Abstract
Thrombin and iron are two major players in intracerebral hemorrhage-induced brain injury and our recent study found that thrombin contributes to hydrocephalus development in a rat model of intraventricular hemorrhage (IVH). This study investigated the role of red blood cell (RBC) lysis and iron in hydrocephalus after IVH. There were three parts to this study. First, male Sprague-Dawley rats received an injection of saline, packed, or lysed RBCs into the right lateral ventricle. Second, rats had an intraventricular injection of iron or saline. Third, the rats received intraventricular injection of lysed RBCs mixed with deferoxamine (0.5 mg in 5 μL saline) or saline. All rats underwent magnetic resonance imaging at 24 hours and were then euthanized for brain edema measurement, western blot analysis, or brain histology. We found that intraventricular injection of lysed RBCs, but not packed RBCs, resulted in ventricular enlargement and marked increases in brain heme oxygenase-1 and ferritin at 24 hours. Intraventricular injection of iron also resulted in ventricular enlargement and ventricular wall damage 24 hours later. Coinjection of deferoxamine reduced lysed RBC-induced ventricular enlargement (P<0.01). These results suggest that iron, a degradation product of hemoglobin, has an important role in hydrocephalus development after IVH.
Keywords: deferoxamine, hydrocephalus, intracerebral hemorrhage, iron, red blood cell
Introduction
Intraventricular hemorrhage (IVH) is often associated with intracerebral hemorrhage (ICH) and subarachnoid hemorrhage.1, 2, 3 Hydrocephalus frequently occurs after IVH, developing in up to 55% of IVH patients.1
Although it is well known that IVH is associated with hydrocephalus, the underlying mechanisms are still not clear. Evidence suggests a relationship between the incidence of posthemorrhagic hydrocephalus and the amount of intraventricular blood. In ICH, thrombin formation, red blood cell (RBC) lysis, and iron toxicity have a major role in brain injury after hemorrhage. Our recent study showed that thrombin contributes to IVH-induced hydrocephalus.4
There have been few studies evaluating the relationship between iron released from blood into cerebrospinal fluid (CSF) and hydrocephalus; however, there is some clinical evidence of a link between iron and posthemorrhagic hydrocephalus. Nonprotein-bound iron was found in CSF from preterm infants with posthemorrhagic ventricular dilatation compared with infants without IVH.5 In subarachnoid hemorrhage patients, a high level of the endogenous iron chelator ferritin in CSF is an independent predictive factor for chronic hydrocephalus.6 In addition, iron and ferritin deposition are found in ependymal or subependymal location after neonatal IVH.7, 8, 9 Our recent studies indicate that iron may have a role in hydrocephalus development.10, 11
In the current study, we investigated whether RBC lysis and iron cause hydrocephalus in adult rats.
Materials and methods
Animal Preparation and Intraventricular Injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. The University of Michigan has an Animal Welfare Assurance on file with the Office for Protection from Research Risks and is fully accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies follow the Guide for The Care and Use of Laboratory Animals (National Research Council) and comply with the ARRIVE guidelines for reporting in vivo experiments. A total of 70 male Sprague-Dawley rats (3 to 4 months old; Charles River Laboratories, Portage, MI, USA), weighing 250 to 350 g, were used in this study. Animals were anesthetized with pentobarbital (50 mg/kg, intraperitoneally) and the right femoral artery was catheterized to monitor arterial blood pressure, blood pH, PaO2, PaCO2, hematocrit, and glucose levels. Core body temperature was maintained at 37.5°C with a feedback-controlled heating pad. Rats were then positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). A cranial burr hole (1 mm) was drilled and a 26-gauge needle was inserted stereotaxically into the right lateral ventricle (coordinates: 0.6 mm posterior, 4.5 mm ventral, and 1.6 mm lateral to the bregma). Packed or lysed RBCs were obtained as previously described.12 Briefly, autologous blood was washed in saline, and packed RBCs were obtained by centrifugation and discarding the supernatant. Red blood cells were lysed by freezing the packed RBCs in liquid nitrogen (8 minutes) followed by thawing at 37°C (5 minutes) three times. Saline, iron, packed, or lysed RBCs were infused into right lateral ventricle over 10 minutes by a micro infusion pump (World Precision Instruments Inc., Sarasota, FL, USA). The needle was removed after injection, the burr hole was filled with bone wax, and the skin incision was closed with sutures. A total of five rats died within 24 hours of injection of lysed RBCs.
