Abstract
CaMKIIγ, the predominant CaMKII isoform in mouse eggs, controls egg activation by regulating cell cycle resumption. In this study we further characterize the involvement and specificity of CaMKIIγ in mouse egg activation. Using exogenous expression of different cRNAs in Camk2g−/− eggs, we show that the other multifunctional CaM kinases, CaMKI, and CaMKIV, are not capable of substituting CaMKIIγ to initiate cell cycle resumption in response to a rise in intracellular Ca2+. Exogenous expression of Camk2g or Camk2d results in activation of nearly 80% of Camk2g−/− MII eggs after stimulation with SrCl2, which does not differ from the incidence of activation of wild-type eggs expressing exogenous Egfp. In contrast, none of the Camk2g−/− MII eggs expressing Camk1 or Camk4 activate in response to SrCl2 treatment. Expression of a constitutively active form of Camk4 (ca-Camk4), but not Camk1, triggers egg activation. EMI2, an APC/C repressor, is a key component in regulating egg activation downstream of CaMKII in both Xenopus laevis and mouse. We show that exogenous expression of either Camk2g, Camk2d, or ca-Camk4, but not Camk1, Camk4, or a catalytically inactive mutant form of CaMKIIγ (kinase-dead) in Camk2g−/− mouse eggs leads to almost complete degradation (~90%) of exogenously expressed EMI2 followed by cell cycle resumption. Thus, degradation of EMI2 following its phosphorylation specifically by CaMKII is mechanistically linked to and promotes cell cycle resumption in MII eggs.
Keywords: CaMKII, egg activation, Ca2+ signaling, EMI2, meiosis
Introduction
The calcium ion (Ca2+) is a key regulator of numerous cellular functions in a myriad of cell types and tissues. These functions include cell cycle progression, cell proliferation, fertilization, neuronal plasticity, neurotransmission, gene expression, and cell death.1,2 The main sensor of Ca2+ is the small calcium-binding protein calmodulin (CaM). The Ca2+/CaM complex binds and regulates a number of proteins, including ion channels and protein kinases. Among these Ca2+/CaM-dependent protein kinases (CaMKs) are myosin light chain kinase (MLCK), phosphorylase kinase, and eEF2-kinase (also known as CaMKIII), which are dedicated kinases, i.e., they phosphorylate a single substrate. On the other hand, the multifunctional CaMK family, which comprises CaMKI, CaMKII, CaMKIV, and CaMK kinase (CaMKK), can phosphorylate many different substrates in a wide range of cell types.3
CaMKII is a 12-subunit holoenzyme comprised of homo- or heteromers of α, β, γ, and δ subunits encoded by 4 different genes. CaMKI, CaMIV, and CaMKK, on the other hand, are monomeric and form the CaM kinase cascade, in which CaMKK phosphorylates and activates CaMKI and CaMKIV.4 The CaMK family shares considerable sequence homology and domain structure. They all have an N-terminal catalytic domain and a central auto-inhibitory and Ca2+/CaM-binding domain. CaMKII also has a unique C-terminal association domain involved in multimerization of the holoenzyme.5 In terms of substrate specificity, the consensus sequences for phosphorylation by CaMKI, CaMKII, and CaMKIV are quite similar, and hence these kinases sometimes phosphorylate the same substrates (for example cAMP-response element-binding protein [CREB]).5
One of the processes regulated by Ca2+ and CaM kinases is egg activation, a coordinated series of events that convert a fully differentiated egg into a cleavage-stage totipotent embryo.6 Upon sperm–egg binding and fusion, a sperm-specific phospholipase C (PLCζ) triggers an Ins(1,4,5)P3-mediated increase in intracellular Ca2+, which activates CaMKII and leads to metaphase II exit.7,8 In vertebrates, metaphase II arrest is maintained by the activity of maturation-promoting factor (MPF), a heterodimer of cyclin B1 and cyclin-dependent kinase 1 (CDK1). Meiotic resumption requires the degradation of cyclin B by the anaphase-promoting complex/cyclosome (APC/C). The endogenous meiotic inhibitor 2 (EMI2) is a protein that binds to and inhibits the APC/C activator CDC20. Upon fertilization, EMI2 is degraded, leading to APC/C activation, cyclin B degradation, and exit from metaphase II.9 The signaling pathway that connects fertilization with EMI2 degradation has been elucidated in frog. In Xenopus eggs, the calcium rise triggered by sperm activates CaMKII, which phosphorylates EMI2 and creates a site for phosphorylation by Xenopus Polo-like kinase 1 (Plx1). EMI2 phosphorylation by Plx1 in turn leads to EMI2 degradation, resulting in APC/C activation and exit from meiotic arrest.9 Expression of a constitutively active form of CaMKIIα in mouse eggs results in cyclin B1 and securin degradation,10 but the role of CaMKII in mediating EMI2 degradation in mouse oocytes remains unknown.
