Abstract
This study evaluates the durability of a novel tissue engineered blood vessel (TEBV) created by seeding a natural vascular tissue scaffold (decellularized human saphenous vein allograft) with autologous adipose-derived stem cells (ASC) differentiated into endothelial-like cells. Previous work with this model revealed the graft to be thrombogenic, likely due to inadequate endothelial differentiation as evidenced by minimal production of nitric oxide (NO). To evaluate the importance of NO expression by the seeded cells, we created TEBV using autologous ASC transfected with the endothelial nitric oxide synthase (eNOS) gene to produce NO. We found that transfected ASC produced NO at levels similar to endothelial cell (EC) controls in vitro and capable of causing vasorelaxation of aortic specimens ex vivo. TEBV (n=5) created with NO-producing ASC and implanted as interposition grafts within the aorta of rabbits remained patent for two months and demonstrated a non-thrombogenic surface compared to unseeded controls (n=5). Despite the xenograft nature of the scaffold, TEBV structure remained well-preserved in seeded grafts. In sum, this study demonstrates that up-regulation of NO expression within adult stem cells differentiated towards an endothelial-like cell imparts a non-thrombogenic phenotype and highlights the importance of NO production by cells to be used as endothelial cell substitutes in vascular tissue engineering applications.
Keywords: Adult stem cells, blood vessel prosthesis, nitric oxide synthase, differentiation, endothelium, eNOS transfection
1. Introduction
Given the limited availability of autologous saphenous vein (Conte et al., 1998; Campbell et al., 2007) and inferior durability of synthetic conduits (McLarty et al., 1998; Szilagyi et al., 1979) for small-diameter arterial bypass surgery, stem cell-based tissue engineering may offer an alternative for patients with coronary or peripheral arterial disease. Several cell types have been identified as endothelial cell substitutes to line the luminal surface of a tissue engineered blood vessel including circulating endothelial progenitor cells (EPC) (Sreerekha et al., 2006; Bu X et al., 2010; Vartanian et al., 2009; Serrano et al., 2008; Shirota et al., 2003) and bone marrow-derived mesenchymal stem cells (BMSC) (Lim SH et al., 2008; Wu et al., 2008; Hjortnaes et al., 2010). While several groups report success with these cell types, decreased availability with advanced in age and co-morbidity, along with harvest difficulties detract from their use in clinical applications (Hoetzer et al., 2007; Dragoo et al., 2003; Siddiq et al., 2009; Magri et al., 2007).
Adipose tissue provides a source of autologous stem cells in large quantity via a minimally invasive procedure with low donor discomfort (DiMuzio et al., 2006; DiMuzio et al., 2007; Schaner et al., 2004; Zhang et al., 2010; McIlhenny et al., 2010; Harris et al., 2010; Harris et al., 2011; Miranville et al., 2004; Planat-Benard et al., 2004; Cao et al., 2005). Human adipose-derived stem cells (ASC) acquire various endothelial characteristics following culture in Endothelial Cell Growth Supplement (ECGS) and exposure to fluid shear stress. Specifically, these cells align in the direction of fluid flow, take up acetylated low density lipoproteins (Ac-LDL), form tube-like structures when plated on extra-cellular matrix proteins (Matrigel), and express endothelial proteins such as Platelet-Endothelial Cell Adhesion Molecule (PECAM-1, or CD31) (Fischer et al., 2009; Zhang et al., 2010). Additionally, EC-differentiated ASC have been used create a tissue engineered blood vessel (TEBV): following seeding onto a decellularized vascular scaffold and flow conditioning up to physiologic arterial shear stress, ASC form a neointima with alignment of cells in the direction of flow (McIlhenny et al., 2010).
Despite the efforts of several groups, there has been limited to no success in promoting ASC to express endothelial nitric oxide synthase (eNOS), a molecule important to the function of the endothelium. Endothelial NOS catalyzes the production of nitric oxide (NO) via enzymatic oxidation of L-arginine to L-citrulline (Sessa 2004). Nitric oxide promotes vasodilation (Furchgott et al., 1980; Vallance et al., 1989), protection against intimal hyperplasia via inhibition of smooth muscle cell proliferation (Garg et al., 1989), and inhibition of platelet adhesion/aggregation (Azuma et al., 1986; Radomski et al., 1987). Previous report by our group demonstrated differentiation of ASC towards the EC-phenotype, but with variable and/or minimal eNOS expression; in vivo evaluation of these cells revealed that they were mildly thrombogenic, possibly related to the lack of eNOS expression (Fischer et al., 2009). Given these critical properties, we hypothesize that eNOS expression by the differentiated stem cell would improve its success as an endothelial cell substitute in vascular tissue engineering.
In this report, we demonstrate successful expression of eNOS in ASC following adenoviral transfection. Transfection initiates eNOS mRNA production, yielding eNOS protein which generates functional NO gas. This gas is bioactive, as evidenced by induction of vascular smooth muscle relaxation. Finally, a TEBV lined with eNOS-expressing ASC differentiated to an EC-like phenotype implanted in vivo demonstrates a non-thrombogenic phenotype and preserved graft structure.
