Abstract
In this contribution to the series of reflective essays celebrating the 25th anniversary of The FASEB Journal, our task is to assess the growth of research on the biology of aging during this period and to suggest where we might be heading during the next 25 yr. A review of the literature suggests a healthy acceleration of progress during the past decade, perhaps largely due to progress on the genetics of longevity of model organisms. Progress on the genetics of health span in these model organisms has lagged, however. Research on the genetic basis of the remarkable interspecific variations in life span has only recently begun to be seriously addressed. The spectacular advances in genomics should greatly accelerate progress. Research on environmental effects on life span and health span needs to be accelerated. Stochastic variations in gene expression in aging have only recently been addressed. These can lead to random departures from homeostasis during aging.—Martin, G. M. The biology of aging: 1985–2010 and beyond.
Keywords: dietary restriction, evolutionary biology, epigenetic drift, geriatric pathology, health span, life span
Given current demographic trends and the associated health care costs of our aging societies (1), it is hard to imagine a more compelling area of biomedical research than basic research on the biology and pathobiology of aging. Intrinsic biological aging is the major risk factor for virtually all of the major diseases of the developed societies—including Alzheimer's disease, Parkinson's disease, Lewy body dementia, frontotempral dementias, strokes, peripheral neuropathies, age-related macular degeneration, ocular cataracts, presbycusis, type 2 diabetes mellitus, osteoporosis, osteoarthritis, sarcopenia, all forms of arteriosclerosis, benign prostatic hyperplasia, and most types of cancer. As we celebrate the 25th anniversary of The FASEB Journal, we should assess what those years have taught us about the what, why, and how of biological aging—especially the how question, as one requires detailed information on mechanisms in order to invent rational interventions. We should also make some educated guesses as to where to put our money to best use over the next 25 yr. These are formidable tasks, especially given the recent funding crisis at the National Institute on Aging (2). Because of space constraints, one cannot possibly provide a comprehensive analysis. I therefore apologize in advance to my many colleagues whose important research I could not review in these pages.
A SAMPLING OF THE LITERATURE, 1985–2010
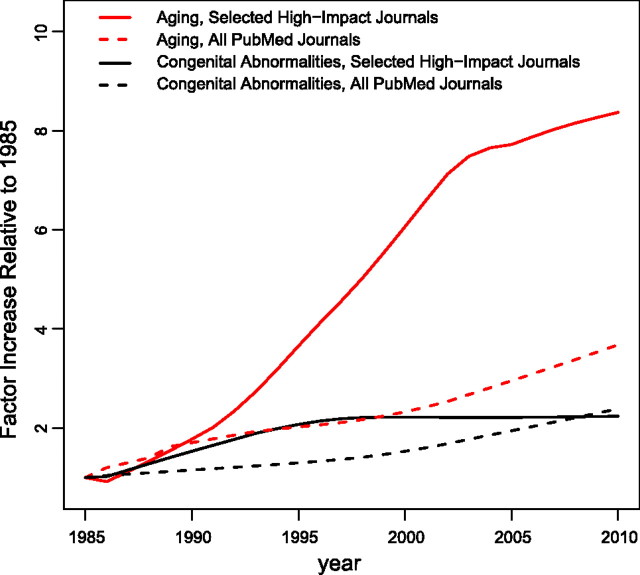
Figure 1 summarizes the results of an August 16, 2011 search of PubMed for the major topic medical subject headings (MeSH) for the term “aging” and 5 major generalist, high-impact journals (Cell, FASEB Journal, Nature, Proceedings of the National Academy of Sciences of the USA, and Science) for the years 1985–2010. This was a very small sample indeed of all references on this subject to all journals indexed in PubMed during that period. The trends over time proved to be informative, however, as they are consistent with my prospective hypothesis—namely, that our field of research did not significantly attract the attention of these leading generalist journals until recent years. What happened to get their attention? One important development was a surge of research on the genetic modulation of longevity in tractable model organisms (Caenorhabditis elegans, Drosophila melanogaster, Mus musculus domesticus), fueled, in part, by the vision of the late Joshua Lederberg and the generosity of Mr. Larry Ellison, who organized the Ellison Medical Foundation some 15 yr ago to fund basic research in aging, much of it with a genetic theme (http://www.ellisonfoundation.org/index.jsp).
Figure 1.
