Abstract
Within blood vessels, endothelial cell–cell and cell–matrix adhesions are crucial to preserve barrier function, and these adhesions are tightly controlled during vascular development, angiogenesis, and transendothelial migration of inflammatory cells. Endothelial cellular signaling that occurs via the family of Rho GTPases coordinates these cell adhesion structures through cytoskeletal remodelling. In turn, Rho GTPases are regulated by GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs). To understand how endothelial cells initiate changes in the activity of Rho GTPases, and thereby regulate cell adhesion, we will discuss the role of Rho GAPs and GEFs in vascular biology. Many potentially important Rho regulators have not been studied in detail in endothelial cells. We therefore will first overview which GAPs and GEFs are highly expressed in endothelium, based on comparative gene expression analysis of human endothelial cells compared with other tissue cell types. Subsequently, we discuss the relevance of Rho GAPs and GEFs for endothelial cell adhesion in vascular homeostasis and disease.
Keywords: Cdc42, Rac, Rho GTPase, VE-cadherin, angiogenesis, inflammation, integrin
Introduction
The endothelial monolayer covers the luminal side of blood and lymphatic vessels and functions as a physical barrier that preserves vascular integrity. Endothelial cells make adhesive contacts with the extracellular matrix (ECM) as well as homotypic adhesions between neighboring cells. Throughout embryonic development, strictly regulated formation and breakdown of adhesion complexes determines tissue shapes and boundaries.1-4 In adults, these adhesions are essential to regulate and maintain the barrier function of the endothelium. Moreover, the activity and content of endothelial cell adhesion structures are highly regulated during angiogenesis and inflammatory responses.5-8
Cell–matrix and cell–cell adhesion complexes
Endothelial cell–matrix interactions, in particular those mediated by integrins, are crucial for vascular development and angiogenesis as they mediate adhesion to, and migration through, the vascular ECM.5 Besides their structural anchoring role, integrins modulate angiogenic growth factor- and inflammatory cytokine-induced signaling pathways through increased receptor clustering and recruitment of signaling molecules that control cell behavior.9,10 Changes in the composition, deposition, or rigidity of the vascular ECM are transmitted through integrin-based complexes to alter cellular signaling pathways,11 and when such changes are prolonged they cause permanent perturbation of endothelial functions, as occurs during age-related cardiovascular disease or chronic inflammation.
The vascular barrier, required to control leakage of solutes and traffic of circulating cells, is maintained by endothelial adherens and tight junctions, which critically depend on cell–cell adhesion mediated by the VE-cadherin complex. Cell–cell adhesions are destabilized by vascular permeability factors like vascular endothelial growth factor (VEGF), thrombin, and tumor necrosis factor α (TNFα), or by transmigrating leukocytes that stimulate signaling pathways, which transiently destabilize the VE-cadherin complex.6,8,12 When the formation of endothelial cell–cell adhesion structures is impaired, vascular permeability increases, which contributes to the pathogenesis of chronic inflammation, edema, or acute lung injury. Regulation of cell–cell adhesions also occurs at the onset of angiogenesis; angiogenic growth factors destabilize endothelial cell–cell junctions and thereby initiate sprouting from pre-existing vessels. In contrast, at later stages when new vessels are formed, cell–cell adhesions need to tighten to re-establish vessel integrity.7,13
Despite the spatially distinct locations of cell–ECM vs. cell–cell adhesions in endothelial cells, there is intimate crosstalk between integrins and cadherins.14 The integrin–cadherin crosstalk largely depends on their shared signaling pathways that control adhesion, in which Rho GTPases play a central role, as well as on the organization of the actomyosin cytoskeleton that tightly associates with both cell–ECM adhesions and cell–cell junctions.15-20 This is also clear during mechanotransduction, when integrins transmit mechanical signals from stiffening ECM toward the actomyosin cytoskeleton.21 This, in turn, destabilizes cell–cell adhesions, and increases permeability of endothelial monolayers.22,23 Moreover, cell–matrix and cell–cell adhesions also cluster various signaling molecules that trigger or enhance signaling by small GTPases that control the actomyosin cytoskeleton.24-28
Regulation of Rho GTPases in endothelial cell adhesion
In this review, we focus on the regulation of Rho GTPases. These are members of the Ras superfamily of small GTPases that act as molecular switches controlling the actomyosin cytoskeleton and cell adhesion.29,30 The regulation of Rap GTPase signaling and its role in endothelial cell adhesion will be discussed in detail elsewhere (Pannekoek et al., Cell Adhesion and Migration, this issue). Small GTPases cycle between active GTP-bound and inactive GDP-bound conformations. This cycle is regulated by guanine nucleotide exchange factors (GEFs) that activate, and GTPase activating proteins (GAPs) that inactivate Rho GTPases.31 Rho GTPases, comprising 20 family members, transduce signals from receptors on the plasma membrane to intracellular effector proteins. The best-studied Rho GTPases regulating cell adhesion are Rho, Rac, and Cdc42. Differences in the spatiotemporal activation of these Rho GTPases is particularly important as they locally drive the formation of actin fibers, stimulate actomyosin contraction, or promote polymerization of branched actin in membrane protrusions through effector proteins such as Rho-associated kinase (ROCK), Diaphanous-related formins (Dia), or Arp2/3, respectively.32,33 In endothelial cells, vascular permeability factors, for instance thrombin or TNFα, regulate RhoA to enhance actomyosin contraction and remodeling of cell–cell adhesions, supporting immune cells to cross the endothelium, and reach inflamed tissue. Another example of growth factor-induced regulation of Rho GTPases is the activation of Rac1 by VEGF and basic fibroblast growth factor (bFGF) to generate protrusions in leading endothelial tip cells that guide newly formed sprouts during angiogenesis.34-36 Many other studies show that RhoA-mediated signaling stimulates actomyosin contractility and weakens the endothelial barrier function, whereas increased Rac1-mediated signaling improves barrier function (reviewed in detail in refs. 34 and 36) (Fig. 1). In general, during angiogenesis, activation of RhoA-mediated actomyosin contractility underlies the onset of sprout formation from pre-existing vessels, whereas Rac1 and Cdc42 signaling contribute to migration and invasion of sprouts by stimulating membrane protrusions (Fig. 1).35
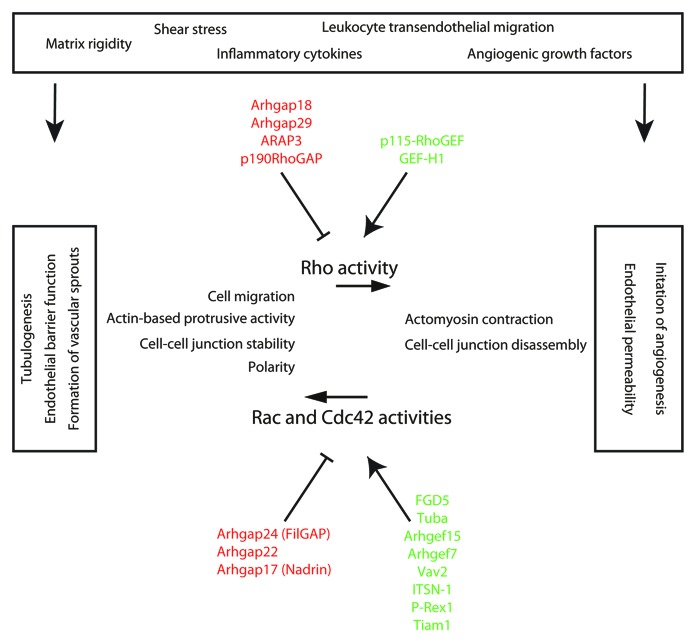
Figure 1. Endothelial functions regulated by Rho GAP and GEF activities. This simplified scheme summarizes the endothelial function of a selection of key GAPs (highlighted in red) and GEFs (highlighted in green) that control the activity of Rho, Rac, and Cdc42 in response to external cues, such as increased extracellular matrix rigidity, changes in shear stress, inflammatory cytokines, angiogenic growth factors, or signals that derive from leukocyte transendothelial migration events. Upon activation, the indicated Rho GAPs and GEFs regulate RhoA signaling toward actomyosin contractility and cell–cell junction disassembly, which underlies the onset of angiogenesis from existing vessels and induction of endothelial permeability. Activated GAPs and GEFs of Rac/Cdc42 regulate their signaling toward actin-based protrusions during cell migration or cell–cell junction stabilization, which is important for endothelial barrier function and formation of vascular sprouts. The control of activity of Cdc42 regulates cellular polarity and lumen formation during tubulogenesis.
Given the fact that Rho GTPase signaling, and especially their local and time-dependent activation-inactivation cycle, is crucial for endothelial cells to maintain vascular barrier function and control dynamic cell behavior during angiogenesis and inflammation, it is surprising that we know so little about the specific role of their regulators, the GAPs and GEFs, in endothelial cell adhesion.
Endothelial GAPs and GEFs
The Rho GEF family, also called the Dbl family, consists of approximately 80 members. The GEF proteins usually contain a Dbl homology (DH) domain and a pleckstrin homology (PH) domain that is important for plasma membrane localization, where the activation of Rho GTPases takes place. GEF DH domains interact with Rho GTPase switch regions, modifying their conformation, resulting in release of GDP. This allows free GTP to bind the GTPase, inducing the switch to the active conformation. The Rho GAPs, comprising approximately 65 members, enhance the intrinsic GTP hydrolysis activity of Rho GTPases and stabilize the GDP-bound switch regions.31,37
The known functions of several endothelial Rho GAPs and GEFs fit in a simplified scheme where they control activation of Rac and Cdc42 GTPases to establish actin-based protrusions during cell migration, cell–cell junction stabilization, or cell polarization in response to external cues (Fig. 1). These processes are particularly important for the formation of new vascular sprouts, during endothelial barrier formation or tube/lumen formation of developing vessels, respectively. Many of the known RhoA regulators control RhoA activity toward actomyosin contraction and concomitant cell–cell junction destabilization, which underlies the onset of angiogenesis and is the major cause for endothelial permeability induction. Because of their overlapping functions, the question arises whether Rho GAPs and GEFs have non-redundant roles during regulation of endothelial cell adhesion. Here, we will provide a complete overview of all the Rho GAPs and GEFs that are highly expressed in endothelial cells, as well as ubiquitously expressed GAPs and GEFs with known endothelial functions, followed by a discussion on the individual relevance of these regulators for endothelial cell adhesion in vascular homeostasis and disease. This overview shows that Rho GAPs and GEFs contain many overlapping functions, but clearly also have distinct and local functions in endothelial cells.
Identifying Rho GAPs and GEFs that are highly expressed in endothelium
Many potentially important Rho regulators have not yet been studied in endothelial cells in detail. To uncover which GAPs and GEFs are highly expressed in endothelium, and therefore, may be relevant for regulation of endothelial cell adhesion, we performed comparative gene expression analysis using genome-wide mRNA profiles of human umbilical vein endothelial cells (HUVECs) that were deposited in the NCBI-GEO database (http://www.ncbi.nlm.nih.gov/gds). Expression levels of Rho GAPs and GEFs were determined based on 17 Affymetrix arrays derived from nine independent and well-defined expression studies available in the public domain. The absolute expression values were related to their levels in many other “normal” human tissues. For this, we used the Roth-504 Affymetrix data set (“The Human Body Index”), the largest normal human tissue data set in the public domain. It contains 504 high-quality genome-wide transcription profiles representing 95 different human tissues, including low-passage, non-stimulated, non-recombinant HUVECs (see Table S1 and legends for details concerning the complete gene expression analysis and calculations). The comparison revealed that 17 different Rho GAPs and 20 Rho GEFs are highly expressed in HUVECs (Fig. 2), at levels over 5-fold increased compared with other tissues. Because the results suggest that these highly expressed GAPs and GEFs are important, we will first discuss their role in endothelial cell adhesion. Obviously, regulation of Rho GTPase signaling and adhesion is not only a matter of transcriptional control, and we will therefore also discuss the role of other Rho GAPs and GEFs that are ubiquitously expressed and described to regulate important endothelial functions.