2.0 Materials and Methods
2.1. Stem Cell Isolation and Culture
Adipose tissue was obtained via peri-umbilical liposuction of patients undergoing elective vascular surgery at Thomas Jefferson University Hospital. All patients were consented and donations conducted under an Institutional Review Board-approved protocol. ASC were isolated from a total of 25 human donors and characterized as previously described (DiMuzio et al., 2006; Fischer et al., 2009) yielding an adult stem cell population with a CD13+29+90+31−45-phenotype. Isolated ASC were differentiated by culture in M199 media (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Gemini BioProducts, West Sacramento, CA), HEPES buffer (1M, Fisher; Pittsburgh, PA), heparin (Elkins-Sinn, Cherry Hill, NJ), antibiotic/antimycotic solution (Mediatech), and Endothelial Cell Growth Supplement (ECGS, 6μg/mL, BD Biosciences, San Jose, CA) for a period of two weeks. Human umbilical vein endothelial cells (HUVEC) and human dermal microvascular endothelial cells (HDMEC) were used as positive controls.
2.2. Adenoviral Vector Construction
Adenoviral vectors were created using the ViraPower Adenoviral Expression System (Invitrogen, Carlsbad, CA) as per manufacturer’s instructions. The ViraPower system allows for the creation of a virus which lacks the entire E1 region resulting in an adenovirus which is replication incompetent and does not integrate into the host genome. These properties result in a transient expression. Two adenoviral vectors were created; an eNOS-containing (experimental) and GFP-containing (control) vector. Full length eNOS cDNA (Accession BC069465) was contained within a pPCR-Script Amp SK (+) plasmid (Open Biosystems, Item MHS1768-9144029). The phrGFP-1 plasmid was obtained from the laboratory of Vickram Srinivas, Thomas Jefferson University, and Department of Orthopaedic Research.
2.3. Adenoviral transfection
Prior to transfection, ASC were washed with PBS and fresh media was added. Adenovirus expressing eNOS gene (Ad-eNOS) was added directly to cell cultures at the indicated multiplicity of infection (MOI). The MOI indicates the ratio of target cells to viral plaque-forming units. Cultures were incubated overnight followed by media exchange. The effects of transfection were evaluated at 12, 24, 48 and 72 hours post-transfection as well as up to 3wk.
2.4. RT-PCR and qPCR
Total RNA was isolated from cultured cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified using spectrophotometry. Reverse Transcription Polymerase Chain Reaction (RT-PCR) was executed in a one-step method utilizing Illustra Ready-To-Go RT-PCR Beads (GE Healthcare, Piscataway, NJ). Electrophoresis was performed on 2% agarose gel treated with ethidium bromide and visualized using ultraviolet light.
Quantitative-PCR was performed using TaqMan Fast Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with Applied Biosystems 7500 Fast System. PCR primers targeting human eNOS and Taq-Man probes were also obtained from Applied Biosystems. The housekeeping gene GAPDH was amplified to normalize for variance in input RNA.
2.5. Flow cytometry
Following transfection, cells were characterized for expression of eNOS by flow cytometry. The cells were fixed with Cytofix (BD Biosciences), washed twice in Perm/Wash Buffer (BD Bioscience) and then stained with 2μg/ml of either FITC-anti NOS3 (Santa Cruz Biotechnology, Inc) or isotype control FITC-anti IgG1 (BD Bioscience). Quantitative analysis was performed using FACScalibur flow cytometer (BD Bioscience) and FlowJo software.
2.6. Western Blot
Experimental cell cultures were harvested with EDTA (50mM, Promega) to preserve integrin integrity. Cell pellets were treated with RIPA buffer containing protease and phosphatase inhibitors (Thermo Scientific, Waltham, MA). Cell debris was removed via centrifugation. Protein concentration was quantified with a bicinchoninic acid (BCA) colorimetric protein assay (Thermo Scientific). Protein was size separated on 8% Tris-Glycine gels (Invitrogen) and transferred to PVDF membranes (Invitrogen). Blots were blocked in fat-free milk for 1h. Primary antibodies were diluted in fat-free milk and incubated overnight with agitation at 4°C. Antibodies were purchased from Santa Cruz: NOS3 (C-20), 1:1000, α-tubulin (B-7), 1:1000. HRP-conjugated secondary antibodies were used at a concentration of 1:10000. Blots were developed with a chemiluminescent detection system (Immobilon Western/Millipore).
2.7. Nitric Oxide Production
Nitric oxide production was measured using a Model 280i Nitric Oxide Analyzer (NOA) (Sievers Instruments, GE Analytical, Boulder, CO). The NOA is a high-sensitivity detector which measures nitric oxide via a gas-phase chemiluminescent reaction between NO and O3. The instrument was used as per manufacturer’s instructions. Cell monolayers were treated with 10uM bradykinin acetate (Sigma) in serum free M199 to induce NO production. NO generation was measured as a function of bradykinin treatment time (t=0, 0.5, 1, 2, 3, 5, 10min), Ad-eNOS MOI (0, 0.1, 1, 3, 10, 30, 100, 1000), and bradykinin dose (1, 10, 100μM).