Factor increase (normalized relative to 1985) of PubMed publications with MeSH search word “aging” in either selected high-impact journals (Science, Nature, Cell, Proceedings of the National Academy of Sciences of the USA, and FASEB Journal; solid red line) or in all PubMed journals (dashed red line). For comparison, the factor increase in PubMed publications (relative to 1985) is shown for the MeSH search words “congenital abnormalities” (CA) either in selected high-impact journals (above; solid black line) or in all PubMed journals (black dotted line). The rate of increase for aging publications in the selected journals is greater than that for aging publications among all PubMed journals by a highly significant factor of 3.3 (P<1E-10). The rate of increase of aging publications among the selected journals is significantly different from that for CA publications in these journals (P=2E-10). After 1997, the rate of increase of CA publications in these selected journals is not significantly different from 0 (P=0.27). The rate of increase in citations for “aging” among all PubMed journals is not significantly different compared to the rate of increase of citations for CA in all PubMed journals (P=0.07). Methods: comparison of rates of change in publication rates over time was done with simple linear regression and a linear spline with one change point at 1996. The lowess smoother was used to produce smooth functions using the R statistical package (http://www.r-project.org).
Figure 1 also includes what might be considered as a control: a comparable PubMed search using the Major Topic MeSH subject of “congenital abnormalities.” A statistical comparison of the slopes of the two curves suggests that our field of biological aging has been developing more rapidly.
THE DISCOVERY OF THE FIRST PUBLIC MODULATION OF LONGEVITY
In the early 1980s, Tom Johnson set out to disprove conclusions made by his colleague Michael Klass. The Klass experiments suggested that a single gene mutation could extend the life span of an organism (in this case, C. elegans). To his surprise, Johnson and his undergraduate student (D. B. Freidman) managed to map the first “longevity gene,” age-1 (3). The Ruvkun laboratory cloned this gene in 1996; it coded a phosphatidylinositol-3-OH kinase (4). The Kenyon laboratory had previously cloned the receptor locus for this pathway (5). The laboratories of Cynthia Kenyon, Gary Ruvkun, Pam Larson, Adam Antebi, Tom Johnson and others elaborated on these gene actions, most notably regarding a downstream transcription factor, daf16. Remarkably, long-lived mutants in homologous signaling pathways (IGF-1/insulin signaling) were found in D. melanogaster (6, 7) and in dwarf mice (reviewed in ref. 8). More recently, variants in this pathway have been associated with unusual longevity in Homo sapiens. A particularly compelling observation has been the association with variants at a locus (FOXO3a) that codes for a transcription factor comparable to daf16, as there have been several confirmations (reviewed in ref. 9). Thus was established the first biochemical genetic pathway capable of modulating the life spans of diverse organisms.
REPLICATIVE SENESCENCE AND THE BIOLOGY OF TELEMORES: AGING RESEARCH WINS ITS FIRST NOBEL PRIZE!
The pioneering research of Leonard Hayflick and Paul Moorhead on the limited replicative life spans of human somatic cells (10) was revitalized by the demonstration, by Woody Wright, Jerry Shay, and colleagues, that such cells could be essentially “immortalized” via transfected telomerase (11). Interest in this area also was enhanced by Judy Campisi and colleagues, who developed both theoretical and experimental lines of evidence consistent with the view that the limited life span of human somatic cells had tumor suppressor functions, for which a price had to be paid, however, during aging. The accumulated postreplicative senescent cells were shown to secrete large amounts of cytokines, mitogens, and enzymes capable of altering extracellular matrix, thus, paradoxically, enhancing the development of neoplasia (reviewed in ref. 12). There has been increasing evidence that senescent cells accumulate in vivo, including assays of tissues in aging nonhuman primates (13).
Elizabeth Blackburn, Carol Greider, and Jack Szostak, the 2009 Nobel laureates, were quite aware of the implications of their research on telomerase for the biology of aging (14). Carol Greiger, in particular, had been quite active in this field. This is perhaps also a reason for the upturn in interest in the field that many of us now refer to as biogerontology.
