gamma networks can interact with each other. Synchronization of transient gamma bursts has multiple meanings, including phase-phase, phase-amplitude, and amplitude-amplitude coupling (Figure 5) (see Cross-Frequency Phase Coupling, sidebar below). Phase-phase synchrony between identical frequency oscillators that emerges at two (or multiple) locations can occur by phase locking (Figure 5b). The magnitude of such synchrony is typically measured by phase coherence. A second form of synchrony refers to the covariation of gamma power at two (or multiple) locations, also known as amplitude or power comodulation (Figure 5c). In this latter case, phase constancy between the gamma waves may or may not be present (Figure 5c,d). Instead, the power (amplitude) envelopes of the gamma bursts are correlated (comodulation of power). Power-power synchrony of gamma rhythms can be effectively brought about by joint phase biasing of the power of gamma oscillations by a slower rhythm, known as cross-frequency phase-amplitude (CFPA) coupling or nested oscillations (Figure 5c,d,e) (Bragin et al. 1995, Schroeder & Lakatos 2009, Canolty & Knight 2010, Fell & Axmacher 2011). The third type of synchrony occurs when there is a relatively constant relationship between the gamma phase and the phase of a modulating slower rhythm (Figure 6e), known as cross-frequency phase-phase (CFPP), or n:m, coupling (Tass et al. 1998). Cross-frequency coupling can take place within or across structures. In practice, each relationship should be investigated with care because even stochastic signals can occasionally yield spurious coupling.
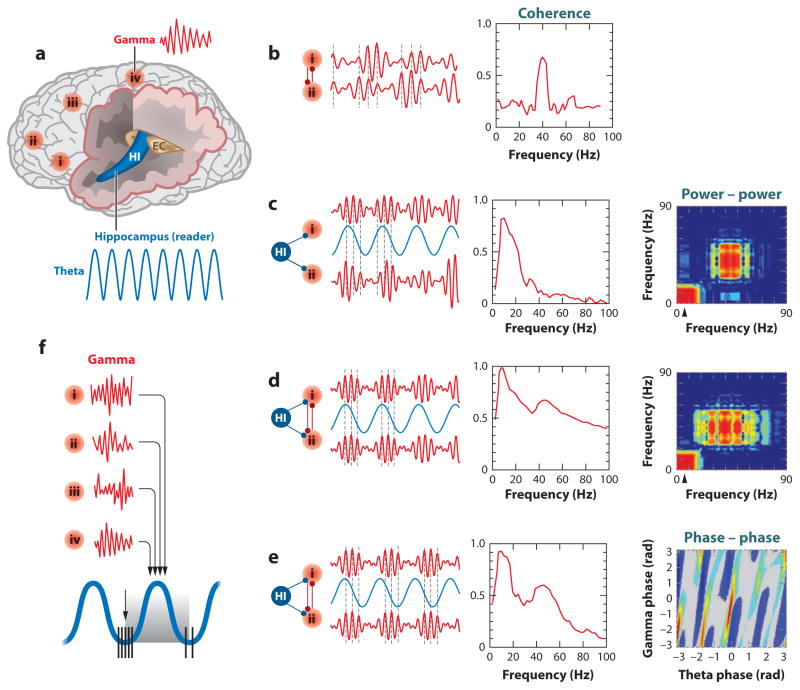
Figure 5.
Oscillatory coupling mechanisms. (a) Schematic view of the human brain showing hot spots of transient gamma oscillations (i–iv) and theta oscillation in the hippocampus (HI); entorhinal cortex (EC). Oscillators of the same and different kind (e.g., theta, gamma) can influence each other in the same and different structures, thereby modulating the phase, amplitude, or both. (b) Phase-phase coupling of gamma oscillations between two areas. Synthetic data used for illustration purposes. Coherence spectrum (or other, more specific, phase-specific measures) between the two signals can determine the strength of phase coupling. (c) Cross-frequency phase-amplitude coupling. Although phase coupling between gamma waves is absent, the envelope of gamma waves at the two cortical sites is modulated by the common theta rhythm. This can be revealed by the power-power correlation (comodugram; right). (d) Gamma phase-phase coupling between two cortical sites, whose powers are modulated by the common theta rhythm. Both gamma coherence and gamma power-power coupling are high. (e) Cross-frequency phase-phase coupling. Phases of theta and gamma oscillations are correlated, as shown by the phase-phase plot of the two frequencies. (f) Hippocampal theta oscillation can modulate gamma power by its duty cycle at multiple neocortical areas so that the results of the local computations are returned to the hippocampus during the accrual (“readiness”) phase of the oscillation. a and f, after Buzsáki (2010); b–e, after Belluscio et al. (2012).