2.8. Bioassay Measurements
Rat abdominal aorta was removed following euthanasia by carbon dioxide asphyxiation. Tissue was dissected free of adherent tissue and cut into ring segment segments 3mm in length. The dissection was carried out in an oxygenated physiological saline solution (PSS) containing (mM): NaCl, 118.1; KCl, 3.0; CaCl2, 1.8; MgSO4, 1.2; KH2PO4, 1.0; NaHCO3, 27.3; glucose, 10.0; and pyruvic acid, 2.5, pH 7.4. Host endothelial cells were denuded mechanically from the aortic lumen to prevent endogenous NO-mediated relaxation. To measure vascular reactivity, three ring segments were mounted for isometric tension studies on a wire myograph (two were used to test relaxation in response to conditioned medium from eNOS-transfected cells, one for control). The ring segments were mounted horizontally in separate muscle chambers on two stainless steel pins (127 μm radius); one pin attached to a movable support and the other to a stationary support. The water-jacketed chambers were filled with PSS (10 mL), maintained at 37°C and continuously aerated with 95% O2/5% CO2. Before experimentation, each ring segment was stretched to optimal passive tension and equilibrated in PSS for 1h. Passive tension was achieved by mounting the ring and stretching it until the force began to increase. During preconditioning, if the force dropped to zero, additional strain was added. Isometric force was measured with Grass FT .03 force transducers and recorded on a Macintosh (G4) PC using A/D conversion provided by a MacLab interface.
Smooth muscle contraction was achieved through the use of norepinephrine (1 nM −10μM). ASC cultured in 6-well plates seven days following eNOS transfection (MOI=1000) were washed three times with PBS. 1mL of 10uM bradykinin in serum free M199 was added to a well of ASC and allowed to incubate with gentle swirling for 2min. 250uL of conditioned media was removed from the well and added to the organ bath to induce smooth muscle relaxation. Nontransfected ASC served as negative control.
2.9. Matrigel tube formation assay
ASC cultured for 2 weeks in EGM-2 medium were plated on top of Matrigel substrate (BD Biosciences) and incubated at 370C in a 5% CO2 for up to 12 h. Formation of cord-like structures was observed by phase-contrast microscopy.
2.10. TEBV construction and in vivo testing
Rabbit autologous ASC were isolated from dorsal fat pads harvested under general anesthesia and processed as described above for human tissue. A total of five animals were used, and each harvest resulted in a viable cell line that was used for the creation of a TEBV (n=5). Following one week of culture in ECGS-containing media, rabbit ASC were transfected with Ad-eNOS (MOI=1000) and incubated for 24h. TEBV were created by seeding ASC upon the luminal surface of decellularized human saphenous vein scaffolds, as previously reported (McIlhenny et al., 2010). The TEBV underwent one week of flow conditioning, where upon shear was increased linearly (1.5dynes/cm2 at 0.2 Pa/day) until physiologic shear was achieved. Grafts were transported from the lab in sterile containers to the animal operating facility.
Five engineered vessels were tested in vivo utilizing a rabbit abdominal aortic interposition graft model. All procedures were conducted under Thomas Jefferson University Institutional Animal Care and Use Committee approved protocols which conformed to NIH animal use standards. In 10 male New Zealand White rabbits, we implanted five TEBV and five control (non-seeded) grafts. Following induction of general anesthesia, the infrarenal abdominal aorta was carefully dissected from surrounding structures through a mid-line incision. Intravenous heparin (100U/kg) was administered and the aorta was clamped proximally and distally. Interposition grafts were sutured in place with end-to-end anastomoses performed with running 7-0 Prolene (Monofilament polypropylene, Ethicon, Inc., Somerville, NJ) suture. Integrity of the graft anastomoses was assured and the abdomen was closed in layers.
Grafts were visualized at two week intervals with duplex ultrasound. Eight weeks post-implant, grafts were re-exposed and pressure-fixed (100mmHg) with 4% paraformaldehyde (250cc over 30min) and harvested en bloc. All of the five engineered graft (TEBV) and five control graft were analyzed. Grafts were imaged with high resolution photography to evaluate the luminal surface for gross thrombin formation. The luminal surface was further imaged with scanning electron microscopy (SEM) and laser confocal microscopy, as previously described (McIlhenny et al., 2010). Tissue was paraffin embedded, sectioned (8 μm), and histologically prepared using hematoxylin and eosin (H&E) as well as phosphotungstic acid hematoxylin (PTAH) staining.
2.11. Laser confocal microscopy
To evaluate cell retention in TEBV luminal surface, grafts were opened longitudinally, stained with Cell Tracker Green, Alexa Fluor 488 phalloidin, and/or propidium iodide (Invitrogen, Carlsbad, CA), as per manufacturer’s instructions, and visualized by laser confocal microscopy (488nm, 543nm).
2.12. Statistical analyses
Data are expressed as mean ± standard deviation. In vitro experiments were compared for statistical difference using a two-tailed Student’s t-test. Differences were considered significant if P<0.05 level.
3. Results
3.1. Efficiency of Ad-eNOS transfection in human ASC
ASC expression of eNOS mRNA following adenoviral transfection correlated with increasing multiplicity of infection and time following transfection (up to 72h) (Figure 1A). Quantification of eNOS mRNA by real-time RT-PCR showed maximal message expression with an MOI of 1000; further increasing the MOI to 3000 did not yield increased expression (Figure 1B). Maximum eNOS message expression remained less than endothelial controls, as HUVEC and HDMEC expressed 2- and 3-fold more mRNA than transfected ASC, respectively. Western blot also confirmed that protein expression correlated with increasing MOI and time following transfection (up to 96h) (Figure 1C). As expected following adenoviral transfection, eNOS expression was transient, with peak mRNA expression at 1wk and complete loss of message by 3wk (this corresponded to three cell doublings in culture) (Figure 1D). FACS analysis determined that transfection efficiency at an MOI of 1000 following 1wk of culture was approximately 50% (range = 49-55.3%, n=3; data not shown). Total of 25 human donors ASC were isolated and cultured for use in all of the in vitro experiments. On ASC isolation, in this and our other two studies (Zhang, et al.; Harris, et al.), we found that isolations are essentially 100% efficacious in delivering viable cultures of adipose-derived stem cells.