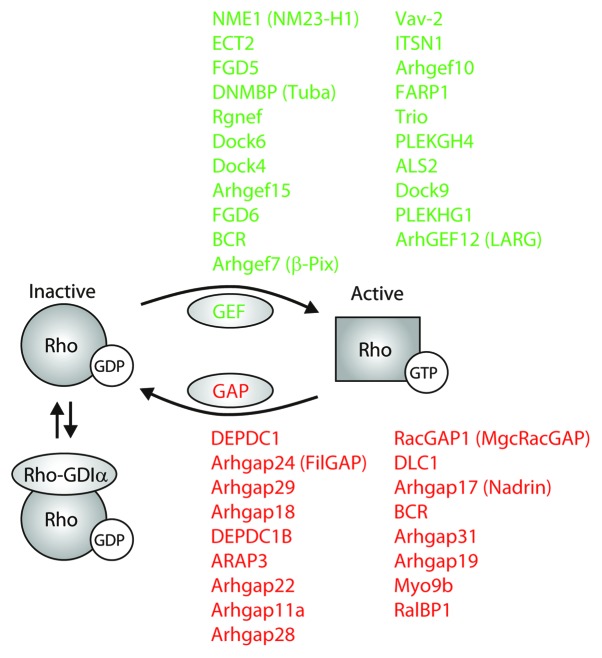
Figure 2. Regulation of Rho GTPase activity by endothelial-enriched GEFs and GAPs. A schematic representation of Rho GTPase regulation by GEFs (green), GAPs (red), and GDIs. Listed are GEFs and GAPs that are above 5-fold higher expressed in endothelial cells compared with cells from other tissues. Rho-GDIα is the only GDI that is strongly expressed in HUVECs.
The roles of Rho GTPase GAPs in endothelium
Highly expressed GAPs
Arhgap24 (FilGAP) and Arhgap22
Among the highest expressed Rho GAPs (Table 1) are Arhgap24 (also known as FilGAP, over 25-fold overexpressed in HUVECs as compared with other tissues) and its close relative Arhgap22 (~11-fold upregulation), both designated as GAPs for Rac1 that are involved in Rho–Rac antagonistic signaling.38 The third member of this protein family, Arhgap25, is never expressed in HUVECs (Table S1). Activation of Rho GTPases at membrane protrusions or at cell–cell adhesions strongly depends on the activity of other Rho GTPases as they suppress each other’s signaling capacity through downstream mediators including ROS, p190RhoGAP, and FilGAP family members.39-41 RhoA-mediated activation of ROCK induces phosphorylation of FilGAP at membrane protrusions, and stimulates its Rac GAP activity. The localization of FilGAP is dependent on its interaction with the cytoskeleton and integrin-associated protein filamin A, which controls cell protrusion activity and cell spreading on ECM-coated surfaces.41,42 Importantly, the complex of FilGAP with filamin A forms a cytoskeletal mechanosensing unit: when mechanical-exerted forces or myosin-dependent contractile forces increase tension on filamin A, its interaction with FilGAP and local FilGAP activity is lost, whereas the interaction with integrins increases.43 This may, in part, explain the force-dependent activation of Rac at integrin-based adhesions that depends on the relocation of FilGAP by filamin A.44 A slightly truncated isoform of FilGAP that lacks the PH domain, called RC-GAP73 or p73RhoGAP, is also expressed in endothelial cells. This isoform is recruited to both integrin- and cadherin-based adhesion complexes, regulates the actomyosin cytoskeleton in a GAP-dependent manner,45 and is important for angiogenesis, based on studies in 3D culture models and in vivo.46 Arhgap22 was described to regulate tumor cell invasive behavior,47 and a PH-domain truncated isoform of Arhgap22, p68RacGAP, was shown to be specifically expressed in endothelial cells, where it controls tube formation on matrigel.48 Thus, inhibition of Rac1 activity by FilGAP or Arhgap22 controls endothelial integrin and cadherin adhesions. This could be involved in mechanotransduction pathways that are relevant during age-related cardiovascular disease when stiffening of the vascular ECM increases tension on integrins, and during inflammation when permeability factors or leukocytes regulate tension on VE-cadherin-based adhesions.22,23,49-51
Table 1. Rho GAPs.
| Gene Name | Alternative names | Relative expression in HUVEC | Rho GTPase targets | Function |
|---|---|---|---|---|
| DEPDC1 | DEP.8; SDP35; DEPDC1A; DEPDC1-V2 | 40.60 | ||
| ARHGAP24 | FilGAP, RC-GAP72, RCGAP72, p73, p73RhoGAP | 25.86 | Rac1, Cdc42 | Regulates endothelial cell migration, tubulogenesis and angiogenesis.46 |
| ARHGAP29 | PARG1, RP11–255E17.1 | 24.97 | RhoA | Regulates endothelial adhesion, spreading and polarization during vascular lumen formation and endothelial barrier function 53, 54. |
| ARHGAP18 | MacGAP, SENEX, bA307O14.2 | 21.52 | RhoA | Role in endothelial capillary tube formation and barrier protective function.56 |
| DEPDC1B | XTP1; BRCC3 | 20.19 | ||
| ARAP3 | CENTD3, DRAG1 | 16.69 | RhoA | Adhesion and migration of endothelial cells during (lymph)angiogenesis.59,63,64 |
| ARHGAP22 | RhoGAP2, RhoGap22, p68RacGAP | 11.16 | Rac1 | Role in endothelial capillary tube formation.48 |
| ARHGAP11a | GAP (1–12); rho GTPase-activating protein 11A; rho-type GTPase-activating protein 11A | 10.73 | ||
| ARHGAP28 | Rho GTPase activating protein 28 | 10.14 | ||
| RACGAP1 | CYK4, HsCYK-4, ID-GAP, MgcRacGAP | 9.97 | Rac1, Cdc42 | |
| DLC1 | Deleted in Liver Cancer 1, ARHGAP7, HP, STARD12, p122-RhoGAP | 7.54 | RhoA, RhoB, RhoC, Cdc42 | |
| ARHGAP17 | PP367; RICH1; WBP15; MST066; MST110; NADRIN; PP4534; RICH1B; MSTP038; MSTP066; MSTP110 | 7.39 | Rac1, Cdc42, RhoA | |
| BCR | 7.39 | RhoA, Rac, Cdc42 | ||
| ARHGAP31 | AOS1, CDGAP | 6.47 | Rac1, Cdc42 | |
| ARHGAP19 | rho GTPase-activating protein 19 | 6.40 | RhoA | |
| MYO9B | myosin-Ixb, CELIAC4, MYR5 | 5.87 | RhoA | |
| RALBP1 | RIP1, RLIP1, RLIP76 | 5.20 | Rac1, Cdc42 | Role in tumor-associated angiogenesis.98 |
Rho GAP and GEF genes with high and robust mRNA expression in HUVECs. These tables show the highest expressed GAPs (Table 1) and GEFs (Table 2) in HUVECs. For each gene, the expression value of the highest expressed transcript is shown in the 3rd column, which is relative to the average expression level in Roth-504. mRNA expression in HUVECs was determined by analysis of Affymetrix U133 Plus 2.0 mRNA genome-wide expression profiles in the public domain. All studies at the NCBI Gene Expression Omnibus (GEO) website (http://www.ncbi.nlm.nih.gov/gds/) on low-passage, non-stimulated, non-recombinant HUVECs with data normalization using the MAS5.0 algorithm (Affymetrix Inc., Santa Barbara, CA) were included. A total of 9 different studies comprising 17 arrays were used for expression analysis, an additional 2 studies comprising 12 arrays were used as validation. Relative gene expression was determined by comparing the average expression over all 17 HUVEC sets with the average expression in all 504 arrays representing 95 different normal human tissues in the Roth “Human Body Index,” the largest study on normal human mRNA expression (NCBI GEO: GSE7307). Only Rho regulatory genes with high expression in 14 or more of the 17 data sets analyzed are shown. The probe-sets shown for each gene detected the highest and widest expression, but all valid probe-sets were analyzed and used for expression analysis. The TranscriptView visualization tool (http://r2.amc.nl) was used to validate probes: probes had to target a unique, anti-sense position in an exon of the target gene. Gene and splice variant specificity was verified by NCBI GENE and BLAST analysis. Full information on the gene expression calculations, probe validations, and GEO studies can be found in Table S1. The Rho GTPases that are targeted by these GAPs and GEFs are indicated in column 4. Column 5 summarizes the known endothelial functions of each Rho regulator including references.
Arhgap29
Arhgap29 is a Rho GAP that is highly expressed in HUVECs (Table 1), and was previously described to be strongly expressed in the vasculature of zebrafish.52 Arhgap29 is a binding partner of Rasip1, an endothelial restricted effector protein of the small GTPase Rap1.53,54 Rasip1-null mutant mice have severely hampered early vascular development; they show edema formation and their vascular lumens do not form properly. This is a consequence of a failure of angioblasts to adhere to ECM and to maintain cell–cell adhesions.54 Depletion of Arhgap29 from human endothelial cells by siRNAs increased RhoA-ROCK-mediated actomyosin activity and lowered Cdc42 and Rac1 activities. As a consequence, the organization of the actin cytoskeleton and integrin-based adhesions, particularly those based on β1 integrins,54 as well as adherens junctions, were strongly perturbed.53 However, loss of Arhgap29 did not induce an increase in biochemically detectable RhoA activity in cell lysates, which might suggest that Arhgap29 is important for local or temporal inhibition of RhoA. In summary, the strong expression of Arhgap29 in endothelial cells is thus important to locally inhibit RhoA-dependent signaling to maintain integrin- and cadherin-based adhesions during vascular development.
Arhgap18
The highly expressed Arhgap18 (21-fold higher than in other tissues) is a RhoA-specific GAP that was recently found to be expressed in HUVECs.55,56 Arhgap18 suppresses RhoA-dependent actin remodeling induced by integrin-mediated adhesion.55 This RhoGAP was described to protect endothelial monolayer integrity during inflammatory responses. Moreover, overexpression of Arhgap18 perturbed HUVEC tube formation on matrigel, presumably by inhibiting RhoA activity.56 To date no other relevant information on Arhgap18 in the context of vascular adhesion is published.
ARAP3
We find ARAP3 highly expressed in human endothelium, over 16-fold compared with other tissue. ARAP3 was recently also reported to be strongly endothelial enriched in mouse vascular development.57 ARAP3 is an effector of PI3K and has GAP activity for both RhoA and Arf6 GTPases.58 The GAP function of ARAP3 is important for actin cytoskeleton organization and focal adhesion distribution, and siRNA-mediated loss of expression showed that ARAP3 is required for platelet-derived growth factor (PDGF)-induced membrane ruffling.59 ARAP3 is also expressed in other cell types, including leukocytes, regulating integrin-dependent adhesion through inhibiting RhoA activity.60-62 Most importantly, mouse studies based on deficiency of ARAP3 or transgenic expression of mutated ARAP3 showed that its expression in endothelial cells is crucial for angiogenesis during development, and depends on phosphoinositide 3-kinase (PI3K)-mediated recruitment to the plasma membrane.63 ARAP3 was recently identified as a strongly downregulated gene in mouse and zebrafish models for lymphatic vascular disorders, and shown to be crucial for lymphangiogenesis by mediating the response of lymphatic endothelial cells to VEGF-C.64 For future research, it would be of interest to study to what extent these defects in angiogenesis are depending on the regulation of either Rho or Arf6 GTPases downstream of ARAP3.
