Abstract
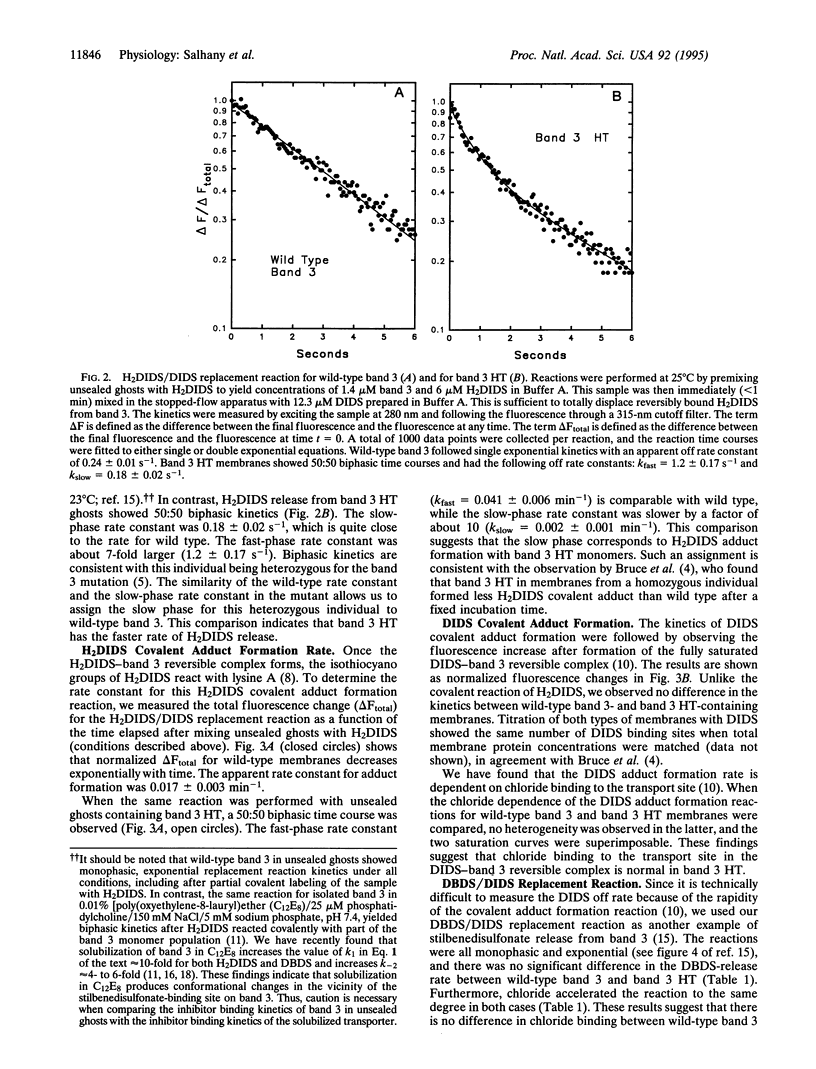
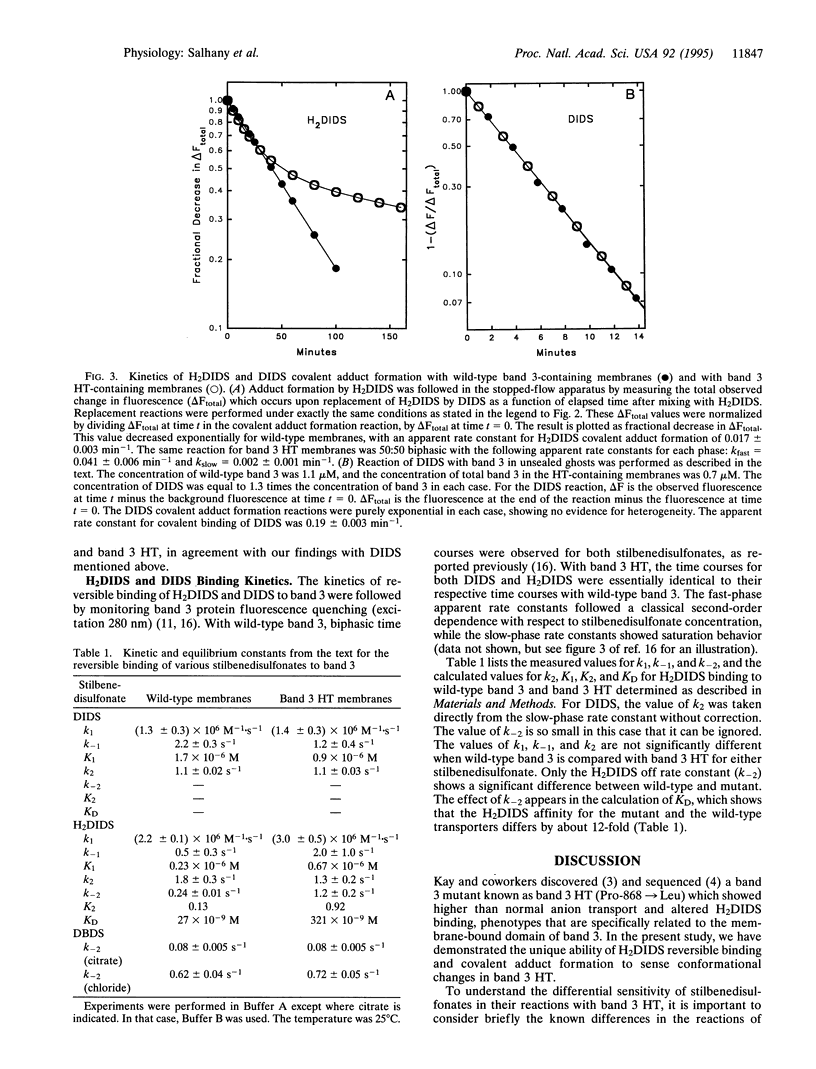
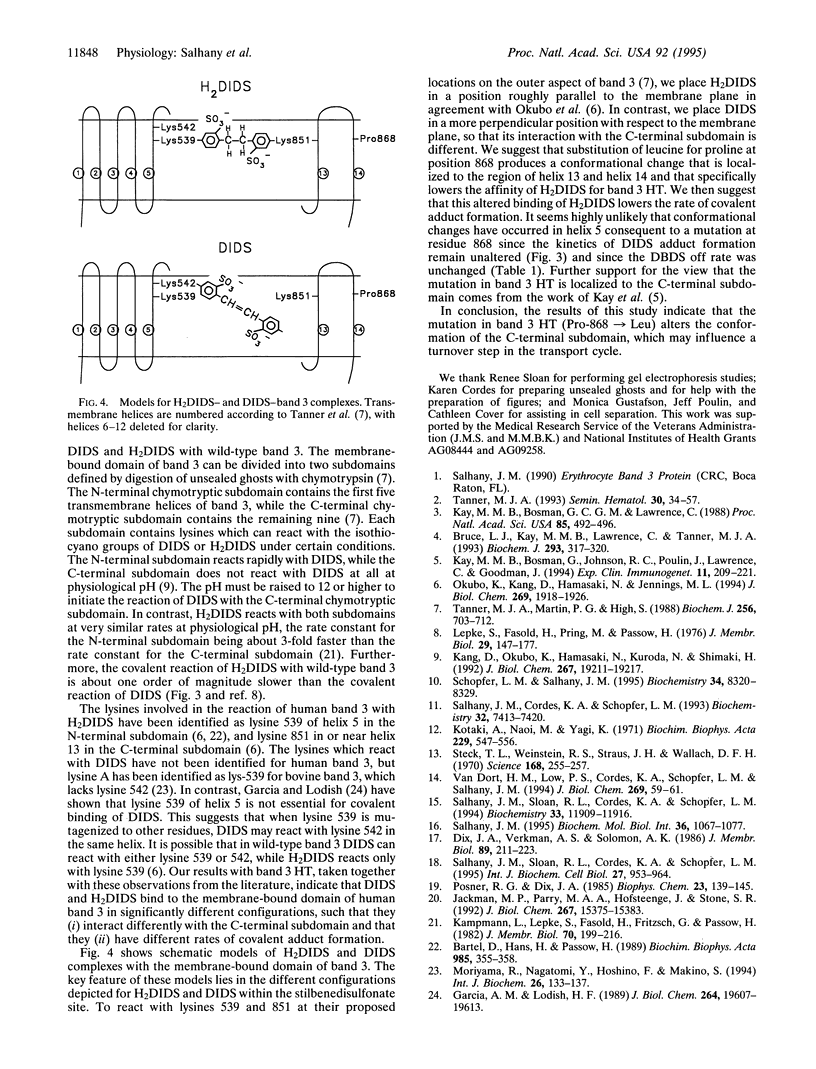
Band 3 HT (Pro-868-->Leu) is a mutant anion exchange protein which has several phenotypic characteristics, including a 2- to 3-fold larger Vmax, and reduced covalent binding of the anion transport inhibitor 4,4'-diisothiocyanodihydrostilbene-2,2'-disulfonate (H2DIDS). We have used fluorescence kinetic methods to study inhibitor binding to band 3 to determine if the point mutation in band 3 HT produces localized or wide-spread conformational changes within the membrane-bound domain of this transporter. Our results show that covalent binding of H2DIDS by band 3 HT is slower by a factor of 10 to 20 compared with the wild-type protein. In contrast, no such difference in the kinetics was observed for covalent binding of 4,4'-diisothiocyanostilbene-2,2'-disulfonate (DIDS). In addition, the kinetics of H2DIDS release from band 3 HT was abnormal, while the kinetics of 4,4'-dibenzamidostilbene-2,2'-disulfonate (DBDS) release showed no difference when compared with the wild-type protein. We conclude that substitution of leucine for proline at position 868 does not perturb the structure of "lysine A" in the membrane-bound domain of band 3 but rather produces an apparently localized conformational change in the C-terminal subdomain of the protein which alters H2DIDS affinity. When combined with the observation of an increased Vmax, these results suggest that protein structural changes at position 868 influence a turnover step in the transport cycle.