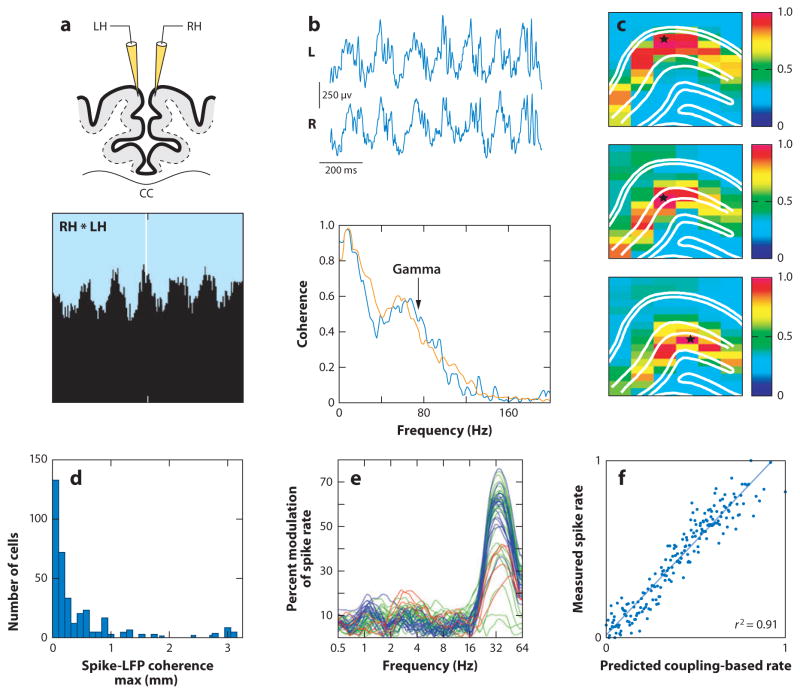
Figure 6.
Long-range synchrony of gamma oscillations. (a) Neurons sharing receptive fields in left (LH) and right (RH) primary visual cortex of the anesthetized cat fire coherently with zero time lag at gamma frequency. (b) Local field potential (LFP) traces from the left (L) and right (R) hippocampal CA1 pyramidal layer of the mouse during running and coherence spectra between the traces during running (orange) and REM sleep (blue). (c) LFP coherence map of gamma (30–90 Hz) in the rat hippocampus during running. Coherence was calculated between the reference site (star) and the remaining 96 recording sites. Note the high coherence values within the same layers (outlined by white lines) and rapid decrease of coherence across layers. (d) Distribution of distances between the unit and LFP recording sites with maximum spike-LFP coherence in the gamma band. Note that, in a fraction of cases, maximum coherence is stronger at large distances between the recorded unit and the LFP. (e) Spike-LFP coherence in the human motor cortex. The probability of spiking correlates with frequency-specific LFP phase of the ipsilateral (blue) and contralateral (green) motor area and contralateral dorsal premotor area (red). (f) The phase-coupling-based spike rate (generated from the preferred LFP–LFP phase-coupling pattern) predicts the measured spike rate. Panels reproduced after (a) Engel et al. (1991), (b) Buzsáki et al. (2003), (c) Montgomery & Buzsáki (2007), (d) Sirota et al. (2008), and (e,f) Canolty et al. (2010).