RacGAP1 and the Rho GEF ECT2
The expression of RacGAP1, also known as CYK4 and MgcRacGAP, is 10-fold higher in HUVECs compared with other cells, and is best known for its Rac-suppressive function as part of the centralspindlin complex that stimulates actomyosin-driven contraction at the cleavage furrow during the final steps of cytokinesis. This occurs in conjunction with recruitment of the RhoA GEF ECT2,65-68 the expression of which is also high in HUVECs (see Table 2). Besides their role in cytokinesis, these Rho GTPase regulators have important extra-mitotic functions. The centralspindlin complex interacts with α-catenin at adherens junctions, thereby recruiting ECT2, to support local RhoA-driven myosin activation and cell–cell junction stabilization in highly polarized epithelial cells.69 Moreover, the inhibition of Rac1 activity by RacGAP1 is important to control cell adhesion and spreading,70 and recruitment of RacGAP1 by the fibronectin-binding integrin α5β1 at protruding membranes greatly enhances the directionality of migration and invasion of tumor cells.71,72 Thus far, not much evidence for endothelial RacGAP1 and ECT2 in vascular biology has been published.
Table 2. Rho GEFs.
| Gene Name | Alternative names | Relative expression in HUVEC | Rho GTPase targets | Function |
|---|---|---|---|---|
| NME1 | NB; AWD; NBS; GAAD; NDKA; NM23; NDPKA; NDPK-A; NM23-H1 | 21.68 | ||
| ECT2 | ARHGEF31 | 19.95 | RhoA, Rac1, Cdc42 | |
| FGD5 | ZFYVE23 | 16.42 | Cdc42 | Involved in VEGF-induced endothelial cell adhesion signaling, cell-cell junction stabilization and associated with vascular development 52, 118–120. |
| DNMBP | TUBA; ARHGEF36; RP11–114F7.3 | 12.63 | Cdc42 | |
| RGNEF | RIP2; p190RHOGEF | 12.08 | RhoA | |
| DOCK6 | AOS2, ZIR1 | 8.45 | Rac1, Cdc42 | |
| DOCK4 | WUGSC:H_GS034D21.1 | 8.36 | Rac1, Rac2 | |
| ARHGEF15 | E5; ARGEF15; Ephexin5; Vsm-RhoGEF | 8.29 | Cdc42, RhoA | Mediator of VEGF-induced retinal angiogenesis.57,134 |
| FGD6 | FYVE, RhoGEF and PH domain-containing protein 6 | 7.49 | ||
| BCR | 7.39 | RhoA | ||
| ARHGEF7 | β-pix, RP11–494P5.1, COOL-1, COOL1, Nbla10314, P50, P50BP, P85, P85COOL1, P85SPR, PAK3, PIXB | 7.32 | Rac1, Cdc42 | Mediator of VEGF-induced permeability.138 |
| VAV2 | vav 2 guanine nucleotide exchange factor | 7.23 | Rac1, RhoA, RhoG, Cdc42 | Mediator of growth factor- or mechanotransduction-induced signaling in control of cell-cell adhesion, migration and angiogenesis.141-143,145,147,148 |
| ITSN1 | ITSN, SH3D1A, SH3P17 | 7.18 | Cdc42 | Pro-survival mediator and promotes vascular integrity.150,151 |
| ARHGEF10 | GEF10 | 7.00 | RhoA, RhoB, RhoC | |
| FARP1 | RP11–111L24.1, CDEP, PLEKHC2, PPP1R75 | 6.43 | Rac1 | |
| TRIO | ARHGEF23, tga | 6.43 | Rac1, RhoG, RhoA | Regulator of endothelial docking structures during transmigration of leukocytes.160 |
| PLEKHG4 | ARHGEF44, PRTPHN1, SCA4 | 6.26 | Rac1, Cdc42, RhoA | |
| ALS2 | ALSJ; PLSJ; IAHSP; ALS2CR6 | 6.25 | Rac1 | |
| DOCK9 | RP11–155N3.2, ZIZ1, ZIZIMIN1 | 5.93 | Cdc42 | |
| PLEKHG1 | D10Ertd733e, Gm521, mKIAA1209 | 5.53 | ||
| ARHGEF12 | Larg, RO2792 | 5.08 | RhoA, RhoC | Mediator of Semaphorin 4D and S1P-induced endothelial signaling.165,166 |
Rho GAP and GEF genes with high and robust mRNA expression in HUVECs. These tables show the highest expressed GAPs (Table 1) and GEFs (Table 2) in HUVECs. For each gene, the expression value of the highest expressed transcript is shown in the 3rd column, which is relative to the average expression level in Roth-504. mRNA expression in HUVECs was determined by analysis of Affymetrix U133 Plus 2.0 mRNA genome-wide expression profiles in the public domain. All studies at the NCBI Gene Expression Omnibus (GEO) website (http://www.ncbi.nlm.nih.gov/gds/) on low-passage, non-stimulated, non-recombinant HUVECs with data normalization using the MAS5.0 algorithm (Affymetrix Inc., Santa Barbara, CA) were included. A total of 9 different studies comprising 17 arrays were used for expression analysis, an additional 2 studies comprising 12 arrays were used as validation. Relative gene expression was determined by comparing the average expression over all 17 HUVEC sets with the average expression in all 504 arrays representing 95 different normal human tissues in the Roth “Human Body Index,” the largest study on normal human mRNA expression (NCBI GEO: GSE7307). Only Rho regulatory genes with high expression in 14 or more of the 17 data sets analyzed are shown. The probe-sets shown for each gene detected the highest and widest expression, but all valid probe-sets were analyzed and used for expression analysis. The TranscriptView visualization tool (http://r2.amc.nl) was used to validate probes: probes had to target a unique, anti-sense position in an exon of the target gene. Gene and splice variant specificity was verified by NCBI GENE and BLAST analysis. Full information on the gene expression calculations, probe validations, and GEO studies can be found in Table S1. The Rho GTPases that are targeted by these GAPs and GEFs are indicated in column 4. Column 5 summarizes the known endothelial functions of each Rho regulator including references.
DLC1
The RhoGAP DLC1 (deleted in liver cancer-1; 7.5-fold upregulated in HUVECs) is crucial for embryonic development.73 DLC1 is described as a tumor suppressor gene, since it is lost in a variety of human cancers. The DLC1 protein shows GAP activity toward RhoA, B, C, and Cdc42 GTPases.74 This GAP activity is required for its regulation of integrin-dependent adhesion and migration, as well as cytoskeletal organization in epithelial cell types. Recruitment of DLC1 to integrin-based adhesion depends on its interaction with tensin proteins75,76 or through interactions with talin and focal adhesion kinase (FAK).77 In addition, an interaction through α-catenin recruits DLC1 to adherens junctions, which stabilizes the cadherin complex in a GAP-dependent manner.78 Interestingly, we find that the closely related DLC2 isoform (also known as STARD13) is clearly expressed in HUVECs (~2.5-fold higher expressed; see Table S1) and mouse studies have previously shown that the expression of DLC2 suppresses experimentally induced angiogenesis.79 The authors further showed that siRNA-mediated loss of DLC2 in HUVECs perturbed adhesion and migration on fibronectin or matrigel in a RhoA-dependent manner. Although there is no experimental evidence for a function of DLC1 in endothelial cells, the fact that the DLC2 isoform does regulate endothelial adhesion, and the finding that DLC1 is highly expressed in HUVECs, suggests that DLC isoforms are important for endothelial cell adhesion.
Arhgap17 and the RhoA GEF Syx
Arhgap17 is also known as Rich1 or Nadrin and is ~7 times upregulated in HUVECs. This GAP regulates Cdc42 and Rac180 and contains an N-Bar domain that targets the protein to convex-curved membranes during lamellipodial protrusion-retraction cycles.81 Arhgap17 associates with the scaffolding protein angiomotin (Amot) at tight junctional complexes to control cell polarity and junction integrity in a GAP-dependent manner.82,83 The regulation of Rho GTPases by Arhgap17 at cell–cell junctions is potentially important during angiogenesis, since Amot and its isoform AmotL1 regulate the migration of tip cells and stability of cell–cell junctions in stalk cells during embryonic sprouting angiogenesis.84,85 Interestingly, Amot family proteins also locally activate RhoA at endothelial cell protrusions during sprouting angiogenesis, through recruitment of the RhoA GEF Plekhg5 (better known as Syx),86,87 which is also expressed in HUVECs (Table S1). Vascular growth factor-induced signaling determines the localization and function of Syx; VEGF promotes the removal of Syx from cell–cell junctions, thereby inducing junction disassembly and endothelial permeability. By contrast, signaling induced by the vascular stabilizer angiopoietin-1 maintains Syx at cell–cell junctions to promote monolayer integrity via the RhoA effector Dia.88 These endothelial functions of Syx appear to be important for sprouting angiogenesis, because Syx-deficient zebrafish and mice display defects in their vascular networks and integrity.87,88
Arhgap31
Arhgap31, also known as CdGAP, is a GAP for Cdc42 and Rac1.89 CdGAP localizes at focal adhesions and its activity, which is dependent on integrin-mediated adhesion, controls directional membrane protrusions, cell migration, and invasion.90,91 In epithelial cells, the activity of CdGAP is inhibited by ERK-mediated phosphorylation, which increases Cdc42 activity at tight junctions and induces their disassembly.92 Although there is no experimental data on a role for CdGAP in endothelial cells, the Arhgap31 gene shows a genetic association with increased risk for coronary artery disease.93
Ralbp1
Ralbp1 is an effector of R-Ras and Ral GTPases that contains a GAP-like domain, which inhibits activity of Cdc42 and Rac1.94-96 The GAP domain is required for its interaction with R-Ras, for Ras-mediated Rac1 activation, and cell spreading.97 However, this occurs independently of Ralbp1 enzymatic GAP activity, but depends on recruitment and signaling via R-Ras. Although it is unclear if the GAP function of Ralbp1 controls cell adhesion, the expression of this multifunctional protein in endothelial cells is involved in tumor-associated angiogenesis in vivo.98 Moreover, auto-antibodies directed against Ralbp1 were detected in sera of patients that suffer from immune-mediated endothelial dysfunction, suggesting that Ralbp1 is associated with cardiovascular disease.99 To understand the role of Ralbp1 in endothelial cells, it would be interesting to investigate which specific adhesion events are regulated by Ralbp1 during angiogenesis.
Other highly expressed Rho GAPs
Myo9b is a GAP for RhoA that is recruited to regions of actin polymerization at membrane protrusions, depending on the activity of its own myosin motor domain. In addition, Myo9b is required for directional migration.100,101 Myo9b is crucial for tight junction formation and intestinal epithelial barrier formation,102 but no role in endothelial cells has been reported to date. Arhgap19 was recently identified as one of the repressed targets of miR-24,103 a microRNA enriched in cardiac endothelial cells after ischemia that inhibits angiogenesis in mouse models of myocardial infarction.104 Suppressing Arhgap19 expression enhanced keratinocyte differentiation and reorganizes the actin cytoskeleton, cell–matrix adhesions, and cell–cell junctions.103 Nothing is known about its putative role in endothelial cells. In addition, we find several other endothelial-enriched Rho GAPs, like DEPDC1, DEPDC1b, Arhgap11a, and Arhgap28, of which we know very little in the context of cell adhesion and vascular biology.