Full text
PDF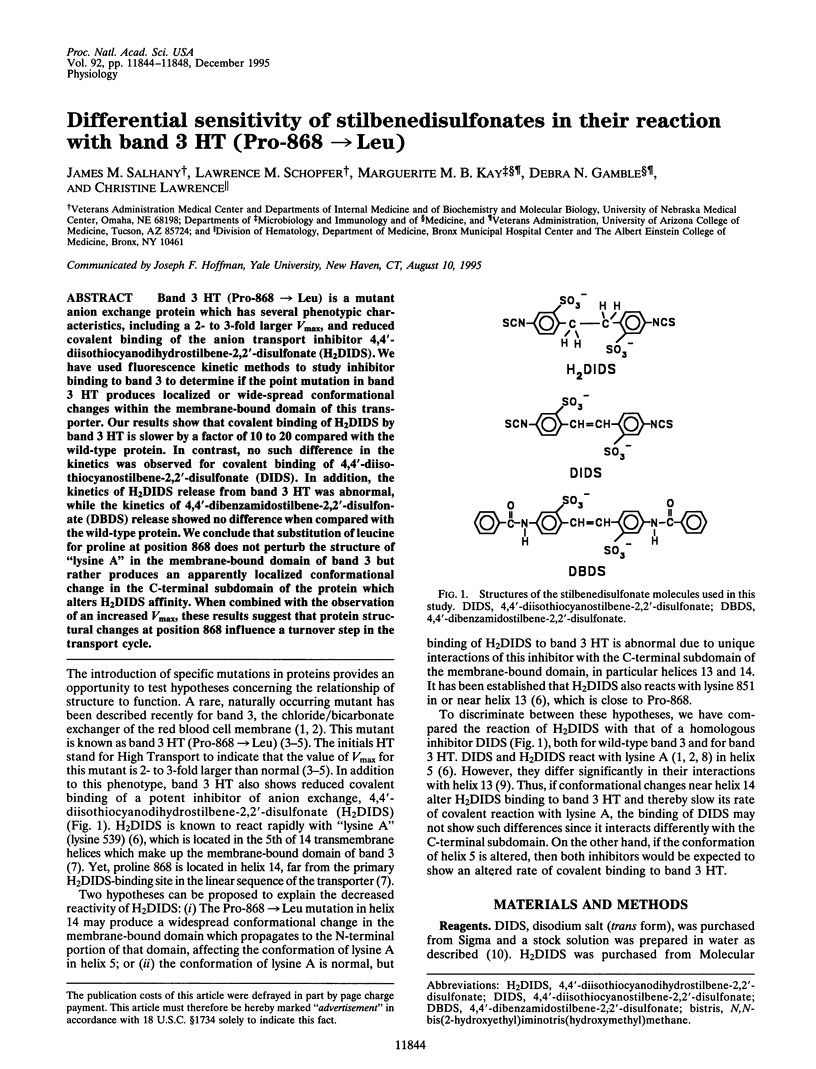

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel D., Hans H., Passow H. Identification by site-directed mutagenesis of Lys-558 as the covalent attachment site of H2DIDS in the mouse erythroid band 3 protein. Biochim Biophys Acta. 1989 Nov 3;985(3):355–358. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- Bruce L. J., Kay M. M., Lawrence C., Tanner M. J. Band 3 HT, a human red-cell variant associated with acanthocytosis and increased anion transport, carries the mutation Pro-868-->Leu in the membrane domain of band 3. Biochem J. 1993 Jul 15;293(Pt 2):317–320. doi: 10.1042/bj2930317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S., Solomon A. K. Binding of chloride and a disulfonic stilbene transport inhibitor to red cell band 3. J Membr Biol. 1986;89(3):211–223. doi: 10.1007/BF01870665. [DOI] [PubMed] [Google Scholar]
- Garcia A. M., Lodish H. F. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J Biol Chem. 1989 Nov 25;264(33):19607–19613. [PubMed] [Google Scholar]
- Jackman M. P., Parry M. A., Hofsteenge J., Stone S. R. Intrinsic fluorescence changes and rapid kinetics of the reaction of thrombin with hirudin. J Biol Chem. 1992 Aug 5;267(22):15375–15383. [PubMed] [Google Scholar]
- Kampmann L., Lepke S., Fasold H., Fritzsch G., Passow H. The kinetics of intramolecular cross-linking of the band 3 protein in the red blood cell membrane by 4,4'-diisothiocyano dihydrostilbene-2,2'-disulfonic acid (H2DIDS). J Membr Biol. 1982;70(3):199–216. doi: 10.1007/BF01870563. [DOI] [PubMed] [Google Scholar]
- Kang D., Okubo K., Hamasaki N., Kuroda N., Shiraki H. A structural study of the membrane domain of band 3 by tryptic digestion. Conformational change of band 3 in situ induced by alkali treatment. J Biol Chem. 1992 Sep 25;267(27):19211–19217. [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Bosman G., Johnson R. C., Poulin J., Lawrence C., Goodman J. Molecular basis of human band-3 mutation associated with increased anion transport. Exp Clin Immunogenet. 1994;11(4):209–221. [PubMed] [Google Scholar]