Experimental Groups
There were three parts to this study. First, 30 μL of saline, packed, or lysed RBCs was injected into the right lateral ventricle. All rats were euthanized at 24 hours for western blot analysis (n=12) or immunohistochemistry (n=12) after T2-weighted magnetic resonance imaging (MRI). Second, rats received an intraventricular injection of FeCl3 (2 mmol/L, 50 μL, n=6) or saline (50 μL) (n=6), and had an MRI scan after 24 hours. In the third part, rats received a 30-μL injection of lysed RBCs (n=14) mixed with 5 μL of deferoxamine (100 mg/mL) or the same volume of saline (n=15) into the right lateral ventricle. Rats were euthanized at 24 hours for brain edema and brain histology after MRI scans.
Magnetic Resonance Imaging and Ventricular Volume Measurement
Imaging was performed in a 7.0-T Varian MR scanner (183-mm horizontal bore; Varian, Palo Alto, CA, USA). Rats were anesthetized with 2% isoflurane/air mixture throughout the MRI examination. A T2 fast spin-echo sequence (repetition time/echo time=4,000/60 ms) was performed. The field of view was 35 × 35 mm, and the matrix was 256 × 128 mm. A total of 25 coronal slices (0.5 mm thick) were acquired to cover the entire axis of the lateral ventricles. All image analysis was performed using NIH Image J (NIH, Bethesda, MD, USA). Lateral ventricular volumes were calculated from T2 images as described previously.13 Both lateral ventricles were outlined and the areas were measured. Ventricular volume was obtained by combining the ventricle area over all slices incorporating the lateral ventricles and multiplying by section thickness (0.5 mm). A blinded observer performed all the measurements. It is important to differentiate the edematous white matter from the lateral ventricles on T2 MRI. Lateral ventricle volumes were measured on T2 images based on the brain anatomy and were confirmed by brain histology.
Western Blot Analysis
Western blot analysis was performed as previously described.10 The brains were perfused with saline before decapitation. The periventricular zone (PVZ) (∼1 mm thick brain tissue around the ventricle) was sampled. Protein concentration was determined by the Bio-Rad protein assay kit (Hercules, CA, USA) and 50 μg protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a Hybond-C pure nitrocellulose membrane (Amersham, Pittsburgh, PA, USA). The primary antibodies were polyclonal rabbit anti-rat heme oxygenase-1 (HO-1) IgG (1:2,000 dilution; StressGen, Farmingdale, NY, USA), rabbit anti-ferritin heavy chain polyclonal antibody (1:2,000 dilution; Cell Signaling Technology, Danvers, MA, USA) and goat anti-ferritin light chain polyclonal antibody (1:2,000 dilution; Abnova, Taipei, Taiwan). The antigen–antibody complexes were visualized by chemiluminescence system (Denville Scientific Inc., Metuchen, NJ, USA) and exposed to photosensitive film. The relative densities of bands were analyzed with NIH Image J.
Immunohistochemistry
Rats were anesthetized with pentobarbital (100 mg/kg, intraperitoneally) and perfused with 4% paraformaldehyde in 0.1 mol/L pH 7.4 phosphate-buffered saline. The brains were removed and kept in 4% paraformaldehyde for 4 to 6 hours, then immersed in 30% sucrose for 3 to 4 days at 4°C. The brains were embedded in optimal cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA, USA) and 18-μm-thick slices were cut using a cryostat. Immunohistochemistry was performed using the avidin–biotin complex technique as previously described.10 The primary antibodies were polyclonal rabbit anti-human ferritin IgG (DAKO, Glostrup, Denmark; 1:400 dilution) and polyclonal rabbit anti-rat HO-1 IgG (StressGen, 1:400 dilution).
Electron Microscopy
Electron microscopy was performed as previously described.14 Rats were anesthetized and subjected to intracardiac perfusion with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L Sorensen's buffer (pH 7.4). The brains were removed and a 1-mm-thick coronal brain slice was cut with a blade ∼4 mm from the frontal pole. The ipsilateral ventricular wall tissue was sampled and immersed in the same fixative overnight at 4°C. Samples were then postfixed with 1.0% OsO4 and dehydrated in graded ethyl alcohol. After complete dehydration, samples were infiltrated with propylene oxide, embedded in Epon, and sectioned. Ultra-thin sections were then stained with uranyl acetate and Reynold's lead citrate, and evaluated using Philips CM 100 TEM (Hillsboro, OR, USA) and digitally imaged using a Hamamatsu (Hamamatsu City, Shizuoka, Japan) ORCA-HR camera.