Non-enriched Rho GAPs with endothelial functions
Because GAPs and GEFs are not only regulated by transcription, we summarized the function of important non-enriched (in HUVECs) Rho regulators in Table 3 based on literature, and we overview the results from a Gene Ontology-based functional annotation search of all Rho regulators in Table S2. The Rho GAP expression profile of HUVECs shows for instance that p190RhoGAP (Arhgap35), an inhibitor of RhoA, is not differently expressed in endothelial cells compared with other cell types (Table S1). However, the expression of p190RhoGAP in endothelial cells is crucial for angiopoietin-1-induced cell–cell adhesion and endothelial barrier function, as it mediates angiopoietin-1-induced antagonistic signaling of active Rac1 toward RhoA.40,105 Also many cytokine-dependent signaling pathways that temporary perturb the endothelial barrier function require p190RhoGAP to suppress RhoA activity at later phases, and to recover endothelial monolayer integrity.106-108 In addition, p190RhoGAP activity toward RhoA was shown to be required for semaphorin-induced chemorepulsion of HUVECs.109 Expression of p190RhoGAP is also needed to control balancing of transcription factors that govern retinal neovascularization.110 Thus, it is clear that p190RhoGAP and six other GAPs (see Table 3) regulate endothelial functions. This emphasizes that the importance of endothelial Rho GAPs or GEFs is not only determined by their level of expression, but also strongly relies on local growth factor- or cytokine-induced changes in enzymatic activity.
Table 3. Non-enriched Rho regulators with known endothelial function.
| Gene Name | Alternative names | Function |
|---|---|---|
| ARAP1 | CENTD2 | Highly enriched in renal vasculature. 207 |
| ARHGAP35 | GRF-1, GRLF1, P190-A, P190A, p190ARhoGAP, p190RhoGAP | Modulates cell adhesion and F-actin organization to protect barrier function and mediates transcriptional control of retinal angiogenesis. 105 - 110 |
| ARHGAP5 | GFI2, RhoGAP5, p190-B, p190BRhoGAP | Extracellular matrix degradation. 208 |
| OCRL | RP3–454M7.1, INPP5F, LOCR, NPHL2, OCRL-1, OCRL1 | Expressed in Schlemm's canal ocular endothelial cells. 209 |
| PIK3R1 | AGM7, GRB1, p85, p85-ALPHA | Regulates migration of endothelial tip cells during zebrafish angiogenesis and controls lymphangiogenesis in mice. 210 , 211 |
| PIK3R2 | MPPH, P85B, p85, p85-BETA | Negatively regulates VEGF signaling in sprouting angiogenesis and vascular integrity. 212 |
| STARD13 | RP11–81F11.1, ARHGAP37, DLC2, GT650, LINC00464 | Mediates endothelial adhesion and migration and suppresses experimentally-induced angiogenesis in mouse models. 79 |
| ARHGEF1 | GEF1, LBCL2, LSC, P115-RHOGEF, SUB1.5 | Inducer of endothelial permeability via regulation of cell adhesion.174-178 |
| ARHGEF11 | RP11–356J7.2, GTRAP48, PDZ-RHOGEF | Mediator of Semaphorin 4D-induced endothelial signaling in adhesion and migration.165 |
| ARHGEF17 | P164RHOGEF, TEM4, p164-RhoGEF | Important for endothelial migration and monolayer integrity via cytoskeletal-dependent regulation of cell adhesion.180,181 |
| ARHGEF2 | RP11–336K24.3, GEF, GEF-H1, GEFH1, LFP40, P40 | Regulator of endothelial permeability induced by agonists or by mechanical strain.183,184 |
| ARHGEF26 | CSGEF, HMFN1864, SGEF | Regulator of endothelial docking structures during transmigration of leukocytes.186,187 |
| ARHGEF4 | ASEF, ASEF1, GEF4, STM6 | Involved in experimentally-induced angiogenesis in mouse models.202 |
| ARHGEF6 | RP3–527F8.4, COOL2, Cool-2, MRX46, PIXA, α-PIX, alphaPIX | Regulator of endothelial migration and adhesion.213 |
| DOCK1 | RP11–82L9.1, DOCK180, ced5 | Regulates polarized membrane protrusions and endothelial migration during vascular development.188,189 |
| ELMO1 | CED-12, CED12, ELMO-1 | Regulates polarized membrane protrusions and endothelial migration during vascular development.188,190 |
| FGD1 | RP1–112K5.1, AAS, FGDY, MRXS16, ZFYVE3 | Regulates TGF-β-induced endothelial podosome formation and extracellular matrix degradation.214 |
| ITSN2 | PRO2015, SH3D1B, SH3P18, SWA, SWAP | Caveola endocytosis.152 |
| PLEKHG5 | RP4–650H14.3, CMTRIC, DSMA4, GEF720, Syx, Tech | Regulator of endothelial cell migration, cell-cell adhesion and angiogenesis.86-88 |
| PREX1 | P-REX1 | Regulator of cell adhesion, Weibel-Palade body secretion, and required for inflammation-induced permeability.191,193,194 |
| PREX2 | 6230420N16Rik, DEP.2, DEPDC2, P-REX2 | Regulator of endothelial cell migration.192 |
| TIAM1 | T-lymphoma invasion and metastasis-inducing protein 1; TIAM-1 | Regulation of endothelial cell-cell adhesion and redox signaling.145,197-201 |
| VAV3 | VAV-3; guanine nucleotide exchange factor VAV3; vav 3 oncogene | Mediator of ephrin-induced angiogenesis and tumor-associated angiogenesis.147,148 |
Non-enriched Rho regulators with known endothelial function. This table shows the GAPs (bold) and GEFs (unbold) that are not specifically enriched in endothelium based on gene expression analysis, but which are, based on literature, known to function in endothelial cells. Gene name, alternative names and a summary of their endothelial function including references are shown.
The Roles of Rho GTPase GEFs in Endothelium
Highly expressed GEFs
NME1
Based on our analysis, NME1, also known as nucleoside diphosphate kinase 1 or NM23-H1, is the most abundantly expression-enriched “GEF” in HUVECs (21-fold higher, Table 2). In epithelial cell types, NME1 is shown to control integrin-mediated adhesion and migration/invasion,111 and regulation of its expression is associated with many tumor types. In solid cancer, NME1 usually functions as a tumor suppressor,112 but in the pediatric solid cancer, neuroblastoma high levels of NME1 correlate with a poor prognosis.113 There is no direct proof that NME1 can exchange guanine nucleotides on GTPases, but it has been described that NME1 regulates other GEFs, such as Dbl, that in turn activate Cdc42.114,115 Moreover, in epithelial cells, NME1 regulates ARF6-mediated endocytosis of cadherin-based cell–cell junctions, via inhibition of Rac1.116 In neuronal cells, NME1 interacts with α-catenin and suppresses Rac1-GTP at cell–cell junctions by inactivating the GEF Tiam1.117 Thus, NME1 is an important Rho GTPase regulator, as a GEF or as a scaffolding protein, but whether NME1 is involved in endothelial adhesion remains to be determined.
FGD5 and FGD6
Two other highly upregulated GEFs in HUVECs are FGD5, 16-fold, and FGD6, 7-fold (Table 2). Interestingly, FGD5, among other proteins, is transcriptionally regulated during vascular development in zebrafish by the ETS-family transcription factor Etv2, suggesting that FGD5 is involved in vascular development.52 Moreover, FGD5 is specifically expressed in endothelial cells, and siRNA-mediated knockdown of FGD5 in HUVECs showed that this GEF is required for VEGF-induced Cdc42 activation, endothelial cell migration, and monolayer integrity.118 Very recently, it was demonstrated that Rap1 activates FGD5 at endothelial cell–cell junctions, increasing local Cdc42 activity that signals to myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), which stabilizes the actin network at tightening junctions.119 The local regulation of endothelial cell–matrix and cell–cell adhesion by FGD5 appears important during angiogenesis, as evidenced by the finding that FGD5 inhibits neo-vascularization in mice in a Cdc42-dependent manner.120 This GEF may be important for other endothelial processes that depend on Cdc42-mediated adhesion signaling, such as polarization and lumen formation during tubulogenesis. To date, the function of its family member FGD6, which is highly expressed in HUVECs, is unknown.
DNMBP/Tuba
The upregulated GEF DNMBP, also known as Tuba, specifically exchanges GTP on Cdc42, but not on Rac1 or RhoA, and recruits the GTPase effectors N-WASP and Ena/VASP to remodel the actin cytoskeleton.121 Tuba itself is recruited to cell–cell adhesions by ZO-1, and its actin remodeling function is important to organize the association of the F-actin cytoskeleton to adherens junctions at the apical part of epithelial monolayers that are under high mechanical tension.122 The GEF activity of Tuba at cell–cell junctions is crucial for polarization during epithelial 3D cyst formation,123-125 a process that closely relates to lumen formation during angiogenesis and vasculogenesis. Whether Tuba regulates cell adhesion in endothelial cells is still unknown.
Rgnef/p190RhoGEF
Rgnef, also known as p190RhoGEF, is a GEF that interacts with Rho, but not with Rac or Cdc42,126 and controls adhesion-induced RhoA activation by fibronectin-binding integrins.127 Part of this adhesion-induced GTPase signaling depends on a scaffolding function of Rgnef that promotes FAK activation, whereas the GEF activity of Rgnef toward Rho is especially important to activate another focal adhesion protein, paxillin, downstream of fibronectin–integrin binding.128 Interestingly, Rgnef recruitment to the leading edge of migrating tumor cells corresponds with local activation of RhoC, which sustains directionality of protrusion activity by remodeling of the actin cytoskeleton.129 Because Rgnef is also highly expressed in HUVECs, we speculate that this GEF might control endothelial cell adhesion to the vascular ECM.
DOCK family
Members of the DOCK family, DOCK4, 6, and 9, are found to be highly expressed in HUVECs. However, no literature on the role of these DOCKs in endothelial cells is available. DOCK4 can activate Rac1 and is involved in neurite differentiation and extension.130 DOCK6 has dual specificity for Rac1 and Cdc42, whereas DOCK9 can only activate Cdc42, both GEFs are involved in neuronal development.131-133 Future research will have to reveal the possible functions of these GEFs in the vasculature.
Arhgef15
Interestingly, two recent studies indicate that the novel GEF Arhgef15 (also known as Ephexin5 or Vsm-RhoGEF) is an endothelial-enriched gene during mouse vascular development. By studying Arhgef15-null mutant mice, both investigations demonstrate that this GEF is required for vessel stabilization during retinal vascularization as it mediates signaling from VEGF towards Cdc42.57,134
Arhgef7/β-Pix/COOL-1
According to our analysis, Arhgef7 is highly expressed in HUVECs. Arhgef7, better known as β-p21-activated kinase (PAK)-interacting exchange factor (β-Pix) or COOL-1, regulates Cdc42 and Rac1 GTPases at membrane protrusions.135 The abundance of β-Pix at focal adhesions decreases when myosin-dependent tension rises, and β-Pix was shown to negatively affect actomyosin contractility-mediated adhesion maturation, whereas it is important for Rac1 activation at nascent adhesions at the front of membrane protrusions that are associated with low myosin activity.136 In addition to its GEF function, β-Pix serves to target Rac1 to these membrane ruffles.137 Interestingly, β-pix localizes at endothelial cell–cell junctions, and the interaction of β-Pix with the GTPase effector PAK is needed for VEGF-induced permeability.138 Many other reports imply a role for β-Pix in adhesion and membrane dynamics in the context of cell migration (reviewed in ref. 139), suggesting that activation of Cdc42 and Rac1 by β-Pix might also be relevant for migratory behavior of endothelial cells for instance during angiogenesis.