Figure 1. Efficiency of Ad-eNOS transfection in human ASC.
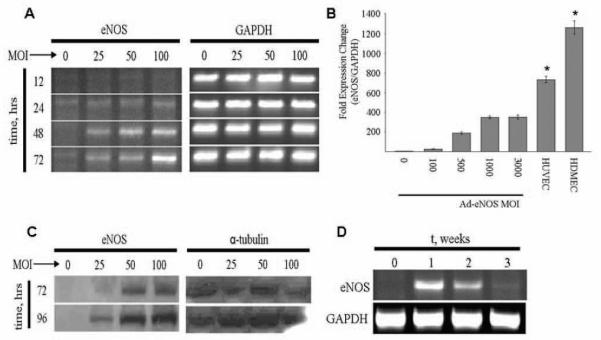
(A) RT-PCR analysis of eNOS mRNA expression in ASC after Ad-eNOS transfection. Increasing adenoviral multiplicity of infection (MOI) yielded increased eNOS mRNA expression through the 72h time point. (B) Quantification RT-PCR analysis of eNOS mRNA level in Ad-eNOS transfected ASC versus endothelial controls. (*: P< 0.05 vs. HUVEC and HDMEC control, n = 3 individual experiment per condition; Mean ± SD are reported). (C) Western Blot analysis of eNOS protein expression in ASC 72 and 96h after Ad-eNOS transfection. (D) RT-PCR analysis of eNOS mRNA expression in ASC 3wk following eNOS transfection.
3.2. Effect of eNOS transfection on NO production in human ASC
While the expression of eNOS protein from ASC represents significant improvement in the ASC endothelial-like profile, the production of NO (the functional aspect of transfection) is not guaranteed. To evaluate NO production, ASC transfected with eNOS were stimulated with bradykinin acetate. Figure 2A reveals NO production by ASC 1wk after transfection as a function of the MOI. At a MOI of 1000 (which corresponded to peak eNOS message expression), ASC interestingly produced NO (247±10nM) more than HUVEC (107±42nM) controls, and similar to HDMEC (288±29nM) controls (n=3 cell lines; P<0.05). Further characterization of NO production by eNOS-transfected ASC reveals that the cells continue to produce NO over 10min past stimulation with a single dose of bradykinin (10uM; n=3 cell lines; Figure 2B). Additionally, NO production appeared dependent on the dose of bradykinin (n=3 cell lines; Figure 2C).
Figure 2. Effect of eNOS transfection on NO production in human ASC.
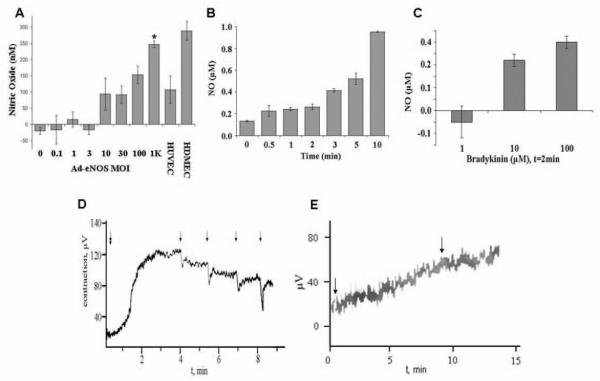
(A) NO production in ASC 1wk following Ad-eNOS transfection as a function of increasing adenoviral multiplicity of infection (MOI) (*: p<0.05 vs. HUVEC control, n=3). (B) Production of NO by eNOS-transfected ASC (MOI=1000) following bradykinin (10uM) stimulation demonstrates that gas production over 10min (n=3 individual experiment per condition; Mean ± SD are reported). (C) Production of NO by eNOS transfected ASC (MOI=1000) is related to bradykinin concentration (n=3 individual experiment per condition; Mean ± SD are reported). (D) The NO produced by the transfected ASC (MOI=1000) is bioactive. Shown is a representative graph of aortic rings (n=2) denuded of EC contract after stimulation with norepinephrine (double arrow, early time point). Norepinephrine was added after stretching the rings to optimal passive tension. The rings appeared to contract maximally approximately 2min after the addition of norepinephrine. During this time period, bradykinin was added to the ASC cultures; after two minutes of bradykinin culture, the conditioned medium from the eNOS-transfected ASC cultures was serially applied to the rings (four times), producing relaxation of the rings denuded of endothelium. (E) In a parallel control experiment (n=1), conditioned media from GFP-transfected ASC failed to produce relaxation (second arrow).
To determine the bioactivity of NO produced by transfected ASC, a denuded arterial ring contraction assay was performed (Figure 2D). Freshly harvested rat aortic rings (3mm in length) were denuded of endothelium (to remove influence of native EC-derived NO), mounted onto a wire myograph, and stimulated with norepinephrine to produce smooth muscle cell contraction. After stimulation with bradykinin (10uM x 2min), conditioned medium from eNOS-transfected ASC cultures added to the muscle bath resulted in relaxation of the aortic rings (n=2). Subsequent doses of conditioned medium produced similar results. Addition of conditioned media from control GFP-transfected ASC failed to produce relaxation (n=1; Figure 2E).