Brain Water Content
The determination of brain water content was performed as described previously.15 Briefly, the rat brain was removed after euthanasia and divided into left hemisphere, right hemisphere, and cerebellum. After weighing, the samples were dried in a gravity oven for 48 hours. The water content was determined as (wet weight−dry weight)/wet weight.
Statistical Analysis
Values are given as mean±S.D. Student's t-test or analysis of variance (ANOVA) was used for analyzing the data. The differences were considered as significant at P<0.05.
Results
Physiologic parameters of rat, including mean arterial blood pressure, blood gas, hematocrit, blood pH, and glucose were monitored and controlled in normal ranges. Mortality in the lysed erythrocyte group was 19% (5 of 27) and zero in all other groups.
Lysed Red Blood Cells But Not Packed Red Blood Cells Result in Hydrocephalus
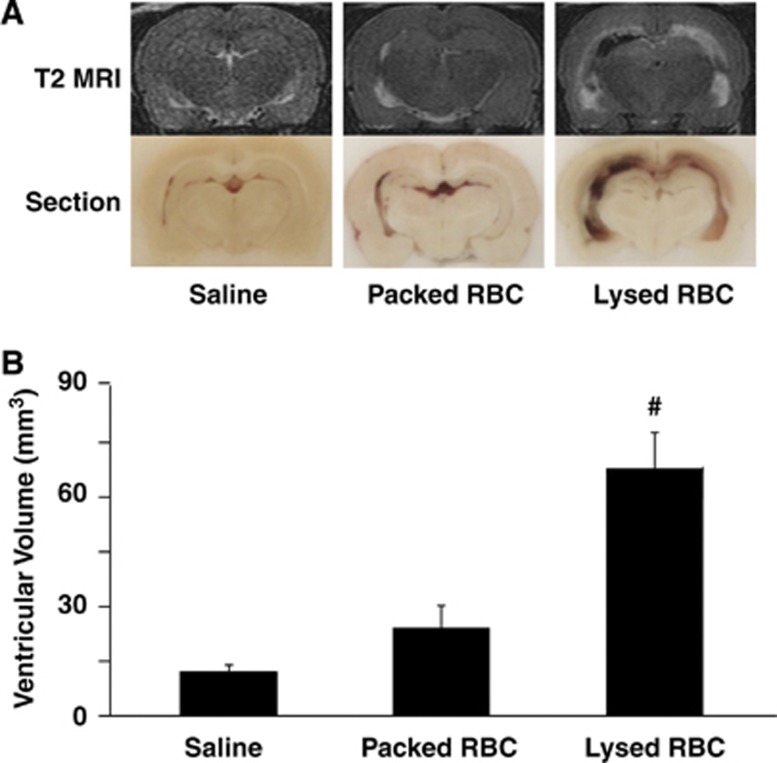
To examine the effect of erythrocyte lysis on hydrocephalus development, saline, packed RBCs, or lysed RBCs were injected into the right lateral ventricle. Injection of lysed RBCs resulted in significantly larger ventricular volumes (lateral ventricle volume 67.9±10.0 mm3) compared with injection of packed RBCs (24.1±6.2 mm3, P<0.01) and saline (P<0.01) at 24 hours (Figure 1). Although there was a trend for injection of packed RBCs to induce slight ventricular dilation compared with saline injection, this did not reach a statistical significance (P>0.05, Figure 1).
Figure 1.
(A) T2-weighted magnetic resonance imaging (MRI) scans and coronal sections at 24 hours after intraventricular injection of saline, packed, or lysed red blood cells (RBCs). (B) Lateral ventricular volumes determined from MRI scans in the same groups. Values are means±s.d., n=6, #P<0.01 vs. saline and packed RBC groups by analysis of variance (ANOVA).