Vav family
The Vav family consists of three members, of whom Vav-2 is highly expressed in HUVECs (7-fold upregulated). Vav-1 is strictly expressed in hematopoietic cells, whereas Vav-3 is ubiquitously expressed, including the endothelium. The Vav GEFs can exchange GDP for GTP on several Rho-GTPases, including Rac1, RhoA, RhoG, and Cdc42.37,140 Seye and colleagues were the first to demonstrate Vav-2 expression in endothelial cells, and they reported that activation of the VEGF receptor 2 (VEGFR-2), downstream of a P2Y2 nucleotide receptor, results in phosphorylation of Vav-2 and corresponding activation of RhoA and Rac1.141 Other labs subsequently confirmed that VEGFR-2 promotes Src-dependent phosphorylation of Vav-2 and activates Rac1.142,143 VEGF-induced phosphorylation of Vav-2 directly affects the affinity of Vav-2 for a nucleotide-free mutant of Rac1, which proves that its GEF activity is enhanced.142,144 Activation of Pak by Rac1-GTP, in turn, results in phosphorylation of residues in the cytoplasmic domain of VE-cadherin, which accounts for its internalization, and thereby, enhances endothelial permeability.143 Interestingly, mechanosensing of endothelial cells in response to fluid shear flow rapidly enhances Rac1 activation, which depends on phosphorylation of Vav-2.145 Vav-2 phosphorylation was also increased when endothelial cells were plated on stiff ECM surfaces (21.5 kPa) compared with softer ones (1.72 kPa), which correlated in this study with enhanced activation of RhoA.146 Moreover, Vav-2 and Vav-3 interact with the EphA2 receptor, and studies using Vav-2/Vav-3-null mutant mice show that these GEFs are crucial mediators of Ephrin-induced Rac1 activation, endothelial cell migration, and angiogenesis,147 as well as tumor-associated angiogenesis.148 Thus, the expression of Vav GEFs, Vav-2 in particular, is important to regulate GTPase signaling that controls cell adhesion during angiogenesis.
ITSN (Intersectin)
Both ITSN-1 and ITSN-2 are expressed in HUVECs (Table 2; Table S1). Intersectins are expressed as long (l) or short (s) isoforms, associate with endocytosis-related components, and act as GEF for Cdc42.149 Expression of ITSN-1 is an important pro-survival signal for endothelial cells,150 whereas reducing expression of this protein in vivo perturbs vascular integrity and induces lung injury.151 Whether these phenomena relate to the GEF function of ITSN-1 is unclear. The GEF function of ITSN-2L was shown to be required for Cdc42 activation and actin remodeling in support of caveolae-induced endocytosis.152
Trio
The GEF Trio was originally identified as a binding partner of the transmembrane tyrosine phosphatase LAR.153 Since it contains three domains with putative enzymatic activity, it was named Trio. Trio is a large, 350 kDa protein that encompasses two DH-PH GEF units with different specificities. The N-terminal DH-PH cassette mediates GDP for GTP exchange on Rac1 and RhoG, whereas the C-terminal DH-PH cassette activates RhoA.153-155 In addition, Trio contains a serine kinase domain and spectrin repeats that may further contribute to its function.156 For instance, the kinase domain interacts with FAK, and Trio may thereby regulate focal adhesion dynamics at membrane protrusions.157 Trio was shown to interact with E-cadherin, and its GEF activity regulates Rac1 activity at epithelial adherens junctions.158 The interaction of Trio with the actin-binding protein filamin is important to support membrane protrusion activity.159 In endothelial cells, Trio is recruited to the intercellular adhesion molecule-1 (ICAM-1) upon leukocyte binding. Subsequently, the first GEF domain of Trio becomes activated and induces formation of so-called docking structures around adherent leukocytes, which are crucial for leukocyte transmigration.160 In a complementary study, we found that expression of Trio is upregulated by the inflammatory cytokine TNFα, and that Trio protein expression in endothelial cells of patients suffering from severe rheumatoid arthritis is higher than in patients with milder arthritis.161 The increased endothelial Trio-mediated Rac1 activity upregulates vascular cell adhesion molecule-1 (VCAM-1) expression and this further contributes to an inflamed endothelial phenotype. The potential importance of Trio for endothelial cell–matrix and cell–cell adhesion has not been reported in detail yet.
Arhgef12/LARG
Arhgef12, better known as Leukemia-associated Rho GEF (LARG), is a specific GEF for Rho, but not for Rac or Cdc42.162 LARG is crucial for RhoA signaling downstream of Gαq in mouse fibroblasts.163 LARG is also particularly important, in conjunction with the GEF-H1, to transmit mechanical force-induced integrin signaling toward RhoA that mediates cytoskeletal reinforcement.164 Furthermore, LARG interacts with plexin-B1 in endothelial cells, which underlies semaphorin 4D-induced Rho signaling in control of endothelial angiogenic behavior.165 Signaling downstream of the sphingosine-1-phosphate receptor 2, LARG inhibits sprouting of HUVEC spheroids in 3D collagen matrices; siRNA-mediated loss of LARG reduces RhoC activation.166 Thus, LARG plays distinct roles in endothelial cells that are relevant in the context of Rho GTPase signaling and adhesion. Moreover, expression of LARG in vascular smooth muscle cells is a crucial element of G-protein-mediated contractile signaling that underlies vascular remodeling and cardiovascular disease.167,168
Other highly expressed Rho GEFs
Breakpoint cluster region (Bcr), a protein that contains both GEF and GAP domains, is highly expressed in HUVECs, according to our analysis, and is described as an upstream regulator of RhoA activity, stress fibers, and focal adhesion formation in keratinocytes.169 In addition, Bcr has GAP activity toward Rac, Cdc42, and RhoA GTPases, which indicates that this Rho regulator is multifunctional.170 Recent data suggest that the neurological disorder-associated GEF PLEKHG4 activates Rac1, RhoA, and Cdc42, and thereby, organizes the actin cytoskeleton and membrane protrusions.171 The GEF amyotrophic lateral sclerosis 2 (ALS2), also known as Alsin, is present within neuron growth cones and activates Rac1 to promote neurite outgrowth.172 In addition, ALS2 is reported to activate the GTPase Rab5 and mutations in this GEF are thought to account for motor neuron diseases.173 So far, no direct evidence for a role of Bcr, PLEKHG4, or ALS2 in endothelial cells is available. The potential role for vascular biology of three other Rho GEFs that are enriched in HUVECs, Arhgef10, PLEKHG1, and FARP1, is completely unknown.
Non-enriched Rho GEFs with endothelial functions
A summary of the function of all non-enriched (in HUVECs) Rho GEFs that are described to function in endothelial cells can be found in Table 3. Here, we briefly discuss the role of a few important Rho GEFs from this list.
Arhgef1/p115-RhoGEF
The GEF p115-RhoGEF, through its phosphorylation by protein kinase C α (PKCα), is responsible for thrombin-induced RhoA activation and downstream signaling toward the actomyosin cytoskeleton promoting endothelial permeability.174-176 Similarly, p115-RhoGEF mediates lipopolysaccharide (LPS)-induced RhoA activation and permeability in brain endothelial cells.177 Others recently implicated p115-RhoGEF in crosstalk between endothelial integrin and cadherin adhesions, as conditional deletion of FAK in mouse endothelium enhanced the interaction of p115-RhoGEF with RhoA, reduced junctional integrity, and induced lung injury.178 In summary, current literature indicates that p115-RhoGEF regulates agonist-induced destabilization of endothelial cell–cell junctions.
Arhgef17/ TEM4
Recently, the function of the actin-binding GEF TEM4, also known as Arhgef17, was characterized in biochemical experiments that show its activity toward RhoA, B, and C.179 In endothelial cells, TEM4 appears critical for organization of the actin cytoskeleton and integrin-based adhesions at membrane protrusions of migrating cells.180 This work was followed up by Ngok and co-workers, who showed that TEM4 associates with the cadherin complex at endothelial cell–cell junctions in confluent monolayers, independent of F-actin binding, whereas TEM4 remains associated with the F-actin cytoskeleton in cells cultured at low density.181 The authors conclude that TEM4 expression in HUVECs is needed for efficient adherens junction formation, tube formation on matrigel, and endothelial barrier function.181
GEF-H1 (Arhgef2)
GEF-H1 is a RhoA-specific exchange factor that is regulated by microtubule binding, and couples microtubule dynamics to Rho-GTPase signaling in a variety of cellular events.182 In endothelial cells, GEF-H1 is described to regulate agonist-induced endothelial cell permeability.183 Moreover, the presence of GEF-H1 is crucial for the mechanotransductory response of pulmonary arterial cells to cyclic stretching.184 The importance of GEF-H1 in endothelial menchanotransduction can be explained by the finding that GEF-H1, together with the GEF LARG, is required for cytoskeletal reinforcement induced by mechanical tension on fibronectin-binding integrins.164 Increased stiffening of the vascular ECM occurs during aging, suggesting that integrin-dependent regulation of GEF-H1 activity may underlie the increased permeability and inflammatory phenotype of endothelial monolayers in stiffening vessels during atherosclerosis development.
Arhgef26/ SGEF
Arhgef26, or SH3-GEF (SGEF), is widely expressed and specifically activates RhoG, but not Rac1 or RhoA.185 SGEF is strongly implicated in the induction of endothelial docking structures that are required for transendothelial migration of leukocytes.186 SGEF-null mutant mice appear healthy and fertile, and its deficiency in combination with a lack of ApoE results in much smaller, Western diet-induced atherosclerotic lesions.187 This result could be attributed to a reduced capacity of aortic endothelial cells to form docking structures leading to a reduced number of leukocytes accumulating at early atherosclerotic lesions. These findings suggest that SGEF plays an important role in the heterotypic interaction between endothelial cells and leukocytes during vascular inflammation.
ELMO/DOCK180
In endothelial cells, DOCK180 (also known as DOCK1), together with its adaptor protein Engulfment-and-celL-MOtility (ELMO) is required for endothelial cells to adapt to changes in shear flow and Rac1-dependent polarized membrane protrusions.188 This function of the atypical Rac1 GEF complex ELMO/DOCK180 appears important for endothelial cell migration during development, as indicated by in vivo knockout studies that show cardiovascular defects upon functional deletion of DOCK180 or ELMO.189,190
P-Rex family
Both phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger (P-Rex)1 and P-Rex2 are expressed in HUVECs. Moreover, expression of the P-Rex1 and P-Rex2b isoforms in endothelial cells mediate stromal cell-derived factor 1 (SDF-1)-induced Rac1 signaling that controls cell adhesion and migration.191,192 P-Rex1 was recently shown to act downstream of TNFα-induced Rac1 signaling controlling cell–cell junctions, as P-Rex-null mutant mice display enhanced lung permeability defects when challenged with TNFα or LPS.193 P-Rex1 expression is also required for Rac1-dependent secretion of Weibel-Palade bodies, endothelial storage vesicles that contain von Willebrand factor.194
Tiam1
The role of Tiam1 is extensively studied in epithelial cells, where it controls cell–matrix and cell–cell adhesion through its GEF and/or scaffolding function for Rac1.195 Mice deficient for Tiam1 are healthy and develop a normal vasculature.196 Expression of Tiam1 or its close homolog Tiam2 in HUVECs was not clearly detectable on mRNA level (Table S1). However, studies in VE-cadherin-null mutant endothelial cells show that the presence of VE-cadherin does control junctional localization of Tiam1, concomitant with persistent Rac1 activation.197 Also, in pulmonary endothelial cells, OxPAPC (a barrier protective agent) induced the translocation of Tiam1 to cell–cell junctions, where it supports OxPAPC-induced barrier enhancement.198 Most likely, the stabilizing effect of Tiam1 on cell–cell junctions depends on cortical F-actin rearrangements.199 In microvascular endothelial cells, it was recently shown that the localization of Tiam1 at cell–cell junctions, as well as its barrier protective function, depends on the production of nitric oxide, an important intermediate for vascular permeability factors.200 In contrast, during TNFα-induced permeability of HUVEC monolayers, the PI3K p110α subunit was shown to promote the association of Tiam1 with the VE-cadherin complex, which in this case is thought to destabilize cell–cell junctions.201 Lastly, Tiam1 was recently described to be part of a polarity complex that locally recruits and activates Rac1 in response to fluid shear flow.145 Taken together, these studies demonstrate that, despite its low mRNA expression in HUVECs, Tiam1 does regulate endothelial cell–cell adhesion.