3.3. Differentiation of ASC and vascular graft creation in the rabbit model
After demonstrating effectiveness of eNOS transfection in human ASC in vitro, we then developed our animal model. All five autologous rabbit stem cell (rASC) isolations were successful, and transfected with Ad-eNOS (MOI=1000); subsequent eNOS mRNA expression was confirmed by RT-PCR (Figure 3A). After differentiation in ECGS-containing medium and transfection, rASC newly demonstrated the endothelial characteristics of alignment in the direction of applied shear stress (1.5 dynes/cm2 at 0.2 Pa, 48h) (Figure 3B) and cord formation upon plating on Matrigel (Figure 3C). TEBV were created by seeding the lumen of a natural vascular tissue scaffold (decellularized human saphenous vein) with rabbit ASC transfected with eNOS, as previously described by us (Schaner et al., 2004; Fischer et al., 2009; McIlhenny et al., 2010; and above). After flow conditioning within bioreactor (0-9dynes at 0.1 Pa over 5d), confocal microscopy revealed complete luminal coverage of the graft surface and alignment of ASC in the direction of flow (Figure 3D). This TEBV, composed of the vascular scaffold seeded with differentiated and transfected autologous rabbit ASC, was then evaluated in vivo by implantation into the donating rabbit’s aorta as an interposition graft.
Figure 3. Characterization of ASC and vascular graft creation within the rabbit model.
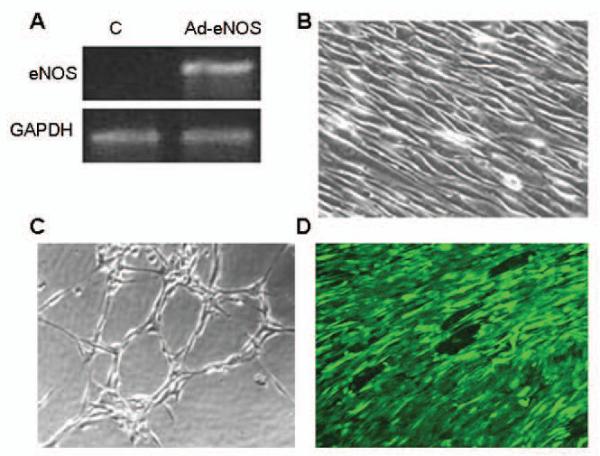
(A) RTPCR analysis of eNOS mRNA expression rabbit ASC differentiated towards and EC lineage before (control, C) and after (Ad-eNOS) eNOS transfection. (B) Phase contrast photomicrograph of rabbit ASC differentiated towards an EC lineage after application of shear stress (1.5 dyne/cm2 at 0.2 Pa, 48h) demonstrating alignment of the cells in the direction of shear. (C) Phase contrast photomicrograph of EC-differentiated rabbit ASC following seeding onto Matrigel demonstrating cord formation indicative of angiogenic potential. (D) Laser confocal micrograph of the luminal surface of a vascular scaffold seeded with differentiated and transfected autologous rabbit ASC and flow conditioned for 5d demonstrates the adherence and alignment of the seeded cells (Cell Tracker Green).
3.4. In vivo transplantation
Previous in vivo evaluation of a TEBV composed of autologous ASC (but not transfected with eNOS, and hence not NO-producing) revealed that the lumen of the graft was not adequately anti-thrombogenic (Fischer et al., 2009). Building upon this work, we now tested the effect of eNOS transfection of the seeded, autologous ASC in vivo. TEBV seeded with autologous ASC differentiated and transfected with eNOS (n=5) and unseeded controls (n=5) were implanted as interposition grafts into the infra-renal abdominal aorta of rabbits. Duplex ultrasound performed bi-weekly demonstrated that all grafts (TEBV and controls) remained patent throughout the two month observation period and were well-incorporated into the surrounding tissue (Figure 4A). No graft ruptures or anastomotic false aneurysms were observed.
Figure 4. Characterization of TEBV in vivo.
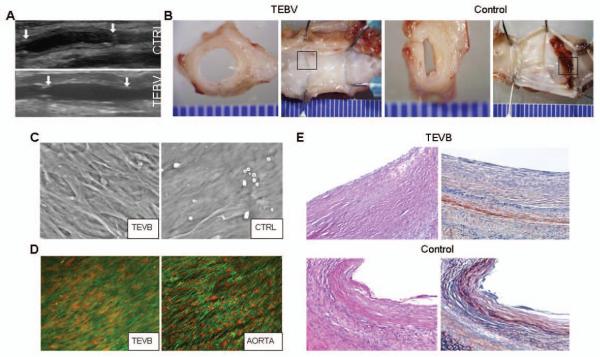
(A) Duplex ultrasound of TEBV and control grafts (CTRL) 2wk following implantation as interposition grafts within rabbit infra-renal aorta. (B) Representative gross examination of TEBV and control graft 8wk after implantation (views of the mid-graft cut transversely and the entire graft splayed open longitudinally). (C) Scanning electron micrograph of the luminal surface of an ASC-seeded TEVB and unseeded control graft (CTRL) 8wk after implantation. (D) Laser confocal micrograph (actin stain, green; nuclear stain, red) of a TEVB 8wk after implantation demonstrates the presence of a confluent, aligned layer of cells on the luminal surface of the graft. For comparison, staining of the adjacent native aorta is shown. (E) Photomicrographs of grafts 8wks after implantation (H&E, left; PTAH, right). The TEBV is free of significant fibrin formation on its luminal surface (fibrin stains red upon staining with PTAH). Representative results are shown from one TEBV and one control graft. Similar results were obtained from another 4 animals.