Phase Coherence of Gamma Rhythms in Distant Networks
If multiple cell assemblies in disparate brain areas need to be synchronized, how can they be engaged in coherent gamma oscillations given the long axon conduction delays of pyramidal cells? Solid evidence for coherent gamma oscillations in distant networks is scarce; perhaps the best-established case is interhemispheric synchronization. Multiple units with similar receptive fields in the left and right primary visual cortex can display coherent gamma-range oscillations (Figure 6a). Similarly, gamma oscillations in homologous hippocampal layers in the two hemispheres display high coherence (Figure 6b). In both cases, phase synchrony is mediated by interhemispheric axon tracts, given that severing these conduits abolishes the synchrony. The high interhemispheric coherence and task-dependent inter-regional gamma synchrony (Engel et al. 1991, Roelfsema et al. 1997, Chrobak & Buzsáki 1998, Rodriguez et al. 1999, Tallon-Baudry et al. 2001, Montgomery et al. 2008) can be contrasted with the fast decrease of gamma coherence across different layers (Figure 6c), owing to the noncoherent relationships among the inputs. The importance of anatomical connectivity, as opposed to physical distance, can explain the occasionally high gamma coherence between spikes and LFP at distant sites (Figure 6d) and the gamma timescale covariations of firing rates of spatially distant neurons (Figure 6e, f).
Temporal coordination between spatially separated oscillators can be established by axon collaterals of pyramidal cells (Traub et al. 1996a, Whittington et al. 2000, Bibbig et al. 2002), interleaving assemblies (Vicente et al. 2008), or long-range interneurons (Buzsáki et al. 2004). In each case, conduction delays are the primary problem because the differing delays between the different gamma inputs can destabilize the rhythm (Ermentrout & Kopell 1998), and the extra interneuron spikes brought about by the excitatory collaterals from the oscillating regions can decelerate the oscillation frequency in the target network. Reciprocal coupling between oscillators in the two hemispheres (Figure 6b) can alleviate the phase-shift problem and result in 0 phase-lag synchrony, provided that the conduction delays are short enough (<4–8 ms) and that synchrony is assessed over multiple cycles (Traub et al. 2002).
Long-range interneurons may be another candidate substrate for establishing gamma synchrony (Figure 7) (Buzsáki et al. 2004). These interneurons distribute their axon terminals over multiple regions and layers of the cortex and even across the hemispheres (Sik et al. 1994, Gulyás et al. 2003, Tomioka et al. 2005, Jinno et al. 2006). Importantly, distally projecting axons of long-range interneurons have several-fold thicker axons and larger diameter myelin sheaths than do pyramidal cells (Figure 7c,d), allowing for considerably faster axon conduction velocity (Jinno et al. 2006). In the I-I gamma model, replacing just 10–20% of the basket synapses with synapses of fast-conducting long-range interneurons could achieve global-phase synchrony (Figure 7a,b) (Buzsáki et al. 2004). An obvious advantage of the hybrid basket, long-range interneuron network is that synchrony among local and distributed cell assemblies can be tuned selectively by differentially targeting the two interneuron types.
Figure 7.
Coupling of gamma oscillators by long-range interneurons. (a) Oscillations in a network with locally connected interneurons. The network is essentially asynchronous. (Upper panel) Spike raster of 4000 neurons; (lower panel) the population firing rate. (b) Oscillations in a network with local interneurons (B) and long-range interneurons (LR; power-law connectivity). Note clear oscillatory rhythm. (c) Cross-section of the axon of a long-range CA1 GABAergic interneuron projecting toward the subiculum/entorhinal cortex. In comparison, neighboring axons of pyramidal cells are also shown (d). Reproduced from Buzsáki et al. (2004) (a,b) and from Jinno et al. (2006) (b,c).
Brain-Wide Synchronization of Gamma Oscillations by Slower Rhythms
Slower temporal coordination among gamma oscillators may be achieved by modulating the gamma power by the phase of slower rhythms (Figure 6). Compared with faster oscillators, slower oscillators involve more neurons in a larger volume (Von Stein & Sarnthein 2000) and are associated with larger membrane potential changes because in longer time windows spikes of many more upstream neurons can be integrated (Hasenstaub et al. 2005, Quilichini et al. 2010).