STEM CELLS AND REGENERATIVE MEDICINE: ON “WAKING UP” OUR AGING ENDOGENOUS STEM CELLS AND REPROGRAMMING OUR TERMINALLY DIFFERENTIATED CELLS
Regenerative medicine and stem cell centers have been sprouting around the country like weeds, in part because of the hope that progress in this area will expedite clinical translations, certainly to include geriatric patients with diverse degenerative disorders. A particularly promising line of research emerged from the laboratory of Tom Rando at Stanford University (Stanford, CA, USA), an institution that has taken a major leadership role in this field. The Rando team used the old technique of parabiosis to demonstrate that factors derived from the circulating blood of a young mouse to its older partner could substantially ameliorate or bypass a deficient response to injury of skeletal muscle, a response that is mediated by satellite cells, the adult stem cells of skeletal muscle (15). There is, therefore, the potential for the development of small molecular weight compounds that, when injected, would be capable of waking up particular families of endogenous stem cells. Equally exciting is more recent work from the laboratory of Helen Blau, also of Stanford University. These investigators have shown that siRNA knockdowns of just two loci (Rb and an alternative reading frame of Ink4a) was sufficient to induce the dedifferentiation of postreplicative skeletal muscle myocytes to form mitotically active myoblasts capable of once again making multinucleated skeletal muscle (16).
While the potential clinical applications to regenerative medicine are enormous, of equal importance is the potential to unravel the pathogenesis of a wide range of genetically modulated degenerative geriatric disorders using induced pleuripotent stem cells from individual donors. In the short 4 yr since the Yamanaka laboratory opened up this field of research (17), there have been numerous methodological enhancements, a recent example being a very exciting innovation from the laboratory of Derrick Rossi (Children's Hospital, Boston, MA, USA) involving the use of engineered RNA (18).
GOOD NEWS FROM RED WINE AND FROM EASTER ISLAND
Our red wine story begins with experiments with budding yeast, starting with research in the Massachusetts Institute of Technology (Cambridge, MA, USA) laboratory of Leonard Guarente (19–22). Sirtuins are a family of NAD+-dependent protein deacetylases. Their importance in the modulation of longevity was established with deletion and overexpression experiments in yeast and in C. elegans (23). Resveratrol (3,5,4′-trihydroxystilbene), a compound present in red wine, initially considered to activate a sirtuin, was shown to substantially ameliorate the fatty livers and life spans of obese, diabetic mice (24). It did not, however, enhance the longevity of mice in an exceptionally well designed study carried out by three independent laboratories using genetically heterogeneous mice and two different doses of resveratrol (25). It remains to be seen, however, whether more potent related compounds will be shown to enhance the life spans and health spans of mammals. We shall also require more information on the mechanisms of action of such compounds, as recent work has failed to confirm that they activate SIRT1, a putative substrate (26).
An even more exciting line of research had its origins in Easter Island (Rapa Nui), where a species of Streptomycin in a soil sample led to the discovery of a macrolide, rapamycin, a drug commonly used as an immunosuppressant in connection with kidney transplantations. In 2006, rapamycin was shown to enhance the replicative life span of yeast cells via inhibition of the target of rapamycin (TOR) signal transduction pathway (27). In 2009, the group of three laboratories mentioned above for their investigation of resveratrol (and for a confirmatory study using rapamycin) initiated a study of the effects of rapamycin on the life span of mice. By the time this group could invent a suitable vehicle for oral administration of the agent, their mouse cohorts were already 20 mo old, equivalent to ∼60 yr old humans. Fortunately, they proceeded to administer the agent at those late ages and discovered a significant increase in maximum life span (28). Neoplasms, particularly lymphomas, are the major causes of death, even in these 4-way-cross mice. Given the evidence that rapamycin has antiprolferative properties (e.g., (29), it is conceivable that the life span extension was caused by a slowing of the rate of growth of neoplasms in these aging mice.
DIETARY RESTRICTION AND ENHANCED LONGEVITY: ON THE POSSIBLE ROLE OF SNIFFING ONE'S FOOD
Caloric restriction has been shown to enhance the life spans of an astonishing range of animal species, from yeast to mammals (30). Since dietary restriction of methionine has also been shown to enhance life span (31), it is prudent to refer to this phenomenon as dietary restriction rather than caloric restriction. Like virtually everything else in life, however, one has to consider the relationship of the response to the dose of the intervention. This has been well established in the case of fruit flies (32). The standard paradigm of dietary restriction for laboratory mice, however, has for many years been to decrease the amount of food provided to the experimental group to 40% of that eaten by control animals fed ad libitum. Only recently has a systematic study been initiated to determine how various murine genotypes would fare under such a regime. To the surprise of many investigators, a remarkable range of variable responses, from decreased to increased life spans, was observed among a series of recombinant inbred strains (33). Those genotypes with enhanced life spans were better at retaining adipose tissue (34). It will be of interest to discover which specific types of fat depots were involved. Some evolutionary biologists have argued that dietary restriction, apart from avoiding obesity, is unlikely to be an efficacious modality of life-span extension in human beings (35). While a complete life table of the results of dietary restriction in a primate species is not yet available, the most recent publication on this subject, involving Rhesus monkeys, does support both enhanced life spans and health spans when these animals are restricted by 30% (36). (The animals could not tolerate a 40% restriction of food.) While the published photograph of a lean, restricted animal did suggest a more youthful phenotype, the animal looked a lot meaner than the well-fed control.