The only CaMKII isoform present in mouse eggs is CaMKIIγ.11,12 Genetic ablation and knockdown experiments demonstrate that CaMKIIγ is essential for meiotic resumption in mouse eggs.11,13 Female mice lacking CaMKIIγ are infertile, due to their inability to exit the metaphase II arrest. Nevertheless, cortical granule exocytosis and the zona pellucida block to polyspermy occur. This function of CaMKII is not isoform-specific, because CaMKIIδ can rescue the phenotype of CaMKIIγ-null eggs.11
In the present study, we set out to characterize further the specificity of CaMKII in triggering egg activation. We find that neither CaMKI nor CaMKIV can rescue the egg activation defects observed in CaMKIIγ-null eggs, and that the reason for this inability is likely that these 2 kinases cannot phosphorylate EMI2. Interestingly, a constitutively active form of CaMKIV, but not CaMKI, can rescue the phenotype of CaMKIIγ-null eggs and trigger degradation of EMI2.
Results and Discussion
CaMKII γ and δ, but not CaMKI or CaMKIV, can trigger cell cycle resumption in mouse eggs
We previously showed that Camk2g-null eggs fail to exit metaphase II arrest and that meiotic resumption in these eggs could be rescued by expression of exogenous Camk2g cRNA.11 This rescue, however, was not isoform-specific, because it could also be achieved by expressing CaMKIIδ, whose transcript is present in MII eggs at trace levels (~500-fold less than the γ isoform).11 In the present study, we addressed whether other multifunctional CaM kinases, namely CaMKI and CaMKIV, which are involved in cell cycle regulation in other cell types, are capable of mediating cell cycle resumption in Camk2g−/− eggs in response to a Ca2+ rise.
The transcript levels of Camk1 and Camk4 were determined in full-grown mouse oocytes using real-time RT-PCR (Fig. S1). Camk4 mRNA levels are comparable to Camk2d transcript levels (~1.5% of Camk2g transcript levels). Camk1 mRNA is virtually undetectable. We prepared cDNA constructs encoding CaMKI, CaMKIV, CaMKIIγ, and CaMKIIδ containing EGFP at their C-terminal end (Fig. 1A); cRNAs were made by in vitro transcription, and their concentrations were adjusted to produce the same level of expression based on quantification of the EGFP signal in injected eggs (Fig. 1B and C). As expected, the extent of activation of Camk2g−/− eggs in the presence of CaMKIIγ or CaMKIIδ following SrCl2 treatment (75.6% and 81.5%, respectively) was similar to that in wild-type eggs injected with Egfp cRNA (79.4%). In contrast, none of the Camk2g−/− eggs activated when Camk1 or Camk4 cRNAs were injected (Fig. 1B and D). This result demonstrated that CaMKII, but not other CaM kinases, is capable of functionally integrating the Ca2+ signal during egg activation.
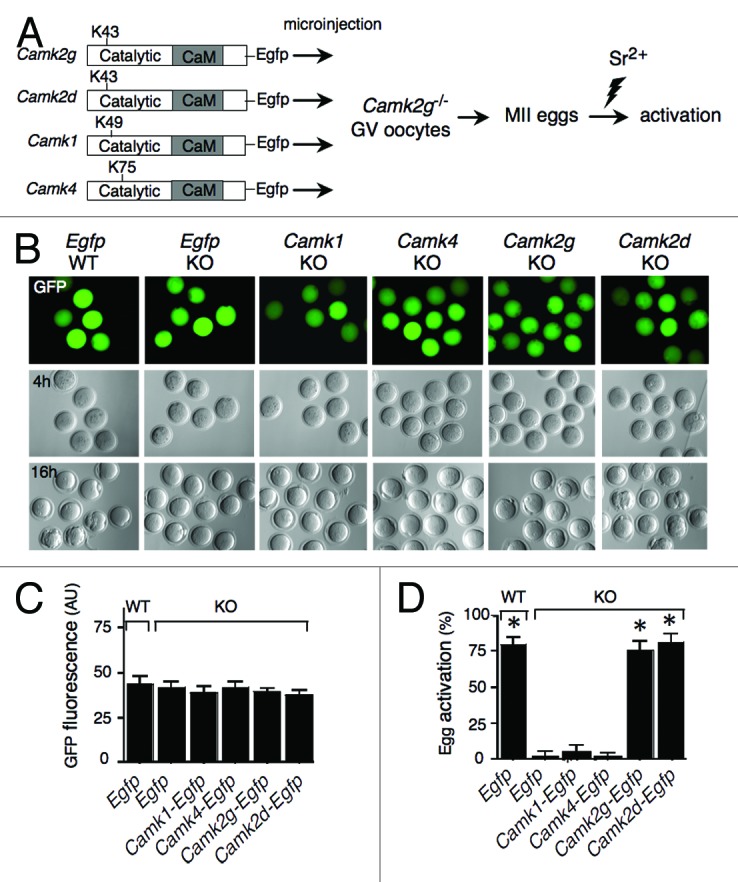
Figure 1. CaMKIIγ and δ, but not CaMKI or CaMKIV, can trigger MII exit in mouse eggs. (A) Schematic representation of the experimental procedure. cRNAs encoding EGFP or EGFP fused to different CaM kinases were microinjected into Camk2g−/− (KO) GV oocytes. As a control, Egfp cRNA was also injected into wild-type (WT) oocytes. Indicated are the catalytic and Ca2+/CaM-binding (CaM) domains, as well as the catalytic site (K43, K49, or K75) of each CaM kinase. Injected oocytes were matured in vitro, parthenogenetically activated