Intraventricular Injection of Lysed Red Blood Cells Results in Upregulation of Periventricular Heme Oxygenase-1 and Ferritin
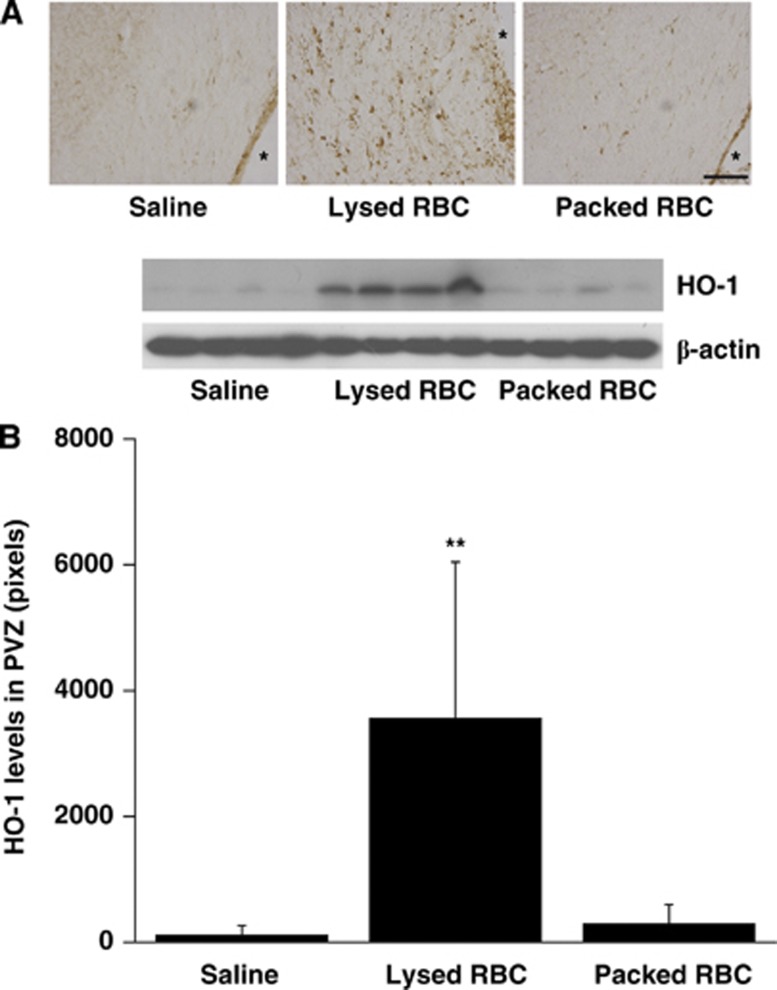
The expression of HO-1, a key enzyme for heme degradation, was evaluated in periventricular tissue. There were many more periventricular HO-1-positive cells after injection of lysed RBCs than after saline or packed RBC injection. Heme oxygenase-1-1-positive cells were mostly gliallike (Figure 2). HO-1 protein levels in the periventricular area were significantly increased in rats receiving lysed RBCs (3,566±2,481 pixels vs. 115±141 pixels in saline group and 295±311 pixels in the packed RBC group, P<0.05, Figure 2).
Figure 2.
Heme oxygenase-1 (HO-1) immunoreactivity (A) and protein levels (B) in the periventricular zone (PVZ) 24 hours after injection of saline, packed red blood cells (RBCs) and lysed RBCs into the right ventricle, n=4, **P<0.05 vs. saline and packed RBC groups by analysis of variance (ANOVA). Scale bar=100 μm, * indicates lateral ventricle.
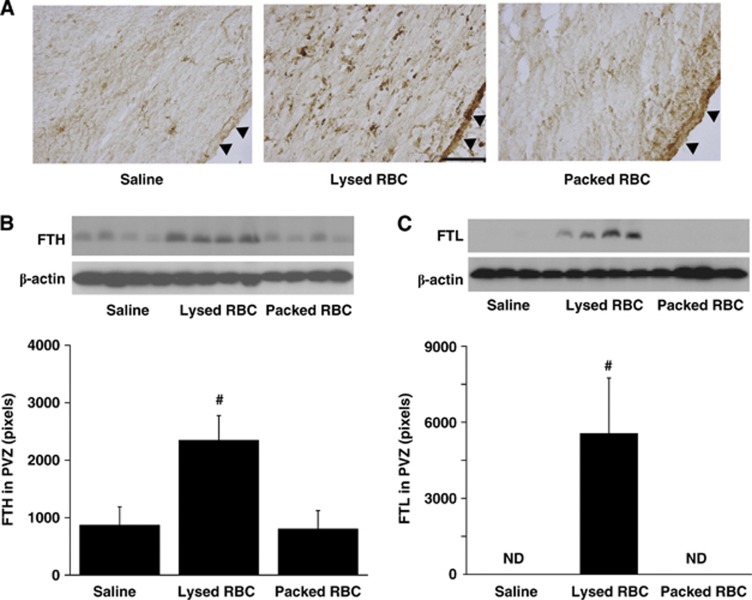
Ferritin was also investigated in the periventricular area 24 hours after injection as it is an iron storage protein. There was a marked increase in periventricular ferritin immunoreactivity after injection of lysed RBCs (Figure 3A) compared with saline or packed RBC injection. Most ferritin-positive cells were also gliallike although there was also evidence of expression in the ependyma. Ferritin upregulation in the PVZ was confirmed by western blotting. Injection of lysed RBCs resulted in higher protein levels of ferritin heavy chain (2,350±429 pixels vs. 800±326 pixels in packed RBC group, P<0.01, Figure 3B) and ferritin light chain (P<0.01, Figure 3C), compared with saline or packed RBCs.