Remarkably, research with D. melanogaster and C. elegans points to a major role for the perception of food, via olfactory clues, in the regulation of the response to dietary restriction (37–41). Our friend, the mTOR pathway, is among the biochemical genetic downstream sensors involved in these complex responses (40). Activation of a FOXO-daf16 transcription factor also appears to be involved (42).
THE EVOLUTIONARY BIOLOGICAL THEORY OF AGING: STILL THE BEST EXPLANATION FOR WHY WE AGE
There is widespread agreement among my colleagues that aging occurs because of the decline in the force of natural selection after the peak of reproduction. Excellent expositions of this idea can be found in books by Michael R. Rose (43) and Steven N. Austad (44). Two dominant mechanisms are widely supported. The first, attributed to J. B. S. Haldane and Peter Medawar, has been referred to as mutation accumulation. It should not be confused with somatic mutation, as it refers to inherited or constitutional mutations that do not reach some phenotypic level of expression until middle age or beyond, when the force of natural selection has become greatly attenuated. There is a very large potential catalog of such mutant alleles, some highly penetrant and others somewhat “leaky,” providing one general explanation for why clinicians see such striking variations for patterns of aging among genetically heterogeneous species such as Homo sapiens. The second general mechanism has been referred to by the late George C. Williams as antagonistic pleiotropy. Like most selected alleles, these are gene variants that enhance reproductive fitness early in the life course. Some such alleles, however, exhibit deleterious effects late in the life span. An oft-cited example, as noted above, would be classes of gene action responsible for replicative senescence. This is thought to provide protection against cancer. Paradoxically, however, accumulations of replicatively senescent cells later in the life course are thought to enhance carcinogenesis via the secretion of mitogens, proinflammatory cytokines and substances that alter the structure of stromal tissues [the senescent associated secretory phenotype (SASP); ref. 12]. The evolutionary biological theory of why we age also provides clues to how we age; for a brief review, see ref. 45. A detailed analysis of the numerous theories on how we age is beyond the scope of this essay, but in the next section, we shall briefly address one of the most venerable of these theories: the free radical theory of aging and its associated focus on mitochondrial structure and function.
HOW THE MITOCHONDRIAL FREE RADICAL THEORY OF AGING HAS FARED OVER THE YEARS
When our ancient eukaryotic ancestors decided to take in bacterial boarders, it seemed like a pretty good deal, as they were good at stoking the furnaces. Alas, they did not foresee the possibility that these creatures, which became our mitochondria, might also create some household problems. While the electron transport enzymatic machinery contributed by both the mitochondrial and nuclear genomes does an excellent job with the quadrivalent reduction of oxygen, there is a small degree of univalent reduction, leading to reactive oxygen species, the most dangerous member of which is the hydroxyl radical, created by the Fenton reaction. In 1956, Denham Harman suggested that such “free radicals” could be responsible for biological aging (46). Since then, numerous papers have provided evidence both for and against this theory. To make a very long story very short, the reader is directed to four recent independent critical reviews that seriously question the validity of this theory (47–50). Despite the best efforts of evolution to optimize the stability of mitochondrial proteins (51), mitochondrial abnormalities nonetheless appear to be playing important roles in a variety of neurodegenerative disorders of aging (52). The loss of protein homeostasis in general (proteostasis) appears to be a key feature of many neurodegenerative diseases of late life (53).