CFPA coupling between gamma and other rhythms within the same and different brain regions has been well documented, including modulation by theta (Figure 3c) (Buzsáki et al. 1983; Soltesz & Deschênes 1993; Bragin et al. 1995; Chrobak & Buzsáki 1998; Wang 2002; Mormann et al. 2005; Canolty et al. 2006; Demiralp et al. 2007; Tort et al. 2008, 2010; Colgin et al. 2009; Griesmayr et al. 2010), alpha (Palva et al. 2005, Cohen et al. 2009), spindle (Peyrache et al. 2011), delta (Lakatos et al. 2005), slow (Hasenstaub et al. 2005, Isomura et al. 2006), and ultraslow (Leopold et al. 2003) oscillations (Buzsáki 2006, Jensen & Colgin 2007, Schroeder & Lakatos 2009, Canolty & Knight 2010, Fell & Axmacher 2011). Because perisomatic basket cells contribute to both gamma and theta rhythms by firing theta-rhythm-paced bursts of spikes at gamma frequency, it has been hypothesized that fast-firing basket cells may play a key role in cross-frequency coupling (Buzsáki et al. 1983, Bragin et al. 1995). This is plausible because several other types of interneurons are often entrained by slower oscillations and they inhibit basket cells (Freund & Buzsáki 1996, Klausberger & Somogyi 2008). A prediction of this hypothesis is that temporal coordination by the basket cells also introduces a CFPP (i.e., phase-phase or n:m) coupling relationship between theta and gamma oscillations (Figure 5e). It may well be that CFPP mechanisms underlie CFPA coupling in most situations, but convincing demonstration of clear phase-phase coupling is hampered by the lack of adequate methods to quantify cross-frequency interactions and reliably track the true phase of nonharmonic oscillators (Tort et al. 2010, Belluscio et al. 2012).
The cross-frequency coupling of rhythms forms a multiscale timing mechanism (Buzsáki & Draguhn 2004, Jensen & Colgin 2007, Schroeder & Lakatos 2009, Canolty & Knight 2010, Fell & Axmacher 2011). Computational models have explored the potential theoretical advantages of such cross-frequency coupling (Lisman & Idiart 1995, Varela et al. 2001, Lisman 2005, Neymotin et al. 2011). The hierarchy of phase-amplitude-coupled rhythms is an effective mechanism for segmentation and linking of spike trains into cell assemblies (“letters”) and assembly sequences (neural “words”) (Buzsáki 2010).
Several studies have examined the relationship between cross-frequency coupling of gamma oscillations and cognitive processes. The magnitude of theta-gamma coupling in the hippocampal region varied with working memory load in patients implanted with depth electrodes (Axmacher et al. 2010). The strength of theta-gamma coupling in the hippocampus and striatum of the rat was affected by task demands (Tort et al. 2008, 2009). Similarly, the magnitude of CFPA coupling between a 4-Hz oscillation and gamma power in the pre-frontal cortex increased in the working memory phase of a choice task (Fujisawa & Buzsáki 2011). In an auditory task, gamma power in the frontal and temporal sites was phase-locked mainly to theta oscillations, whereas over occipital areas phase modulation was strongest by the alpha rhythm in a visual task (Voytek et al. 2010). Increased CFPP coupling between alpha and beta/gamma oscillations correlates with the difficulty of arithmetic mental tasks in the human magnetoencephalogram (Palva et al. 2005), whereas in another study working memory was correlated with theta-gamma synchrony (Griesmayr et al. 2010).
Cross-frequency coupling between slow rhythms and gamma oscillations can support a “reader-initiated” mechanism for information exchange (Sirota et al. 2008). For example, the hippocampal theta rhythm can entrain local gamma oscillations in multiple cortical areas. During its duty cycle, the theta output can phase align gamma oscillations that emerge in numerous activated neocortical local circuits (Figure 5f). In turn, the cell assemblies associated with the transient gamma bursts can address hippocampal networks in the accrual phase of the theta cycle, corresponding to the most sensitive, plastic state (Huerta & Lisman 1995), and can combine neocortical information into a condensed hippocampal representation.