Other potentially relevant endothelial Rho GEFs
Although we do not find Arhgef4 (ASEF) expression in HUVECs, its endothelial expression in mice was shown to regulate cell migration in response to the angiogenic factors bFGF and VEGF, and Arhgef4 is critical for experimental-induced angiogenesis.202 This illustrates that Rho regulators may be differentially expressed between human and mouse, or reflects differences between endothelial cells derived from distinct vascular beds. Several other GEFs are described to regulate endothelial functions, see Table 3 for further details and references.
Conclusions and Perspectives
The regulation of Rho GTPase activity by GAPs and GEFs at the plasma membrane enables the coordination of endothelial cell adhesion during vascular development, homeostasis, and disease. In addition to the direct regulation of their activity, Rho GTPases interact with guanine nucleotide dissociation inhibitors (GDIs), regulators that sequester GDP-bound Rho GTPases in the cytoplasm, and thereby, prevent their activation and degradation.203 Of the Rho GDI family members, only the expression of Arhgdia, known as RhoGDIα, is high in HUVECs (Table S1). RhoGDIα is important for many cellular functions: null-mutant mice display multiple organ abnormalities, including increased endothelial permeability and edema in the lungs.204,205
Taking together the available evidence on the role of Rho GTPase regulators in endothelial cells, it becomes clear that they all take part in a tightly regulated and highly dynamic signaling network. Depending on the vascular growth factor or on the subcellular localization of GAPs or GEFs, distinct vascular processes (i.e., barrier function, angiogenesis) are regulated. Context-dependent control of cell–cell adhesion is clearly exemplified by different effects of the angiogenic growth factor VEGF that removes the Rho GEF Syx from cell–cell adhesions, inducing endothelial permeability, whereas the angiostatic factor angiopoietin-1 induces signaling that maintains Syx at cell–cell junctions and stabilizes monolayer integrity. At this moment it is too premature to summarize the specific functions of the many GAPs and GEFs in a coherent model that describes their role in endothelial cell adhesion events, because the available data that deals with local recruitment or upstream regulation of GAPs and GEFs is still limited.
In some occasions, the role of a Rho GTPase in cell adhesion seems even contradictory. For instance, inactivation of Cdc42 by Cdgap at tight junctions in MDCK cells in response to the hepatocyte growth factor (HGF) disassembles cell–cell adhesions,92 whereas activation of Cdc42 by FGD5 at adherens junctions in HUVECs in response to cyclic adenosine monophosphate (cAMP)-elevating G protein-coupled receptor-induced signaling strengthens cell–cell junctions and enhances monolayer integrity.119 There are possible explanations for such differences that relate to the cellular architecture; e.g., adherens and tight junction distribution in endothelial cells is different from the polarized junctional build up in epithelial cells. Another explanation might be that parallel-activated signaling pathways are completely different; in this example, HGF-induced changes in cell–cell adhesion are compared with more rapid events induced by cAMP signaling. A similar mechanism may explain why FGD5-induced Cdc42 activation is required for cAMP-induced junction strengthening, but also needed for VEGF-induced permeability.118 To achieve complete understanding of the specific and non-redundant roles of all Rho GAPs and GEFs in endothelial cell adhesion, we rely on challenging future studies that focus on combining the live imaging of spatiotemporal activation of Rho GTPases with local recruitment and activation of GEFs or GAPs in a physiologically relevant, vascular experimental set up.
We highlighted the role of Rho regulators that are highly expressed in HUVECs to indicate which GAPs or GEFs may be important for endothelial cell adhesion. We wish to emphasize that regulation of GAPs and GEFs occurs not only at the level of their expression, but also strongly depends on their localization, upstream regulators, and on protein modifications such as phosphorylation. Another interesting avenue to pursue would be to investigate if the level of expression of certain Rho GAPs or GEFs occurs in arterial or venous endothelial cells specifically, possibly explaining their different permeability properties.206
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
S.H. is supported by a VENI grant from the Netherlands Organization for Scientific Research (NWO; project 863.10.003). We thank Dr Peter L Hordijk for critical reading and helpful comments.
References
- 1.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 2.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Takahashi Y. Tissue morphogenesis coupled with cell shape changes. Curr Opin Genet Dev. 2010;20:443–7. doi: 10.1016/j.gde.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Papusheva E, Heisenberg CP. Spatial organization of adhesion: force-dependent regulation and function in tissue morphogenesis. EMBO J. 2010;29:2753–68. doi: 10.1038/emboj.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 6.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 8.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–54. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Mettouchi A. The role of extracellular matrix in vascular branching morphogenesis. Cell Adh Migr. 2012;6:528–34. doi: 10.4161/cam.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr Opin Hematol. 2012;19:206–11. doi: 10.1097/MOH.0b013e3283523df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–87. doi: 10.1161/01.RES.0000039537.73816.E5. [DOI] [PubMed] [Google Scholar]
- 12.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci. 2011;124:1183–93. doi: 10.1242/jcs.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 16.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–33. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–22. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013;126:403–13. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 20.Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s) Curr Opin Cell Biol. 2013;25:39–46. doi: 10.1016/j.ceb.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 21.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–64. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol. 2011;300:C146–54. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–14. doi: 10.1016/S0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 25.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–74. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 27.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–26. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormack J, Welsh NJ, Braga VM. Cycling around cell-cell adhesion with Rho GTPase regulators. J Cell Sci. 2013;126:379–91. doi: 10.1242/jcs.097923. [DOI] [PubMed] [Google Scholar]
- 29.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 30.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 31.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 35.Bryan BA, D’Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–65. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/S1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 37.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura F. FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration. Biochem J. 2013;453:17–25. doi: 10.1042/BJ20130290. [DOI] [PubMed] [Google Scholar]
- 39.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–39. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 40.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–41. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 41.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–14. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 42.Nieves B, Jones CW, Ward R, Ohta Y, Reverte CG, LaFlamme SE. The NPIY motif in the integrin beta1 tail dictates the requirement for talin-1 in outside-in signaling. J Cell Sci. 2010;123:1216–26. doi: 10.1242/jcs.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–3. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shifrin Y, Arora PD, Ohta Y, Calderwood DA, McCulloch CA. The role of FilGAP-filamin A interactions in mechanoprotection. Mol Biol Cell. 2009;20:1269–79. doi: 10.1091/mbc.E08-08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavelin I, Geiger B. Characterization of a novel GTPase-activating protein associated with focal adhesions and the actin cytoskeleton. J Biol Chem. 2005;280:7178–85. doi: 10.1074/jbc.M411990200. [DOI] [PubMed] [Google Scholar]
- 46.Su ZJ, Hahn CN, Goodall GJ, Reck NM, Leske AF, Davy A, Kremmidiotis G, Vadas MA, Gamble JR. A vascular cell-restricted RhoGAP, p73RhoGAP, is a key regulator of angiogenesis. Proc Natl Acad Sci U S A. 2004;101:12212–7. doi: 10.1073/pnas.0404631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 48.Aitsebaomo J, Wennerberg K, Der CJ, Zhang C, Kedar V, Moser M, Kingsley-Kallesen ML, Zeng GQ, Patterson C. p68RacGAP is a novel GTPase-activating protein that interacts with vascular endothelial zinc finger-1 and modulates endothelial cell capillary formation. J Biol Chem. 2004;279:17963–72. doi: 10.1074/jbc.M311721200. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Sniadecki NJ, Chen CS. Mechanical Forces in Endothelial Cells during Firm Adhesion and Early Transmigration of Human Monocytes. Cell Mol Bioeng. 2010;3:50–9. doi: 10.1007/s12195-010-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–52. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez GA, Veldman MB, Zhao Y, Burgess S, Lin S. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci U S A. 2013;110:11427–32. doi: 10.1073/pnas.1306595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011;20:526–39. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda M, Hasegawa H, Hyodo T, Ito S, Asano E, Yuang H, Funasaka K, Shimokata K, Hasegawa Y, Hamaguchi M, et al. ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol Biol Cell. 2011;22:3840–52. doi: 10.1091/mbc.E11-04-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman PR, Hahn CN, Grimshaw M, Lu Y, Li X, Brautigan PJ, Beck K, Stocker R, Vadas MA, Gamble JR. Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood. 2010;116:4016–24. doi: 10.1182/blood-2009-11-252700. [DOI] [PubMed] [Google Scholar]
- 57.Takase H, Matsumoto K, Yamadera R, Kubota Y, Otsu A, Suzuki R, Ishitobi H, Mochizuki H, Kojima T, Takano S, et al. Genome-wide identification of endothelial cell-enriched genes in the mouse embryo. Blood. 2012;120:914–23. doi: 10.1182/blood-2011-12-398156. [DOI] [PubMed] [Google Scholar]
- 58.Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, et al. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell. 2002;9:95–108. doi: 10.1016/S1097-2765(02)00434-3. [DOI] [PubMed] [Google Scholar]
- 59.Krugmann S, Andrews S, Stephens L, Hawkins PT. ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J Cell Sci. 2006;119:425–32. doi: 10.1242/jcs.02755. [DOI] [PubMed] [Google Scholar]
- 60.I ST, Nie Z, Stewart A, Najdovska M, Hall NE, He H, Randazzo PA, Lock P. ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J Cell Sci. 2004;117:6071–84. doi: 10.1242/jcs.01526. [DOI] [PubMed] [Google Scholar]
- 61.Gambardella L, Anderson KE, Nussbaum C, Segonds-Pichon A, Margarido T, Norton L, Ludwig T, Sperandio M, Hawkins PT, Stephens L, et al. The GTPase-activating protein ARAP3 regulates chemotaxis and adhesion-dependent processes in neutrophils. Blood. 2011;118:1087–98. doi: 10.1182/blood-2010-10-312959. [DOI] [PubMed] [Google Scholar]
- 62.Moon MY, Kim HJ, Kim JG, Lee JY, Kim J, Kim SC, Choi IG, Kim PH, Park JB. Small GTPase Rap1 regulates cell migration through regulation of small GTPase RhoA activity in response to transforming growth factor-β1. J Cell Physiol. 2013;228:2119–26. doi: 10.1002/jcp.24383. [DOI] [PubMed] [Google Scholar]
- 63.Gambardella L, Hemberger M, Hughes B, Zudaire E, Andrews S, Vermeren S. PI3K signaling through the dual GTPase-activating protein ARAP3 is essential for developmental angiogenesis. Sci Signal. 2010;3:ra76. doi: 10.1126/scisignal.2001026. [DOI] [PubMed] [Google Scholar]