PROGRESS ON HUMAN PROGEROID SYNDROMES
The author's interest in the biology of aging began with a study of the Werner syndrome, an autosomal recessive disorder resulting in the prototypic human segmental progeroid syndrome (54). After many years of work collecting pedigrees from around the world, the gene was shown to code for a member of the RecQ family of helicases (55). The helicase and exonuuclease functions of the WRN protein participate in many aspects of DNA metabolism, including maintenance of telomere structure and homology-dependent recombination (56). The gene for the Hutchinson-Gilford progeria syndrome (an autosomal dominant mutation) was cloned in the laboratory of Francis Collins in 2003 (57). Virtually all cases are caused by a point mutation in the C-terminal domain of the LMNA gene that activates a cryptic splice donor site, resulting in an isoform with a 50-aa deletion known as progerin. Progerin retains a transient post-translational modification (farnesylation), as it lacks a recognition site for the cleavage event that removes this modification. As a result, progerin mislocalizes within the nucleus, resulting in nuclear distortions and alterations in gene expression (56). Of the many phenotypic features of the Hutchinson-Gilford progeria syndrome, atherosclerosis is by far the most clinically significant. Reports that progerin accumulates in human tissues during normative aging (reviewed in ref. 58) are therefore of great potential importance. Also of great interest is a recent report pointing to a collaboration of progerin and telomere dysfunction in the genesis of the replicative senescence of normal diploid human fibroblasts (58).
LOOKING AHEAD
The striking success of research on the genetic modulation of life spans in model organisms has not yet been matched by investigations of the maintenance of structure and function in various organs (i.e., health span). The laboratory of Monica Driscoll has pioneered such studies by demonstrating marked alterations in the skeletal muscles of C. elegans as they age (59), but much more work is needed in worms, flies, and even in mice, where such studies are more advanced. We also require much more sensitive and specific assays for a range of human physiological functions during aging; it is nowadays hard to find professional physiologists with such interests (60).
There is considerable enthusiasm among my colleagues for the potential power of comparative gerontology to elucidate mechanisms that have evolved to slow intrinsic rates of biological aging in exceptionally long lived species. For example, we now have the full genome sequences of our nearest relative, the chimpanzee, to compare with that of our own sequence. Are there secrets to be revealed from these genomes as to why we live about twice as long as chimps? Remarkably prescient research by Mary Claire King and the late Allan Wilson suggested, some 36 yr ago, that phenotypic differences between these two species are likely determined more by variations in the regulation of gene expression than by variations in the structure of individual proteins, which exhibited very small variations among these two species for the subset examined (61). The field of neurobiology has been the first to benefit from this line of research (62). Biogerontologists, however, are currently focusing on the newly available genome sequence of the naked mole rat. This rodent is about the same size as laboratory mouse, but lives ∼10 times as long (63). A large number of necropsies on old naked mole rats have been performed by Rochelle Buffenstein (University of Texas Health Science Center, San Antonio, TX, USA) and her colleagues, but not a single neoplasm has been observed. This is in stark contrast to the laboratory mouse, most of which die of cancers (predominately lymphomas). We look forward to the results of genomic studies using appropriate phylogenetic comparisons.
In comparison with research on mutagens, carcinogens, and teratogens, there is very little research on “gerontogens”—environmental agents with the potential to accelerate the ages of onset or rates of development of aging and associated components of the senescent phenotype (64). A cogent example is cigarette smoking, which influences a large number of aging phenotypes (65). Exposure to gerontogens might be particularly significant during prenatal and neonatal development.
Except for studies of somatic mutation (the frequency of which rises substantially during aging; ref. 66), investigations of the role of stochastic events in the determination of health span and life span have been neglected. This seems strange, given the fact that generations of researchers have been very much aware that, despite every effort to control the genotype and the environment of their experimental organisms, every life-span experiment reveals marked differences in the life spans of individual organisms. The best available model for such studies is C. elegans. As a hermaphrodite, every diploid locus is driven to homozygosity. It can also be grown in axenic medium with excellent control of environmental parameters. Yet, even in long-lived mutants, there are individuals with shorter life spans than those of wild type parentals (67). Strong evidence that these variations are stochastic comes from experiments from the laboratory of Thomas Johnson (68).
Epigenetic drifts in gene expression have been shown to be among the reasons identical human twins differ as they age (69). Increased variegations of gene expression with age among individual cardiomyocytes have also been established in the aging mouse heart (70). Are such epigenetic variegations mainly due to environmental influences? Do they results from thermodynamic noise? Are the degrees of variegation under genetic control, and, if so, are the rates of variegation and drift related to different degrees of biological aging (71)? If the latter explanation is correct, a deeper knowledge of its mechanisms might lead to interventions that could moderate the degree of drift and, perhaps, associated alterations in physiological homeostasis. Such departures from homeostasis are at the heart of what we define as biological aging.
