Abstract
Amyotrophic lateral sclerosis (ALS) can result in the locked-in state (LIS), characterized by paralysis, and eventual respiratory failure, compensated by artificial ventilation,1 or the completely LIS (CLIS), with additional total paralysis of eye muscles. Brain–computer interfaces (BCIs) have been used to allow paralyzed people to regain basic communication,2 although current EEG-based BCIs have not succeeded with CLIS patients.3 We present Class IV case evidence to establish that communication in the CLIS is possible with a metabolic BCI based on near-infrared spectroscopy (NIRS).
Amyotrophic lateral sclerosis (ALS) can result in the locked-in state (LIS), characterized by paralysis, and eventual respiratory failure, compensated by artificial ventilation,1 or the completely LIS (CLIS), with additional total paralysis of eye muscles. Brain–computer interfaces (BCIs) have been used to allow paralyzed people to regain basic communication,2 although current EEG-based BCIs have not succeeded with CLIS patients.3 We present Class IV case evidence to establish that communication in the CLIS is possible with a metabolic BCI based on near-infrared spectroscopy (NIRS).
Case report.
In August 2012, a 67-year-old woman (video 1 on the Neurology® Web site at Neurology.org) was admitted to CERES, an institution for the care of apallic patients affiliated with Tübingen University, over a period of 4 weeks. The patient had been diagnosed with bulbar sporadic ALS in May 2007, as locked-in in 2009, and as completely locked in May 2010, based on the diagnosis of experienced neurologists. Her husband reported that she had lost the ability to communicate, which was last possible by using her eyes, approximately 27 months prior to admission (table e-1; e-Patient). She had been artificially ventilated since September 2007, fed through a percutaneous endoscopic gastrostomy tube since October 2007, and was in home care. No communication with eye movements, other muscles, or assistive communication devices was possible at the time of admission (score 0 of 48 on the ALS Functional Rating Scale–Revised)4 (video 2). Her cognitive functions were assessed by an extensive neurophysiologic examination based on event-related brain potentials (e-Assessment; figure e-1), suggesting intact cognitive functioning. Before this study, training using a similar paradigm with an EEG-based BCI did not result in stable successful communication.5 A second 2-week admission (second period) and a third 4-week period at home took place in 2013. The study was approved by the ethics committee of the Medical Faculty of Tübingen University and informed consent was obtained from the legal guardian.
Methods.
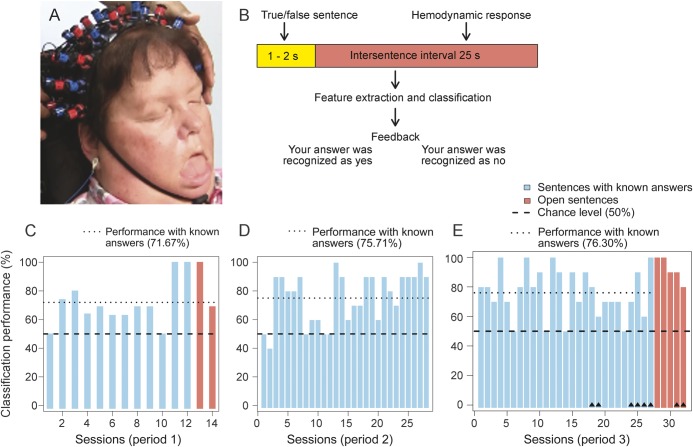
Functional activations of the cerebral cortex to auditory processing of correct or incorrect statements were assessed with NIRS, which can be applied at the bedside, is comparatively low cost, and detects slow changes in hemodynamic brain responses (figure 1A). We employed blocks containing 10–20 auditorily presented sentences (factually true or false), with intersentence intervals (ISI) of 25 seconds, needed for the corresponding hemodynamic response to develop. There were sentences with known answers (e.g., “You were born in Hamburg”), and sentences with unknown answers, termed open sentences (e.g., “You want to be moved from left to right”). The patient was instructed to think “yes” or “no” after each sentence. Sentences were recorded with the voice of the husband (the primary caregiver), but in some sessions a person unknown to the patient spelled the questions to control for any bias. Feedback was given after the ISI: “Your answer was recognized as Yes,” or “Your answer was recognized as No” (figure 1B) depending on online classification (videos 2–4). We preprocessed the time-series data of the measured changes, extracted segments corresponding to the ISI of 25 seconds for each sentence (data point), and employed binary classification of yes vs no brain responses using support vector machines (e-SVM).
Figure 1. Communication with a completely locked-in patient using bedside near-infrared spectroscopy.