Gross examination of the explants revealed that the luminal surface of each TEBV (n=5) was smooth, without evidence of thrombosis, and visually congruent with adjacent native aorta (Figure 4B); conversely, each control graft, despite remaining patent, demonstrated thrombin staining and/or gross thrombus formation within the lumen.
Visualization of the graft lumens en face via scanning electron (SEM) revealed a confluent lining of cells within the TEBV; conversely, the luminal surfaces of the unseeded controls were devoid of significant cell coverage (Figure 4C). Further, the cells resident upon the luminal surface of the TEBV aligned in the direction of arterial blood flow, similar to EC resident within the native aorta (Figure 4D).
Histological examination of the TEBV showed an intact luminal cell layer without evidence of fibrin formation; conversely, the presence of fibrin was confirmed on the luminal surface of unseeded grafts (Figures 4E). Both the TEBV and unseeded control scaffolds appeared thickened compared to the native arterial structure; unfortunately, quantification of hyperplasia was not possible as the margins of the control grafts were indistinct with the surrounding tissue layers.
4. Discussion
The main findings of this study suggest the importance of NO production within the luminal surface of a tissue-engineered blood vessel. Herein, we demonstrated the successful transfection of ASC with the eNOS gene, with subsequent expression of the gene products at the message and protein levels. Second, transfection also produced significant amounts of NO, which was demonstrated to be responsive to receptor-mediated stimulation (bradykinin) and bioactive (as evidenced by its relaxation of vascular smooth muscle). Finally, we demonstrated the use of these cells as endothelial cell substitutes in vivo. Taken together, these data demonstrate a method for creation and improved success of a tissue-engineered blood vessel composed of autologous adult stem cells.
Our group recently demonstrated differentiation of ASC towards the EC-phenotype, but with variable and/or minimal eNOS message or protein expression (Fischer et al., 2009). Other investigators have demonstrated the acquisition of EC traits by ASC (Miranville et al., 2004; Planat-Benard et al., 2004). With specific regards to eNOS, Cao et al (Cao et al., 2005) reported the expression of eNOS message within ASC following three days of culture in VEGF and bFGF-containing medium; however, protein expression or the generation of NO were not reported. Similarly, while Ning et al (Ning et al., 2009) exhibited eNOS immunofluorescent eNOS staining in ASC following culture in bFGF-containing medium, they did not corroborate these results with transcript level or functional analyses. Overall, despite several investigations, it appears that the expression of eNOS and its product NO has been difficult to achieve reliably in ASC.
In this report, we evaluated the function of ASC as endothelial cell substitutes after differentiation in ECGS-containing medium and following adenoviral transfection with eNOS, circumventing the shortcomings of prior attempts to impart eNOS expression in ASC. The described methods successfully promoted eNOS message and protein expression. Although quantification of eNOS message revealed ASC expression to be less than endothelial controls, the production of NO gas was equal to or in excess of endothelial controls, suggesting that only moderate amounts of eNOS protein may be necessary for EC-comparable amounts of NO to be produced by stem cells.
In 2006, both Kanki-Horimoto and Zhang demonstrated effective adenoviral transfection of bone marrow-derived stem cells with eNOS (Kanki-Horimoto et al., 2006; Zhang et al., 2006). The results indicate NOS activity via the enzymatic conversion of L-[3H] arginine to L-[3H] citrulline; however, eNOS message, protein and NO gas production were unreported. This group also explored the use of these cells in seeding the lumen of an ePTFE graft with success, yet reported no in vivo data to date. In 2007, Bivalacqua et al also transfected bone marrow-derived stem cells with eNOS using an adenoviral vector with success (Bivalacqua et al., 2007); in vivo data suggested that eNOS expressing stem cells improved penile function of rats when injected into the corpra cavernosum. Herein, we report for the first time the successful transfection of eNOS into adipose-derived stem cells, with documented eNOS message and protein expression and most importantly the generation of significant concentrations of biologically active NO.
Nitric oxide plays a pivotal role in the maintenance of normal vascular homeostasis and the regulation of systemic blood pressure (Vallance et al., 1989). In addition, it is clear that NO has a number of other important functions in the vessel wall, including inhibition of platelet aggregation and adhesion molecule expression, prevention of smooth muscle proliferation and modulation of vascular growth, and prevention of coagulation and thrombosis (Freedman et al., 1997; Jeremy et al., 1999; Loscalzo, 2001). Thus, given NO’s anti-thrombogenic properties, we hypothesized that the successful expression of bioactive NO by ASC may contribute positively to their role as an endothelial cell substitute in the creation of a TEBV.