with 10 mM SrCl2 (Sr2+) for 3 h, and scored for activation by pronucleus formation. (B) Paired EGFP fluorescence (top) and DIC images following injection of the cRNAs depicted in (A) into Camk2g−/− (KO) or Camk2g+/+ (WT) GV oocytes. The concentration of the different CaMK constructs was adjusted to yield similar levels of GFP fluorescence 4 h after injection (upper panel). The lower panel shows the presence of pronuclei in the control and Camk2g- and Camk2d-injected eggs 16 h after activation. A representative image of 4 experiments is shown. Magnification: 200×. (C) Comparable expression levels of the different constructs. The concentration of the different CaMK constructs was adjusted to yield similar levels of GFP fluorescence upon injection. The fluorescence was quantified using ImageJ software. The experiment was conducted 4 times, and the data are expressed as the mean ± SEM; at least 6 oocytes were analyzed in each group. (D) Egg activation, as measured by pronuclear formation, in the different groups 16 h after exposure to 10 mM SrCl2. The data are expressed as the mean ± SEM. Statistical analysis was performed using ANOVA. *P < 0.0001 vs. KO eggs injected with Egfp cRNA.
Expression of Camk2g and Camk2d, but not Camk1 or Camk4, in Camk2g−/− eggs leads to degradation of exogenously expressed EMI2
The signaling pathway that connects fertilization with EMI2 degradation has been elucidated in the frog, but whether this degradation pathway is conserved in mouse is not fully established. Although EMI2 degradation during mouse egg activation precedes cyclin B destruction,14,15 the role of EMI2 phosphorylation in targeting EMI2 degradation in mouse remains unknown. The phosphodegron responsible for xEmi2 degradation is poorly conserved in mouse,16 but mutations in a different region of mouse EMI2 that resembles a phosphodegron block EMI2 degradation.12 In fact, mouse EMI2 contains a putative CaMKII phosphorylation site (T176), whose mutation to alanine prevents EMI2 degradation in response to SrCl2.12 EMI2, however, is not phosphorylated by CaMKII in vitro.12 Thus, either mouse EMI2 is not a substrate of CaMKII or interaction of CaMKII with other proteins may be required for EMI2 phosphorylation by CaMKII.
To ascertain whether the unique capacity of CaMKII to initiate cell cycle resumption in mouse eggs is due to its ability to trigger EMI2 degradation, we co-injected mCherry-tagged Emi2 cRNA with different CaM kinase cRNAs into Camk2g−/− eggs (Fig. 2A). Exogenous expression of either Camk2g or Camk2d in Camk2g−/− eggs led to almost complete degradation of exogenous EMI2 (13.0% and 8.9% of EMI2 remained in those groups, respectively, compared with the EMI2 amount remaining in the control Egfp group) within an hour following SrCl2 activation. In contrast, EMI2 loss did not occur when either Camk1 or Camk4 cRNAs were injected (Fig. 2B and D). This result suggested that the inability of CaMKI and CaMKIV to trigger cell cycle resumption is due to their inability to phosphorylate EMI2, and thereby target EMI2 for degradation. This finding also suggested that EMI2 degradation is a key component of the egg activation machinery in mouse oocytes, and a unique function of CaMKIIγ (and potentially CaMKIIδ) is to promote EMI2 degradation in a short time window during egg activation.