Figure 3.
Ferritin immunoreactivity (A) and protein levels of ferritin heavy chain (FTH, B) and ferritin light chain (FTL, C) in the periventricular zone (PVZ) 24 hours after intraventricular injection of saline, packed, and lysed red blood cells (RBCs). Values are mean±s.d., n=4, #P<0.01 vs. saline or packed RBCs groups by analysis of variance (ANOVA), ND, not detectable. Scale bar=100 μm. Arrowheads indicate ependymal surface.
Intraventricular Injection of Iron Results in Ventricular Enlargement and Ependymal Cell Injury
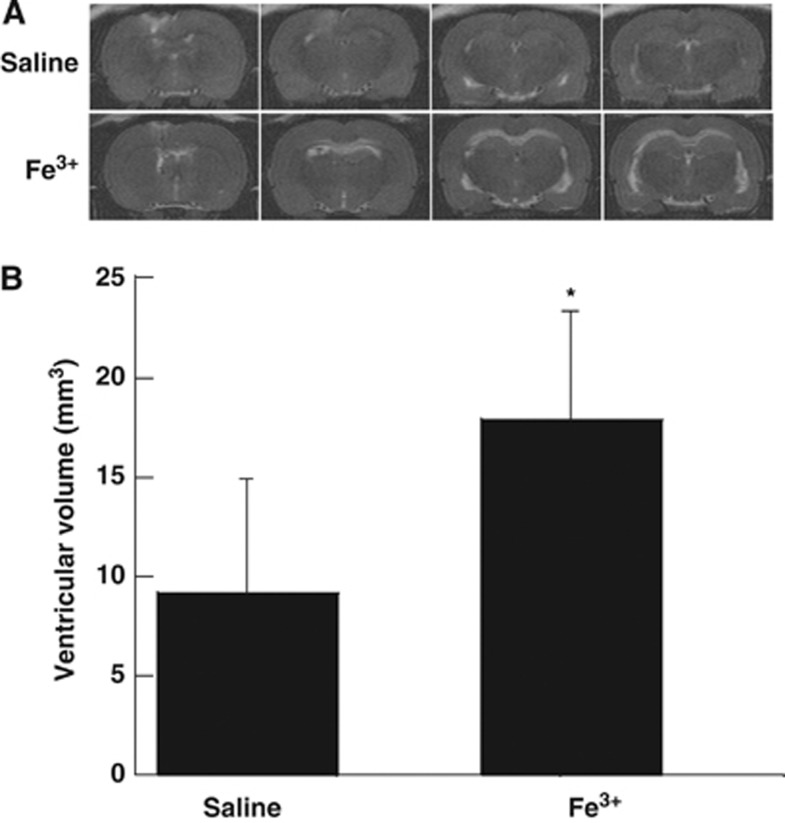
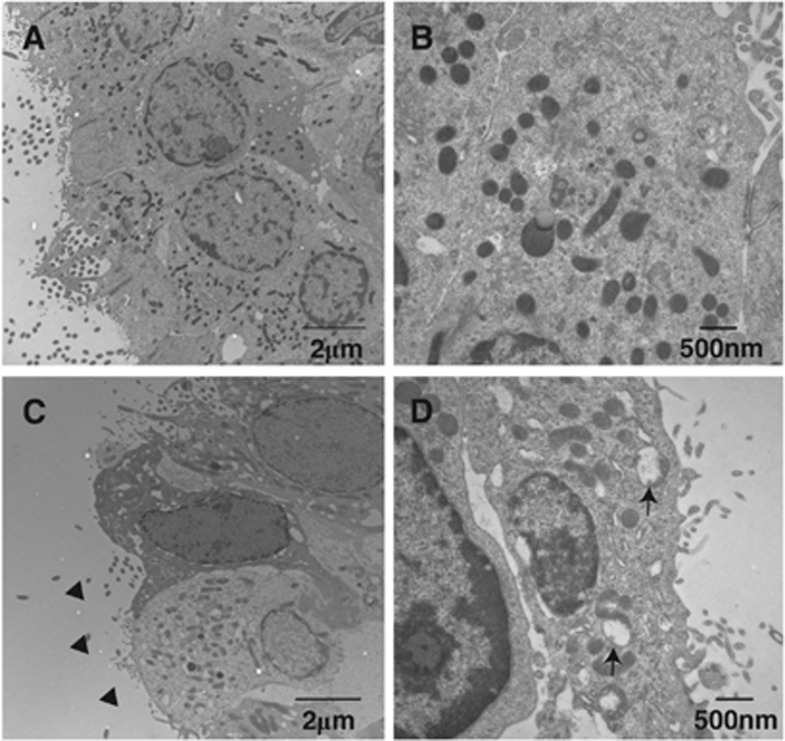
To evaluate the effect of iron on hydrocephalus development, rats received an injection of either 50 μL iron (Fe3+) or saline into the right lateral ventricle. Twenty-four hours after injection, T2 MRI showed a significant bilateral ventricular enlargement in the iron-injected group (17.9±5.7 mm3) compared with saline-injected rats (9.2±5.7 mm3, P<0.01, Figure 4). Electron microscopy shows damage to the ependymal cells with mitochondrial swelling, loss of cilia, and nuclear karyorrhexis in the iron-injected animals compared with control (Figure 5).
Figure 4.