Several lines of evidence support this hypothesis. Previous implantation TEBV with autologous ASC that did not express eNOS demonstrated luminal thrombosis (Fischer LJ, et al., 2009). In this report, grafts implanted with eNOS-producing cells were subsequently demonstrated to have a non-thrombogenic, confluent monolayer of cells upon their luminal surface. These cells are presumed to be those seeded at the time of graft creation, as unseeded control grafts did not demonstrate significant luminal cell coverage. The monolayer of cells on the TEBV, while aligned in the direction of flow similar to endothelium, was morphologically different from native endothelial cells, further supporting the notion that their represent the seeded ASC. Given that the cells on the TEBV were likely the seeded, eNOS-producing ASC, the lack of thrombus observed on these grafts compared to unseeded grafts (and historically to non-transfected ASC grafts) is directly due to the expression of NO secondary to transfection.
The in vitro data suggested that eNOS expression was limited to the first three weeks following transfection. At least two possibilities exist as to why transfection conferred a protective effect beyond this time point. First, we have observed up-regulation of other endothelial cell genes after eNOS transfection including CD31 (PECAM-1), KDR (Flk-1/VEGF receptor 2), and Flt-1 (VEGF receptor 1) that suggest that the presence of this enzyme, or NO itself, may further improve endothelial differentiation of ASC and subsequent function in vivo (unpublished results). Second, it is likely that the in vivo environment, one mainly characterized by fluid shear stress, improved endothelial differentiation of ASC. We have previously demonstrated in vitro the positive effect of shear on ASC, consistent with this mechanism (Fischer et al., 2009; Zhang et al., 2010). In sum, it is possible that forced NO expression was important for prevention of thrombosis early on, after which intrinsic endothelial characteristics of differentiated ASC took over. We do acknowledge that an important control in the current experiments to help elucidate this mechanism would have been the inclusion of a non-transfected (but EC-differentiated) ASC graft; as noted, we previously performed these experiments (Fischer et al., 2009) and in the interest of animal use reduction, we elected to compare the current results with these controls historically.
Given the atheroprotective and anti-hyperplastic properties of NO (Dias RG et al., 2011; Cui B, et al., 2011), it was originally hoped that NO production by the seeded stem cells might reduce graft hyperplasia. Although the graft scaffolding was decellularized to remove foreign antigen and significantly reduce immunogenicity (Meyers RL et al. 2006; Madden R, et al. 2002), our model ultimately employed a xenograft scaffold (human vein scaffold implanting into rabbit aorta) prone to hyperplasia. Hyperplasia did not appear to be significantly altered by eNOS-transfected ASC seeding. Unfortunately, histological evaluation of this important graft healing property was precluded by our inability to identify a clear graft edge within the control group. The observation of hyperplasia within the TEBV suggests that protection may require a more long-lasting production of NO (in contrast to the non-thrombogenic properties of the neointima). Possible mechanisms for any protective effect afforded by the seeded cells against hyperplasia, at least perhaps within the first three weeks, might include inhibition of cell migration by NO as well as reduced growth factor and cytokine production secondary to confluent coverage of the scaffold basement membrane.
In summary, although our group and others have demonstrated the acquisition of endothelial characteristics by ASC in response to various growth factors and shear force, consistent expression of eNOS message, protein or function is lacking. In this study, the forced expression of eNOS, and production of bioactive NO, within ASC appeared to improve their function as endothelial cell substitutes, highlighting the importance of eNOS expression in the creation of a successful tissue-engineered blood vessel.
Acknowledgements
This study was supported by the National Institutes of Health grant K08 HL076300-02 (PJD), and the American Vascular Association Lifeline Foundation (PJD).
References
- Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Yan Y, Zhang Z, et al. Properties of extracellular matrix-like scaffolds for the growth and differentiation of endothelial progenitor cells. J Surg Res. 2010;164(1):50–57. doi: 10.1016/j.jss.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Deng W, Kendirci M, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292(3):H1278–90. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12:43–45. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Campbell JH. Development of tissue engineered vascular grafts. Curr Pharm Biotechnol. 2007;8:43–50. doi: 10.2174/138920107779941426. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- Cui B, Huang L, Fang Y, et al. Transplantation of endothelial progenitor cells overexpressing endothelial nitric oxide synthase enhances inhibition of neointimal hyperplasia and restores endothelium-dependent vasodilatation. Microvasc Res. 2011;81(1):143–50. doi: 10.1016/j.mvr.2010.09.009. [DOI] [PubMed] [Google Scholar]
- DiMuzio P, Fischer L, McIlhenny S, et al. Development of a tissue-engineered bypass graft seeded with stem cells. Vascular. 2006;14:338–342. doi: 10.2310/6670.2006.00058. [DOI] [PubMed] [Google Scholar]
- DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg. 2007;45(Suppl A):A99–103. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21(4):622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Dias RG, Negrão CE, Krieger MH. Nitric oxide and the cardiovascular system: cell activation, vascular reactivity and genetic variant. Arq Bras Cardiol. 2011;96(1):68–75. [PubMed] [Google Scholar]