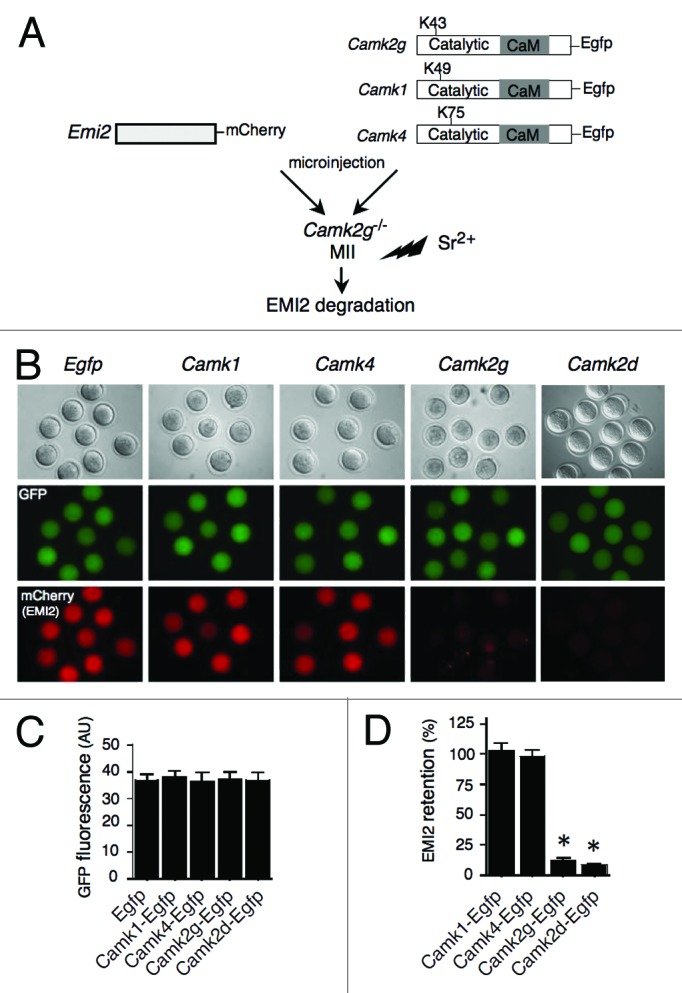
Figure 2. CaMKIIγ or δ, but not CaMKI or CaMKIV, can trigger EMI2 degradation in mouse eggs. (A) Schematic representation of the experimental procedure. Camk2g−/− MII eggs were co-injected with mCherry-tagged Emi2 cRNA and a cRNA encoding either EGFP or EGFP fused to different CaM kinases. After culture for 3 h, the injected eggs were activated with 10 mM SrCl2 for 1 h, and examined for fluorescence. (B) Paired images show DIC (top panel) microscopy of MII eggs, as well as EGFP (green) and mCherry (red) epifluorescence. Magnification: 200×. Representative images of 4 experiments are shown. (C) Quantitative adjustment of expression of different constructs by GFP signal. (D) The histogram shows retention of EMI2 after expression of different forms of CaM kinase, and the data are expressed as the amount of EMI2 in CaM kinase-injected groups relative to its amount in EGFP-injected control. The experiment was performed 3 times, and the data are expressed as mean ± SEM; at least 7 oocytes were analyzed in each group. Statistical analysis was performed using ANOVA. *P < 0.0001 vs. eggs injected with Egfp cRNA.
The catalytic activity of CaMKIIγ is necessary for EMI2 degradation
Our results suggested that mouse EMI2 is likely a CaMKIIγ substrate. To address this possibility, we utilized a catalytically inactive form of CaMKIIγ, in which K43 was replaced by methionine (K43M; Fig. 3A).2 Expression of K43M in Camk2g−/− eggs to the same level as wild-type Camk2g (adjusted by GFP signal; Fig. 3B and C) did not result in EMI2 degradation (Fig. 3B and D). Thus, the catalytic activity of CaMKIIγ is essential for EMI2 degradation, i.e., EMI2 is likely a substrate for CaMKII.