Acknowledgments
The author thanks Mr. Brian Park for PubMed searches and Dr. Mary Emond (Department of Biostatistics, University of Washington, Seattle, WA, USA) for statistical analysis of the data of Fig. 1. Supported in part by NIH grants R24CA078088, P01AG001751 and R01AG019711.
REFERENCES
- 1.Olshansky S. J., Goldman D. P., Zheng Y., Rowe J. W. (2009) Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. , 842–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadman M. (2010) Funding crisis hits US ageing research. Nature , 148. [DOI] [PubMed] [Google Scholar]
- 3.Friedman D. B., Johnson T. E. (1988) A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics , 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris J. Z., Tissenbaum H. A., Ruvkun G. (1996) A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature , 536–539 [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. (1993) A C-elegans mutant that lives twice as long as wild-type. Nature , 461–464 [DOI] [PubMed] [Google Scholar]
- 6.Clancy D. J., Gems D., Harshman L. G., Oldham S., Stocker H., Hafen E., Leevers S. J., Partridge L. (2001) Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science , 104–106 [DOI] [PubMed] [Google Scholar]
- 7.Tatar M., Kopelman A., Epstein D., Tu M. P., Yin C. M., Garofalo R. S. (2001) A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science , 107–110 [DOI] [PubMed] [Google Scholar]
- 8.Bartke A., Brown-Borg H. (2004) Life extension in the dwarf mouse. Curr. Top. Dev. Biol. , 189–225 [DOI] [PubMed] [Google Scholar]
- 9.Ziv E., Hu D. (2011) Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res. Rev. , 201–204 [DOI] [PubMed] [Google Scholar]
- 10.Hayflick L., Moorhead P. S. (1961) The serial cultivation of human diploid cell strains. Exp. Cell Res. , 585–621 [DOI] [PubMed] [Google Scholar]
- 11.Bodnar A. G., Ouellette M., Frolkis M., Holt S. E., Chiu C. P., Morin G. B., Harley C. B., Shay J. W., Lichtsteiner S., Wright W. E. (1998) Extension of life-span by introduction of telomerase into normal human cells. Science , 349–352 [DOI] [PubMed] [Google Scholar]
- 12.Coppe J. P., Desprez P. Y., Krtolica A., Campisi J. (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. , 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbig U., Ferreira M., Condel L., Carey D., Sedivy J. M. (2006) Cellular senescence in aging primates. Science , 1257. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn E. H., Greider C. W., Szostak J. W. (2006) Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. , 1133–1138 [DOI] [PubMed] [Google Scholar]
- 15.Conboy I. M., Conboy M. J., Wagers A. J., Girma E. R., Weissman I. L., Rando T. A. (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature , 760–764 [DOI] [PubMed] [Google Scholar]
- 16.Pajcini K. V., Corbel S. Y., Sage J., Pomerantz J. H., Blau H. M. (2010) Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell , 198–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell , 663–676 [DOI] [PubMed] [Google Scholar]
- 18.Warren L., Manos P. D., Ahfeldt T., Loh Y. H., Li H., Lau F., Ebina W., Mandal P. K., Smith Z. D., Meissner A., Daley G. Q., Brack A. S., Collins J. J., Cowan C., Schlaeger T. M., Rossi D. J. (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell , 618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy B. K., Austriaco N. R., Zhang J. S., Guarente L. (1995) Mutation in the silencing gene Sir4 can delay aging in Saccharomyces-cerevisiae. Cell , 485–496 [DOI] [PubMed] [Google Scholar]
- 20.Kennedy B. K., Gotta M., Sinclair D. A., Mills K., McNabb D. S., Murthy M., Pak S. M., Laroche T., Gasser S. M., Guarente L. (1997) Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S-cerevisiae. Cell , 381–391 [DOI] [PubMed] [Google Scholar]
- 21.Kaeberlein M., McVey M., Guarente L. (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. , 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature , 795–800 [DOI] [PubMed] [Google Scholar]
- 23.Tissenbaum H. A., Guarente L. (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature , 227–230 [DOI] [PubMed] [Google Scholar]