MULTIPLE GAMMA RHYTHMS
Cross-frequency coupling can assist with the separation of gamma sub-bands (Tort et al. 2008, Colgin et al. 2009). In the hippocampal CA1 region, wavelet analysis identified three distinct gamma bands: (a) slow gamma (gammaS, 30–50 Hz) on the descending phase, (b) mid-frequency gamma (gammaM, 50–90 Hz) near the peak, and (c) fast gamma (gammaF, or epsilon band, 90–140 Hz) near the trough of the theta cycle (Figure 8) (Tort et al. 2010, Belluscio et al. 2012). Support for the different origins of gamma sub-bands is provided by their differential distribution in the different depths of the CA1 pyramidal layer and in different segments of the subiculum (Belluscio et al. 2012, Jackson et al. 2011). It is likely that the slow and mid-gamma band distinction applies to other brain regions as well (Kay 2003).
Figure 8.
Multiple gamma sub-bands. Wavelet power between 30 and 150 Hz as a function of waveform-based theta cycle phases. Note the different theta-phase preference of mid-frequency (M) (gammaM, 50–90 Hz, near theta peak) and slow (S) (gammaS, 30–50 Hz on the descending phase of theta) gamma oscillations. Note also the dominance of fast (F) (gammaF, or epsilon band, 90–150 Hz) at the trough of theta. After Belluscio et al. (2012).
WHEN GAMMA POWER IS NOT A RHYTHM.
A caveat in many studies is the lack of a disciplined and quantified analysis of gamma oscillations. To identify true gamma oscillations, appropriate statistics should be applied to demonstrate periodicity (Muresan et al. 2008, Burns et al. 2011, Ray & Maunsell 2011), and additional experiments are needed to distinguish between a power increase resulting from genuine oscillations and an increase resulting from greater spiking activity (Jarvis & Mitra 2001, Crone et al. 2006, Montgomery et al. 2008, Whittingstall & Logothetis 2009, Quilichini et al. 2010, Belluscio et al. 2012, Ray & Maunsell 2011). This is especially important for higher frequencies, such as the epsilon band, but spike-afterdepolarization and -hyperpolarization components can also contribute to the gamma band power. Although spike contamination to oscillatory power can be a nuisance, by using proper analytical methods, spike power can be exploited as a proxy for the assessment of neuronal outputs even in recordings of subdural local field potentials. Studying the temporal features of such high-frequency events may provide clues about oscillatory events that modulate them, even in situations when invasive unit recordings are not an option.
Previous works have distinguished only low and high gamma sub-bands (Csicsvari et al. 1999, Ray & Maunsell 2011) with the high sub-band defined as 60–140 Hz (Canolty et al. 2006, Colgin et al. 2009). Because power in the mid-gamma (50–90 Hz) and epsilon (90–150 Hz) bands is associated with different phases of theta oscillation (Figure 8) and is likely generated by different mechanisms (Belluscio et al. 2012), lumping these bands together is not justified on physiological grounds. Future studies, therefore, should distinguish sub-bands of gamma oscillations and carefully separate true and spurious gamma rhythms (see When Gamma Power is Not a Rhythm, sidebar above).
To conclude, although the word “rhythm” readily conjures up the picture of a clock, gamma rhythms occur in relatively short bursts and are quite variable in frequency, typically associated with stochastic firing of single neurons. The LFP gamma reflects largely the balancing act of excitation and inhibition, i.e., the active mode of a local circuit. Future studies on gamma oscillations will continue to inform us about the complex dynamics of brain circuits.
Supplementary Material
SUMMARY POINTS.
Transient cell assemblies may be organized into gamma-wave cycles.
Perisomatic inhibition by PV basket cells is essential for gamma oscillations.
Gamma oscillations are short-lived and emerge from the coordinated interactions of excitation and inhibition. Thus, LFP gamma can be used to identify active operations of local circuits.
Network gamma oscillations may coexist with highly irregular firing of pyramidal neurons.
Different sub-bands of gamma oscillations can coexist or occur in isolation.
Long-range interneurons may be critical for gamma-phase synchrony in different brain regions
Cross-frequency coupling is an effective mechanism for functionally linking active cortical circuits.
Genuine gamma oscillations should be distinguished from mere increases of gamma-band power and/or increased spiking activity.