(A) T2-weighted magnetic resonance imagings (MRIs) showing ventricular enlargement 24 hours after intraventricular iron but not saline injection. (B) Quantification of lateral ventricular volume. Values are expressed as the means±s.d., *P<0.05 vs. saline group by Student's t-test.
Figure 5.
Electron micrographs showing the ultrastructure of ependymal cells of the ipsilateral right ventricle at 24 hours after 50 μL FeCl3 injection (C, D) or control (A, B). Note mitochondrial swelling (arrows), nuclear karyorrhexis, and cilia loss (arrowheads) after iron injection.
Deferoxamine Attenuates Lysed Red Blood Cell-Induced Ventricular Enlargement
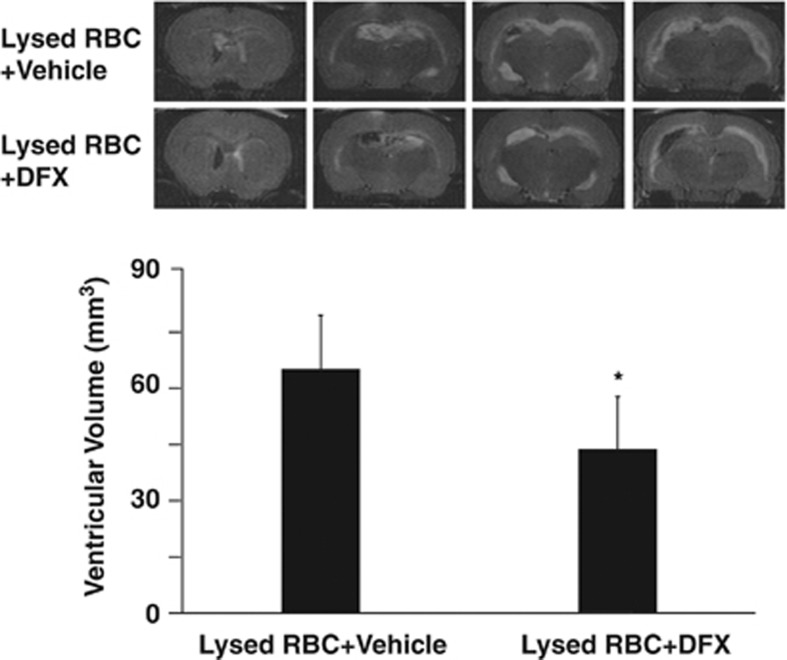
Deferoxamine, an iron chelator was used to evaluate the role of iron in ventricular enlargement after intraventricular injection of lysed RBCs. Coinjection of deferoxamine with lysed RBC reduced ventricular enlargement (47.8±17.8 vs. 59.9±15.7 mm3 in saline coinjection group, P<0.05, Figure 6). There was a tendency that lysed RBC-induced brain edema was less in deferoxamine-treated group (brain water content in the ipsilateral hemisphere: 79.8±0.6% vs. 80.5±0.7% in the saline coinjection group, n=6, P>0.05).