- 24.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature , 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller R. A., Harrison D. E., Astle C. M., Baur J. A., Boyd A. R., de Cabo R., Fernandez E., Flurkey K., Javors M. A., Nelson J. F., Orihuela C. J., Pletcher S., Sharp Z. D., Sinclair D., Starnes J. W., Wilkinson J. E., Nadon N. L., Strong R. (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Med. Sci. , 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacholec M., Bleasdale J. E., Chrunyk B., Cunningham D., Flynn D., Garofalo R. S., Griffith D., Griffor M., Loulakis P., Pabst B., Qiu X., Stockman B., Thanabal V., Varghese A., Ward J., Withka J., Ahn K. (2010) SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. , 8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers R. W., Kaeberlein M., Caldwell S. D., Kennedy B. K., Fields S. (2006) Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. , 174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature , 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao R. D., Buckner J. C., Sarkaria J. N. (2004) Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Curr. Cancer Drug. Targets. , 621–635 [DOI] [PubMed] [Google Scholar]
- 30.Everitt A. V., Rattan S. I. S., Le Couteur D. G., de Cabo R. eds (2010) Calorie restriction, aging and longevity, Springer, New York [Google Scholar]
- 31.Miller R. A., Buehner G., Chang Y., Harper J. M., Sigler R., Smith-Wheelock M. (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell , 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partridge L., Piper M. D., Mair W. (2005) Dietary restriction in Drosophila. Mech. Ageing Dev. , 938–950 [DOI] [PubMed] [Google Scholar]
- 33.Liao C. Y., Rikke B. A., Johnson T. E., Diaz V., Nelson J. F. (2010) Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell , 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson J. F., Liao C. Y., Rikke B. A., Johnson T. E., Gelfond J. A. L., Diaz V. (2011) Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell , 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bourg E. (2010) Predicting whether dietary restriction would increase longevity in species not tested so far. Ageing Res. Rev. , 289–297 [DOI] [PubMed] [Google Scholar]
- 36.Colman R. J., Anderson R. M., Johnson S. C., Kastman E. K., Kosmatka K. J., Beasley T. M., Allison D. B., Cruzen C., Simmons H. A., Kemnitz J. W., Weindruch R. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science , 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apfeld J., Kenyon C. (1999) Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature , 804–809 [DOI] [PubMed] [Google Scholar]
- 38.Bishop N. A., Guarente L. (2007) Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature , 545–549 [DOI] [PubMed] [Google Scholar]
- 39.Libert S., Zwiener J., Chu X., Vanvoorhies W., Roman G., Pletcher S. D. (2007) Regulation of Drosophila life span by olfaction and food-derived odors. Science , 1133–1137 [DOI] [PubMed] [Google Scholar]
- 40.Poon P. C., Kuo T. H., Linford N. J., Roman G., Pletcher S. D. (2010) Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. , e1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith E. D., Kaeberlein T. L., Lydum B. T., Sager J., Welton K. L., Kennedy B. K., Kaeberlein M. (2008) Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC. Dev. Biol. , 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greer E. L., Dowlatshahi D., Banko M. R., Villen J., Hoang K., Blanchard D., Gygi S. P., Brunet A. (2007) An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. , 1646–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose M. R. (1991) Evolutionary biology of aging, Oxford University Press, New York [Google Scholar]
- 44.Austad S. N. (1997) Why we age : what science is discovering about the body's journey through life, John Wiley & Sons, New York [Google Scholar]
- 45.Martin G. M. (2007) Modalities of gene action predicted by the classical evolutionary biological theory of aging. Ann. N. Y. Acad. Sci. , 14–20 [DOI] [PubMed] [Google Scholar]
- 46.Harman D. (1956) Aging: a theory based on free radical and radiation chemistry. J. Gerontol. , 298–300 [DOI] [PubMed] [Google Scholar]
- 47.Alexeyev M. F. (2009) Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. , 5768–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hekimi S., Lapointe J. (2010) When a theory of aging ages badly. Cell. Mol. Life Sci. , 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez V. I., Bokov A., Van Remmen H., Mele J., Ran Q. T., Ikeno Y., Richardson A. (2009) Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta , 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ristow M., Schmeisser S. (2011) Extending life span by increasing oxidative stress. Free Radic. Biol. Med. , 327–336 [DOI] [PubMed] [Google Scholar]