Acknowledgments
We thank R. Canolty, J. Csicsvari, A. Engel, P. Fries, P. Jonas, N. Kopell, J. Maunsell, A. Peyrache, S. Ray, R.D. Traub, and M. Whittington for their comments on the manuscript as well as S. Bressler and W. Freeman for providing historical documents on gamma oscillations. Supported by the National Institutes of Health (NS034994; MH54671); the Human Frontier Science Program; the J.D. McDonnell Foundation; Zukunftskolleg, University of Konstanz, Germany (G.B.) and by the National Institutes of Health (R01 MH062349) and the Kavli Foundation (X.J.W.).
Glossary
- Gamma oscillations
synchronous network rhythm in 30–90 Hz that is minimally defined by an autocorrelation function and/or continuous Gabor transform
- LFP
local field potential
- GABA
gamma-aminobutyric acid
- I-I model
synchronization by mutual inhibition between interneurons
- E-I model
synchronization by an excitatory-inhibitory loop, primarily realized by the reciprocal interaction between pyramidal neurons and interneurons
- Resonance
phenomenon describing a neuron or a neural circuit that is maximally responsive to an oscillatory input at a preferred frequency
- Cross-frequency phase-amplitude (CFPA) coupling
phenomenon in which the amplitude of a faster oscillation is modulated by the phase of a slower rhythm
- Cross-frequency phase-phase coupling (CFPP)
phenomenon in which the phase of a faster oscillation is coupled to multiple phases of a slower rhythm
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
György Buzsáki, Email: Gyorgy.Buzsaki@nyumc.org.
Xiao-Jing Wang, Email: xjwang@yale.edu.
LITERATURE CITED
- Adrian E. Olfactory reactions in the brain of the hedgehog. J Physiol. 1942;100:459–73. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardid S, Wang X-J, Gomez-Cabrero D, Compte A. Reconciling coherent oscillation with modulation of irregular spiking activity in selective attention: gamma-range synchronization between sensory and executive cortical areas. J Neurosci. 2010;30(8):2856–70. doi: 10.1523/JNEUROSCI.4222-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107:3228–33. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32(2):423–35. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Uber das Elektroenkephalogramm des Menschen. Arch Pyschiatr Nervenkr. 1929;87:527–70. [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–96. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bibbig A, Traub R, Whittington M. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–54. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- Borgers C, Kopell N. Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput. 2003;15(3):509–38. doi: 10.1162/089976603321192059. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Brown P, Kupsch A, Magill PJ, Sharott A, Harnack D, Meissner W. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol. 2002;177(2):581–85. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- Brunel N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J Comput Neurosci. 2000;8(3):183–208. doi: 10.1023/a:1008925309027. [DOI] [PubMed] [Google Scholar]
- Brunel N, Hakim V. Fast global oscillations in networks of integrate-and-fire neurons with low firing rates. Neural Comput. 1999;11:1621–71. doi: 10.1162/089976699300016179. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–30. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA. 2011;108(27):11262–67. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl D, Harris K, Hormuzdi S, Monyer H, Buzsáki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–18. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368(6474):823–28. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Burns SP, Xing D, Shapley RM. Is gamma-band activity in the local field potential of v1 cortex a “clock” or filtered noise? J Neurosci. 2011;31(26):9658–64. doi: 10.1523/JNEUROSCI.0660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. New York: Oxford Univ. Press; 2006. [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68(3):362–85. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116(1):201–11. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5(4):504–10. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–29. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Geisler C, Henze DA, Wang X-J. Interneuron diversity series: circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–93. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–71. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Canolty R, Edwards E, Dalal S, Soltani M, Nagarajan S, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–28. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Ganguly K, Kennerley SW, Cadieu CF, Koepsell K, et al. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci USA. 2010;107(40):17356–61. doi: 10.1073/pnas.1008306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–15. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–67. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–98. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Fell J. Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. J Cogn Neurosci. 2009;21(2):390–402. doi: 10.1162/jocn.2008.21020. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–57. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Crone N, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–95. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise K, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–22. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Das NN, Gastaut H. Variations de l’activite electrique du cerveau, du coeur et des muscles squellettiques au cours de la meditation et de l’extase yogique. Electroencephalogr Clin Neurophysiol Suppl. 1955;6:211. [Google Scholar]
- Demiralp T, Bayraktaroglu Z, Lenz D, Junge S, Busch NA, et al. Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int J Psychophysiol. 2007;64(1):24–30. doi: 10.1016/j.ijpsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Paré D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol. 1999;81(4):1531–47. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- Economo MN, White JA. Membrane properties and the balance between excitation and inhibition control gamma-frequency oscillations arising from feedback inhibition. PLoS Comp Biol. 2011;8(1):e1002354. doi: 10.1371/journal.pcbi.1002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Engel AK, König P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–79. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Ermentrout G, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci USA. 1998;95:1259–64. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12(2):105–18. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24(43):9658–68. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–89. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mass Action in the Nervous System. New York: Academic; 1975. [Google Scholar]