- 64.Kartopawiro J, Bower NI, Karnezis T, Kazenwadel J, Betterman KL, Lesieur E, Koltowska K, Astin J, Crosier P, Vermeren S, et al. Arap3 is dysregulated in a mouse model of hypotrichosis-lymphedema-telangiectasia and regulates lymphatic vascular development. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt518. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 65.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, Oegema K. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–6. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yüce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–82. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–14. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 69.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–28. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bastos RN, Penate X, Bates M, Hammond D, Barr FA. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J Cell Biol. 2012;198:865–80. doi: 10.1083/jcb.201204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacquemet G, Green DM, Bridgewater RE, von Kriegsheim A, Humphries MJ, Norman JC, Caswell PT. RCP-driven α5β1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J Cell Biol. 2013;202:917–35. doi: 10.1083/jcb.201302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquemet G, Morgan MR, Byron A, Humphries JD, Choi CK, Chen CS, Caswell PT, Humphries MJ. Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J Cell Sci. 2013;126:4121–35. doi: 10.1242/jcs.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durkin ME, Avner MR, Huh CG, Yuan BZ, Thorgeirsson SS, Popescu NC. DLC-1, a Rho GTPase-activating protein with tumor suppressor function, is essential for embryonic development. FEBS Lett. 2005;579:1191–6. doi: 10.1016/j.febslet.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 74.Kim TY, Vigil D, Der CJ, Juliano RL. Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility. Cancer Metastasis Rev. 2009;28:77–83. doi: 10.1007/s10555-008-9167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC, Braverman R, Yamada KM, Popescu NC, Papageorge AG, Lowy DR. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci U S A. 2007;104:9012–7. doi: 10.1073/pnas.0703033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao YC, Si L, deVere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007;176:43–9. doi: 10.1083/jcb.200608015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G, Du X, Vass WC, Papageorge AG, Lowy DR, Qian X. Full activity of the deleted in liver cancer 1 (DLC1) tumor suppressor depends on an LD-like motif that binds talin and focal adhesion kinase (FAK) Proc Natl Acad Sci U S A. 2011;108:17129–34. doi: 10.1073/pnas.1112122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tripathi V, Popescu NC, Zimonjic DB. DLC1 interaction with α-catenin stabilizes adherens junctions and enhances DLC1 antioncogenic activity. Mol Cell Biol. 2012;32:2145–59. doi: 10.1128/MCB.06580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y, Chen NT, Shih YP, Liao YC, Xue L, Lo SH. DLC2 modulates angiogenic responses in vascular endothelial cells by regulating cell attachment and migration. Oncogene. 2010;29:3010–6. doi: 10.1038/onc.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richnau N, Aspenström P. Rich, a rho GTPase-activating protein domain-containing protein involved in signaling by Cdc42 and Rac1. J Biol Chem. 2001;276:35060–70. doi: 10.1074/jbc.M103540200. [DOI] [PubMed] [Google Scholar]
- 81.Galic M, Jeong S, Tsai FC, Joubert LM, Wu YI, Hahn KM, Cui Y, Meyer T. External push and internal pull forces recruit curvature-sensing N-BAR domain proteins to the plasma membrane. Nat Cell Biol. 2012;14:874–81. doi: 10.1038/ncb2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–48. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 83.Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–40. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21:2055–68. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng Y, Vertuani S, Nyström S, Audebert S, Meijer I, Tegnebratt T, Borg JP, Uhlén P, Majumdar A, Holmgren L. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res. 2009;105:260–70. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- 86.Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–53. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garnaas MK, Moodie KL, Liu ML, Samant GV, Li K, Marx R, Baraban JM, Horowitz A, Ramchandran R. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in Vivo. Circ Res. 2008;103:710–6. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol. 2012;199:1103–15. doi: 10.1083/jcb.201207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamarche-Vane N, Hall A. CdGAP, a novel proline-rich GTPase-activating protein for Cdc42 and Rac. J Biol Chem. 1998;273:29172–7. doi: 10.1074/jbc.273.44.29172. [DOI] [PubMed] [Google Scholar]
- 90.LaLonde DP, Grubinger M, Lamarche-Vane N, Turner CE. CdGAP associates with actopaxin to regulate integrin-dependent changes in cell morphology and motility. Curr Biol. 2006;16:1375–85. doi: 10.1016/j.cub.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 91.Wormer D, Deakin NO, Turner CE. CdGAP regulates cell migration and adhesion dynamics in two-and three-dimensional matrix environments. Cytoskeleton (Hoboken) 2012;69:644–58. doi: 10.1002/cm.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Togawa A, Sfakianos J, Ishibe S, Suzuki S, Fujigaki Y, Kitagawa M, Mellman I, Cantley LG. Hepatocyte Growth Factor stimulated cell scattering requires ERK and Cdc42-dependent tight junction disassembly. Biochem Biophys Res Commun. 2010;400:271–7. doi: 10.1016/j.bbrc.2010.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, Hauser ER, Shah SH, Pericak-Vance MA, Haynes C, Crosslin D, Harris M, Nelson S, Hale AB, Granger CB, et al. Peakwide mapping on chromosome 3q13 identifies the kalirin gene as a novel candidate gene for coronary artery disease. Am J Hum Genet. 2007;80:650–63. doi: 10.1086/512981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem. 1995;270:22473–7. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 95.Cantor SB, Urano T, Feig LA. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–84. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park SH, Weinberg RA. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–55. [PubMed] [Google Scholar]
- 97.Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J Cell Biol. 2006;174:877–88. doi: 10.1083/jcb.200603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee S, Wurtzel JG, Singhal SS, Awasthi S, Goldfinger LE. RALBP1/RLIP76 depletion in mice suppresses tumor growth by inhibiting tumor neovascularization. Cancer Res. 2012;72:5165–73. doi: 10.1158/0008-5472.CAN-12-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Margutti P, Matarrese P, Conti F, Colasanti T, Delunardo F, Capozzi A, Garofalo T, Profumo E, Riganò R, Siracusano A, et al. Autoantibodies to the C-terminal subunit of RLIP76 induce oxidative stress and endothelial cell apoptosis in immune-mediated vascular diseases and atherosclerosis. Blood. 2008;111:4559–70. doi: 10.1182/blood-2007-05-092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van den Boom F, Düssmann H, Uhlenbrock K, Abouhamed M, Bähler M. The Myosin IXb motor activity targets the myosin IXb RhoGAP domain as cargo to sites of actin polymerization. Mol Biol Cell. 2007;18:1507–18. doi: 10.1091/mbc.E06-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanley PJ, Xu Y, Kronlage M, Grobe K, Schön P, Song J, Sorokin L, Schwab A, Bähler M. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Natl Acad Sci U S A. 2010;107:12145–50. doi: 10.1073/pnas.0911986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chandhoke SK, Mooseker MS. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol Biol Cell. 2012;23:2468–80. doi: 10.1091/mbc.E11-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amelio I, Lena AM, Viticchiè G, Shalom-Feuerstein R, Terrinoni A, Dinsdale D, Russo G, Fortunato C, Bonanno E, Spagnoli LG, et al. miR-24 triggers epidermal differentiation by controlling actin adhesion and cell migration. J Cell Biol. 2012;199:347–63. doi: 10.1083/jcb.201203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–30. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 105.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–8. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 106.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J Biol Chem. 2013;288:18290–9. doi: 10.1074/jbc.M112.432757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 108.Chava KR, Tauseef M, Sharma T, Mehta D. Cyclic AMP response element-binding protein prevents endothelial permeability increase through transcriptional controlling p190RhoGAP expression. Blood. 2012;119:308–19. doi: 10.1182/blood-2011-02-339473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 110.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–8. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fournier HN, Albigès-Rizo C, Block MR. New insights into Nm23 control of cell adhesion and migration. J Bioenerg Biomembr. 2003;35:81–7. doi: 10.1023/A:1023450008347. [DOI] [PubMed] [Google Scholar]
- 112.Leone A, McBride OW, Weston A, Wang MG, Anglard P, Cropp CS, Goepel JR, Lidereau R, Callahan R, Linehan WM, et al. Somatic allelic deletion of nm23 in human cancer. Cancer Res. 1991;51:2490–3. [PubMed] [Google Scholar]
- 113.Hailat N, Keim DR, Melhem RF, Zhu XX, Eckerskorn C, Brodeur GM, Reynolds CP, Seeger RC, Lottspeich F, Strahler JR, et al. High levels of p19/nm23 protein in neuroblastoma are associated with advanced stage disease and with N-myc gene amplification. J Clin Invest. 1991;88:341–5. doi: 10.1172/JCI115299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murakami M, Meneses PI, Knight JS, Lan K, Kaul R, Verma SC, Robertson ES. Nm23-H1 modulates the activity of the guanine exchange factor Dbl-1. Int J Cancer. 2008;123:500–10. doi: 10.1002/ijc.23568. [DOI] [PubMed] [Google Scholar]
- 115.Murakami M, Meneses PI, Lan K, Robertson ES. The suppressor of metastasis Nm23-H1 interacts with the Cdc42 Rho family member and the pleckstrin homology domain of oncoprotein Dbl-1 to suppress cell migration. Cancer Biol Ther. 2008;7:677–88. doi: 10.4161/cbt.7.5.5665. [DOI] [PubMed] [Google Scholar]
- 116.Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4:929–36. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka M, Kuriyama S, Aiba N. Nm23-H1 regulates contact inhibition of locomotion, which is affected by ephrin-B1. J Cell Sci. 2012;125:4343–53. doi: 10.1242/jcs.104083. [DOI] [PubMed] [Google Scholar]
- 118.Kurogane Y, Miyata M, Kubo Y, Nagamatsu Y, Kundu RK, Uemura A, Ishida T, Quertermous T, Hirata K, Rikitake Y. FGD5 mediates proangiogenic action of vascular endothelial growth factor in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:988–96. doi: 10.1161/ATVBAHA.111.244004. [DOI] [PubMed] [Google Scholar]
- 119.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol. 2013;202:901–16. doi: 10.1083/jcb.201301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng C, Haasdijk R, Tempel D, van de Kamp EH, Herpers R, Bos F, Den Dekker WK, Blonden LA, de Jong R, Bürgisser PE, et al. Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation. 2012;125:3142–58. doi: 10.1161/CIRCULATIONAHA.111.064030. [DOI] [PubMed] [Google Scholar]
- 121.Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, Serna DM, Sondek J, Gertler FB, De Camilli P. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–43. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- 122.Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–46. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–9. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kovacs EM, Verma S, Thomas SG, Yap AS. Tuba and N-WASP function cooperatively to position the central lumen during epithelial cyst morphogenesis. Cell Adh Migr. 2011;5:344–50. doi: 10.4161/cam.5.4.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gebbink MF, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–13. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller NL, Lawson C, Chen XL, Lim ST, Schlaepfer DD. Rgnef (p190RhoGEF) knockout inhibits RhoA activity, focal adhesion establishment, and cell motility downstream of integrins. PLoS One. 2012;7:e37830. doi: 10.1371/journal.pone.0037830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller NL, Lawson C, Kleinschmidt EG, Tancioni I, Uryu S, Schlaepfer DD. A non-canonical role for Rgnef in promoting integrin-stimulated focal adhesion kinase activation. J Cell Sci. 2013;126:5074–85. doi: 10.1242/jcs.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bravo-Cordero JJ, Sharma VP, Roh-Johnson M, Chen X, Eddy R, Condeelis J, Hodgson L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J Cell Sci. 2013;126:3356–69. doi: 10.1242/jcs.123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xiao Y, Peng Y, Wan J, Tang G, Chen Y, Tang J, Ye WC, Ip NY, Shi L. The atypical guanine nucleotide exchange factor Dock4 regulates neurite differentiation through modulation of Rac1 GTPase and actin dynamics. J Biol Chem. 2013;288:20034–45. doi: 10.1074/jbc.M113.458612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyamoto Y, Yamauchi J, Sanbe A, Tanoue A. Dock6, a Dock-C subfamily guanine nucleotide exchanger, has the dual specificity for Rac1 and Cdc42 and regulates neurite outgrowth. Exp Cell Res. 2007;313:791–804. doi: 10.1016/j.yexcr.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 132.Meller N, Irani-Tehrani M, Kiosses WB, Del Pozo MA, Schwartz MA. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol. 2002;4:639–47. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- 133.Kuramoto K, Negishi M, Katoh H. Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J Neurosci Res. 2009;87:1794–805. doi: 10.1002/jnr.21997. [DOI] [PubMed] [Google Scholar]
- 134.Kusuhara S, Fukushima Y, Fukuhara S, Jakt LM, Okada M, Shimizu Y, Hata M, Nishida K, Negi A, Hirashima M, et al. Arhgef15 promotes retinal angiogenesis by mediating VEGF-induced Cdc42 activation and potentiating RhoJ inactivation in endothelial cells. PLoS One. 2012;7:e45858. doi: 10.1371/journal.pone.0045858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim S, Lee SH, Park D. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J Biol Chem. 2001;276:10581–4. doi: 10.1074/jbc.C000806200. [DOI] [PubMed] [Google Scholar]