- 51.Kitazoe Y., Kishino H., Hasegawa M., Matsui A., Lane N., Tanaka M. (2011) Stability of mitochondrial membrane proteins in terrestrial vertebrates predicts aerobic capacity and longevity. Genome Biol Evol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Przedborski S., Schon E. A. (2011) Mitochondria: the next (neurode) generation. Neuron , 1033–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglas P. M., Dillin A. (2010) Protein homeostasis and aging in neurodegeneration. J. Cell Biol. , 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epstein C. J., Martin G. M., Schultz A. L., Motulsky A. G. (1966) Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) , 177–221 [DOI] [PubMed] [Google Scholar]
- 55.Yu C. E., Oshima J., Fu Y. H., Wijsman E. M., Hisama F., Alisch R., Matthews S., Nakura J., Miki T., Ouais S., Martin G. M., Mulligan J., Schellenberg G. D. (1996) Positional cloning of the Werner's syndrome gene. Science , 258–262 [DOI] [PubMed] [Google Scholar]
- 56.Kudlow B. A., Kennedy B. K., Monnat R. J. (2007) Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat. Rev. Mol. Cell. Biol. , 394–404 [DOI] [PubMed] [Google Scholar]
- 57.Eriksson M., Brown W. T., Gordon L. B., Glynn M. W., Singer J., Scott L., Erdos M. R., Robbins C. M., Moses T. Y., Berglund P., Dutra A., Pak E., Durkin S., Csoka A. B., Boehnke M., Glover T. W., Collins F. S. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature , 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao K., Blair C. D., Faddah D. A., Kieckhaefer J. E., Olive M., Erdos M. R., Nabel E. G., Collins F. S. (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. , 2833–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herndon L. A., Schmeissner P. J., Dudaronek J. M., Brown P. A., Listner K. M., Sakano Y., Paupard M. C., Hall D. H., Driscoll M. (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature , 808–814 [DOI] [PubMed] [Google Scholar]
- 60.Martin G. M. (2002) Help wanted: physiologists for research on aging. Sci. Aging Knowledge Environ. , vp2. [DOI] [PubMed] [Google Scholar]
- 61.King M. C., Wilson A. C. (1975) Evolution at 2 levels in humans and chimpanzees. Science , 107–116 [DOI] [PubMed] [Google Scholar]
- 62.Haussler D., Pollard K. S., Salama S. R., Lambert N., Lambot M. A., Coppens S., Pedersen J. S., Katzman S., King B., Onodera C., Siepel A., Kern A. D., Dehay C., Igel H., Ares M., Vanderhaeghen P. (2006) An RNA gene expressed during cortical development evolved rapidly in humans. Nature , 167–172 [DOI] [PubMed] [Google Scholar]
- 63.Buffenstein R., Edrey Y. H., Hanes M., Pinto M., Mele J. (2011) Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. , 41–53 [DOI] [PubMed] [Google Scholar]
- 64.Martin G. M. (1987) Interactions of aging and environmental agents: the gerontological perspective. Prog. Clin. Biol. Res. , 25–80 [PubMed] [Google Scholar]
- 65.Bernhard D., Moser C., Backovic A., Wick G. (2007) Cigarette smoke–an aging accelerator? Exp. Gerontol. , 160–165 [DOI] [PubMed] [Google Scholar]
- 66.Martin G. M., Ogburn C. E., Colgin L. M., Gown A. M., Edland S. D., Monnat R. J. (1996) Somatic mutations are frequent and increase with age in human kidney epithelial cells. Hum. Mol. Genet. , 215–221 [DOI] [PubMed] [Google Scholar]
- 67.Kirkwood T. B., Feder M., Finch C. E., Franceschi C., Globerson A., Klingenberg C. P., LaMarco K., Omholt S., Westendorp R. G. (2005) What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech. Ageing Dev. , 439–443 [DOI] [PubMed] [Google Scholar]
- 68.Rea S. L., Wu D., Cypser J. R., Vaupel J. W., Johnson T. E. (2005) A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. , 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fraga M. F., Ballestar E., Paz M. F., Ropero S., Setien F., Ballestar M. L., Heine-Suner D., Cigudosa J. C., Urioste M., Benitez J., Boix-Chornet M., Sanchez-Aguilera A., Ling C., Carlsson E., Poulsen P., Vaag A., Stephan Z., Spector T. D., Wu Y. Z., Plass C., Esteller M. (2005) Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U. S. A. , 10604–10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bahar R., Hartmann C. H., Rodriguez K. A., Denny A. D., Busuttil R. A., Dolle M. E., Calder R. B., Chisholm G. B., Pollock B. H., Klein C. A., Vijg J. (2006) Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature , 1011–1014 [DOI] [PubMed] [Google Scholar]
- 71.Martin G. M. (2009) Epigenetic gambling and epigenetic drift as an antagonistic pleiotropic mechanism of aging. Aging Cell , 761–764 [DOI] [PubMed] [Google Scholar]