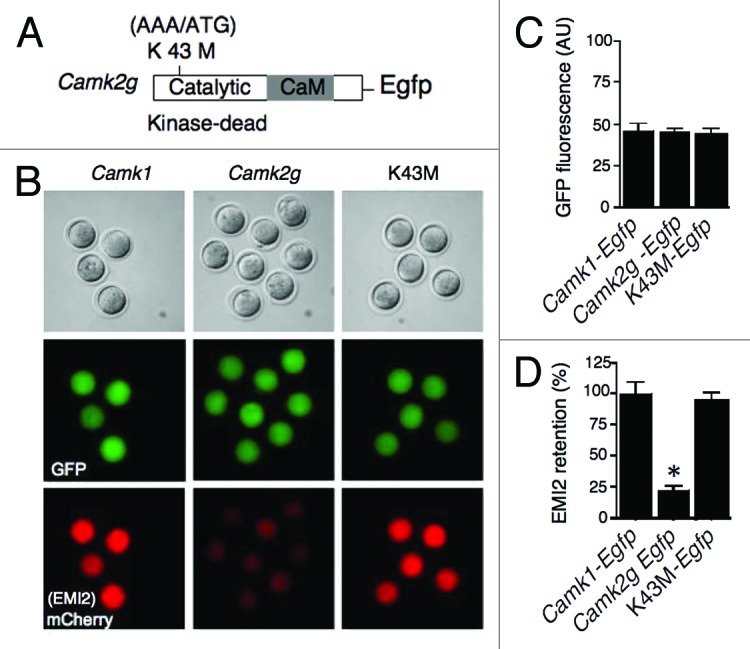
Figure 3. The catalytic activity of CaMKIIγ is necessary for EMI2 degradation. (A) Schematic representation of the mutation strategy to produce a catalytically inactive form (kinase dead) of CaMKIIγ. Lysine 43 was mutated to methionine (K43M). (B) Representative images for the assessment of EMI2 stability in different groups based on mCherry signal. Experimental procedures were the same as described in Figure 2. (C) Quantitative adjustment of expression of different constructs by GFP signal. (D) Quantification of the amount of EMI2 retained in eggs. The data are expressed as the amount of EMI2 in Camk2-injected groups relative to its amount in Camk1-injected control. The experiment was performed 3 times, and the data are expressed as mean ± SEM; at least 4 oocytes were analyzed in each group. Statistical analysis was performed using ANOVA (*P < 0.0001).
Expression of a constitutively active form of CaMKIV, but not CaMKI, in Camk2g−/− eggs leads to cell cycle resumption and degradation of exogenously expressed EMI2
Considering that CaMKIIγ shares some sequence homology with CaMKI and CaMKIV, particularly within the catalytic domain, and that these 3 kinases share several substrates, we wondered whether the failure of Camk2g−/− eggs to degrade EMI2 in the presence of CaMKI or CaMKIV was due to an insufficient activity of these kinases in the egg. Because CaMKI and CaMKIV require the upstream activator CaMKK for full activity, and based on publically available microarray data that this kinase seems to be absent in mouse oocytes, exogenously expressed CaMKI and IV may not be able to efficiently phosphorylate EMI2.
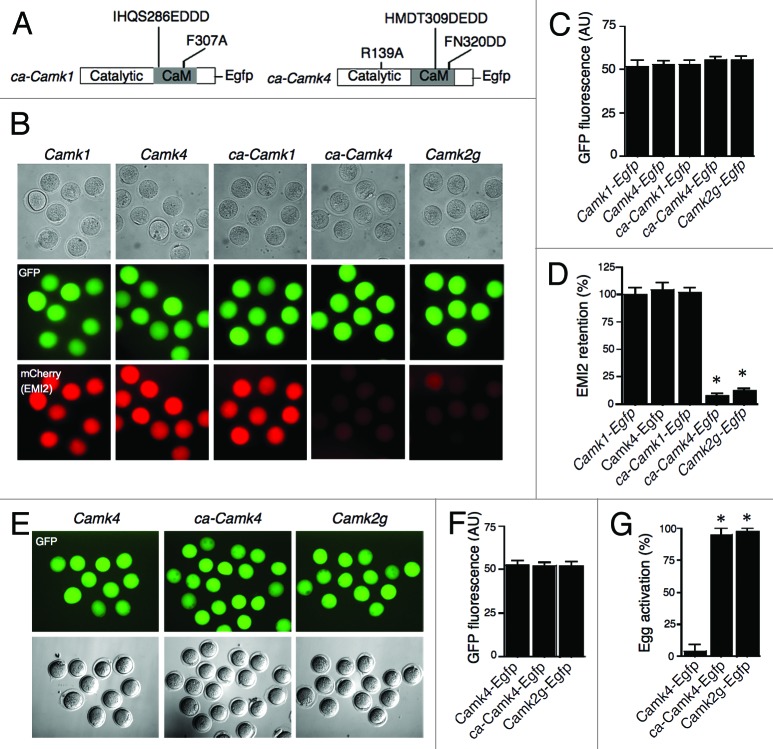
To investigate the last scenario we expressed constitutively active mutated forms of Camk1 (ca-Camk1) and Camk4 (ca-Camk4) that do not require activation by CaMKK17,18 (Fig. 4A). Expression of ca-Camk4 in Camk2g−/− eggs led to a substantial reduction (>90%) of exogenous EMI2, similar to the extent of EMI2 reduction observed in eggs injected with Camk2g cRNA (Fig. 4B–D). No EMI2 degradation, however, was observed in Camk2g−/− eggs expressing ca-Camk1 cRNA. Although it is formally possible that the expressed enzyme was inactive, this possibility is minimized because the cRNA was translated in a natural environment that should support proper protein folding. Further analysis of egg activation following SrCl2 treatment showed that essentially all (95%) Camk2g−/− eggs expressing ca-Camk4 formed pronuclei (Fig. 4E–G), which was similar to the incidence of pronuclear formation among eggs injected with Camk2g (97.5%). This result suggested that CaMKIV, but not CaMKI, could potentially phosphorylate EMI2 to trigger its degradation. Although we cannot exclude the possibility that exogenous expression of ca-CaMKIV can result in off-target phosphorylation of EMI2 due to a loss of substrate specificity, this possibility is minimized, because similar levels of ca-Camk1 cRNA did not trigger degradation of EMI2 in mouse eggs. In a similar vein, because CaMKI overexpression does not trigger EMI2 degradation, and all of the different CaMK isoforms were expressed to similar levels (based on fluorescence intensity), the likelihood that their overexpression led to egg activation is minimized.