Figure 6.
T2-weighted magnetic resonance imaging (MRI) showing lateral ventricle volumes 24 hours after intraventricular injected of lysed red blood cells (RBCs) mixed with DFX or saline. Values are means±s.d., n=8 to 9, *P<0.05 vs. saline-treated group by Student's t-test.
Discussion
The major findings of this study are (1) intraventricular injection of lysed RBCs but not packed RBCs resulted in hydrocephalus; (2) lysed RBCs upregulated brain HO-1 and ferritin levels; (3) intraventricular injection of iron also caused hydrocephalus; and (4) iron chelation with deferoxamine reduced lysed RBC-induced hydrocephalus. Our previous studies have shown that IVH resulted in prolonged hydrocephalus.10, 16 In this proof-of-concept study, hydrocephalus was determined at 24 hours to avoid brain tissue loss-related ventricle enlargement.
Blood components, including thrombin and iron, have a major role in brain injury after ICH.17, 18 Our most recent study has shown that thrombin and thrombin receptor are involved in IVH-induced hydrocephalus.4 The current study found that RBC lysis and iron toxicity can cause hydrocephalus. These results suggest a major role of blood components in hydrocephalus development after IVH. Indeed, it is possible that the effects of iron and thrombin may combine to exacerbate IVH-induced injury, as found in ICH.19 In this proof-of-concept study, deferoxamine was given by intraventricular injection. Our previous study has shown that systemic use of deferoxamine can reduce IVH-induced hydrocephalus.10 Future experiments should determine therapeutic time window of systemic deferoxamine treatment and whether combination therapy is needed for IVH-induced hydrocephalus.
Iron levels in plasma are low, however, iron concentrations are very high in RBCs.20 When RBCs start to lyse, hemoglobin degradation can result in iron release. The perihematomal iron concentration could reach as high as 10 mmol/L, which causes marked brain damage.21 The form of the iron within heme depends on whether there is oxyhemoglobin (Fe2+) or methemoglobin (Fe3+). In initial experiments, we examined the effect of intraventricular Fe2+ and Fe3+, both caused brain injury. The current study focused on Fe3+. High levels of methemoglobin have been reported in CSF after IVH.22, 23 We will continue to pursue the potential role of Fe2+ in IVH-induced brain injury in other studies, but this is hampered by a lack of specific chelators available for ferrous iron.
There is increasing evidence that iron is involved in brain injury after cerebral hemorrhage.17, 18 It is hypothesized that lysis of RBCs within the hemorrhage cause release of hemoglobin, which is metabolized by heme oxygenases resulting in the release of iron. In a rat IVH model, we have found that iron deposition after IVH is present in the first day and upregulation of brain ferritin lasts for several weeks.10 It is well known that brain iron overload can stimulate free radical production and cause oxidative injury, including lipid peroxidation and DNA damage.18, 24, 25 The current study shows that injection of lysed RBCs, but not packed RBCs, can mimic the acute ventricular enlargement induced by IVH.10
Red blood cell lysate contains hemoglobin and its components iron and heme, and although multiple factors could have a role in ventricular enlargement, we show that coinjection with an iron chelator, deferoxamine, reduces ventricular enlargement induced by lysed RBCs. In addition injection of FeCl3 also results in ventricular enlargement further supporting the role of iron in posthemorrhagic hydrocephalus. This pinpoints the importance of iron in hydrocephalus after RBC lysis. It should be noted that two proteins that have crucial roles in iron regulation, HO-1 and ferritin, were markedly increased in the PVZ after injection of lysed RBCs. Heme oxygenase-1 is involved in heme metabolism and the release of iron and ferritin is the major endogenous cellular iron chelator.25 An upregulation of ferritin after injection of lysed RBCs or IVH10, 26 may limit iron-induced brain injury. Indeed, upregulation in the ependyma may limit the penetration of iron into the rest of the brain. Whether HO-1 may be a therapeutic target in IVH, by limiting the rate of iron release, has not been studied. There is, though, evidence in ICH that HO inhibitors reduce injury.27
Iron chelation may have multiple effects on IVH-induced brain injury. Our finding that iron can mimic the effect of IVH on ventricular dilation indicates a direct effect of iron (and iron chelation) on the brain in the absence of RBCs. However, we have found that iron chelation alters the rate of hematoma resolution28 and there are other components of RBCs that may contribute to brain injury (e.g., carbonic anhydrase,29).