- Fischer LJ, McIlhenny S, Tulenko T, et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152(1):157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98(5):2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JE, Loscalzo J, Barnard MR, et al. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Investig. 1997;100:350–356. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortnaes J, Gottlieb D, Figueiredo JL, et al. Intravital molecular imaging of small-diameter tissue-engineered vascular grafts in mice: a feasibility study. Tissue Eng Part C Methods. 2010;16(4):597–607. doi: 10.1089/ten.tec.2009.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoetzer GL, Van Guilder GP, Irmiger HM, et al. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- Holmes K, Roberts OL, Thomas AM, et al. Vascular endothelial growth factor receptor-2: structure, function, intracellular signaling and therapeutic inhibition. Cell Signa. 2007;19(10):2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Zhang P, Abdollahi H, et al. Availability of Adipose-Derived Stem Cells in Patients Undergoing Vascular Surgical Procedures. J Surg Res. 2010;163(2):105–12. doi: 10.1016/j.jss.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LJ, Abdollahi H, Zhang P, et al. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2011;168(2):306–14. doi: 10.1016/j.jss.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540(1-3):7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Rowe D, Emsley AM, et al. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- Kanki-Horimoto S, Horimoto H, Mieno S, et al. Synthetic vascular prosthesis impregnated with mesenchymal stem cells overexpressing endothelial nitric oxide synthase. Circulation. 2006;114(1 Suppl):I327–30. doi: 10.1161/CIRCULATIONAHA.105.001586. [DOI] [PubMed] [Google Scholar]
- Lim SH, Cho SW, Park JC, et al. Tissue-engineered blood vessels with endothelial nitric oxide synthase activity. J Biomed Mater Res B Appl Biomater. 2008;85(2):537–546. doi: 10.1002/jbm.b.30977. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Nitric oxide insufficiency, platelet activation and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- McLarty AJ, Phillips MR, Holmes DR, Jr, et al. Aortocoronary bypass grafting with expanded polytetrafluoroethylene: 12-year patency. Ann Thorac Surg. 1998;65:1442–1444. doi: 10.1016/s0003-4975(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Magri D, Fancher TT, Fitzgerald TN, et al. Endothelial progenitor cells: a primer for vascular surgeons. Vascular. 2007;15(6):384–94. doi: 10.2310/6670.2007.00058. [DOI] [PubMed] [Google Scholar]
- Martin ND, Schaner PJ, Tulenko TN, et al. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17–23. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- McIlhenny SE, Hager ES, Grabo DJ, et al. Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by upregulation of the alpha5beta1 integrin. Tissue Eng Part A. 2010;16(1):245–255. doi: 10.1089/ten.tea.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RL, Lowichik A, Kraiss LW, et al. Aortoiliac reconstruction in infants and toddlers: replacement with decellularized branched pulmonary artery allograft. J Pediatr Surg. 2006;41(1):226–9. doi: 10.1016/j.jpedsurg.2005.10.086. [DOI] [PubMed] [Google Scholar]
- Madden R, Lipkowitz G, Benedetto B, et al. Decellularized cadaver vein allografts used for hemodialysis access do not cause allosensitization or preclude kidney transplantation. Am J Kidney Dis. 2002;40(6):1240–3. doi: 10.1053/ajkd.2002.36892. [DOI] [PubMed] [Google Scholar]
- Ning H, Liu G, Lin G, et al. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6(4):967–79. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Szilagyi DE, Hageman JH, Smith RF, et al. Autogenous vein grafting in femoropopliteal atherosclerosis: the limits of its effectiveness. Surgery. 1979;86:836–851. [PubMed] [Google Scholar]
- Sreerekha PR, Krishnan LK. Cultivation of endothelial progenitor cells on fibrin matrix and layering on dacron/polytetrafluoroethylene vascular grafts. Artif Organs. 2006;30:242–249. doi: 10.1111/j.1525-1594.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Serrano MC, Pagani R, Ameer GA, et al. Endothelial cells derived from circulating progenitors as an effective source to functional endothelialization of NaOH-treated poly(epsilon-caprolactone) films. J Biomed Mater Res A. 2008;87(4):964–971. doi: 10.1002/jbm.a.31728. [DOI] [PubMed] [Google Scholar]
- Shirota T, He H, Yasui H, et al. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9(1):127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- Siddiq S, Pamphilon D, Brunskill S, et al. Bone marrow harvest versus peripheral stem cell collection for haemopoietic stem cell donation in healthy donors. Cochrane Database Syst Rev. 2009;1:CD006406. doi: 10.1002/14651858.CD006406.pub2. [DOI] [PubMed] [Google Scholar]
- Schaner PJ, Martin ND, Tulenko TN, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117(Pt.2):2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Taite LJ, Yang P, Jun HW, et al. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008;84(1):108–16. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Vartanian KB, Kirkpatrick SJ, McCarty OJ, et al. Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J Biomed Mater Res A. 2009;91(2):528–539. doi: 10.1002/jbm.a.32225. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier J, Moncada S. Nitric oxide synthesized from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier JG, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;21:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Wu YF, Zhang J, Gu YQ, et al. Reendothelialization of tubular scaffolds by sedimentary and rotative forces: a first step toward tissue-engineered venous graft. Cardiovasc Revasc Med. 2008;9(4):238–247. doi: 10.1016/j.carrev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Wu S, Wang X, Guo L, Zi J. Adenovirus mediated endothelial nitric oxide synthase gene transfer prevents restenosis of vein grafts. ASAIO J. 2004;50(3):272–277. doi: 10.1097/01.mat.0000124842.79638.ce. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qi H, Wang H, et al. Engineering of vascular grafts with genetically modified bone marrow mesenchymal stem cells on poly (propylene carbonate) graft. Artif Organs. 2006;30(12):898–905. doi: 10.1111/j.1525-1594.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Moudgill N, Hager E, et al. Endothelial Differentiation of Adipose-Derived Stem Cells from Elderly Patients with Cardiovascular Disease. Stem cell & Dev. 2010;20(6):977–988. doi: 10.1089/scd.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]