Figure 4. Expression of ca-CaMKIV in CaMKIIγ−/− eggs triggers degradation of exogenous EMI2 and cell cycle resumption. (A) Schematic representation of the mutation strategy to produce constitutively active forms of CaMKI (ca-CaMKI) and IV (ca-CaMKIV). (B) Representative images for the assessment of EMI2 stability in different groups based on mCherry signal. Experimental procedures were the same as described in Figure 2. (C) Quantitative adjustment of expression of different constructs by GFP signal. (D) Quantification of the amount of EMI2 retained in Camk2g−/− eggs. Camk1 group was used as a control, since the experiments depicted in Figure 2 had established that exogenous EMI2 remains essentially intact in the presence of CaMKI. The data are expressed as the amount of EMI2 in Camk4, ca-Camk1, ca-Camk4, and Camk2g groups relative to the amount in Camk1 group. (E–G) Activation of Camk2g−/− eggs expressing different CaMK constructs. Experimental procedures were the same as described in Figure 1. *P < 0.0001 vs. KO eggs injected with Camk1 cRNA. (E) The expression of Camk4, ca-Camk4, and Camk2g was adjusted to similar level by the intensity of GFP signal. Activation of eggs was assessed by PN formation 8 h after the onset of SrCl2 treatment. Magnification: 200×. (F) Quantitative adjustment of expression of different constructs by GFP signal. The data are expressed as the mean ± SEM (G) Egg activation, as measured by pronuclear formation, in the different groups 8 h after exposure to 10 mM SrCl2. The data are expressed as the mean ± SEM. Statistical analysis was performed using ANOVA. *P < 0.0001.
Camk2a and Camk2b are not expressed in oocytes, consistent with Camk2a being neuron-specific19 and Camk2b being primarily neuronal.20Camk2d and Camk2g are expressed in many tissues,20 and hence the virtual absence of Camk2d expression in oocytes is striking in light of the ability of CaMKIIδ to activate Camk2g−/− eggs to a similar extent as CaMKIIγ under the experimental conditions used here. CaMKIIγ has a lower Kd for CaM than CaMKIIδ, and is activated by lower concentrations of CaM than CaMKIIδ,21 and therefore is predicted to be more sensitive to changes in intracellular Ca2+ concentration. These properties, coupled with CaMKIIγ possessing a lower intrinsic autophosphorylating activity, which would render the enzyme insensitive to changes in intracellular Ca2+, may enable CaMKIIγ’s activity to oscillate better with the transient increases in intracellular Ca2+ that ensue following fertilization22 and drive the events of egg activation.23
It is perplexing, however, why oocytes express only the γ isoform, given the centrality of exit from metaphase II arrest following fertilization to reproduction, and do not express another form, e.g., CaMKIIδ, that could, in principle, provide sufficient compensatory function to support development in the absence of sufficient amounts of CaMKIIγ. Preservation of duplicated genes has been proposed to occur only by either neo-functionalization or sub-functionalization (i.e., purely redundant copies must be lost).24 Maintenance of Camk2d would therefore reflect that it functions similarly but not identically to Camk2g. Thus, there is no apparent a priori reason that Camk2d should not be expressed in oocytes and available to serve in a compensatory fashion should an egg possess an insufficient amount of CaMKIIγ. It is also unlikely that expressing both Camk2d and Camk2g would be deleterious for egg activation, because the incidence of SrCl2-induced activation following injection of Camk2g−/− eggs with cRNAs encoding both Camk2d and Camk2g was 86% (12/14). Lastly, although CaMKIIδ can activate Camk2g−/− eggs, the developmental competence of these activated eggs beyond pronuclear formation could be compromised due to differences in substrate specificity of the different CaMKII isoforms.