Acute hydrocephalus is mostly considered as being the result of reduced CSF absorption. For example, in a prior study from our group on a rat IVH model, hydrocephalus was associated with a threefold increase in CSF absorption resistance suggesting a physical block in the CSF drainage pathways.16 However, in the current study, injection of both lysed RBCs and FeCl3 induced acute hydrocephalus. Neither is likely to cause an acute physical blockage of the CSF drainage pathways. Another possibility is the iron-related hydrocephalus may result from ependymal cell injury. Ependymal cells overlie the cerebral ventricles and their apical surface is covered by cilia that beat in a coordinated manner to facilitate CSF microcirculation. There is an increasing body of evidence that genetic mutants that affect ependymal cilia structure or function also cause hydrocephalus.30, 31, 32 Our results showed that iron can cause ependymal cell death and loss of cilia suggesting the possibility that ependymal cell injury may result in defective CSF dynamics and aggravate hydrocephalus. It is difficult to translate from studies on genetic mutants to acute injury and more studies are needed on the relationship between acute ependymal changes and hydrocephalus.
In conclusion, this study provides evidence that products produced by erythrocyte lysis and, particularly, iron can induce hydrocephalus and may be a therapeutic target for IVH. The effects of erythrocyte lysate and iron in inducing hydrocephalus may be related to ependymal damage.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants NS-057539, NS-073959, NS079157, NS084049, and NS-007222 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the stich trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–1538. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D, Macdonald R, Huo D, Goldenberg F, Novakovic R, Frank J, et al. Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg. 2007;107:261–265. doi: 10.3171/JNS-07/08/0261. [DOI] [PubMed] [Google Scholar]
- Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34:489–494. doi: 10.1038/jcbfm.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res. 2001;49:208–212. doi: 10.1203/00006450-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Muramatsu M, Tanaka K, Fujiwara H, Kojima T, Taki W. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol. 2006;253:1170–1176. doi: 10.1007/s00415-006-0184-1. [DOI] [PubMed] [Google Scholar]
- Rypens F, Avni EF, Dussaussois L, David P, Vermeylen D, Van Bogaert P, et al. Hyperechoic thickened ependyma: sonographic demonstration and significance in neonates. Pediatr Radiol. 1994;24:550–553. doi: 10.1007/BF02012729. [DOI] [PubMed] [Google Scholar]
- Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999;91:80–84. doi: 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- Fukumizu M, Takashima S, Becker LE. Glial reaction in periventricular areas of the brainstem in fetal and neonatal posthemorrhagic hydrocephalus and congenital hydrocephalus. Brain Dev. 1996;18:40–45. doi: 10.1016/0387-7604(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44:547–550. doi: 10.1161/STROKEAHA.112.662312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–1863. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Xi G, Jin H, He Y, Keep RF, Hua Y. Thrombin-induced autophagy: a potential role in intracerebral hemorrhage. Brain Res. 2011;1424:60–66. doi: 10.1016/j.brainres.2011.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Karabiyikoglu M, Hua Y, Keep RF, Xi G. Ischemic preconditioning attenuates brain edema after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3:180–187. doi: 10.1007/s12975-012-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir Suppl. 2006;96:207–211. doi: 10.1007/3-211-30714-1_45. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Park JW, Hua Y, Hoff JT, Keep RF. Holo-transferrin and thrombin can interact to cause brain damage. Stroke. 2005;36:348–352. doi: 10.1161/01.STR.0000153044.60858.1b. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127:2030–2038. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Gram M, Sveinsdottir S, Ruscher K, Hansson SR, Cinthio M, Akerstrom B, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J Neuroinflammation. 2013;10:100. doi: 10.1186/1742-2094-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. Methemoglobin–it's not just blue: a concise review. Am J Hematol. 2007;82:134–144. doi: 10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Gong Y, Tian H, Xi G, Keep R, Hoff J, Hua Y. Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochirur Suppl. 2006;96:232–236. doi: 10.1007/3-211-30714-1_50. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2013;4:546–553. doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Hua Y, Wang J, Keep RF, Xi G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res. 2012;3:130–137. doi: 10.1007/s12975-011-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, et al. A deficiency in rfx3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci. 2006;24:1020–1030. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavino C, Richard S. Patched1 haploinsufficiency impairs ependymal cilia function of the quaking viable mice, leading to fatal hydrocephalus. Mol Cell Neurosci. 2011;47:100–107. doi: 10.1016/j.mcn.2011.03.004. [DOI] [PubMed] [Google Scholar]