- 136.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–69. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007;18:2346–55. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol. 2008;19:234–44. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004;16:1–11. doi: 10.1016/S0898-6568(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 141.Seye CI, Yu N, González FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–86. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 142.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–97. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 144.García-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–37. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J Cell Biol. 2013;201:863–73. doi: 10.1083/jcb.201207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeh YT, Hur SS, Chang J, Wang KC, Chiu JJ, Li YS, Chien S. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS One. 2012;7:e46889. doi: 10.1371/journal.pone.0046889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol. 2006;26:4830–42. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brantley-Sieders DM, Zhuang G, Vaught D, Freeman T, Hwang Y, Hicks D, Chen J. Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol Cancer Res. 2009;7:615–23. doi: 10.1158/1541-7786.MCR-08-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hunter MP, Russo A, O’Bryan JP. Emerging Roles for Intersectin (ITSN) in Regulating Signaling and Disease Pathways. Int J Mol Sci. 2013;14:7829–52. doi: 10.3390/ijms14047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Predescu SA, Predescu DN, Knezevic I, Klein IK, Malik AB. Intersectin-1s regulates the mitochondrial apoptotic pathway in endothelial cells. J Biol Chem. 2007;282:17166–78. doi: 10.1074/jbc.M608996200. [DOI] [PubMed] [Google Scholar]
- 151.Bardita C, Predescu D, Justice MJ, Petrache I, Predescu S. In vivo knockdown of intersectin-1s alters endothelial cell phenotype and causes microvascular remodeling in the mouse lungs. Apoptosis. 2013;18:57–76. doi: 10.1007/s10495-012-0762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Klein IK, Predescu DN, Sharma T, Knezevic I, Malik AB, Predescu S. Intersectin-2L regulates caveola endocytosis secondary to Cdc42-mediated actin polymerization. J Biol Chem. 2009;284:25953–61. doi: 10.1074/jbc.M109.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–52. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- 155.Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–39. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- 156.van Rijssel J, van Buul JD. The many faces of the guanine-nucleotide exchange factor trio. Cell Adh Migr. 2012;6:482–7. doi: 10.4161/cam.21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Medley QG, Buchbinder EG, Tachibana K, Ngo H, Serra-Pagès C, Streuli M. Signaling between focal adhesion kinase and trio. J Biol Chem. 2003;278:13265–70. doi: 10.1074/jbc.M300277200. [DOI] [PubMed] [Google Scholar]
- 158.Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, Tsukita S, Tsukita S. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol. 2011;193:319–32. doi: 10.1083/jcb.201009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–92. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 160.van Rijssel J, Kroon J, Hoogenboezem M, van Alphen FP, de Jong RJ, Kostadinova E, Geerts D, Hordijk PL, van Buul JD. The Rho-guanine nucleotide exchange factor Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell. 2012;23:2831–44. doi: 10.1091/mbc.E11-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van Rijssel J, Timmerman I, Van Alphen FP, Hoogenboezem M, Korchynskyi O, Geerts D, Geissler J, Reedquist KA, Niessen HW, Van Buul JD. The Rho-GEF Trio regulates a novel pro-inflammatory pathway through the transcription factor Ets2. Biol Open. 2013;2:569–79. doi: 10.1242/bio.20134382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, Sondek J, Caligiuri MA, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem. 2001;276:27145–51. doi: 10.1074/jbc.M103565200. [DOI] [PubMed] [Google Scholar]
- 163.Booden MA, Siderovski DP, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol Cell Biol. 2002;22:4053–61. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–24. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 166.Del Galdo S, Vettel C, Heringdorf DM, Wieland T. The activation of RhoC in vascular endothelial cells is required for the S1P receptor type 2-induced inhibition of angiogenesis. Cell Signal. 2013;25:2478–84. doi: 10.1016/j.cellsig.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 167.Althoff TF, Albarrán Juárez J, Troidl K, Tang C, Wang S, Wirth A, Takefuji M, Wettschureck N, Offermanns S. Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J Exp Med. 2012;209:2277–90. doi: 10.1084/jem.20120350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 169.Dubash AD, Koetsier JL, Amargo EV, Najor NA, Harmon RM, Green KJ. The GEF Bcr activates RhoA/MAL signaling to promote keratinocyte differentiation via desmoglein-1. J Cell Biol. 2013;202:653–66. doi: 10.1083/jcb.201304133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chuang TH, Xu X, Kaartinen V, Heisterkamp N, Groffen J, Bokoch GM. Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc Natl Acad Sci U S A. 1995;92:10282–6. doi: 10.1073/pnas.92.22.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gupta M, Kamynina E, Morley S, Chung S, Muakkassa N, Wang H, Brathwaite S, Sharma G, Manor D. Plekhg4 is a novel Dbl family guanine nucleotide exchange factor protein for rho family GTPases. J Biol Chem. 2013;288:14522–30. doi: 10.1074/jbc.M112.430371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tudor EL, Perkinton MS, Schmidt A, Ackerley S, Brownlees J, Jacobsen NJ, Byers HL, Ward M, Hall A, Leigh PN, et al. ALS2/Alsin regulates Rac-PAK signaling and neurite outgrowth. J Biol Chem. 2005;280:34735–40. doi: 10.1074/jbc.M506216200. [DOI] [PubMed] [Google Scholar]
- 173.Otomo A, Hadano S, Okada T, Mizumura H, Kunita R, Nishijima H, Showguchi-Miyata J, Yanagisawa Y, Kohiki E, Suga E, et al. ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum Mol Genet. 2003;12:1671–87. doi: 10.1093/hmg/ddg184. [DOI] [PubMed] [Google Scholar]
- 174.Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–8. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- 175.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 176.Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-α signals P115RhoGEF phosphorylation and RhoA activation in TNF-α-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation. 2011;8:28. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xiaolu D, Jing P, Fang H, Lifen Y, Liwen W, Ciliu Z, Fei Y. Role of p115RhoGEF in lipopolysaccharide-induced mouse brain microvascular endothelial barrier dysfunction. Brain Res. 2011;1387:1–7. doi: 10.1016/j.brainres.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 178.Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol. 2013;305:L291–300. doi: 10.1152/ajplung.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mitin N, Rossman KL, Der CJ. Identification of a novel actin-binding domain within the Rho guanine nucleotide exchange factor TEM4. PLoS One. 2012;7:e41876. doi: 10.1371/journal.pone.0041876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mitin N, Rossman KL, Currin R, Anne S, Marshall TW, Bear JE, Bautch VL, Der CJ. The RhoGEF TEM4 Regulates Endothelial Cell Migration by Suppressing Actomyosin Contractility. PLoS One. 2013;8:e66260. doi: 10.1371/journal.pone.0066260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ngok SP, Geyer R, Kourtidis A, Mitin N, Feathers R, Der C, Anastasiadis PZ. TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J Cell Sci. 2013;126:3271–7. doi: 10.1242/jcs.123869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–9. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 183.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–8. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 184.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837–48. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ellerbroek SM, Wennerberg K, Arthur WT, Dunty JM, Bowman DR, DeMali KA, Der C, Burridge K. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell. 2004;15:3309–19. doi: 10.1091/mbc.E04-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, García-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–93. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Samson T, van Buul JD, Kroon J, Welch C, Bakker EN, Matlung HL, van den Berg TK, Sharek L, Doerschuk C, Hahn K, et al. The guanine-nucleotide exchange factor SGEF plays a crucial role in the formation of atherosclerosis. PLoS One. 2013;8:e55202. doi: 10.1371/journal.pone.0055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zaidel-Bar R, Kam Z, Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci. 2005;118:3997–4007. doi: 10.1242/jcs.02523. [DOI] [PubMed] [Google Scholar]
- 189.Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, Ding G, Noda M, Murata Y, Tanaka Y, et al. DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res. 2010;107:1102–5. doi: 10.1161/CIRCRESAHA.110.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Epting D, Wendik B, Bennewitz K, Dietz CT, Driever W, Kroll J. The Rac1 regulator ELMO1 controls vascular morphogenesis in zebrafish. Circ Res. 2010;107:45–55. doi: 10.1161/CIRCRESAHA.109.213983. [DOI] [PubMed] [Google Scholar]
- 191.Carretero-Ortega J, Walsh CT, Hernández-García R, Reyes-Cruz G, Brown JH, Vázquez-Prado J. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol Pharmacol. 2010;77:435–42. doi: 10.1124/mol.109.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li Z, Paik JH, Wang Z, Hla T, Wu D. Role of guanine nucleotide exchange factor P-Rex-2b in sphingosine 1-phosphate-induced Rac1 activation and cell migration in endothelial cells. Prostaglandins Other Lipid Mediat. 2005;76:95–104. doi: 10.1016/j.prostaglandins.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 193.Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res. 2012;111:1517–27. doi: 10.1161/CIRCRESAHA.112.273078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van Hooren KW, van Breevoort D, Fernandez-Borja M, Meijer AB, Eikenboom J, Bierings R, Voorberg J. Phosphatidylinositol-3,4,5-triphosphate-dependent Rac exchange factor 1 (PREX1) regulates epinephrine induced exocytosis of Weibel-Palade bodies. J Thromb Haemost. 2013:27. doi: 10.1111/jth.12460. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 195.Mertens AE, Roovers RC, Collard JG. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–6. doi: 10.1016/S0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 196.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–71. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 197.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–89. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol. 2007;211:608–17. doi: 10.1002/jcp.20966. [DOI] [PubMed] [Google Scholar]
- 199.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–56. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 200.Di LA, et al. eNOS derived nitric oxide regulates endothelial barrier function via VE cadherin and Rho GTPases. J Cell Sci. 2013:17. doi: 10.1242/jcs.115972. ( Forthcoming. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188:863–76. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kawasaki Y, Jigami T, Furukawa S, Sagara M, Echizen K, Shibata Y, Sato R, Akiyama T. The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis. J Biol Chem. 2010;285:1199–207. doi: 10.1074/jbc.M109.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–83. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 205.Gorovoy M, Neamu R, Niu J, Vogel S, Predescu D, Miyoshi J, Takai Y, Kini V, Mehta D, Malik AB, et al. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ Res. 2007;101:50–8. doi: 10.1161/CIRCRESAHA.106.145847. [DOI] [PubMed] [Google Scholar]
- 206.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Doblinger E, Höcherl K, Mederle K, Kattler V, Walter S, Hansen PB, Jensen B, Castrop H. Angiotensin AT1 receptor-associated protein Arap1 in the kidney vasculature is suppressed by angiotensin II. Am J Physiol Renal Physiol. 2012;302:F1313–24. doi: 10.1152/ajprenal.00620.2011. [DOI] [PubMed] [Google Scholar]
- 208.Guegan F, Tatin F, Leste-Lasserre T, Drutel G, Genot E, Moreau V. p190B RhoGAP regulates endothelial-cell-associated proteolysis through MT1-MMP and MMP2. J Cell Sci. 2008;121:2054–61. doi: 10.1242/jcs.025817. [DOI] [PubMed] [Google Scholar]
- 209.Luo N, Kumar A, Conwell M, Weinreb RN, Anderson R, Sun Y. Compensatory Role of Inositol 5-Phosphatase INPP5B to OCRL in Primary Cilia Formation in Oculocerebrorenal Syndrome of Lowe. PLoS One. 2013;8:e66727. doi: 10.1371/journal.pone.0066727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–29. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mouta-Bellum C, Kirov A, Miceli-Libby L, Mancini ML, Petrova TV, Liaw L, Prudovsky I, Thorpe PE, Miura N, Cantley LC, et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85alpha, p55alpha, and p50alpha. Dev Dyn. 2009;238:2670–9. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Singh NK, Kundumani-Sridharan V, Rao GN. 12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation. Blood. 2011;118:5701–12. doi: 10.1182/blood-2011-04-347468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Daubon T, Buccione R, Génot E. The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in transforming growth factor β-stimulated aortic endothelial cells. Mol Cell Biol. 2011;31:4430–41. doi: 10.1128/MCB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.