As noted above, although Camk2g−/− female mice are infertile, they appear morphologically normal (although their behavior has not been assessed). This observation raises the intriguing question whether CaMKIIγ is only required for metaphase II exit, i.e., only necessary for about 30–60 min post-insemination, in the life history of a female mouse. This proposal could be tested, in principle, by inseminating Camk2g−/− eggs with Camk2g−/− sperm and promoting metaphase II exit and egg activation by treating the inseminated eggs with TPEN, a Zn2+-chelating agent,25 which leads to EMI2 degradation, and assessing whether an offspring is born following embryo transfer.
Materials and Methods
Animals
Generation of CaMKIIγ−null mice has been described previously.11 All mouse experiments were performed in strict accordance with the National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Use and Care Committee of the University of Pennsylvania (protocol number 803766).
Collection and culture of oocytes and eggs
Collection of germinal vesicle (GV)-intact, full-grown oocytes and metaphase II (MII) eggs was performed as previously described.11 All cell cultures were done in 5% CO2 in humidified air at 37 °C. cRNA-injected GV oocytes were cultured in CZB medium26 supplemented with 2.5 µM milrinone (to inhibit germinal vesicle breakdown) for 2 h, washed, and then cultured in milrinone-free CZB for 16 h to allow meiotic maturation. The resulting MII eggs were briefly examined for GFP fluorescence and activated using 10 mM SrCl2 in Ca2+/Mg2+-free CZB supplemented with 5 µg/ml cytochalasin B (to obtain diploid parthenogenotes and prevent fragmentation) for 3 h followed by another 3 h of culture in CZB supplemented with cytochalasin B only. The eggs were then washed and transferred to KSOM27 for further culture. Activation was scored by pronucleus formation and/or cleavage. cRNA-injected in vivo-derived MII eggs were cultured in CZB for 3 h and activated with 10 mM SrCl2 in Ca2+/Mg2+-free CZB for 1 h. The eggs were then used for live fluorescence imaging.
cDNA constructs, cRNA preparation, and microinjection
Plasmids encoding full-length CaMKIIγ (the isoform containing a 21-amino acid insert in the variable domain, which is between the CaM-binding domain and association domain28) and CaMKIIδ in pT7TS were described in reference 11. Full-length human Camk1 and Camk4 in pSG5 vector with an N-terminal FLAG tag were kindly provided by Anthony Means, Duke University School of Medicine. Full-length Camk2g, Camk2d, Camk1, and Camk4 were excised from their original plasmids and subcloned into a pIVT plasmid29 containing Egfp to generate fusion proteins that express EGFP at their C-terminal end. Sequence errors in the original cDNAs were corrected by site-directed mutagenesis using the QuikChange Lightning Multi Site-Directed Mutagenesis Kit (Agilent Technologies, 210515; http://www.genomics.agilent.com/en/Site-Directed-Mutagenesis/QuikChange-Lightning/?cid=AG-PT-175&tabId=AG-PR-1162).
Full-length mouse Emi2 cDNA was generated by RT-PCR of MII egg total RNA using primers described in reference 14. An SmaI-XhoI Emi2 fragment was inserted into a pIVT-mCherry plasmid to produce Emi2 with a C-terminal mCherry fusion. A catalytically inactive form of CaMKIIγ, K43M, constitutively active CaMKI (ca-CaMKI [IHQS286EDDD, F307A]) and constitutively active CaMKIV (ca-CaMKIV [R139A, HMDT309DEDD, FN320DD]) were produced by site-directed mutagenesis using the QuikChange Lightning Multi Site-Directed Mutagenesis Kit. The cDNAs of all kinase mutants were sequenced and were confirmed to be free of errors.
All constructs were linearized and in vitro transcribed using the mMESSAGE mMACHINE T7 kit (Life Technologies, AM1344; http://www.lifetechnologies.com/order/catalog/product/AM1344), according to the manufacturer’s protocol. All Camk and Egfp cRNAs were re-suspended at a concentration of 1.5 mg/mL (the actual concentration was adjusted by titration to provide similar amount of expression of different cRNAs estimated by the intensity of GFP fluorescence), and Emi2 cRNA was re-suspended at a concentration of 0.5 mg/mL in TE buffer and stored at −80 °C prior to use for microinjection. GV oocytes or MII eggs were injected with ~5 pl of the corresponding cRNA solution. In the EMI2 decay experiments, MII eggs were microinjected with ~5 pl of a mixture (1/1) of Emi2 cRNA and either Camk2g, Camk2d, Camk1, Camk4, K43M, ca-Camk1, ca-Camk4, or Egfp cRNA; the injections were performed as previously described.29
Protein fluorescence imaging
For quantitative adjustment of expression of injected cRNAs, GFP fluorescence was briefly examined in live eggs using epifluorescence microscopy; images were captured with a MicroMAX camera (Roper Scientific) driven by MetaMorph image analysis software (Molecular Devices). When appropriate, the eggs were transferred to KSOM for further culture.