- Freeman WJ. Definitions of state variables and state space for brain-computer interface: Part 1. Multiple hierarchical levels of brain function. Cogn Neurodyn. 2007;1:3–14. doi: 10.1007/s11571-006-9001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–63. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72(1):153–65. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Brunel N, Wang X-J. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. J Neurophysiol. 2005;94(6):4344–61. doi: 10.1152/jn.00510.2004. [DOI] [PubMed] [Google Scholar]
- Gibson J, Beierlein M, Connors B. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9(6):807–15. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, et al. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562(Pt. 1):131–47. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM. Synchronous oscillations in neuronal systems: mechanisms and functions. J Comput Neurosci. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–13. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel A, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–37. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Griesmayr B, Gruber WR, Klimesch W, Sauseng P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiol Learn Mem. 2010;93(2):208–15. doi: 10.1016/j.nlm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17(9):1861–72. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Miles R, Sík A, Tóth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366(6456):683–87. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62(2):171–89. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann E, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–37. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–19. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Halgren E, Babb TL, Crandall PH. Responses of human limbic neurons to induced changes in blood gases. Brain Res. 1977;132:43–63. doi: 10.1016/0006-8993(77)90705-3. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzski G. Organization of cell assemblies in the hippocampus. Nature. 2003;4(24):552–56. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick D. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–35. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–95. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15(5):1053–63. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52(5):871–82. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Jackson J, Goutagny R, Williams S. Fast and slow γ rhythms are intrinsically and independently generated in the subiculum. J Neurosci. 2011;31(34):12104–17. doi: 10.1523/JNEUROSCI.1370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–49. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Andrews HL. Brain potentials and voluntary muscle activity in man. J Neurophysiol. 1938;1:87–100. [Google Scholar]
- Jensen O, Colgin L. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci. 2007;11:267–69. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Theta/gamma networks with slow NMDA channels learn sequences and encode episodic memory: role of NMDA channels in recall. Learn Mem. 1996;3(2–3):264–78. doi: 10.1101/lm.3.2-3.264. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton L, Dalezios Y, Roberts J, et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2006;27:8790–804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S. Foundations of Cellular Neurophysiology. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- Kisvárday ZF, Beaulieu C, Eysel UT. Network of GABAergic large basket cells in cat visual cortex (area 18): implication for lateral disinhibition. J Comp Neurol. 1993;327(3):398–415. doi: 10.1002/cne.903270307. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB. Mechanisms of phase-locking and frequency control in pairs of coupled neural oscillators. In: Fielder B, editor. Handbook on Dynamical Systems. New York: Elsevier; 2002. pp. 3–54. [Google Scholar]
- Kopell N, Ermentrout G, Whittington M, Traub R. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–72. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94(3):1904–11. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–95. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Leger JF, Stern EA, Aertsen A, Heck D. Synaptic integration in rat frontal cortex shaped by network activity. J Neurophysiol. 2005;93:281–93. doi: 10.1152/jn.00067.2003. [DOI] [PubMed] [Google Scholar]
- Leopold D, Murayama Y, Logothetis N. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–33. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Leung LS. Nonlinear feedback model of neuronal populations in hippocampal CAl region. J Neurophysiol. 1982;47:845–68. doi: 10.1152/jn.1982.47.5.845. [DOI] [PubMed] [Google Scholar]
- Lewis D, Hashimoto T, Volk D. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci. 2003;23(6):2058–68. doi: 10.1523/JNEUROSCI.23-06-02058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–22. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–15. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96(26):15222–27. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton W, Sejnowski T. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol. 1991;66:1059–79. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275(5297):209–13. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268(5216):1503–6. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Mann E, Suckling J, Hájos N, Greenfield S, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–17. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–15. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early γ oscillations synchronize developing thalamus and cortex. Science. 2011;334:226–29. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsáki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14(9):1174–81. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104(36):14495–500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Sirota A, Buzsáki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–41. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kalra R, Aihara K, Robinson H. Recurrent synaptic input and the timing of gamma-frequency-modulated firing of pyramidal cells during neocortical “UP” states. J Neurosci. 2008;28:1871–81. doi: 10.1523/JNEUROSCI.3948-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, et al. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15(7):890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- Muresan R, Jurjut O, Moca V, Singer W, Nikolic D. The oscillation score: an efficient method for estimating oscillation strength in neuronal activity. J Neurophysiol. 2008;99:1333–53. doi: 10.1152/jn.00772.2007. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89(12):5670–74. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neymotin SA, Lazarewicz MT, Sherif M, Contreras D, Finkel LH, Lytton WW. Ketamine disrupts theta modulation of gamma in a computer model of hippocampus. J Neurosci. 2011;31(32):11733–43. doi: 10.1523/JNEUROSCI.0501-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Recce M. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Palva J, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–72. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsáki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–28. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Battaglia FP, Destexhe A. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc Natl Acad Sci USA. 2011;108:17207–12. doi: 10.1073/pnas.1103612108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529:205–13. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Deschánes M. Voltage-dependent 40-Hz oscillations in the rat reticular thalamic neurons in vivo. Neuroscience. 1992;51:245–58. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12:801–7. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini P, Sirota A, Buzsáki G. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J Neurosci. 2010;30(33):11128–42. doi: 10.1523/JNEUROSCI.1327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397(6718):430–33. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, König P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385(6612):157–61. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior TJ, Huxter JR, Allen K, O’Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28(9):2274–86. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15(10):6651–65. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265(5179):1722–24. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray C. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–86. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–97. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschênes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21(20):RC177. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tass P, Rosenblum MG, Weule J, Kurths J, Pikovsky A, et al. Detection of n:m phase locking from noisy data: application to magnetoencephalography. Phys Rev Lett. 1998;81:3291–94. [Google Scholar]
- Tateno T, Robinson HP. Integration of broadband conductance input in rat somatosensory cortical inhibitory interneurons: an inhibition-controlled switch between intrinsic and input-driven spiking in fast-spiking cells. J Neurophysiol. 2009;101(2):1056–72. doi: 10.1152/jn.91057.2008. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: Are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–32. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21(6):1587–600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106:20942–47. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 2008;105:20517–22. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R, Bibbig A, LeBeau F, Buhl E, Whittington M. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–78. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, et al. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13(1):1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Collins SB, Buzsáki G, Jefferys JGR. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996b;493:471–84. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996a;383(6601):621–24. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Tukker J, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type–specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–89. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vicente R, Gollo LL, Mirasso CR, Fischer I, Pipa G. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc Natl Acad Sci USA. 2008;105:17157–62. doi: 10.1073/pnas.0809353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–13. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J. Ionic basis for intrinsic 40 Hz neuronal oscillations. NeuroReport. 1993;5:221–24. doi: 10.1097/00001756-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Wang X-J. Fast burst firing and short-term synaptic plasticity: a model of neocortical chattering neurons. Neuroscience. 1999;89:347–62. doi: 10.1016/s0306-4522(98)00315-7. [DOI] [PubMed] [Google Scholar]
- Wang X-J. Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. J Neurophysiol. 2002;87:889–900. doi: 10.1152/jn.00135.2001. [DOI] [PubMed] [Google Scholar]
- Wang X-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–13. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J, Rinzel J. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput. 1992;4:84–97. [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron. 2009;64:281–89. doi: 10.1016/j.neuron.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–15. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38(3):315–36. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2009;106(9):3561–66. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
RELATED RESOURCES
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Engel A, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- György Buzsáki’s Web page. http://www.med.nyu.edu/buzsakilab/
- Uhlhaas P, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–36. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Xiao-Jing Wang’s Web page. http://wang.medicine.yale.edu/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.