- Kotaki A., Naoi M., Yagi K. A diaminostilbene dye as a hydrophobic probe for proteins. Biochim Biophys Acta. 1971 Mar 23;229(3):547–556. doi: 10.1016/0005-2795(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Moriyama R., Nagatomi Y., Hoshino F., Makino S. Amino acid sequences around exofacial proteolytic cleavage sites of band 3 from bovine and porcine erythrocytes. Int J Biochem. 1994 Jan;26(1):133–137. doi: 10.1016/0020-711x(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Okubo K., Kang D., Hamasaki N., Jennings M. L. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4'-diisothiocyanodihydrostilbene-2,2'-disulfonic acid) molecule. J Biol Chem. 1994 Jan 21;269(3):1918–1926. [PubMed] [Google Scholar]
- Posner R. G., Dix J. A. Temperature dependence of anion transport inhibitor binding to human red cell membranes. Biophys Chem. 1985 Nov;23(1-2):139–145. doi: 10.1016/0301-4622(85)80072-7. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Cordes K. A., Schopfer L. M. Kinetics of conformational changes associated with inhibitor binding to the purified band 3 transporter. Direct observation of allosteric subunit interactions. Biochemistry. 1993 Jul 27;32(29):7413–7420. doi: 10.1021/bi00080a011. [DOI] [PubMed] [Google Scholar]
- Salhany J. M. Effect of chloride on the binding kinetics of various stilbenedisulfonates to band 3. Biochem Mol Biol Int. 1995 Aug;36(5):1067–1077. [PubMed] [Google Scholar]
- Salhany J. M., Sloan R. L., Cordes K. A., Schopfer L. M. Kinetic evidence for ternary complex formation and allosteric interactions in chloride and stilbenedisulfonate binding to band 3. Biochemistry. 1994 Oct 4;33(39):11909–11916. doi: 10.1021/bi00205a029. [DOI] [PubMed] [Google Scholar]
- Schopfer L. M., Salhany J. M. Characterization of the stilbenedisulfonate binding site on band 3. Biochemistry. 1995 Jul 4;34(26):8320–8329. doi: 10.1021/bi00026a013. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol. 1993 Jan;30(1):34–57. [PubMed] [Google Scholar]
- Van Dort H. M., Low P. S., Cordes K. A., Schopfer L. M., Salhany J. M. Calorimetric evidence for allosteric subunit interactions associated with inhibitor binding to band 3 transporter. J Biol Chem. 1994 Jan 7;269(1):59–61. [PubMed] [Google Scholar]