The amount of EMI2 in cRNA-injected eggs following SrCl2 activation was quantified by the intensity of mCherry signal on a Leica DMI4000B confocal scanning microscope (Leica Microsystems) equipped with a 20 × 1.4 NA objective and differential interference contrast (DIC) optics. Excitation at 540/25 nm was used for mCherry fluorescence detection and at 480/30 nm for GFP fluorescence. IPLab software (BD Biosciences) was used for image acquisition. NIH ImageJ software (National Institutes of Health) was used to quantify the intensity of fluorescence. The data were analyzed using Prism 4 software (Graph Pad Software).
RNA Isolation and quantitative real-time RT-PCR
Total RNA was extracted from 20 full-grown oocytes using the Picopure RNA Isolation Kit (Life Technologies, KIT0204; http://www.lifetechnologies.com/order/catalog/product/KIT0204), according to the manufacturer’s instructions. Reverse transcription and real-time PCR were performed as described.30 The TaqMan gene expression assays used (Life Technologies) were as follows: Mm00437967_m1 (CaMKIIα), Mm00432296_m1 (CaMKIIβ), Mm00618054_m1 (CaMKIIγ), Mm00499266_m1 (CaMKIIδ), Mm00519436_m1 (CaMKI), and Mm01135329_m1 (CaMKIV).
Statistical analysis
All experiments were independently replicated at least 3 times. Data were analyzed by ANOVA using Prism software in which a post-hoc Dunnett test was employed. Images presented are representative of the outcomes obtained in the replicate experiments.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by National Institutes of Health Grant HD 022732 (to R.M.S.).
References
- 1.Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–8. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 2.Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–56. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 3.Skelding KA, Rostas JAP, Verrills NM. Controlling the cell cycle: the role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle. 2011;10:631–9. doi: 10.4161/cc.10.4.14798. [DOI] [PubMed] [Google Scholar]
- 4.Wayman GA, Tokumitsu H, Davare MA, Soderling TR. Analysis of CaM-kinase signaling in cells. Cell Calcium. 2011;50:1–8. doi: 10.1016/j.ceca.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- 6.Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/S0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- 7.Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–73. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–79. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt A, Rauh NR, Nigg EA, Mayer TU. Cytostatic factor: an activity that puts the cell cycle on hold. J Cell Sci. 2006;119:1213–8. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]
- 10.Madgwick S, Levasseur M, Jones KT. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci. 2005;118:3849–59. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- 11.Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A. 2010;107:81–6. doi: 10.1073/pnas.0912658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry ACF. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010;137:3281–91. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H-Y, Minahan K, Merriman JA, Jones KT. Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development. 2009;136:4077–81. doi: 10.1242/dev.042143. [DOI] [PubMed] [Google Scholar]
- 14.Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–45. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry AC, Verlhac MH. Second meiotic arrest and exit in frogs and mice. EMBO Rep. 2008;9:246–51. doi: 10.1038/embor.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayman GA, Kaech S, Grant WF, Davare M, Impey S, Tokumitsu H, Nozaki N, Banker G, Soderling TR. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J Neurosci. 2004;24:3786–94. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokumitsu H, Brickey DA, Glod J, Hidaka H, Sikela J, Soderling TR. Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. Identification of a brain CaM-kinase IV kinase. J Biol Chem. 1994;269:28640–7. [PubMed] [Google Scholar]
- 19.Takaishi T, Saito N, Tanaka C. Evidence for distinct neuronal localization of gamma and delta subunits of Ca2+/calmodulin-dependent protein kinase II in the rat brain. J Neurochem. 1992;58:1971–4. doi: 10.1111/j.1471-4159.1992.tb10079.x. [DOI] [PubMed] [Google Scholar]
- 20.Bayer KU, Löhler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–54. doi: 10.1016/S0169-328X(99)00131-X. [DOI] [PubMed] [Google Scholar]
- 21.Gaertner TR, Kolodziej SJ, Wang D, Kobayashi R, Koomen JM, Stoops JK, Waxham MN. Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:12484–94. doi: 10.1074/jbc.M313597200. [DOI] [PubMed] [Google Scholar]
- 22.Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003;258:464–74. doi: 10.1016/S0012-1606(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 23.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–91. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 24.Ohno S. Evolution by gene duplication. Springer-Verlag, 1970. [Google Scholar]
- 25.Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry ACF. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development. 2010;137:2659–69. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- 26.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–88. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 27.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–8. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry AC. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010;137:3281–91. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol. 2007;312:321–30. doi: 10.1016/j.ydbio.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knott JG, Gardner AJ, Madgwick S, Jones KT, Williams CJ, Schultz RM. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol. 2006;296:388–95. doi: 10.1016/j.ydbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.