(A) Brain oxygenation and deoxygenation changes in hemoglobin were recorded with an ETG-4000 Optical Topography System (Hitachi Medical Co., Tokyo, Japan) covering the sensorimotor cortex and temporal areas. The figure shows the patient with the near-infrared spectroscopy sensors and receivers attached to her head. (B) The structure of sentences, as presented by the near-infrared spectroscopy–based brain–computer interface. (C) Classification performance based on the questions with known answers in period 1, which consisted of 12 sessions spread over 14 days with 105 true and 95 false sentences. Sessions with questions requiring a known answer are blue. Sessions 13 and 14 contained 20 open questions that are orange. (D) Classification performance of each session with known answers in period 2 (28 sessions distributed across 8 days with 140 true and 140 false sentences). (E) Classification performance of each session with known answers in period 3 (27 sessions spread over 2 weeks with 135 true and 135 false sentences). Sessions 28 to 32 contained 50 open questions. Sessions marked with a small black triangle consisted of sentences recorded with an unfamiliar voice; all other sessions were recorded with the voice of the husband. The classification results of the open sentences in parts 1 and 3 were derived from the assumed correct answers the husband had noted before the sessions. The session sequence for sessions with known answers follows the numbers below the respective bars. The sessions with open answers were interspersed in time between the sessions with known answers in period 3 but were moved to the end of the figure to underscore their importance. The chance level is marked by a black horizontal line; the average performance level is marked in blue.
Results.
Classification of the patient's NIRS data from the 25-second period after sentence presentation, when the patient had to think the answer, yielded significantly different deoxygenation levels for the yes and no answers across all 3 training periods (figure e-2). The percentage of correct answers was 71.67% (t11 = 4.73), 75.71% (t27 = 7.38), and 76.30% (t26 = 8.43) for the yes and no answers across the 3 periods, all significantly different from chance, p < 0.01 (figure 1, C–E, table e-2). The positive predictive value was 80.9% and the negative predictive value was 72.9%. The classification of the sentence-induced change in deoxygenation prior to the ISI yielded only chance-level results, suggesting that brain changes related to sentence presentation alone did not create the yes/no differentiation. Sessions 8 and 12 of the third period were separated by 11 days and contained the same questions with known answers. Both sessions resulted in 100% correct classification. Sessions 28 and 29, also separated by 11 days, contained the same open questions and resulted in 100% correct answers (e-Replication).
Discussion.
Using NIRS, we obtained significantly above chance-level answers in a CLIS patient over an extended period of time. The overall performance of 76.30% in the last training period and the 100% correct performance in some sessions suggest that this is a viable method to reestablish communication in CLIS patients with a high test-retest stability. To generalize from a single case to all CLIS patients is not possible, but the accessibility and simplicity of NIRS methodology and the use of standard free access algorithms such as SVM should encourage and facilitate exact replications. Metabolic BCIs might thus break the unbearable “silence” of CLIS.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Prof. Kenji Kansaku from the National Rehabilitation Center for Persons with Disabilities, Tokorozawa, Japan, for providing electrodes, and Dr. Bin Xia and Katja Kraemer for help in preparing the experiment.
Footnotes
Editorial, page 1852
Supplemental data at Neurology.org
Author contributions: G. Gallegos-Ayala and N. Birbaumer designed the brain–computer interface and performed the training. A. Furdea wrote the program for the assessment and analysis. K. Takano contributed to training, assessment, and analysis in the second part of the study. C.A. Ruf organized the training sessions and edited the report. G. Gallegos-Ayala, H. Flor, and N. Birbaumer wrote the report.
Study funding: Deutsche Forschungsgemeinschaft (DFG, Koselleck project), Bundesministerium für Bildung und Forschung (01GQ0831, 16SV5838K), Brain Products, Gilching, Germany, and the Baden-Württemberg Stiftung. The Article Processing Charge was paid by UKT Tuebingen (Prof. Birbaumer).
Disclosure: G. Gallegos-Ayala is supported by The National Secretary of Higher Education, Science, Technology, and Innovation (SENESCYT), Ecuador. A. Furdea, K. Takano, C. Ruf, and H. Flor report no disclosures relevant to the manuscript. N. Birbaumer is supported by Brain Products, Gilching, Germany, the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung, and the Baden-Württemberg Stiftung. Go to Neurology.org for full disclosures.
References
- 1.Hayashi H, Oppenheimer EA. ALS patients on TPPV: totally locked-in state, neurologic findings and ethical implications. Neurology 2003;61:135–137 [DOI] [PubMed] [Google Scholar]
- 2.Birbaumer N, Ghanayim N, Hinterberger T, et al. A spelling device for the paralysed. Nature 1999;398:297–298 [DOI] [PubMed] [Google Scholar]
- 3.Ramos Murguialday A, Hill J, Bensch M, et al. Transition from the locked in to the completely locked-in state: a physiological analysis. Clin Neurophysiol 2011;122:925–933 [DOI] [PubMed] [Google Scholar]
- 4.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21 [DOI] [PubMed] [Google Scholar]
- 5.De Massari D, Ruf CA, Furdea A, et al. Brain communication in the locked-in state. Brain 2013;136:1989–2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.